INTRODUCTION
Esophageal cancer (EC) is a common malignancy of the digestive system with 604000 new cases and 544000 new deaths per year[1]. EC can be classified into two major subtypes: Esophageal adenocarcinoma (EAC) and esophageal squamous cell carcinoma (ESCC). ESCC is the major subgroup, making up almost 90 percent of total cases, and common in East to Central Asia[2]. Under multi-modality management, esophagectomy, endoscopic treatment, radiotherapy, and chemotherapy are the main theraputic strategies for patients with EC. The standard treatment for locally advanced EC uses neoadjuvant radiotherapy followed by esophagectomy; however, the 5-year overall survival rate for EC is less than 20%[3,4].
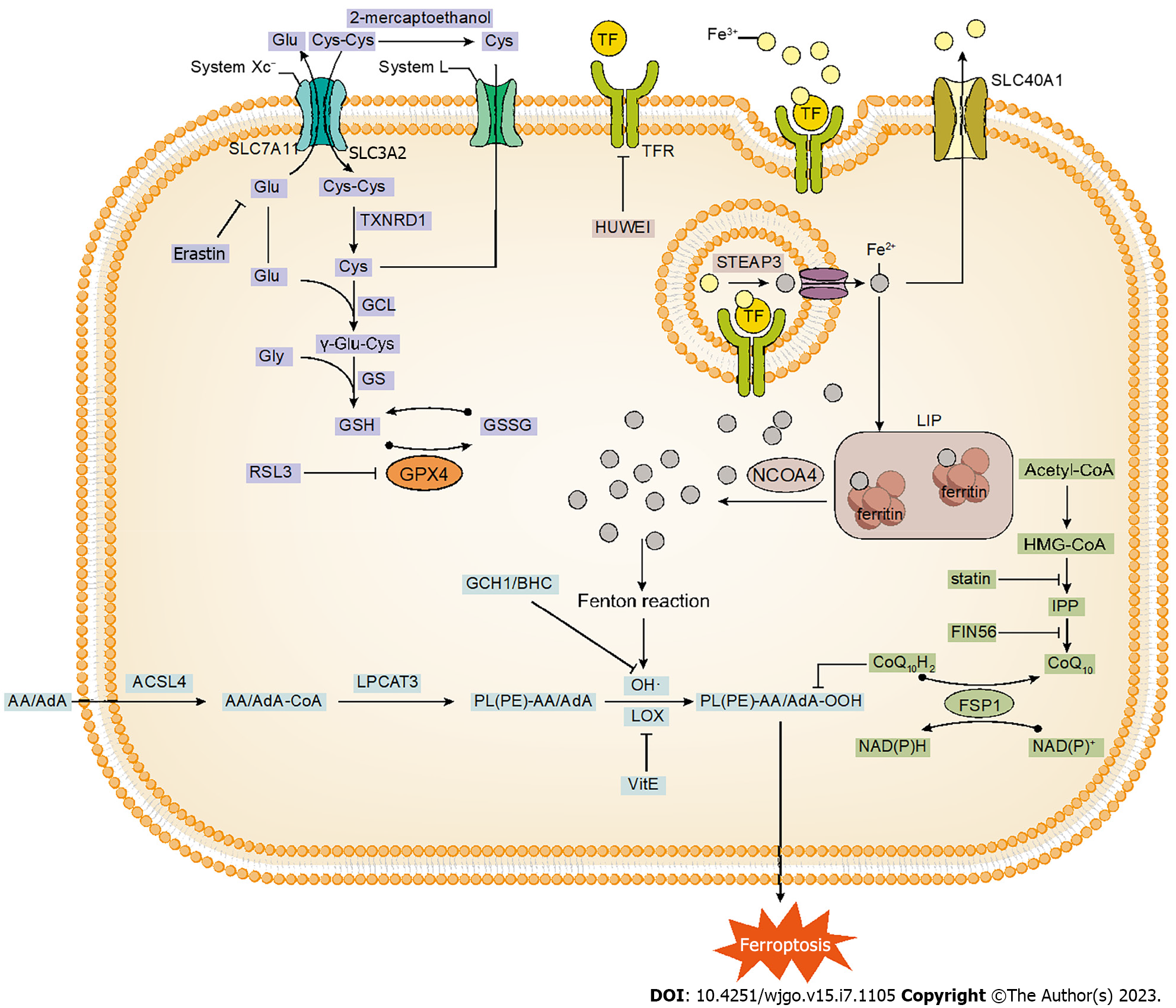
Iron-dependent form of regulated cell death, known as ferroptosis, was first proposed by Dixon et al[5] in 2012. Ferroptosis results in smaller intracellular mitochondria, increased membrane density, and reduced cristae, accompanied by the accumulation of lipid peroxides combined with the production of reactive oxygen species (ROS). Initially, it was considered that inhibition of cystine uptake abnormalized the antioxidant system of cells, leading to iron-dependent oxidative death[5]. Later studies have confirmed that ferroptosis can be induced through various pathways such as glutathione peroxidase 4 (GPX4) inhibition, intracellular iron accumulation, oxidative attack by lipoxygenase (LOX), and targeting of NRF2[6-9] (Figure 1). A study in 2021 showed that 5-aminolevulinic acid (5-ALA) treatment inhibited GPX4 and HMOX1 overexpression, promoting ferroptosis in ESCC[10]. In the same year, Zhang et al[11] demonstrated that oridonin (Ori) inhibited glutathione synthesis in EC cells, thereby inducing ferroptosis to exert anticancer activity. Thus, targeting ferroptosis genes and related pathways may promote further research progress to control tumor growth.
Figure 1 The process of ferroptosis, mainly including glutathione metabolism, iron metabolism and lipid peroxidation reaction.
More recently, immunotherapy in EC has become a hot topic. Immunotherapy complements the conventional treatment options, which shows great promise in unresectable or metastatic EC[12]. Immune checkpoint blockade (ICB) is increasingly at the front line of cancer treatment, often as monotherapy or in combination with radiotherapy. Immune checkpoints, such as programmed cell death 1 ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4), can be expressed on the surface of cancer cells and act tumor immune escape by binding to receptors on the surface of immune cells[13]. Based on this principle, commercially available immune checkpoint blockers (ICIs) include anti-CTLA-4 and anti-programmed cell death 1 (PD-1)/PD-L1[13]. Currently, PD-1 inhibitors have been utilized as first-line immunotherapy for advanced metastatic EC[14,15]. However, there are still limits to identify patients who would be most likely to benefit from ICB therapy.
The tumor immune microenvironment (TIME) can promote tumor progression as well as resist the immune response. Tumor immunosuppressive cells, regulatory T (Treg) cells, M2 macrophages, and migratory dendritic cells (DCs), play an important role in the immune escape of tumors[16]. Therefore, targeting these cells may enhance the antitumor immune effect. For example, Xu et al[17] found that Treg-specific ablation of GPX4 inhibited tumor growth and simultaneously enhanced antitumor immunity. One study reported that CD8+ T cells release Interferon-gamma (IFNγ), which promotes lipid peroxidation and ferroptosis in tumor cells[18]. This study, the first to connect immunity and ferroptosis at once, is a breakthrough discovery. In 2022, Luo et al[19] improved the effectiveness of radiotherapy in ESCC patients by targeting SCD1 to induce ferroptosis in ESCC cells and confer immunogenicity. It appears that the application of ferroptosis in immunotherapy for EC patients deserves further exploration.
In this review, we illustrate the research progress of ferroptosis in EC and try to explore the key role of ferroptosis in EC immunotherapy to provide novel directions and ideas for the treatment of EC.
THE MECHANISM OF FERROPTOSIS
Glutathione metabolism
Glutathione, a tripeptide containing γ-amide bond and sulfhydryl group, consists of glutamic acid, cysteine and glycine. Glutathione exists in both reduced (GSH) and oxidized (GSSG) forms, whereas GSH predominating in the physiological state. As an important member of the intracellular antioxidant system, GSH can directly quench oxidized substances while being oxidized itself to GSSG[20]. GPX4 as the fourth member of the selenium-containing GPX family, converts hydrogen peroxide (H2O2) and lipid hydroperoxide (Lipid-OOH), important drivers of ferroptosis, into water (H2O) and lipid alcohol (Lipid-OH)[20]. This process effectively prevents the generation of toxic ROS and attenuates the occurrence of ferroptosis. Therefore, the reduction of GPX4 Levels in intracellular due to depletion of GSH can effectively induce the intracellular ferroptosis response. A 2014 study demonstrated that the lethality of all 12 ferroptosis inducers was affected by GPX4 expression and knockdown[6]. Affinity-based chemoproteomics displayed that RSL3 [(1S,3R)-RSL3] can bind specifically to GPX4, inhibit GPX4 enzyme activity, and lead to the accumulation of intracellular peroxides, thus triggering ferroptosis[6].
System Xc− is a glutamate/cystine antiporter on cell surface, composed of SLC7A11 and SLC3A2. Cystine and glutamate are exchanged in and out of the cell in an equal proportion via System Xc−, and then intracellular cystine is rapidly reduced to cysteine by thioredoxin reductase 1 (TXNRD1). Cysteine is the rate-limiting substrate for the synthesis of GSH. The GSH biosynthesis consists of two main steps: (1) Glutamate and cysteine synthesize dipeptide (γ-glutamylcysteine) catalyzed by glutamate-cysteine ligase (GCL); and (2) glutathione synthetase (GS) catalyzes the synthesis of GSH from dipeptide and glycine[21]. Consequently, it follows that acting on System Xc− on the cell membrane becomes the primary mechanism for inducing ferroptosis. Erastin[5], sorafenib[22] can interfere with cystine uptake through targeted inhibition of the SLC7A11 subunit of System Xc−, thus affecting GSH synthesis. High concentrations of extracellular glutamate also affect System Xc−, preventing cystine input and leading to GSH depletion[23].
In addition, 2-mercaptoethanol converts extracellular cystine to cysteine and cysteine transfers into the cell via the L system, bypassing System Xc− to participate in the synthesis of GSH[24]. In this way, certain cells can resist erastin-induced ferroptosis. In the study of Hayano et al[25], the loss of cysteinyl-tRNA synthetase induced the transsulfuration pathway and suppressed the ferroptosis induced by erastin.
The potential role of iron
Iron is an essential element for various organisms, and the deficiency or excess of iron can facilitate the development of various diseases. Accumulation of Fe2+ causes peroxidation of cells, further leading to cell death. Fe2+ generates ROS driving the production of lipid peroxides (LPO), which induces ferroptosis, mainly through Fenton reaction with H2O2: Fe2+ + H2O2 → Fe3+ + (OH)- + OH·[26,27]. In addition to the production of LPO by the Fenton reaction, excess iron also triggers the LPO generator through the GSH and iron redox coupling sequence to induce LPO independent with the Fenton reaction, triggering ferroptosis[28].
The balance of iron metabolism is maintained through four major processes: uptake, storage, utilization, and export. An imbalance in any of these processes can lead to a disruption of iron metabolism. Plasma Fe3+ binds to transferrin (TF) on the cell membrane, and TF carries Fe3+ further to the transferrin receptor (TFR)[29]. Subsequent endocytosis of vesicles formed by the plasma membrane transports the TF-Fe3+ complex into the cell. Fe3+ can be reduced intracellularly to Fe2+ by the iron reductase STEAP3[29]. The study showed that knockdown of TF led to reduced cell death and ROS after treatment and inhibited the induction of ferroptosis following siramesine and lapatinib treatment of breast cancer cells[7]. Targeted inhibition of TFR1 by HUWE1 significantly reduces ferroptosis in acute liver injury[30]. On the contrary, aberrant TF and ferritin upregulation triggers iron accumulation via IRP2-induced upregulation of TFR1[31]. Therefore, TF and TFR contribute to iron uptake and regulation of ferroptosis occurrence.
Fe2+ in the cytoplasm enters the labile iron pool (LIP) and the excess iron stably binds to ferritin in the form of Fe3+. Whereas, nuclear receptor coactivator 4 (NCOA4) mediates ferritin phagocytosis and delivers ferritin to autolysosome, thereby allowing lysosomes to degrade ferritin and release iron transport proteins[32], suggesting the vital role of ferritin in ferroptosis. Hypoxia reduces intracellular free iron and increases mitochondrial ferritin expression, which can effectively protect from ferroptosis[33]. SLC40A1 mainly mediates iron export[29].
Lipid peroxidation reaction
Lipid peroxidation is a class of oxidative degradation of lipid reaction in which ROS reacts with the carbon-carbon double bonds of unsaturated lipids in biological membranes, leading to cellular damage. The final products of lipid peroxidation include malondialdehyde (MDA), and 4-hydroxynonenal (4-HNE). It most often affects polyunsaturated fatty acids (PUFAs), because they contain multiple double bonds. Exogenous monounsaturated fatty acids (MUFAs), on the other hand, inhibit the occurrence of lipid peroxidation reactions by a specific mechanism, whereby MUFAs promote the replacement of PUFAs from plasma membrane phospholipids in an ACSL3-dependent manner[34]. Arachidonic acid (AA) and adrenic acid (AdA) are the major PUFAs during ferroptosis. At present, lipid peroxidation mainly acts on esterified PUFAs rather than free PUFAs. Acyl-CoA synthetase long-chain family member 4 (ACSL4) and lysophosphatidylcholine acyltransferase 3 (LPCAT3) have been confirmed to be the key determinants of ferroptosis susceptibility[35,36]. ACSL4 transfers CoA to AA/AdA to form AA/AdA-CoA, while AA/AdA-CoA is esterified to PL(PE)-AA/AdA catalyzed by LPCAT3[37].
Phospholipids containing polyunsaturated fatty acyl groups (PL-PUFAs) can be oxidized by both enzymatic and non-enzymatic pathways. The non-enzymatic reaction occurs mainly through autoxidation of the Fenton reaction, which consists of three steps: (1) The free radicals (such as OH·) attack the hydrogen atom from the methylene carbon of PL-PUFA, which subtracts hydrogen from PL-PUFA, forming phospholipid free radicals (PL·); (2) PL· then reacts with oxygen to form phospholipid peroxide radical (PLOO·); and (3) PLOO· extracts the hydrogen atom from another PUFA to form phospholipid hydroperoxide (PLOOH). Unfortunately, the mechanism of the enzymatic reaction is not clear. One study has shown that the enzymatic reaction emphasizes the role of the LOX family[9]. LOX is a family of non-heme, iron-containing enzymes, and most LOXs consume free fatty acids as substrates. In contrast, 15-LOX directly oxygenates PL(PE)-AA/AdA to produce hydroperoxides, which functions in LPO and ferroptosis[37]. Tocopherols and tocotrienols strongly inhibit LOX and prevent ferroptosis, suggesting a role of the vitamin E family in the regulation of ferroptosis[37]. In addition, the knockdown of 15-LOX mediated by specific inhibitors and siRNA significantly suppressed cell death induced by erastin and RSL3[38]. Further, there could be alternative studies explaning the mechanism that cytochrome P450 oxidoreductase (POR) may promote lipid peroxidation by accelerating the cycling between Fe2+ and Fe3+ in the heme component of cytochrome P450 (CYP)[39].
Other metabolic pathways
Originally named apoptosis-inducing factor mitochondrial 2 (AIFM2), ferroptosis inhibitory protein 1 (FSP1) acts as the NAD(P)H-dependent oxidoreductase mediating the reduction of extra-mitochondrial coenzyme Q (CoQ), thus inhibiting lipid peroxidation reactions[40]. Acted as the lipophilic radical-trapping antioxidant (RTA), reduced CoQ captures lipid radicals as well as elevates the endogenous amounts of α-tocopherol[40,41]. CoQ, an effector molecule of FSP1, is a class of lipid-soluble quinones with side chains composed of different numbers of isoprene units. Normally found in the human body, CoQ10 containing 10 isoprene units is an important fat-soluble antioxidant in vivo and plays an important role in the anti-lipid peroxidation of ferroptosis. The synthesis of CoQ10 results in following steps: synthesis of the benzoquinone ring and isoprene side chains, assembly of CoQ, and modification of the benzoquinone ring[42]. Isoprene side chains are synthesized via the mevalonate (MVA) pathway. 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) is the key intermediate in the MVA pathway, and HMG-CoA undergoes a series of enzymatic reactions to form isopentenyl diphosphate (IPP), which in turn synthesizes isoprene side chains[42,43]. The target site of action of statins is HMG-CoA reductase, which can secondarily inhibit the synthesis of CoQ10 and also prevent the maturation of selenocysteine tRNA and the synthesis of GPX4[44]. As a consequence, besides their cholesterol-lowering activity, statins exert the induction of ferroptosis in tumors. In addition to degrading GPX4, FIN56 binds and activates squalene synthase, and farnesyl pyrophosphate (FPP) (an intermediate in CoQ10 synthesis by IPP) is correspondingly reduced, thereby inhibiting the CoQ10 synthesis process and enhancing susceptibility to ferroptosis[45].
Tetrahydrobiopterin (BH4), acted as RTA, protects cells from oxidation, regulates their redox status, and plays a synergistic role together with vitamin E[46]. Guanosine triphosphate cyclic hydrolase (GCH1), the rate-limiting enzyme for BH4 synthesis, was identified by the genome-wide CRISPR screen as a GPX4-independent ferroptosis suppressor gene[47]. Dihydrofolate reductase (DHFR) catalyzes the reduction of BH2 to BH4. The methotrexate (MTX) blocking DHFR acts in concert with GPX4 to induce ferroptosis[46].
Nuclear factor red lineage 2-related factor (NRF2) is a redox-sensitive transcription factor that may facilitate antioxidation and exert resistance to ferroptosis. NRF2 targets several antioxidant genes: NAD(P)H, glutathione S-transferase (GST), γ-glutamylcysteine synthase, heme oxygenase-1 (HO-1), etc.[48]. Keap1 can bind to NRF2 and plays a central role in ferroptosis by participating in iron and ROS metabolism through the p62-Keap1-NRF2 pathway[8].
THE FERROPTOSIS RESEARCH PROGRESS IN THE TREATMENT OF EC
The prognosis of EC predicted by ferroptosis-related genes
A systematic analysis by Zhu et al[49] determined that four ferroptosis-related genes (FRGs) (CARS1, GCLM, GLS2, and EMC2) held prognostic value for overall survival (OS) in EAC patients. GLS2, CARS1, and EMC2 all induced ferroptosis and were positively correlated with the prognosis of patients, whereas GCLM as the EAC oncogene exerted a suppressive effect on ferroptosis[49]. Lu et al[50] identified 45 FRGs in ESCC patients, among which SCP2, mitogen-activated protein kinase (MAPK), and PRKAA1 possessed strong prognostic value for ferroptosis. Seven FRGs (ALOX12, ALOX12B, ANGPTL7, DRD4, MAPK9, SLC38A1 and ZNF419) were selected as biomarkers in the study of Song et al[51], where ANGPTL7, MAPK9 and ZNF419 were risk genes, while ALOX12, ALOX12B, DRD4 and SLC38A1 were protective genes. Zhao et al[52] identified six signature genes from 117 genes for iron metabolism and ferroptosis associated with prognosis, CNV, TMB and immune cell infiltration in ESCC (PRNP, SLC3A2, SLC39A8 and SLC39A14 had a poor prognosis in ESCC patients, in contrast to ATP6V0A1 and LCN2). Lu et al[53] used TCGA and GEO databases to screen out three prognostically related differentially expressed immune-related ferroptosis genes (DDIT3, SLC2A3 and GCH1), all of which were risk factors for EC patients. Recently, centrosomal and spindle pole-associated protein (CSPP1) has been identified as a novel cancer biomarker. Several studies observed increased expression of CSPP1 in EC, whereas overexpression of CSPP1 inhibited ferroptosis while promoting tumor growth[54].
The FRGs screened in these studies possess an independent prognostic value and can predict the prognosis of EC patients. By targeting these genes, the treatment plan for EC patients can be continuously updated and improved. These findings also provide new ideas and perspectives for the clinical treatment of EC.
Therapeutic value of inhibition of the System Xc− on the cell membrane for EC
System Xc − on the cell membrane mainly transfers extracellular cystine into the cell and participates in the subsequent GSH synthesis. Targeting SLC7A11 and SLC3A2 in System Xc– thus offers an effective way to induce ferroptosis and inhibit tumor proliferation and metastasis. The common chemotherapeutic drugs erastin, sulfasalazine, sorafenib, imidazole ketone erastin are based on this mechanism[5,22,55,56]. High levels of SLC7A11 expression are associated with resistance to radiotherapy, and cancer cells survive under the condition of oxidative stress by upregulating SLC7A11 expression, which mediates cystine uptake[57]. SLC7A11 is highly expressed in TE13 and KYSE170 cells, and knockdown of SLC7A11 using siRNA inhibits the G1-S phase, suggesting that SLC7A11 could be an independent prognostic factor for ESCC[58]. Furthermore, knockdown of SLC7A11 makes p53 knockout (p53-KO) cells susceptible to oxidative stress and restores the radiosensitivity of EAC to cancer therapeutic agents[59]. Inhibition of the SLC7A11-glutathione axis reduces cancer growth and overcomes tumor drug resistance. The mechanism of radioresistance mediated by NRF2, a transcription factor that regulates cellular antioxidation, is unknown. A study in 2021 revealed that activated NRF2 may directly bind to the promoter region of SLC7A11, regulate its transcription, and mediate the radioresistance of ESCC through SLC7A11-mediated ferroptosis inhibition[60]. Consequently, targeting the NRF2/SLC7A11/ferroptosis axis makes it possible to combat the drug resistance of EC and even other tumors in the future.
Most studies have focused on SLC7A11, with little research on SLC3A2. Ma et al[61] found that YTHDC2 could act as an endogenous ferroptosis inducer in lung adenocarcinoma (LUAD) and inhibited SLC3A2 via inhibiting HOXA13 in an indirect m6A manner. SLC3A2 facilitates osteosarcoma growth through the regulation of the PI3K/Akt signaling pathway[62]. A study by Li et al[63] similarly elaborated that overexpressed SNHG1 activated the Akt pathway by regulation of SLC3A2, thereby promoting sorafenib resistance. In summary, SLC3A2 may be a promising anticancer target in the future.
Therapeutic role of GPX4 inhibitors in EC
The expression level of GPX4 is significantly elevated in most tumors. The scavenging of cell membrane Lipid-OOH by GPX4 is the main mechanism of resistance to the occurrence of ferroptosis. Current GPX4 inhibitors in use contain (1S,3R)-RSL3, FIN56, and ML210 which inhibit GPX4 activity by binding to the active site of GPX4 or by degrading GPX4[6,45,64]. A study showed that Hsp27 rescued EC stem cells from ferroptosis-induced cell death by upregulating GPX4, which was associated with poor prognosis in EC patients[65]. Western blot results suggested that DNAJB6a overexpression downregulated GPX4 and promoted ferroptosis in ESCC, thus playing an important anticancer role[66]. In the current study, the neuropeptide LGI1 receptor ADAM23 may lead to ferroptosis in ESCC cells due to the depletion of GPX4, SLC3A2 and SLC7A11[67]. Shishido et al[10] verified in RT-PCR and western blot experiments that 5-ALA restrained GPX4 and overexpressed HO-1 in ESCC tissues. 5-ALA induced ferroptosis in ESCC, suggesting a strong anti-tumor effect. In the RNA immunoprecipitation assay, OIP5-AS1 bound to GPX4, while OIP5-AS1 knockdown inhibited EC cell proliferation by downregulating GPX4[68].
Cancer cells in the high mesenchymal state are dependent on GPX4 for survival and are more sensitive to GPX4 inhibitors[69]. Therefore, the ability of GPX4 inhibitors to eliminate drug resistance in cancer cells is often combined with targeted therapy for tumors and radiotherapy as a way to prevent tumor recurrence. However, the combination of multiple drugs often generates greater side effects for patients. Hangauer et al[69] proposed that targeted therapy or chemotherapy before or after treatment with GPX4 inhibitors, an alternating therapy that improves efficacy as well as reduces toxic side effects. Targeting GPX4 provides a new strategy for treating tumors.
Therapeutic role of competing endogenous RNAs associated with EC ferroptosis
Competing endogenous RNAs (ceRNA) is a hot research topic in recent years, which emphasizes a mutual regulatory mechanism between RNAs. CeRNA regulatory network links microRNA (miRNA), mRNA, long non-coding RNA (lncRNA), circular RNA (circRNA), etc. MiRNA silences mRNA by binding to miRNA response elements (MREs) located on mRNA[70]. CeRNAs (mainly lncRNA and circRNA) containing MREs regulate gene expression by competitively binding to miRNAs[70]. As biological markers, ceRNAs perform an active role in targeted cancer therapy[71,72]. It has been reported that miR-27a inhibited the occurrence of ESCC by targeting KRAS[73]. The expression level of miR-27a-3p increases following p53-KO, whereas TP53 is associated with sensitivity to ferroptosis[59,74]. The downregulation of miR-27a reversed multidrug resistance in ESCC[75]. CircBCAR3 (has_circ_0007624) is highly expressed in EC and upregulates transporter protein-1 (TNPO1) through the action of sponging miR-27a-3p, thus promoting EC cell proliferation, migration, invasion and ferroptosis in vitro[76]. Lu et al[53] found that TMEM161B-AS1 also sponged miR-27a-3p and upregulated the expression of GCH1, promoting EC development. This suggested that miR-27a-3p and TMEM161B-AS1 may affect the ferroptosis process in EC by regulating the GCH1/BH4/DHFR axis.
As previously described, ADAM23 induces ferroptosis in ESCC cells. Chen et al[67] investigated the ceRNA regulatory mechanism of ADAM23: ARHGEF26-AS1 increased ADAM23 expression by regulating the miR-372-3p/ADAM23 axis, thus upregulating the ferroptosis pathway. Yao et al[77] demonstrated that circPVT1 accelerated the cancer progression in ESCC by targeting the miR-30a-5p/FZD3 axis. In the experiment, circPVT1 was significantly upregulated in ESCC cells resistant to 5-fluorouracil, and the knockdown of circPVT1 increased ferroptosis by downregulating p-β-linked protein, GPX4 and SLC7A11. A recent study showed that SLC7A11 was regulated by miR-513a-3p, which is mediated by lncRNA BBOX1-AS1 in ESCC cells[78]. Silencing BBOX1-AS1 through miR-513a-3p/SLC7A11 axis could provide new insights into EC therapy. Circ0120816 acts as a sponge for miR-1305 to promote ESCC development, while miR-1305 exerts tumor suppressive effects in ESCC by directly targeting and inhibiting TXNRD1, a key enzyme in GSH synthesis[79]. Targeting circ0120816 may affect the initiation of ferroptosis by inhibiting the synthesis of GSH.
The study of ceRNAs is a hot topic today, which can explain the regulation of genes on a macroscopic level. Targeting any link of the ceRNA axis can interfere with the initiation of ferroptosis in EC. Studying the regulatory network of ceRNAs associated with ferroptosis develops a variety of new pathways for targeted therapy of esophageal tumors.
The role of chemoradiotherapy in ferroptosis on EC
In the case of patients with advanced EC who cannot undergo surgery, radiotherapy along with concurrent chemotherapy is often recommended[80]. However, resistance to radiotherapy often limits the prognosis of EC patients. Radiotherapy or chemotherapy has recently been discovered to induce ferroptosis in cancer patients, while increased ferroptosis enhances patients' sensitivity to radiotherapy. Lei et al[81] found that ionizing radiation (IR) induced expression of the ferroptosis marker gene PTGS2 in EC cells and that 4-HNE levels were elevated in EC cell line FLO-1 cells after IR treatment. Thus, IR induces lipid peroxidation, which makes EC cell lines attenuate the resistance to ferroptosis. Luo et al[19] revealed that MF-438 significantly improved the effectiveness of radiotherapy in ESCC by targeting stearoyl coenzyme desaturase (SCD1) to improve radiosensitivity. The team analyzed that SCD1 was responsible for the production of MUFAs (oleic and palmitoleic acids) which tended to reduce ferroptosis in tumor cells, suggesting a role for SCD1 resistance to ferroptosis. Oleic acid protects melanoma cells derived from lymph nodes from ferroptosis in an ACSL3-dependent manner. Ori, a diterpenoid extracted from rabdosia rubescens, has been demonstrated to possess anticancer activity and is curative in the treatment of EC. As is shown in the study by Fan et al[82], rabdosia rubescens significantly improved the survival rate of patients with early EC, while rabdosia rubescens also enhanced the effect of chemotherapy treatment in patients with advanced EC. It was shown that Ori could block the γ-glutamyl cycle in EC cells TE1 by inhibiting γ-glutamyl transpeptidase 1 (GGT1) activity and also covalently bound to cysteine to form conjugated Ori-cysteine[11]. As a result, Ori inhibits the cystine/GSH/GPX4 axis, thereby inducing ferroptosis and exerting antitumor activity. Brusatol, also a diterpenoid, inhibits the growth of EAC cells by targeting NRF2. And Brusatol alone or in combination with cisplatin (CDDP) induced significant lipid peroxidation and ferroptosis[83]. It is obvious that Brusatol alone or in conjunction with chemotherapy works primarily through ferroptosis. Eprenetapopt, the first TP53 inhibitor, has a better therapeutic effect in combination with the conventional anticancer drug (azacitidine)[84,85]. Fujihara et al[86] observed reduced levels of GSH in EC cells OACM5.1 following non-targeted metabolomic and label-free quantitative proteomic analyses of EC cells OACM5.1 treated with eprenetapopt. Eprenetapopt induces ferroptosis by triggering GSH depletion, thereby limiting tumor proliferation. In addition, the combination of glycine restriction and eprenetapopt significantly suppressed esophageal tumor growth and delayed the onset of disease, thus prolonging overall survival[86].
Some classical anticancer drugs can promote ferroptosis in various processes, such as blocking System Xc-, depleting GSH, affecting GPX4 enzyme activity, etc. Currently, the induction of ferroptosis can inhibit tumor proliferation and effectively improve the prognosis of cancer patients. Chemoradiotherapy combined with ferroptosis treatment could be a novel approach to improve the sensitivity of tumors to chemoradiotherapy.
THE CROSSTALK BETWEEN FERROPTOSIS AND IMMUNOTHERAPY
Immunotherapy has been recognized as a promising modality in cancer treatment, including four major categories: Adoptive cell therapy, ICIs, non-specific immune activators, and cancer vaccines. The mainstream application of ICIs in immunotherapy holds promise. ICIs block CTLA-4 and PD-1/PD-L1 immune checkpoints that limit immune escape of tumors[13]. However, only a minority of patients receive durable and stable therapeutic effects with ICIs, while limited clinical efficacy is the normality for most tumor patients. Recent findings indicate that CD8+ T cells could promote ferroptosis in tumor cells during cancer immunotherapy[18]. The crosstalk between ferroptosis and anti-tumor immunity may lead to new directions in cancer therapy.
TIME includes tumor cells, immune cells, and cytokines, etc. Immune cells such as T cells, B cells, macrophages, and neutrophils have a broad effect on human diseases. Ferroptosis, however, is a double-edged sword for immunotherapy, affecting immune cells in two different ways: promoting immune function on the one hand, weakening it on the other hand[87]. Consequently, the roles of ferroptosis-related pathways on different aspects of immune cell subsets have different effects on prognosis. Ferroptosis can affect the number of immune cells to suppress the immune function of immune cells. It has been found that T cell-specific GPX4-deficient mice, despite normal thymic T cell development, accumulated lipid peroxides and ferroptosis in peripheral T cells due to the lack of GPX4[88]. In contrast, selenium supplementation enhances GPX4 expression in T cells, protects follicular helper T (TFH) cells from ferroptosis, thereby increasing TFH cell numbers and promoting antibody response[89]. Ferroptosis also activates the immune function of immune cells. Cinnamaldehyde dimer (CDC), a GSH-depleting dimer, causes intracellular GSH depletion after the rupture in the cytoplasm. Zhou et al[90] reported that the combination of CDC and sorafenib resulted in a significant enhancement of ferroptosis in "cold" tumors, as well as triggering a strong immune response in vivo by stimulating the maturation of dendritic cells and the initiation of CD8+ T cells.
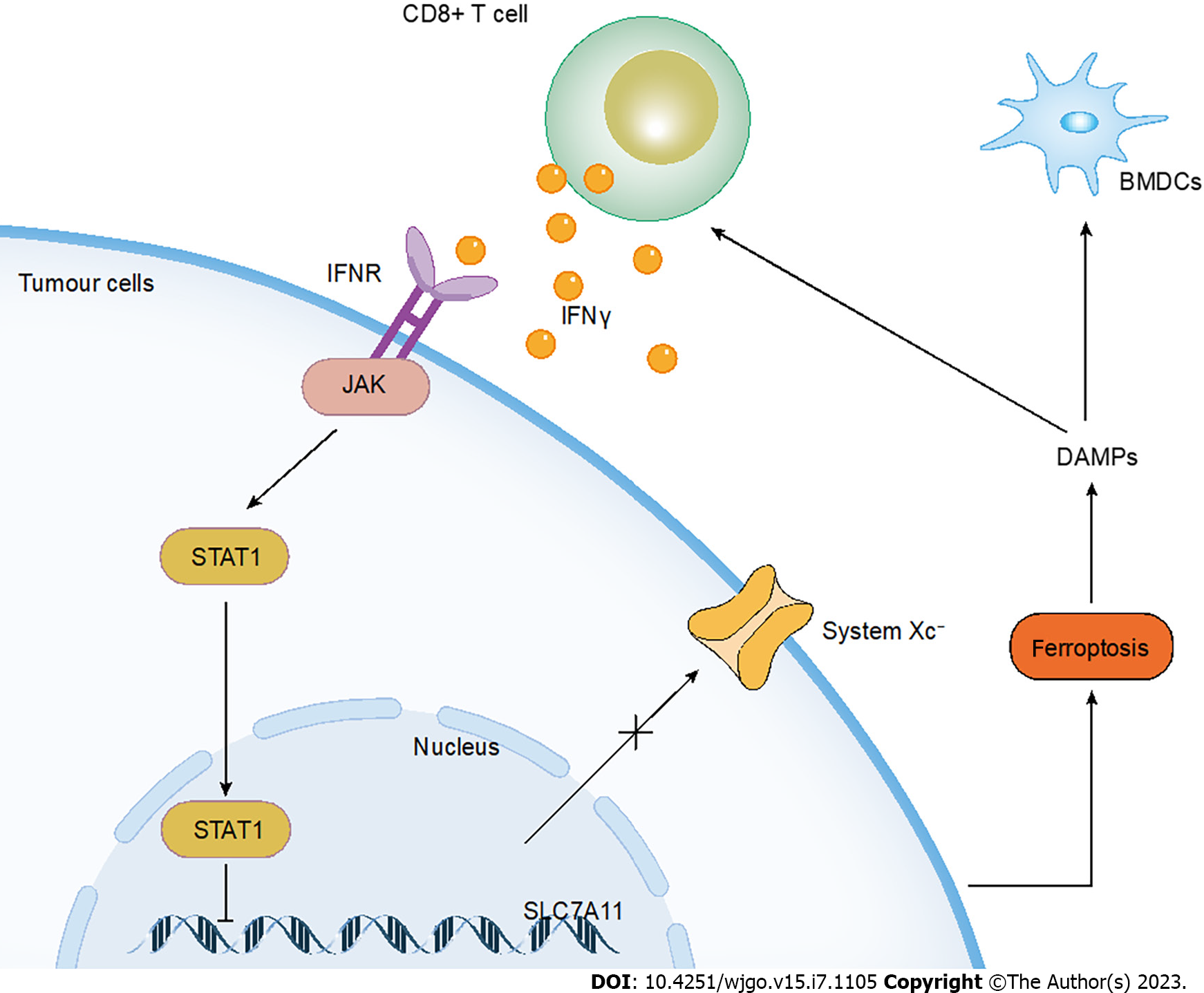
T cells consist of distinct subpopulations such as T helper (Th) cells, cytotoxic CD8+ T lymphocytes (CTLs), Treg cells, etc. CTLs are often considered to release perforin and granzyme and induced FasL-mediated apoptosis so as to kill tumor cells[91]. However, CD8+ T cells have been confirmed to downregulate the expression of two subunits of System Xc-, SLC3A2 and SLC7A11, thus inhibiting cystine uptake of tumor cells and facilitating ferroptosis in tumors. The specific mechanism is that IFNγ released by CD8+ T cells activates the janus kinase (JAK) and signal transducer and activator of transcription (STAT) 1-mediated signaling pathway, whereas STAT1 can bind to the transcriptional start site of SLC7A11[18] (Figure 2). The Ataxia- Telangiectasia mutated gene (ATM) activated after radiotherapy can synergistically suppress SLC7A11 with activated CD8+ T cells[92]. The same team also demonstrated that naive human and mouse CD4 and CD8 T cells were relatively insensitive to cell death induced by erastin or RSL3, whereas tumor cells were susceptible to ferroptosis[18]. IFNγ produced by CD8+ T cells also stimulates ACSL4 and alters lipid patterns of tumor cells, thereby increasing AA incorporation into phospholipids containing C16 and C18 acyl chains. Low doses of AA enhance the antitumor immune effect of ICIs by enhancing tumor ferroptosis[93]. On the other hand, CD36 mediates the uptake of fatty acids on tumor-infiltrating CD8+ T cells, inducing lipid peroxidation and ferroptosis, leading to reduced cytotoxic cytokine production and impaired antitumor capacity. Blocking CD36 restores the antitumor activity of CD8+ T cells and exerts a greater antitumor effect in combination with PD-1 antibodies[94]. Treg cells, known as suppressor T cells at the early stage, exhibit immunosuppressive effects. A recent study showed that deletion of GPX4 mediated ferroptosis of Treg cells in response to T cell receptor (TCR)/CD28 co-stimulation, which inhibited tumor growth and simultaneously enhanced antitumor immunity. Likewise, GPX4-deficient Treg cells mediated the output of mitochondrial superoxide and interleukin-1β (IL-1β) that facilitated Th17 cell responses[17]. Therefore, inducing Treg cells to undergo ferroptosis may become a novel avenue for immunotherapy.
Figure 2 The crosstalk between ferroptosis and immunotherapy.
The IFNγ released by CD8+ T cells activates ferroptosis in tumor cells through the JAK/STAT1 pathway. Ferroptotic tumor cells then release DAMPs which contributes to immune activation. IFN: Interferon; DAMPs: Damage-associated molecular patterns.
Tumor-associated macrophages (TAMs) play a key role in cancer progression, divided into two subtypes: Classically activated M1 macrophages and alternatively activated M2 macrophages. M1 macrophages play an antitumor role; M2 macrophages promote tumor proliferation, invasion, metastasis, and angiogenesis[95]. Hence, the primary object of immunotherapy is to convert M2 macrophages in an immunosuppressed state to M1 macrophages with tumor-killing activity. The ability of macrophages to engulf and remove dead cells contributes to the efficiency of immunotherapy. 1-steaoryl-2-15-HpETE-sn-glycero-3-phosphatidylethanolamine (SAPE-OOH), acted as an "eat me" signal on the surface of ferroptotic cells, targets the TLR2 receptor on macrophages that improves the phagocytosis and clearance ability of macrophages to ferroptotic cells[96]. M1 macrophages produce high NO• production due to a higher content of inducible NO synthase (iNOS) than M2 macrophages. iNOS/NO• can substitute the role of GPX4 to inhibit RSL3-induced ferroptosis. Thus, M1 polarized macrophages are highly resistant to ferroptosis, while M2 macrophages lacking iNOS show high sensitivity to ferroptosis[97]. The application of ferroptosis in M1 macrophages is often used for anticancer therapy. A biomimetic magnetosome composed of Fe3O4 magnetic nanocluster (NC) as the core and TGF-β inhibitor (Ti) and PD-1 antibody (Pa) as the coat enters the tumor and increases H2O2 in polarized M1 macrophages, thus promoting the Fenton reaction with Fe ions released from the magnetosome. The application of this magnetosome promotes ferroptosis in the tumor, and the exposed tumor antigen in turn promotes an immune response[98]. Currently, nanoparticle (NP) is frequently used for ferroptosis and to enhance immune efficacy. Hsieh et al[99] reported that zero-valent-iron nanoparticle (ZVI-NP) enhanced the degradation of NRF2 and triggered ferroptosis in lung cancer cells through oxidative stress and lipid peroxidation. ZVI-NP induced the polarization of TAM towards the M1 phenotype that increased the cytotoxic function of CD8+ T cells and decreased the Treg cell ratio, thereby enhancing antitumor immunity. The biomimetic magnetic nanoparticles Fe3O4-SAS@PLT, triggering ferroptosis pathway by inhibiting System Xc−, that mediated ferroptosis not only induced tumor-specific immune response but also repolarized M2 macrophages to M1 phenotype[100].
DC, a powerful antigen-presenting cell that activates CTL, contributes importantly to the maintenance of the immune environment. As a nuclear receptor involved in the regulation of lipid metabolism, PPARG/PPARγ is responsible for RSL3-induced ferroptosis in DC. Ferroptotic DCs fail to secrete pro-inflammatory cytokines (TNF and IL6) and lose the ability to express MHC class I in response to maturation signals of lipopolysaccharide, and even fail to induce IFNG/IFNγ production by CD8+ T cells[101]. Taken together, ferroptosis occurring in DCs limits antitumor immunity.
Immunogenic cell death (ICD) is the anti-tumor immune response of the organism caused by the conversion of non-immunogenicity to immunogenicity[102]. Cancer cells generate a series of endogenous danger signals during this process, which are called damage-associated molecular patterns (DAMPs). DAMPs mainly include calreticulin (CRT), high mobility group protein 1 (HMGB1), ATP, and so on[103,104]. Lately, HMGB1 is shown to be a DAMP released by ferroptotic cells in an autophagy-dependent manner. Ferroptosis activators, including erastin, sorafenib, RSL3, and FIN56, induce HMGB1 release in both cancer and non-cancer cells[103]. Therefore, probing the immunogenicity of ferroptotic cancer cells broadens the scope of immunogenicity that offers new possibilities for cancer therapy. Efimova et al[105] were the first to demonstrate that ferroptosis-induced ICD in both vivo and vitro. Ferroptotic cells promote phenotypic maturation of bone marrow-derived dendritic cells (BMDCs) and induce vaccination-like effects in immunocompetent mice (Figure 2). Meanwhile, ATP and HMGB1 were shown to be immunogenic signals for early ferroptotic cancer cells[105]. Acidity-activatable dynamic nanoparticles significantly recruited tumor-infiltrating T lymphocytes for IFN γ secretion, sensitizing tumor cells to RSL-3-induced ferroptosis and thus triggering the immunogenicity in ferroptotic tumor cells[106]. Unfortunately, no advanced ferroptotic cancer cells have been observed to be immunogenic. Therefore, the study of the immunogenicity of ferroptotic cancer cells remains a difficult problem today.
THE MECHANISM OF FERROPTOSIS IN THE IMMUNOTHERAPY OF EC
In recent years, breakthroughs have been made in the application of immunotherapy of EC patients. Pembrolizumab (the PD-1 inhibitor) plus chemotherapy (cisplatin and 5-fluorouracil) was approved for the first-line treatment of advanced EC[14]. However, more sufficient evidence-based medical evidence is still needed for immunotherapy in the first-line treatment. In the field of basic cancer research, there have been many studies exploring the role of ferroptosis in cancer immunotherapy. Therefore, the utilization of ferroptosis in immunotherapy provides a new option for the personalized treatment of EC patients. A number of studies exhibited that FRG signature was observed to be closely associated with immunity in EC. Lu et al[50] investigated immune cell infiltration in the tumor microenvironment using the CIBERSORT and ESTIMATE algorithms and found that CD8 T cells, CD4 memory-activated T cells, and M0, M1, and M2 in ESCC patients macrophages were significantly correlated with the screened ferroptosis gene signature[50]. Zhu et al's analysis results showed differences in immune status between high and low risk groups of EAC patients, with higher levels of DC and CD8 + T cells in the low risk group. In addition, the FRGs (GCLM, GLS2) were significantly associated with CD8+ T cells[49]. This suggests to us that ferroptosis may interact with CD8+ T cells to regulate the immune response in EAC patients. In the study by Song et al[51], the ferroptosis signature consisting of seven FRGs was found to be associated with most immune checkpoints, such as MAPK9 interacting with CTLA-4 and SLC38A1 blocking the effectiveness of PD-L1 antibodies. Niu et al[107] evaluated the level of immune cell infiltration for ferroptosis and iron metabolism-associated lncRNAs in TCGA and GEO cohorts, respectively, demonstrating the role of ferroptosis-associated lncRNAs in the regulation of immune infiltration in ESCC[107]. Meanwhile, by studying ten ferroptosis-associated lncRNAs, Zhu et al[108] also revealed significant differences in the TIME of ESCC patients between high- and low-risk groups, as well as the differences in the clinical benefits reaped. Hence, ferroptosis-associated lncRNAs may become immunotherapeutic targets for EC patients. While Lu et al[50] innovatively constructed a prognostic model of EC patients consisting of immune-related FRGs, which closely linked immunity and ferroptosis. The team detected that EC patients in the high-risk group benefited more from ICIs, which provided a personalized immunotherapy regimen for EC patients.
Key genes in the ferroptosis process play an important role in cancer progression, such as GPX4, SLC7A11, and FSP1. Recently, the expression levels of these genes were found to correlate with immune infiltration in pan-cancer. Shi et al[109] found that in EC, GPX4 expression was significantly associated with macrophage and DC infiltration, and AIFM2 expression was significantly associated with CD4 T cell infiltration. Simultaneously, GPX4 expression was positively correlated with the expression levels of monocyte markers (including CD14 and CD115) and M2 macrophage markers (including VSIG4 and MS4A4A) in EC. To summary, there may be a close interaction between GPX4 and macrophages in EC patients.
It seems that the ICD induces antitumor efficacy in EC patients. Zhang et al[110] integrated different cell death signals in ESCC and classified patients into different ICD subtypes. Transcriptomic and proteomic characterization of patients showed that cell death signals, such as ferroptosis, were positively correlated with immune activation in ESCC. A recent study explored the immunogenicity of targeting SCD1-induced ferroptotic ESCC cells. Ferroptotic ESCC cells contribute to DC activation; inhibition of SCD1, a novel ICD inducer, effectively improves the prognosis of ESCC patients[19]. Triggering the immunogenicity of ferroptotic cancer cells could lead to the discovery of new therapeutic targets, a promising therapeutic strategy in patients with EC. However, there are few reports on the immunogenicity of ferroptotic cancer cells in EC. Thus, more experimental studies and clinical validation are required to stimulate the immunogenicity of ferroptotic cancer cells in EC.
CONCLUSION
Patients with advanced EC are often treated with concurrent chemoradiotherapy, plus surgery. However, EC has a poor prognosis due to the development of resistance to chemoradiotherapy. The role of ferroptosis in a variety of cancers has been revealed. Based on the keywords "esophageal cancer" and "ferroptosis", we searched the PubMed database. Most of the studies on ferroptosis in EC are based on FRGs screened by the database, which may have independent prognostic value as protective and risk factors for EC patients, respectively. Some chemotherapeutic agents, such as erastin, sulfasalazine, sorafenib, as well as IR can induce ferroptosis. In turn, targeting essential points in the ferroptosis pathway also induces EC sensitivity to radiotherapy, such as inhibition of SLC7A11 to overcome radioresistance. Ferroptosis combined with radiotherapy could be a viable option for EC therapeutics. Currently, anticancer therapy for EC can promote ferroptosis in cancer cells and inhibit the proliferation of esophageal tumors by targeting System Xc-, GPX4, NRF2, etc. Moreover, the study of the ceRNA regulatory network of ferroptosis in EC may explain the gene regulation process from a macroscopic perspective, thus providing more possibilities for the treatment of EC patients.
Immunotherapy has been reported more frequently in EC. Meanwhile, studies have been conducted to elaborate on the complex mechanisms between ferroptosis and immunotherapy. During our search for articles related to the role of ferroptosis in EC, we identified that there was a close correlation between FRGs and immune infiltration. In addition, key genes in the process of ferroptosis were also associated with immunity. However, little has been reported about the mechanisms of ferroptosis in EC immunotherapy. Only a few reports were revealed to elaborate on the immunogenicity of ferroptotic EC cells. Induction of ferroptosis in cancer cells may promote the expression of their immunogenicity and in turn the anticancer activity of immune cells. Ferroptosis-related immunogenicity seems to broaden the scope of EC immunotherapy, which provides personalized treatment options for EC patients.
Since ferroptosis in EC has been studied mainly by some screening FRGs, future research should focus on the study of ferroptosis regulatory mechanisms. We can focus on one or two genes and explore their mechanisms of action in EC from different histologies (genomics, transcriptomics, proteomics, methylomics, lipidomics and metabolomics), followed by experimental validation. The research on ferroptosis in tumor immunotherapy is still in its infancy. Taken together, it is needed for us to further explore the mechanisms of ferroptosis-related immunogenicity in EC in the future to find more evidence of ferroptosis in the immunotherapy of EC patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fu ZC, China; Hsu C, Taiwan S-Editor: Yan JP L-Editor: A P-Editor: Yu HG