Published online May 15, 2023. doi: 10.4251/wjgo.v15.i5.878
Peer-review started: December 5, 2022
First decision: January 9, 2023
Revised: January 24, 2023
Accepted: April 12, 2023
Article in press: April 12, 2023
Published online: May 15, 2023
Processing time: 157 Days and 17.3 Hours
Improved adenoma detection at colonoscopy has decreased the risk of developing colorectal cancer. However, whether image-enhanced endoscopy (IEE) further improves the adenoma detection rate (ADR) is controversial.
To compare IEE with white-light imaging (WLI) endoscopy for the detection and identification of colorectal adenoma.
This was a multicenter, randomized, controlled trial. Participants were enrolled between September 2019 to April 2021 from 4 hospital in China. Patients were randomly assigned to an IEE group with WLI on entry and IEE on withdrawal (n = 2113) or a WLI group with WLI on both entry and withdrawal (n = 2098). The primary outcome was the ADR. The secondary endpoints were the polyp detection rate (PDR), adenomas per colonoscopy, adenomas per positive colonoscopy, and factors related to adenoma detection.
A total of 4211 patients (966 adenomas) were included in the analysis (mean age, 56.7 years, 47.1% male). There were 2113 patients (508 adenomas) in the IEE group and 2098 patients (458 adenomas) in the WLI group. The ADR in two group were not significantly different [24.0% vs 21.8%, 1.10, 95% confidence interval (CI): 0.99-1.23, P = 0.09]. The PDR was higher with IEE group (41.7%) than with WLI group (36.1%, 1.16, 95%CI: 1.07-1.25, P = 0.01). Differences in mean withdrawal time (7.90 ± 3.42 min vs 7.85 ± 3.47 min, P = 0.30) and adenomas per colonoscopy (0.33 ± 0.68 vs 0.28 ± 0.62, P = 0.06) were not significant. Subgroup analysis found that with narrow-band imaging (NBI), between-group differences in the ADR, were not significant (23.7% vs 21.8%, 1.09, 95%CI: 0.97-1.22, P = 0.15), but were greater with linked color imaging (30.9% vs 21.8%, 1.42, 95%CI: 1.04-1.93, P = 0.04). the second-generation NBI (2G-NBI) had an advantage of ADR than both WLI and the first-generation NBI (27.0% vs 21.8%, P = 0.01; 27.0% vs 21.2.0%, P = 0.01).
This prospective study confirmed that, among Chinese, IEE didn’t increase the ADR compared with WLI, but 2G-NBI increase the ADR.
Core Tip: This study is the biggest randomized controlled trial comparing image-enhanced endoscopy (IEE) with white-light imaging (WLI) over the world, providing the solid evidence. This study provides strong evidence that IEE do not increase adenoma detection rate (ADR) compared with WLI, but second-generation-narrow-band imaging increase the ADR. IEE improved the polyp detection rate without additional withdrawal time.
- Citation: Qi ZP, Xu EP, He DL, Wang Y, Chen BS, Dong XS, Shi Q, Cai SL, Guo Q, Li N, Li X, Huang HY, Li B, Sun D, Xu JG, Chen ZH, Yalikong A, Liu JY, Lv ZT, Xu JM, Zhou PH, Zhong YS. Efficacy of image-enhanced endoscopy for colorectal adenoma detection: A multicenter, randomized trial. World J Gastrointest Oncol 2023; 15(5): 878-891
- URL: https://www.wjgnet.com/1948-5204/full/v15/i5/878.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i5.878
Colorectal cancer is relatively common worldwide, with over 1 million new cases and an estimated 550000 deaths reported in 2018[1]. The 5-year survival rate of advanced colorectal cancer is less than 40%, but detection at an early stage greatly improves the prognosis. Increasing the adenoma detection rate (ADR) by 1.0% can reduce the risk of colorectal cancer by 3.0%[2]. Colonoscopy is an ideal strategy for decreasing the prevalence of colorectal cancer by early detection and endoscopic resection of precancerous lesions. The current standard practice for detecting polyps and adenomas is endoscopy with white-light imaging (WLI), and it has a reported polyp/adenoma miss rate of 26%[3,4]. Given the need for improved detection, image-enhanced endoscopy (IEE) was developed to overcome the limitations of conventional colonoscopy.
IEE includes narrow-band imaging (NBI), flexible spectral imaging color enhancement, linked color imaging (LCI), and i-Scan, which are continually evolving. IEE improves the visualization of mucosal microstructure and microvasculature and the identification of lesions compared with WLI[5]. In a multicenter, randomized, crossover trial comparing LCI and WLI in polyp detection, Min et al[6] reported that the polyp detection rate (PDR) of LCI was 8% higher than that of WLI. A meta-analysis found that the ADR of NBI was significantly higher than that of WLI in patients with the best bowel preparations[7]. However, some studies have reported that NBI did not increase ADR or PDR[8-10]. As the additional benefit of IEE is still controversial, we conducted a prospective, multicenter, randomized, controlled study named the Image-Enhanced Endoscopy in Colonoscopy Screening trail in 4 hospitals in China to compare the ADR of IEE and WLI during colonoscopies. The primary objective was to determine whether IEE detected significantly more adenomas than WLI in patients with elective screening, surveillance, or diagnostic colonoscopy.
Trail design: This prospective, multicenter, randomized, controlled trial was conducted at 4 hospitals in China in following the ethical principles of Declaration of Helsinki (B2019-131R). The study was prepared following the Consolidated Standards of Reporting Trials statement for reporting randomized controlled trials (RCTs)[11], and was registered on the Chinese Clinical Trial Registry (ChiCTR19000
Trial participants: Consecutive eligible patients who were 18-80 years of age and scheduled to undergo colonoscopy were considered eligible for this trial. Patients without bowel preparation or poor bowel preparation indicated by a Boston Bowel Preparation Scale (BBPS) score < 6, or with untreated adenoma in previous examinations, familial polyposis, severe emphysema, interstitial pneumonia, or ischemic heart disease; and those who could not tolerate anesthesia and examination, and patients or their family members who could not understand the conditions and goals of this study were excluded. Eligible patients were informed by the endoscopists about the study aims, procedures, and potential risks. Written informed consent was obtained from all patients.
Randomization: Before colonoscopy withdrawal, eligible patients were randomly assigned 1:1 to the IEE group with WLI on entry and IEE on withdrawal or the WLI group with WLI on both entry and withdrawal. Patients were stratified by age to groups < 50 and ≥ 50 years of age. The investigators used a central customized system (https://uapkd.bioknow.net/#/) to generate random numbers for the group assignments for the eligible patients at each center. Then, the investigators will allocate the patients to different groups based on the results of the customized random system and each center will compete for entry.
Endoscopists and endoscopy equipment: The participating endoscopist at each study center had at least 5 years of work experience in colonoscopy and had rigorous IEE diagnostic training. The endoscopy systems used in this study included CV-260, CV-290 (Olympus) or ELUXEO 7000 (Fujifilm) devices, and high definition colonoscopes was used for all procedures without any mucosal exposure devices.
Endoscopic procedures and histopathology: All patients performed bowel preparations following the local hospital protocol, and conscious sedation was administered according to the judgment of the anesthetist. The endoscope was advanced to the cecum under WLI. Cecal intubation was confirmed by identification of the appendiceal orifice and ileocecal valve or by intubation of the ileum, and the bowel preparation was assessed by the BBPS score obtained during advancement of the endoscope to the cecum. Once cecal intubation was confirmed, the colonoscope was withdrawn to the anus by the assigned method, either IEE or WLI. Detected lesions were evaluated by the Paris morphological classification criteria and removed by the endoscopist[12]. The size and location of lesions were recorded. Withdrawal time were defined as the time from cecal intubation to extraction through the anus and were measured with a stopwatch, excluding the time used for washing of the colonic mucosa, suctioning of fluid, or performance of polypectomy, biopsy, or any other therapeutic maneuvers[13]. The data were recorded on standardized case report forms before being transferred to online electronic report forms (https://wa.zs-hospital.sh.cn/).
Histological samples were fixed in paraffin, processed by standard procedures, and examined by experienced pathologists who were blinded to the study procedures. Histological results were reported following the Vienna classification of gastrointestinal neoplasia[14]. Advanced adenoma was defined as an adenoma ≥ 10 mm in diameter with any villous histology, high-grade dysplasia, or invasive carcinoma[15].
Outcomes: The primary endpoint was ADR, defined as the proportion of patients with at least one detected adenoma of any size[15]. The secondary endpoints were PDR, diminutive ADR, adenomas per colonoscopy, and factors related to adenoma detection. PDR was defined as the proportion of patients with at least one detected polyp. The diminutive ADR included detection of at least one adenoma that was < 5 mm. Screening colonoscopies included those for which there was no diagnostic or surveillance indication. Surveillance colonoscopies included those for which there was no diagnostic indication and were performed in a patient who had a colonoscopy within the previous 10 years or who had a history of polyps or colorectal cancer. Diagnostic colonoscopies were those performed in patients who had one or more symptoms before the procedure[16].
The sample size estimate was based on an ADR of 13.4% by WLI in Chinese populations in previous studies[16]. In our experience, the ADR was around 10% when WLI was used. An increase in the ADR of 3% with IEE was considered clinically significant. Participants were allocated to the experimental and control groups in equal numbers. The Power and Sample Size Calculation program (PASS 2008; NCSS, LLC; https://www.ncss.com), estimated a sample size of 2012 per group using chi-square or Mann-Whitney U tests for comparison, assuming a type I error rate of 5% with 80% power, and a single-sided P < 0.05 for statistical significance. We planned to include 4200 subjects. The sample size was calculated by Dr. Li and Dr. Dong.
Intention-to-treat (see Supplementary Tables 1-7) and per protocol analyses were both conducted. Differences were expressed as relative risk (RR) with 95% confidence intervals (CIs). Continuous variables were tested for normal distribution and reported as means and standard deviation. Normally distributed variables were compared with student’s t-test and non-normally distributed variables were compared with the Mann-Whitney U test. Categorical variables were reported as frequencies and percentages (%), and compared with the χ2 test or Fisher’s exact test when applicable. The χ2 test was used for the analysis of the primary outcome (ADR). RR and 95%CIs were calculated for dichotomous outcomes and for the ADR in the IEE group relative to the WLI group. Secondary dichotomous outcomes and subgroup outcomes were analyzed in the way as the primary outcome. For the safety analysis, the frequency of adverse events and adverse reactions were calculated and analyzed using χ2 or Fisher exact tests. Details of adverse events and adverse reactions were recorded for deep analysis. The analysis was performed with SPSS v.18.0. (IBM Corp. Armonk, NY, United States). All reported P-values were two-sided, and those ≤ 0.05 were considered significant.
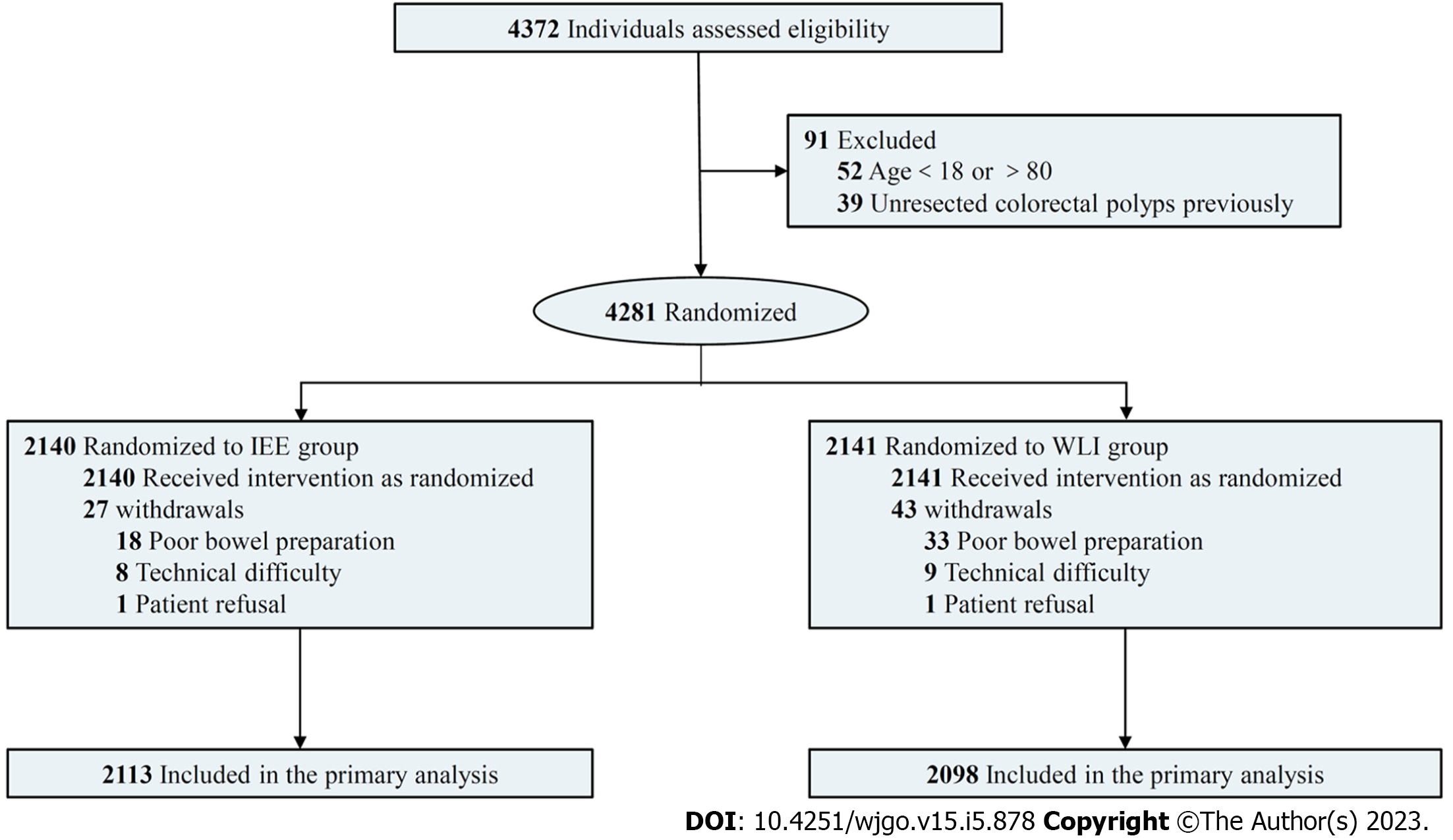
Figure 1 was a flow chart of the trial design and procedures. Between September 2019 and April 2021, 4372 consecutive patients were considered for inclusion, and 91 were excluded because they were < 18 or > 80 years of age or had previously unresected colorectal polyps (Figure 1). The remaining 4281 patients were randomized to the IEE (n = 2140) or WLI (n = 2141) groups. 70 patients failed cecal intubation because of poor bowel preparation, technical difficulties, or intolerance. A total of 4211 patients were included in the analysis, with 2113 in the IEE group and 2098 in the WLI group. No adverse events related to endoscopy occurred. The baseline characteristics of the patients in the two groups were similar (Table 1). The mean age, number of men, colorectal surgery history, and colonoscopy history of IEE and WLI were 56.7 ± 12.9 years and 56.8 ± 13.0 years, 1002 (47.7%) and 982 (46.8%), 149 (7.1%) and 134 (6.4%), 892 (42.2%) and 879 (41.9%), respectively. Between-group differences were not significant (all P > 0.05). The most common colonoscopy indication in both groups was diagnostic, 880(41.6%) patients in the IEE group and 876 (41.8%) in the WLI group (P > 0.05).
| IEE group (n = 2113) | WLI group (n = 2098) | |
| Age (yr) | 56.7 ± 12.9 | 56.8 ± 13.0 |
| Male gender | 1002 (47.7) | 982 (46.8) |
| BMI (kg/m2) | 23.4 ± 3.2 | 23.5 ± 3.2 |
| Comorbidities | ||
| 1468 (69.5) | 1510 (72.0) | |
| 640 (30.3) | 580 (27.6) | |
| 5 (0.2) | 8 (0.4) | |
| Colorectal surgery history | 149 (7.1) | 134 (6.4) |
| Colonoscopy indication | ||
| 880 (41.6) | 876 (41.8) | |
| 613 (29.0) | 601 (28.6) | |
| 620 (29.3) | 621 (29.6) | |
| Colonoscopy history | 892 (42.2) | 879 (41.9) |
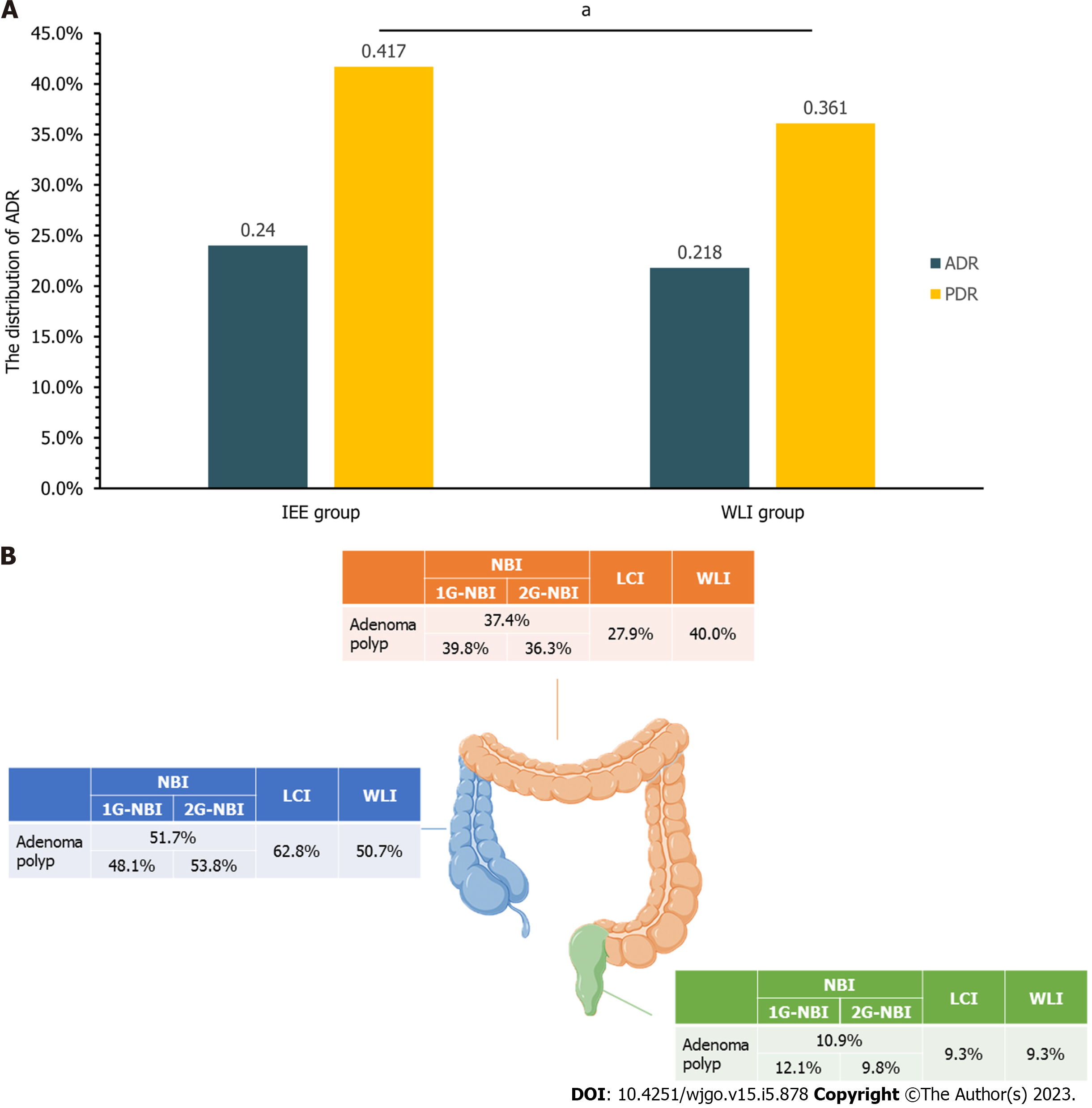
The primary outcome results are shown in Table 2 and Figure 2. A total of 966 adenomas were detected in 4211 patients. 508 adenomas were found in 2113 IEE patients and 458 were found in 2098 WLI patients. The ADR were 24.0% in the IEE patients and 21.8% in the WLI patients (RR = 1.10, 95%CI: 0.99-1.23, P > 0.05). The mean withdrawal time were 7.90 ± 3.42 min in the IEE group and 7.85 ± 3.47 min in the WLI group (P > 0.05). Differences in advanced ADR and diminutive ADR in the two groups were not significant (all P > 0.05). Regardless of age (< 50 or ≥ 50 years of age), sex, and colonoscopy indication, differences in the ADR were not significant (all P > 0.05).
| IEE group (n = 2113) | WLI group (n = 2098) | P value | Relative risk (95%CI) | |
| ADR | 508/2113 (24.0) | 458/2098 (21.8) | 0.09 | 1.10 (0.99-1.23) |
| Advanced ADR | 127/2113 (6.0) | 116/2098 (5.5) | 0.50 | 1.09 (0.85-1.39) |
| Diminutive ADR | 323/2113 (15.3) | 286/2098 (13.6) | 0.13 | 1.12 (0.97-1.30) |
| ADR in different ages | ||||
| 67/586 (11.4) | 54/557 (9.7) | 0.34 | 1.18 (0.84-1.66) | |
| 441/1527 (28.9) | 404/1541 (26.2) | 0.10 | 1.10 (0.98-1.24) | |
| ADR in different gender | ||||
| 288/1002 (28.7) | 254/982 (25.9) | 0.15 | 1.11 (0.96-1.28) | |
| 220/1111 (19.8) | 204/1116 (18.3) | 0.36 | 1.08 (0.91-1.29) | |
| ADR with different indications | ||||
| Diagnostic | ||||
| 26/270 (9.6) | 23/256 (9.0) | 0.80 | 1.07 (0.63-1.83) | |
| 179/610 (29.3) | 166/620 (26.8) | 0.32 | 1.10 (0.92-1.31) | |
| Surveillance | ||||
| 9/104 (8.7) | 7/93 (7.5) | 0.77 | 1.15 (0.45-2.97) | |
| 138/509 (27.1) | 126/508 (24.8) | 0.40 | 1.09 (0.89-1.5) | |
| Screening | ||||
| 32/212 (15.1) | 24/208 (11.5) | 0.28 | 1.31 (0.80-2.14) | |
| 124/408 (30.4) | 112/413 (27.1) | 0.30 | 1.12 (0.90-1.39) | |
| Withdrawal time | 7.90 ± 3.42 | 7.85 ± 3.47 | 0.30 |
The secondary outcomes are shown in Table 3 and Figure 2. A total of 2907 polyps were detected in 4211 patients, including 1588 polyps in 2113 IEE patients and 1319 polyps in 2098 WLI patients. The PDR in the IEE group was significantly greater than that in the WLI group (41.7% vs 36.1%, RR = 1.16, 95%CI: 1.07-1.25, P = 0.01). Pathological evaluation found that adenomas were the most common polyps in both the IEE (43.5%) and WLI (44.7%) groups, and that the difference was not significant (RR = 0.97, 95%CI: 0.90-1.06, P > 0.05). Adenomas per colonoscopy were 0.33 ± 0.68 and 0.28 ± 0.62, respectively in the IEE and WLI groups (P > 0.05). There were also no significant differences in the size, morphology, pathology, or site of the adenoma polyps detected in both groups (all P > 0.05).
| IEE group (n = 2113) | WLI group (n = 2098) | P value | Relative risk (95%CI) | |
| PDR | 882/2113 (41.7) | 757/2098 (36.1) | 0.01 | 1.16 (1.07-1.25) |
| All polyps | 1588 | 1319 | ||
| 426 (26.8) | 341 (25.9) | 0.55 | 1.04 (0.92-1.17) | |
| 179 (11.3) | 150 (11.4) | 0.93 | 0.99 (0.81-1.22) | |
| 690 (43.5) | 590 (44.7) | 0.49 | 0.97 (0.90-1.06) | |
| 10 (0.6) | 11 (0.8) | 0.52 | 0.76 (0.32-1.77) | |
| 262 (16.5) | 211 (16.0) | 0.71 | 1.03 (0.87-1.22) | |
| 16 (1.0) | 13 (1.0) | 0.95 | 1.02 (0.49-2.12) | |
| 5 (0.3) | 3 (0.2) | 0.741 | 1.38 (0.33-5.78) | |
| Adenoma per colonoscopy | 0.33 ± 0.68 | 0.28 ± 0.62 | 0.06 | |
| Adenoma polyp | 690 | 590 | ||
| Size | 0.44 | |||
| 338 (49.0) | 294 (49.8) | 0.98 (0.88-1.10) | ||
| 211 (30.6) | 163 (27.6) | 1.11 (0.93-1.32) | ||
| 141 (20.4) | 133 (22.5) | 0.91 (0.74-1.12) | ||
| Shape | 0.39 | |||
| 72 (10.4) | 76 (12.9) | 0.81 (0.60-1.10) | ||
| 186 (27.0) | 154 (26.1) | 1.03 (0.86-1.24) | ||
| 432 (62.6) | 360 (61.0) | 1.03 (0.94-1.12) | ||
| Pathology | 0.80 | |||
| 670 (97.1) | 569 (96.4) | 1.01 (0.99-1.03) | ||
| 18 (2.6) | 19 (3.2) | 0.81 (0.43-1.53) | ||
| 2 (0.3) | 2 (0.3) | 0.86 (0.12-6.05) | ||
| Site | 0.50 | |||
| 258 (37.4) | 236 (40.0) | 0.94 (0.81-1.07) | ||
| 357 (51.7) | 299 (50.7) | 1.02 (0.92-1.14) | ||
| 75 (10.9) | 55 (9.3) | 1.17 (0.84-1.62) |
The outcomes of the NBI and WLI groups are shown in Tables 4 and 5, Supplementary Table 8 and 9, and Figure 2. The ADR of the two groups were similar (23.7% vs 21.8%, RR = 1.09, 95%CI: 0.97-1.22, P = 0.15). The mean withdrawal time of the two groups were 7.90 ± 3.46 min and 7.85 ± 3.47 min (P > 0.05), and between-group differences in the values of other variables related to ADR were not significant. However, the second-generation NBI (2G-NBI) had an advantage of ADR than both WLI and first-generation NBI (1G-NBI) [27.0% vs 21.8%, RR = 1.24, 95%CI: 1.08-1.42, P = 0.01; 21.2% vs 27.0% (2G), RR = 0.78, 95%CI: 0.67-0.92, P = 0.01]. The mean withdrawal time of them was similar (P > 0.05). And the 2G-NBI was more suitable for small adenoma than WLI and 1G-NBI [17.1% vs 13.6%, RR = 1.26, 95%CI: 1.05-1.51, P = 0.01; 13.5% vs 17.1% (2G), RR = 0.79, 95%CI: 0.64-0.97, P = 0.02]. The PDR in the NBI group was significantly greater than that in the WLI group (41.6% vs 36.1%, RR = 1.15, 95%CI: 1.07-1.25, P < 0.01). There were no significant differences in the size, morphology, pathology, or site of the adenomas detected in the two groups (all P > 0.05). The PDR in the 2G-NBI group was significantly greater than that in both the WLI group (50.7% vs 36.1%, RR = 1.41, 95%CI: 1.29-1.53, P = 0.01), and the 1G-NBI group [34.7% vs 50.7% (2G), RR = 0.68, 95%CI: 0.62-0.76, P = 0.01].
| NBI group (n = 2016) | LCI group (n = 97) | WLI group (n = 2098) | P value1 | RR (95%CI) | P value2 | RR (95%CI) | P value3 | RR (95%CI) | |
| ADR | 478/2016 (23.7) | 30/97 (30.9) | 458/2098 (21.8) | 0.15 | 1.09 (0.97-1.22) | 0.04 | 1.42 (1.04-1.93) | 0.10 | 0.77 (0.56-1.04) |
| Advanced ADR | 122/2016 (6.1) | 5/97 (5.2) | 116/2098 (5.5) | 0.47 | 1.10 (0.86-1.40) | 0.87 | 0.93 (0.39-2.23) | 0.72 | 1.17 (0.49-2.80) |
| Diminutive ADR | 304/2016 (15.1) | 19/97 (19.6) | 286/2098 (13.6) | 0.19 | 1.11 (0.95-1.28) | 0.10 | 1.44 (0.95-2.18) | 0.23 | 0.77 (0.51-1.17) |
| ADR in different ages | |||||||||
| 55/538 (10.2) | 12/48 (25.0) | 54/557 (9.7) | 0.77 | 1.05 (0.74-1.51) | 0.01 | 2.58 (1.49-4.48) | 0.01 | 0.41 (0.24-0.71) | |
| 423/1478 (28.6) | 18/49 (36.7) | 404/1541 (26.2) | 0.14 | 1.09 (0.97-1.23) | 0.10 | 1.40 (0.96-2.04) | 0.22 | 0.78 (0.54-1.14) | |
| ADR in different gender | |||||||||
| 270/953 (28.3) | 18/49 (36.7) | 254/982 (25.9) | 0.22 | 1.10 (0.95-1.27) | 0.09 | 1.42 (0.97-2.08) | 0.21 | 0.77 (0.53-1.13) | |
| 208/1063 (19.6) | 12/48 (25.0) | 204/1116 (18.3) | 0.44 | 1.07 (0.90-1.27) | 0.24 | 1.37 (0.83-2.27) | 0.36 | 0.78 (0.47-1.30) | |
| ADR with different indications | |||||||||
| Diagnostic | |||||||||
| 23/247 (9.3) | 3/23 (13.0) | 23/256 (9.0) | 0.90 | 1.04 (0.60-1.80) | 0.464 | 1.45 (0.47-4.47) | 0.474 | 0.71 (0.23-2.20) | |
| 171/590 (29.0) | 8/20 (40.0) | 166/620 (26.8) | 0.39 | 1.08 (0.90-1.30) | 0.19 | 1.49 (0.86-2.60) | 0.29 | 0.73 (0.42-1.26) | |
| Surveillance | |||||||||
| 8/96 (8.3) | 1/8 (12.5) | 7/93 (7.5) | 0.84 | 1.11 (0.42-2.93) | 0.504 | 1.66 (0.23-11.87) | 0.534 | 0.67 (0.10-4.69) | |
| 134/496 (27.0) | 4/13 (30.8) | 126/508 (24.8) | 0.42 | 1.09 (0.88-1.34) | 0.754 | 1.24 (0.54-2.84) | 0.764 | 0.88 (0.38-2.01) | |
| Screening | |||||||||
| 24/195 (12.3) | 8/17 (47.1) | 24/208 (11.5) | 0.81 | 1.07 (0.63-1.81) | 0.01 | 4.08 (2.17-7.65) | 0.014 | 0.26 (0.14-0.49) | |
| 118/392 (30.1) | 6/16 (37.5) | 112/413 (27.1) | 0.35 | 1.11 (0.89-1.38) | 0.40 | 1.38 (0.72-2.65) | 0.58 | 0.80 (0.42-1.54) | |
| Withdrawal time | 7.90 ± 3.46 | 7.82 ± 2.67 | 7.85 ± 3.47 | 0.47 | 0.02 | 0.05 |
| NBI group (n = 2016) | LCI group (n = 97) | WLI group (n = 2098) | P value1 | RR (95%CI) | P value2 | RR (95%CI) | P value3 | RR (95%CI) | |
| PDR | 839/2016 (41.6) | 43/97 (44.3) | 757/2098 (36.1) | 0.01 | 1.15 (1.07-1.25) | 0.10 | 1.23 (0.98-1.55) | 0.60 | 0.94 (0.75-1.18) |
| All polyps | 1519 | 69 | 1319 | ||||||
| 421 (27.7) | 5 (7.2) | 341 (25.9) | 0.26 | 1.07 (0.95-1.21) | 0.01 | 0.28 (0.12-0.66) | 0.01 | 3.83 (1.64-8.93) | |
| 164 (10.8) | 15 (21.7) | 150 (11.4) | 0.63 | 0.95 (0.77-1.17) | 0.01 | 1.91 (1.19-3.07) | 0.01 | 0.50 (0.31-0.80) | |
| 647 (42.6) | 43 (62.3) | 590 (44.7) | 0.25 | 0.95 (0.88-1.04) | 0.01 | 1.39 (1.15-1.69) | 0.01 | 0.68 (0.56-0.83) | |
| 10 (0.7) | 0 (0) | 11 (0.8) | 0.59 | 0.79 (0.34-1.85) | 1.004 | 1.004 | |||
| 259 (17.1) | 3 (4.3) | 211 (16.0) | 0.45 | 1.07 (0.90-1.26) | 0.01 | 0.27 (0.09-0.83) | 0.01 | 3.92 (1.29-11.93) | |
| 13 (0.9) | 3 (4.3) | 13 (1.0) | 0.72 | 0.87 (0.40-1.87) | 0.044 | 4.41 (1.29-15.12) | 0.034 | 0.20 (0.06-0.68) | |
| 5 (0.3) | 0 (0) | 3 (0.2) | 0.734 | 1.45 (0.35-6.04) | 1.004 | 1.004 | |||
| Adenoma per colonoscopy | 0.32 ± 0.67 | 0.44 ± 0.87 | 0.28 ± 0.62 | 0.11 | 0.03 | 0.10 | |||
| Adenoma polyp | 647 | 43 | 590 | ||||||
| Size | 0.56 | 0.22 | 0.33 | ||||||
| 315 (48.7) | 23 (53.5) | 294 (49.8) | 0.98 (0.87-1.09) | 1.07 (0.80-1.44) | 0.91 (0.68-1.22) | ||||
| 196 (30.3) | 15 (34.9) | 163 (27.6) | 1.10 (0.92-1.31) | 1.26 (0.82-1.94) | 0.87 (0.57-1.33) | ||||
| 136 (21.0) | 5 (11.6) | 133 (22.5) | 0.93 (0.76-1.15) | 0.52 (0.22-1.19) | 1.81 (0.78-4.18) | ||||
| Shape | 0.43 | 0.79 | 1.004 | ||||||
| 68 (10.5) | 4 (9.3) | 76 (12.9) | 0.82 (0.60-1.11) | 0.72 (0.28-1.88) | 1.13 (0.43-2.95) | ||||
| 174 (26.9) | 12 (27.9) | 154 (26.1) | 1.03 (0.86-1.24) | 1.07 (0.65-1.76) | 0.96 (0.59-1.58) | ||||
| 405 (62.6) | 27 (62.8) | 360 (61.0) | 1.03 (0.94-1.12) | 1.03 (0.81-1.31) | 1.00 (0.79-1.26) | ||||
| Pathology | 0.90 | 0.684 | 0.67 | ||||||
| 627 (96.9) | 43 (100) | 569 (96.4) | 1.01 (0.98-1.03) | ||||||
| 18 (2.8) | 0 (0) | 19 (3.2) | 0.86 (0.46-1.63) | ||||||
| 2 (0.3) | 0 (0) | 2 (0.3) | 0.91 (0.13-6.45) | ||||||
| Site | 0.57 | 0.294 | 0.31 | ||||||
| 246 (38.0) | 12 (27.9) | 236 (40.0) | 0.95 (0.83-1.09) | 0.70 (0.43-1.14 | 1.36 (0.83-2.23) | ||||
| 330 (51.0) | 27 (62.8) | 299 (50.7) | 1.01 (0.90-1.12) | 1.24 (0.97-1.58) | 0.81 (0.64-1.04) | ||||
| 71 (11.0) | 4 (9.3) | 55 (9.3) | 1.18 (0.84-1.64) | 1.00 (0.38-2.62) | 1.18 (0.45-3.08) |
As shown in Tables 4 and 5. The ADR was higher in the LCI than in the WLI group (30.9% vs 21.8%, RR = 1.42, 95%CI: 1.04-1.93, P = 0.04) and in the LCI vs the WLI group in screening patients < 50 years of age (47.1% vs 11.5%, RR = 4.08, 95%CI: 2.17-7.65, P = 0.01). The PDR were also not significantly different (44.3% vs 36.1%, RR = 1.23, 95%CI: 0.98-1.55, P = 0.10). In all treatment groups, the proportions of adenomas, hyperplastic polyps, and cancers was higher with LCI than with WLI (all P < 0.05), but the proportions of inflammatory polyps and chronic mucosal inflammation was higher in WLI group (both P < 0.05). The number of adenomas per colonoscopy in the LCI group was more than that of WLI (0.44 ± 0.87 vs 0.28 ± 0.62, P = 0.03) and there were no significant differences in the size, morphology, pathology, or site (all P > 0.05).
The outcomes of the NBI and LCI groups are shown in Tables 4 and 5. The ADR in each group were not significantly different (23.7% vs 30.9%, RR = 0.77, 95%CI: 0.56-1.04, P = 0.10), but the ADR in patient < 50 years of age was lower in the NBI group than in the LCI group (10.2% vs 25.0%, RR = 0.41, 95%CI: 0.24-0.71, P = 0.01). The PDR were also not significantly different (41.6% vs 44.3%, RR = 0.94, 95%CI: 0.75-1.18, P = 0.60). The proportions of adenomatous and hyperplastic polyps, and cancer were higher in with LCI compared with NBI (all P < 0.05), but differences in the proportions of inflammatory polyps and chronic mucosal inflammation were at the contrary (both P < 0.05). There were no significant differences in the size, morphology, pathology, or site of polyps detected by NBI and LCI (all P > 0.05).
IEE was developed to meet the need improve the ADR, but the superiority of IEE is controversial. This randomized trial compared the ADR achieved with IEE and WLI in a large population, which, to the best of our knowledge, largest endoscopy study in China even over the world.
In this study, IEE had a higher ADR than WLI, but the difference was not statistically significant (24.0% vs 21.8%, P = 0.09). The lack of difference may have resulted from the 54.3 percentage of adenomas detected by the 1G-NBI modality, which, in the subgroup analysis had an ADR similar to that of WLI (21.2% vs 21.8%, P = 0.67). The NBI generally required better bowel preparation, as residual debris severely impaired visualization of the colonic mucosa and dim light reduced the recognizability of adenoma, weakening its effect. It was consistent with previous literature reports. One meta-analysis with nine RCTs and 2936 subjects comparing the ADR between 1G-NBI and WLE showed that ADR was similar on both group (36% vs 34%; P = 0.413)[17]; Another meta-analysis also show that 1G-NBI failed to express a better ADR compared with HD-WLE [odds ratio (OR) = 1.01, 95%CI: 0.74- 1.37][8]. However, 2G-NBI having been changed to obtain brighter images than 1G-NBI, even brighter than WLE, to improve ADR. In our subgroup analysis, 2G-NBI depicted a better ADR than WLE as previously reported. An RCT comparing 2G-NBI with WLE showed that the 2G-NBI could detect more adenomas per patient compared with WLE (2.0 vs 1.51, P = 0.031)[18]. One meta-analysis enrolling 11 RCTs, including 3 RCTs using 2G-NBI, showed 2G-NBI had a better ADR than WLE (OR = 1.28; 95%CI: 1.05-1.56, P = 0.02)[7]. The great number of 1G-NBI covered the advantage of 2G-NBI, leading to IEE failed to improve ADR. However, 2G-NBI had a better ADR showed by our subgroup analysis, and it can help improve the quality of colonoscopy.
The experience of endoscopists is known to affect the ADR[5]. Munroe et al[4] showed that the adenoma miss rate of trainees decreased as their experience increased and competency improved during tandem colonoscopy training. In another retrospective study involving 24943 procedures and 14 endoscopists, the number of procedures was independently associated with ADR. Endoscopists with > 1000 procedures had a higher ADR than those with < 200 procedures (OR = 1.51, 95%CI: 1.33-1.71, P = 0.001)[19]. All endoscopists in our study had at least 5 years of colonoscopy experience, and had a higher ADR (21.8%) than the 20% aspirational target recommended by the working group in the United Kingdom and Ireland[20], and most Asian endoscopists (ADR: 8%-20.3%)[16,21,22]. Operator experience might narrow the difference between WLI and IEE. Endoscopist performance may also be affected by the Hawthorne effect. Several studies reported that endoscopists paid more attention during the procedure than usual when they knew they were being monitored[23,24]. The high ADR of our endoscopists might explain the smaller than expected differences between the ADR achieved with WLI and IEE.
Although the difference between the ADR observed with IEE and WLI was similar, the PDR of IEE was higher than that of WLI (41.7% vs 36.1%, P = 0.01), which meant that IEE had a higher sensitivity of polyp detection than WLI. The result is consistent with previous reports that IEE (NBI, i-SCAN, and autofluorescence) benefited polyp detection[25-27]. However, most small non-neoplastic lesions of < 5 mm diameter are benign and need not be removed. Therefore, it is important to distinguish neoplastic from non-neoplastic polyps before endoscopic biopsy to avoid additional treatment-related complications and costs. We were unable to accurately assess the specificity of IEE because of incomplete NBI and LCI classification records, but many studies have previously confirmed that IEE was better than WLI for the differentiation of neoplastic from non-neoplastic polyps[28-30]. A randomized study showed that NBI with magnification had a sensitivity of 90.5% and a specificity of 89.2% for the differentiation of neoplastic and non-neoplastic lesions, which was comparable to magnifying chromoendoscopy. Therefore, IEE detected more polyps and more accurately differentiated neoplastic from non-neoplastic polyps. We recommend that trainees use IEE to reduce messed diagnoses. Although NBI reduced brightness, it significantly improved the visual characterization of polyps. Therefore, senior, expert endoscopy experts can choose white light or IEE mode according to their preference, but for trainees, NBI or LCI mode are recommended to improve the ADR whenever it is difficult to identify lesions in the white light mode.
Subgroup analysis showed 2G-NBI not only had advantage of ADR, but detecting the small adenoma or polyp. Our result revealed that 2G-NBI depicted more small adenomas than WLI and 1G-NBI (P < 0.05). What’s more, the proportion of inflammatory polyps, usually having a small size, was higher in 2G-NBI than others (all P < 0.05). Rex et al[31] recorded that 2G-NBI could demonstrate a better ADR with 5-10 mm than WLI (P = 0.032). Another RCT in 2015 also showed that 2G-NBI might have priority to adenoma with < 5 mm than WLI (P = 0.039)[18]. Combining our data, we recommend 2G-NBI as the major IEE modality.
Subgroup analysis also found a significant difference in the ADR achieved with LCI and WLI (30.9% vs 21.8%, P = 0.04) and the mean number of adenomas per colonoscopy (0.44 ± 0.87 vs 0.28 ± 0.62, P = 0.03). ADR and the mean number of adenomas per colonoscopy are both critical indicators reflecting the quality of colonoscopy[32], and improved performance might be clinically relevant because a highly quality colonoscopy has been associated with an increased ADR[15]. LCI, using an appropriate balance of combined narrow-band short-wavelength light and white light, achieves a bright, clear image, making up for the shortcomings of NBI[33]. Therefore, in clinical practice, when NBI or WLI is too dim to identify a polyp, LCI can be of assistance. Although our results are similar to those of previous studies[6,15], the limit of insufficient sample size in the LCI group could cause bias, which required a larger sample size to provide statistical significance for the ADR. The detection rate for sessile serrated lesions was low, reflecting a different prevalence in Chinese patients[16], and the detailed results was attached in Supplementary Table 10.
When we calculated the sample size, we assumed that the ADR was around 10% when WLI was used. That was far lower than the final result, but it does not impair the reliability of the study, and may even make it more reliable. If the ADR used to calculate the sample size was, then fewer than 4200 subjects were needed. Consequently, the result based on the protocol was reasonable and reliable.
This study strengths included its large sample (4211) which was the largest endoscopic study in China even over the word. In additional, it was the first large, multicenter endoscopic RCT in China, which provided strong evidence with Asian population for guideline development and provided reference for other populations. What’s more, multi-center involved hospitals of different regions and levels in China making the data become more popularize. Furthermore, IEE and WLI procedures had similar withdrawal time, which improving the comparability of ADR between the two groups. Finally, we included two IEE modalities, NBI and LCI, providing a reference for follow-up studies.
The study limitations included a lack of double blinding because of the obvious image characteristics of IEE. Furthermore, the proficiency of different operators in different enhancement modes may have introduced selection bias[34]. Moreover, most patient re-examinations were performed after the study had ended, and the results were not included in the analysis. It was thus difficult to verify the missed diagnosis and misdiagnosis rates of IEE and WLI. In addition, there were objective differences in population distribution and medical conditions in various regions of our country, resulting in different sample sizes of groups enrolled in each center.
In summary, in this RCT performed in an expert setting, IEE did not increase the proportion of patients with at least one detected adenoma compared with WLI. However, the 2G-NBI depicted a better ADR than WLI.
Adenoma detection rate (ADR) as main outcome quality parameter of colonoscopy is under controversial with the use of the image-enhanced endoscopy (IEE). Although there have some randomized controlled trials (RCTs) to compare different IEE with white-light imaging (WLI), the sample is limited and there is still lacking the RCT of IEE with Asian population.
To compare IEE with WLI for the detection and identification of colorectal adenoma and provide the solid outcomes.
To compare IEE with WLI endoscopy for the detection and identification of colorectal adenoma.
We designed a prospective, multicenter, randomized, controlled trial to compere the ADR between the IEE group and WLI group.
The ADR in two group were not significantly different [24.0% vs 21.8%,1.10, 95% confidence interval (CI): 0.99-1.23, P = 0.09]. The polyp detection rate was higher with IEE group (41.7%) than with WLI group (36.1%, 1.16, 95%CI: 1.07-1.25, P = 0.01). Differences in mean withdrawal time (7.90 ± 3.42 min vs 7.85 ± 3.47 min, P = 0.30) and adenomas per colonoscopy (0.33 ± 0.68 vs 0.28 ± 0.62, P = 0.06) were not significant. Subgroup analysis found that with narrow-band imaging (NBI), between-group differences in the ADR, were not significant (23.7% vs 21.8%, 1.09, 95%CI: 0.97-1.22, P = 0.15), but were greater with linked color imaging (30.9% vs 21.8%, 1.42, 95%CI: 1.04-1.93, P = 0.04). The second-generation NBI (2G-NBI) had an advantage of ADR than both WLI and 1G-NBI (27.0% vs 21.8%, P = 0.01; 27.0% vs 21.2.0%, P = 0.01).
This prospective study confirmed that, among Chinese, IEE didn’t increase the ADR compared with WLI, but 2G-NBI increase the ADR. Colonoscopists with low ADR might consider using 2G-NBI for performance.
The efficacy of various modes of IEE still needs to be verified by clinical research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Toskas A, United Kingdom; Trna J, Czech Republic S-Editor: Wang JJ L-Editor: A P-Editor: Zhang YL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55686] [Article Influence: 7955.1] [Reference Citation Analysis (132)] |
| 2. | Corley DA, Jensen CD, Marks AR, Zhao WK, Lee JK, Doubeni CA, Zauber AG, de Boer J, Fireman BH, Schottinger JE, Quinn VP, Ghai NR, Levin TR, Quesenberry CP. Adenoma detection rate and risk of colorectal cancer and death. N Engl J Med. 2014;370:1298-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1552] [Article Influence: 141.1] [Reference Citation Analysis (0)] |
| 3. | van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 878] [Cited by in RCA: 917] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 4. | Munroe CA, Lee P, Copland A, Wu KK, Kaltenbach T, Soetikno RM, Friedland S. A tandem colonoscopy study of adenoma miss rates during endoscopic training: a venture into uncharted territory. Gastrointest Endosc. 2012;75:561-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | McCarty TR, Aihara H. Role of image-enhanced endoscopy: how to improve colorectal polyp detection rates in the coming decade. Gastrointest Endosc. 2020;91:113-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Min M, Deng P, Zhang W, Sun X, Liu Y, Nong B. Comparison of linked color imaging and white-light colonoscopy for detection of colorectal polyps: a multicenter, randomized, crossover trial. Gastrointest Endosc. 2017;86:724-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Atkinson NSS, Ket S, Bassett P, Aponte D, De Aguiar S, Gupta N, Horimatsu T, Ikematsu H, Inoue T, Kaltenbach T, Leung WK, Matsuda T, Paggi S, Radaelli F, Rastogi A, Rex DK, Sabbagh LC, Saito Y, Sano Y, Saracco GM, Saunders BP, Senore C, Soetikno R, Vemulapalli KC, Jairath V, East JE. Narrow-Band Imaging for Detection of Neoplasia at Colonoscopy: A Meta-analysis of Data From Individual Patients in Randomized Controlled Trials. Gastroenterology. 2019;157:462-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 8. | Pasha SF, Leighton JA, Das A, Harrison ME, Gurudu SR, Ramirez FC, Fleischer DE, Sharma VK. Comparison of the yield and miss rate of narrow band imaging and white light endoscopy in patients undergoing screening or surveillance colonoscopy: a meta-analysis. Am J Gastroenterol. 2012;107:363-70; quiz 371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Hazewinkel Y, Tytgat KM, van Leerdam ME, Koornstra JJ, Bastiaansen BA, van Eeden S, Fockens P, Dekker E. Narrow-band imaging for the detection of polyps in patients with serrated polyposis syndrome: a multicenter, randomized, back-to-back trial. Gastrointest Endosc. 2015;81:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Sabbagh LC, Reveiz L, Aponte D, de Aguiar S. Narrow-band imaging does not improve detection of colorectal polyps when compared to conventional colonoscopy: a randomized controlled trial and meta-analysis of published studies. BMC Gastroenterol. 2011;11:100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Schulz KF, Altman DG, Moher D; CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5694] [Cited by in RCA: 6413] [Article Influence: 427.5] [Reference Citation Analysis (108)] |
| 12. | The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc. 2003;58:S3-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1117] [Cited by in RCA: 1318] [Article Influence: 59.9] [Reference Citation Analysis (4)] |
| 13. | Repici A, Badalamenti M, Maselli R, Correale L, Radaelli F, Rondonotti E, Ferrara E, Spadaccini M, Alkandari A, Fugazza A, Anderloni A, Galtieri PA, Pellegatta G, Carrara S, Di Leo M, Craviotto V, Lamonaca L, Lorenzetti R, Andrealli A, Antonelli G, Wallace M, Sharma P, Rosch T, Hassan C. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology. 2020;159:512-520.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 391] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 14. | Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF, Geboes K, Hattori T, Hirota T, Itabashi M, Iwafuchi M, Iwashita A, Kim YI, Kirchner T, Klimpfinger M, Koike M, Lauwers GY, Lewin KJ, Oberhuber G, Offner F, Price AB, Rubio CA, Shimizu M, Shimoda T, Sipponen P, Solcia E, Stolte M, Watanabe H, Yamabe H. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1542] [Article Influence: 61.7] [Reference Citation Analysis (0)] |
| 15. | Houwen BBSL, Hazewinkel Y, Pellisé M, Rivero-Sánchez L, Balaguer F, Bisschops R, Tejpar S, Repici A, Ramsoekh D, Jacobs MAJM, Schreuder RM, Kaminski MF, Rupinska M, Bhandari P, van Oijen MGH, Koens L, Bastiaansen BAJ, Tytgat KM, Fockens P, Vleugels JLA, Dekker E. Linked Colour imaging for the detection of polyps in patients with Lynch syndrome: a multicentre, parallel randomised controlled trial. Gut. 2022;71:553-560. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 16. | Jia H, Pan Y, Guo X, Zhao L, Wang X, Zhang L, Dong T, Luo H, Ge Z, Liu J, Hao J, Yao P, Zhang Y, Ren H, Zhou W, Guo Y, Zhang W, Chen X, Sun D, Yang X, Kang X, Liu N, Liu Z, Leung F, Wu K, Fan D. Water Exchange Method Significantly Improves Adenoma Detection Rate: A Multicenter, Randomized Controlled Trial. Am J Gastroenterol. 2017;112:568-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Dinesen L, Chua TJ, Kaffes AJ. Meta-analysis of narrow-band imaging versus conventional colonoscopy for adenoma detection. Gastrointest Endosc. 2012;75:604-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 18. | Horimatsu T, Sano Y, Tanaka S, Kawamura T, Saito S, Iwatate M, Oka S, Uno K, Yoshimura K, Ishikawa H, Muto M, Tajiri H. Next-generation narrow band imaging system for colonic polyp detection: a prospective multicenter randomized trial. Int J Colorectal Dis. 2015;30:947-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 19. | Dong Z, Sun H, Li B, Zhang Q, Sun K, Wang Z, Qian X, Wang J, Zhan T, Jiang Y, Chen Y, Xu S. Comprehensive evaluation of the learning curve to achieve satisfactory adenoma detection rate. J Gastroenterol Hepatol. 2021;36:1649-1655. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Rees CJ, Thomas Gibson S, Rutter MD, Baragwanath P, Pullan R, Feeney M, Haslam N; British Society of Gastroenterology, the Joint Advisory Group on GI Endoscopy, the Association of Coloproctology of Great Britain and Ireland. UK key performance indicators and quality assurance standards for colonoscopy. Gut. 2016;65:1923-1929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 230] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 21. | Gong D, Wu L, Zhang J, Mu G, Shen L, Liu J, Wang Z, Zhou W, An P, Huang X, Jiang X, Li Y, Wan X, Hu S, Chen Y, Hu X, Xu Y, Zhu X, Li S, Yao L, He X, Chen D, Huang L, Wei X, Wang X, Yu H. Detection of colorectal adenomas with a real-time computer-aided system (ENDOANGEL): a randomised controlled study. Lancet Gastroenterol Hepatol. 2020;5:352-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 261] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 22. | Wang P, Berzin TM, Glissen Brown JR, Bharadwaj S, Becq A, Xiao X, Liu P, Li L, Song Y, Zhang D, Li Y, Xu G, Tu M, Liu X. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: a prospective randomised controlled study. Gut. 2019;68:1813-1819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 547] [Article Influence: 91.2] [Reference Citation Analysis (0)] |
| 23. | Buchner AM, Shahid MW, Heckman MG, Diehl NN, McNeil RB, Cleveland P, Gill KR, Schore A, Ghabril M, Raimondo M, Gross SA, Wallace MB. Trainee participation is associated with increased small adenoma detection. Gastrointest Endosc. 2011;73:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Rogart JN, Siddiqui UD, Jamidar PA, Aslanian HR. Fellow involvement may increase adenoma detection rates during colonoscopy. Am J Gastroenterol. 2008;103:2841-2846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 25. | Bisschops R, Tejpar S, Willekens H, De Hertogh G, Van Cutsem E. Virtual chromoendoscopy (I-SCAN) detects more polyps in patients with Lynch syndrome: a randomized controlled crossover trial. Endoscopy. 2017;49:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | East JE, Suzuki N, Stavrinidis M, Guenther T, Thomas HJ, Saunders BP. Narrow band imaging for colonoscopic surveillance in hereditary non-polyposis colorectal cancer. Gut. 2008;57:65-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Ramsoekh D, Haringsma J, Poley JW, van Putten P, van Dekken H, Steyerberg EW, van Leerdam ME, Kuipers EJ. A back-to-back comparison of white light video endoscopy with autofluorescence endoscopy for adenoma detection in high-risk subjects. Gut. 2010;59:785-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Chang CC, Hsieh CR, Lou HY, Fang CL, Tiong C, Wang JJ, Wei IV, Wu SC, Chen JN, Wang YH. Comparative study of conventional colonoscopy, magnifying chromoendoscopy, and magnifying narrow-band imaging systems in the differential diagnosis of small colonic polyps between trainee and experienced endoscopist. Int J Colorectal Dis. 2009;24:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Guo TJ, Chen W, Chen Y, Wu JC, Wang YP, Yang JL. Diagnostic performance of magnifying endoscopy with narrow-band imaging in differentiating neoplastic colorectal polyps from non-neoplastic colorectal polyps: a meta-analysis. J Gastroenterol. 2018;53:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | Tischendorf JJ, Wasmuth HE, Koch A, Hecker H, Trautwein C, Winograd R. Value of magnifying chromoendoscopy and narrow band imaging (NBI) in classifying colorectal polyps: a prospective controlled study. Endoscopy. 2007;39:1092-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 155] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 31. | Rex DK, Clodfelter R, Rahmani F, Fatima H, James-Stevenson TN, Tang JC, Kim HN, McHenry L, Kahi CJ, Rogers NA, Helper DJ, Sagi SV, Kessler WR, Wo JM, Fischer M, Kwo PY. Narrow-band imaging versus white light for the detection of proximal colon serrated lesions: a randomized, controlled trial. Gastrointest Endosc. 2016;83:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 32. | Park SK, Kim HY, Lee CK, Cha JM, Eun CS, Han DS, Lee BI, Shin JE, Park DI. Comparison of adenoma detection rate and adenoma per colonoscopy as a quality indicator of colonoscopy. Scand J Gastroenterol. 2016;51:886-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Okada M, Sakamoto H, Takezawa T, Hayashi Y, Sunada K, Lefor AK, Yamamoto H. Laterally Spreading Tumor of the Rectum Delineated with Linked Color Imaging Technology. Clin Endosc. 2016;49:207-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Leung WK, Guo CG, Ko MKL, To EWP, Mak LY, Tong TSM, Chen LJ, But DYK, Wong SY, Liu KSH, Tsui V, Lam FYF, Lui TKL, Cheung KS, Lo SH, Hung IFN. Linked color imaging versus narrow-band imaging for colorectal polyp detection: a prospective randomized tandem colonoscopy study. Gastrointest Endosc. 2020;91:104-112.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |