Published online Apr 15, 2023. doi: 10.4251/wjgo.v15.i4.677
Peer-review started: December 24, 2022
First decision: February 10, 2023
Revised: February 20, 2023
Accepted: March 22, 2023
Article in press: March 22, 2023
Published online: April 15, 2023
Processing time: 108 Days and 23.1 Hours
Owing to rarity of disease and lack of prospective studies, data supporting the role of adjuvant chemotherapy in ampulla of Vater (AoV) carcinoma is limited.
To evaluate whether adjuvant chemotherapy cases for AoV carcinoma had better disease-free survival (DFS) rates than cases of observation following curative surgery.
We retrospectively analyzed the association between adjuvant chemotherapy and DFS and overall survival (OS) in patients with stage IB-III AoV carcinoma who underwent curative surgical resection. Fluorouracil-based adjuvant chemotherapy was administered after surgery at the discretion of the physician. Adjusted multivariate regression models were used to evaluate the association between adjuvant chemotherapy and survival outcomes.
Of the total 104 patients who underwent curative surgery, 52 received adjuvant chemotherapy. Multivariate analysis revealed that higher histologic grade [hazard ratio (HR) = 2.24, P = 0.046], advanced tumor stage (HR = 1.85, P = 0.030), and vascular invasion (HR = 2.14, P = 0.010) were associated with shorter DFS. Adjuvant chemotherapy improved DFS compared to the observation group (HR = 0.50, P = 0.015) and tended to be associated with a longer OS, although the difference was not statistically significant (HR = 0.58, P = 0.098).
Among patients with resected AoV carcinoma, the adjuvant chemotherapy group was not associated with a significant survival benefit compared to the observation group. However, on multivariate analysis adjusting for prognostic factors, adjuvant chemotherapy following surgery was an independent prognostic factor for DFS in patients with resected AoV carcinoma. Further studies are needed to investigate the effectiveness of adjuvant chemotherapy according to histologic phenotype.
Core Tip: To date, there is no standard adjuvant treatment for patients with ampulla of Vater (AoV) carcinoma after surgical resection. In this study, we evaluated the effectiveness and safety of adjuvant fluorouracil-based chemotherapy. Of 104 patients investigated, 52 were received 5-fluorouracil-based adjuvant chemotherapy and 52 were observed without any adjuvant treatment. Adjuvant chemotherapy could improve disease-free survival in patients with AoV cancer following surgery, but overall survival was not associated with adjuvant chemotherapy. Treatment related adverse events were manageable. Further studies are warranted to identify patients with resected AoV cancer who might benefit from adjuvant chemotherapy.
- Citation: Park SJ, Shin K, Kim IH, Hong TH, Kim Y, Lee MA. Role of adjuvant chemotherapy on recurrence and survival in patients with resected ampulla of Vater carcinoma. World J Gastrointest Oncol 2023; 15(4): 677-688
- URL: https://www.wjgnet.com/1948-5204/full/v15/i4/677.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i4.677
The ampulla of Vater (AoV) carcinoma arises in the mucosa of the common channel composed of the confluence of the pancreatic and common bile ducts (CBD). Thus, it is difficult to differentiate from malignancies originating in the pancreatic head, distal CBD, or duodenum. AoV carcinoma is a rare form of periampullary cancer, accounting for approximately 7% of cases[1]. Surgical resection remains the primary curative approach for patients with localized AoV carcinoma; however, recurrence is frequent, and the 5-year survival rate following resection is 45%[2].
Various clinicopathological features are known to be associated with recurrence and poor prognosis, including advanced tumor stage, regional lymph node metastasis, poorly differentiated tumors, perineural invasion, and pancreatobiliary histology[3,4]. Given the rarity of this disease, there is absence of large-scale controlled trials investigating adjuvant treatment for patients with AoV cancer. Till date, no standard adjuvant treatment has been accepted globally for resected AoV cancers with a high risk of recurrence. Several previous retrospective studies have shown a significant benefit of adjuvant treatment in selected patients with stage IIB or higher disease[5]. Similarly, patients with regional lymph node involvement may have a survival benefit with adjuvant chemoradiation compared to those with surgery alone[6]. Although there is no clear guideline for adjuvant treatment, concurrent chemoradiation is often performed for patients with resected AoV cancer who have stage IB or higher disease[1]. However, in a previous study that included 113 patients with resected AoV cancer, adjuvant concurrent fluorouracil chemoradiation did not improve survival outcomes or decrease recurrence rates[7]. In terms of adjuvant chemotherapy, European Study Group for Pancreatic Cancer (ESPAC-3), the largest phase III randomized trial to investigate the role of adjuvant chemotherapy in resected periampullary adenocarcinoma, included 428 patients with periampullary carcinoma (297 patients with ampullary cancer) who were randomly assigned to each treatment group: observation, fluorouracil/leucovorin (FL), or gemcitabine. Among patients with AoV cancer, those who received adjuvant gemcitabine treatment had better survival outcomes than those in the observation group[8]. However, data supporting the role of adjuvant chemotherapy from the ESPAC-3 trial are limited because the trial enrolled patients with biliary tract or other periampullary cancers, in addition to AoV cancer. The inclusion of a significant number of patients with stage I or IVA cancer was another limitation.
In this study, we investigated the association between clinicopathological features and recurrence or mortality in patients with resected AoV cancers. Furthermore, we examined the role of adjuvant chemotherapy in patients with resected true AoV cancers.
We reviewed the medical records of patients with histologically confirmed adenocarcinoma of the AoV who underwent curative pancreaticoduodenectomy with regional lymph node dissection at the Catholic University of Korea, Seoul St. Mary’s Hospital between June 01, 2008 and December 31, 2020. Patients aged ≥ 19 years were eligible for this study based on the following criteria: (1) Histologically confirmed adenocarcinoma of the AoV; (2) pathological stage IB-III according to the American Joint Committee of Cancer Staging (AJCC), 8th edition[9]; and (3) recurrence and survival confirmation at the time of data collection. Patients with the following conditions were excluded: (1) Pathological tumor, node, metastasis (TNM) stage IA and IV; (2) no regional lymph node examination; (3) macroscopically remaining tumors (R2 resection); (4) received previous preoperative chemotherapy; (5) did not fully recover from surgery; and (6) newly diagnosed with secondary malignancies after surgery for AoV cancer.
Pancreaticoduodenectomy or pylorus-preserving pancreaticoduodenectomy with standard lymph node dissection was performed at the surgeon’s discretion. The decision to administer adjuvant chemotherapy following surgery and the regimen of adjuvant chemotherapy to be followed were at the physician’s discretion; patients received adjuvant FL or fluorouracil and cisplatin (FP) within 12 wk of surgery. In the FL group, patients received a bolus of 20 mg/m2 leucovorin, followed by a 2-h infusion of 425 mg/m2 fluorouracil for five consecutive days every 28 d for six cycles (24 wk). In the FP group, each cycle consisted of cisplatin at a dose of 70 mg/m2 (day 1 of every cycle) delivered as a 1-h intravenous infusion, followed by fluorouracil at a dose of 1000 mg/m2 (per day) administered by intravenous infusion over 8-h for three consecutive days every 28 d for six cycles (24 wk). The chemotherapy dose and schedule modifications were at the physician’s discretion. The relative dose intensity (RDI) of chemotherapy was defined as the ratio of the delivered dose to the planned dose and presented as a percentage. Adverse events were assessed according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.03.
Patients were evaluated every three months after surgery for two years, every six months for the following three years, and annually thereafter. Imaging evaluations were performed using computed tomography with contrast, and carbohydrate antigen 19-9 (CA 19-9) levels were also checked at every visit. If any evidence suggested the possibility of recurrence, additional imaging or biopsy was performed to confirm recurrence.
Descriptive statistics are reported as proportions and medians with ranges. Categorical variables were compared using the Chi-squared or Fisher’s exact test, and continuous variables were compared using Student’s t-test. Disease-free survival (DFS) was defined as the interval between curative surgery and recurrence or death from any cause. Overall survival (OS) was estimated from the date of surgery to the time of the last follow-up or cancer-related deaths. Survival outcomes were estimated using the Kaplan-Meier method and compared using a two-tailed log-rank test. Multivariable regression based on the Cox proportional hazard model was used to estimate the effect of clinicopathological factors as prognosticators of DFS and OS. Hazard ratios (HR) with 95% confidence intervals (CI) were calculated for each factor. All tests were two-sided, and a P value of less than 0.05 was considered to indicate statistical significance. The number of patients receiving adjuvant treatment and the relative dose intensity of the total doses received are reported. The number of patients experiencing treatment-related adverse events was reported as a percentage of the total number of patients in each treatment group. All statistical analyses were performed using SPSS for Windows (version 24.0; IBM SPSS Inc., Armonk, NY, United States) and GraphPad Prism version 8.0 (GraphPad Software Inc., San Diego, CA, United States).
Between June 1, 2008 to December 31, 2020, 104 patients were eligible for this study. The clinical characteristics and pathological details of the patients are shown in Table 1. The median age was 64 years (range, 49-83) and 58 patients (55.8%) were male. Most patients had negative surgical margins and only two patients (2.0%) had R1 resection status. A total of 30 patients (28.8%) had stage IB, 15 (14.4%) had stage II, and 59 (56.8%) had stage III disease. Preoperative serum CA 19-9 Levels were elevated in 43 patients (41.3%) at the time of diagnosis. Of the 104 patients, half (50.0%) received adjuvant chemotherapy and the other half (50.0%) underwent surgery alone. Patient demographics, such as age, sex, tumor size, histologic grade, and elevated preoperative CA 19-9, were similar between the two groups. With respect to pathological details, patients in the adjuvant chemotherapy group had a more advanced tumor (T3-4, 60.4% vs 39.6%, P = 0.049), node (N1-2, 64.4% vs 35.6%, P = 0.001), and TNM (stage III, 64.4% vs 35.6%, P = 0.002) stage than those in the surgery-alone group. Lymphatic (62.9% vs 37.1%, P = 0.001), vascular (78.3% vs 21.7%, P = 0.002), and perineural (66.7% vs 33.3%, P = 0.030) invasion were also significantly more common in the adjuvant chemotherapy group than in the surgery-alone group.
| Variables | Total (n = 104) | AC (n = 52) | Surgery alone (n = 52) | P value |
| Age, median (range) | 64 (49-83) | 69 (62-83) | 64 (49-83) | 0.924 |
| Gender | ||||
| Male | 58 (55.8) | 33 (56.9) | 25 (43.1) | 0.114 |
| Female | 46 (44.2) | 19 (41.3) | 27 (58.7) | |
| Tumor size (cm), mean ± SD | 2.5 ± 1.1 | 2.6 ± 1.3 | 2.4 ± 1.0 | 0.314 |
| Resection status | ||||
| R0 | 102 (98.0) | 50 (49.0) | 52 (51.0) | 0.495 |
| R1 | 2 (2.0) | 2 (100) | 0 | |
| Histologic grading | ||||
| Grade 1/2 | 94 (90.3) | 45 (47.9) | 49 (52.1) | 0.319 |
| Grade 3 | 10 (9.7) | 7 (70.0) | 3 (30.0) | |
| Tumor stage | ||||
| T1-2 | 56 (53.8) | 23 (41.1) | 33 (58.9) | 0.049 |
| T3-4 | 48 (46.2) | 29 (60.4) | 19 (39.6) | |
| Node stage | ||||
| N0 | 45 (43.3) | 14 (31.1) | 31 (68.9) | 0.001 |
| N1-2 | 59 (56.7) | 38 (64.4) | 21 (35.6) | |
| TNM stage1 | ||||
| Stage IB | 30 (28.8) | 8 (26.7) | 22 (73.3) | 0.002 |
| Stage II | 15 (14.4) | 6 (40.0) | 9 (60.0) | |
| Stage III | 59 (56.8) | 38 (64.4) | 21 (35.6) | |
| Lymphatic invasion | ||||
| No | 42 (40.4) | 13 (31.0) | 29 (69.0) | 0.001 |
| Yes | 62 (59.6) | 39 (62.9) | 23 (37.1) | |
| Vascular invasion | ||||
| No | 81 (77.9) | 34 (42.0) | 47 (58.0) | 0.002 |
| Yes | 23 (22.1) | 18 (78.3) | 5 (21.7) | |
| Perineural invasion | ||||
| No | 74 (71.2) | 32 (43.2) | 42 (56.8) | 0.030 |
| Yes | 30 (28.8) | 20 (66.7) | 10 (33.3) | |
| Preoperative CA19-9 level | ||||
| Within normal (< 40 U/mL) | 45 (43.3) | 22 (48.9) | 23 (51.1) | 0.516 |
| Above normal (≥ 40 U/mL) | 43 (41.3) | 24 (55.8) | 19 (44.2) | |
| Missig data | 16 (15.4) |
The median follow-up time was 30.2 mo (95%CI: 24.16-42.20). Recurrence and cancer-related deaths occurred in 64 (61.5%) and 49 (47.1%) patients, respectively. The median DFS of all patients was 16.4 mo (95%CI: 10.8-22.0) and the DFS rates at 6 mo, 1 year, and 2 years were 84.5% (95%CI: 75.9-90.2), 62.2% (95%CI: 51.7-71.0), and 40.5% (95%CI: 30.3-50.4), respectively. Among patients with confirmed recurrence, most recurrence events (56 patients, 87.5%) occurred within 2 years after surgery. The median OS of all patients was 55.0 mo (95%CI: 38.9-71.0) and estimated OS rates were 94.8% (95%CI: 87.9-97.8) at 1 year, 60.7% (95%CI: 49.4-70.2) at 3 years, and 44.1% (95%CI: 32.1-55.4) at 5 years.
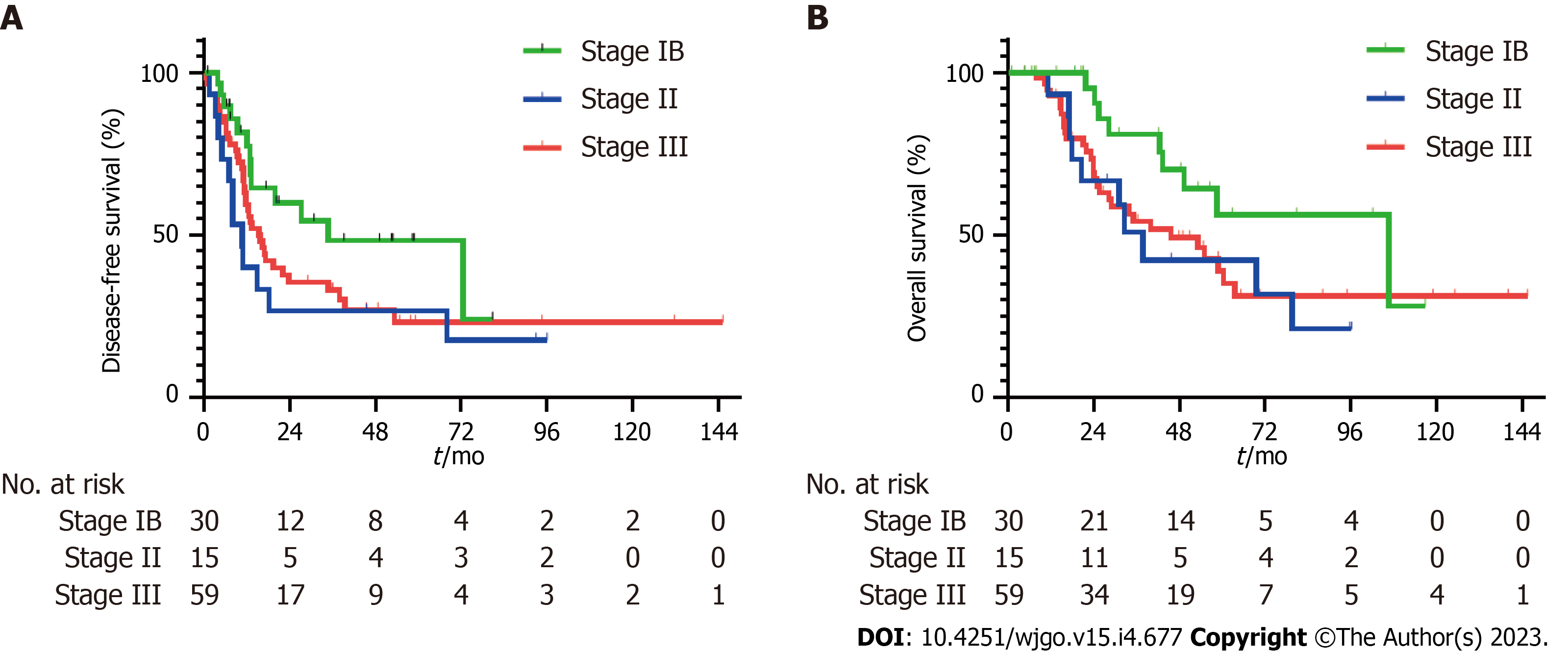
Survival analysis by staging revealed recurrence in 13 (43.3%) of 30 patients with stage IB disease, 12 (80.0%) of 15 patients with stage II disease, and 39 (66.1%) of 59 patients with stage III disease. Cancer-related death events in stages IB, II, and III disease were observed in 9 (30.0%) of 30 patients, 9 (60.0%) of 15 patients, and 30 (50.8%) of 59 patients, respectively. In patients with stage IB disease, the median DFS was 34.8 mo (95%CI: 3.2-66.4) and the DFS rates at 1 year and 2 years were 81.7% (95%CI: 61.3-92.0) and 59.9% (95%CI: 37.9-76.3), respectively (Figure 1A). The median DFS was 10.8 (95%CI: 7.2-14.4) and 15.4 (95%CI: 10.5-20.3) months in patients with stage II and III disease, respectively (Figure 1A). DFS rates at 1 year and 2 years in patients with stage II disease were 40.0% (95%CI: 16.5-62.8) and 26.7% (95%CI: 8.3-49.7), and 59.4% (45.4-70.9) and 35.5% (95%CI: 22.8-48.4) in patients with stage III disease. The median DFS was numerically better in patients with a lower stage of disease than in those with a higher stage, although the difference was not statistically significant (Stage IB vs II, P = 0.062; stage IB vs III, P = 0.094; stage II vs III, P = 0.405). The median OS of patients with stage IB, II, and III disease was 106.7 (95%CI: 36.9-176.5), 37.8 (95%CI: 27.1-48.5), and 45.6 (95%CI: 23.5-67.8) mo, respectively (Figure 1B). Estimated survival rates at 3 and 5 years were 81.0% (95%CI: 56.9-92.4) and 56.3% (95%CI: 30.1-76.0) for stage IB, 50.8% (95%CI: 23.1-73.1) and 42.3% (95%CI: 16.5-66.2) for stage II, and 54.2% (95%CI: 39.3-66.9) and 39.0% (95%CI: 23.9-53.9) for stage III disease, respectively.
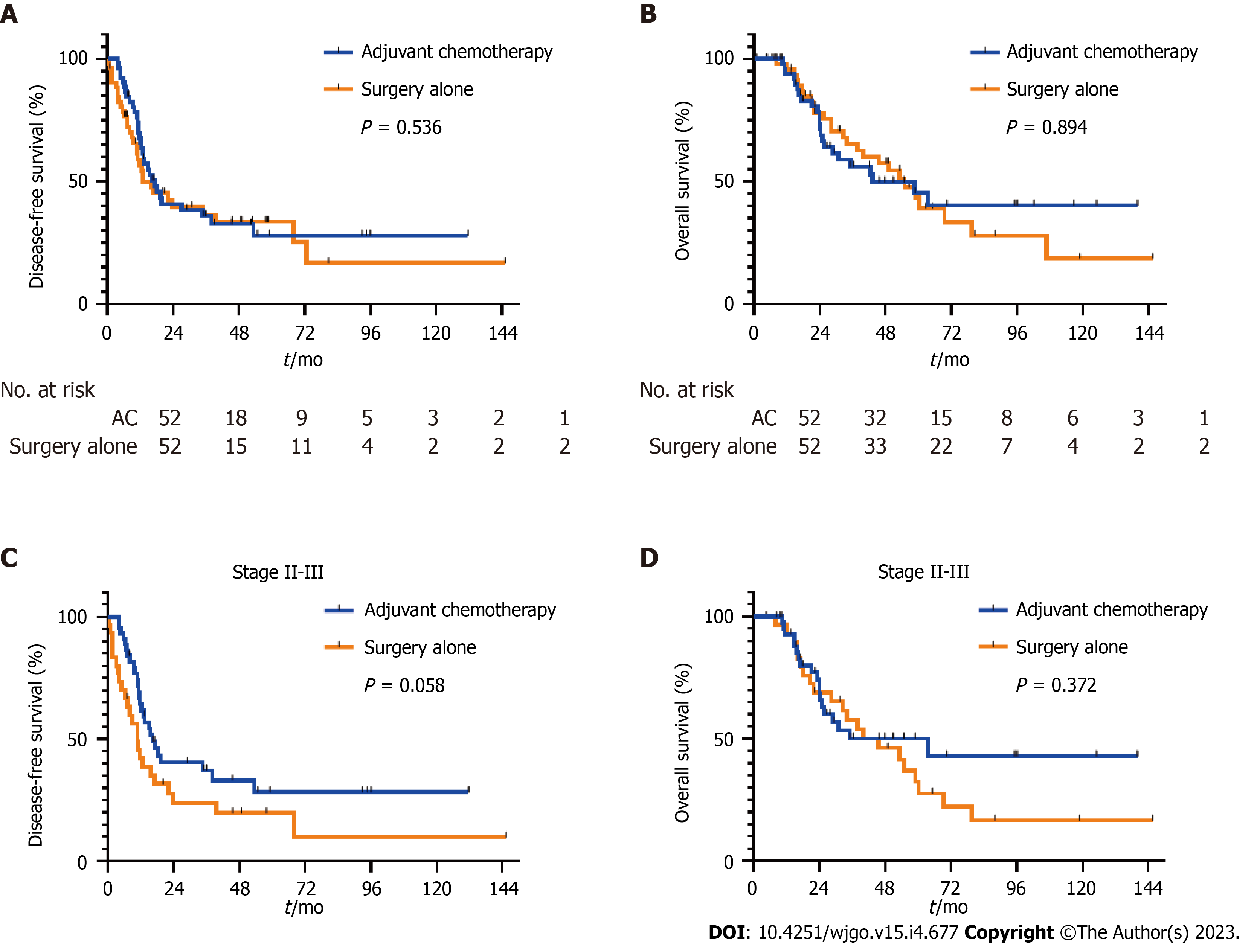
The median DFS was 17.2 mo (95%CI: 11.0-23.3) in the adjuvant chemotherapy group, as compared with 13.0 mo (95%CI: 2.38-23.7) in the surgery-alone group (P = 0.536, Figure 2A). DFS rates at 6 mo, 1 year, and 2 years were 90.4% (95%CI: 78.4-95.9), 67.7% (95%CI: 52.7-78.9), and 40.8% (95%CI: 26.6-54.5), respectively in the adjuvant chemotherapy group, as compared with 78.5% (95%CI: 64.5-87.9), 56.6% (95%CI: 41.4-69.3), and 39.6% (95%CI: 25.2-53.6), respectively in the surgery-alone group. On multivariable analysis, patients who received adjuvant chemotherapy had longer DFS than those treated with surgery alone (HR = 0.50; 95%CI: 0.29-0.88; P = 0.015, Table 2). In addition, poorly differentiated histology (HR = 2.24; 95%CI: 1.02-4.95; P = 0.046), advanced tumor stage (HR = 1.85; 95%CI: 1.06-3.22; P = 0.030), and vascular invasion (HR = 2.14; 95%CI: 1.20-3.79; P = 0.010) were associated with shorter DFS after multivariate analysis (Table 2). The median OS was 43.3 mo (95%CI: 15.2-71.4) in the adjuvant chemotherapy group, as compared with 55.0 mo (95%CI: 39.8-70.1) in the surgery-alone group (P = 0.894, Figure 2B). The OS rate at 3 years was 56.1% (95%CI: 39.9-69.5) in the adjuvant chemotherapy group and 65.3% (95%CI: 49.0-77.5) in the surgery-alone group. Multivariate analysis showed that older age at diagnosis (HR = 2.16, 95%CI: 1.18-3.98, P = 0.013) and higher tumor stage (HR = 2.50, 95%CI: 1.31-4.80, P = 0.006) were significantly associated with poor survival outcomes; however, adjuvant chemotherapy was not significantly associated with OS (HR = 0.58, 95%CI: 0.30-1.11, P = 0.098, Table 3). In stage II or III patients, although the difference was not significant, the median DFS was numerically better in the adjuvant chemotherapy group than in the surgery-alone group (median DFS 16.4 mo vs 10.9 mo; P = 0.058, Figure 2C). The median OS did not differ between two groups (median OS 63.5 mo vs 40.0 mo, P = 0.372, Figure 2D).
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age ≥ 70 yr (vs < 70 yr) | 1.57 (0.91-2.70) | 0.102 | ||
| Histologic grade 3 (vs grade 1-2) | 2.66 (0.98-7.18) | 0.054 | 2.24 (1.02-4.95) | 0.046 |
| Tumor stage 3 or 4 (vs stage 1 or 2) | 1.99 (1.21-3.28) | 0.007 | 1.85 (1.06-3.22) | 0.030 |
| Nodal metastasis (vs none) | 1.24 (0.76-2.03) | 0.390 | 1.10 (0.63-1.95) | 0.736 |
| Lymphatic invasion (vs none) | 1.76 (1.07-2.90) | 0.025 | 1.49 (0.80-2.80) | 0.212 |
| Vascular invasion (vs none) | 2.98 (1.53-5.80) | 0.001 | 2.14 (1.20-3.79) | 0.010 |
| Perineural invasion (vs none) | 1.47 (0.85-2.55) | 0.164 | 0.97 (0.55-1.71) | 0.901 |
| Received AC (vs none) | 0.85 (0.52-1.40) | 0.536 | 0.50 (0.29-0.88) | 0.015 |
| Variables | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age ≥ 70 yr (vs < 70 yr) | 2.66 (1.40-5.07) | 0.003 | 2.16 (1.18-3.98) | 0.013 |
| Histologic grade 3 (vs grade 1-2) | 1.91 (0.67-5.49) | 0.228 | 1.93 (0.77-4.81) | 0.159 |
| Tumor stage 3 or 4 (vs stage 1 or 2) | 2.32 (1.31-4.11) | 0.004 | 2.50 (1.31-4.80) | 0.006 |
| Nodal metastasis (vs none) | 1.36 (0.77-2.39) | 0.284 | 1.09 (0.58-2.04) | 0.799 |
| Lymphatic invasion (vs none) | 1.50 (0.84-2.65) | 0.168 | 1.23 (0.62-2.45) | 0.553 |
| Vascular invasion (vs none) | 2.89 (1.35-6.20) | 0.006 | 1.93 (0.96-3.88) | 0.064 |
| Perineural invasion (vs none) | 2.17 (1.13-4.17) | 0.020 | 1.27 (0.66-2.45) | 0.477 |
| Received AC (vs none) | 1.04 (0.59-1.82) | 0.018 | 0.58 (0.30-1.11) | 0.098 |
The median treatment duration was 24.1 wk (range, 7.9-29.0) in the FL group and 24.1 wk (range, 7.7-29.6) in the FP group (Table 4). The median number of cycles was six (range, 2-6) in both the FL and FP groups. The RDI was 0.83 (range, 0.27-1.00) in the FL group and 0.85 (range, 0.33-1.00) in the FP group. A total of 20 patients (66.7%) in the FL group and 16 patients (72.7%) in the FP group received all planned cycles of chemotherapy. Permanent treatment discontinuation due to intolerance occurred in five patients (16.7%) in the FL group and four patients (18.3%) in the FP group.
| FL (n = 30) | FP (n = 22) | |
| Duration of treatment, weeks, median (range) | 24.1 (7.9-29.0) | 24.1 (7.7-29.6) |
| Cycles of drug administration, median (range) | 6 (2-6) | 6 (2-6) |
| Relative dose intensity, mean (range) | 0.83 (0.27-1.00) | 0.85 (0.33-1.00) |
| Received all cycles of chemotherapy, n (%) | 20 (66.7) | 16 (72.7) |
| Dose reduction or interruption, n (%) | 10 (33.3) | 10 (45.5) |
| Discontinued adjuvant chemotherapy, n (%) | 10 (33.3) | 6 (27.3) |
| Relapse | 5 (16.7) | 2 (9.0) |
| Intolerance | 5 (16.7) | 4 (18.3) |
Grade 3 or 4 adverse events were reported in 2 of 30 patients (6.7%) in the FL group and in 7 of 22 patients (31.8%) in the FP group (P = 0.033, Table 5). No serious adverse events resulted in death in either group. Grade 3 or 4 hematological toxicities were observed only in the FP group (n = 5, 22.7%). The most frequent adverse events were stomatitis (n = 11, 36.7%), fatigue (n = 9, 30.0%), and anemia (n = 8, 26.7%) in the FL group whereas fatigue (n = 19, 86.4%), nausea (n = 19, 86.4%), and neutropenia (n = 14, 63.6%) were the most frequent adverse events in the FP group. All grades of adverse events, including nausea, fatigue, neutropenia, and thrombocytopenia, were observed significantly more frequently in the FP group than in the FL group.
| Adverse event | FL (n = 30) | FP (n = 22) | P value1 | ||
| Grade 1-2 | Grade 3-4 | Grade 1-2 | Grade 3-4 | ||
| Stomatitis | 11 (36.7) | 0 | 3 (13.6) | 0 | 0.112 |
| Nausea | 6 (20.0) | 0 | 19 (86.4) | 0 | < 0.001 |
| Vomiting | 1 (3.3) | 0 | 4 (18.2) | 0 | 0.149 |
| Diarrhea | 2 (6.6) | 1 (3.3) | 0 | 0 | 0.253 |
| Fatigue | 8 (26.7) | 1 (3.3) | 19 (86.4) | 0 | < 0.001 |
| Neutropenia | 3 (10.0) | 0 | 10 (45.5) | 4 (18.2) | < 0.001 |
| Febrile neutropenia | 0 | 0 | 0 | 2 (9.0) | 0.174 |
| Anemia | 8 (26.7) | 0 | 10 (45.5) | 1 (4.5) | 0.084 |
| Thrombocytopenia | 2 (6.6) | 0 | 8 (36.4) | 0 | 0.012 |
| Increased AST/ALT level | 4 (13.3) | 0 | 0 | 0 | 0.128 |
In this trial involving patients with stage IB-III resected adenocarcinoma of the AoV, adjuvant chemotherapy was significantly associated with longer DFS than surgery alone in multivariate analysis; however, there was no significant association with OS. Discontinuation of treatment due to intolerance to chemotherapy occurred at a similar rate in both groups. As there is no clear evidence of clinical benefit with the use of fluorouracil-based chemotherapy in combination with cisplatin, the FL regimen, which is associated with lower toxicity, may be a more feasible option for elderly patients or those with a relatively poor performance status.
Given the rarity of this disease, the availability of large-scale randomized controlled trials investigating adjuvant treatments is limited, and as a result, there is currently no consensus regarding the effectiveness of adjuvant treatments following surgery. Although the ESPAC-3 trials demonstrated a survival benefit with adjuvant chemotherapy in a multivariable analysis, the heterogeneous clinicopathological features of the study population and the modest effect of the adjuvant chemotherapy warrant further investigation[8]. Previous retrospective studies have suggested that patients who received adjuvant chemotherapy tended to have better DFS and OS compared to those who did not receive adjuvant treatment; however, these differences were not statistically significant[4,10]. The studies were limited by the inclusion of a relatively large number of stage IA patients and the use of various adjuvant chemotherapy regimens, which may have influenced the outcomes. Patients with stage IA disease were excluded from this study as they are unlikely to experience relapse and do not typically receive adjuvant treatment. During the same period as the study, 33 patients were diagnosed with stage IA AoV cancer, and only two (6.0%) of these patients experienced recurrence. These findings may aid in the selection of patients who would benefit from adjuvant chemotherapy following surgery.
Contrary to expectations, there was no significant difference in DFS and OS between stage IB-II and stage III disease in this study. Furthermore, lymph node metastasis, a known risk factor for recurrence and poor survival, was not associated with recurrence or cancer-related death in multivariate analysis. These unexpected results may be due to the higher proportion of patients with stage III disease receiving adjuvant chemotherapy than those with stage IB or II disease. Moreover, according to the AJCC 8th staging system, involvement of one or more lymph nodes in the pathological findings, regardless of tumor stage, is indicative of at least stage III disease. For optimal node staging, it is important to harvest sufficient lymph nodes during surgery; however, in this study, a relatively low number of lymph nodes was harvested in patients with stage IB or II. In patients with pancreatic cancer, it has been recommended that at least 11-17 Lymph nodes be examined to provide accurate nodal staging[11,12]. However, less than 11 Lymph nodes were harvested in 28 (62.2%) of 45 patients with stage IB or II from this study; this result suggests that the stage of a significant portion of patients with stage IB or II might have been underestimated. A significant proportion of stage IB or II patients were understaged and did not receive adjuvant chemotherapy. These might be potential confounders that influence the effectiveness of adjuvant chemotherapy.
In the absence of consensus guidance for adjuvant treatment for physicians in real-world settings, there has been a tendency to determine the treatment regimen for patients with AoV cancer based on whether the tumor had an intestinal or pancreatobiliary histological subtype[13,14]. Notably, AoV cancer with pancreato-biliary phenotype has a worse outcome than those with intestinal phenotype[3,15]. According to the ESPAC-3 trial, there were no statistically significant differences in response to adjuvant treatment between the group of patients with pancreato-biliary phenotype and the group of intestinal phenotype[8]. However, more than half of the patients (162 of 297 patients, 54.5%) were classified as having an intermediate phenotype and were excluded from the analysis; therefore, further studies are needed to evaluate the treatment strategy according to the histologic subtypes. For patients with resected pancreatic ductal adenocarcinoma, combination chemotherapy with fluorouracil, leucovorin, irinotecan, and oxaliplatin (modified FOLFIRINOX), and gemcitabine with capecitabine have been used as standard adjuvant treatments following surgery[16,17]. For patients with resected biliary tract cancer, adjuvant capecitabine following surgery can improve OS according to the per-protocol analysis[18]. These published prospective studies suggest that fluorouracil-based chemotherapy might have a role in resected AoV carcinoma with a pancreatobiliary phenotype. Fluorouracil-based adjuvant treatment might also provide benefits for AoV carcinoma with an intestinal phenotype because FL, FL with oxaliplatin (FOLFOX), or capecitabine with oxaliplatin (CAPOX) have already been used as standard adjuvant treatments in patients with colon cancer, which is similar to the intestinal type of AoV cancer[19,20]. In addition, chemotherapy regimens, such as gemcitabine plus cisplatin or CAPOX, which have shown survival benefit in patients with advanced AoV cancer, could be considered as one of the adjuvant chemotherapy options[21,22].
Our study has some limitations. First, this study was conducted at a single institution and had a retrospective design. Second, differences in clinicopathological factors between the two groups hampered comparison of the adjuvant chemotherapy efficacy. Adjuvant chemotherapy was associated with better DFS, but not OS, which is one of the limitations. However, the DFS rate seems to be more suitable than the OS rate for evaluating the efficacy of adjuvant chemotherapy. OS could be affected by various factors, such as whether palliative chemotherapy was performed after recurrence, the pattern of recurrence (local or systemic), and the availability of local treatment.
In summary, among patients with resected AoV carcinoma, fluorouracil-based adjuvant chemotherapy was not associated with a better survival outcome in the primary analysis, compared with surgery alone. However, multivariate analysis demonstrated that better DFS was statistically associated with adjuvant chemotherapy. Further investigations are warranted to identify patients with resected AoV cancer who might benefit from adjuvant chemotherapy.
Surgical resection is the primary curative approach for patients with localized Ampulla of Vater (AoV) carcinoma, but recurrence is frequent. There is no standard adjuvant treatment globally accepted for resected AoV carcinoma.
A significant number of surgically resected AoV carcinoma patients experience recurrence, and there is a great unmet need because standard adjuvant treatment has not been established.
The purpose of this study was to determine the correlation between fluorouracil-based adjuvant chemotherapy and prognosis in surgically resected AoV carcinoma patients.
The association between adjuvant chemotherapy and survival outcomes in patients with stage IB-III AoV carcinoma who underwent surgical resection was analyzed. The administration of fluorouracil-based adjuvant chemotherapy after surgery was determined by the physician's discretion. Adjusted multivariate regression models were utilized to evaluate the correlation between adjuvant chemo
After curative surgery for AoV carcinoma, 52 patients received adjuvant chemotherapy. Multivariate analysis showed that advanced tumor stage, higher histologic grade, and vascular invasion were linked with shorter disease-free survival (DFS). Adjuvant chemotherapy improved DFS and was linked with a longer overall survival, although this was not statistically significant.
Overall, our study found no significant survival benefit of fluorouracil-based adjuvant chemotherapy in patients with resected AoV carcinoma. However, multivariate analysis revealed a positive association between adjuvant chemotherapy and improved DFS. Further research is needed to identify subgroups of resected AoV cancer patients who may benefit from adjuvant chemotherapy.
This study evaluated a relatively homogenous population with a consistent chemotherapy regimen, which is considered a strength in contrast to most retrospective studies that included heterogeneous populations and used inconsistent adjuvant treatment regimens. Patients with tumors that invade beyond the sphincter of Oddi could be considered for adjuvant treatment following surgery as a considerable proportion of stage IB patients experience relapses. These findings will aid in identifying appropriate candidates for adjuvant treatment in patients with AoV carcinoma.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: South Korea
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Buanes T, Norway; Yu F, China S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Ahn DH, Bekaii-Saab T. Ampullary cancer: an overview. Am Soc Clin Oncol Educ Book. 2014;112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 89] [Article Influence: 8.9] [Reference Citation Analysis (1)] |
| 2. | Feng J, Wu R, Zhang G, Yang Z, Zhang L. Prognostic importance of numbers of retrieved lymph nodes and positive lymph nodes for ampulla of vater cancer (AVC) in 2347 patients from the Surveillance, Epidemiology, and End Results (SEER) database. PLoS One. 2021;16:e0244987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 3. | Kim WS, Choi DW, Choi SH, Heo JS, You DD, Lee HG. Clinical significance of pathologic subtype in curatively resected ampulla of vater cancer. J Surg Oncol. 2012;105:266-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Kim JH, Jeong JH, Ryoo BY, Kim KP, Chang HM, Oh D, Song TJ, Lee SS, Seo DW, Lee SK, Kim MH, Park Y, Kwon JW, Hwang DW, Lee JH, Lee W, Kim SC, Yoo C, Song KB. Adjuvant Chemotherapy for Resected Ampulla of Vater Carcinoma: Retrospective Analysis of 646 Patients. Cancer Res Treat. 2021;53:424-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Bonet M, Rodrigo A, Vázquez S, Carrizo V, Vilardell F, Mira M. Adjuvant therapy for true ampullary cancer: a systematic review. Clin Transl Oncol. 2020;22:1407-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 6. | Bhatia S, Miller RC, Haddock MG, Donohue JH, Krishnan S. Adjuvant therapy for ampullary carcinomas: the Mayo Clinic experience. Int J Radiat Oncol Biol Phys. 2006;66:514-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Sikora SS, Balachandran P, Dimri K, Rastogi N, Kumar A, Saxena R, Kapoor VK. Adjuvant chemo-radiotherapy in ampullary cancers. Eur J Surg Oncol. 2005;31:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Neoptolemos JP, Moore MJ, Cox TF, Valle JW, Palmer DH, McDonald AC, Carter R, Tebbutt NC, Dervenis C, Smith D, Glimelius B, Charnley RM, Lacaine F, Scarfe AG, Middleton MR, Anthoney A, Ghaneh P, Halloran CM, Lerch MM, Oláh A, Rawcliffe CL, Verbeke CS, Campbell F, Büchler MW; European Study Group for Pancreatic Cancer. Effect of adjuvant chemotherapy with fluorouracil plus folinic acid or gemcitabine vs observation on survival in patients with resected periampullary adenocarcinoma: the ESPAC-3 periampullary cancer randomized trial. JAMA. 2012;308:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 457] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 9. | Liao X, Zhang D. The 8th Edition American Joint Committee on Cancer Staging for Hepato-pancreato-biliary Cancer: A Review and Update. Arch Pathol Lab Med. 2021;145:543-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 10. | Kim HS, Jang JY, Yoon YS, Park SJ, Kwon W, Kim SW, Han HS, Han SS, Park JS, Yoon DS. Does adjuvant treatment improve prognosis after curative resection of ampulla of Vater carcinoma? J Hepatobiliary Pancreat Sci. 2020;27:721-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Huebner M, Kendrick M, Reid-Lombardo KM, Que F, Therneau T, Qin R, Donohue J, Nagorney D, Farnell M, Sarr M. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Ashfaq A, Pockaj BA, Gray RJ, Halfdanarson TR, Wasif N. Nodal counts and lymph node ratio impact survival after distal pancreatectomy for pancreatic adenocarcinoma. J Gastrointest Surg. 2014;18:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 13. | Westgaard A, Tafjord S, Farstad IN, Cvancarova M, Eide TJ, Mathisen O, Clausen OP, Gladhaug IP. Pancreatobiliary versus intestinal histologic type of differentiation is an independent prognostic factor in resected periampullary adenocarcinoma. BMC Cancer. 2008;8:170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 14. | Sessa F, Furlan D, Zampatti C, Carnevali I, Franzi F, Capella C. Prognostic factors for ampullary adenocarcinomas: tumor stage, tumor histology, tumor location, immunohistochemistry and microsatellite instability. Virchows Arch. 2007;451:649-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Chang DK, Jamieson NB, Johns AL, Scarlett CJ, Pajic M, Chou A, Pinese M, Humphris JL, Jones MD, Toon C, Nagrial AM, Chantrill LA, Chin VT, Pinho AV, Rooman I, Cowley MJ, Wu J, Mead RS, Colvin EK, Samra JS, Corbo V, Bassi C, Falconi M, Lawlor RT, Crippa S, Sperandio N, Bersani S, Dickson EJ, Mohamed MA, Oien KA, Foulis AK, Musgrove EA, Sutherland RL, Kench JG, Carter CR, Gill AJ, Scarpa A, McKay CJ, Biankin AV. Histomolecular phenotypes and outcome in adenocarcinoma of the ampulla of vater. J Clin Oncol. 2013;31:1348-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 16. | Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, Choné L, Francois E, Artru P, Biagi JJ, Lecomte T, Assenat E, Faroux R, Ychou M, Volet J, Sauvanet A, Breysacher G, Di Fiore F, Cripps C, Kavan P, Texereau P, Bouhier-Leporrier K, Khemissa-Akouz F, Legoux JL, Juzyna B, Gourgou S, O'Callaghan CJ, Jouffroy-Zeller C, Rat P, Malka D, Castan F, Bachet JB; Canadian Cancer Trials Group and the Unicancer-GI-PRODIGE Group. FOLFIRINOX or Gemcitabine as Adjuvant Therapy for Pancreatic Cancer. N Engl J Med. 2018;379:2395-2406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1427] [Cited by in RCA: 1932] [Article Influence: 276.0] [Reference Citation Analysis (0)] |
| 17. | Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, Faluyi O, O'Reilly DA, Cunningham D, Wadsley J, Darby S, Meyer T, Gillmore R, Anthoney A, Lind P, Glimelius B, Falk S, Izbicki JR, Middleton GW, Cummins S, Ross PJ, Wasan H, McDonald A, Crosby T, Ma YT, Patel K, Sherriff D, Soomal R, Borg D, Sothi S, Hammel P, Hackert T, Jackson R, Büchler MW; European Study Group for Pancreatic Cancer. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017;389:1011-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1327] [Cited by in RCA: 1385] [Article Influence: 173.1] [Reference Citation Analysis (0)] |
| 18. | Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, Anthony A, Corrie P, Falk S, Finch-Jones M, Wasan H, Ross P, Wall L, Wadsley J, Evans JTR, Stocken D, Praseedom R, Ma YT, Davidson B, Neoptolemos JP, Iveson T, Raftery J, Zhu S, Cunningham D, Garden OJ, Stubbs C, Valle JW, Bridgewater J; BILCAP study group. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20:663-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 806] [Article Influence: 134.3] [Reference Citation Analysis (0)] |
| 19. | André T, Boni C, Navarro M, Tabernero J, Hickish T, Topham C, Bonetti A, Clingan P, Bridgewater J, Rivera F, de Gramont A. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109-3116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1502] [Cited by in RCA: 1637] [Article Influence: 102.3] [Reference Citation Analysis (0)] |
| 20. | Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, Meyerhardt JA, Vernerey D, Yamanaka T, Boukovinas I, Meyers JP, Renfro LA, Niedzwiecki D, Watanabe T, Torri V, Saunders M, Sargent DJ, Andre T, Iveson T. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018;378:1177-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 679] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 21. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3144] [Article Influence: 209.6] [Reference Citation Analysis (1)] |
| 22. | Overman MJ, Varadhachary GR, Kopetz S, Adinin R, Lin E, Morris JS, Eng C, Abbruzzese JL, Wolff RA. Phase II study of capecitabine and oxaliplatin for advanced adenocarcinoma of the small bowel and ampulla of Vater. J Clin Oncol. 2009;27:2598-2603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 156] [Article Influence: 9.8] [Reference Citation Analysis (0)] |