Published online Oct 15, 2024. doi: 10.4251/wjgo.v16.i10.4092
Revised: August 26, 2024
Accepted: September 6, 2024
Published online: October 15, 2024
Processing time: 58 Days and 9.6 Hours
Intrahepatic cholangiocarcinoma (ICC) is a malignant liver tumor that is cha
To establish 1-year and 3-year prognostic models for ICC after surgical resection.
Immunohistochemical staining was performed for CD4, CD8, CD20, pan-cytokeratin (CK), and CD68 in tumors and paired adjacent tissues from 141 patients with ICC who underwent curative surgery. Selection of variables was based on regression diagnostic procedures and goodness-of-fit tests (PH assumption). Clinical parameters and pathological diagnoses, combined with the distribution of immune cells in tumors and paired adjacent tissues, were utilized to establish 1- and 3-year prognostic models.
This is an important application of immune cells in the tumor microenvironment. CD4, CD8, CD20, and CK were included in the establishment of our prognostic model by stepwise selection, whereas CD68 was not significantly associated with the prognosis of ICC. By integrating clinical data associated with ICC, distinct prognostic models were derived for 1- and 3-year survival outcomes using variable selection. The 1-year prediction model yielded a C-index of 0.76 95% confidence interval (95%CI): 0.65-0.87 and the 3-year prediction model produced a C-index of 0.69 (95%CI: 0.65-0.73). Internal validation yielded a C-index of 0.761 (95%CI: 0.669-0.853) for the 1-year model and 0.693 (95%CI: 0.642-0.744) for the 3-year model.
We developed Cox regression models for 1-year and 3-year survival predictions of patients with ICC who underwent resection, which has positive implications for establishing a more comprehensive prognostic model for ICC based on tumor immune microenvironment and immune cell changes in the future.
Core Tip: We present a comprehensive study investigating the prognostic significance of immune cell infiltration and clinical parameters in patients with surgically resected intrahepatic cholangiocarcinoma (ICC). Our research elucidates the intricate interplay between immune cell composition within the tumor microenvironment and clinical outcomes in patients with ICC, offering valuable insights into the development of prognostic predictors and potential therapeutic strategies for this cha
- Citation: Wang ZR, Zhang CZ, Ding Z, Li YZ, Yin JH, Li N. Establishing prognostic models for intrahepatic cholangiocarcinoma based on immune cells. World J Gastrointest Oncol 2024; 16(10): 4092-4103
- URL: https://www.wjgnet.com/1948-5204/full/v16/i10/4092.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v16.i10.4092
Intrahepatic cholangiocarcinoma (ICC), the second most prevalent malignant liver cancer, continues to have an increasing incidence[1]. Despite the expanding array of therapeutic options available for ICC, surgical resection remains the main treatment. However, most cases of ICC are diagnosed at advanced stages, meaning surgical resection is effective in only approximately 20% of patients[2]. Moreover, 80% of surgically treated patients face recurrence risk, with a 5-year survival rate of 17%-35%[3]. Existing prognostic models for ICC, including CA199 and TNM staging, have proven ineffective owing to the genetic complexity and heterogeneity of tumor tissues[4,5]. Therefore, the absence of efficient methods for predicting and enhancing survival rates underscores the practical significance of establishing an effective prognostic monitoring model for patients with ICC.
Tumor-associated immune cells play a pivotal role in the tumor microenvironment (TME) and tumor development. CD4, CD8, CD20, pan-cytokeratin (CK), and CD68 are crucial in tumor development[6]. Elevated levels of CD8+ T cells in ICC are an independent prognostic factor that often correlates with improved overall survival (OS)[7]. In addition, exhaustion of CD8+ T cells can predict the efficacy of immune checkpoint inhibitors in treating hepatocellular carcinoma[8]. A high ratio of CD8+ to CD20+ cells is associated with prolonged disease-free survival. Compared with high levels of CD8+ or CD20+ cells alone, patients with a combined high distribution of tumoral CD8+ and CD20+ cells exhibit extended recurrence-free survival (RFS)[9]. CD20 is a marker on the surface of a subtype of B cells that predominantly infiltrate the tumor margin of ICC. Higher densities of CD20+ B cells are associated with improved OS and RFS[10]. The detection of CD4+ cells and regulatory T cells in preoperative peripheral blood can be potential immunological prognostic indicators for patients undergoing curative resection for hepatocellular carcinoma[11].
Increasing evidence highlights the pivotal role of tumor immune cells in ICC and serves as a crucial survival prognostic indicator. However, clinical studies related to surgically treated patients with ICC have frequently focused on prognostic models based on clinical indicators, overlooking the role of the TME in ICC prognosis. In this study, we comprehensively analyzed the distribution of CD4, CD8, CD20, and CK in tumors and paired adjacent tissues along with preoperative blood examination parameters and postoperative pathological diagnoses. Our objective was to establish robust models for predicting the OS of patients with ICC. The models underwent meticulous internal validation to ensure authenticity.
Tumor tissues and paired adjacent tissues were collected from 141 patients with ICC who underwent surgical resection at Shanghai Eastern Hepatobiliary Surgery Hospital between August 2001 and July 2010. Immunohistochemical analyses for CK18 and CK19 were conducted at the Department of Pathology, Shanghai Eastern Hepatobiliary Surgery Hospital. Clinical, pathological, and demographic characteristics of the patients were retrospectively analyzed. OS, defined as the time from surgery to death, was the primary endpoint of survival. The criteria[12] were negative tumor resection margins and an absence of prior malignancies. The exclusion criteria were incomplete clinical data and loss to follow-up. Two experts independently confirmed pathological diagnoses. Follow-up data collected until August 2016 had a median duration of 27 months (range: 4-79 months). Clinical parameters included: (1) Demographic details (sex, age, height, weight, hepatitis history, smoking, drinking, etc.); (2) Tumor pathology indicators (size, number, microvascular invasion, tumor cell differentiation, lymph node invasion); and (3) Blood metrics [complete blood count, biochemistry, hepatitis B virus (HBV) panel, etc.]. The study protocol conformed to the 1975 Declaration of Helsinki and was approved by the ethics committee of Shanghai Eastern Hepatobiliary Surgery Hospital. Signed informed consent was obtained from all participants in the study.
Tissue sections (4-5 μm thickness) were cut from paraffin-embedded blocks, mounted on glass slides, deparaffinized, and rehydrated. The slides were heated in citrate buffer (pH 6.0) for 10-20 minutes, cooled, and washed with phosphate-buffered saline. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 minutes. Non-specific sites were blocked with 5% bovine serum albumin for 1 hour. Sections were incubated overnight at 4 °C with primary antibodies (all from Boster, Shanghai, China)-anti-CD4 (BM4263), anti-CD8 (BM0216), anti-CD20 (M03780-4), anti-CK (M30944), and anti-CD68 (BA3628). After washing, the slides were incubated with Alexa Fluor-conjugated secondary antibodies for 1 hour in the dark. The nuclei were stained with DAPI for 5 minutes. Visualization was performed using a laser confocal microscope (TCS SP5; Leica, Wetzlar, Germany). All immunofluorescence staining of tissue chips was conducted by Boster Biological Technology Company (Shanghai, China).
Statistical analyses were conducted using SPSS software (version 25.0; SPSS, Inc., Chicago, IL, United States) and R version 4.1.2. The Pearson χ2 test was used to assess single-variable predictive indicators from immunohistochemistry. Kaplan-Meier analysis generated OS curves, with the log-rank test used for evaluating differences. The “pROC” package, featuring time-dependent receiver operating characteristic (ROC) curves and the Youden index, was used for validating predictive indicators. Predictors were identified through the Variable Selection for Cox Proportional Hazards Model, incorporating stepwise selection and Akaike Information Criterion (AIC) analysis, and prognostic models were con
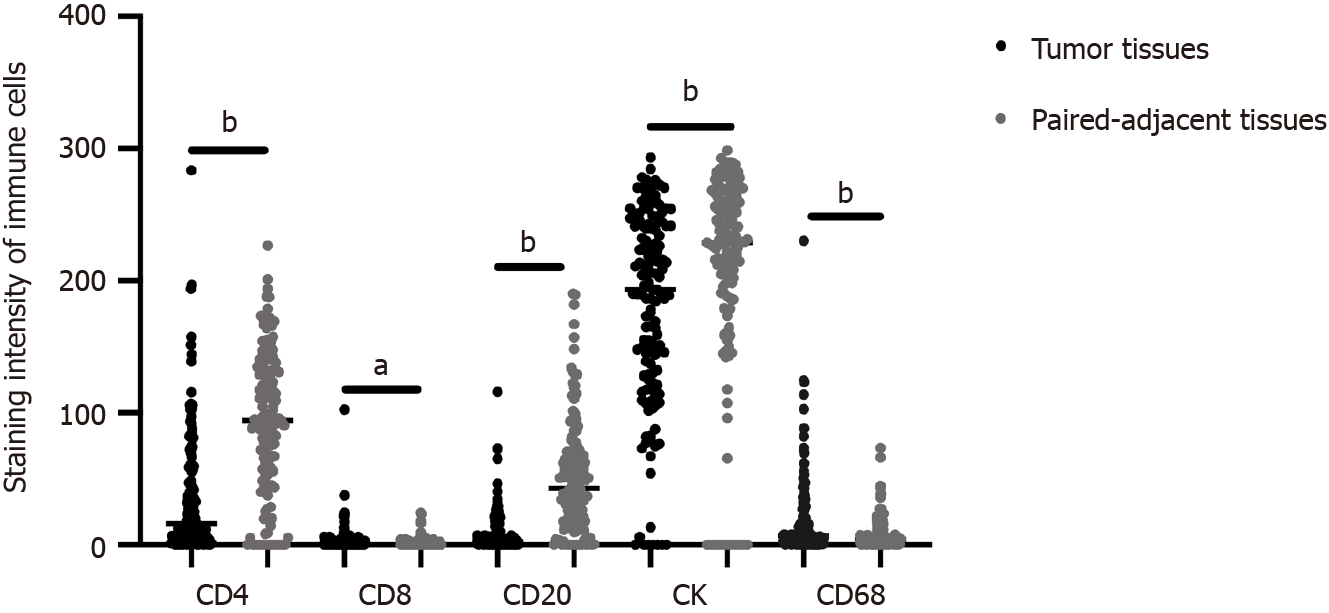
Infiltration of representative immune cells, such as CD4, CD8, CD20, CK, and CD68, in the TME is known to play a crucial role in predicting cancer prognosis[6,13]. However, research relating to ICC is lacking. Therefore, we conducted multiplex immunofluorescence staining of these immune cells in ICC to analyze their impact on prognosis of this malignancy. The distribution of CD4, CD8, CK, CD20, and CD68 in cancerous and paired adjacent tissues of patients with ICC is illustrated in Figure 1. In addition, Table 1 summarizes the correlations between baseline characteristics and OS in the patients with ICC.
| Patient characteristic | 1-year survival | 1-year death | P value | 3-year survival | 3-year death | P value |
| Age | ||||||
| > 53 years | 63 | 8 | 0.237 | 31 | 40 | 0.335 |
| < 53 years | 58 | 13 | 25 | 45 | ||
| Sex | ||||||
| Male | 80 | 16 | 0.388 | 35 | 61 | 0.248 |
| Female | 40 | 5 | 21 | 24 | ||
| HBV | ||||||
| Yes | 54 | 12 | 0.304 | 27 | 39 | 0.786 |
| No | 66 | 9 | 29 | 46 | ||
| Smoking | ||||||
| Yes | 28 | 5 | 11 | 9 | 24 | 0.095 |
| No | 92 | 16 | 47 | 61 | ||
| Drinking | ||||||
| Yes | 31 | 5 | 0.844 | 10 | 26 | 0.09 |
| No | 89 | 16 | 46 | 59 | ||
| Family history | ||||||
| Yes | 11 | 3 | 0.4391 | 4 | 10 | 0.369 |
| No | 109 | 18 | 52 | 75 | ||
| WBC | ||||||
| Abnormal | 22 | 5 | 0.5541 | 10 | 17 | 0.752 |
| Normal | 98 | 16 | 46 | 68 | ||
| ALT | ||||||
| Abnormal | 38 | 10 | 0.155 | 20 | 28 | 0.734 |
| Normal | 82 | 11 | 36 | 57 | ||
| AST | ||||||
| Abnormal | 39 | 12 | 0.03 | 18 | 33 | 0.419 |
| Normal | 81 | 9 | 38 | 52 | ||
| AFP | ||||||
| > 400 μg/L | 12 | 2 | 11 | 7 | 7 | 0.407 |
| < 400 μg/L | 108 | 19 | 49 | 78 | ||
| CEA | ||||||
| Abnormal | 22 | 4 | 11 | 9 | 17 | 0.556 |
| Normal | 98 | 17 | 47 | 68 | ||
| CA199 | ||||||
| Abnormal | 57 | 11 | 0.68 | 24 | 44 | 0.3 |
| Normal | 63 | 10 | 32 | 41 | ||
| Child-Pugh | ||||||
| Group A | 62 | 18 | 0.004 | 56 | 80 | 0.002 |
| Group B | 58 | 3 | 0 | 15 | ||
| Tumor necrosis | ||||||
| Yes | 49 | 17 | 0.001 | 29 | 63 | 0.077 |
| No | 71 | 4 | 27 | 32 | ||
| Tumor metastasis | ||||||
| Yes | 58 | 17 | 0.006 | 47 | 69 | 0.112 |
| No | 62 | 4 | 9 | 26 |
Stepwise selection of variables for inclusion in the Cox proportional hazards model revealed significant correlation between CD4, CD8, CD20, and CK and the prognosis of postoperative ICC patients. The antigen presentation function of CD68 is recognized[14]; however, in this study, univariate and multivariate Cox regression analysis for factors affecting the survival of ICC in 141 patients enrolled in cohorts found that CD68 was not significant for either 1- or 3-year survival after resection (Tables 2 and 3). Previous studies have suggested that CD68 expression may vary among different stromal types in ICC, and that CD68 might be associated with prognosis in patients without metastasis, with higher CD68 expression linked to poorer outcomes[15]. However, in our study, after variable selection, CD68 was found to have a weaker association with prognosis compared to CD4, CD8, CD20, and CK markers and was therefore not included in our models. Consequently, CD4, CD8, CD20, and CK levels were chosen to establish the Cox proportional hazards model for ICC. The prognostic value of individual immune cells (CD4, CD8, CD20, and CK) was subsequently investigated in a clinical cohort of 141 patients with ICC. By employing time-dependent ROC analysis, the optimal cutoff values for CD4, CD8, CD20, and CK were determined. The prognostic significance of immune markers, such as CD20, paired adjacent tissue CD4, and paired adjacent tissue CD8 in ICC was further explored with survival prognosis. In addition, aspartate aminotransferase (AST) expression correlated with 1-year survival prognosis (P = 0.001), and Child-Pugh staging was associated with 1-year (P = 0.001) and 3-year (P = 0.001) survival prognoses. Postoperative pathological features, including tumor necrosis (P = 0.03) and metastasis (P = 0.01), exhibited high correlations with 3-year survival prognosis.
| Variable | No. of participants (n = 141) | Univariate analysis HR (95%CI) | P value | Multivariate analysis HR (95%CI) | P value |
| AST | 0.002 | 0.002 | |||
| Normal | 81 | 0.20 (0.07, 0.56) | 0.18 (0.06, 0.53) | ||
| Abnormal | 60 | 1 | 1 | ||
| Child-Pugh | < 0.001 | < 0.001 | |||
| Group A | 136 | 1 | 1 | ||
| Group B | 5 | 9.35 (2.74, 31.94) | 68.29 (9.34, 499.16) | ||
| CD20 | 0.009 | < 0.001 | |||
| High | 5 | 1 | 1 | ||
| Low | 136 | 0.19 (0.06, 0.64) | 0.08 (0.02, 0.29) | ||
| Paired adjacent tissues CD20 | 0.08 | - | |||
| High | 90 | 1 | |||
| Low | 51 | 2.14 (0.91, 5.03) | |||
| CD4 | 0.14 | - | |||
| High | 65 | 1 | |||
| Low | 76 | 0.52 (0.21, 1.24) | |||
| Paired adjacent tissues CD4 | 0.39 | 0.05 | |||
| High | 75 | 1 | 1 | ||
| Low | 66 | 0.68 (0.28, 1.65) | 0.39 (0.16, 0.98) | ||
| CD8 | 0.29 | - | |||
| High | 94 | 1 | |||
| Low | 47 | 1.59 (0.67, 3.78) | |||
| Paired adjacent tissues CD8 | 0.46 | 0.002 | |||
| High | 37 | 1 | 1 | ||
| Low | 104 | 7.75 (1.04, 57.78) | 57.49 (4.44, 744.84) | ||
| CK | 0.23 | - | |||
| High | 16 | 0.51 (0.17, 1.53) | |||
| Low | 125 | 1 | |||
| Paired adjacent tissues CK | 0.07 | - | |||
| High | 133 | 1 | |||
| Low | 8 | 3.06 (0.90, 10.40) | |||
| CD68 | 0.12 | - | |||
| High | 9 | 0.38 (0.11, 1.29) | |||
| Low | 132 | 1 | |||
| Paired adjacent tissues CD68 | 0.10 | - | |||
| High | 52 | 0.49 (0.21, 1.15) | |||
| Low | 89 | 1 |
| Variable | No. of participants (n = 141) | Univariate analysis HR (95%CI) | P value | Multivariate analysis HR (95%CI) | P value |
| Tumor necrosis | 0.01 | 0.03 | |||
| 0 | 50 | 1 | 1 | ||
| 0.5 | 6 | 1.31 (1.03, 1.62) | 1.26 (1.13, 1.41) | ||
| 1 | 85 | 1.93 (1.20, 3.12) | 1.73 (1.06, 2.84) | ||
| Tumor metastasis | < 0.001 | 0.008 | |||
| No | 20 | 1 | 0.47 (0.27, 0.82) | ||
| Yes | 121 | 2.53 (1.48, 4.32) | 1 | ||
| Child-Pugh | 0.001 | < 0.001 | |||
| Group A | 136 | 1 | 0.17 (0.07, 0.46) | ||
| Group B | 5 | 4.49 (1.80, 11.22) | 1 | ||
| CD20 | 0.03 | - | |||
| High | 61 | 1 | |||
| Low | 80 | 1.65 (1.06, 2.58) | |||
| Paired adjacent tissues CD20 | 0.74 | - | |||
| High | 83 | 1 | |||
| Low | 58 | 1.48 (0.96, 2.26) | |||
| CD4 | 0.09 | - | |||
| High | 16 | 1 | |||
| Low | 125 | 2.04 (0.89, 4.69) | |||
| Paired adjacent tissues CD4 | 0.15 | 0.02 | |||
| High | 100 | 1 | 2.01 (1.13, 3.57) | ||
| Low | 41 | 0.70 (0.43, 1.14) | 1 | ||
| CD8 | 0.16 | - | |||
| High | 73 | 1 | |||
| Low | 68 | 1.35 (0.88, 2.07) | |||
| Paired adjacent tissues CD8 | 0.01 | < 0.001 | |||
| High | 84 | 1 | 0.4 (0.25, 0.67) | ||
| Low | 57 | 1.75 (1.15, 2.68) | 1 | ||
| CK | 0.01 | 0.04 | |||
| High | 112 | 1 | 0.58 (0.35, 0.96) | ||
| Low | 29 | 1.86 (1.15, 3.02) | 1 | ||
| Paired adjacent tissues CK | 0.16 | - | |||
| High | 104 | 1 | |||
| Low | 37 | 1.39 (0.88, 2.19) | |||
| CD68 | 0.30 | - | |||
| High | 113 | 1 | |||
| Low | 28 | 0.74 (0.42, 1.31) | |||
| Paired adjacent tissues CD68 | 0.35 | - | |||
| High | 130 | 1 | |||
| Low | 11 | 0.65 (0.26, 1.60) |
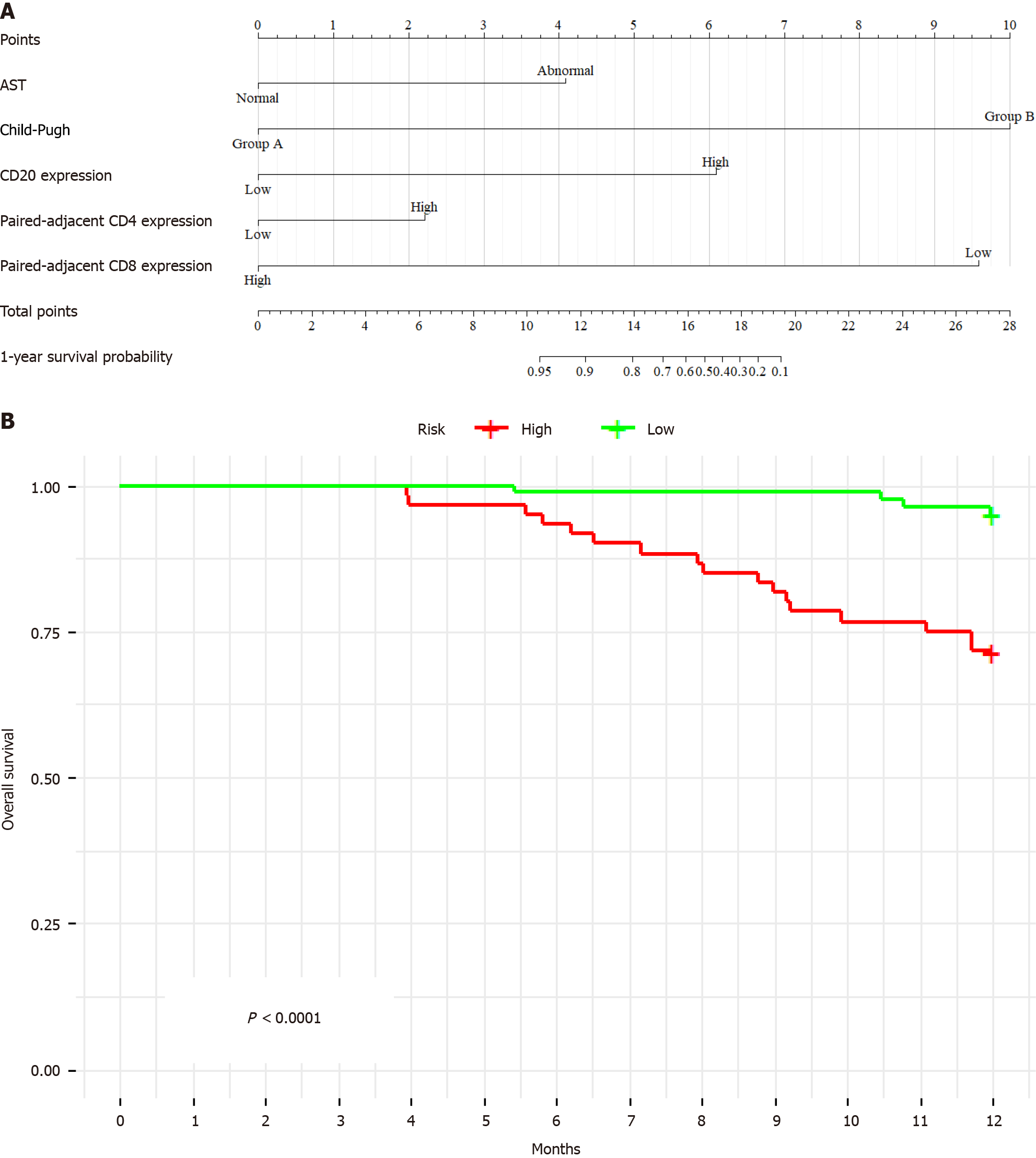
Utilizing variable selection for the Cox proportional hazards model and incorporating stepwise selection and AIC analysis, a prognostic model was developed. The model highlighted that AST (P = 0.001), Child-Pugh (P = 0.001), and the expression of CD20 (P = 0.001), paired adjacent tissues CD4 (P = 0.05), and paired adjacent tissues CD8 (P = 0.002) had the highest predictive value for 1-year OS of ICC. Simultaneously, prior to establishing the Cox proportional hazards model, we conducted normality tests and found that the key variables included in the model exhibited a trend towards normal distribution (P > 0.05), which aligns with the assumptions of linear regression.
This Cox proportional hazards model was significant for short-term prognosis (P < 0.001), as shown in Figure 2A. The derived formula of the model is “total points = 8.943957 × AST + 10 × Child-Pugh + 8.046718 × CD20 + 7.799139 × paired-adjacent tissues CD4 + 1.036407 × paired adjacent tissues CD8”. Validation in the test cohort confirmed the predictive capacity of the model for early survival prognosis in patients with ICC. Furthermore, internal validation revealed a C-index of 0.76 [95% confidence interval (95%CI): 0.65-0.87], indicating excellent validation efficiency. Kaplan-Meier analysis demonstrated a significantly lower survival time for high-risk patients than for low-risk patients (P < 0.001) (Figure 2B).
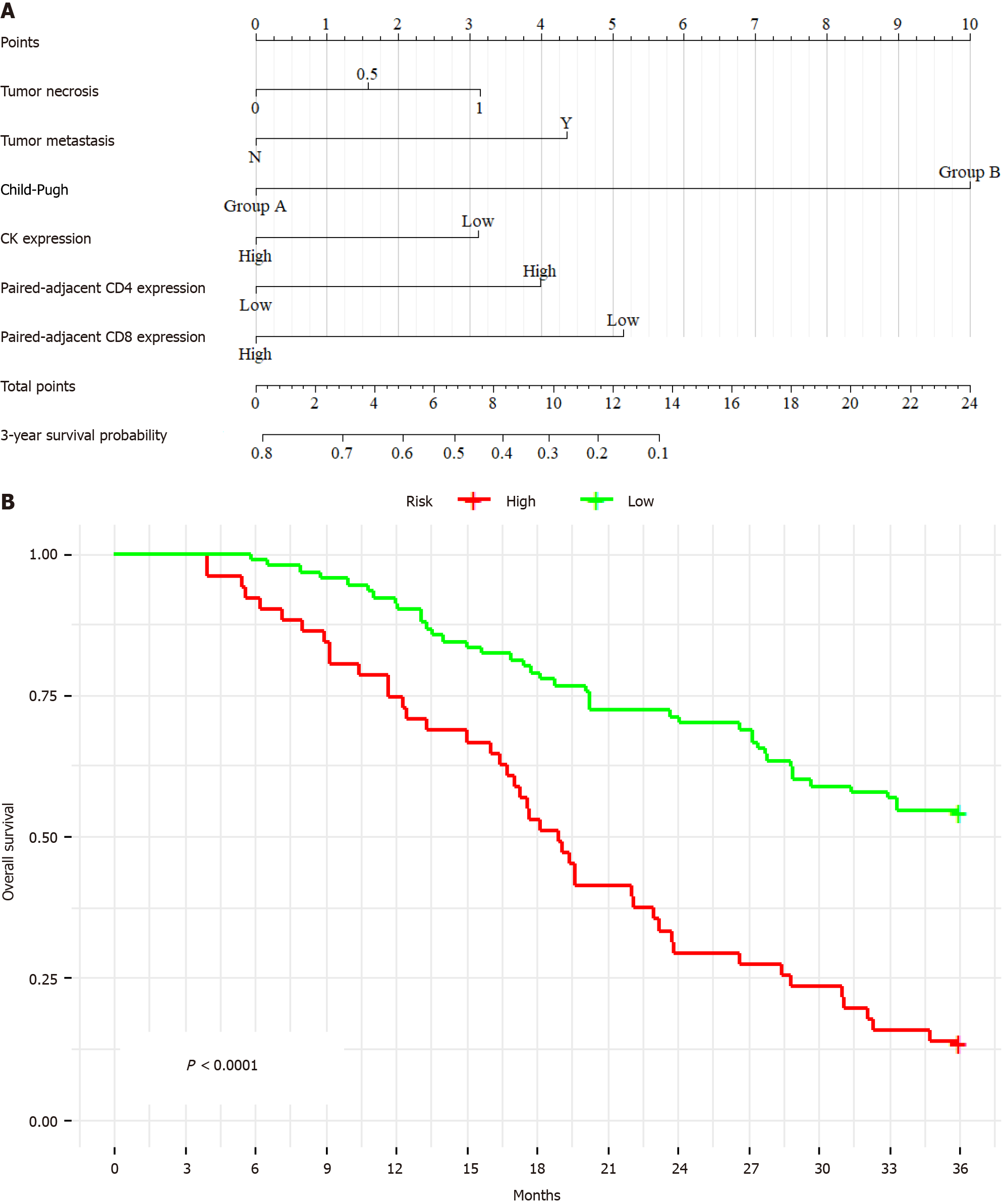
Variable selection for the 3-year survival Cox proportional hazards model incorporated expression of paired adjacent tissues CD8 (P = 0.001), paired adjacent tissues CD4 (P = 0.02), CK (P = 0.04), and a series of crucial clinical factors (including tumor necrosis (P = 0.03), tumor metastasis (P = 0.01), and Child-Pugh (P = 0.001)) into the model. The key variables included in the model passed the normality tests, demonstrating a trend towards normal distribution (P > 0.05), which meets the conditions for linear regression. The established model is significant for long-term prognosis (P < 0.001), as illustrated in Figure 3A. The derived formula of the model is “total points = 3.142282 × tumor necrosis + 4.352792 × tumor metastasis + 10 × Child-Pugh - 3.111830 × CK + 3.992448 × paired adjacent tissues CD4 - 5.153573 × paired adjacent tissues CD8”. Internal validation yielded a C-index of 0.69 (95%CI: 0.65-0.73), indicating permissible validation efficiency. Kaplan-Meier analysis revealed a significantly lower survival time for high-risk patients than for low-risk patients (P < 0.001) (Figure 3B).
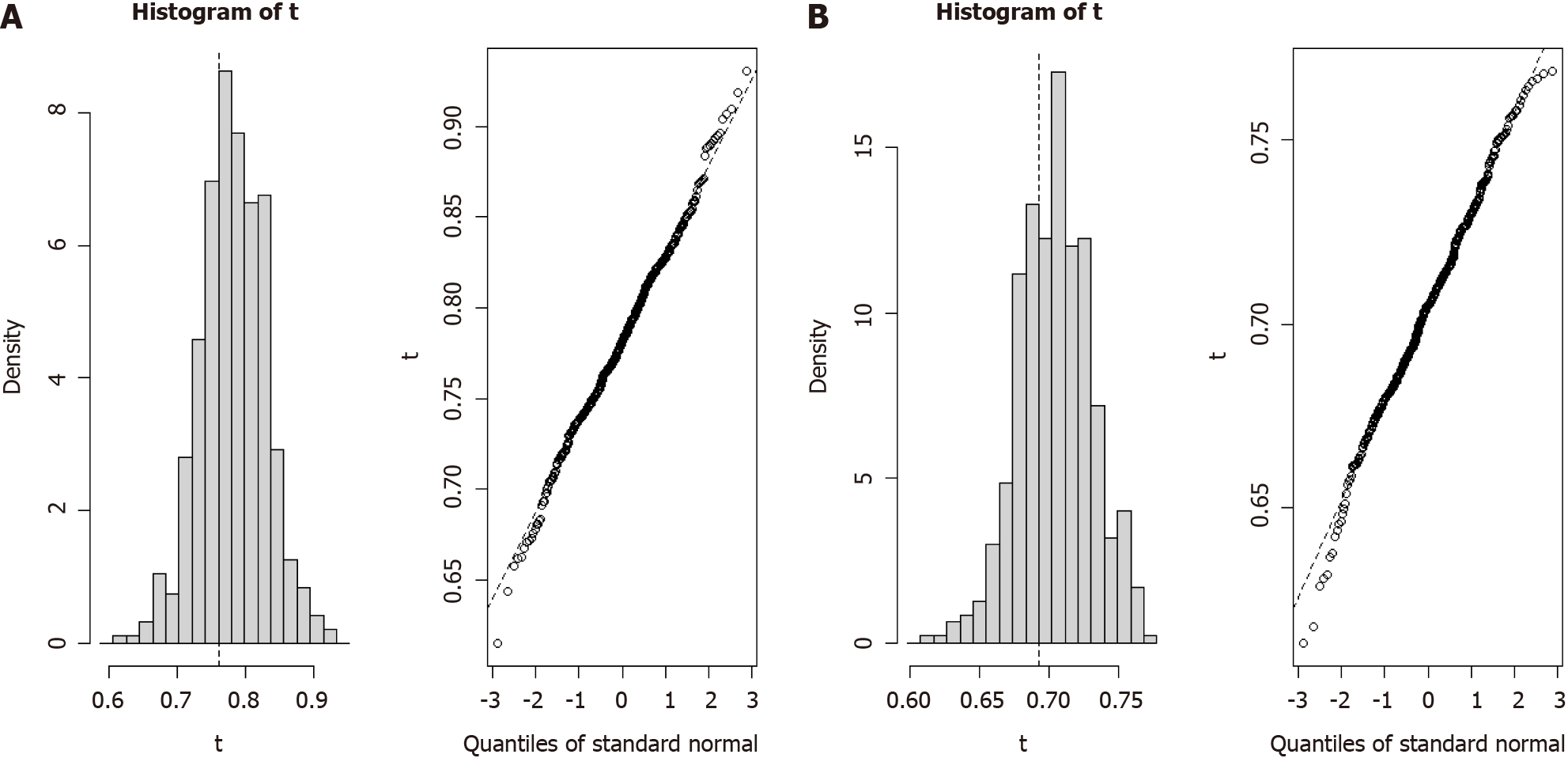
The robustness of the two Cox proportional hazards models was explored through bootstrap analysis, which involved resampling with replacement from the original dataset. We estimated the distribution of population parameters by generating multiple bootstrap sample sets and assessed the predictive performance of the models. After 500 iterations of resampling from the original data, the 1-year predictive model exhibited a C-index of 0.761 (95%CI: 0.669-0.853) (Figure 4A) and the 3-year predictive model produced a C-index of 0.693 (95%CI: 0.642-0.744) (Figure 4B). These results confirmed the reliability and predictive accuracy of our prognostic survival models for patients with ICC.
ICC, a highly malignant liver tumor, presents considerable challenges in treatment and management. Besides well-known risk factors such as smoking, alcohol consumption, and HBV, conditions such as recurrent suppurative cholangitis or liver fluke may also trigger ICC. The effects of these factors on immune cells within the TME and on patient outcomes warrant deeper investigation[16]. Immune cells associated with tumors are critical in the prognosis of ICC, with the composition and distribution of immune cells in cancerous and adjacent tissues being closely linked to the survival prognosis of patients with ICC. For example, CD8+ cells in paired adjacent tissues are significant in predicting short-term and long-term survival outcomes for ICC[17]. The infiltration of CD8+ cells in paired adjacent tissues is closely associated with the survival prognosis of patients with ICC, consistent with the findings of other studies. CD8+ T cells, also known as cytotoxic T cells, are generally regarded as favorable prognostic markers in various tumors[7]. CD20+ cells, which are a subtype of B cells, also showed correlation with better OS[10]. In addition, CD68+ expression-an important marker of macrophages-has been suggested to be associated with the prognosis of ICC[18]. Our analysis did not show a significant strong correlation with the prognosis. This discrepancy may be attributed to the limited sample size, inclusion of patients at advanced stages of ICC during data collection, and different treatment methods used in our study. Future research should focus on expanding the sample size to further investigate this relationship between CD68 and prognosis of ICC.
The TME has multiple regulatory mechanisms for the expression of immune cells, including increased immunosuppressive metabolites and enzymes, malnutrition, hypoxia, increased acidity, large amounts of extracellular ATP and adenosine, dysregulated bioenergy and purinergic signals, and ion imbalance[19], which leads to significant differences in the composition of immune cells between tumors and adjacent tissues. Furthermore, complex interactions between tumor cells and immune cells may influence the function and distribution of immune cells in the TME[20]. For a more accurate prediction of the survival prognosis of patients with ICC, we employed sophisticated statistical methods and model validation in this study. By combining clinical indicators, postoperative pathological indicators, and the distribution of immune cells, effective nomograms predicting 1-year and 3-year survival for patients with ICC were established. Thus, this study provides a new and comprehensive prognostic tool for ICC. However, our findings do require further validation, particularly in other independent cohorts, to ensure the robustness of the model in predicting prognosis and survival. Moreover, other potential prognostic indicators, such as tumor-related genetic variations, metabolites, and their interactions with immune cells, merit further investigation.
In conclusion, this study offers a novel perspective for understanding and predicting ICC prognosis. Immune cells, particularly those in cancerous and adjacent tissues, may serve as new biomarkers for assessing ICC prognosis. Compared with existing prognostic indicators and models for ICC, our model based on immune cells and their interactions with tumors may provide more accurate predictions and facilitate personalized treatment plans for patients with ICC. From a clinical practice perspective, our model involves performing immunofluorescence staining for multiple markers on the cancerous and adjacent tissues of patients with ICC who have undergone surgical resection, and then conducting prognostic analysis. Nowadays, Immunofluorescence technology has now reached a high level of maturity and is widely adopted, owing to its strong feasibility and ease of implementation.
This study had certain limitations. First, as a retrospective study, the data analyzed were historical, and diagnostic and treatment standards, techniques, medications, and disease management strategies may have changed between the studied cohort and current patients with ICC. There may be some biases when using future collected ICC samples to validate models. In addition, the sample size was relatively small at 141 participants, potentially introducing bias into the conclusions. However, the establishment of early and late prognosis models for patients with ICC undergoing surgery remains clinically significant. Consequently, expanding the sample size and performing comprehensive analyses is essential for improving understanding of the prognostic factors that influence ICC.
We developed Cox regression models for 1-year and 3-year survival predictions of patients with ICC who underwent resection, which has positive implications for establishing a more comprehensive prognostic model for ICC based on tumor immune microenvironment and immune cell changes in the future.
The authors would like to acknowledge the patients and their families for their assistance.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11065] [Cited by in RCA: 12186] [Article Influence: 1523.3] [Reference Citation Analysis (3)] |
| 2. | Scott A, Wong P, Melstrom LG. Surgery and hepatic artery infusion therapy for intrahepatic cholangiocarcinoma. Surgery. 2023;174:113-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 3. | Buettner S, Galjart B, van Vugt JLA, Bagante F, Alexandrescu S, Marques HP, Lamelas J, Aldrighetti L, Gamblin TC, Maithel SK, Pulitano C, Margonis GA, Weiss M, Bauer TW, Shen F, Poultsides GA, Marsh JW, IJzermans JNM, Groot Koerkamp B, Pawlik TM. Performance of prognostic scores and staging systems in predicting long-term survival outcomes after surgery for intrahepatic cholangiocarcinoma. J Surg Oncol. 2017;116:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 4. | Moazzam Z, Alaimo L, Endo Y, Lima HA, Ruzzenente A, Guglielmi A, Aldrighetti L, Weiss M, Bauer TW, Alexandrescu S, Poultsides GA, Maithel SK, Marques HP, Martel G, Pulitano C, Shen F, Cauchy F, Koerkamp BG, Endo I, Cloyd J, Ejaz A, Pawlik TM. Combined Tumor Burden Score and Carbohydrate Antigen 19-9 Grading System to Predict Outcomes Among Patients with Intrahepatic Cholangiocarcinoma. J Am Coll Surg. 2023;236:804-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 5. | Zhao C, He C, Lu J, Huang X, Chen C, Lin X. Progression Patterns and Post-Progression Survival in Recurred Intrahepatic Cholangiocarcinoma Patients: A Novel Prognostic Nomogram Based on Multicenter Cohorts. Front Oncol. 2022;12:832038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Mori H, Bolen J, Schuetter L, Massion P, Hoyt CC, VandenBerg S, Esserman L, Borowsky AD, Campbell MJ. Characterizing the Tumor Immune Microenvironment with Tyramide-Based Multiplex Immunofluorescence. J Mammary Gland Biol Neoplasia. 2020;25:417-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 7. | Chen L, Huang H, Huang Z, Chen J, Liu Y, Wu Y, Li A, Ge J, Fang Z, Xu B, Zheng X, Wu C. Prognostic values of tissue-resident CD8(+)T cells in human hepatocellular carcinoma and intrahepatic cholangiocarcinoma. World J Surg Oncol. 2023;21:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 8. | Hsu CL, Ou DL, Bai LY, Chen CW, Lin L, Huang SF, Cheng AL, Jeng YM, Hsu C. Exploring Markers of Exhausted CD8 T Cells to Predict Response to Immune Checkpoint Inhibitor Therapy for Hepatocellular Carcinoma. Liver Cancer. 2021;10:346-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 92] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 9. | Trailin A, Červenková L, Ambrozkiewicz F, Ali E, Kasi P, Pálek R, Hošek P, Třeška V, Daum O, Tonar Z, Liška V, Hemminki K. T- and B-Cells in the Inner Invasive Margin of Hepatocellular Carcinoma after Resection Associate with Favorable Prognosis. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Shi JY, Gao Q, Wang ZC, Zhou J, Wang XY, Min ZH, Shi YH, Shi GM, Ding ZB, Ke AW, Dai Z, Qiu SJ, Song K, Fan J. Margin-infiltrating CD20(+) B cells display an atypical memory phenotype and correlate with favorable prognosis in hepatocellular carcinoma. Clin Cancer Res. 2013;19:5994-6005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 176] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 11. | Chen B, Lu M, Chen Q, Zou E, Bo Z, Li J, Zhao R, Zhao J, Yu Z, Chen G, Wu L. Systematic profiling of mitochondria-related transcriptome in tumorigenesis, prognosis, and tumor immune microenvironment of intrahepatic cholangiocarcinoma: a multi-center cohort study. Front Genet. 2024;15:1430885. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Pocock SJ, Clayton TC, Altman DG. Survival plots of time-to-event outcomes in clinical trials: good practice and pitfalls. Lancet. 2002;359:1686-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 362] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 13. | García-Martínez E, Gil GL, Benito AC, González-Billalabeitia E, Conesa MA, García García T, García-Garre E, Vicente V, Ayala de la Peña F. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 2014;16:488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 200] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 14. | Atanasov G, Hau HM, Dietel C, Benzing C, Krenzien F, Brandl A, Wiltberger G, Matia I, Prager I, Schierle K, Robson SC, Reutzel-Selke A, Pratschke J, Schmelzle M, Jonas S. Prognostic significance of macrophage invasion in hilar cholangiocarcinoma. BMC Cancer. 2015;15:790. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 15. | Ye YH, Xin HY, Li JL, Li N, Pan SY, Chen L, Pan JY, Hu ZQ, Wang PC, Luo CB, Sun RQ, Fan J, Zhou J, Zhou ZJ, Zhou SL. Development and Validation of a Stromal-Immune Signature to Predict Prognosis in Intrahepatic Cholangiocarcinoma. Clin Mol Hepatol. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Liau MYQ, Toh EQ, Shelat VG. Opisthorchis viverrini-Current Understanding of the Neglected Hepatobiliary Parasite. Pathogens. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 6.5] [Reference Citation Analysis (1)] |
| 17. | Deng M, Li SH, Fu X, Yan XP, Chen J, Qiu YD, Guo RP. Relationship between PD-L1 expression, CD8+ T-cell infiltration and prognosis in intrahepatic cholangiocarcinoma patients. Cancer Cell Int. 2021;21:371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 33] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 18. | Zheng Y, Huang N, Kuang S, Zhang J, Zhao H, Wu J, Liu M, Wang L. The clinicopathological significance and relapse predictive role of tumor microenvironment of intrahepatic cholangiocarcinoma after radical surgery. Cancer. 2023;129:393-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 19. | Verma NK, Wong BHS, Poh ZS, Udayakumar A, Verma R, Goh RKJ, Duggan SP, Shelat VG, Chandy KG, Grigoropoulos NF. Obstacles for T-lymphocytes in the tumour microenvironment: Therapeutic challenges, advances and opportunities beyond immune checkpoint. EBioMedicine. 2022;83:104216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 20. | Zheng BH, Ma JQ, Tian LY, Dong LQ, Song GH, Pan JM, Liu YM, Yang SX, Wang XY, Zhang XM, Zhou J, Fan J, Shi JY, Gao Q. The distribution of immune cells within combined hepatocellular carcinoma and cholangiocarcinoma predicts clinical outcome. Clin Transl Med. 2020;10:45-56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |