Published online May 15, 2023. doi: 10.4251/wjgo.v15.i5.776
Peer-review started: December 28, 2022
First decision: January 9, 2023
Revised: January 23, 2023
Accepted: April 21, 2023
Article in press: April 21, 2023
Published online: May 15, 2023
Processing time: 135 Days and 0.6 Hours
The relevance of constipation to the development and progression of colorectal cancer (CRC) is currently a controversial issue. Studies have shown that changes in the composition of the gut microbiota, a condition known as ecological imbalance, are correlated with an increasing number of common human diseases, including CRC and constipation. CRC is the second leading cause of cancer-related deaths worldwide, and constipation has been receiving widespread attention as a risk factor for CRC. Early colonoscopy screening of constipated patients, with regular follow-ups and timely intervention, can help detect early intestinal lesions and reduce the risks of developing colorectal polyps and CRC. As an important regulator of the intestinal microenvironment, the gut microbiota plays a critical role in the onset and progression of CRC. An increasing amount of evidence supports the thought that gut microbial composition and function are key determinants of CRC development and progression, with alterations inducing changes in the expression of host genes, metabolic regulation, and local and systemic immunological responses. Furthermore, constipation greatly affects the composition of the gut microbiota, which in turn influences the susceptibility to intestinal diseases such as CRC. However, the crosstalk between the gut microbiota, constipation, and CRC is still unclear.
Core Tip: The changes in the composition of the gut microbiota are correlated with an increasing number of common human diseases, including colorectal cancer (CRC) and constipation. CRC is the second leading cause of cancer-related deaths worldwide, and constipation has been receiving widespread attention as a risk factor for CRC. An increasing amount of evidence supports the thought that gut microbial composition and function are key determinants of CRC development and progression, with alterations inducing changes in the expression of host genes, metabolic regulation, and local and systemic immunological responses.
- Citation: Wang LW, Ruan H, Wang BM, Qin Y, Zhong WL. Microbiota regulation in constipation and colorectal cancer. World J Gastrointest Oncol 2023; 15(5): 776-786
- URL: https://www.wjgnet.com/1948-5204/full/v15/i5/776.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i5.776
There are approximately 100 trillion microbial cells in the gut microbiota, including a varied mix of bacteria, fungi, protozoa, and viruses[1]. Most gut bacteria form complex networks that are important mediators of tissue homeostasis, inflammation, and tumor development[2]. Despite regularly being described to as the “forgotten organ,” the symbiotic equilibrium of the gut microbiota plays a crucial role in maintaining host health[3]. The gut microbiota is involved in a variety of physiological activities in the host, such as the fermentation of food components, production of short-chain fatty acids (SCFAs), regulation of immune function, regulation of the growth and differentiation of intestinal epithelial cells (IEC), bile salt metabolism, and production of vitamins and other protective substances. It also acts as a biological barrier to prevent the adhesion and invasion of pathogenic and potentially pathogenic bacteria. This intricate ecosystem not only involves a passive colonizer of the gut, but it also facilitates engagement with the host through a variety of interactions that support a number of physiological functions, including nutrition absorption, immunity, metabolism, and tissue development[4,5].
Colorectal cancer (CRC) is the third most prevalent type of cancer, accounting for nearly 2 million new cases each year, and is the second leading cause of cancer-related deaths globally[6]. As with many other diseases, the onset and progression of CRC are due to a combination of hereditary and environmental factors. The microbiota is an essential environmental component that contributes to the development of cancers such as colorectal, liver, biliary tract, and breast cancers[7]. The microbiota in the colorectum interacts with IEC to obtain energy and regulate the body's immune response; consequently, its role in colorectal carcinogenesis is of great interest.
Constipation is a common gastrointestinal disorder and a common symptom in patients with cancer. It is characterized by scanty stools, hardened stools, or difficulty passing stools, and may occur alone or secondary to other diseases[8]. Constipation is a common problem for 16% of individuals overall and 33.5% of seniors (60-101 years)[9]. Disruption of the intestinal microbial community (ecological dysbiosis) can lead to various changes in host pathophysiology, resulting in functional gastrointestinal disorders, particularly constipation[10].
It is widely accepted that a variety of variables, including heredity, the environment, and chronic inflammation, contribute to the etiology of CRC[11]. Moreover, inflammation is a recognized driver of CRC development[2,12]. The gut microbiota can affect inflammatory processes in the digestive system as part of its interaction with the host immune system. When feces from patients with CRC were instilled into sterile, carcinogen-fed mice, the gut microbiome promoted the synthesis of chemokines, which increased histological inflammation and the expression of inflammatory genetic markers[13]. This is because the gut microbiota stimulates the production of chemokines (e.g., CCL5, CXCL9, CXCL10, CCL17, and CCL20) through tumorigenic cancer cells, thus favoring the recruitment of beneficial T cells into tumor tissue[14]. The microbiome induces multiple cases of inflammation and activates oncogenic pathways, resulting in increased cytokine expression during inflammation.
The intestinal mucosal barrier usually keeps the immune cells and gut microbiota apart. IECs make up the single layer of the intestinal mucosal barrier, which is joined by tight junctions[15]. The intestinal mucosal barrier is extremely permeable in both humans and CRC mouse models[16]. Increased susceptibility to CRC due to dextran sodium sulfate-induced colitis disrupts the function of the intestinal mucosal barrier[17]. The mucosal barrier in rats is compromised by ammonia, a product of the intestinal microbiota, which has also been associated with an increase in colonic adenomas[18]. Sulfides are toxic to colon cells and inhibit butyrate oxidation, which can damage the barrier of the colon cell[19]. Notably, even some metabolites can enhance the mucosal barrier function of the intestine. SCFAs are essential nutrients for IEC, which encourage the proliferation and differentiation of these cells and maintain the integrity of the intestinal epithelium[20].
Pattern recognition receptors (PRRs) enable communication between the immune system and microbiota by recognizing specific molecular patterns associated with pathogens[21]. In animal models, these PRRs are present among those associated with coliform-associated carcinogenesis, including Toll-like receptors (TLRs)[22], nucleotide-binding oligomerization-like receptors, retinol-induced gene-I-like receptors[23], and melanoma 2-like receptors[24]. When myeloid differentiation factor 88 (MyD88), a crucial bridge protein required for TLR signaling, is activated, invasive commensal bacteria and their components bind to the TLRs on tumor-infiltrating myeloid cells[25]. This in turn triggers the synthesis of downstream pro-inflammatory cytokines, including IL-23, IL-17A, IL-6, IL22, IL-1β, and TNF-α[14,16,25]. These cytokines promote malignant progression by enhancing cell proliferation, aggressiveness, and resistance to apoptosis. Ultimately, they stimulate the signaling pathways for nuclear factor-κB (NF-κB) and activator of transcription 3 (STAT3), which enhances tumor cell growth[26,27]. Additionally, commensal bacteria and their metabolites boost the expression of IL-17C in transformed IECs via TLR/MyD88-dependent signaling. IL-17C promotes tumor cell survival and carcinogenesis by inducing the expressions of B-cell lymphoma-2 and B-cell lymphoma-xL in IECs in an autocrine manner[25].
According to previous studies, oncogenesis can be undertaken using a number of bacteria, such as Fusobacterium nucleatum (F. nucleatum) adhesion and the invasion of colonic epithelial cells, to regulate oncogenic and inflammatory responses through FadA antigen binding to E-calmodulin on IECs to activate β-linked proteins[28]. Through the activation of TLR-4 signaling to NF-κB and the upregulation of miR-21 expression, myeloid cell infiltration is induced in tumors, and cancer cell proliferation and tumor progression are promoted[29].
In addition to the inflammatory immune mechanisms of gut microbes, the gut microbiota is capable of producing proteins, molecules, and secondary metabolites that are especially harmful to DNA. Host DNA can directly interact with and be modified by these products[30]. Bacteria produce two well-defined genotoxins: Cytolethal distending toxin (CDT) and colistin[31]. Several enteric pathogens, including Salmonella, Escherichia and Campylobacter spp. produce CDT, which induces double-stranded DNA breaks through its deoxyribonuclease activity[32,33]. In the form of a deoxyribonuclease I-like protein, CDT exhibits DNA enzyme activity and regulates cell cycle development[34]. This toxin causes eukaryotic cells to stagnate in the G2 /M transition phase of the cell cycle, which stops the division of eukaryotic cells, but the cytoplasm continues to grow and expand. At last, the nucleus was seriously damaged and chromatin was obviously broken or completely disappeared[35]. Bacteroides fragilis (B. fragilis) toxins can lead to CRC progression by inducing mutations, damaging DNA, and ultimately damaging the epithelial cell genome[36]. Upon IECs exposure, B. fragilis toxin binds to specific IEC receptors and rapidly cleaves the extracellular structural domain of E-calmodulin, leading to complete degradation of E-calmodulin[37]. Subsequently, β-linked protein/T-cell factor-dependent transcriptional activation induces transcription and translation of the c-Myc oncogene and sustained cell proliferation[38].Furthermore, both B. fragilis toxins and Enterococcus faecalis reactive oxygen species have been linked to strand breaks and chromosomal aberrations in vitro[39,40]. Because small-molecule inhibitors that target the production of Escherichia coli (E. coli) toxins have been demonstrated to reduce the tumor burden in mouse models, their binding or inactivation may have therapeutic or preventative effects on CRC[40].
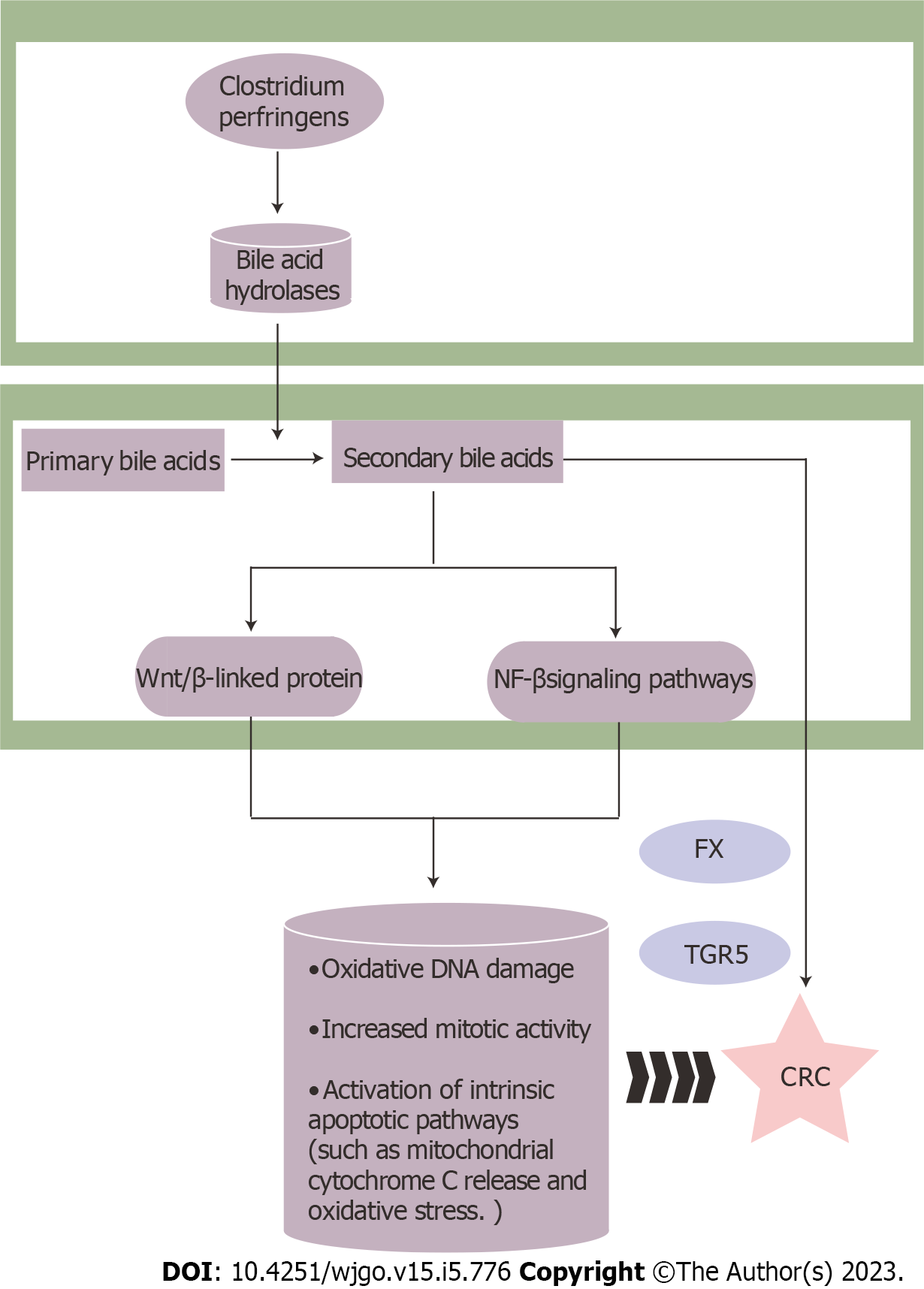
Besides, Clostridium perfringens belongs to the genus Clostridium, which produces bile acid hydrolases that catalyze the production of secondary bile acids (such as deoxycholic acid and lithocholic acid. Increased secondary bile acids levels activate the Wnt/β-linked protein and NF-κB signaling pathways, resulting in oxidative DNA damage, increased mitotic activity, and activation of intrinsic apoptotic pathways such as mitochondrial cytochrome C release and oxidative stress[41,42]. Secondary bile acids also influence CRC by activating the bile acid receptors G protein-coupled bile acid receptor 5 (TGR5) and farnesoid X receptor (FXR)[43,44] (Figure 1).
It is widely accepted that gut dysfunction, such as intestinal fluid transport, intestinal peristalsis, mucus production, and intestinal nerve conduction disorders, is the primary cause of constipation[45]. However, several recent studies have demonstrated that the gut microbiota and its metabolism play a significant role in the physiology and pathology of constipation. They have the capacity to change intestinal secretion and the microenvironment by interacting with the immune system, enteric nervous system (ENS), and central nervous system[46]. Therefore, gut microbiota may cause intestinal motility disorders through complex mechanisms, but the key underlying mechanisms are still under investigation.
In a constipated state, intestinal motility and secretion can become abnormal if the gut microbiota and metabolism are disrupted[47]. Simultaneously, the host modulates the gut microbiota via a variety of PRRs. In terms of the regulation of gastrointestinal motility, most TLRs are expressed in gut microbial components and gastrointestinal sensory components[48,49]. TLRs can communicate directly with bacterial components to make it easier for gastrointestinal cells and the gut microbiota to work together. For example, there is evidence of the expression of TLR2 in intestinal smooth muscle cells, neurons, glial cells, and interstitial cells of Cajal (ICCs). By binding to TLR2 from the gut microbiota, lipopeptides, peptidoglycan, and lipophilin acid trigger the release of glial cell line-derived neurotrophic factor via NF-κB and p38 mitogen-activated protein kinase (MAPK) signaling, maintain ENS and neurogenesis neurons, and exert anti-inflammatory effects to improve gastrointestinal motility in a manner that is not dependent on MyD88[48,50].
Of the receptors previously described, TLR4 is the most suitable for recognizing lipopolysaccharides (LPS) generated from the gut microbiota, along with TLR2. When LPS binds to TLR4 expressed on myeloid macrophages (MM), it induces the production of bone morphogenetic protein 2 (BMP2), which improves gastrointestinal motility. Enteric neurons generate colony-stimulating factor 1 in conjunction with BMP2, which facilitates MM homeostasis and regulates gastrointestinal motility[51]. As a result, the gut microbiota participates in and regulates the crosstalk between the MM and gut neurons, thereby influencing gastrointestinal dynamics. Nevertheless, higher concentrations of LPS expressed on ICCs, cause them to bind to TLR4 and inhibit pacemaker activity in ICC via the MAPK and NF-κB signaling pathways, thereby suppressing gastrointestinal motility and leading to reduced fecal production and prolonged defecation[52].
Furthermore, gut microbes can interact with ENS not only through TLRS but also through the intestinal serotonin network to promote the functional maturation of the enteric neural network. This promoted the synthesis and release of serotonin (5-HT) through the action of SCFAs on enterochromaffin cells[53]. 5-HT is a key regulator of gastrointestinal motility and secretion, and consists mainly of 5-HT1, 2, 3, 4, and 7 isoforms, all of which have the ability to act directly on the various receptors on epithelial cells, smooth muscle cells, and enteric neurons, thereby affecting smooth muscle relaxation and contraction[54,55]. Notably, 5-HT is also a major product of tryptophan metabolism. The dysregulation of tryptophan metabolites significantly contributes to the etiology of colonic dysmotility[56,57]. The creation of indole-3-methanol by the microbiota stimulates aryl hydrocarbon receptors in myenteric neurons, allowing them to respond to the microbial environment in the lumen. It also triggers neuron-specific effector mechanisms and the expression of colonic motility[58].
Similarly, the intestinal flora produces gas, which has a significant impact on intestinal motility. Methane, hydrogen, hydrogen sulfide, and carbon dioxide are among the gases generated by gut microorganisms in the digestive tract. In the gastrointestinal system, unabsorbed carbohydrates are fermented by bacteria, producing these byproducts. In fact, the lactulose hydrogen breath test reveals a substantial link between constipation-predominant irritable bowel syndrome and excessive methane levels[59]. The most prevalent methanogenic bacterium in the human gut is Methanobacterium smegmatis (M. smegmatis)[60]. A clinical study showed that M. smegmatis was overgrown in the intestines of constipated patients with elevated methane levels[61]. In addition, nitrate or nitrite from the gut lumen can serve as raw material for the production of NO by gut microbes[62]. It has been established that NO is an inhibitory neurotransmitter that may contribute to reduced gastrointestinal smooth muscle tension and diminished gastrointestinal motility.
Bile acids function as physiological laxatives to modify water and electrolyte transport in the intestinal lumen, as well as to regulate intestinal motility. Bile acids stimulate the TGR5 in enterochromaffin cells and myelinated neurons, releasing 5-HT and calcitonin gene-related peptides[63]. Several studies have shown that ileal bile acid-transport protein inhibitors significantly reduce bowel passage time, and improve constipation symptoms when compared with placebos[64,65]. Gastrointestinal flora modulates the gut microbiota, regulates the synthesis of hepatic bile acids, and promotes the participation of pro-bile acids in various chemical reactions in the body, thereby increasing the diversity of bile acid derivatives[66].
Consequently, the development of functional maturation of the ENS, and the reduction of colonic motility issues, may be aided by microecological management that directly targets specific TLR and 5-HT signaling pathways. At the same time, these findings support the hypothesis that the metabolism of Trp under the control of the gut microbiome is involved in host-microbiota crosstalk and gastrointestinal motility fine-tuning. This suggests that Trp metabolism may be a viable therapeutic target for gastrointestinal motility.
Because the etiologies of constipation and CRC are similar, it is unknown whether constipation and the emergence of CRC are causally related. Several hypothetical mechanisms may be behind the associations observed in this study. It has been theorized that lower bowel motility, and correspondingly longer transport time, in constipated patients would increase the risk of CRC due to prolonged exposure of the colonic mucosa to fecal carcinogens. Second, it has been suggested that constipation may accelerate the onset of CRC by causing immunological abnormalities and gene mutations or deletions via the disruption of intestinal microecology. Furthermore, harmful compounds released by microbial cells are thought to spread to other regions of the body, leading to the development, initiation, or progression of cancer[67,68]. Additionally, any relationship with constipation may be due to inverse causality; in other words, CRC may cause constipation before the clinical manifestation of cancer. Eventually, although CRC is more likely to be detected later than constipation is usually detected, the two conditions may be separate but converging disorders caused by similar underlying risk factors. Therefore, although constipation is not an indication for a colonoscopy, it should be considered in specific individuals (e.g., those over 50 years of age) for colon cancer screening[8].
While associations have been drawn between constipation and CRC in prior studies, the results are contradictory[69,70]. Generally, these studies were often constrained by selection bias, recollection bias, and self-reported data on constipation. The specific relationship between constipation and CRC is not fully understood, and in this context, the gut microbiota may be the key to solving this mystery.
The type and amount of gut microbiota and their metabolites differ between patients with constipation and the healthy population. The abundances of lactococci, rumenococci, E. coli, and Staphylococcus aureus in the intestinal flora were considerably higher in the stool of patients with constipation, whereas the abundances of bifidobacteria and lactobacilli were significantly lower, resulting in severe dysbiosis[71,72]. The abundance of Bacillus spp. in the colonic mucosa is significantly higher not only in patients with constipation, but also in those with CRC[73,74]. In addition, when constipation occurs, because dry, hard stools remain in the colon for an extended period of time, they easily consume the mucus of the loose external mucus layer of the intestine. This creates an opportunity for imbalances in the gut microbiota to invade the internal mucus layer, thereby inducing an immune response and causing inflammation, which is a necessary trigger for CRC[47].
The presence of B. fragilis, E. coli, and F. nucleatum in the intestine may also induce the abnormal expression of pro-oncogenes and oncogenes, as well as abnormal mismatch chromosome repair. By doing so, it may trigger cellular heterogeneous hyperplasia and adenomatous polyps, and contribute to the emergence and spread of CRC[75-77]. The increased abundance of F. nucleatum and E. coli may be involved in colorectal carcinogenesis and development by activating the Wnt and NF-κB signaling pathways, which promote the release of chemokines, adhesion molecules, and pro-inflammatory cytokines. These pathways can even induce chromosomal instability and the abnormal methylation of CpG islands to mediate immune cell aggregation, thereby inducing apoptosis and regulating the tumor immune microenvironment. Notably, altered abundance of E. fragilis can induce signal transducer and STAT3 activation in colonic epithelial cells, and Enterococcus faecalis can induce the formation of reactive oxygen species, related oxidative stress, and DNA damage. This in turn may cause cell proliferation, apoptosis, and abnormal immune responses, leading to colorectal tumor development[78].
Simultaneously, aberrant metabolites of the gut microbiota caused by intestinal microecological dysregulation are also involved in the development and progression of colorectal diseases. In addition to anti-inflammatory and immunomodulatory effects, moderate amounts of butyrate can enhance the defense of the gastrointestinal mucosal barrier. They can also lessen colon cancer cell propagation and migration by increasing the production of mucin-encoding genes; activating the activity of heat shock proteins, trefoil factors, antimicrobial peptides, and glutaminyl transferases; and inhibiting histone deacetylases[79]. However, excessive amounts of butyrate not only inhibit the release of mucin by intestinal cup cells and encourage the absorption of water and electrolytes from the colon, but they also inhibit the contraction of colonic smooth muscle and reduce the movement of the colon. This leads to constipation and may even promote the proliferation of tumor cells or increase the activity of β-catenin, thereby increasing the risk of tumor development[80].
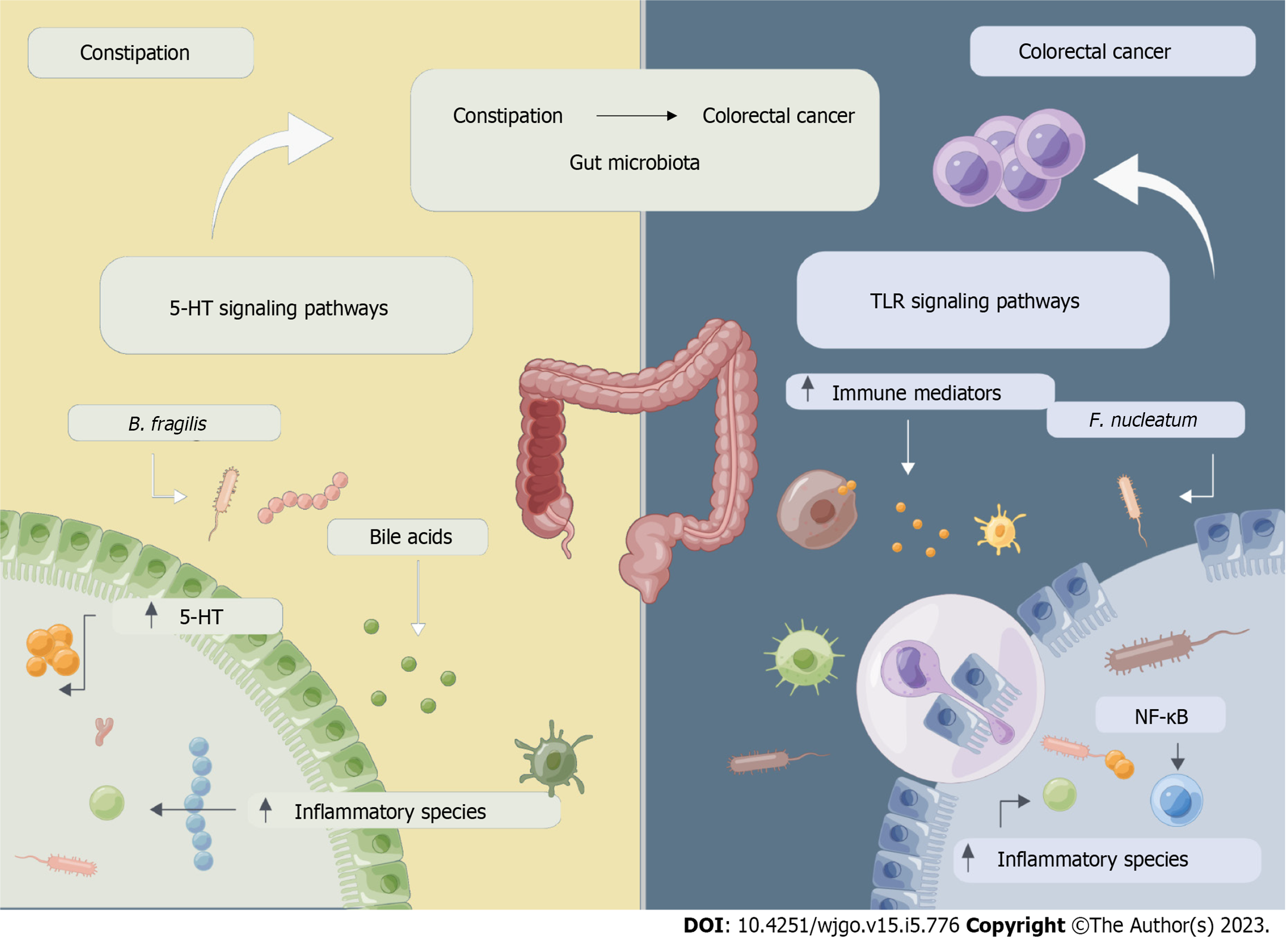
Constipation may be involved in the process of CRC development and progression via a mechanism that may involve changes in the composition of the flora, and abnormalities in its metabolites caused by dysbiosis of the intestinal flora, leading to intestinal motility dysfunction and/or abnormalities in the immune microenvironment, as shown in Figure 2. The pro-tumorigenic effects of individual cytokines are context-dependent and significantly affected by synergistic effects in a complex cytokine environment.
The crosstalk between the gut microbiota, constipation, and CRC, and their specific mechanisms of action, are still poorly understood. Nevertheless, they also provide a wealth of new ideas and prospective targets for the prevention and treatment of CRC. The direction of relevant gut microbial research is still dominated by animal studies, and there remain numerous obstacles to be overcome in clinical treatment owing to individual variations, tumor staging, and cross-species translation. To further understand the relationship between the gut microbiota, constipation, and CRC, ongoing preclinical and clinical research is required.
The future study design is as follows: Subjects first need to be pretreated with fecal sample sequencing and macrogenome sequencing, and oral antibiotics to deplete the natural gut microbes. Patients then undergo fecal transplantation and periodic fecal testing with sigmoid biopsy and tumor biopsy at appropriate times to observe the effects of fecal transplantation on constipation and CRC and assess the safety, feasibility, and impact of fecal transplantation on the intestinal microenvironment in patients with constipation and CRC. Future studies should clarify which patients can receive fecal transplants and which donor gut microbes are effective. In addition, the timing of antibiotic pre-treatment all need to be further investigated. Gut microbiota may soon become a potent tool in the battle against CRC.
According to the latest guidelines on constipation, although constipation itself is not an indication of colonoscopy, patients with severe chronic constipation or alarm symptoms should consider colonoscopy to screen for CRC. In addition, CRC screening is not a "one size fits all" concept due to the variable incidence of recognized CRC risk factors. It is now recognized that those people identified as having a greater risk for CRC, such as those with a family history of CRC or CRC-associated genetic illnesses, should be examined at a younger age and using colonoscopy. The guidelines recommend that colonoscopy be started before age 50 or even at age 45 for patients with associated risk factors, or after age 50 if there are no associated risk factors and timely interventions should be made to reduce the risk of developing colorectal polyps and CRC to prevent disease progression.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Feng S, China; Wismayer R, Uganda S-Editor: Zhang H L-Editor: A P-Editor: Zhao S
| 1. | Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, Nielsen T, Pons N, Levenez F, Yamada T, Mende DR, Li J, Xu J, Li S, Li D, Cao J, Wang B, Liang H, Zheng H, Xie Y, Tap J, Lepage P, Bertalan M, Batto JM, Hansen T, Le Paslier D, Linneberg A, Nielsen HB, Pelletier E, Renault P, Sicheritz-Ponten T, Turner K, Zhu H, Yu C, Jian M, Zhou Y, Li Y, Zhang X, Qin N, Yang H, Wang J, Brunak S, Doré J, Guarner F, Kristiansen K, Pedersen O, Parkhill J, Weissenbach J; MetaHIT Consortium, Bork P, Ehrlich SD, Wang J. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9101] [Cited by in RCA: 7831] [Article Influence: 522.1] [Reference Citation Analysis (4)] |
| 2. | West NR, McCuaig S, Franchini F, Powrie F. Emerging cytokine networks in colorectal cancer. Nat Rev Immunol. 2015;15:615-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 280] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 3. | O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1690] [Cited by in RCA: 1785] [Article Influence: 93.9] [Reference Citation Analysis (2)] |
| 4. | Bosch TC, McFall-Ngai MJ. Metaorganisms as the new frontier. Zoology (Jena). 2011;114:185-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | Bozkurt HS, Quigley EM, Kara B. Bifidobacterium animalis subspecies lactis engineered to produce mycosporin-like amino acids in colorectal cancer prevention. SAGE Open Med. 2019;7:2050312119825784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64447] [Article Influence: 16111.8] [Reference Citation Analysis (176)] |
| 7. | Sepich-Poore GD, Zitvogel L, Straussman R, Hasty J, Wargo JA, Knight R. The microbiome and human cancer. Science. 2021;371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 731] [Article Influence: 182.8] [Reference Citation Analysis (0)] |
| 8. | Bharucha AE, Pemberton JH, Locke GR 3rd. American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 554] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 9. | Bharucha AE, Wald A. Chronic Constipation. Mayo Clin Proc. 2019;94:2340-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 10. | Chassard C, Dapoigny M, Scott KP, Crouzet L, Del'homme C, Marquet P, Martin JC, Pickering G, Ardid D, Eschalier A, Dubray C, Flint HJ, Bernalier-Donadille A. Functional dysbiosis within the gut microbiota of patients with constipated-irritable bowel syndrome. Aliment Pharmacol Ther. 2012;35:828-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 11. | Qing F, Xie T, Xie L, Guo T, Liu Z. How Gut Microbiota Are Shaped by Pattern Recognition Receptors in Colitis and Colorectal Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883-899. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8437] [Cited by in RCA: 8170] [Article Influence: 544.7] [Reference Citation Analysis (0)] |
| 13. | Wong SH, Zhao L, Zhang X, Nakatsu G, Han J, Xu W, Xiao X, Kwong TNY, Tsoi H, Wu WKK, Zeng B, Chan FKL, Sung JJY, Wei H, Yu J. Gavage of Fecal Samples From Patients With Colorectal Cancer Promotes Intestinal Carcinogenesis in Germ-Free and Conventional Mice. Gastroenterology. 2017;153:1621-1633.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 448] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 14. | Cremonesi E, Governa V, Garzon JFG, Mele V, Amicarella F, Muraro MG, Trella E, Galati-Fournier V, Oertli D, Däster SR, Droeser RA, Weixler B, Bolli M, Rosso R, Nitsche U, Khanna N, Egli A, Keck S, Slotta-Huspenina J, Terracciano LM, Zajac P, Spagnoli GC, Eppenberger-Castori S, Janssen KP, Borsig L, Iezzi G. Gut microbiota modulate T cell trafficking into human colorectal cancer. Gut. 2018;67:1984-1994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 195] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 15. | Lee TC, Huang YC, Lu YZ, Yeh YC, Yu LC. Hypoxia-induced intestinal barrier changes in balloon-assisted enteroscopy. J Physiol. 2018;596:3411-3424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, Datz C, Feng Y, Fearon ER, Oukka M, Tessarollo L, Coppola V, Yarovinsky F, Cheroutre H, Eckmann L, Trinchieri G, Karin M. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1035] [Cited by in RCA: 1038] [Article Influence: 79.8] [Reference Citation Analysis (0)] |
| 17. | Tanaka T, Kohno H, Suzuki R, Hata K, Sugie S, Niho N, Sakano K, Takahashi M, Wakabayashi K. Dextran sodium sulfate strongly promotes colorectal carcinogenesis in Apc(Min/+) mice: inflammatory stimuli by dextran sodium sulfate results in development of multiple colonic neoplasms. Int J Cancer. 2006;118:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Windey K, De Preter V, Verbeke K. Relevance of protein fermentation to gut health. Mol Nutr Food Res. 2012;56:184-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 371] [Cited by in RCA: 454] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 19. | Nguyen LH, Ma W, Wang DD, Cao Y, Mallick H, Gerbaba TK, Lloyd-Price J, Abu-Ali G, Hall AB, Sikavi D, Drew DA, Mehta RS, Arze C, Joshi AD, Yan Y, Branck T, DuLong C, Ivey KL, Ogino S, Rimm EB, Song M, Garrett WS, Izard J, Huttenhower C, Chan AT. Association Between Sulfur-Metabolizing Bacterial Communities in Stool and Risk of Distal Colorectal Cancer in Men. Gastroenterology. 2020;158:1313-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 20. | Wang Q, Xu R. Data-driven multiple-level analysis of gut-microbiome-immune-joint interactions in rheumatoid arthritis. BMC Genomics. 2019;20:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016;16:341-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1428] [Cited by in RCA: 2242] [Article Influence: 280.3] [Reference Citation Analysis (1)] |
| 22. | Kesselring R, Glaesner J, Hiergeist A, Naschberger E, Neumann H, Brunner SM, Wege AK, Seebauer C, Köhl G, Merkl S, Croner RS, Hackl C, Stürzl M, Neurath MF, Gessner A, Schlitt HJ, Geissler EK, Fichtner-Feigl S. IRAK-M Expression in Tumor Cells Supports Colorectal Cancer Progression through Reduction of Antimicrobial Defense and Stabilization of STAT3. Cancer Cell. 2016;29:684-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 23. | Zhu H, Xu WY, Hu Z, Zhang H, Shen Y, Lu S, Wei C, Wang ZG. RNA virus receptor Rig-I monitors gut microbiota and inhibits colitis-associated colorectal cancer. J Exp Clin Cancer Res. 2017;36:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Man SM, Zhu Q, Zhu L, Liu Z, Karki R, Malik A, Sharma D, Li L, Malireddi RK, Gurung P, Neale G, Olsen SR, Carter RA, McGoldrick DJ, Wu G, Finkelstein D, Vogel P, Gilbertson RJ, Kanneganti TD. Critical Role for the DNA Sensor AIM2 in Stem Cell Proliferation and Cancer. Cell. 2015;162:45-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 271] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 25. | Song X, Gao H, Lin Y, Yao Y, Zhu S, Wang J, Liu Y, Yao X, Meng G, Shen N, Shi Y, Iwakura Y, Qian Y. Alterations in the microbiota drive interleukin-17C production from intestinal epithelial cells to promote tumorigenesis. Immunity. 2014;40:140-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 26. | Kortylewski M, Xin H, Kujawski M, Lee H, Liu Y, Harris T, Drake C, Pardoll D, Yu H. Regulation of the IL-23 and IL-12 balance by Stat3 signaling in the tumor microenvironment. Cancer Cell. 2009;15:114-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 388] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 27. | Wang K, Kim MK, Di Caro G, Wong J, Shalapour S, Wan J, Zhang W, Zhong Z, Sanchez-Lopez E, Wu LW, Taniguchi K, Feng Y, Fearon E, Grivennikov SI, Karin M. Interleukin-17 receptor a signaling in transformed enterocytes promotes early colorectal tumorigenesis. Immunity. 2014;41:1052-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 266] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 28. | Rubinstein MR, Wang X, Liu W, Hao Y, Cai G, Han YW. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe. 2013;14:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1511] [Cited by in RCA: 1658] [Article Influence: 138.2] [Reference Citation Analysis (1)] |
| 29. | Yang Y, Weng W, Peng J, Hong L, Yang L, Toiyama Y, Gao R, Liu M, Yin M, Pan C, Li H, Guo B, Zhu Q, Wei Q, Moyer MP, Wang P, Cai S, Goel A, Qin H, Ma Y. Fusobacterium nucleatum Increases Proliferation of Colorectal Cancer Cells and Tumor Development in Mice by Activating Toll-Like Receptor 4 Signaling to Nuclear Factor-κB, and Up-regulating Expression of MicroRNA-21. Gastroenterology. 2017;152:851-866.e24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 711] [Article Influence: 88.9] [Reference Citation Analysis (1)] |
| 30. | Barrett M, Hand CK, Shanahan F, Murphy T, O'Toole PW. Mutagenesis by Microbe: the Role of the Microbiota in Shaping the Cancer Genome. Trends Cancer. 2020;6:277-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 31. | Fang Y, Yan C, Zhao Q, Xu J, Liu Z, Gao J, Zhu H, Dai Z, Wang D, Tang D. The roles of microbial products in the development of colorectal cancer: a review. Bioengineered. 2021;12:720-735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 32. | Tremblay W, Mompart F, Lopez E, Quaranta M, Bergoglio V, Hashim S, Bonnet D, Alric L, Mas E, Trouche D, Vignard J, Ferrand A, Mirey G, Fernandez-Vidal A. Cytolethal Distending Toxin Promotes Replicative Stress Leading to Genetic Instability Transmitted to Daughter Cells. Front Cell Dev Biol. 2021;9:656795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | He Z, Gharaibeh RZ, Newsome RC, Pope JL, Dougherty MW, Tomkovich S, Pons B, Mirey G, Vignard J, Hendrixson DR, Jobin C. Campylobacter jejuni promotes colorectal tumorigenesis through the action of cytolethal distending toxin. Gut. 2019;68:289-300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 34. | Lara-Tejero M, Galán JE. A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science. 2000;290:354-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 403] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 35. | Bezine E, Vignard J, Mirey G. The cytolethal distending toxin effects on Mammalian cells: a DNA damage perspective. Cells. 2014;3:592-615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Allen J, Sears CL. Impact of the gut microbiome on the genome and epigenome of colon epithelial cells: contributions to colorectal cancer development. Genome Med. 2019;11:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 132] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 37. | Wu S, Rhee KJ, Zhang M, Franco A, Sears CL. Bacteroides fragilis toxin stimulates intestinal epithelial cell shedding and gamma-secretase-dependent E-cadherin cleavage. J Cell Sci. 2007;120:1944-1952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 213] [Cited by in RCA: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 38. | Wu S, Morin PJ, Maouyo D, Sears CL. Bacteroides fragilis enterotoxin induces c-Myc expression and cellular proliferation. Gastroenterology. 2003;124:392-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 292] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 39. | Wang X, Huycke MM. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology. 2007;132:551-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 179] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 40. | Goodwin AC, Destefano Shields CE, Wu S, Huso DL, Wu X, Murray-Stewart TR, Hacker-Prietz A, Rabizadeh S, Woster PM, Sears CL, Casero RA Jr. Polyamine catabolism contributes to enterotoxigenic Bacteroides fragilis-induced colon tumorigenesis. Proc Natl Acad Sci U S A. 2011;108:15354-15359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 442] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 41. | Nusse R, Clevers H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell. 2017;169:985-999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2031] [Cited by in RCA: 3103] [Article Influence: 387.9] [Reference Citation Analysis (0)] |
| 42. | Jia W, Xie G, Jia W. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat Rev Gastroenterol Hepatol. 2018;15:111-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1355] [Cited by in RCA: 1250] [Article Influence: 178.6] [Reference Citation Analysis (0)] |
| 43. | Gadaleta RM, Garcia-Irigoyen O, Moschetta A. Bile acids and colon cancer: Is FXR the solution of the conundrum? Mol Aspects Med. 2017;56:66-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 44. | Sun L, Cai J, Gonzalez FJ. The role of farnesoid X receptor in metabolic diseases, and gastrointestinal and liver cancer. Nat Rev Gastroenterol Hepatol. 2021;18:335-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 258] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 45. | Zhao Q, Chen YY, Xu DQ, Yue SJ, Fu RJ, Yang J, Xing LM, Tang YP. Action Mode of Gut Motility, Fluid and Electrolyte Transport in Chronic Constipation. Front Pharmacol. 2021;12:630249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 46. | Pan R, Wang L, Xu X, Chen Y, Wang H, Wang G, Zhao J, Chen W. Crosstalk between the Gut Microbiome and Colonic Motility in Chronic Constipation: Potential Mechanisms and Microbiota Modulation. Nutrients. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 76] [Reference Citation Analysis (0)] |
| 47. | Yang L, Wang Y, Zhang Y, Li W, Jiang S, Qian D, Duan J. Gut microbiota: a new avenue to reveal pathological mechanisms of constipation. Appl Microbiol Biotechnol. 2022;106:6899-6913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 48. | Brun P, Giron MC, Qesari M, Porzionato A, Caputi V, Zoppellaro C, Banzato S, Grillo AR, Spagnol L, De Caro R, Pizzuti D, Barbieri V, Rosato A, Sturniolo GC, Martines D, Zaninotto G, Palù G, Castagliuolo I. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology. 2013;145:1323-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 233] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 49. | Anitha M, Vijay-Kumar M, Sitaraman SV, Gewirtz AT, Srinivasan S. Gut microbial products regulate murine gastrointestinal motility via Toll-like receptor 4 signaling. Gastroenterology. 2012;143:1006-16.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 50. | Yarandi SS, Kulkarni S, Saha M, Sylvia KE, Sears CL, Pasricha PJ. Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice. Gastroenterology. 2020;159:200-213.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 51. | Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 363] [Cited by in RCA: 505] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 52. | Zuo DC, Choi S, Shahi PK, Kim MY, Park CG, Kim YD, Lee J, Chang IY, So I, Jun JY. Inhibition of pacemaker activity in interstitial cells of Cajal by LPS via NF-κB and MAP kinase. World J Gastroenterol. 2013;19:1210-1218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 53. | Reigstad CS, Salmonson CE, Rainey JF 3rd, Szurszewski JH, Linden DR, Sonnenburg JL, Farrugia G, Kashyap PC. Gut microbes promote colonic serotonin production through an effect of short-chain fatty acids on enterochromaffin cells. FASEB J. 2015;29:1395-1403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 570] [Cited by in RCA: 898] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 54. | Mawe GM, Hoffman JM. Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol. 2013;10:473-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 800] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 55. | Sikander A, Rana SV, Prasad KK. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin Chim Acta. 2009;403:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 197] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 56. | Zhang X, Yang H, Zheng J, Jiang N, Sun G, Bao X, Lin A, Liu H. Chitosan oligosaccharides attenuate loperamide-induced constipation through regulation of gut microbiota in mice. Carbohydr Polym. 2021;253:117218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 57. | Gao K, Mu CL, Farzi A, Zhu WY. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv Nutr. 2020;11:709-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 58. | Obata Y, Castaño Á, Boeing S, Bon-Frauches AC, Fung C, Fallesen T, de Agüero MG, Yilmaz B, Lopes R, Huseynova A, Horswell S, Maradana MR, Boesmans W, Vanden Berghe P, Murray AJ, Stockinger B, Macpherson AJ, Pachnis V. Neuronal programming by microbiota regulates intestinal physiology. Nature. 2020;578:284-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 235] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 59. | Sahakian AB, Jee SR, Pimentel M. Methane and the gastrointestinal tract. Dig Dis Sci. 2010;55:2135-2143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 60. | Dridi B, Henry M, El Khéchine A, Raoult D, Drancourt M. High prevalence of Methanobrevibacter smithii and Methanosphaera stadtmanae detected in the human gut using an improved DNA detection protocol. PLoS One. 2009;4:e7063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 256] [Cited by in RCA: 276] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 61. | Takakura W, Pimentel M, Rao S, Villanueva-Millan MJ, Chang C, Morales W, Sanchez M, Torosyan J, Rashid M, Hosseini A, Wang J, Leite G, Kowalewski E, Mathur R, Rezaie A. A Single Fasting Exhaled Methane Level Correlates With Fecal Methanogen Load, Clinical Symptoms and Accurately Detects Intestinal Methanogen Overgrowth. Am J Gastroenterol. 2022;117:470-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 62. | Koch CD, Gladwin MT, Freeman BA, Lundberg JO, Weitzberg E, Morris A. Enterosalivary nitrate metabolism and the microbiome: Intersection of microbial metabolism, nitric oxide and diet in cardiac and pulmonary vascular health. Free Radic Biol Med. 2017;105:48-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 63. | Alemi F, Poole DP, Chiu J, Schoonjans K, Cattaruzza F, Grider JR, Bunnett NW, Corvera CU. The receptor TGR5 mediates the prokinetic actions of intestinal bile acids and is required for normal defecation in mice. Gastroenterology. 2013;144:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 264] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 64. | Wong BS, Camilleri M. Elobixibat for the treatment of constipation. Expert Opin Investig Drugs. 2013;22:277-284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Nakajima A, Seki M, Taniguchi S, Ohta A, Gillberg PG, Mattsson JP, Camilleri M. Safety and efficacy of elobixibat for chronic constipation: results from a randomised, double-blind, placebo-controlled, phase 3 trial and an open-label, single-arm, phase 3 trial. Lancet Gastroenterol Hepatol. 2018;3:537-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 113] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 66. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1678] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 67. | Garrett WS. Cancer and the microbiota. Science. 2015;348:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 901] [Cited by in RCA: 968] [Article Influence: 96.8] [Reference Citation Analysis (1)] |
| 68. | Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1231] [Article Influence: 102.6] [Reference Citation Analysis (2)] |
| 69. | Staller K, Olén O, Söderling J, Roelstraete B, Törnblom H, Song M, Ludvigsson JF. Chronic Constipation as a Risk Factor for Colorectal Cancer: Results From a Nationwide, Case-Control Study. Clin Gastroenterol Hepatol. 2022;20:1867-1876.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 70. | Guérin A, Mody R, Fok B, Lasch KL, Zhou Z, Wu EQ, Zhou W, Talley NJ. Risk of developing colorectal cancer and benign colorectal neoplasm in patients with chronic constipation. Aliment Pharmacol Ther. 2014;40:83-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 81] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 71. | Khalif IL, Quigley EM, Konovitch EA, Maximova ID. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig Liver Dis. 2005;37:838-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (2)] |
| 72. | Guo M, Yao J, Yang F, Liu W, Bai H, Ma J, Ma X, Zhang J, Fang Y, Miao Y, Sun J, Zhang Y, Zhao H. The composition of intestinal microbiota and its association with functional constipation of the elderly patients. Future Microbiol. 2020;15:163-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 73. | Parthasarathy G, Chen J, Chen X, Chia N, O'Connor HM, Wolf PG, Gaskins HR, Bharucha AE. Relationship Between Microbiota of the Colonic Mucosa vs Feces and Symptoms, Colonic Transit, and Methane Production in Female Patients With Chronic Constipation. Gastroenterology. 2016;150:367-79.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 283] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 74. | Kostic AD, Gevers D, Pedamallu CS, Michaud M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, Baselga J, Liu C, Shivdasani RA, Ogino S, Birren BW, Huttenhower C, Garrett WS, Meyerson M. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1199] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 75. | Nosho K, Sukawa Y, Adachi Y, Ito M, Mitsuhashi K, Kurihara H, Kanno S, Yamamoto I, Ishigami K, Igarashi H, Maruyama R, Imai K, Yamamoto H, Shinomura Y. Association of Fusobacterium nucleatum with immunity and molecular alterations in colorectal cancer. World J Gastroenterol. 2016;22:557-566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 292] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (3)] |
| 76. | Galan-Ros J, Ramos-Arenas V, Conesa-Zamora P. Predictive values of colon microbiota in the treatment response to colorectal cancer. Pharmacogenomics. 2020;21:1045-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Peng C, Ouyang Y, Lu N, Li N. The NF-κB Signaling Pathway, the Microbiota, and Gastrointestinal Tumorigenesis: Recent Advances. Front Immunol. 2020;11:1387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 78. | Hnatyszyn A, Hryhorowicz S, Kaczmarek-Ryś M, Lis E, Słomski R, Scott RJ, Pławski A. Colorectal carcinoma in the course of inflammatory bowel diseases. Hered Cancer Clin Pract. 2019;17:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 79. | O'Keefe SJ. Diet, microorganisms and their metabolites, and colon cancer. Nat Rev Gastroenterol Hepatol. 2016;13:691-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 772] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 80. | Guo M, Li Z. Polysaccharides isolated from Nostoc commune Vaucher inhibit colitis-associated colon tumorigenesis in mice and modulate gut microbiota. Food Funct. 2019;10:6873-6881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 78] [Article Influence: 15.6] [Reference Citation Analysis (0)] |