Published online May 15, 2023. doi: 10.4251/wjgo.v15.i5.892
Peer-review started: January 13, 2023
First decision: January 21, 2023
Revised: February 1, 2023
Accepted: April 7, 2023
Article in press: April 7, 2023
Published online: May 15, 2023
Processing time: 119 Days and 0.7 Hours
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and has a high mortality. However, the treatment options for advanced HCC are limited to tyrosine kinase inhibitors, such as sorafenib and lenvatinib. Since previous regimens have an insufficient efficacy, the combination therapy of atezolizumab and bevacizumab (Ate/Bev) has been investigated, which showed an improvement in progression-free and overall survival. However, the adverse events of this combination therapy in advanced HCC have not been established. Herein, we report a novel case of an unresectable HCC and acute respiratory distress syndrome (ARDS) after a combination therapy of Ate/Bev.
An 82-year-old male visited our outpatient clinic for an incidentally detected liver mass. Liver magnetic resonance imaging and enhanced chest computed tomography (CT) were performed, which showed arterial hyperenhancement with washout in delayed phase suggesting HCC, and a well-defined metastatic solid nodule, respectively. F-18 fluorodeoxyglucose positron emission tomo
Clinicians should be aware of severe pneumonitis due to the immune-related adverse events of this combination therapy, and patients should be closely monitored after therapy.
Core Tip: Nowadays, the combination therapy of atezolizumab and bevacizumab is recommended as the first-line systemic treatment for advanced hepatocellular carcinomas. A global phase III study and recent real-world studies demonstrated rare life-threatening adverse events of atezolizumab and bevacizumab. However, our patient underwent acute respiratory distress syndrome and finally died three days after treatment. To the best of our knowledge, this is the first case of severe respiratory failure resulting in death with a very short interval from atezolizumab and bevacizumab administration. Therefore, we suggest close monitoring of lung toxicity after therapy.
- Citation: Cho SH, You GR, Park C, Cho SG, Lee JE, Choi SK, Cho SB, Yoon JH. Acute respiratory distress syndrome and severe pneumonitis after atezolizumab plus bevacizumab for hepatocellular carcinoma treatment: A case report. World J Gastrointest Oncol 2023; 15(5): 892-901
- URL: https://www.wjgnet.com/1948-5204/full/v15/i5/892.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i5.892
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third leading cause of cancer-related mortality globally[1]. HCC can be treated with various options depending on the tumor stage, remaining liver function, performance status score, and tumor burden[2]. Early-stage HCC has curative treatment options, such as surgical resection, radiofrequency ablation, or liver transplantation. However, only 10%–20% of patients are candidates for curative therapies, and 80% of patients have unresectable HCCs that can only be treated with locoregional therapies, such as transarterial chemoembolization, transarterial radioembolization (TARE), radiotherapy, or palliative management, including systemic therapy[3]. Despite the various locoregional therapies, many patients eventually progress to advanced stages of HCC. Therefore, systemic therapy for managing advanced HCC is clinically significant.
Since 2008, sorafenib, an oral multi-kinase inhibitor, has been used as a first-line systemic chemotherapy based on the SHARP trial, which demonstrated the increased overall survival of patients with advanced HCC compared with placebo-treated advanced HCC patients[4]. In 2018, lenvatinib, another oral multi-kinase inhibitor, was approved as a first-line therapy for advanced HCC according to the REFLECT trial, which demonstrated its non-inferiority over sorafenib in terms of overall survival[5]. However, in large-scale real-world GIDEON study, which comprised of 3202 HCC patients, the presented median overall survival was 13.6 mo in Child-Pugh A group and 5.2 mo in the Child-Pugh B group. The ELEVATOR study, which investigated the real-world efficacy of lenvatinib, showed that the median overall survival was 10.7 mo in the Child-Pugh A group and 5.3 mo in the Child-Pugh B group. Efforts have been devoted to improve the clinical outcomes of patients with advanced HCC undergoing systemic chemotherapy.
With the introduction of various immune checkpoint inhibitors (ICIs), several clinical trials had attempted to verify their treatment efficacy. The CheckMate 459 trial compared the overall survival between nivolumab and sorafenib, of which nivolumab treatment did not significantly improve overall survival[6]. Also, the KEYNOTE-240 trial compared the overall survival and progression free survival between pembrolizumab and placebo in advanced HCC patients who were previously treated with sorafenib, and failed to reach statistical significance[7]. The IMbrave150 trial investigated the efficacy of the combination therapy of atezolizumab, an anti-PD-L1 antibody, and bevacizumab, a vascular endothelial growth factor (VEGF) -targeting antibody, for treating advanced HCC. The progression-free and overall survival were longer in the combination therapy group than in the sorafenib treatment group, with no differences in grade 3 or 4 adverse events[8,9]. Based on these results, many clinical guidelines have been updated to include atezolizumab and bevacizumab as a first-line systemic treatment for advanced HCC[2]. However, regarding the safety of atezolizumab and bevacizumab, few reports have described the adverse events, especially drug-related pneumonitis.
Herein, we present the novel case of a patient who had an unresectable HCC and experienced acute respiratory distress syndrome (ARDS) due to severe pneumonitis three days after being treated with atezolizumab and bevacizumab.
An 82-year-old male visited our outpatient clinic for an incidentally detected liver mass discovered on abdominal ultrasonography.
He denied fever, night sweats, weight loss, and abdominal pain.
The patient was on medication for hypertension and diabetes mellitus for 20 and 10 years, respectively. He also had underlying liver cirrhosis of an unknown cause, which was diagnosed 13 years ago.
The patient had no family history.
There were no abnormal findings on physical examination.
The laboratory workup showed the following: platelet count, 510 × 109/L (reference range, 130–450 × 109/L); total bilirubin, 0.63 mg/dL (reference range, 0.22–1.3 mg/dL); aspartate aminotransferase level, 37 U/L (reference range, 10–37 U/L); alanine aminotransferase level, 46 U/L (reference range, 10–37 U/L); albumin level, 4.0 g/dL (reference range, 3.5–5.2 g/dL); and prothrombin time, 0.97 international normalized ratio (INR; reference range, 0.8–1.2 INR). In the serum laboratory examinations for viral hepatitis, the hepatitis B surface antigen and hepatitis C antibody were negative, whereas the hepatitis B core antibody and hepatitis B surface antibody were positive, implying a prior hepatitis B viral infection. The alpha-fetoprotein level was 84.97 IU/mL, and the protein level induced by vitamin K absence-II (PIVKA-II) was 60232 mAU/mL.
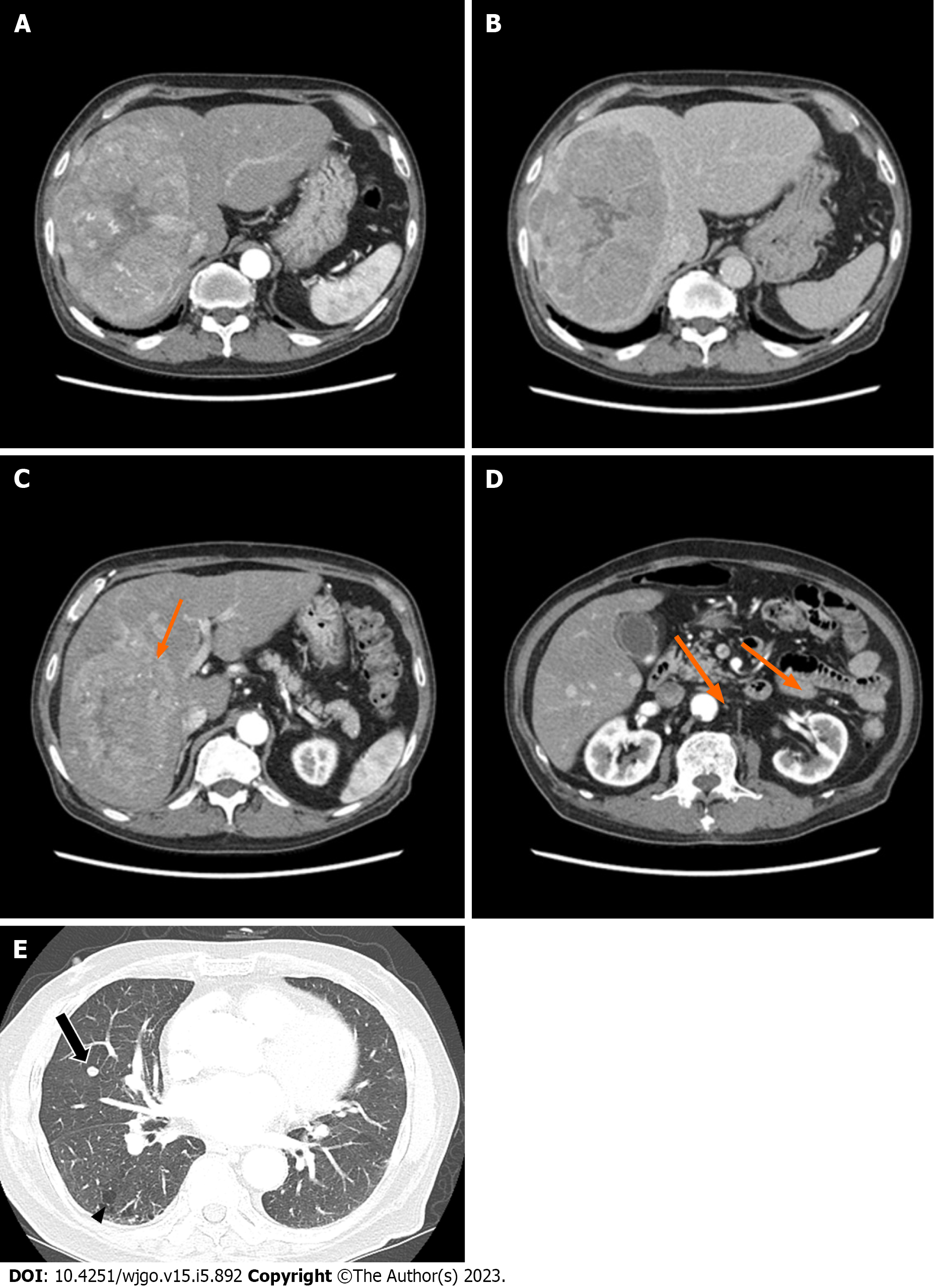
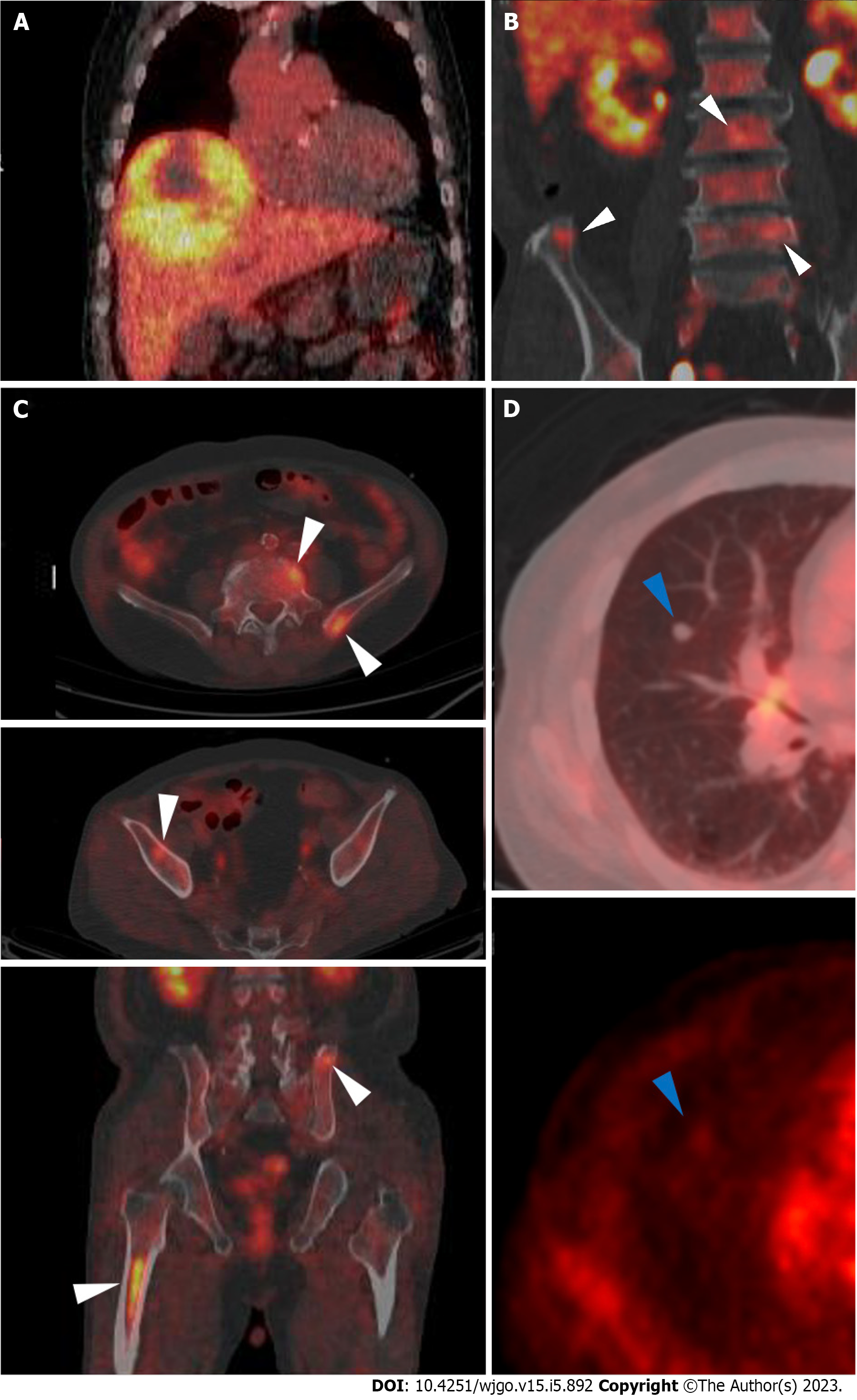
Abdominal CT revealed a 16 cm × 11 cm × 10 cm mass with early arterial enhancement and early washout in the delayed phase. Several daughter nodules were found around the main mass. Ascites was not detected (Figure 1). Liver magnetic resonance imaging with Gadoxetate disodium (Primovist®) and enhanced chest CT were performed as the staging workup for the HCC, which showed showed arterial hyperenhancement with washout assessed in the portal venous phase in the enhanced T1-weighted images, and a 7-mm well-defined solid nodule suspected to be a metastatic nodule in the right middle lobe with interstitial lung abnormality (ILA) findings, such as bilateral subpleural reticulation and non-emphysematous cysts with traction bronchiectasis, respectively (Figure 1). Although the patient did not have respiratory symptoms such as dyspnea, pulmonary function tests were performed for ILA evaluation. His forced vital capacity (FVC) and 1-second forced expiratory volume (FEV1) were 1.87 L (52.4% of predicted value) and 1.39 L (63.2% of predicted value), respectively. The FEV1/FVC ratio was 109.7% of predicted value that showed restrictive patterns of lung disease. Because of the suspicious malignant metastatic pulmonary lesions, F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET)-CT was performed to thoroughly evaluate the extrahepatic metastasis, which showed multiple hypermetabolic lesions in both iliac bones, lumbar vertebrae, and the right femur (Figure 2). A solid nodule in the right middle lobe also had mild focal hypermetabolic activity compared to the surrounding lung parenchyma with a maximum standardized uptake value of 1.0.
The patient was diagnosed with compensated liver cirrhosis, Child-Pugh A (score, 5) and HCC with multiple extrahepatic metastases [modified Union for International Cancer Control Stage (mUICC) IVB, Barcelona Clinic Liver Cancer (BCLC) C]. His performance status score was 0. He was willing to receive anticancer treatment. Although the patient demonstrated an extrahepatic metastasis, TARE of the primary intrahepatic HCC in the right lobe was planned to address the high tumor burden of the mass and to debulk it. Systemic therapy was administered for the remaining lesions.
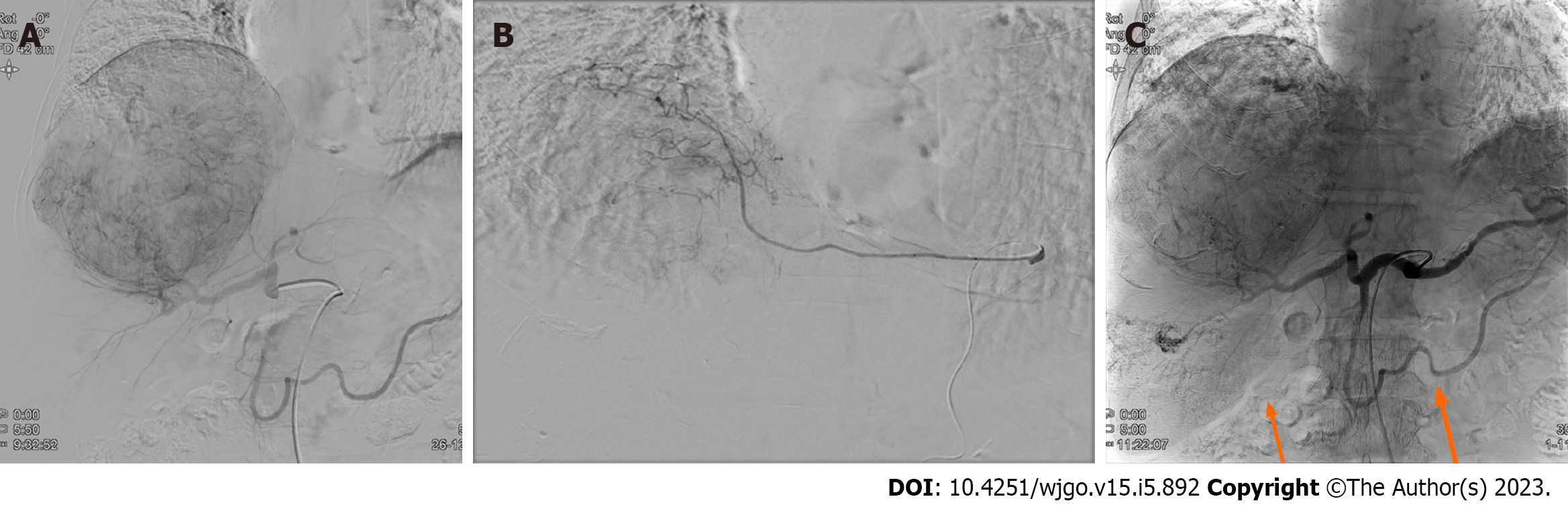
Prior to the TARE, a Technetium-99m macro aggregated albumin (99mTc-MAA) perfusion lung scan was performed to assess the hepatopulmonary shunt. The calculated lung shunt fraction was only 8.44%, and the TARE was conducted as scheduled. A hypervascular mass (about 15 cm) with several daughter nodules in the right hepatic lobe fed by branches of the right hepatic, left hepatic, and left inferior phrenic arteries was examined via angiography. An Yttrium-90 (Y90) infusion was performed by feeding the branches of the right hepatic artery, and the mean dose volumes were 108.419 Gy on the tumor, 85.543 Gy on the liver, and 0.873 Gy on both lungs (0.714 and 0.159 Gy on the right and left lungs, respectively). Additionally, chemoembolization using a doxorubicin-lipiodol mixture was performed through the branches of the right hepatic artery, while bland embolization of the left inferior phrenic artery was simultaneously conducted (Figure 3).
Postoperatively, the patient’s general condition was tolerable without abdominal pain, and no complications, such as liver failure or post-embolization syndrome, were observed. The patient was discharged after two days. A restaging baseline CT was performed 34 d after the initial TARE and before the systemic therapy. Abdominal CT showed more necrotic changes in the known primary HCC lesion, with some sparse lipiodol uptake in several daughter nodules. Chest CT showed no remarkable changes in the 7-mm metastatic nodule. Since the patient’s general condition was tolerable, systemic therapy using the combination of atezolizumab and bevacizumab was administered the day after the CT scan. For the systemic therapy, 1200 mg of atezolizumab and 870 mg (15 mg/kg body weight) of bevacizumab were infused for 60 and 90 min, respectively. No acute side effects occurred during the injections, and the patient returned home in the same day.
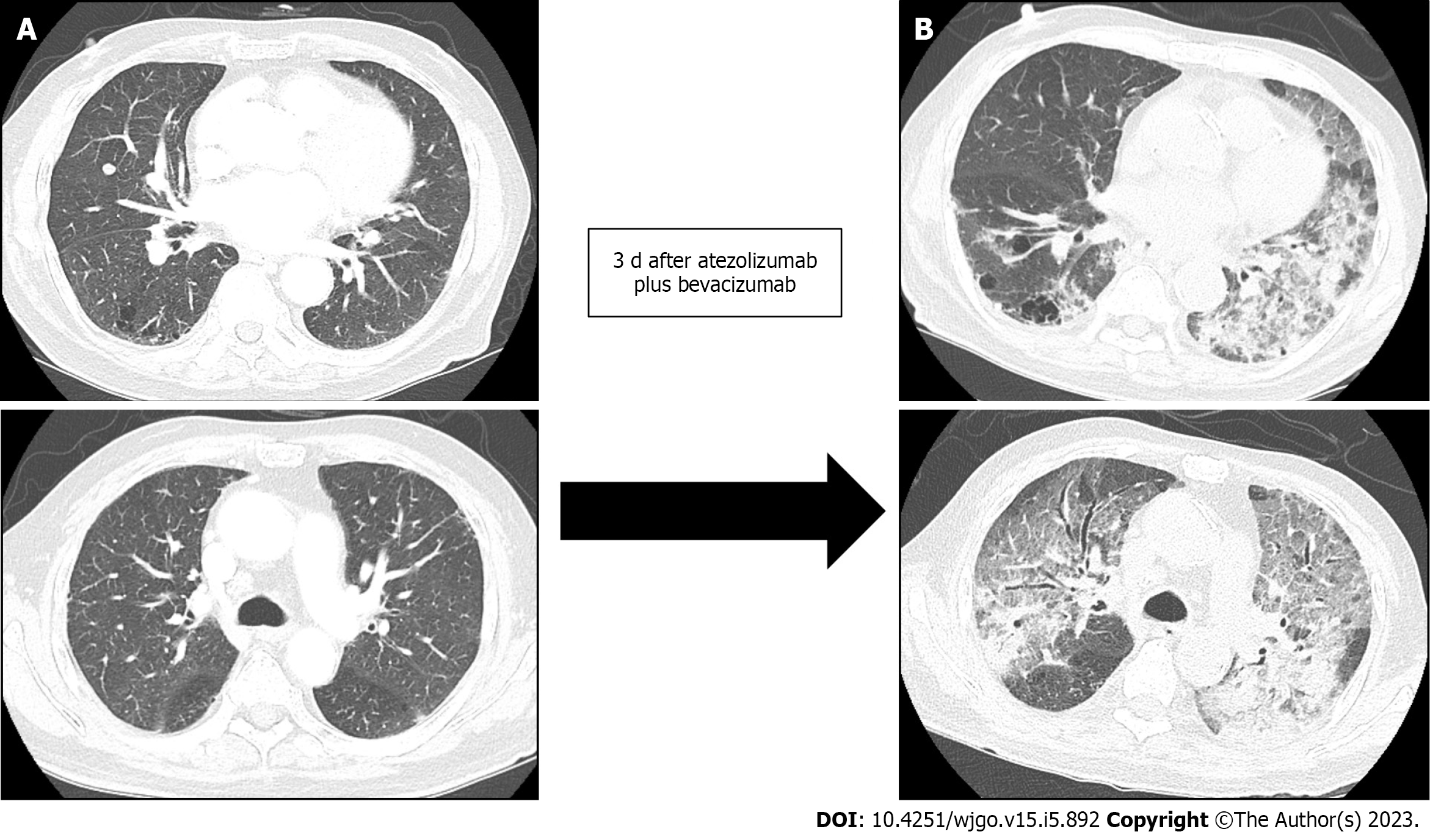
However, three days after the combination therapy of atezolizumab and bevacizumab, the patient visited the emergency room for severe dyspnea (Modified Medical Research Council Dyspnea Grade 4). The serum laboratory workup showed that the total bilirubin was 0.49 mg/dL (reference range, 0.22–1.3 mg/dL), aspartate aminotransferase level was 59 U/L (reference range, 10–37 U/L), and alanine aminotransferase level was 36 U/L (reference range, 10–37 U/L). The arterial blood gas test showed hypoxemia [partial pressure of oxygen (pO2), 84 mmHg] under 0.9 of the fraction of inspired oxygen (FiO2) level. Chest CT revealed newly detected diffused ground-glass opacities with bilateral septal thickening and consolidations (Figure 4). Infectious pneumonia was ruled out due to lack of related symptoms, such as fever or purulent sputum, and negative microbiological test (e.g., sputum culture and respiratory virus exam) results. Although oxygen was administered via a high-flow nasal cannula, the P/F ratio was only 74; therefore, the patient was diagnosed with ARDS based on the overall examination results. The patient was immediately admitted to the intensive care unit after tracheal intubation. Low tidal volume with high positive end-expiratory pressure, sedative agents combined with a neuromuscular blocker, and a systemic steroid (125 mg of methylprednisolone per day) were promptly applied to manage the ARDS. However, the patient’s P/F ratio worsened, and he did not recover from the hypoxia. Unfortunately, he expired 31 h after being admitted.
The paradigm in the systemic treatment of HCC has shifted from conventional tyrosine kinase inhibitors (TKI) to a combination of an ICI with a TKI or another ICI[10]. According to the IMBrave150 trial, a pivotal study on the use of atezolizumab and bevacizumab for unresectable HCCs, the progression-free survival of this combination treatment was better than that of sorafenib[8]. Follow-up results demonstrated a meaningful survival benefit of the combination therapy of atezolizumab and bevacizumab[9]. Therefore, the HCC treatment guidelines have been updated, including the combination therapy of atezolizumab and bevacizumab as a first-line therapy for advanced HCCs[2,11]. Conventional TKI agents, such as sorafenib, lenvatinib, and cabozantinib, are considered second-line treatments or alternatives to the first-line treatment for ineligible patients for the combination therapy of atezolizumab and bevacizumab.
In addition to treatment efficacy, the safety profile of drug administration is an essential concern. Conventional TKI agents have various treatment-related adverse events, such as diarrhea, hypertension, proteinuria, and skin toxicities, such as rash, desquamation, and hand-foot skin reactions[12]. The IMBrave 150 trial showed a better safety profile for atezolizumab and bevacizumab compared with that of sorafenib. The most common grade 3 or grade 4 adverse events with an incidence of more than 10% in the IMBrave150 trial were hypertension (15.2%), increased aspartate aminotransferase levels (7.0%), and increased alanine aminotransferase levels (3.6%), which were mostly consistent with the follow-up study. Although 1.2% of the patients experienced grade 1–2 pneumonitis, no patients with severe grade toxicity were found. This finding was consistent with the follow-up study (2.0% of patients had grade 1–2 pneumonitis).
Considering the timeline of the combination therapy of atezolizumab and bevacizumab into clinical practice, few real-world studies regarding the treatment efficacy and safety profile have been completed, reporting conflicting results. D'Alessio et al[13] conducted a multi-national, retrospective study on 202 patients with HCC treated with the combination of atezolizumab and bevacizumab. They observed that 1.0% of the patients experienced treatment-related pneumonitis of more than grade 3. Tada et al[14] compared the results of the combination of atezolizumab and bevacizumab in elderly (≥ 75 years old) and nonelderly (< 75 years old) HCC patients, and revealed that the treatment efficacy and safety did not differ between the groups, and reported no drug-related pneumonitis. However, Ng et al[15] performed a study on the immune-related adverse events (irAEs) associated with the use of ICIs, including atezolizumab, in advanced HCCs, and found that pneumonitis occurred in 3.0%–10.0% of the patients (2.4% of which had pneumonitis of grade 3 or higher) and the pneumonitis was lethal in 0.2%–2.0% of the patients. Additionally, Endo et al[16] recently reported two fatal cases after applying atezolizumab plus bevacizumab on patients with pre-existing lung diseases. One patient showed honeycomb lungs before treatment and died 5 d after treatment. Another patient previously underwent right lower lobectomy due to lung adenocarcinoma. She died after 11 courses of treatment.
Pneumonitis is an uncommon but possible adverse event closely related to the use of various ICIs in HCC patients. The KEYNOTE-240 trial studied the use of pembrolizumab, an anti-PD-1 monoclonal antibody, in HCC patients, and reported an occurrence of pneumonitis of any grade in 18.3% of the patients, and grade 3 or 4 pneumonitis in 7.2% of the patients[17]. Lung-related irAEs due to ICIs have been investigated in other malignancies, such as lung cancer, renal cell cancer, and melanoma[18]. Lung-related irAEs should be managed based on the grade of pneumonitis. In patients with grade 3 or grade 4 pneumonitis with severe symptoms and life-threatening respiratory compromise, ICI use must be permanently discontinued, and glucocorticoids and empirical antibiotics must be administered[19]. Discontinuing ICI therapy and initiating systemic steroids are highly effective treatments for ICI-related pneumonitis. However, ICI-induced pneumonitis accounts for 35.0% of PD-1/PD-L1 inhibitor-related deaths and may have a similar fatal clinical course to that of our case[20].
Although the associated factors for predicting the development of pneumonitis have been investigated, limited data are available. Decreased pulmonary function and a history of smoking likely increase the risk of pneumonitis[21]. Concurrent radiation therapy, combination immunotherapy, and previous high-dose chemotherapy have also been proposed as potential risk factors[22]. Furthermore, the presence of ILAs is associated with the risk of complications from medical interventions, such as chemotherapy and surgery[23]. Chest CT done on our patient before atezolizumab and bevacizumab therapy showed ILA findings, which may have influenced the rapid onset of ARDS. Although our patient underwent combination therapy of ICI and TKI a meta-analysis by Nishino et al[24] reported significantly higher incidences of all-grade pneumonitis (6.6% vs 1.6%; P < 0.001) and grade 3 or higher pneumonitis (1.5% vs 0.2%; P = 0.001) in the ICI-combination therapy group than in the monotherapy group. Despite varying reported onset times of pneumonitis after treatment initiation (range, 9 d–19 mo)[21], the median time to onset is reported to be shorter when a combination therapy of ICIs is used[25,26]. The presenting symptoms of ICI-induced pneumonitis are nonspecific, including dyspnea (53.0%), cough (35.0%), and fever (12.0%)[17], and the incidence level for these risk factors in clinical practice in terms of atezolizumab and bevacizumab combination therapy is not yet established. Therefore, clinicians must be aware of the possibilities and characteristics of immune-related pneumonitis associated with these treatments.
This study has some limitations. First, thorough post-treatment pulmonary pathological and laboratory examinations may have clarified the patient’s diagnosis; however, a biopsy was not eligible because of the rapid course of the ARDS, and the family did not want an autopsy. Additionally, several serum biomarkers, such as Krebs von den Lungen-6 (KL-6), were related to the diagnosis of interstitial lung disease. If the results of the biomarkers were available, an acute exacerbation of interstitial lung disease could have been ruled out; unfortunately, these laboratory tests were not performed. Second, radiation pneumonitis due to the initial TARE likely occurred. However, the calculated radiation dose to both lungs was 0.873 Gy (left lung, 0.159 Gy; right lung, 0.714 Gy), which was lower than the recommended dose for preventing pneumonitis[27]. Considering that the CT results were from three days before the combination therapy of atezolizumab and bevacizumab, the possibility of radiation pneumonitis due to the TARE was substantially low. However, although respiratory symptoms such as cough, dyspnea and immune-associated pneumonia are well-known ICI-related adverse events, fatal ARDS with an extremely short duration from therapy, as was in our case, has not been reported; therefore, our case report may be of value.
Our case implies that the combination therapy of atezolizumab and bevacizumab to treat HCCs might cause fatal pneumonitis leading to ARDS; however, the benefit of this treatment could outweigh the irAEs in terms of survival. Additionally, the severe adverse events of the newly introduced atezolizumab and bevacizumab therapy are uncommon. However, clinicians should be aware of the possible lung toxicity caused by this treatment, especially in newly developed respiratory symptoms of patients. Once the diagnosis is made, management based on the severity of pneumonitis is highly required. Furthermore, future studies should verify the risk factors of developing pneumonitis to identify high-risk patient groups.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lin J, China; Martin M, France S-Editor: Liu JH L-Editor: A P-Editor: Zhang XD
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64456] [Article Influence: 16114.0] [Reference Citation Analysis (176)] |
| 2. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2578] [Article Influence: 859.3] [Reference Citation Analysis (59)] |
| 3. | Belghiti J, Kianmanesh R. Surgical treatment of hepatocellular carcinoma. HPB (Oxford). 2005;7:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 118] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, Schwartz M, Porta C, Zeuzem S, Bolondi L, Greten TF, Galle PR, Seitz JF, Borbath I, Häussinger D, Giannaris T, Shan M, Moscovici M, Voliotis D, Bruix J; SHARP Investigators Study Group. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10258] [Article Influence: 603.4] [Reference Citation Analysis (2)] |
| 5. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3816] [Article Influence: 545.1] [Reference Citation Analysis (1)] |
| 6. | Yau T, Park JW, Finn RS, Cheng AL, Mathurin P, Edeline J, Kudo M, Harding JJ, Merle P, Rosmorduc O, Wyrwicz L, Schott E, Choo SP, Kelley RK, Sieghart W, Assenat E, Zaucha R, Furuse J, Abou-Alfa GK, El-Khoueiry AB, Melero I, Begic D, Chen G, Neely J, Wisniewski T, Tschaika M, Sangro B. Nivolumab vs sorafenib in advanced hepatocellular carcinoma (CheckMate 459): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022;23:77-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 738] [Article Influence: 184.5] [Reference Citation Analysis (0)] |
| 7. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1338] [Article Influence: 267.6] [Reference Citation Analysis (0)] |
| 8. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4673] [Article Influence: 934.6] [Reference Citation Analysis (2)] |
| 9. | Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Ma N, Nicholas A, Wang Y, Li L, Zhu AX, Finn RS. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76:862-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 951] [Article Influence: 317.0] [Reference Citation Analysis (0)] |
| 10. | Su TH, Hsu SJ, Kao JH. Paradigm shift in the treatment options of hepatocellular carcinoma. Liver Int. 2022;42:2067-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Kudo M, Kawamura Y, Hasegawa K, Tateishi R, Kariyama K, Shiina S, Toyoda H, Imai Y, Hiraoka A, Ikeda M, Izumi N, Moriguchi M, Ogasawara S, Minami Y, Ueshima K, Murakami T, Miyayama S, Nakashima O, Yano H, Sakamoto M, Hatano E, Shimada M, Kokudo N, Mochida S, Takehara T. Management of Hepatocellular Carcinoma in Japan: JSH Consensus Statements and Recommendations 2021 Update. Liver Cancer. 2021;10:181-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 450] [Article Influence: 112.5] [Reference Citation Analysis (0)] |
| 12. | Bellmunt J, Eisen T, Fishman M, Quinn D. Experience with sorafenib and adverse event management. Crit Rev Oncol Hematol. 2011;78:24-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | D'Alessio A, Fulgenzi CAM, Nishida N, Schönlein M, von Felden J, Schulze K, Wege H, Gaillard VE, Saeed A, Wietharn B, Hildebrand H, Wu L, Ang C, Marron TU, Weinmann A, Galle PR, Bettinger D, Bengsch B, Vogel A, Balcar L, Scheiner B, Lee PC, Huang YH, Amara S, Muzaffar M, Naqash AR, Cammarota A, Personeni N, Pressiani T, Sharma R, Pinter M, Cortellini A, Kudo M, Rimassa L, Pinato DJ. Preliminary evidence of safety and tolerability of atezolizumab plus bevacizumab in patients with hepatocellular carcinoma and Child-Pugh A and B cirrhosis: A real-world study. Hepatology. 2022;76:1000-1012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 167] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 14. | Tada T, Kumada T, Hiraoka A, Hirooka M, Kariyama K, Tani J, Atsukawa M, Takaguchi K, Itobayashi E, Fukunishi S, Tsuji K, Ishikawa T, Tajiri K, Ochi H, Yasuda S, Toyoda H, Ogawa C, Nishimura T, Hatanaka T, Kakizaki S, Shimada N, Kawata K, Tanaka T, Ohama H, Nouso K, Morishita A, Tsutsui A, Nagano T, Itokawa N, Okubo T, Arai T, Imai M, Naganuma A, Koizumi Y, Nakamura S, Joko K, Iijima H, Hiasa Y; Real-life Practice Experts for HCC (RELPEC) Study Group and the Hepatocellular Carcinoma Experts from 48 clinics in Japan (HCC 48) Group. Safety and efficacy of atezolizumab plus bevacizumab in elderly patients with hepatocellular carcinoma: A multicenter analysis. Cancer Med. 2022;11:3796-3808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Ng KYY, Tan SH, Tan JJE, Tay DSH, Lee AWX, Ang AJS, Wong LWJ, Choo SP, Tai DW, Lee JJX. Impact of Immune-Related Adverse Events on Efficacy of Immune Checkpoint Inhibitors in Patients with Advanced Hepatocellular Carcinoma. Liver Cancer. 2022;11:9-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Endo K, Kuroda H, Oikawa T, Ito Y, Abe T, Kooka Y, Kakisaka K, Miyasaka A, Sugai T, Matsumoto T. Immune Checkpoint Inhibitor-Related Pneumonia in Unresectable Hepatocellular Carcinoma: Two Fatal Cases under Atezolizumab plus Bevacizumab. Liver Cancer. 2022;11:572-575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Hsu C, Rimassa L, Sun HC, Vogel A, Kaseb AO. Immunotherapy in hepatocellular carcinoma: evaluation and management of adverse events associated with atezolizumab plus bevacizumab. Ther Adv Med Oncol. 2021;13:17588359211031141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7:306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 779] [Article Influence: 129.8] [Reference Citation Analysis (0)] |
| 19. | Brahmer JR, Lacchetti C, Schneider BJ, Atkins MB, Brassil KJ, Caterino JM, Chau I, Ernstoff MS, Gardner JM, Ginex P, Hallmeyer S, Holter Chakrabarty J, Leighl NB, Mammen JS, McDermott DF, Naing A, Nastoupil LJ, Phillips T, Porter LD, Puzanov I, Reichner CA, Santomasso BD, Seigel C, Spira A, Suarez-Almazor ME, Wang Y, Weber JS, Wolchok JD, Thompson JA; National Comprehensive Cancer Network. Management of Immune-Related Adverse Events in Patients Treated With Immune Checkpoint Inhibitor Therapy: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1714-1768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2245] [Cited by in RCA: 2578] [Article Influence: 368.3] [Reference Citation Analysis (0)] |
| 20. | Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, Rathmell WK, Ancell KK, Balko JM, Bowman C, Davis EJ, Chism DD, Horn L, Long GV, Carlino MS, Lebrun-Vignes B, Eroglu Z, Hassel JC, Menzies AM, Sosman JA, Sullivan RJ, Moslehi JJ, Johnson DB. Fatal Toxic Effects Associated With Immune Checkpoint Inhibitors: A Systematic Review and Meta-analysis. JAMA Oncol. 2018;4:1721-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1108] [Cited by in RCA: 1840] [Article Influence: 262.9] [Reference Citation Analysis (0)] |
| 21. | Kalisz KR, Ramaiya NH, Laukamp KR, Gupta A. Immune Checkpoint Inhibitor Therapy-related Pneumonitis: Patterns and Management. Radiographics. 2019;39:1923-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 105] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 22. | Nishino M, Ramaiya NH, Hatabu H, Hodi FS, Armand PF. PD-1 inhibitor-related pneumonitis in lymphoma patients treated with single-agent pembrolizumab therapy. Br J Haematol. 2018;180:752-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Hatabu H, Hunninghake GM, Richeldi L, Brown KK, Wells AU, Remy-Jardin M, Verschakelen J, Nicholson AG, Beasley MB, Christiani DC, San José Estépar R, Seo JB, Johkoh T, Sverzellati N, Ryerson CJ, Graham Barr R, Goo JM, Austin JHM, Powell CA, Lee KS, Inoue Y, Lynch DA. Interstitial lung abnormalities detected incidentally on CT: a Position Paper from the Fleischner Society. Lancet Respir Med. 2020;8:726-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 365] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 24. | Nishino M, Giobbie-Hurder A, Hatabu H, Ramaiya NH, Hodi FS. Incidence of Programmed Cell Death 1 Inhibitor-Related Pneumonitis in Patients With Advanced Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016;2:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 560] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 25. | Gosangi B, McIntosh L, Keraliya A, Irugu DVK, Baheti A, Khandelwal A, Thomas R, Braschi-Amirfarzan M. Imaging features of toxicities associated with immune checkpoint inhibitors. Eur J Radiol Open. 2022;9:100434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Delaunay M, Cadranel J, Lusque A, Meyer N, Gounant V, Moro-Sibilot D, Michot JM, Raimbourg J, Girard N, Guisier F, Planchard D, Metivier AC, Tomasini P, Dansin E, Pérol M, Campana M, Gautschi O, Früh M, Fumet JD, Audigier-Valette C, Couraud S, Dalle S, Leccia MT, Jaffro M, Collot S, Prévot G, Milia J, Mazieres J. Immune-checkpoint inhibitors associated with interstitial lung disease in cancer patients. Eur Respir J. 2017;50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 281] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 27. | Weber M, Lam M, Chiesa C, Konijnenberg M, Cremonesi M, Flamen P, Gnesin S, Bodei L, Kracmerova T, Luster M, Garin E, Herrmann K. EANM procedure guideline for the treatment of liver cancer and liver metastases with intra-arterial radioactive compounds. Eur J Nucl Med Mol Imaging. 2022;49:1682-1699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 112] [Article Influence: 37.3] [Reference Citation Analysis (0)] |