Published online Jun 15, 2023. doi: 10.4251/wjgo.v15.i6.1062
Peer-review started: January 13, 2023
First decision: February 9, 2023
Revised: February 23, 2023
Accepted: April 23, 2023
Article in press: April 23, 2023
Published online: June 15, 2023
Processing time: 152 Days and 15.5 Hours
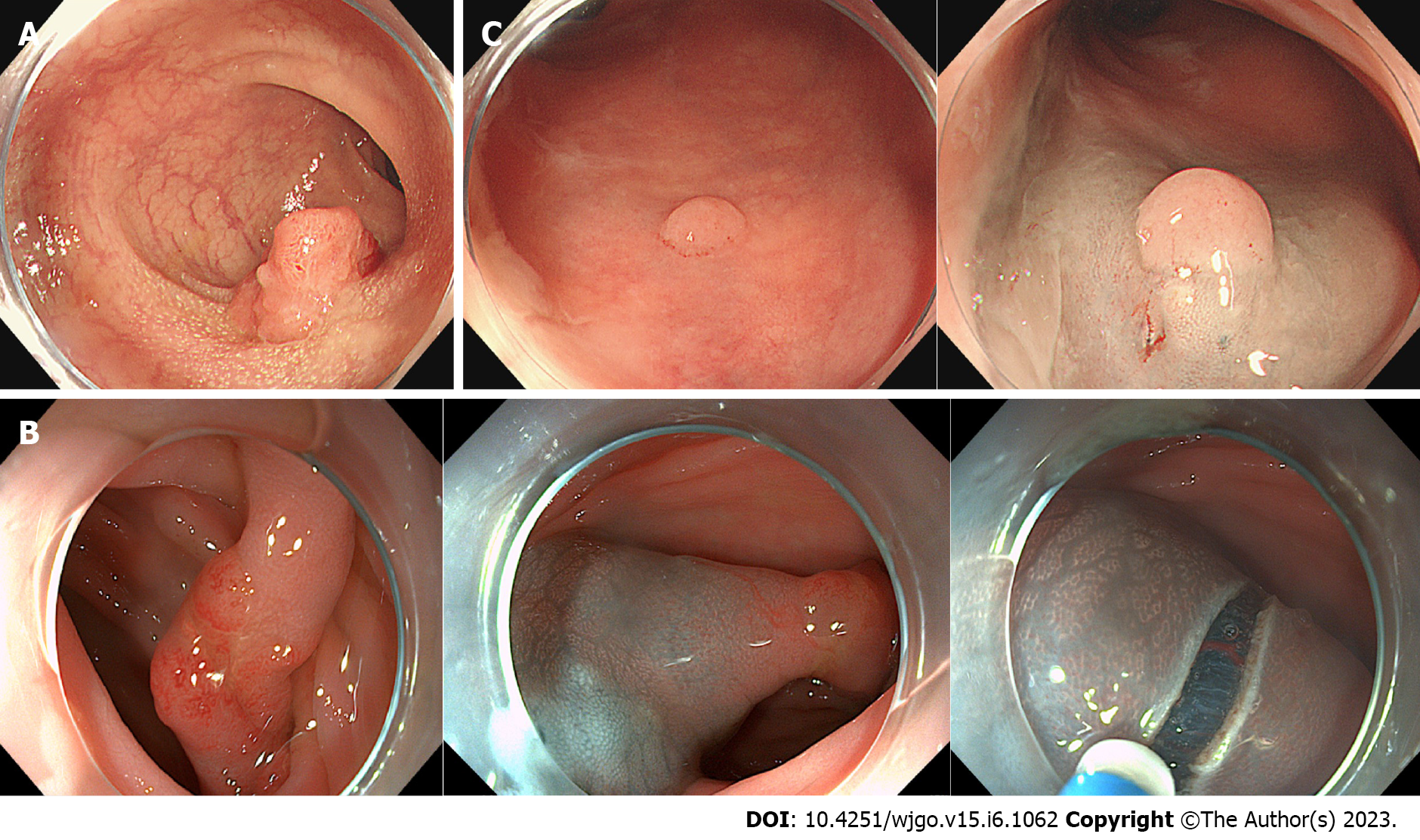
Chicken skin mucosa (CSM) surrounding colon polyps is a common endoscopic finding with pale yellow-speckled mucosa during a colonoscopy screening. Although reports about CSM surrounding small colorectal cancer are scarce, and its clinical significance in intramucosal and submucosal cancers is unclear, previous studies have suggested it could be an endoscopic predictive marker for colonic neoplastic and advanced polyps. Currently, because of the inaccurate preoperative evaluation by endoscopists, many small colorectal cancers, particularly lesions with a diameter < 2 cm, are improperly treated. Therefore, more effective methods are required to better assess the depth of the lesion before treatment.
To explore potential markers of small colorectal cancer early invasion under white light endoscopy, providing patients with better treatment alternatives.
This retrospective cross-sectional study included 198 consecutive patients [233 early colorectal cancers (ECCs)] who underwent endoscopy or surgical proce
The submucosal carcinoma (SM stage) was larger than the mucosal carcinoma (M stage) with a significant difference (17.2 ± 4.1 vs 13.4 ± 4.6 mm, P < 0.01). M- and SM-stage cancers were common in the left colon; however, no significant differences were found between them (151/196, 77% and 32/37, 86.5%, respectively, P = 0.199). The endoscopic features of colorectal cancer revealed that CSM, depressed areas with clear boundaries, and erosion or ulcer bleeding were more common in the SM-stage cancer group than in the M-stage cancer group (59.5% vs 26.2%, 46% vs 8.7%, and 27.3% vs 4.1%, respectively, P < 0.05). CSM prevalence in this study was 31.3% (73/233). The positive rates of CSM in flat, protruded, and sessile lesions were 18% (11/61), 30.6% (30/98), and 43.2% (32/74), respectively, with significant differences (P = 0.007).
CSM-related small colorectal cancer was primarily located in the left colon and could be a predictive marker of submucosal invasion in the left colon.
Core Tip: Chicken skin mucosa (CSM) surrounding colorectal polyps is a relatively common clinical feature. Previous studies have found that it could be an endoscopic predictor of neoplastic and advanced colorectal polyps. However, it is unclear whether it is associated with early colorectal cancer or invasion. In our study, CSM-related small colorectal cancer was mainly found in the distal colon; this could be a potential predictive marker of submucosal invasion cancers located in the left colon. Since these cancers cannot be treated as a normal polyp, biopsy snare, cold-snare polypectomy, and cold-snare endoscopic mucosal resection may not be the appropriate options.
- Citation: Zhang YJ, Wen W, Li F, Jian Y, Zhang CM, Yuan MX, Yang Y, Chen FL. Chicken skin mucosa surrounding small colorectal cancer could be an endoscopic predictive marker of submucosal invasion. World J Gastrointest Oncol 2023; 15(6): 1062-1072
- URL: https://www.wjgnet.com/1948-5204/full/v15/i6/1062.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i6.1062
Abnormal colorectal adenomatous mucosa is widely used as an indication to screen for colorectal cancer early. Chicken skin mucosa (CSM) is a mucosal anomaly defined by a pale-yellow speckled pattern in the colon and rectum observed under conventional white light endoscopy. It was first reported in 1998[1] and is characterized by fat accumulation in the lamina propria macrophages. Previous studies suggest that CSM is caused by colonic intestinal metaplasia, toxic factors from damaged intraluminal mucosa, or previous mild damage. Recent studies have observed that it may effectively predict colorectal adenoma and adenoma carcinogenesis[2,3]. However, its significance in intramucosal and submucosal cancers is unclear. Therefore, we retrospectively analyzed the endoscopic results of 233 patients with early colorectal cancer (ECC) (< 20 mm in diameter), including the tumor location, morphology, CSM features, and other conventional white light endoscopic findings, to improve endoscopists’ understanding of the white light endoscopic features of invasive colorectal cancer (< 20 mm diameter) to prevent misdiagnosis and reduce non-curative resection, thereby improving patient outcomes.
CSM is a common endoscopic finding during a colonoscopy screening; it is a mucosal anomaly characterized by a pale-yellow speckled pattern surrounding the colon and rectum polyps observed before or after injection under conventional white light endoscopy. Patients aged between 18 and 85 years who underwent screening, surveillance, or diagnostic colonoscopy at the Digestive Endoscopy Center of Chengdu Second People’s Hospital between January 2021 and August 2022 were enrolled in this retrospective study. ECCs were screened based on the pathological results after endoscopic or surgical operation. These are cancers with an invasion depth limited to the mucosa and submucosa, regardless of lymph node metastasis.
Our study complied with the diagnostic criteria of the Japanese Colorectal Cancer Research Association[4]. Intramucosal carcinoma refers to cellular or structural atypia to a certain extent, and the lesions are limited to the mucosal layer, confining the focus to the mucosal layer. Submucosal carcinoma (SM-stage cancer) refers to lesions where atypical cells break through the muscularis mucosae and infiltrate the submucosa.
The inclusion criteria were as follows: (1) Receiving endoscopic or surgical treatment, including endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD) or surgical treatment, and pathologically confirmed colorectal cancer; and (2) a lesion diameter < 2 cm.
The exclusion criteria were as follows: (1) A history of colorectal carcinoma (CRC) or inflammatory bowel disease; (2) familial adenomatous polyposis; (3) poor bowel preparation; and (4) a history of malignancy at other sites.
Standard colonoscopes were used throughout (CF H260AI, CF Q260AI, or CF Q290AI, Olympus Limited, Tokyo, Japan), and lesion size was measured in vivo using open biopsy forceps with a deployed diameter of 7 mm (QYQ-AXC2.3X2300, Changchun Huichun Medical Devices, China).
Endoscopic findings: The endoscopic imaging data of the patients were reviewed, and when the record was unclear or controversial, an experienced doctor reconfirmed. The lesion location and size, morphological classification under endoscopy, and morphological indexes for predicting ECC depth reported in previous literature were analyzed.
(1) Location: Tumors were either in the right (including the cecum, ascending colon, transverse colon, or splenic flexure) or left (including the descending colon, sigmoid colon, and rectum) colon; and (2) Morphology: According to the Paris classification[4], there were three types of tumors based on their endoscopic morphological characteristics, including protruded (0-I), flat (0-II), and depressed (0-III) types. According to whether the lesions had no pedicle, type 0-I polyp was classified into pedunculated and sessile lesions.
The following parameters[5-7] facilitated ECC depth prediction: CSM, fold convergency, loss of lobulation, surface fullness, a depressed area with a clear boundary, a deeper red mucosal color, erosion or ulcer bleeding, and stalk swelling[5-8]. The relationship between our results and ECC depth was determined using statistical analysis.
Cure criterion: Tumor invasion limited to the mucosal layer is referred to as mucosal carcinoma (M-stage cancer), and invasion into the submucosal layer without muscularis propria invasion is known as SM-stage cancer. With a boundary of the submucosal membrane (SM) of 1000 μm, the penetration depth to the inferior margin of the mucosal layer was defined as superficial submucosal membrane carcinoma (SM1), and that of > 1000 μm was defined as deep SM-stage cancer (SM2 and SM3).
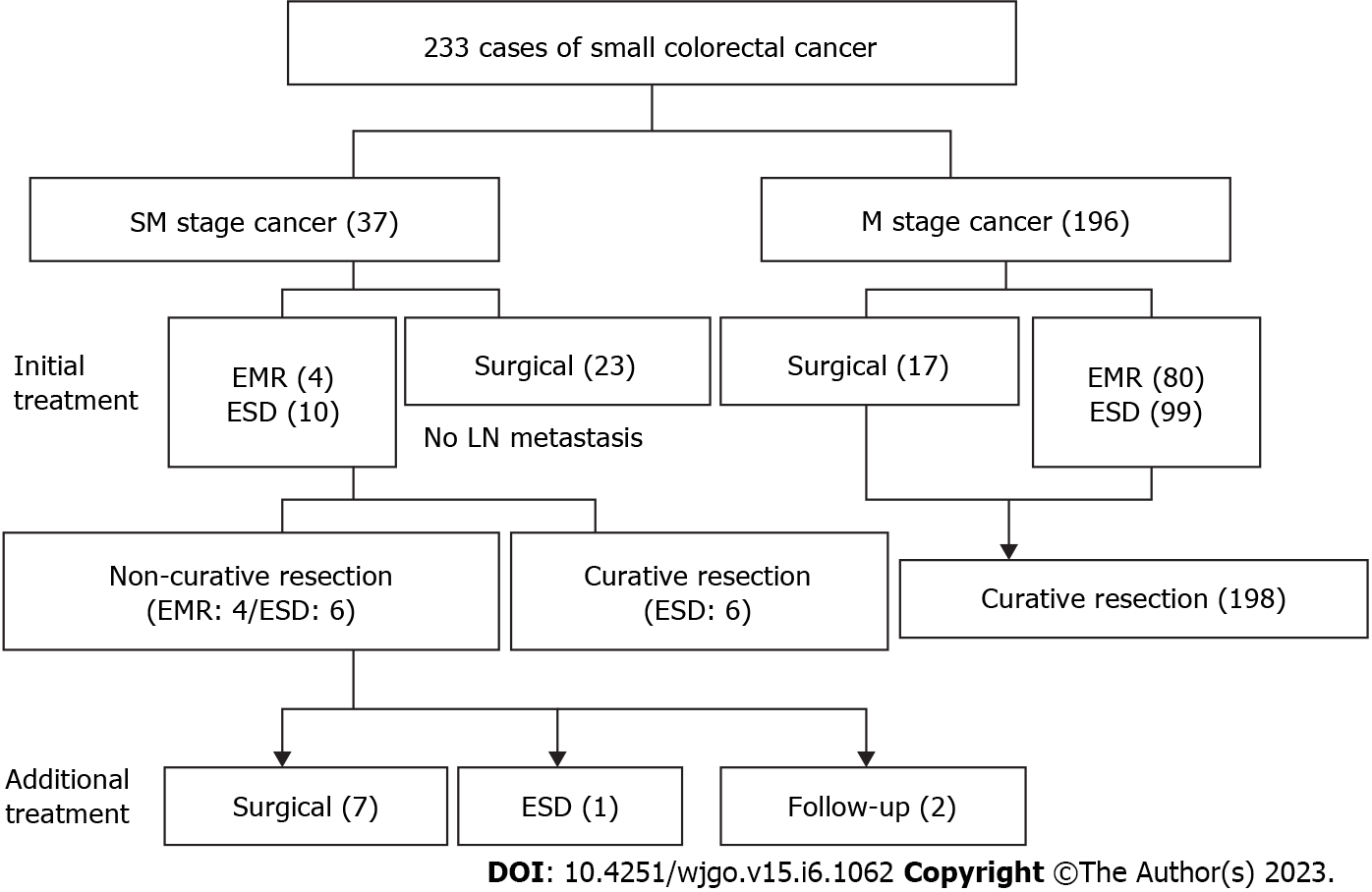
All the cases in this study were adenocarcinomas, and the submucosal invasion depth was measured in SM-stage cancer specimens resected using ESD. The requirements for curative resection were as follows: (1) R0 resection; (2) an invasion depth of M- or SM-stage cancer of < 1 mm from the muscularis mucosa, or an invasion depth of pedunculated SM-stage cancer of < 3 mm from the Haggitt’s level II; (3) no lymphatic or vascular vessel invasion; and (4) well- and moderately-differentiated adenocarcinoma. However, failure to meet any of the criteria above resulted in non-curative resection (Figure 1).
The statistical review of the study was performed by a biomedical statistician. Statistical analysis was performed using IBM SPSS Statistics, Version 23.0 (IBM Corp., Armonk, NY, United States) based on the pathological diagnosis. Continuous variables are expressed as mean ± SD. This study’s primary outcome was to evaluate the different endoscopic findings among small M- and SM-stage cancers. Patients’ baseline characteristics were analyzed using descriptive statistics. Furthermore, enumeration data are expressed as percentages or rates (%). Fisher’s exact test, the χ2 test, and Student’s t-test were used to analyze the patients’ basic characteristics, and the endoscopy revealed differences in CRC with different immersion depths. Statistical significance was considered at P < 0.05. Multivariate analysis was performed using logistic regressions. We included the data that had a level of significance at P < 0.05 or approximately 0.05 in the bivariate analysis as independent variables. This study’s statistical methods were reviewed by Yang Y and Chen FL from the Department of Chengdu Medical College Statistics.
Between January 2021 and August 2022, 233 ECC cases (198 patients) were diagnosed under endoscopy and pathologically confirmed at our hospital. There were 133 males and 65 females, with a male-to-female ratio of 2.05:1. The patients’ ages were 29-85 years (median, 62.2 years). There were 196 and 37 cases of M- and SM-stage cancers, respectively, with a ratio of 5.3:1. M- and SM-stage cancers were commonly observed in the left colon. The diameter of M-stage cancer was 1.34 ± 0.46 cm, and that of SM-stage cancer was significantly larger (1.72 ± 0.41 cm). The diameters of all SM-stage cancers were > 1 cm, and a statistical difference was observed between the groups (Table 1).
| SM (37) | M (196) | P value | ||
| Age (mean ± SD, yr) | 66.9 ± 9.6 | 61.24 ± 11.1 | 0.06 | |
| Sex | Male | 25 (67.6) | 110 (66.3) | 0.879 |
| Female | 12 (32.4) | 56 (33.7) | ||
| Size (mm) | 17.2 ± 4.1 | 13.4 ± 4.6 | < 0.001 | |
| Location | Right colon | 5 (13.5) | 45 (23) | 0.199 |
| Left colon | 32 (86.5) | 151 (77) | ||
The CSM prevalence in this study was 31.3% (73/233). The positive rates of CSM in flat, pedun
| CSM (+) 73 | CSM (-) 160 | P value | ||
| M | 51 (69.86) | 145 (90.63) | < 0.001 | |
| SM | 22 (30.14) | 15 (9.37) | ||
| Morphology | Flat (IIa, LST) | 11 (15.07) | 50 (31.25) | 0.007 |
| Pedunculated (Isp, Ip) | 30 (41.1) | 68 (42.5) | ||
| Sessile (Is, Is + IIc) | 32 (43.84) | 42 (26.25) | ||
| Location | Left colon | 66 (90.41) | 117 (73.13) | 0.03 |
| Right colon | 7 (9.59) | 43 (26.88) | ||
| Size | ≥ 10 mm | 65 (89.04) | 124 (77.5) | 0.037 |
| < 10 mm | 8 (10.96) | 36 (22.5) | ||
Based on the endoscopic morphological classification, elevated lesions (73.8%, 172/233) were more common than flat lesions (26.2%, 61/233). In this study, the proportions of flat and sessile lesions were higher than that of M-stage cancer; however, no significant difference was observed between the types at different depths of invasion. The number of laterally spreading tumors (LSTs) included in 0-IIA lesions and SM-stage cancer was low and could not be further stratified. In addition, the 0-IS + 0-IIC type was classified as 0-IS type, 0-IIA + 0-IIC type as 0-IIA type, and 0-IIC + 0-IIA type as IIc type (Table 3).
| Total | SM | M | P value | |
| Morphology | 233 | 37 | 196 | 0.252 |
| Flat | 61 (26.18) | 12 (32.43) | 49 (25) | |
| Pedunculated | 98 (42.06) | 11 (29.73) | 87 (44.39) | |
| Sessile | 74 (31.76) | 14 (37.84) | 60 (30.61) |
CSM is a depressed area with clear boundaries, erosion, or ulcer bleeding, and these features are more common in SM-stage cancers. In total, 59.5% (22/37) of cases had “chicken skin” changes in the basal mucosa around the SM-stage cancer lesion; however, the corresponding number in M-stage cancer was 26.2% (51/196), and the difference was significant (P < 0.001). In 45.9% (17/37) of SM-stage cancers, local depressions with clear boundaries were observed, whereas these were observed in 8.7% (17/196) of M-stage cancers, and the difference was significant (P < 0.001).
The proportion of local erosion or ulcer bleeding in M-stage cancer was 4.1% (8/196), whereas it was 27.3% (10/37) in SM-stage cancer, and the difference was significant (P < 0.01). There were 98 0-Ip and 0-Isp lesions, including 87 M- and 11 SM-stage tumors. Stalk swelling was observed in 13.8% (12/87) and 54.6% (6/11) of M- and SM-stage tumor cases, respectively. The P value of Fisher’s exact probability analysis was 0.04. However, its significance should be further explored using data from a larger sample. Therefore, if “chicken skin” changes and local depressions with clear boundaries, local erosion, or ulcer bleeding are observed under white light endoscopy, possible lesion invasion of the submucosa should be considered. However, no significant difference was observed in the proportion of mucosal fold convergency, loss of lobulation, surface fullness, and deeper red mucosal color between M- and SM-stage cancers (P > 0.05) (Table 4). Logistic regression results showed that CSM, a depressed area with clear boundaries, erosion, or ulcer bleeding, and size (≥ 10 mm) were independent risk factors for submucosal invasion in ECC (Table 5).
| Endoscopic finding | SM (37) | M (196) | P value |
| (All) CSM | 22 (59.5) | 51 (26.2) | < 0.001 |
| (All) Ulceration or errhysis | 10 (27.3) | 8 (4.1) | < 0.001 |
| (All) Demarcated depressed area | 17 (46) | 17 (8.7) | < 0.001 |
| (All) Deeper red mucosal color | 26 (70.3) | 130 (66.3) | 0.354 |
| (P, S) Loss of lobulation | 5/15 (33.3) | 30/145 (20.7) | 0.323 |
| (P) Stalk swelling | 6/11 (54.6) | 12/87 (13.8) | 0.04 |
| (F) Fold convergency | 2/49 (4.1) | 4/12 (33.3) | 0.11 |
| (P, S) Fullness | 28/147 (19) | 8/25 (32) | 0.14 |
| Variable | OR (95%CI) | P value |
| Size (≥ 10 mm/10 mm) | 3.89 (1.35-11.26) | 0.01 |
| CSM (+/-) | 2.54 (1.14-5.95) | 0.04 |
| Ulceration or errhysis (+/-) | 5.44 (1.64-17) | 0.006 |
| Demarcated depressed area (+/-) | 5.82 (2.31-14.6) | 0.01 |
| Stalk swelling | 1.03 (0.26-4.06) | 0.97 |
Further stratification based on the growth morphology of the lesions revealed that the positive rates of CSM in flat-type SM- and M-stage cancers were 41.64% (5/12) and 12.24% (6/49), respectively, with a significant difference (P = 0.03).
The positive rates of CSM in protruded-type SM- and M-stage cancers were 63.64% (7/11) and 26.44% (23/87), respectively, with a significant difference (P = 0.03). Furthermore, the positive rates of CSM in sessile-type SM- and M-stage cancers were 71.43% (10/14) and 36.67% (23/87), respectively, with a statistical difference (P = 0.02) (Table 6). According to the results of logistic regressions, submucosal invasion, anatomical position (left colon), and growth morphology (pedunculated or sessile) were independent risk factors for CSM changes in ECCs (Table 7).
| Total | SM | M | P value | |
| Morphology | 233 | 37 | 196 | 0.252 |
| Flat | CSM (+) | 5 (41.67) | 6 (12.24) | 0.03 |
| CSM (-) | 7 (58.33) | 43 (87.76) | ||
| Protruded | CSM (+) | 7 (63.64) | 23 (26.44) | 0.03 |
| CSM (-) | 4 (36.36) | 64 (73.56) | ||
| Sessile | CSM (+) | 10 (71.43) | 22 (36.67) | 0.02 |
| CSM (-) | 4 (28.57) | 38 (63.33) |
| Variable | OR (95%CI) | P value |
| SM/M | 3.76 (1.7-8.3) | 0.01 |
| Location (left/right colon) | 2.45 (1.03-5.82) | 0.04 |
| Size (≥ 10 mm/< 10 mm) | 1.15 (0.59-1.41) | 0.09 |
| Morphology (flat/pedunculated/sessile) | 1.54 (0.79-3.06) | |
| 1.66 (0.85-3.27) | 0.03 |
According to statistics, the incidence and mortality rates of CRC rank third and second in the incidence and death spectrums, respectively, of malignant tumors[9]. The CRC incidence and mortality rates also have an increasing trend in China. However, early diagnosis and intervention can considerably improve the quality of life and the 5-year survival rate of patients with CRC. Endoscopic treatment is feasible for colorectal precancerous lesions, M-stage cancer, and colorectal cancer confined to SM1. However, surgical treatment is necessary for patients with an invasion depth exceeding a third of the upper submucosa due to the high lymph node metastasis rate[10]. Therefore, an accurate judgment of tumor nature, size, and depth of invasion under endoscopy is a vital prerequisite for making tumor treatment choices. Combining narrowband imaging and magnifying endoscopy has been consistently recognized for identifying and diagnosing early tumors, with improved detection rates of early and advanced cancers[11]. However, many primary hospitals lack the conditions for routinely using magnifying endoscopy and can only screen using conventional white light endoscopy. Furthermore, the current understanding of such lesions is insufficient, particularly those with a diameter < 2 cm. Before the pathological tissue evaluation, it is challenging to judge the nature and invasion depth of lesions under conventional white light endoscopy, which may lead endoscopists to select inappropriate treatment; however, it cannot achieve curative resection. The definition of colorectal cancer differs between Western countries and Japan, and this study referred to the Japanese Society for Colon Cancer Research and Vienna classification criteria[4,12]. M-stage cancer refers to a certain degree of cellular or structural atypia where the lesion is confined to the mucosal layer. In contrast, SM-stage cancer refers to lesions where abnormal cells break through the muscularis mucosa and infiltrate the submucosa. In SM-stage carcinoma, if the immersion depth exceeds 1000 μm of the submucosal layer, there is a metastasis risk of 6%-12%[13]. At this time, endoscopic resection cannot attain curative standards; therefore, it is unsuitable for treatment, and clinicians should pay more attention. Endoscopists frequently select treatment based on the location, shape, and endoscopic appearance of the lesion[14]. In this study, the most common sites of ECCs were the rectum and sigmoid colon, consistent with the common site of colorectal cancer in the left colon. Moreover, the lesion diameter affects the depth of ECCs invasion. The diameter of SM-stage cancers was significantly larger than that of M-stage cancers, and there was a significant difference between the groups. Furthermore, the SM-stage cancer diameter was > 1 cm, similar to the conclusion of a previous study[5]. According to the literature, the lesion morphology of ECC detected in Western countries is mainly the hump type (0-I type). Conversely, this study’s results were similar to the protruded type (0-I type). The proportion of protruded type (0-I type) lesions was 73.82%, possibly because hump-type lesions are easier to detect than flat types during colonoscopy. In this study, the proportions of flat, pedunculated, and sessile lesions in ECCs with different depths of invasion were similar, without significant differences. Because of the few 0-IIa, 0-IIa + 0-IIc, 0-IIc + 0-IIa, and LST lesions, further detailed stratification and comparison were not performed.
According to the literature, CSM, fold convergency, loss of lobulation, surface fullness, a depressed area with a clear boundary, deeper red mucosal color, erosion, or ulcer bleeding, and stalk swelling under standard white light endoscopy are commonly used to analyze the depth of invasion of ECC. In this study, the sessile lesions, left hemicolons, and lesions > 1 cm had a higher positive rate of CSM, which is consistent with the results of previous studies[1-3].
CSM is an endoscopic finding with uniform yellow-white spots around the lesion base, and the corresponding pathological manifestation is fat accumulation in the lamina propria macrophages. It is similar to the endoscopic appearance of gastric xanthoma. CSM is not the same as white spots, which are associated with invasive cancer, and is effective in inhibiting the progression of lesions with high malignant potential[15]. The CSM was classified into two based on their characteristics in related research[3]. Type 1 CSM was obvious under a white light endoscope and could be confirmed before injection, which was similar to white spots. However, type 2 differed from white spots and was cer
The histopathology revealed foam cells filled with lipids in the mucosa. Furthermore, it is regarded as a compensatory response to polyp growth[16]. However, a study in 2012 reported that the expression levels of proliferation markers (ki-67, COX-2) were higher in adenocarcinomas and adenomas with CSM[17]. A recent study revealed that CSM is associated with carcinogenesis and its progression[18]. Macrophages are important immune cells vital to cancer pathophysiology progression. The stool is retained in the left colon for a longer time; therefore, because of the retaining stool, bacteria, and macrophages, more bowel inflammation is experienced. Therefore, the abnormal inflammation or increased expression of inflammatory genes may have resulted in tumorigenesis despite the lack of macrophages revealing CSM.
Currently, for the endoscopic resection of lesions without signs of submucosal invasion, cold snare polypectomy is recommended for lesions smaller than 10 mm; EMR is recommended for non-pedunculated lesions larger than 20 mm according to the European Society of Gastrointestinal Endoscopy clinical guidelines and the United States Multi-Society Task Force[19,20]. However, the optimal resection method of 10-20 mm (medium size) remains controversial, and whether hot or cold resection is preferable remains unclear. Recently, some studies have demonstrated that cold-snare EMR can be safely performed en bloc for 10-14 mm colorectal adenomas without severe adverse events. However, the histological complete resection rate and submucosal layer found in the resected specimens were 63.8% and 25.0%, respectively[21]. For early-stage small colorectal cancer with submucosal invasion, this resection is non-curative. Therefore, it is crucial to determine whether the ECC has a submucosal invasion before the surgical choice of treatment and the prognosis of patients. Our study can be useful in this regard.
Previous studies have revealed that surface fullness is an important characteristic of ECC; however, this study’s results reveal that it is not vital to determine whether the tumor invades the submucosa. The primary reason could be that the surface fullness mainly reflects tumor growth expansion. When it progresses to a certain stage, with tumor volume increase, particularly deep infiltration, the surface tumor cells may have different extents of necrosis, resulting in different degrees of well-defined surface depressions. Fold convergency and loss of lobulation showed no differences in ECCs with different depths of invasion, whereas the P value of the difference in the stalk swelling in ECCs with different depths of invasion was approximately 0.05, which may be due to the small sample size of this study, which requires further discussion.
This study had some limitations. First, we did not further measure the specific submucosal infiltration depth, distinguishing SM1, SM2, and SM3 for surgical resection and EMR resection of patients with submucosal infiltration. Therefore, this may have impacted the preoperative evaluation and treatment selection. Second, we did not define surveillance colonoscopy after EMR and ESD. The current European and United States guidelines recommended a 3-year surveillance colonoscopy for patients with adenoma larger than 10 mm; hence, the true complete resection rate and the necessity of additional treatment in this study require our regular review and further evaluation following the recommendations in the guidelines. Third, this study was a single-center, retrospective clinical analysis. In addition, the sample size was limited, and the study’s single-center nature may have caused bias. Therefore, using a larger sample size and multi-center clinical study is necessary to elucidate the relationship between endoscopic appearance and immersion depth from the perspective of fine cell segmentation.
CSM has a clinicopathological value for predicting deep immersion in early CRC in the left colon. It can enable endoscopists to better identify the submucosal infiltrates of ECC, thereby providing patients with more appropriate treatment options.
Currently, endoscopists frequently decide the treatment of colorectal polyps according to the characteristics of white light endoscopy, and polyps smaller than 2 cm usually do not receive adequate attention, leading to their inappropriate treatment.
Chicken skin mucosa (CSM) surrounding colon polyps is a common endoscopic finding that could be an endoscopic predictive marker of submucosal invasion. Therefore, we should consider the lesions with such characteristics and adopt more appropriate treatment.
To explore potential markers of small colorectal cancer early invasion under white light endoscopy, We found that CSM was more common in early submucosal invasive carcinoma than intramucosal carcinoma. Therefore, a more aggressive treatment should be used rather than cold-snare polypectomy or cold-snare endoscopic mucosal resection.
This retrospective cross-sectional study included 198 consecutive patients [233 early colorectal cancers (ECCs)] who underwent endoscopy or surgical procedures. Logistic regression analysis was used to examine the relationship between morphological characteristics, size, CSM prevalence, and invasion depth of ECC under white light endoscopy.
CSM, a depressed area with clear boundaries, erosion, or ulcer bleeding, and size (≥ 10 mm) were independent risk factors for submucosal invasion in ECC. We should consider the lesions with such characteristics and adopt more appropriate treatment.
CSM has a clinicopathological value for predicting deep immersion in early colorectal carcinoma in the left colon.
We will further expand our research to test our conclusions from various geographic, ethnic, and other factors.
Provenance and peer review: Unsolicited article; Externally peer-reviewed.
Peer-review model: Single-blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ichita C, Japan; Musa Y, Nigeria; Osera S, Japan S-Editor: Yan JP L-Editor: A P-Editor: Yuan YY
| 1. | Shatz BA, Weinstock LB, Thyssen EP, Mujeeb I, DeSchryver K. Colonic chicken skin mucosa: an endoscopic and histological abnormality adjacent to colonic neoplasms. Am J Gastroenterol. 1998;93:623-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Chung EJ, Lee JY, Choe J, Chang HS, Kim J, Yang DH, Ye BD, Byeon JS, Kim KJ, Yang SK, Kim JH, Myung SJ. Colonic Chicken Skin Mucosa is an Independent Endoscopic Predictor of Advanced Colorectal Adenoma. Intest Res. 2015;13:318-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 3. | Lee YM, Song KH, Koo HS, Lee CS, Ko I, Lee SH, Huh KC. Colonic Chicken Skin Mucosa Surrounding Colon Polyps Is an Endoscopic Predictive Marker for Colonic Neoplastic Polyps. Gut Liver. 2022;16:754-763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 4. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Takiuchi H, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 597] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 5. | Park W, Kim B, Park SJ, Cheon JH, Kim TI, Kim WH, Hong SP. Conventional endoscopic features are not sufficient to differentiate small, early colorectal cancer. World J Gastroenterol. 2014;20:6586-6593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Li N, Jin P, Yu DL, Yang L, Xie H, Kan Q, He YQ, Sheng JQ. The relationship between morphological characteristics of early colorectal cancer under the white light endoscopy and its infiltration depth. Zhonghua Xiaohua Neijing Zazhi. 2016;33. |
| 7. | Wen CC, Zhou S, Liu H, Wu DQ, Xu XR. Endoscopic findings combined with Ki67 for evaluating submucosal invasion of early colorectal cancer. J Tongji Univ (Med Sci). 2022;43. |
| 8. | Bugajski M, Kaminski MF, Orlowska J, Mroz A, Pachlewski J, Rupinski M, Zagorowicz E, Rawa T, Regula J. Suspicious macroscopic features of small malignant colorectal polyps. Scand J Gastroenterol. 2015;50:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64684] [Article Influence: 16171.0] [Reference Citation Analysis (177)] |
| 10. | Ikematsu H, Yoda Y, Matsuda T, Yamaguchi Y, Hotta K, Kobayashi N, Fujii T, Oono Y, Sakamoto T, Nakajima T, Takao M, Shinohara T, Murakami Y, Fujimori T, Kaneko K, Saito Y. Long-term outcomes after resection for submucosal invasive colorectal cancers. Gastroenterology. 2013;144:551-9; quiz e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 11. | Gonai T, Kawasaki K, Nakamura S, Yanai S, Akasaka R, Sato K, Toya Y, Asakura K, Urushikubo J, Fujita Y, Eizuka M, Uesugi N, Sugai T, Matsumoto T. Microvascular density under magnifying narrow-band imaging endoscopy in colorectal epithelial neoplasms. Intest Res. 2020;18:107-114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Bray C, Bell LN, Liang H, Collins D, Yale SH. Colorectal Cancer Screening. WMJ. 2017;116:27-33. [PubMed] |
| 13. | Dumoulin FL, Hildenbrand R. Endoscopic resection techniques for colorectal neoplasia: Current developments. World J Gastroenterol. 2019;25:300-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 47] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 14. | Shaukat A, Kaltenbach T, Dominitz JA, Robertson DJ, Anderson JC, Cruise M, Burke CA, Gupta S, Lieberman D, Syngal S, Rex DK. Endoscopic Recognition and Management Strategies for Malignant Colorectal Polyps: Recommendations of the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2020;159:1916-1934.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 15. | Iwai T, Imai K, Hotta K, Ito S, Yamaguchi Y, Kawata N, Tanaka M, Kakushima N, Takizawa K, Ishiwatari H, Matsubayashi H, Ono H. Endoscopic prediction of advanced histology in diminutive and small colorectal polyps. J Gastroenterol Hepatol. 2019;34:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Nowicki MJ, Subramony C, Bishop PR, Parker PH. Colonic chicken skin mucosa: association with juvenile polyps in children. Am J Gastroenterol. 2001;96:788-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Guan J, Zhao R, Zhang X, Cheng Y, Guo Y, Wang L, Mi L, Liu F, Ma X, Li B. Chicken skin mucosa surrounding adult colorectal adenomas is a risk factor for carcinogenesis. Am J Clin Oncol. 2012;35:527-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Yang M, McKay D, Pollard JW, Lewis CE. Diverse Functions of Macrophages in Different Tumor Microenvironments. Cancer Res. 2018;78:5492-5503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 308] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 19. | Ferlitsch M, Moss A, Hassan C, Bhandari P, Dumonceau JM, Paspatis G, Jover R, Langner C, Bronzwaer M, Nalankilli K, Fockens P, Hazzan R, Gralnek IM, Gschwantler M, Waldmann E, Jeschek P, Penz D, Heresbach D, Moons L, Lemmers A, Paraskeva K, Pohl J, Ponchon T, Regula J, Repici A, Rutter MD, Burgess NG, Bourke MJ. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2017;49:270-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 559] [Cited by in RCA: 766] [Article Influence: 95.8] [Reference Citation Analysis (0)] |
| 20. | Kaltenbach T, Anderson JC, Burke CA, Dominitz JA, Gupta S, Lieberman D, Robertson DJ, Shaukat A, Syngal S, Rex DK. Endoscopic Removal of Colorectal Lesions-Recommendations by the US Multi-Society Task Force on Colorectal Cancer. Gastrointest Endosc. 2020;91:486-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 21. | Yabuuchi Y, Imai K, Hotta K, Ito S, Kishida Y, Yoshida M, Kawata N, Kakushima N, Takizawa K, Ishiwatari H, Matsubayashi H, Aizawa D, Oishi T, Imai T, Ono H. Efficacy and safety of cold-snare endoscopic mucosal resection for colorectal adenomas 10 to 14 mm in size: a prospective observational study. Gastrointest Endosc. 2020;92:1239-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |