Published online Apr 15, 2023. doi: 10.4251/wjgo.v15.i4.617
Peer-review started: December 4, 2022
First decision: December 26, 2022
Revised: January 9, 2023
Accepted: March 8, 2023
Article in press: March 8, 2023
Published online: April 15, 2023
Processing time: 128 Days and 17.6 Hours
Hepatocellular carcinoma (HCC) is a common malignant tumor that affecting many people's lives globally. The common risk factors for HCC include being overweight and obese. The liver is the center of lipid metabolism, synthesizing most cholesterol and fatty acids. Abnormal lipid metabolism is a significant feature of metabolic reprogramming in HCC and affects the prognosis of HCC patients by regulating inflammatory responses and changing the immune microenvironment. Targeted therapy and immunotherapy are being explored as the primary treatment strategies for HCC patients with unresectable tumors. Here, we detail the specific changes of lipid metabolism in HCC and its impact on both these therapies for HCC. HCC treatment strategies aimed at targeting lipid metabolism and how to integrate them with targeted therapy or immunotherapy rationally are also presented.
Core Tip: This review systematically summarizes the aberrant changes of lipid metabolism in hepatocellular carcinoma (HCC), and for the first time expounds the impact of lipid metabolism on HCC targeted therapy and immunotherapy. It vividly displayed the changes of lipid metabolism in HCC and the targets of some reagents by drawing figures, and summarized the impact of lipid metabolism related reagents on HCC targeted therapy and immunotherapy through table.
- Citation: Feng XC, Liu FC, Chen WY, Du J, Liu H. Lipid metabolism of hepatocellular carcinoma impacts targeted therapy and immunotherapy. World J Gastrointest Oncol 2023; 15(4): 617-631
- URL: https://www.wjgnet.com/1948-5204/full/v15/i4/617.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i4.617
Liver cancer, a common malignancy, has documented an increasing incidence over the years. In 2020, there were 910000 new cases of liver cancer worldwide and 830000 people died of liver cancer with the global number of patients and deaths ranking sixth and third positions, respectively[1]. Hepatocellular carcinoma (HCC), accounting for nearly 90% of total cases, is the most frequently occurring type of liver cancer[2]. HCC has many risk factors related to its occurrence, including virus hepatitis, nonalcoholic fatty liver disease (NAFLD), alcoholic hepatitis, and some genetic metabolic diseases like Wilson disease, alpha1-antitrypsin deficiency, and hereditary tyrosinemia type I[3,4].
HCC has a poor prognosis, and many patients have no obvious early symptoms, leading to untimely diagnosis. To date, radical resection and liver transplantation are still the only curative treatment for HCC patients. However, many patients are already in the advanced stage (Barcelona Clinic Liver Cancer B-D stage) when they are initially diagnosed with HCC, making them unable to undergo radical surgery[5]. The increasing shortage of liver donors and resources also makes liver transplantation a treatment strategy that only a few HCC patients can receive. It is worth noting that systemic treatment, like molecular targeted therapy and immunotherapy has brought fresh hope to HCC patients. Phase I/II/III clinical trial reports provide strong evidence for these above drugs in treating HCC[6]. Since its approval in 2007, sorafenib has been the only targeted therapy drug for advanced HCC patients for a long time. In recent years, the introduction of lenvatinib and immunotherapy such as the anti-programmed cell death protein 1/programmed death ligand 1 (anti-PD-1/PD-L1), belonging to immune checkpoint inhibitors (ICIs), has enriched the therapeutic strategy for HCC patients with unresectable tumors. Although these treatments have documented certain achievements, they are far from satisfactory. Current targeted therapies aim to inhibit tumor blood supply or directly inhibit tumor growth by affecting proliferation related signal pathways However, these targets do not have a significant and direct impact on tumor metabolism[7].
Metabolic reprogramming is an important malignancy feature that significantly impacts tumor’s occurrence, proliferation, and invasion[8]. Compared with normal liver cells, the metabolism of HCC cells also documents many changes. Among these alterations, abnormal lipid metabolism is a significant aspect. On the one hand, aberrant lipid metabolism helps HCC cells obtain more energy to meet their rapid growth, proliferation, and metastasis needs. On the other hand, such altered lipid metabolism products and intermediates impact cell signal transmission and the formation of cell structures[9]. This review mainly discusses the abnormal changes in lipid metabolism in HCC and their impacts on targeted therapy and immunotherapy of HCC to explore the potential clinical application value of targeting lipid metabolism in treating HCC.
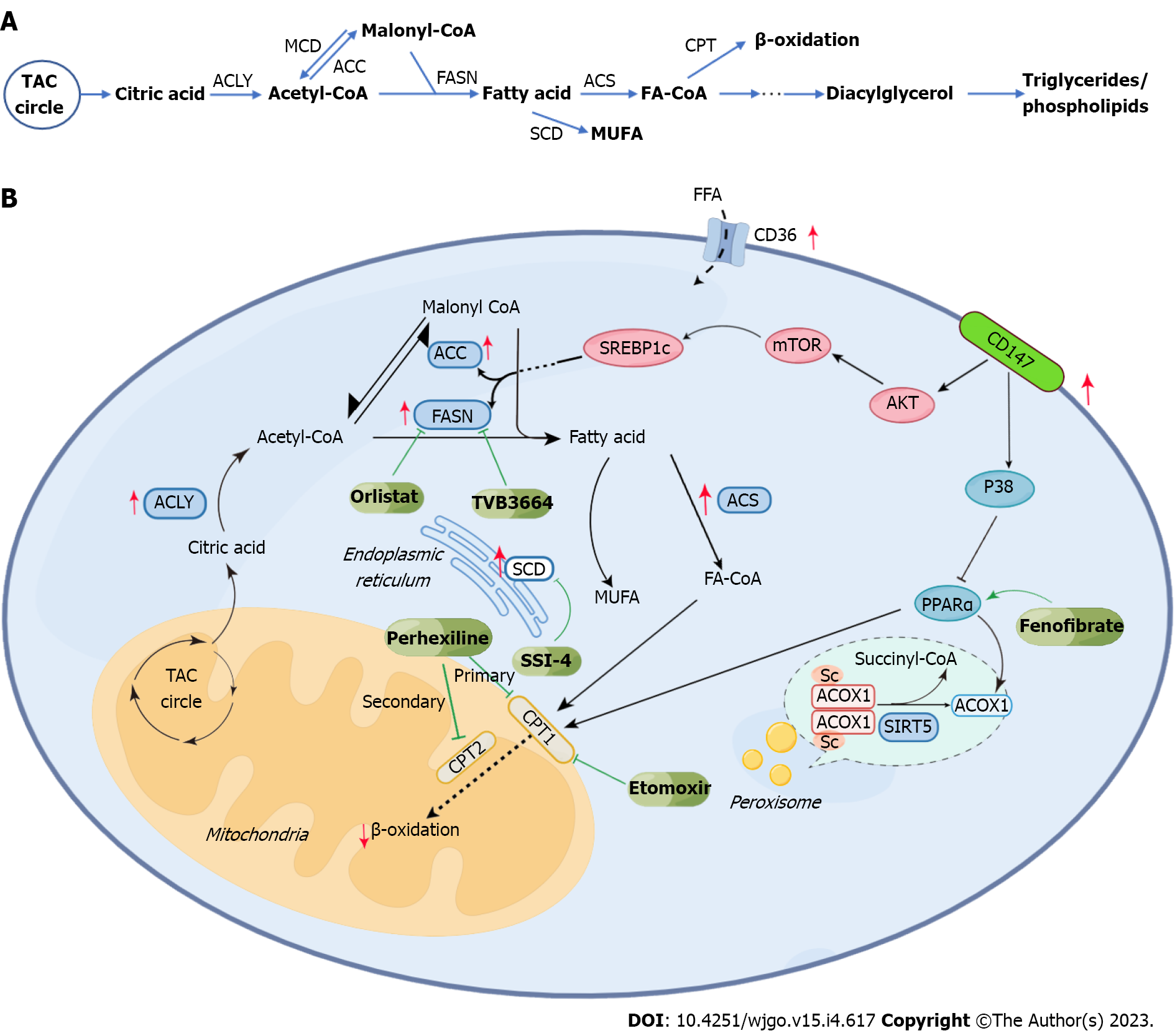
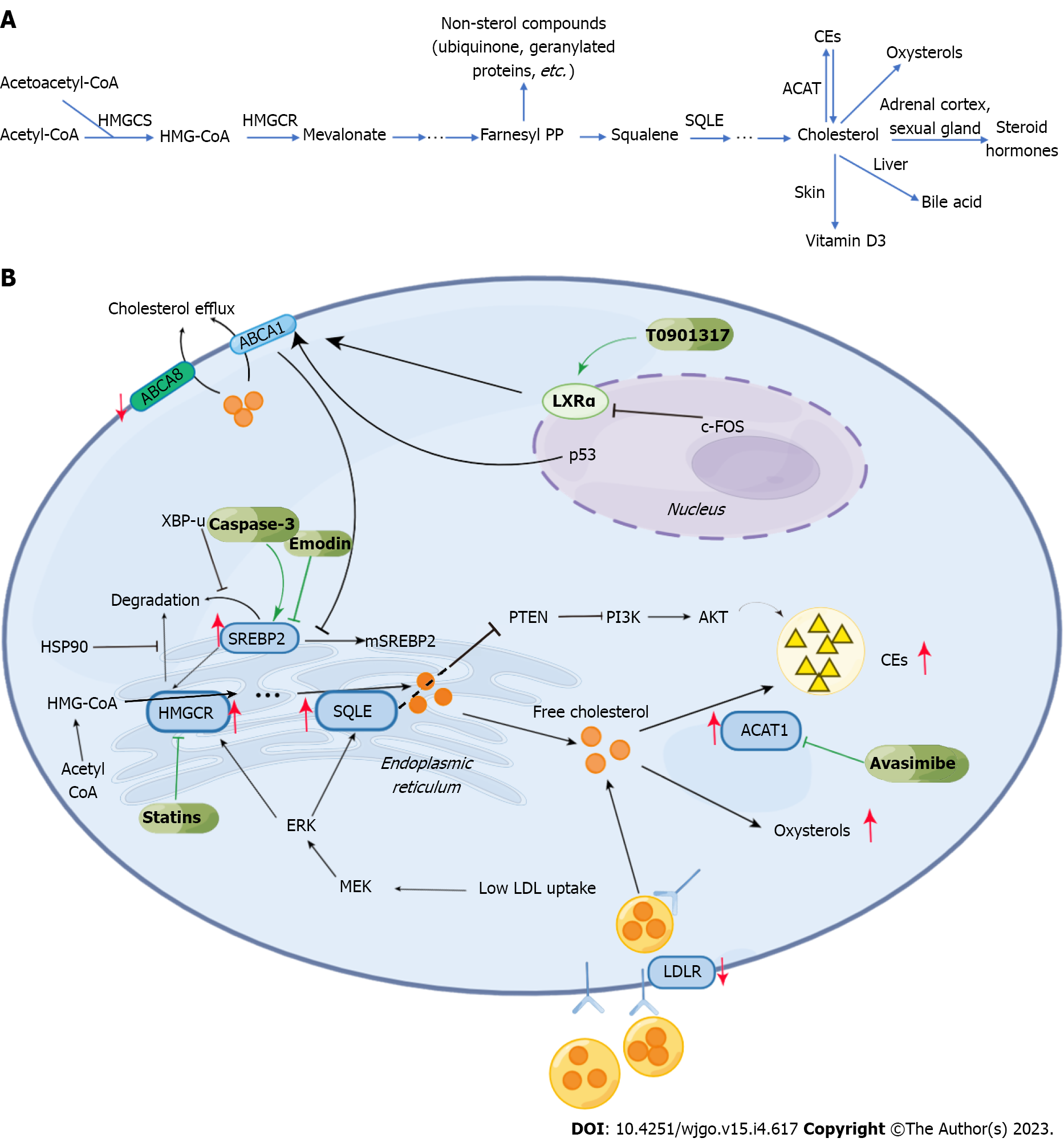
In HCC, abnormal lipid metabolism mainly manifests as changes in lipid uptake and efflux, upregulation of endogenous lipid synthesis, enhanced cholesterol esterification, and changes in lipid oxidation (Figures 1 and 2). These above changes closely correlate with tumor survival, growth, proliferation, and metastasis.
HCC cells can promote their growth and proliferation by increasing the uptake of extracellular fatty acids (FAs). FA uptake by cancer cells is an active transport process dependent on the fatty acid translocase (FAT, also called CD36) on the cell membrane surface[10]. CD36 is abnormally expressed in HCC and promotes tumor metastasis and epithelial-mesenchymal transformation (EMT) by increasing FA uptake, thereby promoting tumor progression[11]. There is a mixed opinion on the exogenous cholesterol uptake of HCC. In many malignant tumors, cholesterol uptake is significantly increased, related to the high expression of low-density lipoprotein receptor (LDLR)[12]. However, some studies have found that the expression of LDLR in HCC cells is significantly lower than that in peri-tumorous normal cells around the tumor, thus leading to a decrease in LDL uptake of HCC cells. The upregulation of intracellular cholesterol biosynthesis may cause this phenomenon. Interestingly, the low expression of LDLR can increase cholesterol synthesis in HCC cells by activating MEK/ERK signaling pathway[13]. Cholesterol efflux, as opposed to extracellular uptake, is also downregulated in HCC. Further, the low expression of ATP binding cassette subfamily A member 8, transporters responsible for cholesterol efflux on cell membranes, in HCC is correlated with poor prognosis[14]. P53, a tumor suppressor gene, upregulates the expression of ABCA1, inhibiting sterol regulatory element binding protein (SREBP) maturation by affecting cholesterol transport[15].
The synthesis of FAs in HCC cells is abnormally higher, closely related to the aberrant expression of some key enzymes in this process. Synthesis of FA occurs in the cytoplasm. To begin with, citric acid generated by the tricarboxylic acid cycle forms acetyl coenzyme A (acetyl-CoA) upon the catalysis by ATP citrate lyase (ACLY). It has been reported that ACLY is highly expressed in HCC. Inhibition of ACLY can improve drug resistance to sorafenib[16]. Acetyl-CoA then forms malonyl coenzyme A (malonyl-CoA) by the action of acetyl-CoA carboxylase (ACC). Upregulation of ACC expression is significantly related to the poor prognosis of HCC. The survival of ACC-expressing rats was improved by an ACC inhibitor alone or combined with sorafenib[17]. Malonyl-CoA and acetyl-CoA form FA upon catalysis by fatty acid synthase (FASN). Overexpression of FASN promotes the carcinogenesis of HCC. TVB compounds, FASN inhibitors, can potentially treat HCC[18]. Stearoyl-CoA desaturase (SCD) then catalyzes the conversion of saturated fatty acids into monounsaturated fatty acids. HCC patients with high expression of SCD1 often have a worse prognosis[19].
In HCC, the synthesis of cholesterol is also upregulated. High cholesterol levels in the liver give rise to nonalcoholic steatohepatitis (NASH) and promote its further development into HCC[20]. Such abnormal upregulation of cholesterol synthesis is linked to some key pathways and molecules. Blocking the SREBP pathway can inhibit cholesterol synthesis and slow down the progress of HCC by inhibiting inflammation[21]. 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), a key enzyme in cholesterol biosynthesis, in the ER, is subjected to precise regulation by SREBP2[22]. The unspliced X-box binding protein 1 was shown to inhibit the degradation of SREBP2, which can activate the transcription of HMGCR. This pathway promotes cholesterol biosynthesis in HCC cells, contributing to tumorigenesis and progression[23]. In addition, Heat shock protein 90 can directly interact with HMGCR and inhibit its degradation from promoting the pathway of cholesterol biosynthesis and, thereby, HCC[24]. A report showed an overexpression of squalene epoxidase (SQLE), another rate-limiting enzyme in cholesterol biosynthesis, which promoted tumor cell proliferation in NAFLD-HCC[25].
Increased cholesterol esterification is another crucial aspect of abnormal cholesterol metabolism documented in HCC. Cholesterol esters (CEs) are a storage form of cholesterol occurring in cells and are of especially great significance for the energy supply of tumor cells[26]. Reports show the overexpression of sterol-O-acyltransferase 1 (SOAT1; also called ACAT1), a key enzyme of cholesterol esterification, as a characteristic feature that promotes tumor proliferation[27]. Interestingly, such a high expression of ACAT1 in CD8+T cells inhibits their cellular functions and thereby helps tumor cell survival indirectly. The ACAT1 inhibitor, avasimibe, was found to directly inhibit HCC incidence and its progress by interfering with energy metabolism. Furthermore, it was found to boost tumor immunity by enhancing the function of cytotoxic lymphocytes[28]. Moreover, studies have shown that the loss of expression of Phosphatase and tensin homolog deleted on Chromosome 10 (PTEN) increases CE accumulation, which leads to the progress of viral hepatitis and HCC[29]. The SQLE inhibits PTEN, and its influence on CEs is closely related to the AKT pathway[25,30].
β-oxidation of FAs (FAO), occurring in the mitochondria, is significantly downregulated in HCC. Before β-oxidation, FAs must be converted into bioactive Fatty Acyl-CoA (FA-CoA) upon activation by acyl-CoA synthetases (ACS). HCCs have documented a significant overexpression of ACS, especially long-chain acyl-CoA synthetase 4 (ACSL4) and this overexpression promotes the progress of HCC[31]. The carnitine palmitoyl transfer (CPT) system is essential for FA-CoA to enter mitochondria from the cytoplasm. There are two kinds of CPT, CPT1, which is located in the outer mitochondrial membrane, and CPT2, which is located in the inner mitochondrial membrane. Overexpression of CPT1 in NASH leads to extensive activation of oncogenic signals, thereby promoting tumorigenesis and proliferation[32]. In addition, low expression of CPT2 observed in HCC leads to its escape from lipotoxicity by inhibiting the activation of JNK mediated by Src. The subsequent accumulation of acylcarnitine can promote the development of HCC by activating STAT3[33]. Acyl-CoA oxidase 1 (ACOX1) is a key rate limiting enzyme that catalyzes the oxidation of long-chain FAs. Studies have shown that loss of ACOX1 expression can promote the occurrence of HCC[34]. Medium chain acyl-CoA dehydrogenase (ACADM) is another enzyme regulating FAO. Studies have shown the significant downregulation of ACADM in HCC, which is related to the abnormal accumulation of SREBP1 caused by the increased expression of caveolin-1[35]. In addition, deacetylase sirtuin 5 (SIRT5) can inhibit the activity of ACOX1 through desuccinylation. A loss of SIRT5 expression in HCC leads to the increased activity of ACOX1 and oxidative damage of DNA[36]. CD147 is a transmembrane glycoprotein with high expression in HCC and reduces the expression of ACOX1 and CTP1A by inhibiting the MAPK pathway, thereby suppressing β-oxidation. In addition, it can also promote the expression of ACC and FASN through the AKT mTOR pathway and promote FA synthesis[37].
Besides being converted into CE for further storage, the cholesterol in cells can also be oxidized into oxysterols. Some studies have shown that c-FOS can downregulate the nuclear Liver X receptor α (LXRα), which regulates the expression level of genes related to cholesterol transport ABCA1 and hence promote the accumulation of oxysterols and HCC occurrence[38,39]. Oxysterols significantly impact the tumor microenvironment (TME), and their high level promotes immunosuppression and assists tumor metastasis[40]. For example, the accumulation of 22OHC was shown to recruit CD11b+Gr1high neutrophils with immunosuppressive functions[41]. In addition, 27OHC is closely related to the depletion of cytotoxic CD8+T cells[42]. 27OHC has also been found to have an apparent cancer-promoting effect in HCC, and its adverse effects could be reversed by targeting glucose-regulated protein 75[43]. 25OHC was found to induce HCC cell metastasis by upregulating fatty acid binding protein 4 (FABP4)[44].
The commonly employed HCC targeting drugs used for clinical treatment include sorafenib, Lenvatinib, and cabozantinib. The peroxisome proliferator-activated receptor (PPAR) signaling pathway is closely related to FA metabolism and controls FAO, synthesis, and desaturation, among other processes[45-47]. The PPAR signaling pathway can raise the stemness of tumor cells to increase the drug resistance of tumor cells to sorafenib and reduce the drug’s efficacy[48]. The drug resistance of tumor-initiating cells (TIC) and sorafenib can be regulated by SCD1, which is overexpressed in HCC to regulate desaturating FAs through the PPAR pathway and regulation of endoplasmic reticulum stress. Reports show that the combined efficacy of SSI-4 (a new SCD1 inhibitor) and sorafenib was significantly better than that of sorafenib alone[49]. Sorafenib resistance is also linked to the genes cytochrome P450 family 8 subfamily B member 1 and nuclear receptor subfamily 1 group H member 3 (NR1H3, LXRα), which can induce the increased expression of SCD while retinoid X receptor β can induce the transcription of NR1H3[39,48,50]. Orlistat, a FASN inhibitor, can regulate fat metabolism by inhibiting FA synthesis, lowering the drug resistance of HCC to sorafenib, and improving drug efficacy[51]. The expression of ACSL4 can predict the sensitivity of sorafenib treatment. A higher level of ACSL4 expression was documented in a sorafenib-sensitive sample patient set than in the non-sensitive group[52]. Fatty acid transport protein-5 (FATP5/SLC27A5) is closely related to FA transport, which can inhibit the invasion, metastasis and EMT of HCC. However, this effect is the opposite as FATP5 has a low expression in HCC. The anticancer mechanism of FATP5 depends on inhibiting AMP-activated protein kinase (AMPK); targeting the FATP5-AMPK axis is promising for individualized treatment of HCC[53]. Furthermore, some studies have shown that the combined use of sorafenib with nuclear factor E2-related factor 2 and thioredoxin reductase 1 inhibitors (brusatol and auranofin) could significantly improve the therapeutic effect in FATP5 deficient HCC cells[54].
In TICs, Toll-like receiver 4 induces NANOG (a unique homeobox transcription factor), which inhibits the mitochondrial oxidative phosphorylation (OXPHS) and activates FAO. This thereby inhibits the oxygen consumption rate and the production of reactive oxygen species, which promotes tumor proliferation and induces chemotherapy resistance in the tumors. Specifically, NANOG has a synergistic effect with PPAR in activating FAO. The drug resistance of HCC to sorafenib is significantly improved when the expression of OXPHS is restored by repressing NANOG or using the FAO inhibitor, etomoxirs[55,56]. Coiled-coil domain containing protein 25 (CCDC25) can potentially mediate liver metastasis of extrahepatic tumors[57], The low expression of CCDC25 in HCC leads to metabolic disorders, including FA alterations, and is significantly related to poor prognosis. However, a contradiction here is that HCCs with low expression of CCDC25 are more sensitive to sorafenib, lapatinib, and gefitinib. The specific mechanism here may involve the process of ferroptosis[58]. Sorafenib also influences lipid metabolism; for example, it can increase the level of erythropoietic acid (EETs) in the blood, and these EETs, in turn, affect the efficacy of sorafenib. According to a study, simultaneous docosahexaenoic acid supplements were recommended for HCC patients undergoing sorafenib treatment to augment their 19,20-erythropoietic acid levels, which can improve the therapeutic effect of sorafenib[59]. Sorafenib can induce tumor cells to upregulate glycolysis during treatment, reducing efficacy. However, the glycolysis upregulation can be inhibited by sodium butyrate (NaBu), a salt of FAs, which regulates the expression of hexokinase 2 and improve the therapeutic effect of sorafenib[60].
In addition to the efficacy as discussed above, lipid metabolism is also closely related to the adverse reactions caused by sorafenib is also related to lipid metabolism. For example, sorafenib-related adverse reactions like the hand-foot skin reaction are significantly related to the low levels of FAA and acylcarnitine in the plasma[61]. Further, patients with significant adverse reactions to sorafenib had higher levels of SREBP-1 in their tumors. The reactions to sorafenib were improved by using Betulin, the inhibitor of SREBP-1, and the curative effect of sorafenib was improved[62].
Lenvatinib significantly affects the metabolism of FAs when treating diseases. The metalloenzyme carbonic anhydrase (CA) is involved in FA biosynthesis and other metabolic processes. It is reported that lenvatinib has the potential to inhibit CA, which can be used to treat obesity[63]. Inhibition of aberrant lipid metabolism may be another potential mechanism of lenvatinib treatment. The efficacy of TVB3664, another FASN inhibitor is limited as a single drug, but it significantly improves the efficacy of cabozantinib and sorafenib in HCC treatment[18]. Tumor necrosis factor-α- induced protein 8 (TNFAIP8) is another molecule related to the metabolism of FAs. When the level of TNFAIP8 increases, it blocks the apoptosis of HCC cells to increase the tumor survival rate and increase the drug resistance of HCC to regorafenib and sorafenib[64].
The combination of atezolizumab and bevacizumab is a first-line clinical treatment for advanced HCC, and its efficacy in treating the unresectable tumor is better than sorafenib[65]. Bevacizumab is a targeted therapy drug that inhibits tumor angiogenesis by regulating vascular endothelial growth factor (VEGF). Studies have shown that the hypoxic environment induced by bevacizumab upregulates FA uptake and transport-related genes such as FABP, thereby promoting the accumulation of lipid droplets in TME, which promotes the survival of tumor cells. When lipid droplet accumulation is blocked by inhibiting FABP, the tumor growth rate decreases significantly[66]. In addition, Pan et al[67] found that FABP5 plays an essential role in the angiogenesis of HCC, and its mechanism is closely related to the activation of VEGF-related pathways. Regulation of lipid metabolism may improve the efficacy of anti-angiogenesis drugs such as bevacizumab[67].
Research has documented that cholesterol metabolism also impacts the targeted treatment of HCC. Simvastatin is a commonly used drug to reduce cholesterol, which itself has the effect of inhibiting tumor growth[68]. A report showed that simvastatin, in combination with sorafenib, significantly increases the sensitivity of HCC to sorafenib[69]. Another study showed that HCC resistance to sorafenib could be significantly addressed by cholesterol-modified agomiR-30a-5p (a miR-30a-5p mimic)[70]. The role of high levels of p90 Ribosomal S6 kinase 2 (RSK2) in promoting tumor invasion and metastasis is known. HCC is closely associated with RSK2 mutations, which activate the MAPK pathway to enhance cholesterol biosynthesis and improve tumor sensitivity to sorafenib[71]. Steroidogenic acute regulatory protein-related lipid transfer domain containing 4 (STARD4) is a key molecule mediating cholesterol transport, which promotes tumor cell proliferation and weakens the response of HCC to sorafenib. SREBP2 upregulates the expression level of STARD4. Knockdown of STARD4 or SREBP2 could increase the drug sensitivity of sorafenib-resistant HCC models to sorafenib[72].
Further, maprotiline, a noradrenergic reuptake blocker, could significantly reduce the phosp
In addition to directly impacting the efficacy of sorafenib, cholesterol can be used as a drug carrier. For instance, a study showed that the lipopolymers formed by polyethylene and cholesterol could be used to deliver sorafenib and other insoluble drugs[82]. Abnormal cholesterol metabolism is also linked to drug resistance to lenvatinib. Such aberrant metabolism often changes the activity of lipid rafts on the cell surface to influence the activity of ATP-binding cassette transporter B1 and subsequently impact lenvatinib drug resistance by enhancing exocytosis[83]. Caspase-3 can regulate the cleavage of SREBP2 to promote the synthesis of cholesterol and subsequently activate the sonic hedgehog signaling pathway to increase the drug resistance of HCC to lenvatinib[84]. Not only does cholesterol play a role in activating this signaling pathway, but so does its metabolite, 25-OHC[85].
A study showed that small lipid nanoparticles (usLNPs) composed of several phospholipids and a highly-selective targeting peptide could efficiently deliver sorafenib to HCC in mice[86]. HCC tumorigenesis and its drug resistance have a close correlation with Sphingolipid metabolism. Sphingomyelin synthase1 (SMS1) was reported to be significantly upregulated in HCC after sorafenib treatment. This SMS1 upregulation reduces the cytotoxicity of sorafenib; hence, SMS1 inhibitor D609 significantly improves the therapeutic effect of sorafenib by lowering Ras activity[87]. Sphingosine kinase 2 (SK2) forms pro-survival sphingosine 1-phosphate (S1P). A study showed that the efficacy of sorafenib could be augmented when used with the SK2 inhibitor ABC294640[88]. Bavituximab, which targets phosphatidylserine, inhibits tumor growth by blocking tumor angiogenesis and activating antitumor immunity. The therapeutic effect of a combination of bavituximab with sorafenib was found to be significantly better than that of sorafenib alone[89]. It has been reported that the level of phosp
Lipid metabolism is closely associated with targeted therapy (Table 1). On the one hand, altered lipid profiles and metabolism significantly impact the efficacy of current targeted therapies. On the other hand, long-term exposure to targeted therapy drugs such as sorafenib may also change the expression level of lipid metabolism related genes. Further, many molecules associated with lipid metabolism have the potential to become therapeutic targets.
| Type of lipid metabolism | Reagents | Specific impact | Ref. | |
| Targeted therapy | Fatty acid | SSI-4 | Blocking SCD1, then enhancing the efficacy of sorafenib by regulating endoplasmic reticulum stress | [49] |
| Orlistat | Improving sorafenib resistance by inhibiting FASN | [51] | ||
| Brusatol | Inhibiting NRF2, then improving the efficacy of sorafenib by improving lipid metabolism disorder and promoting redox homeostasis | [54] | ||
| Auranofin | Inhibiting TXNRD1, then improving the efficacy of sorafenib by improving lipid metabolism disorder and promoting redox homeostasis | [54] | ||
| Etomoxir | Enhancing the efficacy of sorafenib by inhibiting mitochondrial fatty acid oxidation | [55] | ||
| DHA | Enhancing the efficacy of sorafenib by improving 19,20-erythropoietic acid (19,20-EDP) level | [59] | ||
| Sodium butyrate (NaBu) | Improving the curative effect of sorafenib by regulating the expression of hexokinase 2 | [60] | ||
| Betulin | Alleviating the adverse reaction of sorafenib and improving its efficacy by blocking SREBP-1 | [62] | ||
| TVB3664 | Enhancing the efficacy of cabozantinib and sorafenib by inhibiting FASN | [18] | ||
| Cholesterol | Simvastatin | Enhancing the efficacy of sorafenib by inhibiting cholesterol synthesis | [68-69] | |
| Maprotiline | Enhancing the efficacy of sorafenib by inhibiting cholesterol synthesis through reducing the phosphorylation level of SREBP2 | [73] | ||
| Emodin | Enhancing the efficacy of sorafenib by inhibiting cholesterol synthesis through inhibiting SREBP2 | [74] | ||
| Lycorine | Lowering the level of intracellular cholesterol by inhibiting SCAP, then enhancing the efficacy of sorafenib | [76-77] | ||
| T0901317 | Regulating cholesterol efflux by activating LXR, then enhancing the efficacy of sorafenib | [80] | ||
| Caspase-3 | Improve the drug resistance of HCC to lenvatinib by promoting the synthesis of cholesterol | [84] | ||
| Other lipid metabolism | D609 | Inhibiting SMS1, and then enhancing sorafenib efficacy by lowering Ras activity | [87] | |
| ABC294640 | Reducing the formation of S1P by inhibiting SK2, then enhancing the efficacy of sorafenib | [88] | ||
| Bavituximab | Enhancing the efficacy of sorafenib targeting tumor angiogenesis and reactivating antitumor immunity | [89] | ||
| Immunotherapy | Fatty acid | Perhexiline | Prolonging the survival of CD4+ T cell through inhibiting CPT | [103] |
| Fenofibrate | Improving the efficacy of cancer vaccine through activating PPARα in other tumors (worth exploring in HCC) | [104] | ||
| Cholesterol | Avasimibe | Enhancing the function of CD8+T cells by inhibiting ACAT1, thereby improving the therapeutic effect of PD-1 inhibitors (worth exploring in HCC) | [107] | |
| Simvastatin | Its combination with PD-L1 antibody effectively inhibits the proliferation of HCC | [111] |
In TME, molecules related to FA metabolism and their metabolites change the state of tumor immune responses. For instance, CCDC25, which regulates FA metabolism as described above, affects HCC sensitivity to targeted therapy, the infiltration of immune cells, and the expression level of immune checkpoints. A study showed that CCDC25 is positively correlated with the infiltration of CD8+T cells, macrophages, and dendritic cells, while negatively correlated with regulatory T cells (Treg) infiltration and the expression level of immune checkpoints such as PDCD1, CTLA4, and TIGIT. CCDC25 also blocked the immune escape of tumors by upregulating tumor killer cells, downregulating immunosuppressive cells, and inhibiting immune checkpoints[58].
It was reported that the FASN inhibitor TVB3664 could improve the therapeutic effect of cabozantinib and sorafenib; however, its combination with PD-L1 treatment could not effectively inhibit the growth of HCC. This may be due to the specific immunosuppression of HCC[18]. As mentioned above, the combination of atezolizumab and bevacizumab is commonly used in HCC patients, and lipid metabolism impacts the efficacy of bevacizumab. Atezolizumab is a kind of ICIs, which reverses the immunosuppression of T cells by targeting PD-L1 to prevent its interaction with PD-1[93]. Liu et al[94] found that the expression of FABP5 on tumor-derived monocytes is negatively related to the prognosis of HCC patients because it activates the expression of PD-L1 on Treg cells through the JNK-STAT3 pathway, thereby inhibiting tumor immunity[94]. Studies have shown that the use of atezolizumab plus bevacizumab in HCC patients with hepatic steatosis has an excellent therapeutic effect, which is related to the upregulation of PD-L1 induced by high palmitic acid levels[95]. FAO is also the primary pathway by which Treg and M2 macrophages obtain energy, making it an aspect that can enhance the immunosuppressive function of Treg[96,97]. Short-chain fatty acids (SCFAs) can enhance the function of Treg and impair the functions of CD8+T cells. The CD8+T cells/Treg ratio could predict the therapeutic effect of PD-1 inhibitor immunotherapy. It can be inferred that SCFAs can impact the therapeutic effect of PD-1 inhibitors, such as pembrolizumab[98,99]. High levels of FAs also alter the distribution of FAs in tumors. Specifically, the uptake of FAs by cancer cells increases their uptake of FAs while CD8+T cells have no increasing uptake. In addition, the expression of PD-1 on CD8+T cells was significantly reduced, which affects the function of CD8+T cells in TME. Hence, metabolic reprogramming by blocking FA related genes can significantly improve antitumor immunity[100]. For instance, Zhu et al[101] used the TCGA database to assess differential expression genes related to FA metabolism. They built a risk prediction model, which could predict not only patient prognosis, but also the therapeutic effect of anti-PD-1 immunotherapy. When patients’ risk score was lower in this model, the efficacy of anti-PD-1 immunotherapy was found to be better[101]. Cheng et al[102] described two new HCC cell lines. On the one hand, these two cell lines have different lipid metabolism. On the other hand, they have different responses to the immune system. This research result provides a new practical tool for studying the correlation between lipid metabolism and immunotherapy in HCC[102]. The upregulation of the PPARα-mediated CPT gene could promote the apoptosis of CD4+T cells by influencing FAO. The use of the CPT inhibitor, perhexiline, significantly prolonged the survival of CD4+T and inhibited HCC. Therefore, targeting the CPT family may emerge as a new approach for the immunotherapy of HCC[103]. Interestingly, using fenofibrate, an agonist of PPARα, could improve the efficacy of a cancer vaccine in treating tumors, as it increased the metabolism of FAs and reduced the use of glucose in tumors. This accumulated glucose could provide energy for inducing the generation of CD8+T cells by the vaccine. However, this interesting result has not yet been reported in any HCC cell line making this line of research worthy of future exploration[104].
Antitumor immunity mainly depends on cell-mediated immunity that mainly involves T-cell functions. The cellular signal transduction and functions of T cells depend on their membrane lipid structure[105]. Cholesterol is the critical component of membrane lipids with an involvement in the formation of vital T-cell immune synapses and the functions of the T cell receptor[106]. While the cholesterol biosynthesis or its uptake by T cells can enhance their antitumor functions, the upregulation of oxysterols in TME can significantly inhibit the function of T cells through the LXR[22]. Further, combining immunotherapies with cholesterol-esterification inhibitors is a prospective therapeutic strategy. Bioenergy utilization of CD8+T cells was optimized by avasimibe, by inhibiting the cholesterol esterase of T cells from enhancing their effector functions and promoting their proliferation. A study showed that the combination of avasimibe and PD-1 inhibitor documented better efficacy than individual drug use[107]. Furthermore, avasimibe, in combination with a tumor vaccine, had an excellent therapeutic effect on other tumors[108]. Also, avasimibe can be used to optimize the adoptive cell therapies for tumors or hepatitis B virus. For example, combining avasimibe with chimeric antigen receptor-T cell therapy showed better curative efficacy[107,109]. These results make similar research also worth exploring for HCC. Statins have the effect of improving metabolism and are promising anticancer agents. Therefore, combining statins and immunotherapy, such as PD-1 inhibitors, may have surprising therapeutic effects, especially for HCC[110]. Previous research has shown that statins play a significant role in remodeling the immune microenvironment of HCC. For instance, simvastatin can inhibit the capillarization of liver sinusoidal endothelial cells to degrade the matrix environment and inhibit tumor progression by recruiting natural killer T cells. According to a recent study, simvastatin and PD-L1 antibody combination demonstrated a considerable therapeutic effect in HCC[111].
To summarize, the aberrant lipid metabolism of HCC provides energy for tumor growth, and regulates different immune cells to change their functional status, thus jointly affecting the progression of the tumor. Targeting HCC lipid metabolism to enhance tumor immunotherapy is a promising anticancer strategy (Table 1).
HCC has a high incidence rate and poor prognosis, making it a substantial medical burden globally. The search for efficient treatment strategies is ongoing. Researchers should focus their efforts on developing treatments targeting HCC's metabolic pathways. This is because many studies have shown significantly reprogrammed lipid metabolism in HCC cells compared with normal cells. Such metabolic abnormalities are related to the aberrant activation of key enzymes or related pathways of lipid metabolism. Drugs targeting several essential molecules involved in lipid metabolism have been widely studied for further treatment in HCC and have demonstrated promising therapeutic impacts. Sorafenib and lenvatinib are tyrosine kinase inhibitors widely studied and applied to clinical use. While their combination with ICIs such as PD-1 inhibitors has shown better therapeutic effects, it has not yet demonstrated satisfactory results[112]. Whether it is lipid metabolism, cholesterol metabolism or other lipid metabolism types, all these influence the efficacy of targeted therapy or immunotherapy for HCC; for example, blocking PPAR signal pathway-related molecules could improve the therapeutic effect of sorafenib[48], improving immune cell profiles and inhibiting HCC[103]. It will hence be an effective therapeutic approach to combine biological therapies targeting lipid metabolism with the existing targeted therapy and immunotherapy for HCC.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liang X, China; Ota Y, Japan; Xie Y, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64637] [Article Influence: 16159.3] [Reference Citation Analysis (176)] |
| 2. | Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1284] [Cited by in RCA: 1227] [Article Influence: 409.0] [Reference Citation Analysis (41)] |
| 3. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 2905] [Article Influence: 484.2] [Reference Citation Analysis (17)] |
| 4. | McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of Hepatocellular Carcinoma. Hepatology. 2021;73 Suppl 1:4-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 820] [Cited by in RCA: 1343] [Article Influence: 335.8] [Reference Citation Analysis (1)] |
| 5. | Reig M, Forner A, Rimola J, Ferrer-Fàbrega J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V, Salem R, Sangro B, Singal AG, Vogel A, Fuster J, Ayuso C, Bruix J. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J Hepatol. 2022;76:681-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2610] [Article Influence: 870.0] [Reference Citation Analysis (59)] |
| 6. | Luo XY, Wu KM, He XX. Advances in drug development for hepatocellular carcinoma: clinical trials and potential therapeutic targets. J Exp Clin Cancer Res. 2021;40:172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 141] [Article Influence: 35.3] [Reference Citation Analysis (0)] |
| 7. | Huang A, Yang XR, Chung WY, Dennison AR, Zhou J. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5:146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 495] [Article Influence: 99.0] [Reference Citation Analysis (0)] |
| 8. | Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. 2020;368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 1495] [Article Influence: 299.0] [Reference Citation Analysis (0)] |
| 9. | Sangineto M, Villani R, Cavallone F, Romano A, Loizzi D, Serviddio G. Lipid Metabolism in Development and Progression of Hepatocellular Carcinoma. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (1)] |
| 10. | Wang M, Han J, Xing H, Zhang H, Li Z, Liang L, Li C, Dai S, Wu M, Shen F, Yang T. Dysregulated fatty acid metabolism in hepatocellular carcinoma. Hepat Oncol. 2016;3:241-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 11. | Pascual G, Avgustinova A, Mejetta S, Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A, Hueto JA, Bescós C, Di Croce L, Benitah SA. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature. 2017;541:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 1034] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 12. | Garcia-Bermudez J, Baudrier L, Bayraktar EC, Shen Y, La K, Guarecuco R, Yucel B, Fiore D, Tavora B, Freinkman E, Chan SH, Lewis C, Min W, Inghirami G, Sabatini DM, Birsoy K. Squalene accumulation in cholesterol auxotrophic lymphomas prevents oxidative cell death. Nature. 2019;567:118-122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 304] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 13. | Chen Z, Chen L, Sun B, Liu D, He Y, Qi L, Li G, Han Z, Zhan L, Zhang S, Zhu K, Luo Y, Zhang N, Guo H. LDLR inhibition promotes hepatocellular carcinoma proliferation and metastasis by elevating intracellular cholesterol synthesis through the MEK/ERK signaling pathway. Mol Metab. 2021;51:101230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 14. | Cui Y, Liang S, Zhang S, Zhang C, Zhao Y, Wu D, Wang J, Song R, Yin D, Liu Y, Pan S, Liu X, Wang Y, Han J, Meng F, Zhang B, Guo H, Lu Z, Liu L. ABCA8 is regulated by miR-374b-5p and inhibits proliferation and metastasis of hepatocellular carcinoma through the ERK/ZEB1 pathway. J Exp Clin Cancer Res. 2020;39:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Moon SH, Huang CH, Houlihan SL, Regunath K, Freed-Pastor WA, Morris JP 4th, Tschaharganeh DF, Kastenhuber ER, Barsotti AM, Culp-Hill R, Xue W, Ho YJ, Baslan T, Li X, Mayle A, de Stanchina E, Zender L, Tong DR, D'Alessandro A, Lowe SW, Prives C. p53 Represses the Mevalonate Pathway to Mediate Tumor Suppression. Cell. 2019;176:564-580.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 322] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 16. | Sun H, Wang F, Huang Y, Wang J, Zhang L, Shen Y, Lin C, Guo P. Targeted inhibition of ACLY expression to reverse the resistance of sorafenib in hepatocellular carcinoma. J Cancer. 2022;13:951-964. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Lally JSV, Ghoshal S, DePeralta DK, Moaven O, Wei L, Masia R, Erstad DJ, Fujiwara N, Leong V, Houde VP, Anagnostopoulos AE, Wang A, Broadfield LA, Ford RJ, Foster RA, Bates J, Sun H, Wang T, Liu H, Ray AS, Saha AK, Greenwood J, Bhat S, Harriman G, Miao W, Rocnik JL, Westlin WF, Muti P, Tsakiridis T, Harwood HJ Jr, Kapeller R, Hoshida Y, Tanabe KK, Steinberg GR, Fuchs BC. Inhibition of Acetyl-CoA Carboxylase by Phosphorylation or the Inhibitor ND-654 Suppresses Lipogenesis and Hepatocellular Carcinoma. Cell Metab. 2019;29:174-182.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 282] [Article Influence: 47.0] [Reference Citation Analysis (0)] |
| 18. | Wang H, Zhou Y, Xu H, Wang X, Zhang Y, Shang R, O'Farrell M, Roessler S, Sticht C, Stahl A, Evert M, Calvisi DF, Zeng Y, Chen X. Therapeutic efficacy of FASN inhibition in preclinical models of HCC. Hepatology. 2022;76:951-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 19. | Liu HH, Xu Y, Li CJ, Hsu SJ, Lin XH, Zhang R, Chen J, Gao DM, Cui JF, Yang XR, Ren ZG, Chen RX. An SCD1-dependent mechanoresponsive pathway promotes HCC invasion and metastasis through lipid metabolic reprogramming. Mol Ther. 2022;30:2554-2567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 20. | Ioannou GN. The Role of Cholesterol in the Pathogenesis of NASH. Trends Endocrinol Metab. 2016;27:84-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 376] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 21. | Li N, Zhou ZS, Shen Y, Xu J, Miao HH, Xiong Y, Xu F, Li BL, Luo J, Song BL. Inhibition of the sterol regulatory element-binding protein pathway suppresses hepatocellular carcinoma by repressing inflammation in mice. Hepatology. 2017;65:1936-1947. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 22. | Huang B, Song BL, Xu C. Cholesterol metabolism in cancer: mechanisms and therapeutic opportunities. Nat Metab. 2020;2:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 555] [Article Influence: 111.0] [Reference Citation Analysis (0)] |
| 23. | Wei M, Nurjanah U, Herkilini A, Huang C, Li Y, Miyagishi M, Wu S, Kasim V. Unspliced XBP1 contributes to cholesterol biosynthesis and tumorigenesis by stabilizing SREBP2 in hepatocellular carcinoma. Cell Mol Life Sci. 2022;79:472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Dong L, Xue L, Zhang C, Li H, Cai Z, Guo R. HSP90 interacts with HMGCR and promotes the progression of hepatocellular carcinoma. Mol Med Rep. 2019;19:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Liu D, Wong CC, Fu L, Chen H, Zhao L, Li C, Zhou Y, Zhang Y, Xu W, Yang Y, Wu B, Cheng G, Lai PB, Wong N, Sung JJY, Yu J. Squalene epoxidase drives NAFLD-induced hepatocellular carcinoma and is a pharmaceutical target. Sci Transl Med. 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 204] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 26. | Tosi MR, Tugnoli V. Cholesteryl esters in malignancy. Clin Chim Acta. 2005;359:27-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 27. | Zhu Y, Gu L, Lin X, Zhou X, Lu B, Liu C, Li Y, Prochownik EV, Karin M, Wang F. P53 deficiency affects cholesterol esterification to exacerbate hepatocarcinogenesis. Hepatology. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 28. | Yang W, Bai Y, Xiong Y, Zhang J, Chen S, Zheng X, Meng X, Li L, Wang J, Xu C, Yan C, Wang L, Chang CC, Chang TY, Zhang T, Zhou P, Song BL, Liu W, Sun SC, Liu X, Li BL. Potentiating the antitumour response of CD8(+) T cells by modulating cholesterol metabolism. Nature. 2016;531:651-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 740] [Article Influence: 82.2] [Reference Citation Analysis (0)] |
| 29. | Peyrou M, Clément S, Maier C, Bourgoin L, Branche E, Conzelmann S, Kaddai V, Foti M, Negro F. PTEN protein phosphatase activity regulates hepatitis C virus secretion through modulation of cholesterol metabolism. J Hepatol. 2013;59:420-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Yue S, Li J, Lee SY, Lee HJ, Shao T, Song B, Cheng L, Masterson TA, Liu X, Ratliff TL, Cheng JX. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 2014;19:393-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 546] [Cited by in RCA: 676] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 31. | Ndiaye H, Liu JY, Hall A, Minogue S, Morgan MY, Waugh MG. Immunohistochemical staining reveals differential expression of ACSL3 and ACSL4 in hepatocellular carcinoma and hepatic gastrointestinal metastases. Biosci Rep. 2020;40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 44] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 32. | Yang H, Deng Q, Ni T, Liu Y, Lu L, Dai H, Wang H, Yang W. Targeted Inhibition of LPL/FABP4/CPT1 fatty acid metabolic axis can effectively prevent the progression of nonalcoholic steatohepatitis to liver cancer. Int J Biol Sci. 2021;17:4207-4222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 33. | Fujiwara N, Nakagawa H, Enooku K, Kudo Y, Hayata Y, Nakatsuka T, Tanaka Y, Tateishi R, Hikiba Y, Misumi K, Tanaka M, Hayashi A, Shibahara J, Fukayama M, Arita J, Hasegawa K, Hirschfield H, Hoshida Y, Hirata Y, Otsuka M, Tateishi K, Koike K. CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut. 2018;67:1493-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 153] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 34. | Huang J, Viswakarma N, Yu S, Jia Y, Bai L, Vluggens A, Cherkaoui-Malki M, Khan M, Singh I, Yang G, Rao MS, Borensztajn J, Reddy JK. Progressive endoplasmic reticulum stress contributes to hepatocarcinogenesis in fatty acyl-CoA oxidase 1-deficient mice. Am J Pathol. 2011;179:703-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 35. | Ma APY, Yeung CLS, Tey SK, Mao X, Wong SWK, Ng TH, Ko FCF, Kwong EML, Tang AHN, Ng IO, Cai SH, Yun JP, Yam JWP. Suppression of ACADM-Mediated Fatty Acid Oxidation Promotes Hepatocellular Carcinoma via Aberrant CAV1/SREBP1 Signaling. Cancer Res. 2021;81:3679-3692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 69] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 36. | Chen XF, Tian MX, Sun RQ, Zhang ML, Zhou LS, Jin L, Chen LL, Zhou WJ, Duan KL, Chen YJ, Gao C, Cheng ZL, Wang F, Zhang JY, Sun YP, Yu HX, Zhao YZ, Yang Y, Liu WR, Shi YH, Xiong Y, Guan KL, Ye D. SIRT5 inhibits peroxisomal ACOX1 to prevent oxidative damage and is downregulated in liver cancer. EMBO Rep. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 205] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 37. | Li J, Huang Q, Long X, Zhang J, Huang X, Aa J, Yang H, Chen Z, Xing J. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARα pathways. J Hepatol. 2015;63:1378-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 38. | Bakiri L, Hamacher R, Graña O, Guío-Carrión A, Campos-Olivas R, Martinez L, Dienes HP, Thomsen MK, Hasenfuss SC, Wagner EF. Liver carcinogenesis by FOS-dependent inflammation and cholesterol dysregulation. J Exp Med. 2017;214:1387-1409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 39. | Lin CY, Gustafsson JÅ. Targeting liver X receptors in cancer therapeutics. Nat Rev Cancer. 2015;15:216-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 136] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 40. | Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, Yi P, Tang L, Pan Q, Rao S, Liang J, Tang Y, Su M, Luo X, Yang Y, Shi Y, Wang H, Zhou Y, Liao Q. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 717] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 41. | Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, Musumeci A, Chiricozzi E, Trincavelli ML, Daniele S, Martini C, Gustafsson JA, Doglioni C, Feo SG, Leiva A, Ciampa MG, Mauri L, Sensi C, Prinetti A, Eberini I, Mora JR, Bordignon C, Steffensen KR, Sonnino S, Sozzani S, Traversari C, Russo V. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210:1711-1728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 180] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 42. | Baek AE, Yu YA, He S, Wardell SE, Chang CY, Kwon S, Pillai RV, McDowell HB, Thompson JW, Dubois LG, Sullivan PM, Kemper JK, Gunn MD, McDonnell DP, Nelson ER. The cholesterol metabolite 27 hydroxycholesterol facilitates breast cancer metastasis through its actions on immune cells. Nat Commun. 2017;8:864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 304] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 43. | Jin M, Yang Y, Dai Y, Cai R, Wu L, Jiao Y, Zhang Z, Yang H, Zhou Y, Tang L, Li L, Li Y. 27-Hydroxycholesterol is a specific factor in the neoplastic microenvironment of HCC that causes MDR via GRP75 regulation of the redox balance and metabolic reprogramming. Cell Biol Toxicol. 2022;38:311-324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 44. | Wang S, Yao Y, Wang X, Zheng G, Ouyang W, Chen W. 25-HC promotes hepatocellular carcinoma metastasis through up-regulation of TLR4 dependent FABP4. Am J Cancer Res. 2019;9:2140-2155. [PubMed] |
| 45. | Bougarne N, Weyers B, Desmet SJ, Deckers J, Ray DW, Staels B, De Bosscher K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr Rev. 2018;39:760-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 556] [Article Influence: 79.4] [Reference Citation Analysis (0)] |
| 46. | Mao Z, Feng M, Li Z, Zhou M, Xu L, Pan K, Wang S, Su W, Zhang W. ETV5 Regulates Hepatic Fatty Acid Metabolism Through PPAR Signaling Pathway. Diabetes. 2021;70:214-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 47. | Antonosante A, d'Angelo M, Castelli V, Catanesi M, Iannotta D, Giordano A, Ippoliti R, Benedetti E, Cimini A. The Involvement of PPARs in the Peculiar Energetic Metabolism of Tumor Cells. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Feng T, Wu T, Zhang Y, Zhou L, Liu S, Li L, Li M, Hu E, Wang Q, Fu X, Zhan L, Xie Z, Xie W, Huang X, Shang X, Yu G. Stemness Analysis Uncovers That The Peroxisome Proliferator-Activated Receptor Signaling Pathway Can Mediate Fatty Acid Homeostasis In Sorafenib-Resistant Hepatocellular Carcinoma Cells. Front Oncol. 2022;12:912694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 49. | Ma MKF, Lau EYT, Leung DHW, Lo J, Ho NPY, Cheng LKW, Ma S, Lin CH, Copland JA, Ding J, Lo RCL, Ng IOL, Lee TKW. Stearoyl-CoA desaturase regulates sorafenib resistance via modulation of ER stress-induced differentiation. J Hepatol. 2017;67:979-990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 131] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 50. | Fujii H. [PPARs-mediated intracellular signal transduction]. Nihon Rinsho. 2005;63:565-571. [PubMed] |
| 51. | Shueng PW, Chan HW, Lin WC, Kuo DY, Chuang HY. Orlistat Resensitizes Sorafenib-Resistance in Hepatocellular Carcinoma Cells through Modulating Metabolism. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 52. | Feng J, Lu PZ, Zhu GZ, Hooi SC, Wu Y, Huang XW, Dai HQ, Chen PH, Li ZJ, Su WJ, Han CY, Ye XP, Peng T, Zhou J, Lu GD. ACSL4 is a predictive biomarker of sorafenib sensitivity in hepatocellular carcinoma. Acta Pharmacol Sin. 2021;42:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 114] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 53. | Wang MD, Wang NY, Zhang HL, Sun LY, Xu QR, Liang L, Li C, Huang DS, Zhu H, Yang T. Fatty acid transport protein-5 (FATP5) deficiency enhances hepatocellular carcinoma progression and metastasis by reprogramming cellular energy metabolism and regulating the AMPK-mTOR signaling pathway. Oncogenesis. 2021;10:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 54. | Gao Q, Zhang G, Zheng Y, Yang Y, Chen C, Xia J, Liang L, Lei C, Hu Y, Cai X, Zhang W, Tang H, Chen Y, Huang A, Wang K, Tang N. SLC27A5 deficiency activates NRF2/TXNRD1 pathway by increased lipid peroxidation in HCC. Cell Death Differ. 2020;27:1086-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 55. | Chen CL, Uthaya Kumar DB, Punj V, Xu J, Sher L, Tahara SM, Hess S, Machida K. NANOG Metabolically Reprograms Tumor-Initiating Stem-like Cells through Tumorigenic Changes in Oxidative Phosphorylation and Fatty Acid Metabolism. Cell Metab. 2016;23:206-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 298] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 56. | Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell pluripotency. Cell Res. 2007;17:42-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 440] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 57. | Dickson I. NETs promote liver metastasis via CCDC25. Nat Rev Gastroenterol Hepatol. 2020;17:451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 58. | Deng H, Zhang J, Zheng Y, Li J, Xiao Q, Wei F, Han W, Xu X, Zhang Y. CCDC25 may be a potential diagnostic and prognostic marker of hepatocellular carcinoma: Results from microarray analysis. Front Surg. 2022;9:878648. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 59. | Leineweber CG, Pietzner A, Zhang IW, Blessin UB, Rothe M, Schott E, Schebb NH, Weylandt KH. Assessment of the Effect of Sorafenib on Omega-6 and Omega-3 Epoxyeicosanoid Formation in Patients with Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Yu Q, Dai W, Ji J, Wu L, Feng J, Li J, Zheng Y, Li Y, Cheng Z, Zhang J, Wu J, Xu X, Guo C. Sodium butyrate inhibits aerobic glycolysis of hepatocellular carcinoma cells via the c-myc/hexokinase 2 pathway. J Cell Mol Med. 2022;26:3031-3045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 61. | Saito K, Ikeda M, Kojima Y, Hosoi H, Saito Y, Kondo S. Lipid profiling of pre-treatment plasma reveals biomarker candidates associated with response rates and hand-foot skin reactions in sorafenib-treated patients. Cancer Chemother Pharmacol. 2018;82:677-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 62. | Yin F, Feng F, Wang L, Wang X, Li Z, Cao Y. SREBP-1 inhibitor Betulin enhances the antitumor effect of Sorafenib on hepatocellular carcinoma via restricting cellular glycolytic activity. Cell Death Dis. 2019;10:672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 63. | Supuran CT. Anti-obesity carbonic anhydrase inhibitors: challenges and opportunities. J Enzyme Inhib Med Chem. 2022;37:2478-2488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 38] [Reference Citation Analysis (0)] |
| 64. | Niture S, Gyamfi MA, Lin M, Chimeh U, Dong X, Zheng W, Moore J, Kumar D. TNFAIP8 regulates autophagy, cell steatosis, and promotes hepatocellular carcinoma cell proliferation. Cell Death Dis. 2020;11:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4696] [Article Influence: 939.2] [Reference Citation Analysis (2)] |
| 66. | Bensaad K, Favaro E, Lewis CA, Peck B, Lord S, Collins JM, Pinnick KE, Wigfield S, Buffa FM, Li JL, Zhang Q, Wakelam MJO, Karpe F, Schulze A, Harris AL. Fatty acid uptake and lipid storage induced by HIF-1α contribute to cell growth and survival after hypoxia-reoxygenation. Cell Rep. 2014;9:349-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 514] [Article Influence: 46.7] [Reference Citation Analysis (0)] |
| 67. | Pan L, Xiao H, Liao R, Chen Q, Peng C, Zhang Y, Mu T, Wu Z. Fatty acid binding protein 5 promotes tumor angiogenesis and activates the IL6/STAT3/VEGFA pathway in hepatocellular carcinoma. Biomed Pharmacother. 2018;106:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 68. | Huang X, Ma J, Xu J, Su Q, Zhao J. Simvastatin induces growth inhibition and apoptosis in HepG2 and Huh7 hepatocellular carcinoma cells via upregulation of Notch1 expression. Mol Med Rep. 2015;11:2334-2340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 69. | Feng J, Dai W, Mao Y, Wu L, Li J, Chen K, Yu Q, Kong R, Li S, Zhang J, Ji J, Wu J, Mo W, Xu X, Guo C. Simvastatin re-sensitizes hepatocellular carcinoma cells to sorafenib by inhibiting HIF-1α/PPAR-γ/PKM2-mediated glycolysis. J Exp Clin Cancer Res. 2020;39:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 70. | Zhang Z, Tan X, Luo J, Yao H, Si Z, Tong JS. The miR-30a-5p/CLCF1 axis regulates sorafenib resistance and aerobic glycolysis in hepatocellular carcinoma. Cell Death Dis. 2020;11:902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 69] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 71. | Chan LK, Ho DW, Kam CS, Chiu EY, Lo IL, Yau DT, Cheung ET, Tang CN, Tang VW, Lee TK, Wong CC, Chok KS, Chan AC, Cheung TT, Wong CM, Ng IO. RSK2-inactivating mutations potentiate MAPK signaling and support cholesterol metabolism in hepatocellular carcinoma. J Hepatol. 2021;74:360-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 72. | Yue X, Kong Y, Zhang Y, Sun M, Liu S, Wu Z, Gao L, Liang X, Ma C. SREBF2-STARD4 axis confers sorafenib resistance in hepatocellular carcinoma by regulating mitochondrial cholesterol homeostasis. Cancer Sci. 2023;114:477-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 73. | Zheng C, Zhu Y, Liu Q, Luo T, Xu W. Maprotiline Suppresses Cholesterol Biosynthesis and Hepatocellular Carcinoma Progression Through Direct Targeting of CRABP1. Front Pharmacol. 2021;12:689767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 74. | Kim YS, Lee YM, Oh TI, Shin DH, Kim GH, Kan SY, Kang H, Kim JH, Kim BM, Yim WJ, Lim JH. Emodin Sensitizes Hepatocellular Carcinoma Cells to the Anti-Cancer Effect of Sorafenib through Suppression of Cholesterol Metabolism. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 75. | Lee SH, Lee JH, Im SS. The cellular function of SCAP in metabolic signaling. Exp Mol Med. 2020;52:724-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 76. | Sinha RA, Bruinstroop E, Singh BK, Yen PM. Nonalcoholic Fatty Liver Disease and Hypercholesterolemia: Roles of Thyroid Hormones, Metabolites, and Agonists. Thyroid. 2019;29:1173-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 192] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 77. | Li D, Yao Y, Rao Y, Huang X, Wei L, You Z, Zheng G, Hou X, Su Y, Varghese Z, Moorhead JF, Chen Y, Ruan XZ. Cholesterol sensor SCAP contributes to sorafenib resistance by regulating autophagy in hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41:116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 78. | Suk FM, Wang YH, Chiu WC, Liu CF, Wu CY, Chen TL, Liao YJ. Secretory NPC2 Protein-Mediated Free Cholesterol Levels Were Correlated with the Sorafenib Response in Hepatocellular Carcinoma. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 79. | Liu M, Zhou R, Wu X, Xu X, Su M, Yang B. Clinicopathologic charcterization of sorafenib-induced endoplasmic reticulum stress in human liver cancer cells. J Physiol Pharmacol. 2018;69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 80. | Shao W, Zhu W, Lin J, Luo M, Lin Z, Lu L, Jia H, Qin L, Lu M, Chen J. Liver X Receptor Agonism Sensitizes a Subset of Hepatocellular Carcinoma to Sorafenib by Dual-Inhibiting MET and EGFR. Neoplasia. 2020;22:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 81. | Yuan J, Lv T, Yang J, Wu Z, Yan L, Shi Y, Jiang L. HDLBP Promotes Hepatocellular Carcinoma Proliferation and Sorafenib Resistance by Suppressing Trim71-dependent RAF1 Degradation. Cell Mol Gastroenterol Hepatol. 2023;15:307-325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 82. | Monajati M, Tavakoli S, Abolmaali SS, Yousefi G, Tamaddon A. Effect of PEGylation on assembly morphology and cellular uptake of poly ethyleneimine-cholesterol conjugates for delivery of sorafenib tosylate in hepatocellular carcinoma. Bioimpacts. 2018;8:241-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Hu B, Zou T, Qin W, Shen X, Su Y, Li J, Chen Y, Zhang Z, Sun H, Zheng Y, Wang CQ, Wang Z, Li TE, Wang S, Zhu L, Wang X, Fu Y, Ren X, Dong Q, Qin LX. Inhibition of EGFR Overcomes Acquired Lenvatinib Resistance Driven by STAT3-ABCB1 Signaling in Hepatocellular Carcinoma. Cancer Res. 2022;82:3845-3857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 84. | Mok EHK, Leung CON, Zhou L, Lei MML, Leung HW, Tong M, Wong TL, Lau EYT, Ng IOL, Ding J, Yun JP, Yu J, Zhu HL, Lin CH, Lindholm D, Leung KS, Cybulski JD, Baker DM, Ma S, Lee TKW. Caspase-3-Induced Activation of SREBP2 Drives Drug Resistance via Promotion of Cholesterol Biosynthesis in Hepatocellular Carcinoma. Cancer Res. 2022;82:3102-3115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 58] [Reference Citation Analysis (0)] |
| 85. | Corcoran RB, Scott MP. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc Natl Acad Sci U S A. 2006;103:8408-8413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 250] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 86. | Younis MA, Khalil IA, Elewa YHA, Kon Y, Harashima H. Ultra-small lipid nanoparticles encapsulating sorafenib and midkine-siRNA selectively-eradicate sorafenib-resistant hepatocellular carcinoma in vivo. J Control Release. 2021;331:335-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 87. | Lu H, Zhou L, Zuo H, Le W, Hu J, Zhang T, Li M, Yuan Y. Overriding sorafenib resistance via blocking lipid metabolism and Ras by sphingomyelin synthase 1 inhibition in hepatocellular carcinoma. Cancer Chemother Pharmacol. 2021;87:217-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 88. | Beljanski V, Lewis CS, Smith CD. Antitumor activity of sphingosine kinase 2 inhibitor ABC294640 and sorafenib in hepatocellular carcinoma xenografts. Cancer Biol Ther. 2011;11:524-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 89. | Mokdad AA, Zhu H, Beg MS, Arriaga Y, Dowell JE, Singal AG, Yopp AC. Efficacy and Safety of Bavituximab in Combination with Sorafenib in Advanced Hepatocellular Carcinoma: A Single-Arm, Open-Label, Phase II Clinical Trial. Target Oncol. 2019;14:541-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 90. | Abushawish KYI, Soliman SSM, Giddey AD, Al-Hroub HM, Mousa M, Alzoubi KH, El-Huneidi W, Abu-Gharbieh E, Omar HA, Elgendy SM, Bustanji Y, Soares NC, Semreen MH. Multi-Omics Analysis Revealed a Significant Alteration of Critical Metabolic Pathways Due to Sorafenib-Resistance in Hep3B Cell Lines. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 91. | Zhong H, Xiao M, Zarkovic K, Zhu M, Sa R, Lu J, Tao Y, Chen Q, Xia L, Cheng S, Waeg G, Zarkovic N, Yin H. Mitochondrial control of apoptosis through modulation of cardiolipin oxidation in hepatocellular carcinoma: A novel link between oxidative stress and cancer. Free Radic Biol Med. 2017;102:67-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 92. | Wang X, Qiu Z, Dong W, Yang Z, Wang J, Xu H, Sun T, Huang Z, Jin J. S1PR1 induces metabolic reprogramming of ceramide in vascular endothelial cells, affecting hepatocellular carcinoma angiogenesis and progression. Cell Death Dis. 2022;13:768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 93. | Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3965] [Cited by in RCA: 4183] [Article Influence: 380.3] [Reference Citation Analysis (0)] |
| 94. | Liu J, Sun B, Guo K, Yang Z, Zhao Y, Gao M, Yin Z, Jiang K, Dong C, Gao Z, Ye M, Liu J, Wang L. Lipid-related FABP5 activation of tumor-associated monocytes fosters immune privilege via PD-L1 expression on Treg cells in hepatocellular carcinoma. Cancer Gene Ther. 2022;29:1951-1960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 95. | Murai H, Kodama T, Maesaka K, Tange S, Motooka D, Suzuki Y, Shigematsu Y, Inamura K, Mise Y, Saiura A, Ono Y, Takahashi Y, Kawasaki Y, Iino S, Kobayashi S, Idogawa M, Tokino T, Hashidate-Yoshida T, Shindou H, Miyazaki M, Imai Y, Tanaka S, Mita E, Ohkawa K, Hikita H, Sakamori R, Tatsumi T, Eguchi H, Morii E, Takehara T. Multiomics identifies the link between intratumor steatosis and the exhausted tumor immune microenvironment in hepatocellular carcinoma. Hepatology. 2023;77:77-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 102] [Article Influence: 51.0] [Reference Citation Analysis (0)] |
| 96. | Gualdoni GA, Mayer KA, Göschl L, Boucheron N, Ellmeier W, Zlabinger GJ. The AMP analog AICAR modulates the Treg/Th17 axis through enhancement of fatty acid oxidation. FASEB J. 2016;30:3800-3809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 95] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 97. | Chen L, Zhou Q, Liu J, Zhang W. CTNNB1 Alternation Is a Potential Biomarker for Immunotherapy Prognosis in Patients With Hepatocellular Carcinoma. Front Immunol. 2021;12:759565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 98. | Shen S, Khatiwada S, Behary J, Kim R, Zekry A. Modulation of the Gut Microbiome to Improve Clinical Outcomes in Hepatocellular Carcinoma. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 99. | Kumagai S, Togashi Y, Kamada T, Sugiyama E, Nishinakamura H, Takeuchi Y, Vitaly K, Itahashi K, Maeda Y, Matsui S, Shibahara T, Yamashita Y, Irie T, Tsuge A, Fukuoka S, Kawazoe A, Udagawa H, Kirita K, Aokage K, Ishii G, Kuwata T, Nakama K, Kawazu M, Ueno T, Yamazaki N, Goto K, Tsuboi M, Mano H, Doi T, Shitara K, Nishikawa H. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat Immunol. 2020;21:1346-1358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 545] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 100. | Ringel AE, Drijvers JM, Baker GJ, Catozzi A, García-Cañaveras JC, Gassaway BM, Miller BC, Juneja VR, Nguyen TH, Joshi S, Yao CH, Yoon H, Sage PT, LaFleur MW, Trombley JD, Jacobson CA, Maliga Z, Gygi SP, Sorger PK, Rabinowitz JD, Sharpe AH, Haigis MC. Obesity Shapes Metabolism in the Tumor Microenvironment to Suppress Anti-Tumor Immunity. Cell. 2020;183:1848-1866.e26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 475] [Article Influence: 95.0] [Reference Citation Analysis (0)] |
| 101. | Zhu XR, Zhu JQ, Chen YF, Liu YY, Lu JJ, Sun J, Peng SQ, Chen MB, Du YP. Bioinformatics analysis and experimental verification of the prognostic and biological significance mediated by fatty acid metabolism related genes for hepatocellular carcinoma. Front Oncol. 2022;12:972744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 102. | Cheng YH, Ko YC, Ku HJ, Huang CC, Yao YC, Liao YT, Chen YT, Huang SF, Huang LR. Novel Paired Cell Lines for the Study of Lipid Metabolism and Cancer Stemness of Hepatocellular Carcinoma. Front Cell Dev Biol. 2022;10:821224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 103. | Brown ZJ, Fu Q, Ma C, Kruhlak M, Zhang H, Luo J, Heinrich B, Yu SJ, Zhang Q, Wilson A, Shi ZD, Swenson R, Greten TF. Carnitine palmitoyltransferase gene upregulation by linoleic acid induces CD4(+) T cell apoptosis promoting HCC development. Cell Death Dis. 2018;9:620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 104. | Chekaoui A, Ertl HCJ. PPARα Agonist Fenofibrate Enhances Cancer Vaccine Efficacy. Cancer Res. 2021;81:4431-4440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 105. | Kidani Y, Elsaesser H, Hock MB, Vergnes L, Williams KJ, Argus JP, Marbois BN, Komisopoulou E, Wilson EB, Osborne TF, Graeber TG, Reue K, Brooks DG, Bensinger SJ. Sterol regulatory element-binding proteins are essential for the metabolic programming of effector T cells and adaptive immunity. Nat Immunol. 2013;14:489-499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 419] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 106. | Molnár E, Swamy M, Holzer M, Beck-García K, Worch R, Thiele C, Guigas G, Boye K, Luescher IF, Schwille P, Schubert R, Schamel WW. Cholesterol and sphingomyelin drive ligand-independent T-cell antigen receptor nanoclustering. J Biol Chem. 2012;287:42664-42674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 107. | Schmidt NM, Wing PAC, Diniz MO, Pallett LJ, Swadling L, Harris JM, Burton AR, Jeffery-Smith A, Zakeri N, Amin OE, Kucykowicz S, Heemskerk MH, Davidson B, Meyer T, Grove J, Stauss HJ, Pineda-Torra I, Jolly C, Jury EC, McKeating JA, Maini MK. Targeting human Acyl-CoA:cholesterol acyltransferase as a dual viral and T cell metabolic checkpoint. Nat Commun. 2021;12:2814. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 70] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 108. | Pan J, Zhang Q, Palen K, Wang L, Qiao L, Johnson B, Sei S, Shoemaker RH, Lubet RA, Wang Y, You M. Potentiation of Kras peptide cancer vaccine by avasimibe, a cholesterol modulator. EBioMedicine. 2019;49:72-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 109. | Hao M, Hou S, Li W, Li K, Xue L, Hu Q, Zhu L, Chen Y, Sun H, Ju C, Zhang C. Combination of metabolic intervention and T cell therapy enhances solid tumor immunotherapy. Sci Transl Med. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 109] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 110. | Geh D, Anstee QM, Reeves HL. NAFLD-Associated HCC: Progress and Opportunities. J Hepatocell Carcinoma. 2021;8:223-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 111. | Yu Z, Guo J, Liu Y, Wang M, Liu Z, Gao Y, Huang L. Nano delivery of simvastatin targets liver sinusoidal endothelial cells to remodel tumor microenvironment for hepatocellular carcinoma. J Nanobiotechnology. 2022;20:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 71] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 112. | Liu CY, Chen KF, Chen PJ. Treatment of Liver Cancer. Cold Spring Harb Perspect Med. 2015;5:a021535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 257] [Article Influence: 25.7] [Reference Citation Analysis (0)] |