Published online Apr 15, 2023. doi: 10.4251/wjgo.v15.i4.571
Peer-review started: October 15, 2022
First decision: November 2, 2022
Revised: November 11, 2022
Accepted: March 15, 2023
Article in press: March 15, 2023
Published online: April 15, 2023
Processing time: 178 Days and 17.5 Hours
Pancreatic adenocarcinoma (PDAC) is a fatal disease with a 5-year survival rate of 8% and a median survival of 6 mo. In PDAC, several mutations in the genes are involved, with Kirsten rat sarcoma oncogene (90%), cyclin-dependent kinase inhibitor 2A (90%), and tumor suppressor 53 (75%–90%) being the most common. Mothers against decapentaplegic homolog 4 represents 50%. In addition, the self-preserving cancer stem cells, dense tumor microenvironment (fibrous accounting for 90% of the tumor volume), and suppressive and relatively depleted immune niche of PDAC are also constitutive and relevant elements of PDAC. Molecular targeted therapy is widely utilized and effective in several solid tumors. In PDAC, targeted therapy has been extensively evaluated; however, survival improvement of this aggressive disease using a targeted strategy has been minimal. There is currently only one United States Food and Drug Administration-approved targeted therapy for PDAC – erlotinib, but the absolute benefit of erlotinib in combination with gemcitabine is also minimal (2 wk). In this review, we summarize current targeted therapies and clinical trials targeting dysregulated signaling pathways and components of the PDAC oncogenic process, analyze possible reasons for the lack of positive results in clinical trials, and suggest ways to improve them. We also discuss emerging trends in targeted therapies for PDAC: combining targeted inhibitors of multiple pathways. The PubMed database and National Center for Biotechnology Information clinical trial website (www.clinicaltrials.gov) were queried to identify completed and published (PubMed) and ongoing (clinicaltrials.gov) clinical trials (from 2003-2022) using the keywords pancreatic cancer and targeted therapy. The PubMed database was also queried to search for information about the pathogenesis and molecular pathways of pancreatic cancer using the keywords pancreatic cancer and molecular pathways.
Core Tip: Pancreatic adenocarcinoma (PDAC) is a fatal and rare disease with a 5-year survival rate of 8% and a median survival of 6 mo. In PDAC, targeted therapy has been extensively evaluated; however, survival improvement of this aggressive disease using a targeted strategy has been minimal. This manuscript summarizes current targeted therapies and clinical trials targeting dysregulated signaling pathways and components of the PDAC oncogenic process, analyzes possible reasons for the lack of positive results in clinical trials, and suggests ways to improve them. We also discuss emerging trends in targeted therapies for PDAC: combining targeted inhibitors of multiple pathways.
- Citation: Fang YT, Yang WW, Niu YR, Sun YK. Recent advances in targeted therapy for pancreatic adenocarcinoma. World J Gastrointest Oncol 2023; 15(4): 571-595
- URL: https://www.wjgnet.com/1948-5204/full/v15/i4/571.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i4.571
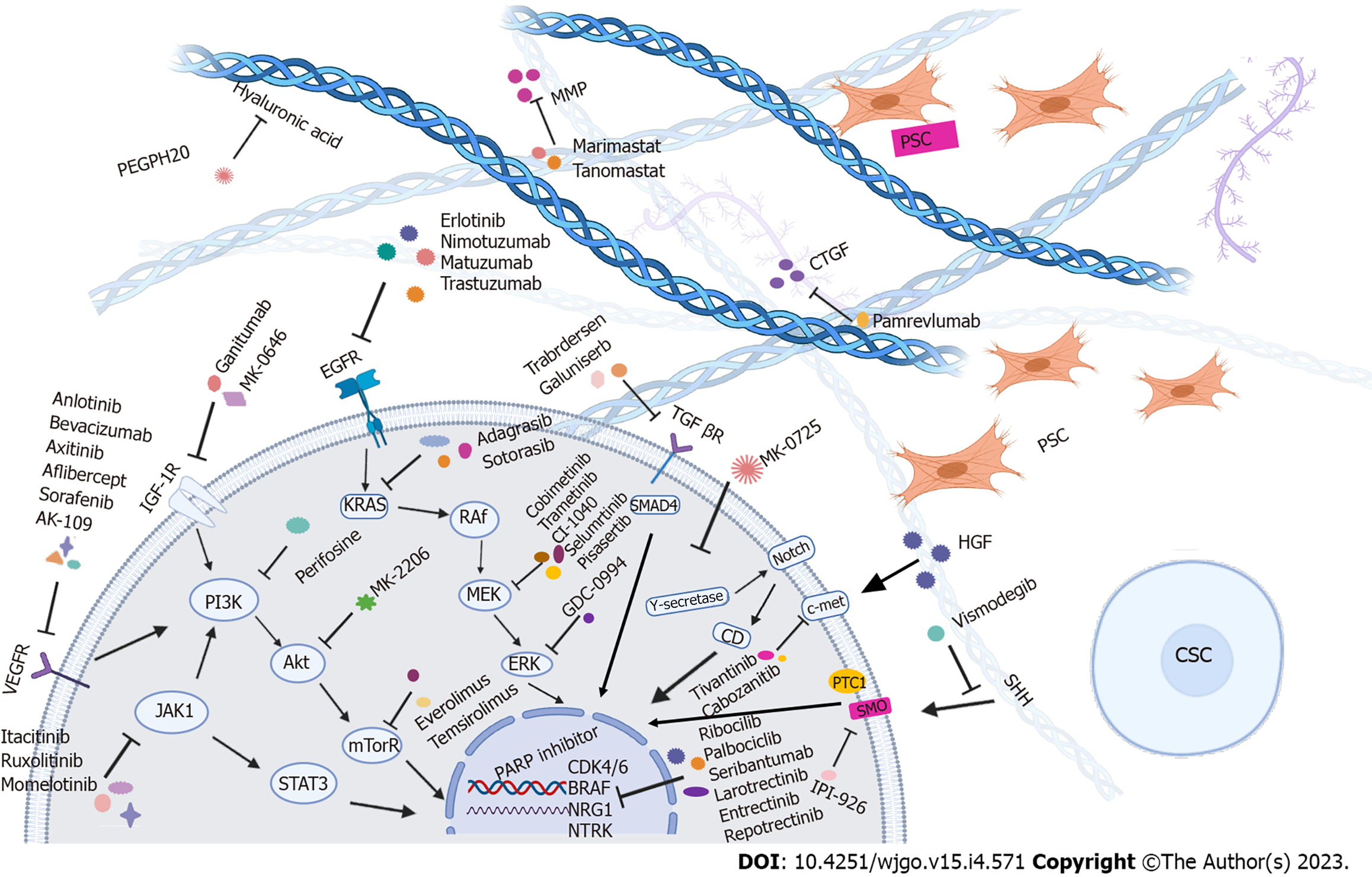
Pancreatic adenocarcinoma (PDAC) is a fatal disease with a 5-year survival rate of 8% and a median survival of 6 mo[1]. It ranks fourth among cancer-related deaths in the United States, and will become the number two cause within a decade[2]. In PDAC, several mutations in the genes are involved, with Kirsten rat sarcoma oncogene (KRAS) (90%), cyclin-dependent kinase inhibitor 2A (CDKN2A) (90%), and tumor suppressor 53 (TP53) (75%–90%) being the most common. Mothers against decapentaplegic homolog 4 (SMAD4) represents 50% (Table 1). In addition, the self-preserving cancer stem cells (CSCs), dense tumor microenvironment (fibrous accounting for 90% of the tumor volume), and suppressive and relatively depleted immune niche of PDAC are also constitutive and relevant elements of PDAC. They are considered significant clinical barriers to successful therapy development, making PDAC one of the most challenging diseases to treat. At present, only surgical resection is a potentially curative treatment for this refractory disease, which shows improvement in survival rates[3,4].
| Target | Frequency of mutation/expression, % |
| KRAS | 95 |
| VEGF | 93 |
| Sonic hedgehog | 70 |
| Notch3 | 69-74 |
| TP53 | 70 |
| NF-kB | 70 |
| IGF-1R | 64 |
| CDKN2A | 60 |
| EGFR | 43-69 |
| Akt/mTOR | 40 |
| SMAD | 40 |
| BRCA1/2 | 1-3 |
| NRG1 fusion | 0.5 |
| NTRK fusion | 0.3 |
Conventional cytotoxic treatments, such as chemotherapy and radiation therapy, have not been successful in improving the chances of survival in pancreatic cancer patients. Since 2011, two combination regimens for metastatic pancreatic cancer have become the gold standard: 5-fluorouracil/leucovorin with irinotecan and oxaliplatin (FOLFIRINOX); and nab-paclitaxel with gemcitabine. With these approaches, response rates range from 23% to 31%, progression-free survival (PFS) rates are 5.5–6.6 mo, and overall survival (OS) is between 8.5 and 11 mo. Single-agent gemcitabine, and its combinations, have failed to provide the expected results, only achieving moderate life expectancy prolongation. However, most patients are diagnosed at the unresectable stage. Therefore, the development of novel and effective therapeutic strategies is vital to improving treatments that are both targeted and personalized.
Imatinib ushered the era of targeted therapies for solid tumors in 2001. Since then, targeted therapies have been approved for renal, colorectal, gastroenteropancreatic neuroendocrine tumors, non-small cell lung cancer, and malignant melanoma[5-9]. There is only one United States Food and Drug Administration (FDA)-approved targeted therapy for PDAC-erlotinib, an epidermal growth factor receptor (EGFR) inhibitor, combined with gemcitabine hydrochloride in patients with metastatic, locally advanced, or unresectable PDAC. However, the absolute benefit of gemcitabine plus erlotinib is also minimal (2 wk)[10].
In this review, we summarize current targeted therapies and clinical trials targeting dysregulated signaling pathways and components of the PDAC oncogenic process, analyze possible reasons for the lack of positive results in clinical trials, and suggest ways to improve them. We also discuss emerging trends in targeted therapies for PDAC: combining targeted inhibitors of multiple pathways. The PubMed database and National Center for Biotechnology Information clinical trial website (www.clinicaltrials.gov) were queried to identify completed and published (PubMed) and ongoing (clinicaltrials.gov) clinical trials (from 2003-2022) using the keywords pancreatic cancer and targeted therapy. The PubMed database was also queried to search for information about the pathogenesis and molecular pathways of pancreatic cancer using the keywords pancreatic cancer and molecular pathways.
Targeted therapy highlights the association between tumor characteristics and individualized treatment response. Biomarkers and genomic mutations may serve as potential targets or prognostic indicators based on the expression of biomarkers. Overall, targeted therapies are based on three main approaches: inhibition of aberrant activation of oncogenes, interference with inactivation of tumor suppressor genes, and exploitation of biological functional defects in specific genes.
Most pancreatic tumors (about 95%) carry RAS mutations, the most common of which are KRAS alterations (85%)[11]. Mutations of KRAS and other genes, such as inactivation of CDKN2A (in about 90% of PDAC cases) and SMAD4/DPC4 (approximately 55%), breast cancer susceptibility gene 2 (BRCA2), MutL homolog 1, or protease serine 1 alterations accumulate throughout the development of tumors. Approximately 50%-70% of PDAC cases carry mutations in the TP53 gene, which occurs in late pancreatic intraepithelial neoplasia and leads to the malignant progression of PDAC[12]. As a result of these mutations, multiple critical processes-related signaling pathways are dysregulated, including apoptosis and cell proliferation. In addition, key molecules and pathways from the tumor and surrounding stroma, such as EGFR-mediated and pro-angiogenic pathways, influence the resistance of PDAC to therapy and are associated with a poor prognosis[13]. A total of 60 mutations in 12 different signaling pathways accompany the occurrence of aberrant ducts in PDAC[14], making targeted therapy a possible way to improve the efficacy of existing therapies (Table 2, Figure 1).
| Target | Study treatment | Phase | Population | No. of patients | mPFS | mOS | Ref. |
| EGFR | GEM + Erlotinib | III | Locally advanced or metastatic PDAC | 285 | 3.75 | 6.24 | Moore et al[10], 2007 |
| GEM + Placebo | 284 | 3.55 | 5.91 | ||||
| EGFR | GEM + Nimotuzumab | IIb | Locally advanced or metastatic PC | 96 | 5.1 | 8.6 | Schultheis et al[50], 2017 |
| GEM + Placebo | 96 | 3.4 | 6 | ||||
| EGFR | GEM + Nimotuzumab | III | K-Ras wild-type, locally advanced or metastatic PC | 46 | 4.2 | 10.9 | Qin et al[51], 2022) |
| GEM + Placebo | 46 | 3.6 | 8.5 | ||||
| ERB2 | GEM + Afatinib | II | Metastatic PC | 79 | 3.9 | 7.3 | Haas et al[57], 2021 |
| GEM + Placebo | 40 | 3.9 | 7.4 | ||||
| VEGF | GEM + axitinib | II | Advanced PC | 69 | 4.2 | 6.9 | Spano et al[67], 2008 |
| GEM | 34 | 3.7 | 5.6 | ||||
| VEGF | GEM + axitinib | III | Advanced PDAC | 314 | 4.4 | 8.5 | Kindler et al[68], 2011 |
| GEM + Placebo | 316 | 4.4 | 8.3 | ||||
| VEGF | GEM + aflibercept | III | Metastatic PC | 271 | 3.7 | 6.5 | Rougier et al[69], 2013 |
| GEM + Placebo | 275 | 5.1 | 7.8 | ||||
| PARP | Veliparib | II | BRCA-mutated PDAC | 16 | 3.1 | 1.7 | Lowery et al[101], 2018 |
| PARP | Olaparib | III | gBRCA1 or BRCA2 mutation and metastatic PC | 92 | 7.4 | 18.9 | Golan et al[103], 2019 |
| Placebo | 62 | 3.8 | 18.1 | ||||
| PARP | Cisplatin and GEM + Veliparib | II | Untreated gBRCA/PALB2+ PDAC with measurable stage III to IV PDAC | 27 | 10.1 | 15.5 | Sohal et al[40], 2020 |
| Cisplatin and GEM | 23 | 9.7 | 16.4 | ||||
| RET | GEM + Vandetanib | II | Locally advanced or metastatic PC | 72 | NA | 8.83 | Middleton et al[107], 2017 |
| GEM + Placebo | 70 | 8.95 | |||||
| Hedgehog | GEM + Vismodegib | II | Metastatic PC | 53 | 4 | 6.9 | Catenacci et al[160], 2015 |
| GEM + Placebo | 53 | 2.5 | 6.1 | ||||
| Hyaluronic acid | mFOLFIRINOX + PEGPH20 | II | Metastatic PDAC | 55 | 4.3 | 7.7 | Ramanathan et al[169], 2019 |
| mFOLFIRINOX | 59 | 6.2 | 14.4 | ||||
| MMP | GEM + Marimastat | NA | Advanced PC | 120 | NA | 5.51 | Bramhall et al[172], 2002 |
| GEM + Placebo | 119 | 5.47 | |||||
| MMPs | Tanomastat | III | Advanced or Metastatic PDAC | 138 | 1.68 | 3.74 | Moore et al[173], 2003 |
| GEM | 139 | 3.5 | 6.59 | ||||
| NOTCH | RO4929097 | II | Previously treated metastatic PDAC | 18 | 1.5 | 4.1 | De Jesus-Acosta et al[190], 2014 |
| NOTCH | GEM + Tarextumab | II | Untreated metastatic PC | 89 | 3.7 | 6.4 | Hu et al[193], 2019 |
| GEM + Placebo | 88 | 5.5 | 7.9 | ||||
| Wnt | GEM and nab-paclitaxel + Ipafricept | Ib | Untreated stage IV PC | 26 | 5.9 | 9.7 | Dotan et al[196], 2020 |
| Autophagy | GEM and nab-paclitaxel + Hydroxychloroquine | II | Advanced PC | 55 | 5.7 | 11.1 | Karasic et al[209], 2019 |
| GEM and nab-paclitaxel | 57 | 6.4 | 12.1 |
KRAS: KRAS oncogenic mutations can be observed in more than 90% of PDAC cases. Unfortunately, in mouse models, the resulting mitogen-activated protein kinase (MAPK) inhibition after KRAS inhibition (or direct blockade of downstream MEK) may further lead to the activation of protein kinase B alpha (Akt), EGFR, human epidermal growth factor receptor 2 (HER2), platelet-derived growth factor receptor α (PDGFRα), and AXL, resulting in the ineffectiveness of such drugs[15]. Therefore, the development of clinically effective KRAS inhibitors has been challenging. Initially, the strategy to target KRAS was to inhibit farnesyltransferase, as farnesylation is critical for RAS activation. A phase II trial (SWOG 9924) evaluated the efficacy of an oral farnesyltransferase inhibitor R115777 as first-line therapy for metastatic PDAC patients, but there was no clinical benefit[16]. A novel alternative strategy for targeting KRAS involves the use of exosomes, or small extracellular vesicles loaded with small interfering RNAs targeting KRASG12D, the most common KRAS mutation in PDAC[17], and was studied in a recent phase I trial (NCT03608631) that included patients with metastatic PDAC (mPDAC).
In addition, the KRASG12C mutation was identified in 2% of PDACs[18], and its molecular inhibitors ARS-1620 and sotorasib have shown preliminary antitumor efficacy in preclinical models[19] and patients with advanced solid tumors[20]. To date, only a small subset of patients carrying the KRASG12C mutation can be treated with FDA-approved sotorasib or adagrasib. The CRYSTAL-1 phase II clinical trial applied adagrasib to patients with KRASG12C-mutated pretreated solid tumors, and 1 PDAC patient achieved a partial response. Phase I/II trials (NCT03785249 and NCT04330664) evaluating the effectiveness of adagrasib are ongoing.
Given the difficulty of directly targeting KRAS, therapies targeting its major downstream effector pathways are in development, including the RAS/rapid accelerated fibrosarcoma (RAF)/MEK/extracellular signal-regulated kinase (ERK) and phosphatidylinositol-3 kinase (PI3K)/phosphoinositide-dependent kinase-1/Akt signaling pathways[21].
RAF/MEK/ERK MAPK pathway: Mitogen-activated extracellular kinases are a component of the RAS/RAF/MEK/ERK pathway and play a key role in proliferation, apoptosis, differentiation, and angiogenesis[22]. ERK1/2 MAPK is phosphorylated and activated after RAF serine/threonine kinase phosphorylates and activates MEK1 and MEK2. Activated ERK subsequently modulates the activity of approximately 160 substrates including transcription factors, protein kinases, phosphatases, and regulators of apoptosis[23]. However, several phase II studies of MEK inhibitors did not show efficacy as monotherapy for PDAC including CI-1040[24], selumetinib[25], pimasertib[26], and trametinib[27]. Most likely, the unsatisfactory results were caused by feedback activation and crosstalk between pathways, resulting in the activation of PI3K/mammalian target of rapamycin (mTOR)/Akt[28].
Mirzoeva et al[29] demonstrated the utility of the combinatorial effect of EGFR plus MEK inhibitors in the epithelial molecular subtype of PDAC. In addition, Brauswetter et al[30] identified specific molecular isoforms with KRAS G12C mutants that responded better to MEK inhibition than the more common G12D variant. Therefore, outcomes can be improved by identifying molecular subtypes and appropriate combination therapy to select the right targeted therapy for the right patient.
However, even considering the abovementioned issues, the MEK inhibitors’ therapeutic effect is still unsatisfactory. It was shown in a phase I trial that afatinib combined with selumetinib, an inhibitor of MEK, had limited anticancer activity in patients with KRAS-mutated solid tumors including pancreatic cancers[31]. Similarly, the results of a phase II trial of selumetinib and MK-2206 (Akt inhibitor) in combination with modified FOLFIRINOX showed that the combination was less effective than FOLFIRONIX in PDAC patients, but had more significant toxicity[32]. The THREAD trial evaluated the efficacy of trametinib and hydroxychloroquine in PDAC patients at different stages of PDAC (NCT03825289).
Currently, approximately 10 clinical trials of MEK1/2 inhibitors targeting PDAC (selumetinib, cobimetinib, and trametinib) are underway, and it is crucial to evaluate the results before considering them for the clinical treatment of PDAC patients.
Furthermore, cobimetinib (MEK inhibitor) or GDC-0994 (ERK1/2 inhibitor) alone only transiently suppresses the MAPK pathway in KRAS mutant cancer cell lines[33,34]. Alternatively, co-targeting MEK and ERK with these drugs demonstrates significant antitumor activity in cancer cells and tumor models with dysregulated MAPK pathways. However, in the clinical setting, combining cobimetinib and GDC-0994 in clinical settings is no longer recommended due to overlapping adverse events (AEs)[35]. Overall, developing inhibitors targeting this pathway is promising, but further research is needed to find more appropriate combinations while reducing AEs.
KRAS wild-type PDAC: As mentioned above, most patients with PDAC have KRAS mutations. In the small subset of patients with KRAS wild-type (WT) PDAC, other mutations, such as neurotrophic receptor tyrosine kinase (NTRK) and neuregulin 1 (NRG1), can initiate PDAC tumorigenesis and be targeted. The incidence of NTRK fusions is 0.3%[36]. Chromosomal rearrangements in the NTRK gene family promote the expression of chimeric rearranged promyosin receptor kinases[37]. It is possible that these chimeric proteins signal through the same MAPK and PI3K/Akt pathways as normal TRK proteins and are involved in tyrosine kinase crosstalk[38]. Therefore, a promising approach for targeted therapy is to address fusions of tropomyosin receptor kinase genes 1, 2, or 3 (NTRK1, 2, 3).
In solid tumors with NTRK gene fusions, regardless of tumor type, larotrectinib, and other TRK inhibitors have shown significant and durable antitumor activity (overall response rate 75%, 95% confidence interval [CI]: 61%-85%)[39]. The latest American Society of Clinical Oncology-Gastro
NRG1 fusions are rare oncogenic drivers, found in approximately 0.2% of all solid tumors[36]. These fusions trigger hyperactivation of ERBB3/HER3, which drives tumor growth and cancer cell survival. Seribantumab is a fully humanized anti-HER3 immunoglobulin G2 (IgG2) monoclonal antibody (mAb) that inhibits tumor growth in NRG1 fusion-driven preclinical models. CRESTONE is a phase II trial of seribantumab in patients with locally advanced or metastatic solid tumors with NRG1 fusions. Preliminary data suggest that seribantumab induces durable responses with a favorable safety profile. These data support the continued evaluation of seribantumab in the CRESTONE study (NCT04383210).
EGFR: EGFR is highly expressed in 30%-50% of PDACs[45-47]. Interestingly, EGFR signaling input is required for pancreatic carcinogenesis even in the presence of an oncogenic KRAS mutation[48,49]. The small molecule erlotinib, a selective inhibitor of EGFR tyrosine kinases, is the first approved targeted therapy in PDAC. In a phase III trial of metastatic PDAC, the combination of gemcitabine and erlotinib improved median OS (mOS) significantly by 0.33 mo (about 10 d) in the entire study population[10].
Nimotuzumab, an anti-EGFR mAb, showed significantly prolonged OS in combination with gemcitabine vs gemcitabine monotherapy in a phase II trial (median PFS 3.2 mo vs 5.5 mo, hazard ratio [HR] 0.55, P = 0.0096; median OS 5.2 mo vs 8.6 mo, HR 0.66, P = 0.034)[50]. A phase III trial (NCT02395016) showed that nimotuzumab in combination with gemcitabine improved OS and PFS in patients harboring KRAS WT with locally advanced or metastatic pancreatic cancer, with significantly longer median OS in the nitrozumab-gemcitabine group (10.9 mo vs 8.5 mo, HR = 0.50, 95%CI: 0.06-0.94; P = 0.025). In addition, median PFS was 4.2 mo in the trial group compared with 3.6 mo in the control group (HR = 0.56, 95%CI: 0.12-0.99; P = 0.013)[51].
Positive trends have been reported for the EGFR inhibitors matuzumab (phase I)[52] and panitumumab in combination with gemcitabine and erlotinib (phase II)[53]. By contrast, the combination of cetuximab and gemcitabine failed to improve OS, with an mOS of 6.3 mo and PFS of 3.4 mo in the combination arm, compared with 5.9 and 3 mo, respectively, in the gemcitabine monotherapy arm[54].
Trastuzumab, a humanized Ab against HER2, has not yet improved the prognosis of pancreatic cancer in clinical trials. The 12-wk PFS rate for trastuzumab in combination with capecitabine was 23.5%, with a median OS of 7.0 mo[55]. Another recombinant humanized mAb against HER2, pertuzumab, has been used to treat solid tumors including pancreatic cancer. Two pancreatic cancer patients showed partial responses with stable disease for 15.3 mo in 1 patient[56]. Afatinib, a second-generation irreversible inhibitor of ERBB receptors (both EGFR and HER2/neu), is approved as monotherapy for the first-line treatment of non-small cell lung cancer (NSCLC) with EGFR mutations and treatment of lung squamous cell carcinoma after failure of platinum-based chemotherapy. A phase II trial conducted by the “Arbeitsgemeinschaft Internistische Onkologie” was designed to evaluate whether the gemcitabine/afatinib combination was more effective than gemcitabine alone in metastatic PDAC. However, adding afatinib to gemcitabine did not improve therapeutic efficacy and was more toxic. Median OS in the combination group was 7.3 and 7.4 mo in the gemcitabine group. The median PFS was identical in both groups (3.9 mo vs 3.9 mo). In addition, AEs were more frequent in the combination group, especially diarrhea (71% vs 13%) and rash (65% vs 5%)[57].
Vascular endothelial growth factor: Overexpression of vascular endothelial growth factor (VEGF) in PDAC is associated with tumor progression and poorer prognosis[58,59]. However, angiogenesis-targeted therapy is clinically ineffective in pancreatic cancer patients. The reason may be that dense stromal tissue with reduced vascular density impedes the delivery of effective drugs. Moreover, the withdrawal of antiangiogenic agents after therapy may be associated with increased tumor aggressiveness and invasion, offsetting the potential therapeutic benefits offered by antiangiogenic agents[60].
Multiple clinical trials of antiangiogenic agents have been conducted to treat PDAC, yet the results have been overwhelmingly disappointing. For PDAC patients, it has shown improvement in PFS in a few clinical trials[61], but no significant prolongation in OS has been observed. Humanized monoclonal antibodies such as bevacizumab have an affinity for circulating VEGF-A, but phase II and III studies have shown no survival advantage for bevacizumab in combination with gemcitabine and erlotinib[61-64]. A meta-analysis concluded that bevacizumab plus gemcitabine treatment elicited only a moderate response rate without survival modifications[65]. Other VEGF inhibitors, such as axitinib and aflibercept, provide no survival advantage[66-69]. Likewise, sorafenib (an inhibitor of VEGFR and RAS/RAF/MAPK signaling) had no additional value for patient survival over gemcitabine[70].
The promising drug in the field is currently anlotinib. Anlotinib is a novel oral tyrosine kinase inhibitor that targets VEGFR, fibroblast growth factor receptor, PDGFR, and c-kit. Compared to the placebo, it improved PFS and OS in a phase III trial in patients with advanced NSCLC[71]. A phase II trial of anlotinib, toripalimab, and nab-paclitaxel in patients with locally advanced/metastatic pancreatic cancer is underway (NCT04718701). A first-in-human phase I study of AK109, an anti-VEGFR2 Ab, in patients with advanced or metastatic solid tumors, including 2 patients with pancreatic cancer (2/40), showed a controlled safety profile and promising antitumor activity (NCT04547205). Two phase II studies of AK109 in combination with AK104 (anti-PD-1/cytotoxic T-lymphocyte-associated protein 4 [CTLA-4] bispecific Ab) are being evaluated in patients with multiple solid tumors (NCT05142423, NCT04982276).
Insulin-like growth factor receptor 1: Insulin-like growth factor receptor 1 (IGF-1R), a transmembrane receptor tyrosine kinase, is overexpressed in pancreatic cancer. Activation of IGF-1R is associated with decreased apoptosis, cancer cell proliferation, and angiogenesis[72,73]. Yet the use of gemcitabine and a single IGF-1R inhibitor alone has not achieved satisfactory clinical results. A phase III clinical trial of the IGF-1R mAb ganitumab showed no improvement in patient survival[74].
A previous study showed that the simultaneous blockade of IGF1R and EGFR/HER2 synergistically inhibited pancreatic tumor growth and eliminated the activation of IRS-1, Akt, and MAPK phosphorylation. Based on this, combining these two inhibitors may prevent drug-resistance reactions caused by monotherapy[75]. A phase I/II study of gemcitabine and erlotinib in combination or not with MK-0646, an IGF1R inhibitor, in advanced pancreatic cancer showed that the combination of MK-0646 with gemcitabine plus erlotinib was tolerable and improved OS but not PFS compared with gemcitabine plus erlotinib[76]. Istiratumab (MM-141), a quadrivalent bispecific Ab recognizing IGF-1R and ERBB3, provided promising results in preclinical studies[77], but its phase II clinical trial was negative[78].
The overexpression of Akt is found in more than 40% of PDAC cases[79,80]. PI3K/Akt/mTOR, as a critical pathway in many aspects of cell growth, survival, and apoptosis, plays an essential role in the occurrence and development of various tumors including PDAC[81]. Dysregulation of this pathway may lead to tumor resistance to chemotherapy[82,83]. It has been documented that activation of Akt is associated with a poor prognosis[84,85]. Inhibition of Akt signaling induces apoptosis and limits tumor growth[86].
Alkyl phospholipid perifosine acts as an inhibitor of Akt and PI3K phosphorylation[87]. Combining perifosine with gemcitabine exhibits synergistic effects on pancreatic cancer cells expressing high levels of phosphorylated Akt, primarily inhibiting tumor migration/invasion and inducing tumor cell apoptosis[88].
Clinical activity of everolimus (mTOR inhibitor) in patients with gemcitabine-refractory pancreatic cancer was limited, with a median PFS of 1.8 mo and median OS of 4.5 mo[89]. Combining everolimus with capecitabine achieved appropriate efficacy, with a mean OS of 8.9 mo (95%CI: 4.6-13.1) and median PFS of 3.6 mo (95%CI: 1.9-5.3)[90]. Temsirolimus is another mTOR inhibitor tested in locally developed or metamorphosic conditions[91,92]. A phase I/II trial evaluating sirolimus, a selective inhibitor of mTOR, enrolls patients with advanced pancreatic cancer (NCT03662412). In addition, other drugs targeting this pathway have been developed such as PI3K inhibitors, BKM120 and BYL179 (NCT02155088); RX-0201 (Akt antisense oligonucleotide inhibitor); and BEZ235 (combined inhibitor of PI3K and mTOR)[93,94].
Germline BRCA mutation is an autosomal dominant mutation associated with an increased risk of breast, gynecologic, colorectal, and pancreatic cancers. In families with germline BRCA2 mutations, the relative risk of pancreatic cancer is 3.5% (95%CI: 1.9–6.6)[95]. Mounting evidence has demonstrated that BRCA1/2 mutant breast and ovarian cancers are susceptible to DNA damage-related therapies, including poly (ADP-ribose) polymerase inhibitors (PARPis) and platinum-based drugs[96].
Clinically, PARPis have shown significant efficacy against other refractory BRCA-mutated solid tumors[97-100]. Olaparib is a PARPi that was effective in a single-arm phase II trial[98]. Veliparib, another PARPi, has modest activity in patients with previously platinum-treated germline BRCA1/2 mutation-positive pancreatic cancer[101]. The RUCAPANC study, which evaluated the PARPi rucaparib, was discontinued during the interim analysis due to a lack of patient response[102].
A phase II trial of niraparib, a highly specific PARP-1 and PARP-2 inhibitor, is currently being conducted in metastatic PDAC patients with somatic or germline defects in multiple DDR genes (NCT05442749). A randomized phase II trial (PARPVAX) of niraparib (nira) vs an immune checkpoint inhibitor, nivolumab (nivo, PD-1 mAb) or ipilimumab (ipi, CTLA-4 mAb), has been evaluated in a non-genomic selected, advanced PDAC patient population that has received at least 16 wk of platinum-based therapy without progression (NCT03553004). Another similar trial showed that compared to nira/nivo, nira/ipi prolonged median PFS as maintenance therapy for advanced PDAC patients with no progressive disease after first-line platinum-based chemotherapy, with an mPFS of 1.9 mo (95%CI: 1.8-1.9) for nira/nivo and 7.6 mo (95%CI: 4.0-11.1) for nira/ipi (NCT03404960).
A prospective phase III trial (POLO, NCT02184195) evaluated olaparib in metastatic PDAC patients with BRCA mutations[103]. The results indicated that metastatic PDAC patients with germline BRCA1 or BRCA2 mutations were significantly less likely to progress after taking olaparib. The trial included 154 patients with germline BRCA mutations whose tumors had not progressed after 16 wk of platinum-based induction chemotherapy. They were randomly assigned: 92 to receive olaparib and 62 to placebo. PFS was significantly longer in the olaparib group compared with the placebo group (median PFS: 7.4 mo vs 3.8 mo, HR = 0.53; P = 0.004). There was no difference in OS between the placebo and olaparib groups, despite the fact that some patients in the placebo group received PARPis as follow-up therapy. The risk of disease progression was reduced by 47% in the olaparib group, and patients treated with olaparib were at least twice as likely to be disease progression-free at 6, 12, 18, and 24 mo as those receiving a placebo. Based on this, National Comprehensive Cancer Network guidelines included olaparib as recommended maintenance therapy for PDAC patients with germline BRCA1/2 mutations, good performance status, metastatic disease, and no disease progression after 4-6 mo of first-line chemotherapy. In addition, the safety of olaparib was also validated in the POLO trial, where patients’ health-related quality of life was assessed and found to remain unchanged with no clinically meaningful deterioration. Grade ≥ 3 or higher AEs occurred in 39.6% of the olaparib group and 23.3% of the placebo group; 5.5% and 1.7% of patients discontinued treatment due to AEs, respectively[99,104].
Multidrug combination therapy is also a promising strategy. Antiangiogenic agents act synergistically with PARP inhibitors, resulting in increased levels of hypoxia and downregulation of homology-driven repair genes[105]. This combination will be further investigated in a phase II trial, including patients with mPDAC (NCT02498613). In addition, an ongoing phase II trial is evaluating the efficacy of olaparib in combination with pembrolizumab (an immunotherapy cancer drug) in patients with BRCA-mutated pancreatic cancer (NCT04548752). A phase II trial evaluating talazoparib in patients with advanced cancer and DNA repair variants is ongoing (NCT04550494).
Genetic abnormalities in the RET proto-oncogene have been reported in PDAC. In phase I trials for pancreatic and biliary tract cancer, vandetanib (a multitargeted tyrosine kinase inhibitor of EGF, VEGFR, and RET) was evaluated in combination with gemcitabine and capecitabine. A 78% disease control rate (> 2 mo), 3 partial responses, and 15 patients with stable disease were observed in this trial[106]. A subsequent phase II trial of vandetanib in combination with gemcitabine vs gemcitabine monotherapy has shown that the combination did not improve OS in advanced PDAC (8.83 mo vs 8.95 mo, HR = 1.21, 80.8%CI: 0.95-1.53; P = 0.303)[107]. In addition, LOXO-292, a selective RET inhibitor, is being investigated in a phase I study (NCT03157128).
TP53 tumor suppressor pathway: Contrary to the role of proto-oncogenes, the role of a tumor suppressor is to suppress tumorigenesis. TP53 is the most frequently inactivated suppressor in PDAC, and TP53 gene alterations are found in approximately 70% of PDAC patients[108,109]. p53 is a transcription factor that regulates the expression of multiple genes. Its biological functions include inhibiting cell proliferation by inducing p21 expression, promoting tumor cell apoptosis, maintaining gene stability, and inhibiting tumor vascularization by stimulating B-cell lymphoma 2-associated X protein expression[110,111]. TP53 reactivators include Zn2+ chelators such as COTI-2, cys-targeting agents such as APR-246 and CP-31398, and other proteins that assist in p53 resilience, inhibit abnormal p53 aggregation, or stabilize p53[112].
A clinical trial of COTI-2 is ongoing in patients with TP53 mutant PDAC (NCT02433626). In addition to reactivation, inhibition of Mouse double minute 2 homolog (MDM2) is another emerging strategy for targeting TP53-mutated tumors. The p62-NRF2-MDM2 axis is involved in tumor progression and programming[113], and MDM2 antagonizes p53 through direct interaction or ubiquitin-dependent degradation[114]. Therefore, inhibition of MDM2 may increase p53 activity and suppress p53-mutant cancers[115]. Recent studies have confirmed the efficacy of MDM2 inhibitors, such as Nutlin, MA242, SP141, and MI-319, in vitro and in vivo[116-119]. MANTRA-2 is a phase II trial evaluating the clinical benefit of Milademetan, a selective MDM2 inhibitor, in MDM2 amplified (copy ≥ 12) TP53-WT solid tumors and is currently recruiting (NCT05012397).
Transforming growth factor/β SMAD4 pathway: Another tumor suppressor gene associated with the pathogenesis of pancreatic cancer is the SMAD4 gene, and approximately 40% of PDAC patients carry SMAD4 mutations[109]. In normal cells, the product of this gene (a 64-kDa protein) plays a role in transforming growth factor beta (TGF-β)-mediated signal transduction, gene transcription, and growth arrest. The TGF-β/SMAD4 signaling pathway mediates tumor-stromal interactions and the epithelial-stromal transition. Evidence suggests that TGF-β inhibitors, including trabedersen and galunisertib, reduce tumor metastasis and invasion in animal models[120,121]. A randomized phase II trial showed that galunisertib in combination with gemcitabine improved OS compared with gemcitabine alone[122]. The combination of galunisertib and durvalumab (programmed death-ligand 1 mAb) has also been studied in metastatic PDAC patients[123]. The sponsor has since terminated further studies of galunisertib due to limited clinical activity. Instead, a new generation of TGF-β pathway inhibitors, such as TGF-βR inhibitors and TGF-β-checkpoint traps, are under development[124,125]. NIS793, a human IgG2 mAb TGF-β antagonist, is in a phase III trial to evaluate the efficacy of NIS793 in treatment-naïve patients with mPDAC (NCT04935359). Furthermore, TGF-β levels are reduced in fibroblasts due to blockade of the angiotensin type III receptor[126,127]. Thus the angiotensin receptor blocker losartan was tested in a preclinical model of pancreatic cancer and subsequently tested in combination with FOLFIRINOX in a phase II trial[128], which enabled R0 resection in 69% (30/49) of patients with locally advanced disease[129]. A randomized phase II trial evaluating losartan in combination with FOLFIRINOX and stereotactic body radiotherapy in neoadjuvant setting is ongoing (NCT03563248).
Dysfunctional CDKN2A and CDK4/6 inhibitors: CDKN2A is a multifunctional gene that creates p16 and p19, arrests the cell cycle at the G1/S checkpoint through a CKD4/6-regulated mechanism[130], and the proteins bind to MDM2 to block the reduction in p53 levels[131]. Approximately 60% of PDAC patients carry CDKN2A mutations, with an odds ratio of 12.33, indicating that germline mutations in CDKN2A are associated with a high risk of developing PDAC[108,109]. CDK4/6 is a potential target for CDKN2A-deficient tumors[132,133]. The CDK4/6 inhibitors ribociclib and palbociclib have shown safety and efficacy in metastatic breast cancer and liposarcoma[134,135]. Additionally, CDK4 inhibitors are efficacious in preclinical models of PDAC[136-139], and a related clinical trial (NCT02501902) is ongoing. Researchers have concluded that CDK4/6 inhibitors alone exert limited antitumor effects and can show greater promise when used in combination with other targeted agents[140]. Mechanistically, CDK4/6 inhibitors block DNA repair mechanisms and increase the sensitivity of PDAC cells to PARPis[141]. PDAC cells are more sensitive to immune checkpoint blockers when CDK4/6 and MEK are inhibited jointly[142]. A phase I clinical trial of palbociclib in combination with the PI3K/mTOR inhibitor gedatolisib in advanced PDAC patients is ongoing (NCT03065062).
Nuclear factor kappa B (NF-κB) is a protein complex involved in cell proliferation, cell adhesion, apoptosis, and inflammatory responses[143]. Overexpression of the NF-κB pathway is reported in approximately 70% of pancreatic cancers[144,145]. Curcumin is a potent inhibitor of this pathway, and its effects have been demonstrated in several in vitro and in vivo pancreatic cancer models[146,147].
Nafamostat mesilate (NM) is a synthetic serine protease inhibitor that inhibits NF-kB activation[148]. NM infusion with gemcitabine for inoperable advanced pancreatic cancer was evaluated in a phase I/II study. The median OS and 1-year survival rates were 10 mo and 40%, respectively[149]. Subsequently, a phase II study of NM/gemcitabine adjuvant chemotherapy showed that gemcitabine combined with local arterial perfusion adjuvant chemotherapy with NM is safe and may be an option in the adjuvant setting after curative surgery for pancreatic cancer[150].
PDAC is characterized by dense fibrous stroma representing up to 90% of the tumor volume. Desmoplasia means excessive proliferation of fibrotic tissue with a modified extracellular matrix providing a protumorigenic environment[151,152]. Pancreatic stellate cells play a major role in stromal responses, and they are closely associated with pancreatic cancer cells[153,154], controlling matrix synthesis, cell growth, migration, and invasion through a diverse set of signaling cascades. In addition, hepatocyte growth factor (HGF) from stromal cells was associated with the growth, angiogenesis, and invasiveness of pancreatic cancer[155]. The pro-fibroproliferative response is accompanied by a relatively avascular tumor microenvironment, followed by hypoperfusion and hypoxia in the cancerous tissue, which leads to the generation of more aggressive tumor subclones[156], altered tumor metabolism, increased glycolysis[157], and decreased chemotherapeutic drug concentrations. Therefore, stroma-specific therapeutic strategies can be developed. One way is to directly target specific components of the extracellular matrix, such as matrix metalloproteinases (MMPs), and the other is to target specific signaling pathways that promote the development of the tumor stroma, such as the Sonic Hedgehog (SHH) pathway.
Hedgehog signaling is an essential pathway for proliferation and survival in embryonic development. In response to hedgehog ligand binding to PATCHED 1 receptor protein in target cells, a signaling cascade is triggered, eliminating the inhibitory effect of Smoothened (SMO), which then enhance tumor progression, metastasis, and tumorigenesis[158].
Combined with gemcitabine, cyclopamine, an SMO antagonist, was shown to reduce metastatic potential in the GEMM (KPC) model of PDAC[159]. A phase II trial of vismodegib (a second-generation SMO inhibitor) combined with gemcitabine had a PFS benefit (4 mo vs 2.5 mo; P = 0.30) but did not improve OS (6.9 mo vs 6.1 mo; P = 0.84)[160]. These results are consistent with another clinical trial (NCT01088815)[161]. In addition, a phase I trial (NCT00878163) enrolled metastatic PDAC patients to evaluate the combination of vismodegib and erlotinib. Although the combination was well tolerated and 20% of patients exhibited stable disease, there was no significant tumor shrinkage effect[162]. Overall, the clinical trials with vismodegib did not meet expectations. Thus, the clinical development of this drug has been discontinued. In another phase II trial, saridegib (an SMO inhibitor) plus gemcitabine had a survival disadvantage (NCT01130142). Nevertheless, when combined with FOLFIRINOX, there was clinical activity with an objective response rate of 67%[163]. The clinical development of this drug was also halted.
The reasons for the disappointing results of hedgehog inhibition could be arising SMO mutations under therapy and compensatory feedback loops leading to a (hyper) activation of the PI3K pathway or downstream targets of the hedgehog pathway (e.g., Gli2)[164,165]. This suggests that targeting both the Hedgehog pathway and PI3K pathway could be used for treating pancreatic cancer, as shown in medulloblastoma[166].
Hyaluronic acid (HA) is a glycosaminoglycan that is abundantly present in the extracellular matrix and contributes to the dense desmoplastic stroma surrounding the tumor. The degradation of HA by hyaluronidase may help disrupt the stroma and enhance drug delivery to the tumor[167]. Recombinant human hyaluronidase (PEGPH20) has been studied in mouse models of pancreatic cancer and was found to degrade HA, reduce interstitial fluid pressure, increase vascular permeability, and enhance doxorubicin delivery to tumors. In combination with gemcitabine, PEGPH20 inhibits tumor growth and prolongs survival[167].
The HALO 202 trial examined improvements in PFS in patients with untreated metastatic PDAC. In this phase II trial, 269 patients were randomized to treatment with PEGPH20 plus nab-paclitaxel/gemcitabine (PAG) vs nab-paclitaxel/gemcitabine (AG). The mPFS was significantly improved in the PAG arm for 6 mo vs 5.3 mo in the AG arm (HR = 0.73; P = 0.045). In patients with > 50% of HA staining, the PAG group had a higher objective response rate (45% vs 31%) and a longer mOS (11.5 mo vs 8.5 mo, HR = 0.96, 95%CI: 0.57-1.61)[168]. The HALO109-301 phase III clinical trial evaluating PEGPH 20 (NCT02715804) was terminated due to unsatisfactory results. In a phase II trial (SWOG S1313) of modified FOLFIRINOX (mFOLFIRINOX) plus PEGPH20 compared with mFOLFIRINOX monotherapy. Ramanathan et al[169] reported an inferior OS when PEGPH20 added to mFOLFIRINOX (7.7 mo [95%CI: 4.6-9.3 mo] vs 14.4 mo [95%CI: 10.1-15.7 mo]). Several phase I/II trials of PEGPH20 combined with programmed cell death protein 1 mAbs and other drugs are currently recruiting patients (NCT03634332, NCT03193190). There may soon be new treatment paradigms for this disease based on the randomized phase III trials of PEGPH20.
MMPs can disrupt the extracellular matrix and basement membrane, thus contributing to tumor invasion, angiogenesis, and metastasis[170]. Marimastat is an MMP inhibitor demonstrating single-agent activity and safety in PDAC patients[171]. However, when combined with gemcitabine, marimastat did not show any clinical benefit or survival advantage, with mOS of 165.5 d in the combination group compared with 164 d in the gemcitabine monotherapy group and 1-year survival rates of 18% and 17%, respectively[172]. Similarly, tanomastat, an MMP inhibitor, did not show any clinical benefit in PDAC compared with gemcitabine[173]. The study ended after a second interim analysis (median OS of 3.74 mo for tanomastat vs 6.59 mo for gemcitabine). Andecaliximab, an mAb targeting MMP9, demonstrated favorable safety and clinical activity in a phase I trial in combination with gemcitabine and nab-paclitaxel in advanced PDAC patients, with an mPFS of 7.8 mo (90%CI: 6.9-11.0), an objective response rate of 44.4% and a median duration of response of 7.6 mo[174].
Connective tissue growth factor (CTGF) is overexpressed in PDAC and is a profibrotic mediator. In a preclinical study, FG-3019, an mAb against CTGF, increased the effectiveness of gemcitabine, resulting in a significant tumor response[175]. A phase II clinical trial for advanced PDAC showed that FG-3019 in combination with gemcitabine and erlotinib was well tolerated, with median PFS and OS of 3.7 and 7.4 mo, respectively[176]. Based on the results of a phase II trial, gemcitabine plus nab-paclitaxel, in combination with FG-3019 or placebo, showed significant improvement in median PFS in the group using FG-3109 (18.4 mo vs 10.4 mo) (NCT02210559). In early 2018, FDA granted a fast-track designation to FG-3019 (pamrevlumab) for treating patients with locally advanced, unresectable PDAC. An ongoing phase III, randomized, double-blind trial is enrolling patients with locally advanced, unresectable PDAC to evaluate the efficacy of receiving gemcitabine in combination with pamrevlumab (NCT03941093).
HGF and its receptor c-MET are vital to the onset and progression of pancreatic cancer. HGF, present on pancreatic stellate cells, increases stromal production and interacts with its ligand, c-MET, on pancreatic cancer cells. This process is vital to the proliferation and migration of pancreatic cancer cells[177].
Among c-MET-targeted therapies, the most advanced clinical development is tivantinib, a c-MET inhibitor in phase III development for various malignancies[178]. A randomized phase II study has been conducted to evaluate the efficacy of tivantinib in combination with gemcitabine in patients with unresectable locally advanced or metastatic untreated pancreatic cancer (NCT00558207). Recently, an HGF-neutralizing Ab, YYB101, has been developed with encouraging preclinical results and has been tested in clinical trials in patients with refractory solid tumors[179]. In addition, NK4, an intramolecular fragment of HGF that targets the HGF/c-MET axis, has demonstrated promising results in vitro and in vivo[180,181].
Cabozantinib, a small molecule inhibitor targeting c-MET and VEGFR-2, is evaluated in a randomized phase II study in several solid tumors, including metastatic pancreatic cancer (NCT01466036). In addition, anti-MET antibodies (emibetuzumab and onartuzumab) have been successfully used in preclinical models of pancreatic cancer[182,183].
CSCs are a unique subset of cells with the potential for self-renewal and differentiation, which can lead to carcinogenesis, progression, metastasis, and drug resistance. Pancreatic CSCs were first described by Li et al[184]; they identified a subpopulation of pancreatic cancer cells expressing CD44, CD24, and epithelial surface antigen (ESA) (CD44+ CD24+ ESA+). CSCs with this phenotype form pancreatic tumors when injected into the tail of orthotopic immunocompromised mice[185]. Wnt/β-catenin, Notch, and activation of the Janus kinase/signal transducer and transcription (JAK/STAT) pathways play a central role in developing pancreatic CSCs[186].
The Notch pathway is an evolutionarily conserved pathway important in mammalian pancreas organogenesis. Upregulation of Notch has been found in PDAC and increases tumorigenesis. Evidence suggests that crosstalk between phytochemicals, microRNAs, and Notch signaling regulates the self-renewal division of CSCs[187]. The intracellular domain of Notch induces proliferative signaling and differentiation by altering gene transcription. The Notch pathway interacts with the Hedgehog, KRAS, and NF-κB pathways[93,188,189].
Since Notch signaling is activated by γ-secretase, γ-secretase inhibitors have been developed as therapeutic agents for the treatment of PDAC. A single-arm phase II trial of the γ-secretase inhibitor RO4929097 was discontinued due to intolerable toxic effects. The 6-mo survival rate is 27.8%, the mOS is 4.1 mo, and median PFS is 1.5 mo[190]. A phase I trial of MK-0725 (a γ-secretase inhibitor) and gemcitabine for PDAC patients, achieved 13 stable disease and one partial response of 19 evaluable patients[191]. Tarextumab is a fully human IgG2 Ab targeting Notch2 and Notch3 receptors[192]. The results of a randomized phase II study evaluating tarextumab in combination with gemcitabine and nab-paclitaxel in patients with untreated metastatic PDAC were suboptimal, without improvement in OS, PFS, or ORR[193].
The WNT pathway is important in cell differentiation and proliferation. In preclinical mouse models, abnormal WNT signaling leads to pancreatic cancer[194].
Vantictumab is an mAb that blocks WNT signaling. Preclinical studies have shown that this Ab reduces cancer stem cell frequency and increases the activity of chemotherapy[195]. The safety and tolerability of vantictumab combined with nab-paclitaxel and gemcitabine are being investigated in a phase Ib dose-escalation study (NCT02005315).
Ipafricept inhibits WNT signaling by acting as a decoy receptor while binding and sequestering WNT ligands. The combination of ipafricept and gemcitabine and nab-paclitaxel was well tolerated in a phase Ib study for patients with untreated stage IV pancreatic cancer, with a median PFS of 5.9 mo and a median OS of 9.7 mo[196].
JAK/STAT pathway has been found in pancreatic cancer[197,198]. Abnormalities in the JAK/STAT pathway directly leads to increased cell transformation, cell proliferation, apoptosis, and angiogenesis. Additionally, STAT3 inhibition results in increased sensitivity to chemotherapy (mainly gemcitabine) and delays tumor progression in PDAC patients[199]. PDAC cell death and proliferation increases when STAT3 inhibitors are administered with chemotherapeutic agents. A phase III trial of evaluating STAT3 inhibitors on PDAC when co-administered with standard chemotherapy regimens has been completed (NCT02231723), but results have not yet been uploaded.
Itacitinib, a selective JAK1 inhibitor, combined with nab-paclitaxel and gemcitabine was evaluated in a phase Ib/II study in patients with advanced solid tumors including locally advanced/metastatic pancreatic cancer patients[200]. The combination therapy demonstrated acceptable safety and clinical activity[201]. However, after an interim analysis of the phase III JANUS 1 and 2 trials of ruxolitinib (JAK1/2 inhibitor) in combination with capecitabine showed no additional clinical benefit of ruxolitinib compared to capecitabine (NCT02117479, NCT02119663), the sponsor prematurely terminated this study on itacitinib on February 11, 2016.
Napabucasin is an investigational, oral agent hypothesized to inhibit multiple oncogenic pathways. Several clinical trials have been initiated to evaluate the safety and efficacy of the drug in various gastrointestinal malignancies[202]. Single-arm phase Ib/II study with napabucasin and nab-paclitaxel plus gemcitabine recruited 59 patients with mPDAC. According to published abstracts, the combination regimen was well tolerated. Among the 50 patients evaluated, the disease control rate was 92%, with 2 complete remissions (4%) and 26 partial responses (52%)[203]. Of all 59 patients enrolled, the 1- and 2-year OS rates were 46% and 13%, respectively. These results led to the further investigation of this treatment combination in the ongoing phase III CanStem111P trial (NCT02993731).
Momelotinib is a JAK1/2 inhibitor with additional activity against TANK-binding kinase 1[204]. Momelotinib was safe and well tolerated in a phase I dose-escalation trial of momelotinib combined with gemcitabine and nab-paclitaxel in patients with previously untreated metastatic PDAC (NCT02101021). However, there was no OS or PFS benefit vs gemcitabine plus nab-paclitaxel in the context of suboptimal engagement of the target. This study does not support momelotinib as a first-line treatment for pancreatic cancer[205].
CSC may be an important target for treatment, but there is still a question of whether targeting them is the best way to counteract their ability to progress, expand and resist treatment in the host environment[206]. Future studies should focus on clonal evolution, especially on monitoring CSC during cancer progression and after treatment.
An autophagy process primarily involves degrading damaged organelles or proteins[207] and enables cells to recycle cellular contents as an internal fuel source during cellular recycling. This process is necessary for pancreatic cancer cells to overcome nutritional deficiencies.
Hydroxychloroquine (HCQ) was one of the first autophagy inhibitors to enter clinical trials. However, HCQ alone did not show significant antitumor effects[208]. According to a randomized phase II study, gemcitabine/nab-paclitaxel with or without HCQ did not improve OS (11.1 mo vs 12.1 mo; P = 0.44) or PFS (5.7 mo vs 6.4 mo; P = 0.25)[209]. In a randomized phase II trial, there was a significant improvement in Evans Grade histopathology and carbohydrate antigen 19-9 response after adding HCQ in the preoperative setting. OS and DFS were not different between groups in this study, nor were AEs or R0 resections[210].
In a recent study, KRAS inhibition and ERK inhibition increased autophagic flux in PDAC[211]. Thus, autophagy inhibitors synergistically act with ERK inhibitors in inhibiting PDAC driven by KRAS mutations[211]. A synergistic antiproliferative effect was observed when autophagy inhibition was combined with MEK1/2 inhibition in PDAC cells as well as patient-derived xenograft models[212]. According to these studies, inhibiting autophagy genetically or pharmacologically may enhance the antitumor effects of other antitumor drugs such as ERK inhibitors and MEK inhibitors in PDAC. Several promising studies have evaluated the combination of autophagy inhibitors and MEK (NCT04132505, NCT03825289) or ERK inhibitors (NCT04386057) in patients with locally advanced or metastatic cancer[213-215].
Adenosine has been identified as an essential regulator of tumor proliferation, survival, and migration. Inhibition of adenosine receptors has been shown to modulate immune responses within the tumor microenvironment, thereby enhancing antitumor effects[216]. Several clinical trials evaluate the safety and efficacy of adenosine A2 receptor antagonists in combination with immunotherapy or cytotoxic therapy in patients with advanced solid tumors including PDAC, Ciforadenant (NCT03454451), and NIR178 (NCT03207867).
Accumulating clinical evidence suggests that overexpression of urokinase-type plasminogen activator (uPA) or its cell surface receptor is closely associated with worse clinicopathological features and poor prognosis in PDAC patients[217]. RHB-107, the only known agent targeting the uPA pathway, was effective in a phase II clinical trial in patients with locally advanced unresectable pancreatic cancer (NCT00499265). RHB-107, combined with gemcitabine, significantly improved 1-year survival by 17% in patients with unresectable PC. In 2017, RHB-107 received an Orphan Drug Designation from the FDA for PDAC adjuvant therapy.
Despite the advances in the last 20 years, pancreatic cancer remains a devastating malignancy with limited options for effective treatment. As mentioned above, the self-preserving CSCs, dense tumor microenvironment, and suppressive and relatively depleted immune niche of PDAC are considered significant clinical barriers to successful therapy development, making it one of the most challenging diseases to target.
Targeting individual molecules is not a good approach. In the currently known studies on the mechanisms of ineffectiveness or resistance of targeted therapies, it is suggested that inhibition of one pathway may lead to activation or compensatory upregulation of others, e.g., inhibition of the PI3K/Akt/mTOR pathway may lead to tumor escape via the MAPK pathway. This suggests to us that, in fact, most clinical trials have also demonstrated that monotherapy of targeted drugs is not feasible. Therefore, combining targeted inhibitors of multiple pathways may be the future targeted therapy research's primary direction. At the same time, in addition to considering drug efficacy, we must consider that a multidrug combination implies a superposition of AEs and toxicity.
Based on the characteristics of pancreatic cancer - dense fibrous stroma, accounting for 90% of the tumor volume, and excessive proliferation of fibrous histochemistry, drugs are not easy to reach the tumor interior. Investigating targeted or cytotoxic drugs that are more accessible to the tumor, or using more efficient delivery methods, such as local arterial delivery, may improve efficacy.
Most of the studies conducted to date have been designed based on gemcitabine activity. Given that gemcitabine is no longer the reference drug, future studies should focus on targeted therapy with either nab-paclitaxel or FOLFIRINOX as the control group, which may improve the results achieved. Furthermore, most studies showed promising results in preclinical evaluations, but the vast majority failed to proceed to more advanced clinical studies due to the lack of positive results. This suggests that better preclinical models should be developed to accurately reflect the tumor characteristics and environment in humans, thereby making clinical trials more relevant to preclinical studies.
PDAC is a very complex entity, joining different molecular particularities and in a dynamic manner, not in a static one. As some guidelines already stated and can be concluded from de data shown here, is very important to spread the genetic and transcriptomic profiling of every PDAC to capture the vulnerabilities of the tumor as far as possible as the way to improve therapeutic results. In conclusion, developing the targeted drug for pancreatic cancer has a long way to go. The complex interactions within targeted biological pathways, the pharmacokinetics of targeted drugs, predictive markers of the targeted drug benefit, and the combined application of targeted drugs still require extensive and in-depth studies.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Caronna R, Italy; Sureda M, Spain S-Editor: Wang JL L-Editor: Filipodia P-Editor: Wang JL
| 1. | Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25:1650-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913-2921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5379] [Cited by in RCA: 5115] [Article Influence: 465.0] [Reference Citation Analysis (0)] |
| 3. | Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, Talamonti MS. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007;110:738-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 346] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 4. | Wörmann SM, Algül H. Risk factors and therapeutic targets in pancreatic cancer. Front Oncol. 2013;3:282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Blumenthal GM, Cortazar P, Zhang JJ, Tang S, Sridhara R, Murgo A, Justice R, Pazdur R. FDA approval summary: sunitinib for the treatment of progressive well-differentiated locally advanced or metastatic pancreatic neuroendocrine tumors. Oncologist. 2012;17:1108-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Cohen MH, Johnson JR, Chattopadhyay S, Tang S, Justice R, Sridhara R, Pazdur R. Approval summary: erlotinib maintenance therapy of advanced/metastatic non-small cell lung cancer (NSCLC). Oncologist. 2010;15:1344-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Lugowska I, Koseła-Paterczyk H, Kozak K, Rutkowski P. Trametinib: a MEK inhibitor for management of metastatic melanoma. Onco Targets Ther. 2015;8:2251-2259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Hamid O. Emerging treatments in oncology: focus on tyrosine kinase (erbB) receptor inhibitors. J Am Pharm Assoc (2003). 2004;44:52-58. [PubMed] |
| 9. | Conti A, Santoni M, Amantini C, Burattini L, Berardi R, Santoni G, Cascinu S, Muzzonigro G. Progress of molecular targeted therapies for advanced renal cell carcinoma. Biomed Res Int. 2013;2013:419176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Moore MJ, Goldstein D, Hamm J, Figer A, Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, Campos D, Lim R, Ding K, Clark G, Voskoglou-Nomikos T, Ptasynski M, Parulekar W; National Cancer Institute of Canada Clinical Trials Group. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2835] [Cited by in RCA: 2768] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 11. | Löhr M, Klöppel G, Maisonneuve P, Lowenfels AB, Lüttges J. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia. 2005;7:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 238] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Scarpa A, Capelli P, Mukai K, Zamboni G, Oda T, Iacono C, Hirohashi S. Pancreatic adenocarcinomas frequently show p53 gene mutations. Am J Pathol. 1993;142:1534-1543. [PubMed] |
| 13. | Cohen MH, Farrell A, Justice R, Pazdur R. Approval summary: imatinib mesylate in the treatment of metastatic and/or unresectable malignant gastrointestinal stromal tumors. Oncologist. 2009;14:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Yachida S, Iacobuzio-Donahue CA. Evolution and dynamics of pancreatic cancer progression. Oncogene. 2013;32:5253-5260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 137] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 15. | Kessler D, Gmachl M, Mantoulidis A, Martin LJ, Zoephel A, Mayer M, Gollner A, Covini D, Fischer S, Gerstberger T, Gmaschitz T, Goodwin C, Greb P, Häring D, Hela W, Hoffmann J, Karolyi-Oezguer J, Knesl P, Kornigg S, Koegl M, Kousek R, Lamarre L, Moser F, Munico-Martinez S, Peinsipp C, Phan J, Rinnenthal J, Sai J, Salamon C, Scherbantin Y, Schipany K, Schnitzer R, Schrenk A, Sharps B, Siszler G, Sun Q, Waterson A, Wolkerstorfer B, Zeeb M, Pearson M, Fesik SW, McConnell DB. Drugging an undruggable pocket on KRAS. Proc Natl Acad Sci U S A. 2019;116:15823-15829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 303] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 16. | Macdonald JS, McCoy S, Whitehead RP, Iqbal S, Wade JL 3rd, Giguere JK, Abbruzzese JL. A phase II study of farnesyl transferase inhibitor R115777 in pancreatic cancer: a Southwest oncology group (SWOG 9924) study. Invest New Drugs. 2005;23:485-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 111] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Kamerkar S, LeBleu VS, Sugimoto H, Yang S, Ruivo CF, Melo SA, Lee JJ, Kalluri R. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature. 2017;546:498-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1167] [Cited by in RCA: 1891] [Article Influence: 236.4] [Reference Citation Analysis (1)] |
| 18. | Zeitouni D, Pylayeva-Gupta Y, Der CJ, Bryant KL. KRAS Mutant Pancreatic Cancer: No Lone Path to an Effective Treatment. Cancers (Basel). 2016;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 126] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 19. | Janes MR, Zhang J, Li LS, Hansen R, Peters U, Guo X, Chen Y, Babbar A, Firdaus SJ, Darjania L, Feng J, Chen JH, Li S, Long YO, Thach C, Liu Y, Zarieh A, Ely T, Kucharski JM, Kessler LV, Wu T, Yu K, Wang Y, Yao Y, Deng X, Zarrinkar PP, Brehmer D, Dhanak D, Lorenzi MV, Hu-Lowe D, Patricelli MP, Ren P. Targeting KRAS Mutant Cancers with a Covalent G12C-Specific Inhibitor. Cell. 2018;172:578-589.e17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 589] [Cited by in RCA: 834] [Article Influence: 119.1] [Reference Citation Analysis (0)] |
| 20. | Hong DS, Fakih MG, Strickler JH, Desai J, Durm GA, Shapiro GI, Falchook GS, Price TJ, Sacher A, Denlinger CS, Bang YJ, Dy GK, Krauss JC, Kuboki Y, Kuo JC, Coveler AL, Park K, Kim TW, Barlesi F, Munster PN, Ramalingam SS, Burns TF, Meric-Bernstam F, Henary H, Ngang J, Ngarmchamnanrith G, Kim J, Houk BE, Canon J, Lipford JR, Friberg G, Lito P, Govindan R, Li BT. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. 2020;383:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1160] [Article Influence: 232.0] [Reference Citation Analysis (0)] |
| 21. | Eser S, Schnieke A, Schneider G, Saur D. Oncogenic KRAS signalling in pancreatic cancer. Br J Cancer. 2014;111:817-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 344] [Cited by in RCA: 391] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 22. | Ng SS, Tsao MS, Nicklee T, Hedley DW. Effects of the epidermal growth factor receptor inhibitor OSI-774, Tarceva, on downstream signaling pathways and apoptosis in human pancreatic adenocarcinoma. Mol Cancer Ther. 2002;1:777-783. [PubMed] |
| 23. | Miksad RA, Schnipper L, Goldstein M. Does a statistically significant survival benefit of erlotinib plus gemcitabine for advanced pancreatic cancer translate into clinical significance and value? J Clin Oncol. 2007;25:4506-7; author reply 4508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Rinehart J, Adjei AA, Lorusso PM, Waterhouse D, Hecht JR, Natale RB, Hamid O, Varterasian M, Asbury P, Kaldjian EP, Gulyas S, Mitchell DY, Herrera R, Sebolt-Leopold JS, Meyer MB. Multicenter phase II study of the oral MEK inhibitor, CI-1040, in patients with advanced non-small-cell lung, breast, colon, and pancreatic cancer. J Clin Oncol. 2004;22:4456-4462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 482] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 25. | Bodoky G, Timcheva C, Spigel DR, La Stella PJ, Ciuleanu TE, Pover G, Tebbutt NC. A phase II open-label randomized study to assess the efficacy and safety of selumetinib (AZD6244 [ARRY-142886]) versus capecitabine in patients with advanced or metastatic pancreatic cancer who have failed first-line gemcitabine therapy. Invest New Drugs. 2012;30:1216-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 185] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 26. | Van Cutsem E, Hidalgo M, Canon JL, Macarulla T, Bazin I, Poddubskaya E, Manojlovic N, Radenkovic D, Verslype C, Raymond E, Cubillo A, Schueler A, Zhao C, Hammel P. Phase I/II trial of pimasertib plus gemcitabine in patients with metastatic pancreatic cancer. Int J Cancer. 2018;143:2053-2064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 27. | Infante JR, Somer BG, Park JO, Li CP, Scheulen ME, Kasubhai SM, Oh DY, Liu Y, Redhu S, Steplewski K, Le N. A randomised, double-blind, placebo-controlled trial of trametinib, an oral MEK inhibitor, in combination with gemcitabine for patients with untreated metastatic adenocarcinoma of the pancreas. Eur J Cancer. 2014;50:2072-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 274] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 28. | Pettazzoni P, Viale A, Shah P, Carugo A, Ying H, Wang H, Genovese G, Seth S, Minelli R, Green T, Huang-Hobbs E, Corti D, Sanchez N, Nezi L, Marchesini M, Kapoor A, Yao W, Francesco ME, Petrocchi A, Deem AK, Scott K, Colla S, Mills GB, Fleming JB, Heffernan TP, Jones P, Toniatti C, DePinho RA, Draetta GF. Genetic events that limit the efficacy of MEK and RTK inhibitor therapies in a mouse model of KRAS-driven pancreatic cancer. Cancer Res. 2015;75:1091-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Mirzoeva OK, Collisson EA, Schaefer PM, Hann B, Hom YK, Ko AH, Korn WM. Subtype-specific MEK-PI3 kinase feedback as a therapeutic target in pancreatic adenocarcinoma. Mol Cancer Ther. 2013;12:2213-2225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Brauswetter D, Gurbi B, Varga A, Várkondi E, Schwab R, Bánhegyi G, Fábián O, Kéri G, Vályi-Nagy I, Peták I. Molecular subtype specific efficacy of MEK inhibitors in pancreatic cancers. PLoS One. 2017;12:e0185687. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 31. | van Brummelen EMJ, Huijberts S, van Herpen C, Desar I, Opdam F, van Geel R, Marchetti S, Steeghs N, Monkhorst K, Thijssen B, Rosing H, Huitema A, Beijnen J, Bernards R, Schellens J. Phase I Study of Afatinib and Selumetinib in Patients with KRAS-Mutated Colorectal, Non-Small Cell Lung, and Pancreatic Cancer. Oncologist. 2021;26:290-e545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 32. | Chung V, McDonough S, Philip PA, Cardin D, Wang-Gillam A, Hui L, Tejani MA, Seery TE, Dy IA, Al Baghdadi T, Hendifar AE, Doyle LA, Lowy AM, Guthrie KA, Blanke CD, Hochster HS. Effect of Selumetinib and MK-2206 vs Oxaliplatin and Fluorouracil in Patients With Metastatic Pancreatic Cancer After Prior Therapy: SWOG S1115 Study Randomized Clinical Trial. JAMA Oncol. 2017;3:516-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 33. | Hatzivassiliou G, Haling JR, Chen H, Song K, Price S, Heald R, Hewitt JF, Zak M, Peck A, Orr C, Merchant M, Hoeflich KP, Chan J, Luoh SM, Anderson DJ, Ludlam MJ, Wiesmann C, Ultsch M, Friedman LS, Malek S, Belvin M. Mechanism of MEK inhibition determines efficacy in mutant KRAS- versus BRAF-driven cancers. Nature. 2013;501:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 244] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 34. | Lito P, Saborowski A, Yue J, Solomon M, Joseph E, Gadal S, Saborowski M, Kastenhuber E, Fellmann C, Ohara K, Morikami K, Miura T, Lukacs C, Ishii N, Lowe S, Rosen N. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell. 2014;25:697-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 35. | Weekes C, Lockhart A, LoRusso P, Murray E, Park E, Tagen M, Singh J, Sarkar I, Mueller L, Dokainish H, Shapiro G, Burris H. A Phase Ib Study to Evaluate the MEK Inhibitor Cobimetinib in Combination with the ERK1/2 Inhibitor GDC-0994 in Patients with Advanced Solid Tumors. Oncologist. 2020;25:833-e1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Christenson ES, Jaffee E, Azad NS. Current and emerging therapies for patients with advanced pancreatic ductal adenocarcinoma: a bright future. Lancet Oncol. 2020;21:e135-e145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 167] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 37. | Nevala-Plagemann C, Hidalgo M, Garrido-Laguna I. From state-of-the-art treatments to novel therapies for advanced-stage pancreatic cancer. Nat Rev Clin Oncol. 2020;17:108-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 38. | Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15:731-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 644] [Cited by in RCA: 1000] [Article Influence: 166.7] [Reference Citation Analysis (0)] |
| 39. | Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo AS, Turpin B, Dowlati A, Brose MS, Mascarenhas L, Federman N, Berlin J, El-Deiry WS, Baik C, Deeken J, Boni V, Nagasubramanian R, Taylor M, Rudzinski ER, Meric-Bernstam F, Sohal DPS, Ma PC, Raez LE, Hechtman JF, Benayed R, Ladanyi M, Tuch BB, Ebata K, Cruickshank S, Ku NC, Cox MC, Hawkins DS, Hong DS, Hyman DM. Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med. 2018;378:731-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1577] [Cited by in RCA: 1920] [Article Influence: 274.3] [Reference Citation Analysis (0)] |
| 40. | Sohal DPS, Kennedy EB, Cinar P, Conroy T, Copur MS, Crane CH, Garrido-Laguna I, Lau MW, Johnson T, Krishnamurthi S, Moravek C, O'Reilly EM, Philip PA, Pant S, Shah MA, Sahai V, Uronis HE, Zaidi N, Laheru D. Metastatic Pancreatic Cancer: ASCO Guideline Update. J Clin Oncol. 2020;JCO2001364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 166] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 41. | Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi D, Besse B, Chawla SP, Bazhenova L, Krauss JC, Chae YK, Barve M, Garrido-Laguna I, Liu SV, Conkling P, John T, Fakih M, Sigal D, Loong HH, Buchschacher GL Jr, Garrido P, Nieva J, Steuer C, Overbeck TR, Bowles DW, Fox E, Riehl T, Chow-Maneval E, Simmons B, Cui N, Johnson A, Eng S, Wilson TR, Demetri GD; trial investigators. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020;21:271-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 574] [Cited by in RCA: 1126] [Article Influence: 187.7] [Reference Citation Analysis (0)] |
| 42. | Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin JD, Federman N, Mascarenhas L, Geoerger B, Dowlati A, Pappo AS, Bielack S, Doz F, McDermott R, Patel JD, Schilder RJ, Tahara M, Pfister SM, Witt O, Ladanyi M, Rudzinski ER, Nanda S, Childs BH, Laetsch TW, Hyman DM, Drilon A. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet Oncol. 2020;21:531-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 694] [Article Influence: 138.8] [Reference Citation Analysis (0)] |
| 43. | Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol. 2019;30 Suppl 8:viii23-viii30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 193] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 44. | Drilon A, Nagasubramanian R, Blake JF, Ku N, Tuch BB, Ebata K, Smith S, Lauriault V, Kolakowski GR, Brandhuber BJ, Larsen PD, Bouhana KS, Winski SL, Hamor R, Wu WI, Parker A, Morales TH, Sullivan FX, DeWolf WE, Wollenberg LA, Gordon PR, Douglas-Lindsay DN, Scaltriti M, Benayed R, Raj S, Hanusch B, Schram AM, Jonsson P, Berger MF, Hechtman JF, Taylor BS, Andrews S, Rothenberg SM, Hyman DM. A Next-Generation TRK Kinase Inhibitor Overcomes Acquired Resistance to Prior TRK Kinase Inhibition in Patients with TRK Fusion-Positive Solid Tumors. Cancer Discov. 2017;7:963-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 332] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 45. | Valsecchi ME, McDonald M, Brody JR, Hyslop T, Freydin B, Yeo CJ, Solomides C, Peiper SC, Witkiewicz AK. Epidermal growth factor receptor and insulinlike growth factor 1 receptor expression predict poor survival in pancreatic ductal adenocarcinoma. Cancer. 2012;118:3484-3493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 46. | Walsh N, Kennedy S, Larkin A, Corkery B, O'Driscoll L, Clynes M, Crown J, O'Donovan N. EGFR and HER2 inhibition in pancreatic cancer. Invest New Drugs. 2013;31:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 47. | Einama T, Ueda S, Tsuda H, Ogasawara K, Hatsuse K, Matsubara O, Todo S, Yamamoto J. Membranous and cytoplasmic expression of epidermal growth factor receptor in metastatic pancreatic ductal adenocarcinoma. Exp Ther Med. 2012;3:931-936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Ardito CM, Grüner BM, Takeuchi KK, Lubeseder-Martellato C, Teichmann N, Mazur PK, Delgiorno KE, Carpenter ES, Halbrook CJ, Hall JC, Pal D, Briel T, Herner A, Trajkovic-Arsic M, Sipos B, Liou GY, Storz P, Murray NR, Threadgill DW, Sibilia M, Washington MK, Wilson CL, Schmid RM, Raines EW, Crawford HC, Siveke JT. EGF receptor is required for KRAS-induced pancreatic tumorigenesis. Cancer Cell. 2012;22:304-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 49. | Navas C, Hernández-Porras I, Schuhmacher AJ, Sibilia M, Guerra C, Barbacid M. EGF receptor signaling is essential for k-ras oncogene-driven pancreatic ductal adenocarcinoma. Cancer Cell. 2012;22:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 316] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 50. | Schultheis B, Reuter D, Ebert MP, Siveke J, Kerkhoff A, Berdel WE, Hofheinz R, Behringer DM, Schmidt WE, Goker E, De Dosso S, Kneba M, Yalcin S, Overkamp F, Schlegel F, Dommach M, Rohrberg R, Steinmetz T, Bulitta M, Strumberg D. Gemcitabine combined with the monoclonal antibody nimotuzumab is an active first-line regimen in KRAS wildtype patients with locally advanced or metastatic pancreatic cancer: a multicenter, randomized phase IIb study. Ann Oncol. 2017;28:2429-2435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 51. | Qin S, Bai Y, Wang Z, Chen Z, Xu R, Xu J, Zhang H, Chen J, Yuan Y, Liu T, Yang L, Zhong H, Chen D, Shen L, Hao C, Fu D, Cheng Y, Yang J, Bai Xh and Li J. Nimotuzumab combined with gemcitabine vs gemcitabine in K-RAS wild-type locally advanced or metastatic pancreatic cancer: A prospective, randomized-controlled, double-blinded, multicenter, and phase III clinical trial. J Clin Oncol. 2022;40:LBA4011-LBA4011. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 52. | Graeven U, Kremer B, Südhoff T, Killing B, Rojo F, Weber D, Tillner J, Unal C, Schmiegel W. Phase I study of the humanised anti-EGFR monoclonal antibody matuzumab (EMD 72000) combined with gemcitabine in advanced pancreatic cancer. Br J Cancer. 2006;94:1293-1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 81] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Halfdanarson TR, Foster NR, Kim GP, Meyers JP, Smyrk TC, McCullough AE, Ames MM, Jaffe JP, Alberts SR. A Phase II Randomized Trial of Panitumumab, Erlotinib, and Gemcitabine Versus Erlotinib and Gemcitabine in Patients with Untreated, Metastatic Pancreatic Adenocarcinoma: North Central Cancer Treatment Group Trial N064B (Alliance). Oncologist. 2019;24:589-e160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 54. | Philip PA, Benedetti J, Corless CL, Wong R, O'Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC, Rivkin SE, Khorana AA, Goldman B, Fenoglio-Preiser CM, Abbruzzese JL, Blanke CD. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605-3610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 499] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 55. | Mihaljevic A, Büchler P, Harder J, Hofheinz R, Gregor M, Kanzler S, Schmiegel W, Heinemann V, Endlicher E, Klöppel G, Seufferlein T, Geissler M. A prospective, non-randomized phase II trial of Trastuzumab and Capecitabine in patients with HER2 expressing metastasized pancreatic cancer. BMC Surg. 2009;9:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Agus DB, Gordon MS, Taylor C, Natale RB, Karlan B, Mendelson DS, Press MF, Allison DE, Sliwkowski MX, Lieberman G, Kelsey SM, Fyfe G. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol. 2005;23:2534-2543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 289] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 57. | Haas M, Waldschmidt DT, Stahl M, Reinacher-Schick A, Freiberg-Richter J, Fischer von Weikersthal L, Kaiser F, Kanzler S, Frickhofen N, Seufferlein T, Dechow T, Mahlberg R, Malfertheiner P, Illerhaus G, Kubicka S, Abdul-Ahad A, Snijder R, Kruger S, Westphalen CB, Held S, von Bergwelt-Baildon M, Boeck S, Heinemann V. Afatinib plus gemcitabine versus gemcitabine alone as first-line treatment of metastatic pancreatic cancer: The randomised, open-label phase II ACCEPT study of the Arbeitsgemeinschaft Internistische Onkologie with an integrated analysis of the 'burden of therapy' method. Eur J Cancer. 2021;146:95-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 58. | Kuehn R, Lelkes PI, Bloechle C, Niendorf A, Izbicki JR. Angiogenesis, angiogenic growth factors, and cell adhesion molecules are upregulated in chronic pancreatic diseases: angiogenesis in chronic pancreatitis and in pancreatic cancer. Pancreas. 1999;18:96-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Seo Y, Baba H, Fukuda T, Takashima M, Sugimachi K. High expression of vascular endothelial growth factor is associated with liver metastasis and a poor prognosis for patients with ductal pancreatic adenocarcinoma. Cancer. 2000;88:2239-2245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 60. | Pàez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Viñals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220-231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1901] [Cited by in RCA: 1899] [Article Influence: 118.7] [Reference Citation Analysis (0)] |
| 61. | Van Cutsem E, Vervenne WL, Bennouna J, Humblet Y, Gill S, Van Laethem JL, Verslype C, Scheithauer W, Shang A, Cosaert J, Moore MJ. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231-2237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 499] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 62. | Kindler HL, Niedzwiecki D, Hollis D, Sutherland S, Schrag D, Hurwitz H, Innocenti F, Mulcahy MF, O'Reilly E, Wozniak TF, Picus J, Bhargava P, Mayer RJ, Schilsky RL, Goldberg RM. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol. 2010;28:3617-3622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 674] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 63. | Crane CH, Winter K, Regine WF, Safran H, Rich TA, Curran W, Wolff RA, Willett CG. Phase II study of bevacizumab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacizumab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol. 2009;27:4096-4102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 64. | Ko AH, Venook AP, Bergsland EK, Kelley RK, Korn WM, Dito E, Schillinger B, Scott J, Hwang J, Tempero MA. A phase II study of bevacizumab plus erlotinib for gemcitabine-refractory metastatic pancreatic cancer. Cancer Chemother Pharmacol. 2010;66:1051-1057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 65. | Tian W, Ding W, Kim S, Xu X, Pan M, Chen S. Efficacy and safety profile of combining agents against epidermal growth factor receptor or vascular endothelium growth factor receptor with gemcitabine-based chemotherapy in patients with advanced pancreatic cancer: a meta-analysis. Pancreatology. 2013;13:415-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Taeger J, Moser C, Hellerbrand C, Mycielska ME, Glockzin G, Schlitt HJ, Geissler EK, Stoeltzing O, Lang SA. Targeting FGFR/PDGFR/VEGFR impairs tumor growth, angiogenesis, and metastasis by effects on tumor cells, endothelial cells, and pericytes in pancreatic cancer. Mol Cancer Ther. 2011;10:2157-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 67. | Spano JP, Chodkiewicz C, Maurel J, Wong R, Wasan H, Barone C, Létourneau R, Bajetta E, Pithavala Y, Bycott P, Trask P, Liau K, Ricart AD, Kim S, Rixe O. Efficacy of gemcitabine plus axitinib compared with gemcitabine alone in patients with advanced pancreatic cancer: an open-label randomised phase II study. Lancet. 2008;371:2101-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 68. | Kindler HL, Ioka T, Richel DJ, Bennouna J, Létourneau R, Okusaka T, Funakoshi A, Furuse J, Park YS, Ohkawa S, Springett GM, Wasan HS, Trask PC, Bycott P, Ricart AD, Kim S, Van Cutsem E. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 320] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 69. | Rougier P, Riess H, Manges R, Karasek P, Humblet Y, Barone C, Santoro A, Assadourian S, Hatteville L, Philip PA. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur J Cancer. 2013;49:2633-2642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 140] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 70. | Gonçalves A, Gilabert M, François E, Dahan L, Perrier H, Lamy R, Re D, Largillier R, Gasmi M, Tchiknavorian X, Esterni B, Genre D, Moureau-Zabotto L, Giovannini M, Seitz JF, Delpero JR, Turrini O, Viens P, Raoul JL. BAYPAN study: a double-blind phase III randomized trial comparing gemcitabine plus sorafenib and gemcitabine plus placebo in patients with advanced pancreatic cancer. Ann Oncol. 2012;23:2799-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 171] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 71. | Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, Cheng Y, He J, Shi Y, Zhao Y, Yu H, Chen W, Luo Y, Wu L, Wang X, Pirker R, Nan K, Jin F, Dong J, Li B, Sun Y. Effect of Anlotinib as a Third-Line or Further Treatment on Overall Survival of Patients With Advanced Non-Small Cell Lung Cancer: The ALTER 0303 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2018;4:1569-1575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 455] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 72. | Neid M, Datta K, Stephan S, Khanna I, Pal S, Shaw L, White M, Mukhopadhyay D. Role of insulin receptor substrates and protein kinase C-zeta in vascular permeability factor/vascular endothelial growth factor expression in pancreatic cancer cells. J Biol Chem. 2004;279:3941-3948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 73. | Liu W, Bloom DA, Cance WG, Kurenova EV, Golubovskaya VM, Hochwald SN. FAK and IGF-IR interact to provide survival signals in human pancreatic adenocarcinoma cells. Carcinogenesis. 2008;29:1096-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 74. | Fuchs CS, Azevedo S, Okusaka T, Van Laethem JL, Lipton LR, Riess H, Szczylik C, Moore MJ, Peeters M, Bodoky G, Ikeda M, Melichar B, Nemecek R, Ohkawa S, Świeboda-Sadlej A, Tjulandin SA, Van Cutsem E, Loberg R, Haddad V, Gansert JL, Bach BA, Carrato A. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann Oncol. 2015;26:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 141] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 75. | Urtasun N, Vidal-Pla A, Pérez-Torras S, Mazo A. Human pancreatic cancer stem cells are sensitive to dual inhibition of IGF-IR and ErbB receptors. BMC Cancer. 2015;15:223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 76. | Abdel-Wahab R, Varadhachary GR, Bhosale PR, Wang X, Fogelman DR, Shroff RT, Overman MJ, Wolff RA, Javle M. Randomized, phase I/II study of gemcitabine plus IGF-1R antagonist (MK-0646) versus gemcitabine plus erlotinib with and without MK-0646 for advanced pancreatic adenocarcinoma. J Hematol Oncol. 2018;11:71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 77. | Fitzgerald JB, Johnson BW, Baum J, Adams S, Iadevaia S, Tang J, Rimkunas V, Xu L, Kohli N, Rennard R, Razlog M, Jiao Y, Harms BD, Olivier KJ Jr, Schoeberl B, Nielsen UB, Lugovskoy AA. MM-141, an IGF-IR- and ErbB3-directed bispecific antibody, overcomes network adaptations that limit activity of IGF-IR inhibitors. Mol Cancer Ther. 2014;13:410-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 82] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 78. | Kundranda M, Gracian AC, Zafar SF, Meiri E, Bendell J, Algül H, Rivera F, Ahn ER, Watkins D, Pelzer U, Charu V, Zalutskaya A, Kuesters G, Pipas JM, Santillana S, Askoxylakis V, Ko AH. Randomized, double-blind, placebo-controlled phase II study of istiratumab (MM-141) plus nab-paclitaxel and gemcitabine versus nab-paclitaxel and gemcitabine in front-line metastatic pancreatic cancer (CARRIE). Ann Oncol. 2020;31:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 79. | Yamamoto S, Tomita Y, Hoshida Y, Morooka T, Nagano H, Dono K, Umeshita K, Sakon M, Ishikawa O, Ohigashi H, Nakamori S, Monden M, Aozasa K. Prognostic significance of activated Akt expression in pancreatic ductal adenocarcinoma. Clin Cancer Res. 2004;10:2846-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 80. | Mirzoeva OK, Hann B, Hom YK, Debnath J, Aftab D, Shokat K, Korn WM. Autophagy suppression promotes apoptotic cell death in response to inhibition of the PI3K-mTOR pathway in pancreatic adenocarcinoma. J Mol Med (Berl). 2011;89:877-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 81. | Jones S, Zhang X, Parsons DW, Lin JC, Leary RJ, Angenendt P, Mankoo P, Carter H, Kamiyama H, Jimeno A, Hong SM, Fu B, Lin MT, Calhoun ES, Kamiyama M, Walter K, Nikolskaya T, Nikolsky Y, Hartigan J, Smith DR, Hidalgo M, Leach SD, Klein AP, Jaffee EM, Goggins M, Maitra A, Iacobuzio-Donahue C, Eshleman JR, Kern SE, Hruban RH, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801-1806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3216] [Cited by in RCA: 3021] [Article Influence: 177.7] [Reference Citation Analysis (0)] |
| 82. | Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4468] [Cited by in RCA: 4583] [Article Influence: 199.3] [Reference Citation Analysis (0)] |
| 83. | Liu D, Zhang Y, Dang C, Ma Q, Lee W, Chen W. siRNA directed against TrkA sensitizes human pancreatic cancer cells to apoptosis induced by gemcitabine through an inactivation of PI3K/Akt-dependent pathway. Oncol Rep. 2007;18:673-677. [PubMed] |
| 84. | Ma J, Sawai H, Matsuo Y, Ochi N, Yasuda A, Takahashi H, Wakasugi T, Funahashi H, Sato M, Takeyama H. IGF-1 mediates PTEN suppression and enhances cell invasion and proliferation via activation of the IGF-1/PI3K/Akt signaling pathway in pancreatic cancer cells. J Surg Res. 2010;160:90-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 85. | Chadha KS, Khoury T, Yu J, Black JD, Gibbs JF, Kuvshinoff BW, Tan D, Brattain MG, Javle MM. Activated Akt and Erk expression and survival after surgery in pancreatic carcinoma. Ann Surg Oncol. 2006;13:933-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 86. | Fei HR, Chen G, Wang JM, Wang FZ. Perifosine induces cell cycle arrest and apoptosis in human hepatocellular carcinoma cell lines by blockade of Akt phosphorylation. Cytotechnology. 2010;62:449-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 87. | Kondapaka SB, Singh SS, Dasmahapatra GP, Sausville EA, Roy KK. Perifosine, a novel alkylphospholipid, inhibits protein kinase B activation. Mol Cancer Ther. 2003;2:1093-1103. [PubMed] |
| 88. | Avan A, Maftouh M, Funel N, Ghayour-Mobarhan M, Boggi U, Peters GJ, Giovannetti E. MET as a potential target for the treatment of upper gastrointestinal cancers: characterization of novel c-Met inhibitors from bench to bedside. Curr Med Chem. 2014;21:975-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Wolpin BM, Hezel AF, Abrams T, Blaszkowsky LS, Meyerhardt JA, Chan JA, Enzinger PC, Allen B, Clark JW, Ryan DP, Fuchs CS. Oral mTOR inhibitor everolimus in patients with gemcitabine-refractory metastatic pancreatic cancer. J Clin Oncol. 2009;27:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 239] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 90. | Kordes S, Klümpen HJ, Weterman MJ, Schellens JH, Richel DJ, Wilmink JW. Phase II study of capecitabine and the oral mTOR inhibitor everolimus in patients with advanced pancreatic cancer. Cancer Chemother Pharmacol. 2015;75:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | Kulke MH, Siu LL, Tepper JE, Fisher G, Jaffe D, Haller DG, Ellis LM, Benedetti JK, Bergsland EK, Hobday TJ, Van Cutsem E, Pingpank J, Oberg K, Cohen SJ, Posner MC, Yao JC. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 92. | Rocha Lima CM, Green MR, Rotche R, Miller WH Jr, Jeffrey GM, Cisar LA, Morganti A, Orlando N, Gruia G, Miller LL. Irinotecan plus gemcitabine results in no survival advantage compared with gemcitabine monotherapy in patients with locally advanced or metastatic pancreatic cancer despite increased tumor response rate. J Clin Oncol. 2004;22:3776-3783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 424] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 93. | Di Marco M, Grassi E, Durante S, Vecchiarelli S, Palloni A, Macchini M, Casadei R, Ricci C, Panzacchi R, Santini D, Biasco G. State of the art biological therapies in pancreatic cancer. World J Gastrointest Oncol. 2016;8:55-66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 24] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 94. | Sheppard K, Kinross KM, Solomon B, Pearson RB, Phillips WA. Targeting PI3 kinase/AKT/mTOR signaling in cancer. Crit Rev Oncog. 2012;17:69-95. [PubMed] |
| 95. | de Mestier L, Danset JB, Neuzillet C, Rebours V, Cros J, Soufir N, Hammel P. Pancreatic ductal adenocarcinoma in BRCA2 mutation carriers. Endocr Relat Cancer. 2016;23:T57-T67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 96. | Luo G, Lu Y, Jin K, Cheng H, Guo M, Liu Z, Long J, Liu C, Ni Q, Yu X. Pancreatic cancer: BRCA mutation and personalized treatment. Expert Rev Anticancer Ther. 2015;15:1223-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69:280-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 784] [Article Influence: 130.7] [Reference Citation Analysis (0)] |
| 98. | Kaufman B, Shapira-Frommer R, Schmutzler RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G, Stemmer SM, Hubert A, Rosengarten O, Steiner M, Loman N, Bowen K, Fielding A, Domchek SM. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol. 2015;33:244-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1325] [Article Influence: 120.5] [Reference Citation Analysis (0)] |
| 99. | Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, Delaloge S, Li W, Tung N, Armstrong A, Wu W, Goessl C, Runswick S, Conte P. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377:523-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 2266] [Article Influence: 283.3] [Reference Citation Analysis (0)] |
| 100. | Mateo J, Carreira S, Sandhu S, Miranda S, Mossop H, Perez-Lopez R, Nava Rodrigues D, Robinson D, Omlin A, Tunariu N, Boysen G, Porta N, Flohr P, Gillman A, Figueiredo I, Paulding C, Seed G, Jain S, Ralph C, Protheroe A, Hussain S, Jones R, Elliott T, McGovern U, Bianchini D, Goodall J, Zafeiriou Z, Williamson CT, Ferraldeschi R, Riisnaes R, Ebbs B, Fowler G, Roda D, Yuan W, Wu YM, Cao X, Brough R, Pemberton H, A'Hern R, Swain A, Kunju LP, Eeles R, Attard G, Lord CJ, Ashworth A, Rubin MA, Knudsen KE, Feng FY, Chinnaiyan AM, Hall E, de Bono JS. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373:1697-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1642] [Cited by in RCA: 1748] [Article Influence: 174.8] [Reference Citation Analysis (0)] |
| 101. | Lowery MA, Kelsen DP, Capanu M, Smith SC, Lee JW, Stadler ZK, Moore MJ, Kindler HL, Golan T, Segal A, Maynard H, Hollywood E, Moynahan M, Salo-Mullen EE, Do RKG, Chen AP, Yu KH, Tang LH, O'Reilly EM. Phase II trial of veliparib in patients with previously treated BRCA-mutated pancreas ductal adenocarcinoma. Eur J Cancer. 2018;89:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 102. | Shroff RT, Hendifar A, McWilliams RR, Geva R, Epelbaum R, Rolfe L, Goble S, Lin KK, Biankin AV, Giordano H, Vonderheide RH, Domchek SM. Rucaparib Monotherapy in Patients With Pancreatic Cancer and a Known Deleterious BRCA Mutation. JCO Precis Oncol. 2018;2018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 110] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 103. | Golan T, Hammel P, Reni M, Van Cutsem E, Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, Reinacher-Schick A, Tortora G, Algül H, O'Reilly EM, McGuinness D, Cui KY, Schlienger K, Locker GY, Kindler HL. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med. 2019;381:317-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1137] [Cited by in RCA: 1614] [Article Influence: 269.0] [Reference Citation Analysis (0)] |
| 104. | Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke GS, Gourley C, Banerjee S, Oza A, González-Martín A, Aghajanian C, Bradley W, Mathews C, Liu J, Lowe ES, Bloomfield R, DiSilvestro P. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N Engl J Med. 2018;379:2495-2505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1940] [Article Influence: 277.1] [Reference Citation Analysis (0)] |
| 105. | Chan N, Bristow RG. "Contextual" synthetic lethality and/or loss of heterozygosity: tumor hypoxia and modification of DNA repair. Clin Cancer Res. 2010;16:4553-4560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 106. | Kessler ER, Eckhardt SG, Pitts TM, Bradshaw-Pierce EL, O'byrant CL, Messersmith WA, Nallapreddy S, Weekes C, Spratlin J, Lieu CH, Kane MA, Eppers S, Freas E, Leong S. Phase I trial of vandetanib in combination with gemcitabine and capecitabine in patients with advanced solid tumors with an expanded cohort in pancreatic and biliary cancers. Invest New Drugs. 2016;34:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 107. | Middleton G, Palmer DH, Greenhalf W, Ghaneh P, Jackson R, Cox T, Evans A, Shaw VE, Wadsley J, Valle JW, Propper D, Wasan H, Falk S, Cunningham D, Coxon F, Ross P, Madhusudan S, Wadd N, Corrie P, Hickish T, Costello E, Campbell F, Rawcliffe C, Neoptolemos JP. Vandetanib plus gemcitabine versus placebo plus gemcitabine in locally advanced or metastatic pancreatic carcinoma (ViP): a prospective, randomised, double-blind, multicentre phase 2 trial. Lancet Oncol. 2017;18:486-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 108. | Knudsen ES, O'Reilly EM, Brody JR, Witkiewicz AK. Genetic Diversity of Pancreatic Ductal Adenocarcinoma and Opportunities for Precision Medicine. Gastroenterology. 2016;150:48-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 109. | Qian ZR, Rubinson DA, Nowak JA, Morales-Oyarvide V, Dunne RF, Kozak MM, Welch MW, Brais LK, Da Silva A, Li T, Li W, Masuda A, Yang J, Shi Y, Gu M, Masugi Y, Bui J, Zellers CL, Yuan C, Babic A, Khalaf N, Aguirre A, Ng K, Miksad RA, Bullock AJ, Chang DT, Tseng JF, Clancy TE, Linehan DC, Findeis-Hosey JJ, Doyle LA, Thorner AR, Ducar M, Wollison B, Laing A, Hahn WC, Meyerson M, Fuchs CS, Ogino S, Hornick JL, Hezel AF, Koong AC, Wolpin BM. Association of Alterations in Main Driver Genes With Outcomes of Patients With Resected Pancreatic Ductal Adenocarcinoma. JAMA Oncol. 2018;4:e173420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 163] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 110. | Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5100] [Cited by in RCA: 5105] [Article Influence: 204.2] [Reference Citation Analysis (0)] |
| 111. | Blandino G, Di Agostino S. New therapeutic strategies to treat human cancers expressing mutant p53 proteins. J Exp Clin Cancer Res. 2018;37:30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 112. | Bykov VJN, Eriksson SE, Bianchi J, Wiman KG. Targeting mutant p53 for efficient cancer therapy. Nat Rev Cancer. 2018;18:89-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 658] [Article Influence: 94.0] [Reference Citation Analysis (0)] |
| 113. | Todoric J, Antonucci L, Di Caro G, Li N, Wu X, Lytle NK, Dhar D, Banerjee S, Fagman JB, Browne CD, Umemura A, Valasek MA, Kessler H, Tarin D, Goggins M, Reya T, Diaz-Meco M, Moscat J, Karin M. Stress-Activated NRF2-MDM2 Cascade Controls Neoplastic Progression in Pancreas. Cancer Cell. 2017;32:824-839.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 107] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 114. | Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3508] [Cited by in RCA: 3651] [Article Influence: 173.9] [Reference Citation Analysis (0)] |
| 115. | Ringshausen I, O'Shea CC, Finch AJ, Swigart LB, Evan GI. Mdm2 is critically and continuously required to suppress lethal p53 activity in vivo. Cancer Cell. 2006;10:501-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 116. | Qin L, Yang F, Zhou C, Chen Y, Zhang H, Su Z. Efficient reactivation of p53 in cancer cells by a dual MdmX/Mdm2 inhibitor. J Am Chem Soc. 2014;136:18023-18033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 117. | Azmi AS, Aboukameel A, Banerjee S, Wang Z, Mohammad M, Wu J, Wang S, Yang D, Philip PA, Sarkar FH, Mohammad RM. MDM2 inhibitor MI-319 in combination with cisplatin is an effective treatment for pancreatic cancer independent of p53 function. Eur J Cancer. 2010;46:1122-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 118. | Wang W, Qin JJ, Voruganti S, Nijampatnam B, Velu SE, Ruan KH, Hu M, Zhou J, Zhang R. Discovery and Characterization of Dual Inhibitors of MDM2 and NFAT1 for Pancreatic Cancer Therapy. Cancer Res. 2018;78:5656-5667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 119. | Wang W, Qin JJ, Voruganti S, Wang MH, Sharma H, Patil S, Zhou J, Wang H, Mukhopadhyay D, Buolamwini JK, Zhang R. Identification of a new class of MDM2 inhibitor that inhibits growth of orthotopic pancreatic tumors in mice. Gastroenterology. 2014;147:893-902.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 120. | Gaspar NJ, Li L, Kapoun AM, Medicherla S, Reddy M, Li G, O'Young G, Quon D, Henson M, Damm DL, Muiru GT, Murphy A, Higgins LS, Chakravarty S, Wong DH. Inhibition of transforming growth factor beta signaling reduces pancreatic adenocarcinoma growth and invasiveness. Mol Pharmacol. 2007;72:152-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 121. | Schlingensiepen KH, Jaschinski F, Lang SA, Moser C, Geissler EK, Schlitt HJ, Kielmanowicz M, Schneider A. Transforming growth factor-beta 2 gene silencing with trabedersen (AP 12009) in pancreatic cancer. Cancer Sci. 2011;102:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 122. | Melisi D, Garcia-Carbonero R, Macarulla T, Pezet D, Deplanque G, Fuchs M, Trojan J, Oettle H, Kozloff M, Cleverly A, Smith C, Estrem ST, Gueorguieva I, Lahn MMF, Blunt A, Benhadji KA, Tabernero J. Galunisertib plus gemcitabine vs. gemcitabine for first-line treatment of patients with unresectable pancreatic cancer. Br J Cancer. 2018;119:1208-1214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 222] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 123. | Melisi D, Oh DY, Hollebecque A, Calvo E, Varghese A, Borazanci E, Macarulla T, Merz V, Zecchetto C, Zhao Y, Gueorguieva I, Man M, Gandhi L, Estrem ST, Benhadji KA, Lanasa MC, Avsar E, Guba SC, Garcia-Carbonero R. Safety and activity of the TGFβ receptor I kinase inhibitor galunisertib plus the anti-PD-L1 antibody durvalumab in metastatic pancreatic cancer. J Immunother Cancer. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 136] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 124. | Akhurst RJ. Targeting TGF-β Signaling for Therapeutic Gain. Cold Spring Harb Perspect Biol. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 166] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 125. | Ravi R, Noonan KA, Pham V, Bedi R, Zhavoronkov A, Ozerov IV, Makarev E, V Artemov A, Wysocki PT, Mehra R, Nimmagadda S, Marchionni L, Sidransky D, Borrello IM, Izumchenko E, Bedi A. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFβ enhance the efficacy of cancer immunotherapy. Nat Commun. 2018;9:741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 222] [Cited by in RCA: 252] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 126. | Diop-Frimpong B, Chauhan VP, Krane S, Boucher Y, Jain RK. Losartan inhibits collagen I synthesis and improves the distribution and efficacy of nanotherapeutics in tumors. Proc Natl Acad Sci U S A. 2011;108:2909-2914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 611] [Article Influence: 43.6] [Reference Citation Analysis (0)] |
| 127. | Cohn RD, van Erp C, Habashi JP, Soleimani AA, Klein EC, Lisi MT, Gamradt M, ap Rhys CM, Holm TM, Loeys BL, Ramirez F, Judge DP, Ward CW, Dietz HC. Angiotensin II type 1 receptor blockade attenuates TGF-beta-induced failure of muscle regeneration in multiple myopathic states. Nat Med. 2007;13:204-210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 561] [Cited by in RCA: 529] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 128. | Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P, Popović Z, Huang P, Bawendi MG, Boucher Y, Jain RK. Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun. 2013;4:2516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 674] [Cited by in RCA: 787] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 129. | Murphy JE, Wo JY, Ryan DP, Clark JW, Jiang W, Yeap BY, Drapek LC, Ly L, Baglini CV, Blaszkowsky LS, Ferrone CR, Parikh AR, Weekes CD, Nipp RD, Kwak EL, Allen JN, Corcoran RB, Ting DT, Faris JE, Zhu AX, Goyal L, Berger DL, Qadan M, Lillemoe KD, Talele N, Jain RK, DeLaney TF, Duda DG, Boucher Y, Fernández-Del Castillo C, Hong TS. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol. 2019;5:1020-1027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 395] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 130. | Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol. 2013;14:518-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 1056] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 131. | Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer. 2016;16:553-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 305] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 132. | Klein ME, Kovatcheva M, Davis LE, Tap WD, Koff A. CDK4/6 Inhibitors: The Mechanism of Action May Not Be as Simple as Once Thought. Cancer Cell. 2018;34:9-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 272] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 133. | O'Leary B, Finn RS, Turner NC. Treating cancer with selective CDK4/6 inhibitors. Nat Rev Clin Oncol. 2016;13:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 595] [Cited by in RCA: 801] [Article Influence: 89.0] [Reference Citation Analysis (0)] |
| 134. | Turner NC, Slamon DJ, Ro J, Bondarenko I, Im SA, Masuda N, Colleoni M, DeMichele A, Loi S, Verma S, Iwata H, Harbeck N, Loibl S, André F, Puyana Theall K, Huang X, Giorgetti C, Huang Bartlett C, Cristofanilli M. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2018;379:1926-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 833] [Article Influence: 119.0] [Reference Citation Analysis (0)] |
| 135. | Dickson MA, Schwartz GK, Keohan ML, D'Angelo SP, Gounder MM, Chi P, Antonescu CR, Landa J, Qin LX, Crago AM, Singer S, Koff A, Tap WD. Progression-Free Survival Among Patients With Well-Differentiated or Dedifferentiated Liposarcoma Treated With CDK4 Inhibitor Palbociclib: A Phase 2 Clinical Trial. JAMA Oncol. 2016;2:937-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 246] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 136. | Heilmann AM, Perera RM, Ecker V, Nicolay BN, Bardeesy N, Benes CH, Dyson NJ. CDK4/6 and IGF1 receptor inhibitors synergize to suppress the growth of p16INK4A-deficient pancreatic cancers. Cancer Res. 2014;74:3947-3958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 137. | Rencuzogulları O, Yerlikaya PO, Gürkan AÇ, Arısan ED, Telci D. Palbociclib, a selective CDK4/6 inhibitor, restricts cell survival and epithelial-mesenchymal transition in Panc-1 and MiaPaCa-2 pancreatic cancer cells. J Cell Biochem. 2020;121:508-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 138. | Chou A, Froio D, Nagrial AM, Parkin A, Murphy KJ, Chin VT, Wohl D, Steinmann A, Stark R, Drury A, Walters SN, Vennin C, Burgess A, Pinese M, Chantrill LA, Cowley MJ, Molloy TJ; Australian Pancreatic Cancer Genome Initiative (APGI), Waddell N, Johns A, Grimmond SM, Chang DK, Biankin AV, Sansom OJ, Morton JP, Grey ST, Cox TR, Turchini J, Samra J, Clarke SJ, Timpson P, Gill AJ, Pajic M. Tailored first-line and second-line CDK4-targeting treatment combinations in mouse models of pancreatic cancer. Gut. 2018;67:2142-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 139. | Dhir T, Schultz CW, Jain A, Brown SZ, Haber A, Goetz A, Xi C, Su GH, Xu L, Posey J 3rd, Jiang W, Yeo CJ, Golan T, Pishvaian MJ, Brody JR. Abemaciclib Is Effective Against Pancreatic Cancer Cells and Synergizes with HuR and YAP1 Inhibition. Mol Cancer Res. 2019;17:2029-2041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 140. | Sherr CJ. A New Cell-Cycle Target in Cancer - Inhibiting Cyclin D-Dependent Kinases 4 and 6. N Engl J Med. 2016;375:1920-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 141. | Salvador-Barbero B, Álvarez-Fernández M, Zapatero-Solana E, El Bakkali A, Menéndez MDC, López-Casas PP, Di Domenico T, Xie T, VanArsdale T, Shields DJ, Hidalgo M, Malumbres M. CDK4/6 Inhibitors Impair Recovery from Cytotoxic Chemotherapy in Pancreatic Adenocarcinoma. Cancer Cell. 2020;37:340-353.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 142. | Knudsen ES, Kumarasamy V, Chung S, Ruiz A, Vail P, Tzetzo S, Wu J, Nambiar R, Sivinski J, Chauhan SS, Seshadri M, Abrams SI, Wang J, Witkiewicz AK. Targeting dual signalling pathways in concert with immune checkpoints for the treatment of pancreatic cancer. Gut. 2021;70:127-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 143. | Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119-127. [PubMed] |
| 144. | Liu D, He M, Yi B, Guo WH, Que AL, Zhang JX. Pim-3 protects against cardiomyocyte apoptosis in anoxia/reoxygenation injury via p38-mediated signal pathway. Int J Biochem Cell Biol. 2009;41:2315-2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 145. | Morran DC, Wu J, Jamieson NB, Mrowinska A, Kalna G, Karim SA, Au AY, Scarlett CJ, Chang DK, Pajak MZ; Australian Pancreatic Cancer Genome Initiative (APGI), Oien KA, McKay CJ, Carter CR, Gillen G, Champion S, Pimlott SL, Anderson KI, Evans TR, Grimmond SM, Biankin AV, Sansom OJ, Morton JP. Targeting mTOR dependency in pancreatic cancer. Gut. 2014;63:1481-1489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 146. | Kunnumakkara AB, Guha S, Krishnan S, Diagaradjane P, Gelovani J, Aggarwal BB. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor-kappaB-regulated gene products. Cancer Res. 2007;67:3853-3861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 451] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 147. | Li L, Braiteh FS, Kurzrock R. Liposome-encapsulated curcumin: in vitro and in vivo effects on proliferation, apoptosis, signaling, and angiogenesis. Cancer. 2005;104:1322-1331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 450] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 148. | Uwagawa T, Li Z, Chang Z, Xia Q, Peng B, Sclabas GM, Ishiyama S, Hung MC, Evans DB, Abbruzzese JL, Chiao PJ. Mechanisms of synthetic serine protease inhibitor (FUT-175)-mediated cell death. Cancer. 2007;109:2142-2153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 149. | Uwagawa T, Misawa T, Tsutsui N, Ito R, Gocho T, Hirohara S, Sadaoka S, Yanaga K. Phase II study of gemcitabine in combination with regional arterial infusion of nafamostat mesilate for advanced pancreatic cancer. Am J Clin Oncol. 2013;36:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 150. | Uwagawa T, Sakamoto T, Yasuda J, Shiozaki H, Furukawa K, Onda S, Gocho T, Shiba H, Yanaga K. Phase II Study of Adjuvant Chemotherapy With Gemcitabine and Nafamostat Mesilate for Pancreatic Cancer. Pancreas. 2021;50:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 151. | Erkan M, Hausmann S, Michalski CW, Fingerle AA, Dobritz M, Kleeff J, Friess H. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 489] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 152. | Erkan M, Reiser-Erkan C, Michalski CW, Kong B, Esposito I, Friess H, Kleeff J. The impact of the activated stroma on pancreatic ductal adenocarcinoma biology and therapy resistance. Curr Mol Med. 2012;12:288-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 153. | Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 352] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 154. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 797] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 155. | Xu D, Matsuo Y, Ma J, Koide S, Ochi N, Yasuda A, Funahashi H, Okada Y, Takeyama H. Cancer cell-derived IL-1α promotes HGF secretion by stromal cells and enhances metastatic potential in pancreatic cancer cells. J Surg Oncol. 2010;102:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 156. | Erkan M, Reiser-Erkan C, Michalski CW, Deucker S, Sauliunaite D, Streit S, Esposito I, Friess H, Kleeff J. Cancer-stellate cell interactions perpetuate the hypoxia-fibrosis cycle in pancreatic ductal adenocarcinoma. Neoplasia. 2009;11:497-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 227] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 157. | Lu X, Kang Y. Hypoxia and hypoxia-inducible factors: master regulators of metastasis. Clin Cancer Res. 2010;16:5928-5935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 535] [Cited by in RCA: 526] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 158. | Amakye D, Jagani Z, Dorsch M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat Med. 2013;19:1410-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 159. | Yao J, An Y, Wie JS, Ji ZL, Lu ZP, Wu JL, Jiang KR, Chen P, Xu ZK, Miao Y. Cyclopamine reverts acquired chemoresistance and down-regulates cancer stem cell markers in pancreatic cancer cell lines. Swiss Med Wkly. 2011;141:w13208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 160. | Catenacci DV, Junttila MR, Karrison T, Bahary N, Horiba MN, Nattam SR, Marsh R, Wallace J, Kozloff M, Rajdev L, Cohen D, Wade J, Sleckman B, Lenz HJ, Stiff P, Kumar P, Xu P, Henderson L, Takebe N, Salgia R, Wang X, Stadler WM, de Sauvage FJ, Kindler HL. Randomized Phase Ib/II Study of Gemcitabine Plus Placebo or Vismodegib, a Hedgehog Pathway Inhibitor, in Patients With Metastatic Pancreatic Cancer. J Clin Oncol. 2015;33:4284-4292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 424] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 161. | De Jesus-Acosta A, Sugar EA, O'Dwyer PJ, Ramanathan RK, Von Hoff DD, Rasheed Z, Zheng L, Begum A, Anders R, Maitra A, McAllister F, Rajeshkumar NV, Yabuuchi S, de Wilde RF, Batukbhai B, Sahin I, Laheru DA. Phase 2 study of vismodegib, a hedgehog inhibitor, combined with gemcitabine and nab-paclitaxel in patients with untreated metastatic pancreatic adenocarcinoma. Br J Cancer. 2020;122:498-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 113] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 162. | McCleary-Wheeler AL, Carr RM, Palmer SR, Smyrk TC, Allred JB, Almada LL, Tolosa EJ, Lamberti MJ, Marks DL, Borad MJ, Molina JR, Qi Y, Lingle WL, Grothey A, Pitot HC, Jatoi A, Northfelt DW, Bryce AH, McWilliams RR, Okuno SH, Haluska P, Kim GP, Colon-Otero G, Lowe VJ, Callstrom MR, Ma WW, Bekaii-Saab T, Hung MC, Erlichman C, Fernandez-Zapico ME. Phase 1 trial of Vismodegib and Erlotinib combination in metastatic pancreatic cancer. Pancreatology. 2020;20:101-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 163. | Ko AH, LoConte N, Tempero MA, Walker EJ, Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci DV, Venook AP, Kindler HL. A Phase I Study of FOLFIRINOX Plus IPI-926, a Hedgehog Pathway Inhibitor, for Advanced Pancreatic Adenocarcinoma. Pancreas. 2016;45:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 181] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 164. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D, Frese KK, Denicola G, Feig C, Combs C, Winter SP, Ireland-Zecchini H, Reichelt S, Howat WJ, Chang A, Dhara M, Wang L, Rückert F, Grützmann R, Pilarsky C, Izeradjene K, Hingorani SR, Huang P, Davies SE, Plunkett W, Egorin M, Hruban RH, Whitebread N, McGovern K, Adams J, Iacobuzio-Donahue C, Griffiths J, Tuveson DA. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2581] [Cited by in RCA: 2531] [Article Influence: 158.2] [Reference Citation Analysis (0)] |
| 165. | Metcalfe C, de Sauvage FJ. Hedgehog fights back: mechanisms of acquired resistance against Smoothened antagonists. Cancer Res. 2011;71:5057-5061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 142] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 166. | Buonamici S, Williams J, Morrissey M, Wang A, Guo R, Vattay A, Hsiao K, Yuan J, Green J, Ospina B, Yu Q, Ostrom L, Fordjour P, Anderson DL, Monahan JE, Kelleher JF, Peukert S, Pan S, Wu X, Maira SM, García-Echeverría C, Briggs KJ, Watkins DN, Yao YM, Lengauer C, Warmuth M, Sellers WR, Dorsch M. Interfering with resistance to smoothened antagonists by inhibition of the PI3K pathway in medulloblastoma. Sci Transl Med. 2010;2:51ra70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 392] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 167. | Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1630] [Article Influence: 125.4] [Reference Citation Analysis (0)] |
| 168. | Hingorani SR, Zheng L, Bullock AJ, Seery TE, Harris WP, Sigal DS, Braiteh F, Ritch PS, Zalupski MM, Bahary N, Oberstein PE, Wang-Gillam A, Wu W, Chondros D, Jiang P, Khelifa S, Pu J, Aldrich C, Hendifar AE. HALO 202: Randomized Phase II Study of PEGPH20 Plus Nab-Paclitaxel/Gemcitabine Versus Nab-Paclitaxel/Gemcitabine in Patients With Untreated, Metastatic Pancreatic Ductal Adenocarcinoma. J Clin Oncol. 2018;36:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 343] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 169. | Ramanathan RK, McDonough SL, Philip PA, Hingorani SR, Lacy J, Kortmansky JS, Thumar J, Chiorean EG, Shields AF, Behl D, Mehan PT, Gaur R, Seery T, Guthrie KA, Hochster HS. Phase IB/II Randomized Study of FOLFIRINOX Plus Pegylated Recombinant Human Hyaluronidase Versus FOLFIRINOX Alone in Patients With Metastatic Pancreatic Adenocarcinoma: SWOG S1313. J Clin Oncol. 2019;37:1062-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 236] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 170. | Cascinu S, Verdecchia L, Valeri N, Berardi R, Scartozzi M. New target therapies in advanced pancreatic cancer. Ann Oncol. 2006;17 Suppl 5:v148-v152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 171. | Bramhall SR, Rosemurgy A, Brown PD, Bowry C, Buckels JA; Marimastat Pancreatic Cancer Study Group. Marimastat as first-line therapy for patients with unresectable pancreatic cancer: a randomized trial. J Clin Oncol. 2001;19:3447-3455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 291] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 172. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 397] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 173. | Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B, Tu D, Ottaway J, Humphrey R, Seymour L; National Cancer Institute of Canada Clinical Trials Group. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296-3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 276] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 174. | Bendell J, Sharma S, Patel MR, Windsor KS, Wainberg ZA, Gordon M, Chaves J, Berlin J, Brachmann CB, Zavodovskaya M, Liu J, Thai D, Bhargava P, Shah MA, Khan SA, Starodub A. Safety and Efficacy of Andecaliximab (GS-5745) Plus Gemcitabine and Nab-Paclitaxel in Patients with Advanced Pancreatic Adenocarcinoma: Results from a Phase I Study. Oncologist. 2020;25:954-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 175. | Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA. CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci U S A. 2013;110:12325-12330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 176. | Picozzi V, Alseidi A, Winter J, Pishvaian M, Mody K, Glaspy J, Larson T, Matrana M, Carney M, Porter S, Kouchakji E, Rocha F, Carrier E. Gemcitabine/nab-paclitaxel with pamrevlumab: a novel drug combination and trial design for the treatment of locally advanced pancreatic cancer. ESMO Open. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 177. | Pothula SP, Xu Z, Goldstein D, Biankin AV, Pirola RC, Wilson JS, Apte MV. Hepatocyte growth factor inhibition: a novel therapeutic approach in pancreatic cancer. Br J Cancer. 2016;114:269-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 178. | Sharma N, Adjei AA. In the clinic: ongoing clinical trials evaluating c-MET-inhibiting drugs. Ther Adv Med Oncol. 2011;3:S37-S50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 179. | Kim ST, Hong JY, Park SH, Park JO, Park YW, Park N, Lee H, Hong SH, Lee SJ, Song SW, Kim K, Park YS, Lim HY, Kang WK, Nam DH, Lee JW, Park K, Kim KM, Lee J. First-in-human phase I trial of anti-hepatocyte growth factor antibody (YYB101) in refractory solid tumor patients. Ther Adv Med Oncol. 2020;12:1758835920926796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 180. | Tomioka D, Maehara N, Kuba K, Mizumoto K, Tanaka M, Matsumoto K, Nakamura T. Inhibition of growth, invasion, and metastasis of human pancreatic carcinoma cells by NK4 in an orthotopic mouse model. Cancer Res. 2001;61:7518-7524. [PubMed] |
| 181. | Qian LW, Mizumoto K, Inadome N, Nagai E, Sato N, Matsumoto K, Nakamura T, Tanaka M. Radiation stimulates HGF receptor/c-Met expression that leads to amplifying cellular response to HGF stimulation via upregulated receptor tyrosine phosphorylation and MAP kinase activity in pancreatic cancer cells. Int J Cancer. 2003;104:542-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 182. | Jin H, Yang R, Zheng Z, Romero M, Ross J, Bou-Reslan H, Carano RA, Kasman I, Mai E, Young J, Zha J, Zhang Z, Ross S, Schwall R, Colbern G, Merchant M. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res. 2008;68:4360-4368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 184] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 183. | Liu L, Zeng W, Wortinger MA, Yan SB, Cornwell P, Peek VL, Stephens JR, Tetreault JW, Xia J, Manro JR, Credille KM, Ballard DW, Brown-Augsburger P, Wacheck V, Chow CK, Huang L, Wang Y, Denning I, Davies J, Tang Y, Vaillancourt P, Lu J. LY2875358, a neutralizing and internalizing anti-MET bivalent antibody, inhibits HGF-dependent and HGF-independent MET activation and tumor growth. Clin Cancer Res. 2014;20:6059-6070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 93] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 184. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2423] [Article Influence: 134.6] [Reference Citation Analysis (0)] |
| 185. | Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di Magliano M, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218-2227.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 186. | Ercan G, Karlitepe A, Ozpolat B. Pancreatic Cancer Stem Cells and Therapeutic Approaches. Anticancer Res. 2017;37:2761-2775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 187. | Nwaeburu CC, Abukiwan A, Zhao Z, Herr I. Quercetin-induced miR-200b-3p regulates the mode of self-renewing divisions in pancreatic cancer. Mol Cancer. 2017;16:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 96] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 188. | Ristorcelli E, Lombardo D. Targeting Notch signaling in pancreatic cancer. Expert Opin Ther Targets. 2010;14:541-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 189. | Okamoto W, Okamoto I, Tanaka K, Hatashita E, Yamada Y, Kuwata K, Yamaguchi H, Arao T, Nishio K, Fukuoka M, Jänne PA, Nakagawa K. TAK-701, a humanized monoclonal antibody to hepatocyte growth factor, reverses gefitinib resistance induced by tumor-derived HGF in non-small cell lung cancer with an EGFR mutation. Mol Cancer Ther. 2010;9:2785-2792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 190. | De Jesus-Acosta A, Laheru D, Maitra A, Arcaroli J, Rudek MA, Dasari A, Blatchford PJ, Quackenbush K, Messersmith W. A phase II study of the gamma secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest New Drugs. 2014;32:739-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 191. | Cook N, Basu B, Smith DM, Gopinathan A, Evans J, Steward WP, Palmer D, Propper D, Venugopal B, Hategan M, Anthoney DA, Hampson LV, Nebozhyn M, Tuveson D, Farmer-Hall H, Turner H, McLeod R, Halford S, Jodrell D. A phase I trial of the γ-secretase inhibitor MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma. Br J Cancer. 2018;118:793-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 192. | Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B, Henner WR, Meisner R, Sato A, Shah J, Tang T, Wallace B, Wang M, Zhang C, Kapoun AM, Lewicki J, Gurney A, Hoey T. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin Cancer Res. 2015;21:2084-2095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 194] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 193. | Hu ZI, Bendell JC, Bullock A, LoConte NK, Hatoum H, Ritch P, Hool H, Leach JW, Sanchez J, Sohal DPS, Strickler J, Patel R, Wang-Gillam A, Firdaus I, Yu KH, Kapoun AM, Holmgren E, Zhou L, Dupont J, Picozzi V, Sahai V, O'Reilly EM. A randomized phase II trial of nab-paclitaxel and gemcitabine with tarextumab or placebo in patients with untreated metastatic pancreatic cancer. Cancer Med. 2019;8:5148-5157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 194. | White BD, Chien AJ, Dawson DW. Dysregulation of Wnt/β-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 394] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 195. | Gurney A, Axelrod F, Bond CJ, Cain J, Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, Lam A, Lazetic S, Ma S, Mitra S, Park IK, Pickell K, Sato A, Satyal S, Stroud M, Tran H, Yen WC, Lewicki J, Hoey T. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc Natl Acad Sci U S A. 2012;109:11717-11722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 481] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 196. | Dotan E, Cardin DB, Lenz HJ, Messersmith W, O'Neil B, Cohen SJ, Denlinger CS, Shahda S, Astsaturov I, Kapoun AM, Brachmann RK, Uttamsingh S, Stagg RJ, Weekes C. Phase Ib Study of Wnt Inhibitor Ipafricept with Gemcitabine and nab-paclitaxel in Patients with Previously Untreated Stage IV Pancreatic Cancer. Clin Cancer Res. 2020;26:5348-5357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 197. | Müller S, Raulefs S, Bruns P, Afonso-Grunz F, Plötner A, Thermann R, Jäger C, Schlitter AM, Kong B, Regel I, Roth WK, Rotter B, Hoffmeier K, Kahl G, Koch I, Theis FJ, Kleeff J, Winter P, Michalski CW. Next-generation sequencing reveals novel differentially regulated mRNAs, lncRNAs, miRNAs, sdRNAs and a piRNA in pancreatic cancer. Mol Cancer. 2015;14:94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 200] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 198. | Lili LN, Matyunina LV, Walker LD, Daneker GW, McDonald JF. Evidence for the importance of personalized molecular profiling in pancreatic cancer. Pancreas. 2014;43:198-211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 199. | Venkatasubbarao K, Peterson L, Zhao S, Hill P, Cao L, Zhou Q, Nawrocki ST, Freeman JW. Inhibiting signal transducer and activator of transcription-3 increases response to gemcitabine and delays progression of pancreatic cancer. Mol Cancer. 2013;12:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 200. | Bissonnette R, Luchi M, Fidelus-Gort R, Jackson S, Zhang H, Flores R, Newton R, Scherle P, Yeleswaram S, Chen X, Menter A. A randomized, double-blind, placebo-controlled, dose-escalation study of the safety and efficacy of INCB039110, an oral janus kinase 1 inhibitor, in patients with stable, chronic plaque psoriasis. J Dermatolog Treat. 2016;27:332-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 201. | Beatty GL, Shahda S, Beck T, Uppal N, Cohen SJ, Donehower R, Gabayan AE, Assad A, Switzky J, Zhen H, Von Hoff DD. A Phase Ib/II Study of the JAK1 Inhibitor, Itacitinib, plus nab-Paclitaxel and Gemcitabine in Advanced Solid Tumors. Oncologist. 2019;24:14-e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 202. | Sonbol MB, Bekaii-Saab T. A clinical trial protocol paper discussing the BRIGHTER study. Future Oncol. 2018;14:901-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 203. | Sonbol MB, Ahn DH, Goldstein D, Okusaka T, Tabernero J, Macarulla T, Reni M, Li CP, O'Neil B, Van Cutsem E, Bekaii-Saab T. CanStem111P trial: a Phase III study of napabucasin plus nab-paclitaxel with gemcitabine. Future Oncol. 2019;15:1295-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 204. | Tyner JW, Bumm TG, Deininger J, Wood L, Aichberger KJ, Loriaux MM, Druker BJ, Burns CJ, Fantino E, Deininger MW. CYT387, a novel JAK2 inhibitor, induces hematologic responses and normalizes inflammatory cytokines in murine myeloproliferative neoplasms. Blood. 2010;115:5232-5240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 190] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 205. | Ng K, Hendifar A, Starodub A, Chaves J, Yang Y, Koh B, Barbie D, Hahn WC, Fuchs CS. Phase 1 dose-escalation study of momelotinib, a Janus kinase 1/2 inhibitor, combined with gemcitabine and nab-paclitaxel in patients with previously untreated metastatic pancreatic ductal adenocarcinoma. Invest New Drugs. 2019;37:159-165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 206. | Lonardo E, Hermann PC, Heeschen C. Pancreatic cancer stem cells - update and future perspectives. Mol Oncol. 2010;4:431-442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 207. | Kang R, Tang D. Autophagy in pancreatic cancer pathogenesis and treatment. Am J Cancer Res. 2012;2:383-396. [PubMed] |
| 208. | Wolpin BM, Rubinson DA, Wang X, Chan JA, Cleary JM, Enzinger PC, Fuchs CS, McCleary NJ, Meyerhardt JA, Ng K, Schrag D, Sikora AL, Spicer BA, Killion L, Mamon H, Kimmelman AC. Phase II and pharmacodynamic study of autophagy inhibition using hydroxychloroquine in patients with metastatic pancreatic adenocarcinoma. Oncologist. 2014;19:637-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 298] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 209. | Karasic TB, O'Hara MH, Loaiza-Bonilla A, Reiss KA, Teitelbaum UR, Borazanci E, De Jesus-Acosta A, Redlinger C, Burrell JA, Laheru DA, Von Hoff DD, Amaravadi RK, Drebin JA, O'Dwyer PJ. Effect of Gemcitabine and nab-Paclitaxel With or Without Hydroxychloroquine on Patients With Advanced Pancreatic Cancer: A Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019;5:993-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 221] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 210. | Zeh HJ, Bahary N, Boone BA, Singhi AD, Miller-Ocuin JL, Normolle DP, Zureikat AH, Hogg ME, Bartlett DL, Lee KK, Tsung A, Marsh JW, Murthy P, Tang D, Seiser N, Amaravadi RK, Espina V, Liotta L, Lotze MT. A Randomized Phase II Preoperative Study of Autophagy Inhibition with High-Dose Hydroxychloroquine and Gemcitabine/Nab-Paclitaxel in Pancreatic Cancer Patients. Clin Cancer Res. 2020;26:3126-3134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 211. | Bryant KL, Stalnecker CA, Zeitouni D, Klomp JE, Peng S, Tikunov AP, Gunda V, Pierobon M, Waters AM, George SD, Tomar G, Papke B, Hobbs GA, Yan L, Hayes TK, Diehl JN, Goode GD, Chaika NV, Wang Y, Zhang GF, Witkiewicz AK, Knudsen ES, Petricoin EF 3rd, Singh PK, Macdonald JM, Tran NL, Lyssiotis CA, Ying H, Kimmelman AC, Cox AD, Der CJ. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat Med. 2019;25:628-640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 484] [Cited by in RCA: 522] [Article Influence: 87.0] [Reference Citation Analysis (0)] |
| 212. | Kinsey CG, Camolotto SA, Boespflug AM, Guillen KP, Foth M, Truong A, Schuman SS, Shea JE, Seipp MT, Yap JT, Burrell LD, Lum DH, Whisenant JR, Gilcrease GW 3rd, Cavalieri CC, Rehbein KM, Cutler SL, Affolter KE, Welm AL, Welm BE, Scaife CL, Snyder EL, McMahon M. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat Med. 2019;25:620-627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 510] [Cited by in RCA: 486] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 213. | Boone BA, Zeh HJ 3rd, Bahary N. Autophagy Inhibition in Pancreatic Adenocarcinoma. Clin Colorectal Cancer. 2018;17:25-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 214. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, Bennouna J, Bachet JB, Khemissa-Akouz F, Péré-Vergé D, Delbaldo C, Assenat E, Chauffert B, Michel P, Montoto-Grillot C, Ducreux M; Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5609] [Article Influence: 400.6] [Reference Citation Analysis (1)] |
| 215. | Dhir M, Zenati MS, Hamad A, Singhi AD, Bahary N, Hogg ME, Zeh HJ 3rd, Zureikat AH. FOLFIRINOX Versus Gemcitabine/Nab-Paclitaxel for Neoadjuvant Treatment of Resectable and Borderline Resectable Pancreatic Head Adenocarcinoma. Ann Surg Oncol. 2018;25:1896-1903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 216. | Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer. 2017;17:709-724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 530] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 217. | Kumar AA, Buckley BJ, Ranson M. The Urokinase Plasminogen Activation System in Pancreatic Cancer: Prospective Diagnostic and Therapeutic Targets. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |