Published online Apr 15, 2023. doi: 10.4251/wjgo.v15.i4.596
Peer-review started: November 19, 2022
First decision: January 9, 2023
Revised: February 12, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: April 15, 2023
Processing time: 143 Days and 23.7 Hours
Many digestive system malignant tumors are characterized by high incidence and mortality rate. Increasing evidence has revealed that the tumor microenvironment (TME) is involved in cancer initiation and tumor progression. Tumor-associated macrophages (TAMs) are a predominant constituent of the TME, and participate in the regulation of various biological behaviors and influence the prognosis of digestive system cancer. TAMs can be mainly classified into the antitumor M1 phenotype and protumor M2 phenotype. The latter especially are crucial drivers of tumor invasion, growth, angiogenesis, metastasis, immunosuppression, and resistance to therapy. TAMs are of importance in the occurrence, development, diagnosis, prognosis, and treatment of common digestive system malignant tumors. In this review, we summarize the role of TAMs in common digestive system malignant tumors, including esophageal, gastric, colorectal, pancreatic and liver cancers. How TAMs promote the development of tumors, and how they act as potential therapeutic targets and their clinical applications are also described.
Core Tip: This review summarizes the role of tumor-associated macrophages (TAMs) in common digestive system malignant tumors, including esophageal, gastric, colorectal, pancreatic and liver cancers. How TAMs promote the development of tumors, and how they act as potential therapeutic targets and their clinical applications are also described.
- Citation: Shen Y, Chen JX, Li M, Xiang Z, Wu J, Wang YJ. Role of tumor-associated macrophages in common digestive system malignant tumors. World J Gastrointest Oncol 2023; 15(4): 596-616
- URL: https://www.wjgnet.com/1948-5204/full/v15/i4/596.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i4.596
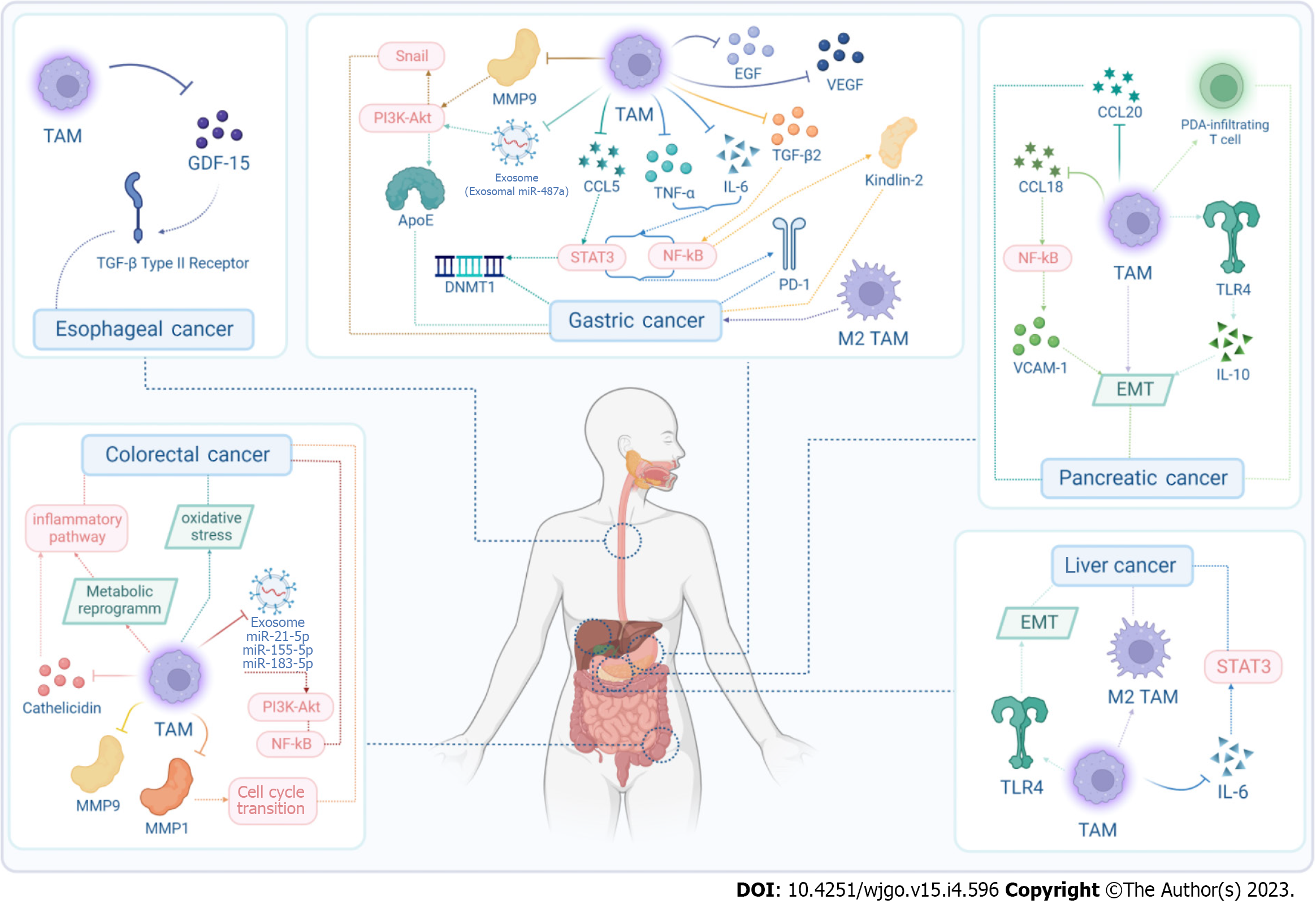
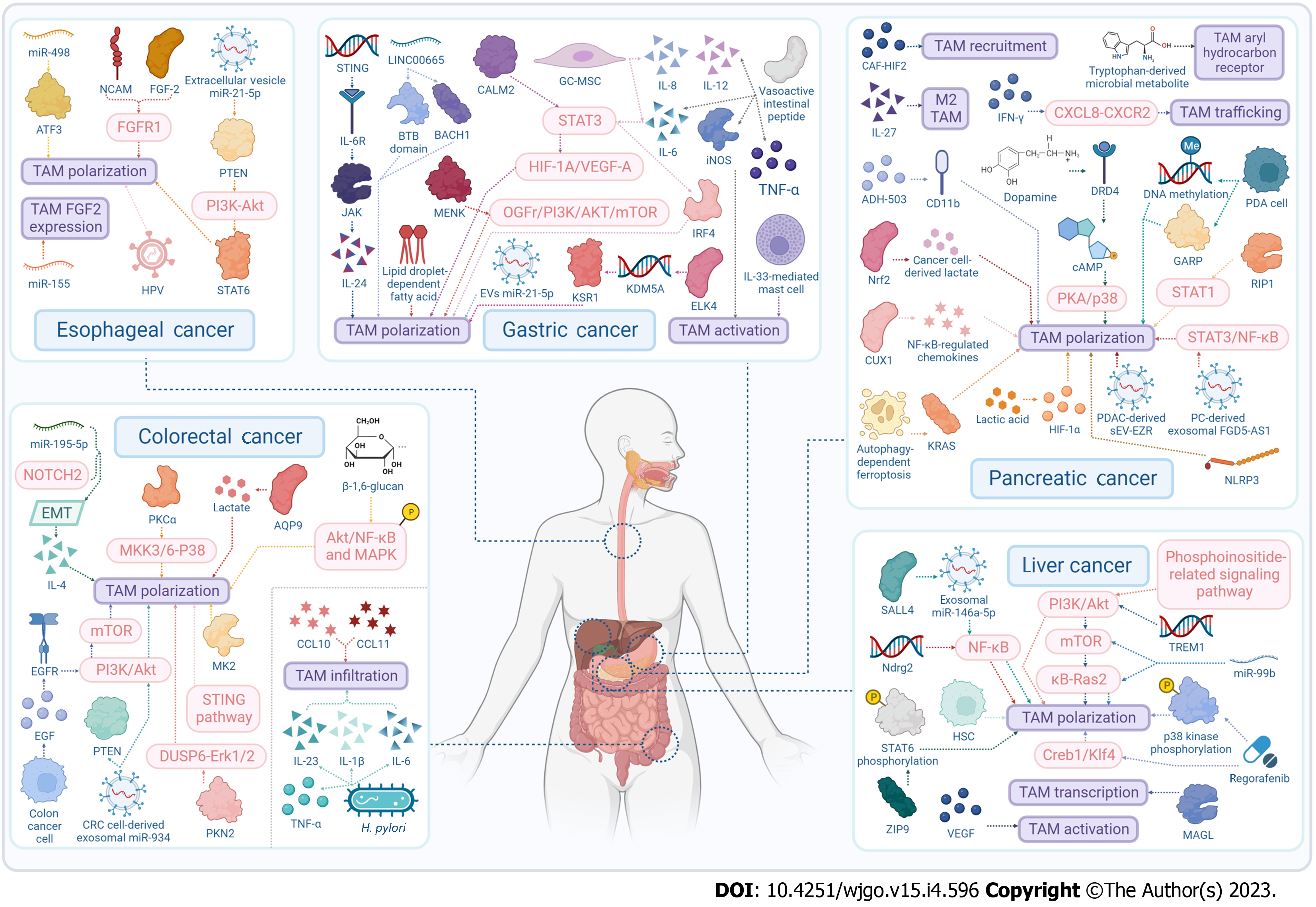
Many digestive system malignant tumors have high incidence and mortality rate, including esophageal cancer (EC), gastric cancer (GC), colorectal cancer (CRC), pancreatic cancer (PC), and liver cancer (LC). There is increasing evidence that the tumor microenvironment (TME), which encompasses the tumor tissue structure comprising stromal cells, is involved in cancer initiation and tumor progression[1-4]. Tumor-associated macrophages (TAMs) as a predominant constituent of the TME, are a special type of macrophages generated by circulating monocytes and recruited into the TME[5]. TAMs are categorized into two functionally contrasting subtypes: Classically activated M1 macrophages and alternatively activated M2 macrophages. TAMs are extensively present in various tumors[6,7], which can participate in the regulation of various biological behaviors and influence the prognosis of digestive system cancers. In this review, we summarize the role of TAMs in EC, GC, CRC, PC and LC. More specifically, we also described how TAMs promote the development of tumors (Figure 1), and how they act as potential therapeutic targets (Figure 2) and their clinical applications.
It was originally believed that macrophages in the TME originated from circulating monocyte precursors in the bone marrow (BM), under the influence of tissue microenvironmental signals. However, other studies suggested a minor splenic[8] and early embryonic[9] contribution to the main proportion of TAMs derived from the BM, validating the coexistence of macrophages with different origins.
In accordance with the commonly accepted theory[10], TAMs can be primarily categorized into the antitumor M1 phenotype (classically activated state) and the protumor M2 phenotype (alternatively activated state), which have contrasting functions. The former has the capacity to remove tumor cells[11] and facilitate tumor cell destruction via initiating cytokine[12] production within the TME and recruitment of immunostimulating leukocytes and tumor cells phagocytosis. On the contrary, M2 macrophages have a central role in propagating tumorigenesis. The function of M2 macrophages includes the removal of debris, promotion of angiogenesis, tissue reconstruction, and injury repair, as well as facilitation of tumorigenesis and progression[6].
Upon recruited to the TME by tumor-secreted stimuli, TAMs undergo M1- or M2-like activation in response[13]. However, as a result of their remarkable plasticity, TAMs can reversibly respond to specific stimuli in the TME and switch from one phenotype to another[14], transition between antitumor M1-like and protumor M2-like phenotypes amidst the immune response. Colegio et al[15] have reported that the hypoxic TME can induce M2-type polarization through the production of tumor-derived lactic acid and hypoxia-inducible factor (HIF)-1α[15]. Many other cytokines can govern M2 polarization, including interleukin (IL)-21[16] and IL-33[17]. TAM plasticity highlights that the reprogramming of TAMs is an attractive potential therapeutic target to inhibit tumor progression.
M2 TAMs are crucial drivers of tumor invasion, growth, angiogenesis, metastasis, immunosuppression, and resistance to therapy[18]. TAMs can propagate tumor progression through upregulation of proteolytic enzymes[19] and in a manner dependent on tumor necrosis factor (TNF)-α and matrix metalloproteinases (MMPs)[20]. TAMs can also express a number of soluble factors[13] and major inflammatory mediators[21], stimulating tumor cell proliferation and survival.
TAMs act in various microenvironments, such as invasive regions where they facilitate cancer cell movement, stromal and perivascular regions where they promote metastasis, and avascular and perivascular regions where hypoxic TAMs induce angiogenesis[18].
Research advances in cancer immunology have led to multifarious strategies for modulation of TAMs for therapeutic applications[22], including strategies to deplete TAMs, inhibit TAM recruitment, influence TAM polarization, and target TAM receptors. M2 TAMs can also contribute to evaluating prognosis, which has been proven to be correlated with poorer outcomes in almost all digestive system malignant tumors[23]. On the contrary, increasing levels of M1 TAMs indicate better prognosis[24], resulting in emerging therapeutic strategies to remove M2 TAMs or alter TAM phenotypes, which can facilitate promising therapeutic benefits.
Numerous studies have shown that TAMs can directly and indirectly dampen the antitumor activity of cytotoxic T lymphocytes (CTLs)[25] and tumor-infiltrating T cells[26] in various tumors[27,28]. Underlying this functional role are molecular mechanisms that initially involve immune checkpoint engagement, which is initially mediated through the expression of molecules like programmed cell death 1 (PD-1) ligand 1 (PD-L1)[29]. In addition, the production of inhibitory cytokines and transcription factors are also implicated in the suppression progress, which mainly include IL-10[28], interferon (IFN)-γ[30], transforming growth factor (TGF)-β[31,32] and HIF-1α[26]. Metabolic activities of TAMs, concerning the consumption of metabolites such as L-arginine[33] and generation of reactive oxygen species, also contribute to suppression of T-cell responses that is either specific to or indep
Specifically in digestive system cancers, TAMs are similarly thought to have mutual modulation with T cells, including but not limited to blocking the recruitment and priming of T cells and resulting in T-cell exclusion within the TME. In GC, TAMs and LAMP3+ dendritic cells (DCs) are involved in mediating T-cell activity and form intercellular interaction hubs with tumor-associated stromal cells[37]. IL-10+ TAM infiltration yielded an immunoevasive TME featured by Treg cell infiltration and CD8+ T-cell dysfunction[38]. In CRC, C1q+ TAMs modulate tumor-infiltrating CD8+ T cells by expressing multiple immunomodulatory ligands in an RNA N6-methyladenosine (m6A)-dependent manner. There is evidence that compensation between TAMs and Forkhead box (Fox)p3+ Treg cells promote tumor progression by limiting antitumor immunity. Decreasing colony-stimulating factor (CSF)1-dependent TAMs led to heightened CD8+ T-cell against tumors, although the impact on tumor growth was restricted by a compensatory rise in Foxp3+ Treg cells[39]. In pancreatic ductal adenocarcinoma (PDAC), receptor-interacting serine/threonine protein kinase (RIP)1 inhibition in TAMs resulted in CTL activation and T helper (Th) cell differentiation toward a mixed Th1/Th17 phenotype[40]. By targeting proliferating tumor-infiltrating macrophages, the infiltration of CD8+ CTL and the spatial redistribution of CD8+ T cells within tumors could be escalated[41]. TAMs are critical regulators in orchestrating epigenetic profile of PDAC-infiltrating T cells towards a protumoral phenotype[42]. In hepatocellular carcinoma (HCC), HCC-derived exosomes instigate macrophages to heighten IFN-γ and TNF-α expression in T cells, while upregulating the expression of inhibitory receptors PD-1 and cytotoxic T-lymphocyte-associated antigen-4[43]. These findings collectively demonstrate that TAMs are central drivers of immunosuppressive TME within digestive system tumors by suppressing T cell mobilization and performance.
TAMs can promote development of EC: TAMs can facilitate a variety of protumorigenic mechanisms in EC (Figure 1 and Table 1). In esophageal squamous cell carcinoma (ESCC), growth differentiation factor 15 derived from TAMs promoted cancer progression via TGF-β type II receptor activation[44].
| Diseases | Factors | Functions | Mechanism | Ref. |
| EC | GDF-15 derived from TAMs | Promoting progression of ESCC | Activating TGF-β type II receptor | [44] |
| GC | TAMs | Promoting peritoneal dissemination of GC | Secreting IL-6 | [53] |
| TAMs | Promoting progression in GC | Polarizing to the M2 phenotype | [54] | |
| TAMs | Supporting peritoneal metastasis | Producing EGF and VEGF | [55] | |
| TNF-alpha and IL-6 secreted by TAMs | Promoting proliferation of GC cells | Activating the NF-κB and STAT3 signaling pathway to regulate PD-L1 expression | [56] | |
| TGFβ2 secreted by TAMs | Promoting the invasion of GC cells | Regulating Kindlin-2 through NF-κB | [57] | |
| CCL5 secreted by TAMs | Promoting the proliferation, invasion and metastasis of GC cells | Stat3 signaling pathway | [58] | |
| TAMs | Influencing omental milky spots and lymph nodes micrometastasis | COX-2/PGE-2/TGF-β/VEGF signal pathways | [59] | |
| TAMs | Promoting epigenetic silencing of tumor suppressor gelsolin, and silence GSN | Upregulation of DNMT1 by CCL5/CCR5/STAT3 signaling | [60] | |
| TAMs | Inducing invasion and poor prognosis in GC | Promoting MMP9 expression | [63] | |
| MMP-9 secreted by TAMs | Suppressing distant metastasis in GC | PI3K/AKT/Snail dependent pathway | [64] | |
| Exosomal miR-487a derived from TAMs | Promoting the proliferation and tumorigenesis in GC | - | [65] | |
| M2 macrophage-derived exosomes | Remodeling the cytoskeleton-supporting migration in recipient GC cells | Mediating an intercellular transfer of ApoE-activating PI3K-Akt signaling pathway | [66] | |
| CRC | TAMs | Potentiating the angiogenic capacity of the TME | Oxidative stress-dependent manner | [91] |
| Metabolic reprogramming in TAMs | Building a bridge between metabolic dysfunction and the onset and progression of CRC | Inflammatory pathways | [92] | |
| M2 macrophage-derived exosomes | Promoting CRC cells’ migration and invasion | MiR-21-5p and miR-155-5p | [93] | |
| Exosomal miR-183-5p Shuttled by M2 TAMs | Promoting the development of colon cancer | THEM4 mediated PI3K/AKT and NF-κB pathways | [94] | |
| MMP1 derived from TAMs | Facilitating colon cancer cell proliferation | Accelerating cell cycle transition from G0/G1 to S and G2/M phase | [95] | |
| M2 TAMs | Inducing colon cancer cell invasion | MMP-9 | [96] | |
| Cathelicidin secreted by TAMs | Promoting the growth of CRC | Recruiting inflammatory cells | [97] | |
| PC | Intraperitoneal TAMs | Promoting peritoneal dissemination and chemoresistance | Inducing EMT | [123] |
| M2 TAMs | Promoting EMT | TLR4/IL-10 signaling | [124] | |
| TAMs | Promoting progression and the Warburg effect | CCL18/NF-Kb/VCAM-1 pathway | [125] | |
| CCL20 secreted by M2 TAMs | Promoting the migration, epithelial-mesenchymal transition, and invasion of pancreatic cancer cells | - | [126] | |
| TAMs | Orchestrating functions PDA-infiltrating T cells | Odulating PDA-infiltrating T cells epigenetic profile towards a pro-tumoral phenotype | [42] | |
| LC | TAMs | Promoting LCSLC self-renewal capability and carcinogenicity | M2 polarization | [151] |
| TAMs | Promoting EMT of Hep3B hepatoma cells | TLR4 | [153] | |
| IL-6 secreted by TAMs | Promoting expansion of these CSCs and tumorigenesis | STAT3 signaling | [154] |
TAMs act as potential therapeutic targets for EC: TAMs might be potential therapeutic targets to prevent EC progression (Table 2). There is evidence supporting that miR-498 inhibits autophagy and M2-like polarization of TAMs in EC via inhibiting murine double minute 2-mediated degradation of activated transcription factor-3[45]. miR-155-regulated fibroblast growth factor (FGF)-2 expression from TAMs inhibited EC cell invasion, migration and proliferation, and blocked vasculature formation[46]. EC-derived extracellular vesicle miR-21-5p upregulated ESCC-derived EVs-miR-21-5p through the phosphatase and tensin homolog (PTEN)/AKT/signal transducers and activator of transcription (STAT)6 pathway, thus disorganizing macrophage polarization through, and contributing to epithelial mesenchymal transition (EMT) of ESCC cells via TGF-β/Smad2 signaling[47]. PTEN induced M2 TAM polarization through the phosphoinositide 3-kinase (PI3K)/AKT cascade, thus enhancing the malignant behavior of tumor-associated vascular endothelial cells and promoting ESCC angiogenesis[48]. Neural-cell-adhesion-molecule- and FGF2-mediated FGFR1 signaling in the TME of EC regulated the survival and migration of TAMs and cancer cells[49]. Human papillomavirus 16 infection can promote an M2 macrophage phenotype, contributing to the invasion and metastasis of ESCC[50].
| Diseases | Factors | Types | Targets | Functions | Mechanism | Ref. |
| EC | MiR-498 | MiRNA | Inhibiting autophagy and M2-like polarization of TAMs in esophageal cancer | - | Inhibiting MDM2-mediated ATF3 degradation | [45] |
| MiR-155 | MiRNA | Regulating TAMs FGF2 expression | Suppressing EC cell proliferation, migration, invasion and inhibiting vasculature formation | - | [46] | |
| EC-Derived Extracellular Vesicle miR-21-5p | MiRNA | Disorganizing macrophages polarization | Contributing to EMT of ESCC cells via | PTEN/AKT/STAT6 pathway | [47] | |
| PTEN | Protein | Inducing M2 TAMs polarization | Enhancing the malignant behavior of TECs, promoting ESCC angiogenesis | Activating the PI3K/AKT signaling pathway | [48] | |
| NCAM- and FGF-2-mediated FGFR1 signaling | Signaling | Regulating the survival and migration of TAMs and cancer cells | - | NCAM knockdown via a suppression of PI3K-Akt and FGFR1 signaling, and rhFGF-2 -through FGFR1 signaling | [49] | |
| HR-HPV; HPV16 infection | Virus | Promoting M2 macrophages phenotype | Promoting the invasion and metastasis of esophageal squamous cell carcinoma | - | [50] | |
| GC | STING | Gene | Promoting TAMs polarizing into pro-inflammatory subtype | Inducing apoptosis of GC cells | IL6R-JAK-L24pathway | [67] |
| LINC00665 | LncRNA | Activating Wnt1 and mediating TAMs M2 polarization | - | Interacting with BTB domain and BACH1 | [68] | |
| CALM2 | Protein | Polarizing TAMs | Facilitating angiogenesis and metastasis of GC | STAT3/HIF-1A/VEGF-A | [69] | |
| MENK | Protein | Skewing macrophages toward M2 phenotype from M1 phenotype | Inducing cells apoptosis | OGFr/PI3K/AKT/Mtor signaling pathway | [70] | |
| ELK4 | Transcription factor | Promoting M2 polarization | Promoting the development of GC | Reducing the PJA2-dependent inhibition of KSR1 by transcriptional activation of KDM5A | [71] | |
| IL-6 | Cytokine | Polarizing the Mφs | Promoting tumor invasion | IL-6/STAT3/IRF4 signaling pathway | [72] | |
| GC-MSCs | Cell | Promoting M2 polarization | Promoting metastasis and EMT in GC | Secreting IL-6 and IL-8 | [73] | |
| Vasoactive intestinal peptide | Protein | Repressing activation of TAMs | - | Regulating TNFα, IL-6, IL-12 and Inos | [74] | |
| Lipid droplet-dependent fatty acid | Fatty acid | Controlling the immune suppressive phenotype of TAMs | - | - | [75] | |
| MiR-151-3p derived from GC exosomes | Exosome | Inducing M2-phenotype polarization | Promoting tumor growth | - | [76] | |
| IL-33-mediated mast cell | Cell | Mobilizing macrophages | Promoting GC | - | [77] | |
| CRC | PKN2 | Protein | Inhibiting M2 phenotype polarization | - | DUSP6-Erk1/2 pathway | [98] |
| AQP9 | Protein | Stimulating M2-like polarization | Promoting colon cancer progression | Transporting lactate | [99] | |
| PKCα | Tumor suppressor | Promoting M1 macrophages polarization | - | MKK3/6-P38 signaling pathway | [100] | |
| MK2 | Protein | Promoting polarization of protumorigenic TAMs | - | - | [101] | |
| MiR-195-5p/NOTCH2-mediated EMT | - | Affecting M2-like TAMs polarization | - | Modulating IL-4 secretion | [102] | |
| CRC cell-derived exosomal miR-934 | Exosome | Inducing M2 macrophages polarization | - | Downregulating PTEN expression and activate the PI3K/AKT signaling pathway | [103] | |
| Stimulator of STING pathway | Signaling pathway | Activating reprogramed TAMs toward the M1 phenotype | - | - | [104] | |
| Colon cancer cell | Cell | Promoting M2 polarization of TAMs | - | Secreting EGF; EGFR/PI3K/AKT/Mtor pathway | [105] | |
| CXCL10 and CXCL11 | Chemokine | Inducing the infiltration of TAMs | Leading to the poor prognosis of CRC | - | [106] | |
| β-1, 6-glucan | Organic compound | Reseting TAMs from M2-like to M1-like phenotype | Inhibiting the viability of colon cancer cells | Increasing the phosphorylation of Akt/NF-κB and MAPK | [107] | |
| H. pylori infection | Becteria | Reducing the infiltration of M2-like TAMs | - | Downregulating TNF-α, IL-1β, IL-6 and IL-23 | [108] | |
| PC | Autophagy-dependent ferroptosis | - | Driving TAMs polarization | - | Releasing and uptaking of oncogenic KRAS protein | [127] |
| RIP1 | Kinase | Reprogramming TAMs | - | STAT1-dependent manner | [40] | |
| Deletion of CAF-HIF2 | Protein | Decreasing the intratumoral recruitment of immunosuppressive M2 macrophages | - | - | [128] | |
| ADH-503 | Small-molecule agonist | Leading to the repolarization of TAMs | - | Partial activation of CD11b | [129] | |
| NLRP3 | Inflammasome | Regulating TAMs polarization | Enhancing lung metastasis of PDAC | - | [130] | |
| IL-27 | Cytokine | Targeting M2 TAMs | Dampening the proliferation, migration and metastasis of PC cells | - | [131] | |
| IFN-γ | Chemokines | Preventing trafficking of TAMs | Improving the efficacy of PD1 blockade therapy in PC | Blocking the CXCL8-CXCR2 axis | [132] | |
| PC-derived exosomal FGD5-AS1 | Exosome | Stimulating M2 macrophages polarization | Promoting proliferation and migration of PC cell | Activating STAT3/NF-κB pathway | [133] | |
| PDAC-derived Sev-EZR | Exosome | Modulating TAMs polarization | Promoting PDAC metastasis | - | [134] | |
| CUX1 | Transcription factor | Mediating M1 polarization | Inhibiting angiogenesis and tumor progression | Downregulating several NF-κB -regulated chemokines | [135] | |
| Tryptophan-derived microbial metabolite | Metabolite | Activating the aryl hydrocarbon receptor in TAMs | Suppressing anti-tumor immunity | - | [136] | |
| Nrf2 | Transcription factor | Stimulating M2 macrophages polarization | Promoting EMT | Activating cancer cell-derived lactate | [137] | |
| Lactic acid | Organic compound | Redistributing M2TAMs subsets | Upregulating PDL1 to assist tumor immune escape | HIF1α signaling pathway | [138] | |
| Activation of DRD4 by DA | Protein | Suppressing the tumor-promoting inflammation of TAMs | - | Decreasing Camp; inhibit the activation of PKA/p38 signal pathway | [139] | |
| PDA cells | Cell | Reprogramming M1-like macrophages | - | GARP-dependent and DNA methylation-mediated mechanism | [140] | |
| LC | Ndrg2 | Gene | Influencing TAMs polarization | - | NF-κB pathway | [155] |
| TREM1knockdown | Gene | Shifting M2 macrophages towards a M1 phenotype | - | Inhibiting PI3K/AKT/Mtor activation | [156] | |
| MiR-99b | MiRNA | Promoting M1 while suppressing M2 macrophages polarization | - | Targeting κB -Ras2 and/or mTOR | [157] | |
| MAGL | Kinase | Promoting the transcription and secretion of inflammatory factors in TAMs | - | - | [158] | |
| - | - | Blocking triggering receptor expressed on myeloid cells-1-positive TAMs | Reversing immunosuppression and anti-PD-L1 resistance in LC | - | [159] | |
| Regorafenib | Multikinase inhibitors | Reversing M2 polarization | - | Suppressing p38 kinase phosphorylation and downregulating Creb1/Klf4 activity in BMDMs | [160] | |
| ZIP9 | Protein | Promoting M2 macrophages polarization | - | Enhancing phosphorylated STAT6 | [161] | |
| Phosphoinositide-related signaling pathway | Signaling pathway | Reprogramming TAMs | - | Enhancing activation of the PI3K/Akt pathway | [162] | |
| Inhibite VEGF signaling pathway | Signaling pathway | Attenuating TAMs activity in liver cancer | - | - | [163] | |
| SALL4-mediated upregulation of exosomal miR-146a-5p | Exosome | Leading to M2-polarized TAMs | - | Activating NF-κB signaling and inducing pro-inflammatory factors | [43] | |
| Activated HSCs | Cell | Converting macrophages to TAMs | - | - | [164] |
Clinical significance of TAMs in EC: Clinically, TAMs are associated with the response of EC to chemotherapy. In patients undergoing neoadjuvant chemotherapy, high infiltration of CD68+/CD163- macrophages can serve as an adverse prognostic factor in esophageal and gastric adenocarcinoma[51,52].
TAMs can promote development of GC: In GC, peritoneal dissemination transpires through an invasive mechanism in which cancer cells directly penetrate the gastric wall and exfoliate into the peritoneal cavity (Table 1). Stimulation with anti-inflammatory triggers (such as TNF-α and IL-6), growth factors [such as epidermal growth factor (EGF), vascular endothelial growth factor (VEGF) and TGF-β2], chemokines [such as chemokine CC ligand (CCL)5], exosomes and enzymes (such as MMP and PI3K/Akt), leads to expression of transcription factors [such as STAT6, nuclear factor (NF)-κB and Snail) (Figure 1). Intraperitoneal TAMs are involved in promoting peritoneal dissemination of GC via secreted IL-6[53] and polarization to the M2 phenotype[54].
Numerous studies have demonstrated that TAMs are capable of express multifarious cytokines and chemokines that promote tumor cell proliferation and viability, including EGF[55], VEGF[55], TNF-α[56], TGF-β2[57], IL-6[56], and CCL5[58]. TAMs can facilitate the development of GC through multiple signal pathways, such as cyclooxygenase-2/prostaglandin E2/TGF-β/VEGF[59], and CCL5/chemokine CC receptor 5/STAT3[60].
It is also reported that TAMs may promote the invasion, metastasis and poor prognosis of GC cells by increasing expression of MMP9 and MMP2[61-63], mechanistically involving the PI3K/AKT/Snail-dependent pathway[64].
As for exosomes like exosomal miR-487a[65] derived from M2 macrophages, they can promote the proliferation and tumorigenesis, and remodel cytoskeleton-supporting migration in GC, through the ApoE-activating PI3K/Akt signaling pathway[66].
TAMs act as potential therapeutic targets for GC: As a potential therapeutic target in GC, TAMs can be reprogrammed into a proinflammatory subtype by targeting many pathways (Table 2), such as the stimulator of interferon genes (STING) pathway[67]. At the RNA level, LINC00665 interfaces with transcription factor BTB domain and CNC homology 1 to activate Wnt1 and mediates M2 polarization of TAMs in GC[68]. Many proteins can also mediate TAM polarization (calmodulin 2[69], methionine enkephalin[70], ETS-like transcription factor 4[71], IL-6[72], and IL-8[73]) and repress TAM activation (vasoactive intestinal peptide[74]), via signaling pathways such as STAT3/HIF-1A/VEGF-A axis[69], opioid growth factor receptor/PI3K/AKT/mammalian target of rapamycin (mTOR) axis[70], and IL-6/STAT3/interferon regulatory factor 4 axis[72], and so on. Lipid-droplet-dependent fatty acid metabolism[75] and miR-151-3p derived from GC exosomes[76] can also control the immunosuppressive phenotype of TAMs.
TAMs can be regulated by other cells, such as tumor-promoting GC-derived mesenchymal stromal cells[74] and IL-33-mediated mast cells[77].
Clinical significance of TAMs in GC: More clinically, TAMs can be used to potentiate localized immunotherapy of GC. For instance, researchers created an injectable hydrogel that can shear-thin and is loaded with polyphyllin II and resiquimod, which can help potentiate localized immunotherapy of GC by repolarizing TAMs[78]. Polyclonal antibody stimulator monotherapy or combined with PD-1 antibody[79], as well as using a natural alkaloid product isolated from sophora alopecuroides. L, sophoridine[80], may decrease the number of immunosuppressive M2-polarized TAMs.
When it comes to chemotherapy, exosomes and other factors could represses the chemosensitivity of gastric tumor cells in a TAM-dependent manner. Exosomal transfer of TAM-derived miR-21 confers cisplatin resistance in GC cells[81]. Yu et al[82] discovered that macrophages can be stimulated into a tumor-protective M2-like phenotype by tumor-derived leukemia inhibitory factor through activation of the STAT3 signaling pathway[82]. 5-Fluorouracil (5-FU) treatment activates HIF-1α in GC cells, leading to the accumulation of M2 TAMs that shield tumor cells from the effects of chemotherapeutic agents[83]. By generating growth differentiation factor 15 to exacerbate fatty acid β-oxidation in tumor cells, the recruited TAMs display the tumor-supporting M2 phenotype and enhance the chemoresistance of GC cells. And inversely polarized M2 macrophages can potentiate 5-FU resistance in tumors via CCL8 and phosphorylation of the Janus kinase 1/STAT3 signaling pathway[84].
The positive correlation between high level of TAMs in tumors and low overall survival of patients has been demonstrated. High density of M2 TAMs was associated with larger tumor size, diffuse Lauren type, poor histological differentiation, deeper tumor invasion, lymph node metastasis, and advanced TNM stage[85]. Abundance of CD163-positive TAMs in early GC[86] as well as CD206+ myeloid-derived TAMs[87] predict te recurrence after curative resection. CD8+ tumor-infiltrating lymphocytes and CD68+ TAMs[88], and high expression of HIF-1α combined with TAM infiltration[89] and coexistence of osteopontin and infiltrating M2 TAMs[90] can serve as a prognostic marker in GC.
TAMs can promote the development of CRC: The protumor role of TAMs in the development of colon carcinoma has been confirmed (Table 1). TAMs work with exosomes, MMP and cathelicidin, concerning signaling pathways (such as PI3K/Akt and NF-κB), cell cycle transition, metabolic reprogramming, inflammatory pathways, and oxidative stress (Figure 1). TAMs potentiate the angiogenic capacity of the TME in an oxidative-stress-dependent manner[91] or by metabolic reprogramming[92].
M2-macrophage-mediated regulation of CRC cell migration and invasion relies on M2-macrophage-derived exosomes, such as miR-21-5p and miR-155-5p[93], which may take effect through downregulating expression of BRG1. Exosomal miR-183-5p transferred by M2 polarized TAMs facilitate colon cancer through targeting thioesterase superfamily member 4-mediated PI3K/AKT and NF-kB pathways[94].
Multiple studies indicated that MMPs, such as MMP1 and MMP9, derived from TAMs may induce colon cancer cell invasion and proliferation[95,96]. It has been demonstrated that cathelicidin secreted by TAMs can promote the growth of CRC in mice by recruiting inflammatory cells such as macrophages into the TME[97].
TAMs act as potential therapeutic targets for CRC: In colon carcinoma, TAM M2 phenotype polarization can be regulated by diverse proteins (Table 2), such as protein kinase N2[98], aquaporin 9[99], tumor suppressor protein kinase (PK)Cα[100], and MAPKAP kinase 2[101]. miR-195-5p/NOTCH2-mediated EMT also affects M2-like TAM polarization by modulating IL-4 secretion in CRC[102], as does CRC-cell-derived exosomal miR-934 by downregulating PTEN expression and activating the PI3K/AKT signaling pathway[103].
The activation of pathways like the STING[104] and EGFR/PI3K/AKT/mTOR axis[105] has some of this same functionality. As chemokines, neuroendocrine-like-cell-derived CXCL10 and CXCL11 expand the infiltration of TAMs, accounting of the poor prognosis of CRC[106].
From the metabolic perspective, β-1,6-glucan resets tumor-supporting M2-like macrophages to tumor-inhibiting M1-like phenotype by activating the phosphorylation of Akt/NF-kB) and mitogen-activated protein kinase[107]. In mice with colitis-associated colorectal tumors, Helicobacter pylori infection quenched infiltration of TAMs, especially M2-like TAMs, while downregulating proinflammatory and protumorigenic factors TNF-α, IL-1β, IL-6, and IL-23[108].
Clinical significance of TAMs in CRC: TAMs with an M2-like phenotype have been associated with immunosuppression and resistance to chemotherapy of CRC. CD206/CD68 ratio[109] functions as a potent prognostic biomarker for predicting postoperative adjuvant chemotherapy in stage II colon cancer. In CRC, high infiltration of CD68+ TAMs[110], as well as type and number of intratumoral macrophages and clever-1(+) vessel density[111] could both function as a favorable prognostic marker.
Several TAM-targeting immunotherapies have been shown to promote antitumor immunity in CRC. A ketogenic diet restrains colon tumors via inducing intratumor oxidative stress through downregulation of MMP9 expression, and facilitating the polarization of TAM towards an M1-like proinflammatory phenotype[112]. By re-educating TAMs in CRC, piceatannol is an effective TGF-β1/TGF-Βr1 pathway inhibitor and TME modulator that inhibits tumor progression and metastasis[113]. Licorne-mediated immunogenic photodynamic therapy synergizes with myeloid-derived suppressor cell (MDSC)-targeting immunotherapy[114], Bte-Pd-Au-R-combined radiophotothermal therapy[115], as well as combination of foretinib and anti-PD-1 antibody immunotherapy[116] significantly inhibited tumor growth via decreasing tumor infiltration or the percentage of M2-like TAMs. Numerous studies have demonstrated that triptolide decreased TAM infiltration and M2 polarization[117] to remodel the colon cancer immune microenvironment through suppressing the sphingosine kinase–sphingosine-1-phosphate signaling pathway[118], or inhibiting tumor-derived CXCL12 via NF-kB and the extracellular signal-regulated protein kinases 1 and 2 axis[119]. Plinabulin[120], a distinct microtubule-targeting chemotherapy, as well as short-course radiotherapy[121], promoted a shift in M2 to M1 TAM polarization.
TAMs can promote development of PC: Pancreatic tumors are characterized by a desmoplastic stroma consisting of fibroblasts, immune cells, and a dense network of collagen fibers. Within this stroma, TAMs are among the most numerous immune cell populations[122]. Their protumorigenic function is predominantly attributed to their capacity to facilitate immune evasion and metastasis (Figure 1 and Table 1).
In PC, intraperitoneal TAMs potentially play a crucial role in promoting peritoneal dissemination and chemoresistance by inducing EMT[123]. Similarly, M2-polarized TAMs enhanced EMT in PC cells partially via Toll like receptor (TLR)-4/IL-10 signaling[124]. TAMs promote progression and the Warburg effect via CCL18/NF-kB/vascular cellular adhesion molecule 1 pathway in PDAC[125]. In addition, CCL20 secreted by M2 macrophages promoted the migration, EMT, and invasion of PC cells[126]. The study indicated a decisive role of TAMs in orchestrating functions of PDAC-infiltrating T cells by modifying their epigenetic profile towards a pro-tumoral phenotype[42].
TAMs act as potential therapeutic targets for PC: TAMs can also act as potential therapeutic targets for PC (Table 2). Autophagy-dependent ferroptosis accelerates TAM polarization via secretion and absorption of oncogenic KRAS protein[127]. Researchers discovered upregulation of RIP-1 in TAMs in PDAC[40]. Deletion of cancer-associated fibroblast HIF-2 significantly decreased the intratumoral recruitment of immunosuppressive M2 macrophages[128]. Fractional activation of CD11b by a small-molecule agonist contributes to TAM repolarization[129]. NLRP3 activation in TAMs enhanced lung metastasis of PDAC through regulation of TAM polarization[130].
By targeting M2-like TAMs, IL-27 dampened the proliferation, migration and metastasis of PC cells and boosted the potency of gemcitabine[131]. IFN-γ is a potential translational strategy to optimize performance of PD-1 blockade therapy in PC by preventing migration of CXCR2+CD68+ macrophages by blocking the CXCL8/CXCR2 axis[132]. PC-derived exosomal FGD5-AS1 induced M2 macrophage polarization via STAT3/NF-κB pathway[133]. The PDAC-derived small extracellular vesicle Ezrin can modulate macrophage polarization and promote PDAC metastasis[134]. Cut like homeobox 1 suppresses handful NF-κB-regulated chemokines like CXCL10, which are linked with M1 polarization and hindrance of angiogenesis and tumor development[135].
In addition, tryptophan-derived microbial metabolites stimulate the aryl hydrocarbon receptor in TAMs to inhibit antitumor immunity[136]. Cancer-cell-derived lactate activates macrophage nuclear factor erythroid 2-related factor 2 (Nrf2), skewing macrophages polarization towards an M2-like phenotype. These educated macrophages then trigger Nrf2 activation in cancer cells, ultimately promoting EMT[137]. Modulation of lactic acid level can redistribute M2 TAMs and upregulate PD-L1 to assist tumor immune escape, possibly through the HIF-1α signaling pathway[138]. Activation of dopamine receptor D4 by dopamine is instrumental in a depletion of cAMP, thereby hindering the activation of the PKA/p38 signaling pathway, ultimately leading to the suppression of tumor-promoting inflammation of TAMs[139].
For PC cells themselves, they render TAMs metabolically reprogrammed through a glycoprotein A repetitions predominant (GARP)-dependent and DNA-methylation-mediated mechanism to adopt a precancerous fate[140].
Clinical significance of TAMs in PC: The association between TAMs and immune response has primarily been observed as a reduction in the immunostimulatory function of TAMs.
An exosome-based dual delivery biosystem was created to improve immunotherapy for PDAC and reverse immunosuppression of M2 TAMs upon disruption of the galectin-9/dectin 1 axis[141]. A TME-responsive micellar system co-loaded with gemcitabine and PI3K inhibitor wortmannin was employed to achieve dual targeting of TAMs and tumor cells, aimed at repolarizing TAMs and improving the chemoimmunotherapy efficacy against PC[142].
Hyaluronic acid nanoparticle-encapsulated miRNA-125b reprogrammed TAMs to an antitumor phenotype in PDAC[143]. M2-TAM-targeting nanomicelles were created to simultaneously deliver PI3K-γ inhibitor NVP-BEZ 235 and CSF-1R-siRNA, leading to specific TAM reprogramming and antitumor immune response activation[144]. A customized nanocomplex through the self-assembling synthetic 4-(phosphonooxy)phenyl-2,4-dinitrobenzenesulfonate and Fe3+, subsequently decorated with hyaluronic acid, jointly repolarized TAMs to deactivate stromal cells and therefore weaken stroma[145]. A reduction-responsive RNAi nanoplatform utilized its reduction-responsive characteristic to rapidly release siRNA, inducing depolarization of TAMs into tumor-inhibiting M1-like phenotype[146].
To aid diagnosis, metabolizable near-infrared-II nanoprobes were applied to dynamic imaging of deep-seated TAMs in PC[147]. DN-ICG nanoprobes were qualified to discern dynamic variation of TAMs stimulated by low-dose radiotherapy and zoledronic acid.
By activating M2-like TAM polarization, atorvastatin mitigates the effect of aspirin on PC development and the chemotherapeutic potency of gemcitabine in PC[148]. Combined blockade of TGF-β1 and granulocyte-macrophage CSF improves chemotherapeutic effects in PC by modulating the TME[149]. In tumor-bearing Klebsiella pneumoniae carbapenemase mice, pharmacological TAM depletion enhanced therapeutic response to gemcitabine[150].
TAMs can promote development of LC: TAMs have been proved to promote the development of LC (Table 1). M2 polarization of TAMs in the TME promotes LC stem-like cell self-renewal capability and carcinogenicity[151,152]. Since TAMs can hasten EMT of Hep3B hepatoma cells, reduction of TLR4 expression in TAMs may attenuate that[153]. TAMs produce IL-6, which promotes expansion of these cancer stem cells and tumorigenesis. Restraint of TAM-stimulated CD44+ cell activity can be attainable by obstructing IL-6 signaling using tocilizumab, a drug approved by the United States Food and Drug Administration (FDA) for the treatment of rheumatoid arthritis[154].
TAMs act as potential therapeutic targets for LC: TAMs have also been found to serve as potential therapeutic targets for LC (Table 2). Researchers demonstrated that loss of Ndrg-2 influenced TAM polarization via the NF-κB pathway[155]. Knocking down triggering receptors expressed on myeloid cells (TREM1) in macrophages quenched the activation of the PI3K/AKT/mTOR pathway in M2 macrophages polarization[156]. Targeted delivery of miR-99b and/or miR-125a into TAMs substantially decelerated the progression of HCC and Lewis lung cancer, particularly following miR-99b delivery[157].
The mechanistic study illustrated that the high expression of monoacylglycerol lipase promoted the transcription and excretion of inflammatory factors such as IL-1β, IL-6 and TNF-α in M2-type TAMs cells[158]. Blocking TREM1-positive TAMs induced by hypoxia reverses immunosuppression and anti-PD-L1 resistance in LC[159]. Regorafenib, a multikinase inhibitor, reversed M2 polarization by suppressing p38 kinase phosphorylation and downstream Creb1/Klf4 activity in BM-derived macrophages[160]. The zinc-regulated transporters, iron-regulated transporter-like protein 9 upregulates phosphorylated STAT6 to facilitate polarization of M2 macrophages while downregulating the phosphorylation of IκBα/β to hinder M1 macrophage polarization[161].
TNF-α-induced protein 8-like 1 redounded arousal of the PI3K/Akt pathway in macrophages by directly attaching to and modulating the metabolism of phosphatidylinositol 4,5-bisphosphate and phosphatidylinositol 3,4,5-trisphosphate[162]. Inhibiting the VEGF signaling pathway was shown to attenuate TAM activity in LC[163]. Sal-like protein-4-mediated upregulation of exosomal miR-146a-5p remodeled macrophages by triggering NF-κB signaling and proinflammatory factors, contributing to M2-type polarization in TAMs[43].
In the TME, activated hematopoietic stem cells transform macrophages to TAMs and respectively stimulate the differentiation of DCs and monocytes into regulatory DCs and MDSCs[164].
Clinical significance of TAMs in LC: To reverse immunosuppressive process, a BisCCL2/5i mRNA nanoplatform was directly evolved, which appreciably ignited the antitumoral M1-type polarization in TAMs and reduced immunosuppression in the TME[165]. Researchers developed a nanoliposome-loaded C6-ceremide (LipC6) to reduce the number of TAMs and their production of reactive oxygen species[166]. LipC6 animated TAM differentiation into M1 phenotype, which engendering a decrease in immunosuppression and an increase in CD8+ T cell activity.
By interference with insulin-like growth factor (IGF)-1 secretion, sorafenib altered macrophage polarization, reduced IGF-1-driven cancer growth in vitro and partially inhibited macrophage activation in vivo[167]. Elevated serum levels of taurocholic acid were associated with reduced sirtuin (SIRT)5 expression and an increase in M2-like TAMs in HCC patient samples. Treatment with cholestyramine, a bile acid sequestrant and FDA-approved medication for hyperlipemia, reversed the implication of SIRT5 deficiency in impelling M2-like polarized TAMs and LC progression[168]. The novel glycyrrhetinic acid-tetramethylpyrazine conjugate TOGA exerted an anti-hepatocarcinogenic effect by attenuating effectiveness of TAMs on tumor cells through a mechanism related to the NF-κB pathway[169].
In the HCC microenvironment, M2 TAMs secreted considerable amounts of IL-17, which suppressed oxaliplatin-induced tumor cell apoptosis by triggering chaperone-mediated autophagy and curtailing cyclin D1 expression[170]. Radiofrequency ablation suppressed protumoral activation of local TAMs[171]. The combination of zwitterionic chito-oligosaccharides (COSs) with a photothermal material impaired the undesirable tumor promotion of TAMs, thus enhancing the outcome of photothermal therapy. Zwitterionic COSs acted as potent immune activators to re-educate TAMs to M1[172].
TAMs play a significant role in digestive system malignant tumors; therefore, TAM modulation is an attractive potential therapeutic target to enhance antitumor immune response and inhibit tumor progression. So far, diverse clinical therapies targeting TAMs have proven to be effective, highlighting the clinical significance of TAMs in digestive system malignant tumors. However, there are still many questions about the characteristics and functions of TAMs in digestive system malignant tumors. Continuous basic, transformation and clinical research may reveal some new prospects, such as how to use TAMs to improve cancer outcomes. Therefore, this is a promising field of cancer treatment, which may provide fruitful results.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caboclo JLF, Brazil; Exbrayat JM, France S-Editor: Fan JR L-Editor: A P-Editor: Zhang XD
| 1. | Bissell MJ, Hines WC. Why don't we get more cancer? Nat Med. 2011;17:320-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1049] [Cited by in RCA: 1157] [Article Influence: 82.6] [Reference Citation Analysis (0)] |
| 2. | Cui J, Tian J, Wang W, He T, Li X, Gu C, Wang L, Wu J, Shang A. IGF2BP2 promotes the progression of colorectal cancer through a YAP-dependent mechanism. Cancer Sci. 2021;112:4087-4099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 3. | Xiang Z, Hua M, Hao Z, Biao H, Zhu C, Zhai G, Wu J. The Roles of Mesenchymal Stem Cells in Gastrointestinal Cancers. Front Immunol. 2022;13:844001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 4. | Wolfe W, Xiang Z, Yu X, Li P, Chen H, Yao M, Fei Y, Huang Y, Yin Y, Xiao H. The Challenge of Applications of Probiotics in Gastrointestinal Diseases. Adv Gut Micro Res. 2023;2023. [DOI] [Full Text] |
| 5. | Zhou J, Tang Z, Gao S, Li C, Feng Y, Zhou X. Tumor-Associated Macrophages: Recent Insights and Therapies. Front Oncol. 2020;10:188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 442] [Article Influence: 88.4] [Reference Citation Analysis (0)] |
| 6. | Ngambenjawong C, Gustafson HH, Pun SH. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv Drug Deliv Rev. 2017;114:206-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 602] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 7. | Wu J, Zhang JR, Qin J. Clinical significance of methylation of E-cadherin and p14ARF gene promoters in skin squamous cell carcinoma tissues. Int J Clin Exp Med. 2014;7:1808-1812. [PubMed] |
| 8. | Shand FH, Ueha S, Otsuji M, Koid SS, Shichino S, Tsukui T, Kosugi-Kanaya M, Abe J, Tomura M, Ziogas J, Matsushima K. Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc Natl Acad Sci U S A. 2014;111:7771-7776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 9. | Hoeffel G, Ginhoux F. Fetal monocytes and the origins of tissue-resident macrophages. Cell Immunol. 2018;330:5-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 281] [Article Influence: 40.1] [Reference Citation Analysis (0)] |
| 10. | Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166-6173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1965] [Cited by in RCA: 2341] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 11. | Evans R, Alexander P. Cooperation of immune lymphoid cells with macrophages in tumour immunity. Nature. 1970;228:620-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 229] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 12. | Perry CJ, Muñoz-Rojas AR, Meeth KM, Kellman LN, Amezquita RA, Thakral D, Du VY, Wang JX, Damsky W, Kuhlmann AL, Sher JW, Bosenberg M, Miller-Jensen K, Kaech SM. Myeloid-targeted immunotherapies act in synergy to induce inflammation and antitumor immunity. J Exp Med. 2018;215:877-893. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 13. | Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, Xu D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front Immunol. 2020;11:1731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 319] [Cited by in RCA: 464] [Article Influence: 92.8] [Reference Citation Analysis (1)] |
| 14. | Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2390] [Cited by in RCA: 2921] [Article Influence: 194.7] [Reference Citation Analysis (0)] |
| 15. | Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, Cyrus N, Brokowski CE, Eisenbarth SC, Phillips GM, Cline GW, Phillips AJ, Medzhitov R. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 2170] [Article Influence: 197.3] [Reference Citation Analysis (0)] |
| 16. | Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. The IL-21 receptor augments Th2 effector function and alternative macrophage activation. J Clin Invest. 2006;116:2044-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 17. | Hazlett LD, McClellan SA, Barrett RP, Huang X, Zhang Y, Wu M, van Rooijen N, Szliter E. IL-33 shifts macrophage polarization, promoting resistance against Pseudomonas aeruginosa keratitis. Invest Ophthalmol Vis Sci. 2010;51:1524-1532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 18. | Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1523] [Cited by in RCA: 1661] [Article Influence: 87.4] [Reference Citation Analysis (2)] |
| 19. | Domagala W, Striker G, Szadowska A, Dukowicz A, Weber K, Osborn M. Cathepsin D in invasive ductal NOS breast carcinoma as defined by immunohistochemistry. No correlation with survival at 5 years. Am J Pathol. 1992;141:1003-1012. [PubMed] |
| 20. | Hagemann T, Robinson SC, Schulz M, Trümper L, Balkwill FR, Binder C. Enhanced invasiveness of breast cancer cell lines upon co-cultivation with macrophages is due to TNF-alpha dependent up-regulation of matrix metalloproteases. Carcinogenesis. 2004;25:1543-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 282] [Article Influence: 13.4] [Reference Citation Analysis (1)] |
| 21. | Caux C, Ramos RN, Prendergast GC, Bendriss-Vermare N, Ménétrier-Caux C. A Milestone Review on How Macrophages Affect Tumor Growth. Cancer Res. 2016;76:6439-6442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (1)] |
| 22. | Malfitano AM, Pisanti S, Napolitano F, Di Somma S, Martinelli R, Portella G. Tumor-Associated Macrophage Status in Cancer Treatment. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 23. | Wang H, Tian T, Zhang J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 230] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 24. | Tan Q, Liu H, Xu J, Mo Y, Dai F. Integrated analysis of tumor-associated macrophage infiltration and prognosis in ovarian cancer. Aging (Albany NY). 2021;13:23210-23232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 25. | DeNardo DG, Ruffell B. Macrophages as regulators of tumour immunity and immunotherapy. Nat Rev Immunol. 2019;19:369-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1310] [Cited by in RCA: 1636] [Article Influence: 272.7] [Reference Citation Analysis (0)] |
| 26. | Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465-7475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 526] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 27. | Lievense LA, Cornelissen R, Bezemer K, Kaijen-Lambers ME, Hegmans JP, Aerts JG. Pleural Effusion of Patients with Malignant Mesothelioma Induces Macrophage-Mediated T Cell Suppression. J Thorac Oncol. 2016;11:1755-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 28. | Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, Daniel D, Hwang ES, Rugo HS, Coussens LM. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 769] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 29. | Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327-1337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 591] [Cited by in RCA: 724] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 30. | Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 987] [Cited by in RCA: 1102] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 31. | Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, Sevillano M, Ibiza S, Cañellas A, Hernando-Momblona X, Byrom D, Matarin JA, Calon A, Rivas EI, Nebreda AR, Riera A, Attolini CS, Batlle E. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 917] [Cited by in RCA: 1334] [Article Influence: 190.6] [Reference Citation Analysis (0)] |
| 32. | Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R, Jhunjhunwala S, Banchereau R, Yang Y, Guan Y, Chalouni C, Ziai J, Şenbabaoğlu Y, Santoro S, Sheinson D, Hung J, Giltnane JM, Pierce AA, Mesh K, Lianoglou S, Riegler J, Carano RAD, Eriksson P, Höglund M, Somarriba L, Halligan DL, van der Heijden MS, Loriot Y, Rosenberg JE, Fong L, Mellman I, Chen DS, Green M, Derleth C, Fine GD, Hegde PS, Bourgon R, Powles T. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554:544-548. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3113] [Cited by in RCA: 3656] [Article Influence: 522.3] [Reference Citation Analysis (1)] |
| 33. | Geiger R, Rieckmann JC, Wolf T, Basso C, Feng Y, Fuhrer T, Kogadeeva M, Picotti P, Meissner F, Mann M, Zamboni N, Sallusto F, Lanzavecchia A. L-Arginine Modulates T Cell Metabolism and Enhances Survival and Anti-tumor Activity. Cell. 2016;167:829-842.e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 716] [Cited by in RCA: 1193] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 34. | Ruffell B, Coussens LM. Macrophages and therapeutic resistance in cancer. Cancer Cell. 2015;27:462-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 902] [Cited by in RCA: 1149] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 35. | Petty AJ, Li A, Wang X, Dai R, Heyman B, Hsu D, Huang X, Yang Y. Hedgehog signaling promotes tumor-associated macrophage polarization to suppress intratumoral CD8+ T cell recruitment. J Clin Invest. 2019;129:5151-5162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 36. | Liu C, Chikina M, Deshpande R, Menk AV, Wang T, Tabib T, Brunazzi EA, Vignali KM, Sun M, Stolz DB, Lafyatis RA, Chen W, Delgoffe GM, Workman CJ, Wendell SG, Vignali DAA. Treg Cells Promote the SREBP1-Dependent Metabolic Fitness of Tumor-Promoting Macrophages via Repression of CD8(+) T Cell-Derived Interferon-γ. Immunity. 2019;51:381-397.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 260] [Article Influence: 43.3] [Reference Citation Analysis (0)] |
| 37. | Sun K, Xu R, Ma F, Yang N, Li Y, Sun X, Jin P, Kang W, Jia L, Xiong J, Hu H, Tian Y, Lan X. scRNA-seq of gastric tumor shows complex intercellular interaction with an alternative T cell exhaustion trajectory. Nat Commun. 2022;13:4943. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 38. | Zhang H, Li R, Cao Y, Gu Y, Lin C, Liu X, Lv K, He X, Fang H, Jin K, Fei Y, Chen Y, Wang J, Liu H, Li H, Zhang H, He H, Zhang W. Poor Clinical Outcomes and Immunoevasive Contexture in Intratumoral IL-10-Producing Macrophages Enriched Gastric Cancer Patients. Ann Surg. 2022;275:e626-e635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 119] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 39. | Gyori D, Lim EL, Grant FM, Spensberger D, Roychoudhuri R, Shuttleworth SJ, Okkenhaug K, Stephens LR, Hawkins PT. Compensation between CSF1R+ macrophages and Foxp3+ Treg cells drives resistance to tumor immunotherapy. JCI Insight. 2018;3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 106] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 40. | Wang W, Marinis JM, Beal AM, Savadkar S, Wu Y, Khan M, Taunk PS, Wu N, Su W, Wu J, Ahsan A, Kurz E, Chen T, Yaboh I, Li F, Gutierrez J, Diskin B, Hundeyin M, Reilly M, Lich JD, Harris PA, Mahajan MK, Thorpe JH, Nassau P, Mosley JE, Leinwand J, Kochen Rossi JA, Mishra A, Aykut B, Glacken M, Ochi A, Verma N, Kim JI, Vasudevaraja V, Adeegbe D, Almonte C, Bagdatlioglu E, Cohen DJ, Wong KK, Bertin J, Miller G. RIP1 Kinase Drives Macrophage-Mediated Adaptive Immune Tolerance in Pancreatic Cancer. Cancer Cell. 2018;34:757-774.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 41. | Yang X, Lin J, Wang G, Xu D. Targeting Proliferating Tumor-Infiltrating Macrophages Facilitates Spatial Redistribution of CD8(+) T Cells in Pancreatic Cancer. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 42. | Borgoni S, Iannello A, Cutrupi S, Allavena P, D'Incalci M, Novelli F, Cappello P. Depletion of tumor-associated macrophages switches the epigenetic profile of pancreatic cancer infiltrating T cells and restores their anti-tumor phenotype. Oncoimmunology. 2018;7:e1393596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 43. | Yin C, Han Q, Xu D, Zheng B, Zhao X, Zhang J. SALL4-mediated upregulation of exosomal miR-146a-5p drives T-cell exhaustion by M2 tumor-associated macrophages in HCC. Oncoimmunology. 2019;8:1601479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 44. | Okamoto M, Koma YI, Kodama T, Nishio M, Shigeoka M, Yokozaki H. Growth Differentiation Factor 15 Promotes Progression of Esophageal Squamous Cell Carcinoma via TGF-β Type II Receptor Activation. Pathobiology. 2020;87:100-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 45. | Baas P, Scherpereel A, Nowak AK, Fujimoto N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, Oulkhouir Y, Bautista Y, Cornelissen R, Greillier L, Grossi F, Kowalski D, Rodríguez-Cid J, Aanur P, Oukessou A, Baudelet C, Zalcman G. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet. 2021;397:375-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 432] [Cited by in RCA: 770] [Article Influence: 192.5] [Reference Citation Analysis (0)] |
| 46. | Wang P, Xu LJ, Qin JJ, Zhang L, Zhuang GH. MicroRNA-155 inversely correlates with esophageal cancer progression through regulating tumor-associated macrophage FGF2 expression. Biochem Biophys Res Commun. 2018;503:452-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 47. | Song J, Yang P, Li X, Zhu X, Liu M, Duan X, Liu R. Esophageal Cancer-Derived Extracellular Vesicle miR-21-5p Contributes to EMT of ESCC Cells by Disorganizing Macrophage Polarization. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 48. | Yang C, Chen C, Xiao Q, Wang X, Shou Y, Tian X, Wang S, Li H, Liang Y, Shu J, Chen K, Sun M. Relationship Between PTEN and Angiogenesis of Esophageal Squamous Cell Carcinoma and the Underlying Mechanism. Front Oncol. 2021;11:739297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 49. | Takase N, Koma Y, Urakawa N, Nishio M, Arai N, Akiyama H, Shigeoka M, Kakeji Y, Yokozaki H. NCAM- and FGF-2-mediated FGFR1 signaling in the tumor microenvironment of esophageal cancer regulates the survival and migration of tumor-associated macrophages and cancer cells. Cancer Lett. 2016;380:47-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 50. | Yuan X, Liu K, Li Y, Zhang AZ, Wang XL, Jiang CH, Liang WH, Zhang HJ, Pang LJ, Li M, Yang L, Qi Y, Zheng Q, Li F, Hu JM. HPV16 infection promotes an M2 macrophage phenotype to promote the invasion and metastasis of esophageal squamous cell carcinoma. Clin Transl Oncol. 2021;23:2382-2393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Sugimura K, Miyata H, Tanaka K, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y. High infiltration of tumor-associated macrophages is associated with a poor response to chemotherapy and poor prognosis of patients undergoing neoadjuvant chemotherapy for esophageal cancer. J Surg Oncol. 2015;111:752-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 52. | Svensson MC, Svensson M, Nodin B, Borg D, Hedner C, Hjalmarsson C, Leandersson K, Jirström K. High Infiltration of CD68+/CD163- Macrophages Is an Adverse Prognostic Factor after Neoadjuvant Chemotherapy in Esophageal and Gastric Adenocarcinoma. J Innate Immun. 2022;14:615-628. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Sakamoto S, Kagawa S, Kuwada K, Ito A, Kajioka H, Kakiuchi Y, Watanabe M, Kagawa T, Yoshida R, Kikuchi S, Kuroda S, Tazawa H, Fujiwara T. Intraperitoneal cancer-immune microenvironment promotes peritoneal dissemination of gastric cancer. Oncoimmunology. 2019;8:e1671760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 54. | Yamaguchi T, Fushida S, Yamamoto Y, Tsukada T, Kinoshita J, Oyama K, Miyashita T, Tajima H, Ninomiya I, Munesue S, Harashima A, Harada S, Yamamoto H, Ohta T. Tumor-associated macrophages of the M2 phenotype contribute to progression in gastric cancer with peritoneal dissemination. Gastric Cancer. 2016;19:1052-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 55. | Song H, Wang T, Tian L, Bai S, Chen L, Zuo Y, Xue Y. Macrophages on the Peritoneum are involved in Gastric Cancer Peritoneal Metastasis. J Cancer. 2019;10:5377-5387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 56. | Ju X, Zhang H, Zhou Z, Chen M, Wang Q. Tumor-associated macrophages induce PD-L1 expression in gastric cancer cells through IL-6 and TNF-ɑ signaling. Exp Cell Res. 2020;396:112315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 90] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 57. | Wang Z, Yang Y, Cui Y, Wang C, Lai Z, Li Y, Zhang W, Mustonen H, Puolakkainen P, Ye Y, Jiang K, Shen Z, Wang S. Tumor-associated macrophages regulate gastric cancer cell invasion and metastasis through TGFβ2/NF-κB/Kindlin-2 axis. Chin J Cancer Res. 2020;32:72-88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 58. | Ding H, Zhao L, Dai S, Li L, Wang F, Shan B. CCL5 secreted by tumor associated macrophages may be a new target in treatment of gastric cancer. Biomed Pharmacother. 2016;77:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 59. | Zhang C, Hu X, Liu XY, Liang P, Zhang J, Cao L, Wang ZL, Liu HR, Yin XG, Dong CY, Wang LM. Effect of tumor-associated macrophages on gastric cancer stem cell in omental milky spots and lymph node micrometastasis. Int J Clin Exp Pathol. 2015;8:13795-13805. [PubMed] |
| 60. | Wang HC, Chen CW, Yang CL, Tsai IM, Hou YC, Chen CJ, Shan YS. Tumor-Associated Macrophages Promote Epigenetic Silencing of Gelsolin through DNA Methyltransferase 1 in Gastric Cancer Cells. Cancer Immunol Res. 2017;5:885-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 61. | Xu J, Yu Y, He X, Niu N, Li X, Zhang R, Hu J, Ma J, Yu X, Sun Y, Ni H, Wang F. Tumor-associated macrophages induce invasion and poor prognosis in human gastric cancer in a cyclooxygenase-2/MMP9-dependent manner. Am J Transl Res. 2019;11:6040-6054. [PubMed] |
| 62. | Zhu JM, Quan XL, Han SC, Fan XJ, Li HM, Liang SS, Chen X, Wang RY, Ji XN. Establishment of a Model of Microencapsulated SGC7901 Human Gastric Carcinoma Cells Cocultured with Tumor-Associated Macrophages. Can J Gastroenterol Hepatol. 2018;2018:3767482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Shen Z, Kauttu T, Seppänen H, Vainionpää S, Ye Y, Wang S, Mustonen H, Puolakkainen P. Both macrophages and hypoxia play critical role in regulating invasion of gastric cancer in vitro. Acta Oncol. 2013;52:852-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Liu L, Ye Y, Zhu X. MMP-9 secreted by tumor associated macrophages promoted gastric cancer metastasis through a PI3K/AKT/Snail pathway. Biomed Pharmacother. 2019;117:109096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 65. | Yang X, Cai S, Shu Y, Deng X, Zhang Y, He N, Wan L, Chen X, Qu Y, Yu S. Exosomal miR-487a derived from m2 macrophage promotes the progression of gastric cancer. Cell Cycle. 2021;20:434-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 66. | Zheng P, Luo Q, Wang W, Li J, Wang T, Wang P, Chen L, Zhang P, Chen H, Liu Y, Dong P, Xie G, Ma Y, Jiang L, Yuan X, Shen L. Tumor-associated macrophages-derived exosomes promote the migration of gastric cancer cells by transfer of functional Apolipoprotein E. Cell Death Dis. 2018;9:434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 284] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 67. | Miao L, Qi J, Zhao Q, Wu QN, Wei DL, Wei XL, Liu J, Chen J, Zeng ZL, Ju HQ, Luo HY, Xu RH. Targeting the STING pathway in tumor-associated macrophages regulates innate immune sensing of gastric cancer cells. Theranostics. 2020;10:498-515. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 68. | Yang B, Su K, Sha G, Bai Q, Sun G, Chen H, Xie H, Jiang X. LINC00665 interacts with BACH1 to activate Wnt1 and mediates the M2 polarization of tumor-associated macrophages in GC. Mol Immunol. 2022;146:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 69. | Mu G, Zhu Y, Dong Z, Shi L, Deng Y, Li H. Calmodulin 2 Facilitates Angiogenesis and Metastasis of Gastric Cancer via STAT3/HIF-1A/VEGF-A Mediated Macrophage Polarization. Front Oncol. 2021;11:727306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 62] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 70. | Wang X, Jiao X, Meng Y, Chen H, Griffin N, Gao X, Shan F. Methionine enkephalin (MENK) inhibits human gastric cancer through regulating tumor associated macrophages (TAMs) and PI3K/AKT/mTOR signaling pathway inside cancer cells. Int Immunopharmacol. 2018;65:312-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 71. | Zheng L, Xu H, Di Y, Chen L, Liu J, Kang L, Gao L. ELK4 promotes the development of gastric cancer by inducing M2 polarization of macrophages through regulation of the KDM5A-PJA2-KSR1 axis. J Transl Med. 2021;19:342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 72. | Su CY, Fu XL, Duan W, Yu PW, Zhao YL. High density of CD68+ tumor-associated macrophages predicts a poor prognosis in gastric cancer mediated by IL-6 expression. Oncol Lett. 2018;15:6217-6224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Li W, Zhang X, Wu F, Zhou Y, Bao Z, Li H, Zheng P, Zhao S. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis. 2019;10:918. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 240] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 74. | Chen L, Yuan W, Chen Z, Wu S, Ge J, Chen J. Vasoactive intestinal peptide represses activation of tumor-associated macrophages in gastric cancer via regulation of TNFα, IL-6, IL-12 and iNOS. Int J Oncol. 2015;47:1361-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 75. | Mok TSK, Wu YL, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I, Kubota K, Lubiniecki GM, Zhang J, Kush D, Lopes G; KEYNOTE-042 Investigators. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393:1819-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1597] [Cited by in RCA: 2411] [Article Influence: 401.8] [Reference Citation Analysis (0)] |
| 76. | Xie S, Zhu Y, Wang S, Yang X, Yuan X, Piao D. [miR-151-3p derived from gastric cancer exosomes induces M2-phenotype polarization of macrophages and promotes tumor growth]. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2022;38:584-589. [PubMed] |
| 77. | Eissmann MF, Dijkstra C, Jarnicki A, Phesse T, Brunnberg J, Poh AR, Etemadi N, Tsantikos E, Thiem S, Huntington ND, Hibbs ML, Boussioutas A, Grimbaldeston MA, Buchert M, O'Donoghue RJJ, Masson F, Ernst M. IL-33-mediated mast cell activation promotes gastric cancer through macrophage mobilization. Nat Commun. 2019;10:2735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 160] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 78. | Yang Y, Yang Y, Chen M, Chen J, Wang J, Ma Y, Qian H. Injectable shear-thinning polylysine hydrogels for localized immunotherapy of gastric cancer through repolarization of tumor-associated macrophages. Biomater Sci. 2021;9:6597-6608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 79. | Smith JP, Cao H, Chen W, Mahmood K, Phillips T, Sutton L, Cato A. Gastrin Vaccine Alone and in Combination With an Immune Checkpoint Antibody Inhibits Growth and Metastases of Gastric Cancer. Front Oncol. 2021;11:788875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 80. | Peng Z, Guan Q, Luo J, Deng W, Liu J, Yan R, Wang W. Sophoridine exerts tumor-suppressive activities via promoting ESRRG-mediated β-catenin degradation in gastric cancer. BMC Cancer. 2020;20:582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Zheng P, Chen L, Yuan X, Luo Q, Liu Y, Xie G, Ma Y, Shen L. Exosomal transfer of tumor-associated macrophage-derived miR-21 confers cisplatin resistance in gastric cancer cells. J Exp Clin Cancer Res. 2017;36:53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 326] [Cited by in RCA: 489] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 82. | Yu S, Li Q, Wang Y, Cui Y, Yu Y, Li W, Liu F, Liu T. Tumor-derived LIF promotes chemoresistance via activating tumor-associated macrophages in gastric cancers. Exp Cell Res. 2021;406:112734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Yu S, Li Q, Yu Y, Cui Y, Li W, Liu T, Liu F. Activated HIF1α of tumor cells promotes chemoresistance development via recruiting GDF15-producing tumor-associated macrophages in gastric cancer. Cancer Immunol Immunother. 2020;69:1973-1987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 84. | He Z, Chen D, Wu J, Sui C, Deng X, Zhang P, Chen Z, Liu D, Yu J, Shi J, Li G, Yao X. Yes associated protein 1 promotes resistance to 5-fluorouracil in gastric cancer by regulating GLUT3-dependent glycometabolism reprogramming of tumor-associated macrophages. Arch Biochem Biophys. 2021;702:108838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 85. | Yin S, Huang J, Li Z, Zhang J, Luo J, Lu C, Xu H. The Prognostic and Clinicopathological Significance of Tumor-Associated Macrophages in Patients with Gastric Cancer: A Meta-Analysis. PLoS One. 2017;12:e0170042. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 86. | Zhou W, Zhang Y, He F, Lv S, Zhang X, Fei C. Abundance of CD163-Positive Tumor-Associated Macrophages in the Early Gastric Cancer Predicts the Recurrence after Curative Resection. Dig Dis. 2020;38:458-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 87. | Wang Z, Dong Z, Zhao G, Ni B, Zhang ZZ. Prognostic role of myeloid-derived tumor-associated macrophages at the tumor invasive margin in gastric cancer with liver metastasis (GCLM): a single-center retrospective study. J Gastrointest Oncol. 2022;13:1340-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 88. | Lu J, Xu Y, Wu Y, Huang XY, Xie JW, Wang JB, Lin JX, Li P, Zheng CH, Huang AM, Huang CM. Tumor-infiltrating CD8+ T cells combined with tumor-associated CD68+ macrophages predict postoperative prognosis and adjuvant chemotherapy benefit in resected gastric cancer. BMC Cancer. 2019;19:920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 89. | Zhang WJ, Chen C, Zhou ZH, Gao ST, Tee TJ, Yang LQ, Xu YY, Pang TH, Xu XY, Sun Q, Feng M, Wang H, Lu CL, Wu GZ, Wu S, Guan WX, Xu GF. Hypoxia-inducible factor-1 alpha Correlates with Tumor-Associated Macrophages Infiltration, Influences Survival of Gastric Cancer Patients. J Cancer. 2017;8:1818-1825. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 90. | Lin CN, Wang CJ, Chao YJ, Lai MD, Shan YS. The significance of the co-existence of osteopontin and tumor-associated macrophages in gastric cancer progression. BMC Cancer. 2015;15:128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 91. | Luput L, Licarete E, Sesarman A, Patras L, Alupei MC, Banciu M. Tumor-associated macrophages favor C26 murine colon carcinoma cell proliferation in an oxidative stress-dependent manner. Oncol Rep. 2017;37:2472-2480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 92. | Scheurlen KM, Billeter AT, O'Brien SJ, Galandiuk S. Metabolic dysfunction and early-onset colorectal cancer - how macrophages build the bridge. Cancer Med. 2020;9:6679-6693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 93. | Lan J, Sun L, Xu F, Liu L, Hu F, Song D, Hou Z, Wu W, Luo X, Wang J, Yuan X, Hu J, Wang G. M2 Macrophage-Derived Exosomes Promote Cell Migration and Invasion in Colon Cancer. Cancer Res. 2019;79:146-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 482] [Article Influence: 68.9] [Reference Citation Analysis (2)] |
| 94. | Zhang S, Li D, Zhao M, Yang F, Sang C, Yan C, Wang Z, Li Y. Exosomal miR-183-5p Shuttled by M2 Polarized Tumor-Associated Macrophage Promotes the Development of Colon Cancer via Targeting THEM4 Mediated PI3K/AKT and NF-κB Pathways. Front Oncol. 2021;11:672684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 95. | Yu J, Xu Z, Guo J, Yang K, Zheng J, Sun X. Tumor-associated macrophages (TAMs) depend on MMP1 for their cancer-promoting role. Cell Death Discov. 2021;7:343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 96. | Vinnakota K, Zhang Y, Selvanesan BC, Topi G, Salim T, Sand-Dejmek J, Jönsson G, Sjölander A. M2-like macrophages induce colon cancer cell invasion via matrix metalloproteinases. J Cell Physiol. 2017;232:3468-3480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 102] [Article Influence: 12.8] [Reference Citation Analysis (3)] |
| 97. | Quan WW, Pan XH, Wu JL, Yao YW, Li D. [Effect and mechanism of antimicrobial peptide cathelicidin secreted by tumor associated macrophage on the growth of colorectal cancer in mice]. Zhonghua Zhong Liu Za Zhi. 2018;40:172-177. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 98. | Cheng Y, Zhu Y, Xu J, Yang M, Chen P, Xu W, Zhao J, Geng L, Gong S. PKN2 in colon cancer cells inhibits M2 phenotype polarization of tumor-associated macrophages via regulating DUSP6-Erk1/2 pathway. Mol Cancer. 2018;17:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 109] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 99. | Shi Y, Yasui M, Hara-Chikuma M. AQP9 transports lactate in tumor-associated macrophages to stimulate an M2-like polarization that promotes colon cancer progression. Biochem Biophys Rep. 2022;31:101317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 100. | Cheng Y, Zhu Y, Xu W, Xu J, Yang M, Chen P, Zhao J, Geng L, Gong S. PKCα in colon cancer cells promotes M1 macrophage polarization via MKK3/6-P38 MAPK pathway. Mol Carcinog. 2018;57:1017-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 101. | Suarez-Lopez L, Sriram G, Kong YW, Morandell S, Merrick KA, Hernandez Y, Haigis KM, Yaffe MB. MK2 contributes to tumor progression by promoting M2 macrophage polarization and tumor angiogenesis. Proc Natl Acad Sci U S A. 2018;115:E4236-E4244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 102. | Lin X, Wang S, Sun M, Zhang C, Wei C, Yang C, Dou R, Liu Q, Xiong B. miR-195-5p/NOTCH2-mediated EMT modulates IL-4 secretion in colorectal cancer to affect M2-like TAM polarization. J Hematol Oncol. 2019;12:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 164] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 103. | Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, Zhang Z, Cai S, Xu Y, Li X, He X, Zhong X, Li G, Chen Z, Li D. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 538] [Article Influence: 107.6] [Reference Citation Analysis (0)] |
| 104. | Lee SJ, Yang H, Kim WR, Lee YS, Lee WS, Kong SJ, Lee HJ, Kim JH, Cheon J, Kang B, Chon HJ, Kim C. STING activation normalizes the intraperitoneal vascular-immune microenvironment and suppresses peritoneal carcinomatosis of colon cancer. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 59] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 105. | Lian G, Chen S, Ouyang M, Li F, Chen L, Yang J. Colon Cancer Cell Secretes EGF to Promote M2 Polarization of TAM Through EGFR/PI3K/AKT/mTOR Pathway. Technol Cancer Res Treat. 2019;18:1533033819849068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 106] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 106. | Zeng YJ, Lai W, Wu H, Liu L, Xu HY, Wang J, Chu ZH. Neuroendocrine-like cells -derived CXCL10 and CXCL11 induce the infiltration of tumor-associated macrophage leading to the poor prognosis of colorectal cancer. Oncotarget. 2016;7:27394-27407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 107. | Cheng H, Sun L, Shen D, Ren A, Ma F, Tai G, Fan L, Zhou Y. Beta-1,6 glucan converts tumor-associated macrophages into an M1-like phenotype. Carbohydr Polym. 2020;247:116715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 108. | Li LN, Liu Y, Zhang HC, Wu T, Dai Y, Wang WH. Helicobacter pylori infection reduces TAMs infiltration in a mouse model of AOM/DSS induced colitis-associated cancer. PLoS One. 2020;15:e0241840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 109. | Feng Q, Chang W, Mao Y, He G, Zheng P, Tang W, Wei Y, Ren L, Zhu D, Ji M, Tu Y, Qin X, Xu J. Tumor-associated Macrophages as Prognostic and Predictive Biomarkers for Postoperative Adjuvant Chemotherapy in Patients with Stage II Colon Cancer. Clin Cancer Res. 2019;25:3896-3907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 101] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 110. | Li J, Li L, Li Y, Long Y, Zhao Q, Ouyang Y, Bao W, Gong K. Tumor-associated macrophage infiltration and prognosis in colorectal cancer: systematic review and meta-analysis. Int J Colorectal Dis. 2020;35:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 111. | Ålgars A, Kemppinen L, Fair-Mäkelä R, Mustonen H, Haglund C, Jalkanen S. Stage I-IV Colorectal Cancer Prognosis Can Be Predicted by Type and Number of Intratumoral Macrophages and CLEVER-1(+) Vessel Density. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 112. | Zhang N, Liu C, Jin L, Zhang R, Wang T, Wang Q, Chen J, Yang F, Siebert HC, Zheng X. Ketogenic Diet Elicits Antitumor Properties through Inducing Oxidative Stress, Inhibiting MMP-9 Expression, and Rebalancing M1/M2 Tumor-Associated Macrophage Phenotype in a Mouse Model of Colon Cancer. J Agric Food Chem. 2020;68:11182-11196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 113. | Chiou YS, Lan YM, Lee PS, Lin Q, Nagabhushanam K, Ho CT, Pan MH. Piceatannol Prevents Colon Cancer Progression via Dual-Targeting to M2-Polarized Tumor-Associated Macrophages and the TGF-β1 Positive Feedback Signaling Pathway. Mol Nutr Food Res. 2022;66:e2200248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 114. | Ding D, Zhong H, Liang R, Lan T, Zhu X, Huang S, Wang Y, Shao J, Shuai X, Wei B. Multifunctional Nanodrug Mediates Synergistic Photodynamic Therapy and MDSCs-Targeting Immunotherapy of Colon Cancer. Adv Sci (Weinh). 2021;8:e2100712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 115. | Huang W, He L, Zhang Z, Shi S, Chen T. Shape-Controllable Tellurium-Driven Heterostructures with Activated Robust Immunomodulatory Potential for Highly Efficient Radiophotothermal Therapy of Colon Cancer. ACS Nano. 2021;15:20225-20241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 116. | Fu Y, Peng Y, Zhao S, Mou J, Zeng L, Jiang X, Yang C, Huang C, Li Y, Lu Y, Wu M, Yang Y, Kong T, Lai Q, Wu Y, Yao Y, Wang Y, Gou L, Yang J. Combination Foretinib and Anti-PD-1 Antibody Immunotherapy for Colorectal Carcinoma. Front Cell Dev Biol. 2021;9:689727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 117. | Li H, Li L, Mei H, Pan G, Wang X, Huang X, Wang T, Jiang Z, Zhang L, Sun L. Antitumor properties of triptolide: phenotype regulation of macrophage differentiation. Cancer Biol Ther. 2020;21:178-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 118. | Li H, Xing X, Zhang X, Li L, Jiang Z, Wang T, Huang X, Wang X, Zhang L, Sun L. Effects of triptolide on the sphingosine kinase - Sphingosine-1-phosphate signaling pathway in colitis-associated colon cancer. Int Immunopharmacol. 2020;88:106892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 119. | Jiang X, Cao G, Gao G, Wang W, Zhao J, Gao C. Triptolide decreases tumor-associated macrophages infiltration and M2 polarization to remodel colon cancer immune microenvironment via inhibiting tumor-derived CXCL12. J Cell Physiol. 2021;236:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 120. | Wang Y, Shen W, Liu S, Zhu G, Meng X, Mao K, Wang J, Yang YG, Xiao C, Sun T. The Host-Defense-Peptide-Mimicking Synthetic Polypeptides Effectively Enhance Antitumor Immunity through Promoting Immunogenic Tumor Cell Death. Macromol Biosci. 2021;21:e2100171. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 121. | Stary V, Wolf B, Unterleuthner D, List J, Talic M, Laengle J, Beer A, Strobl J, Stary G, Dolznig H, Bergmann M. Short-course radiotherapy promotes pro-inflammatory macrophages via extracellular vesicles in human rectal cancer. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 122. | LaRue MM, Parker S, Puccini J, Cammer M, Kimmelman AC, Bar-Sagi D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc Natl Acad Sci U S A. 2022;119:e2119168119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 78] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 123. | Kuwada K, Kagawa S, Yoshida R, Sakamoto S, Ito A, Watanabe M, Ieda T, Kuroda S, Kikuchi S, Tazawa H, Fujiwara T. The epithelial-to-mesenchymal transition induced by tumor-associated macrophages confers chemoresistance in peritoneally disseminated pancreatic cancer. J Exp Clin Cancer Res. 2018;37:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 124. | Liu CY, Xu JY, Shi XY, Huang W, Ruan TY, Xie P, Ding JL. M2-polarized tumor-associated macrophages promoted epithelial-mesenchymal transition in pancreatic cancer cells, partially through TLR4/IL-10 signaling pathway. Lab Invest. 2013;93:844-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 356] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 125. | Ye H, Zhou Q, Zheng S, Li G, Lin Q, Wei L, Fu Z, Zhang B, Liu Y, Li Z, Chen R. Tumor-associated macrophages promote progression and the Warburg effect via CCL18/NF-kB/VCAM-1 pathway in pancreatic ductal adenocarcinoma. Cell Death Dis. 2018;9:453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 180] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 126. | Liu B, Jia Y, Ma J, Wu S, Jiang H, Cao Y, Sun X, Yin X, Yan S, Shang M, Mao A. Tumor-associated macrophage-derived CCL20 enhances the growth and metastasis of pancreatic cancer. Acta Biochim Biophys Sin (Shanghai). 2016;48:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 127. | Dai E, Han L, Liu J, Xie Y, Kroemer G, Klionsky DJ, Zeh HJ, Kang R, Wang J, Tang D. Autophagy-dependent ferroptosis drives tumor-associated macrophage polarization via release and uptake of oncogenic KRAS protein. Autophagy. 2020;16:2069-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 351] [Cited by in RCA: 438] [Article Influence: 87.6] [Reference Citation Analysis (0)] |
| 128. | Garcia Garcia CJ, Huang Y, Fuentes NR, Turner MC, Monberg ME, Lin D, Nguyen ND, Fujimoto TN, Zhao J, Lee JJ, Bernard V, Yu M, Delahoussaye AM, Jimenez Sacarello I, Caggiano EG, Phan JL, Deorukhkar A, Molkentine JM, Saur D, Maitra A, Taniguchi CM. Stromal HIF2 Regulates Immune Suppression in the Pancreatic Cancer Microenvironment. Gastroenterology. 2022;162:2018-2031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 107] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 129. | Panni RZ, Herndon JM, Zuo C, Hegde S, Hogg GD, Knolhoff BL, Breden MA, Li X, Krisnawan VE, Khan SQ, Schwarz JK, Rogers BE, Fields RC, Hawkins WG, Gupta V, DeNardo DG. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci Transl Med. 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 130. | Gu H, Deng W, Zhang Y, Chang Y, Shelat VG, Tsuchida K, Lino-Silva LS, Wang Z. NLRP3 activation in tumor-associated macrophages enhances lung metastasis of pancreatic ductal adenocarcinoma. Transl Lung Cancer Res. 2022;11:858-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 131. | Yao L, Wang M, Niu Z, Liu Q, Gao X, Zhou L, Liao Q, Zhao Y. Interleukin-27 inhibits malignant behaviors of pancreatic cancer cells by targeting M2 polarized tumor associated macrophages. Cytokine. 2017;89:194-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 132. | Zhang M, Huang L, Ding G, Huang H, Cao G, Sun X, Lou N, Wei Q, Shen T, Xu X, Cao L, Yan Q. Interferon gamma inhibits CXCL8-CXCR2 axis mediated tumor-associated macrophages tumor trafficking and enhances anti-PD1 efficacy in pancreatic cancer. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 133. | He Z, Wang J, Zhu C, Xu J, Chen P, Jiang X, Chen Y, Jiang J, Sun C. Exosome-derived FGD5-AS1 promotes tumor-associated macrophage M2 polarization-mediated pancreatic cancer cell proliferation and metastasis. Cancer Lett. 2022;548:215751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 122] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 134. | Chang YT, Peng HY, Hu CM, Huang SC, Tien SC, Jeng YM. Pancreatic cancer-derived small extracellular vesical Ezrin regulates macrophage polarization and promotes metastasis. Am J Cancer Res. 2020;10:12-37. [PubMed] |
| 135. | Kühnemuth B, Mühlberg L, Schipper M, Griesmann H, Neesse A, Milosevic N, Wissniowski T, Buchholz M, Gress TM, Michl P. CUX1 modulates polarization of tumor-associated macrophages by antagonizing NF-κB signaling. Oncogene. 2015;34:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 136. | Hezaveh K, Shinde RS, Klötgen A, Halaby MJ, Lamorte S, Ciudad MT, Quevedo R, Neufeld L, Liu ZQ, Jin R, Grünwald BT, Foerster EG, Chaharlangi D, Guo M, Makhijani P, Zhang X, Pugh TJ, Pinto DM, Co IL, McGuigan AP, Jang GH, Khokha R, Ohashi PS, O'Kane GM, Gallinger S, Navarre WW, Maughan H, Philpott DJ, Brooks DG, McGaha TL. Tryptophan-derived microbial metabolites activate the aryl hydrocarbon receptor in tumor-associated macrophages to suppress anti-tumor immunity. Immunity. 2022;55:324-340.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 363] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 137. | Feng R, Morine Y, Ikemoto T, Imura S, Iwahashi S, Saito Y, Shimada M. Nrf2 activation drive macrophages polarization and cancer cell epithelial-mesenchymal transition during interaction. Cell Commun Signal. 2018;16:54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 146] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 138. | Shan T, Chen S, Chen X, Wu T, Yang Y, Li S, Ma J, Zhao J, Lin W, Li W, Cui X, Kang Y. M2TAM subsets altered by lactic acid promote Tcell apoptosis through the PDL1/PD1 pathway. Oncol Rep. 2020;44:1885-1894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 139. | Liu Q, Zhang R, Zhang X, Liu J, Wu H, Li Y, Cui M, Li T, Song H, Gao J, Zhang Y, Yang S, Liao Q. Dopamine improves chemotherapeutic efficacy for pancreatic cancer by regulating macrophage-derived inflammations. Cancer Immunol Immunother. 2021;70:2165-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 140. | Zhang M, Pan X, Fujiwara K, Jurcak N, Muth S, Zhou J, Xiao Q, Li A, Che X, Li Z, Zheng L. Pancreatic cancer cells render tumor-associated macrophages metabolically reprogrammed by a GARP and DNA methylation-mediated mechanism. Signal Transduct Target Ther. 2021;6:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 141. | Zhou W, Zhou Y, Chen X, Ning T, Chen H, Guo Q, Zhang Y, Liu P, Li C, Chu Y, Sun T, Jiang C. Pancreatic cancer-targeting exosomes for enhancing immunotherapy and reprogramming tumor microenvironment. Biomaterials. 2021;268:120546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 307] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 142. | Li T, Chen D, Liu H, Tao Y, He X, Zang S, Li J, Zhang L, Li M, Liu J, He Q. Spatially targeting and regulating tumor-associated macrophages using a raspberry-like micellar system sensitizes pancreatic cancer chemoimmunotherapy. Nanoscale. 2022;14:13098-13112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 143. | Parayath NN, Hong BV, Mackenzie GG, Amiji MM. Hyaluronic acid nanoparticle-encapsulated microRNA-125b repolarizes tumor-associated macrophages in pancreatic cancer. Nanomedicine (Lond). 2021;16:2291-2303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 144. | Chen S, Liu H, Li T, Huang R, Gui R, Zhang J. Correlation analysis of coagulation dysfunction and liver damage in patients with novel coronavirus pneumonia: a single-center, retrospective, observational study. Ups J Med Sci. 2020;125:293-296. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 145. | Chen Y, Huang Y, Zhou S, Sun M, Chen L, Wang J, Xu M, Liu S, Liang K, Zhang Q, Jiang T, Song Q, Jiang G, Tang X, Gao X, Chen J. Tailored Chemodynamic Nanomedicine Improves Pancreatic Cancer Treatment via Controllable Damaging Neoplastic Cells and Reprogramming Tumor Microenvironment. Nano Lett. 2020;20:6780-6790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 146. | Cao S, Saw PE, Shen Q, Li R, Liu Y, Xu X. Reduction-responsive RNAi nanoplatform to reprogram tumor lipid metabolism and repolarize macrophage for combination pancreatic cancer therapy. Biomaterials. 2022;280:121264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 147. | Luo X, Hu D, Gao D, Wang Y, Chen X, Liu X, Zheng H, Sun M, Sheng Z. Metabolizable Near-Infrared-II Nanoprobes for Dynamic Imaging of Deep-Seated Tumor-Associated Macrophages in Pancreatic Cancer. ACS Nano. 2021;15:10010-10024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 148. | Liu Q, Li Y, Niu Z, Zong Y, Wang M, Yao L, Lu Z, Liao Q, Zhao Y. Atorvastatin (Lipitor) attenuates the effects of aspirin on pancreatic cancerogenesis and the chemotherapeutic efficacy of gemcitabine on pancreatic cancer by promoting M2 polarized tumor associated macrophages. J Exp Clin Cancer Res. 2016;35:33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 149. | Liu Q, Wu H, Li Y, Zhang R, Kleeff J, Zhang X, Cui M, Liu J, Li T, Gao J, Pan B, Wu W, Wang W, Zhou L, Guo J, Dai M, Zhang T, Liao Q, Lu Z, Zhao Y. Combined blockade of TGf-β1 and GM-CSF improves chemotherapeutic effects for pancreatic cancer by modulating tumor microenvironment. Cancer Immunol Immunother. 2020;69:1477-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 150. | Buchholz SM, Goetze RG, Singh SK, Ammer-Herrmenau C, Richards FM, Jodrell DI, Buchholz M, Michl P, Ellenrieder V, Hessmann E, Neesse A. Depletion of Macrophages Improves Therapeutic Response to Gemcitabine in Murine Pancreas Cancer. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 151. | Sun S, Cui Y, Ren K, Quan M, Song Z, Zou H, Li D, Zheng Y, Cao J. 8-bromo-7-methoxychrysin Reversed M2 Polarization of Tumor-associated Macrophages Induced by Liver Cancer Stem-like Cells. Anticancer Agents Med Chem. 2017;17:286-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 152. | Wang G, Chen Z. HBV Genomic Integration and Hepatocellular Carcinoma. Adv Gut Micro Res. 2022;2022:1-7. [DOI] [Full Text] |
| 153. | Yao RR, Wang MM, Wang YH. [Effect of tumor-associated macrophages in promoting epithelial-mesenchymal transition of Hep3B hepatoma cells and related mechanisms]. Zhonghua Gan Zang Bing Za Zhi. 2018;26:305-309. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 154. | Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, Simeone DM, Zou W, Welling TH. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 531] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 155. | Li M, Lai X, Zhao Y, Zhang Y, Li M, Li D, Kong J, Jing P, Li H, Qin H, Shen L, Yao L, Li J, Dou K, Zhang J. Loss of NDRG2 in liver microenvironment inhibits cancer liver metastasis by regulating tumor associate macrophages polarization. Cell Death Dis. 2018;9:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 156. | Chen M, Lai R, Lin X, Chen W, Wu H, Zheng Q. Downregulation of triggering receptor expressed on myeloid cells 1 inhibits invasion and migration of liver cancer cells by mediating macrophage polarization. Oncol Rep. 2021;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 157. | Wang L, Hu YY, Zhao JL, Huang F, Liang SQ, Dong L, Chen Y, Yu HC, Bai J, Yang JM, Fan JY, Feng L, Li SZ, Han H, Qin HY. Targeted delivery of miR-99b reprograms tumor-associated macrophage phenotype leading to tumor regression. J Immunother Cancer. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 158. | Zhu WP, He XG, Zhao YM, Pan Q, Zhang N, Zhou JM, Wang LR, Wang M, Liu ZY, Zhu HX, Wang L. [Effect of monoacylglycerol lipase with proliferation of MHCC97H human liver cancer cells in vivo]. Zhonghua Gan Zang Bing Za Zhi. 2019;27:516-520. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 159. | Wu Q, Zhou W, Yin S, Zhou Y, Chen T, Qian J, Su R, Hong L, Lu H, Zhang F, Xie H, Zhou L, Zheng S. Blocking Triggering Receptor Expressed on Myeloid Cells-1-Positive Tumor-Associated Macrophages Induced by Hypoxia Reverses Immunosuppression and Anti-Programmed Cell Death Ligand 1 Resistance in Liver Cancer. Hepatology. 2019;70:198-214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 160. | Ou DL, Chen CW, Hsu CL, Chung CH, Feng ZR, Lee BS, Cheng AL, Yang MH, Hsu C. Regorafenib enhances antitumor immunity via inhibition of p38 kinase/Creb1/Klf4 axis in tumor-associated macrophages. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 161. | Gou Y, Yang D, Tian T, Zhu X, Zhang R, Ren J, Tu D, Luo Y, Miao Y, Zhao H, Wang Y, Wei B. The Transcription of ZIP9 Is Associated With the Macrophage Polarization and the Pathogenesis of Hepatocellular Carcinoma. Front Immunol. 2022;13:725595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 162. | Cheng Y, Bai F, Ren X, Sun R, Guo X, Liu W, Wang B, Yang Y, Zhang X, Xu Y, Li C, Yang X, Gao L, Ma C, Li X, Liang X. Phosphoinositide-Binding Protein TIPE1 Promotes Alternative Activation of Macrophages and Tumor Progression via PIP3/Akt/TGFβ Axis. Cancer Res. 2022;82:1603-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 163. | Okikawa S, Morine Y, Saito Y, Yamada S, Tokuda K, Teraoku H, Miyazaki K, Yamashita S, Ikemoto T, Imura S, Shimada M. Inhibition of the VEGF signaling pathway attenuates tumorassociated macrophage activity in liver cancer. Oncol Rep. 2022;47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 164. | Ezhilarasan D. Hepatic stellate cells in the injured liver: Perspectives beyond hepatic fibrosis. J Cell Physiol. 2022;237:436-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 165. | Wang Y, Tiruthani K, Li S, Hu M, Zhong G, Tang Y, Roy S, Zhang L, Tan J, Liao C, Liu R. mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv Mater. 2021;33:e2007603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 166. | Li G, Liu D, Kimchi ET, Kaifi JT, Qi X, Manjunath Y, Liu X, Deering T, Avella DM, Fox T, Rockey DC, Schell TD, Kester M, Staveley-O'Carroll KF. Nanoliposome C6-Ceramide Increases the Anti-tumor Immune Response and Slows Growth of Liver Tumors in Mice. Gastroenterology. 2018;154:1024-1036.e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 114] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 167. | Sprinzl MF, Puschnik A, Schlitter AM, Schad A, Ackermann K, Esposito I, Lang H, Galle PR, Weinmann A, Heikenwälder M, Protzer U. Sorafenib inhibits macrophage-induced growth of hepatoma cells by interference with insulin-like growth factor-1 secretion. J Hepatol. 2015;62:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 168. | Sun R, Zhang Z, Bao R, Guo X, Gu Y, Yang W, Wei J, Chen X, Tong L, Meng J, Zhong C, Zhang C, Zhang J, Sun Y, Ling C, Tong X, Yu FX, Yu H, Qu W, Zhao B, Guo W, Qian M, Saiyin H, Liu Y, Liu RH, Xie C, Liu W, Xiong Y, Guan KL, Shi Y, Wang P, Ye D. Loss of SIRT5 promotes bile acid-induced immunosuppressive microenvironment and hepatocarcinogenesis. J Hepatol. 2022;77:453-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 83] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 169. | Wang X, Tan Y, Zhang Y, Xu Z, Xu B, Lei H, Ding C, Cheng S, Wang X, Wei P, Wang Z, Mao Q, Ai C, Hua Q. The novel glycyrrhetinic acid-tetramethylpyrazine conjugate TOGA induces anti-hepatocarcinogenesis by inhibiting the effects of tumor-associated macrophages on tumor cells. Pharmacol Res. 2020;161:105233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 170. | Guo B, Li L, Guo J, Liu A, Wu J, Wang H, Shi J, Pang D, Cao Q. M2 tumor-associated macrophages produce interleukin-17 to suppress oxaliplatin-induced apoptosis in hepatocellular carcinoma. Oncotarget. 2017;8:44465-44476. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 171. | Song X, Li N, Liu Y, Wang Z, Wang T, Tan S, Li C, Qiu C, Gao L, Asano K, Tanaka M, Liang X, Liu X, Ma C. CD169-positive macrophages enhance abscopal effect of radiofrequency ablation therapy in liver cancer. Transl Oncol. 2022;15:101306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 172. | Zhang Y, Sha W, Zhang X, Cheng M, Wu Q, Wang W, Yuan Z. Zwitterionic chitooligosaccharide-modified ink-blue titanium dioxide nanoparticles with inherent immune activation for enhanced photothermal therapy. Biomater Sci. 2019;7:5027-5034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |