Copyright
©The Author(s) 2023.
World J Gastrointest Oncol. Feb 15, 2023; 15(2): 251-267
Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.251
Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.251
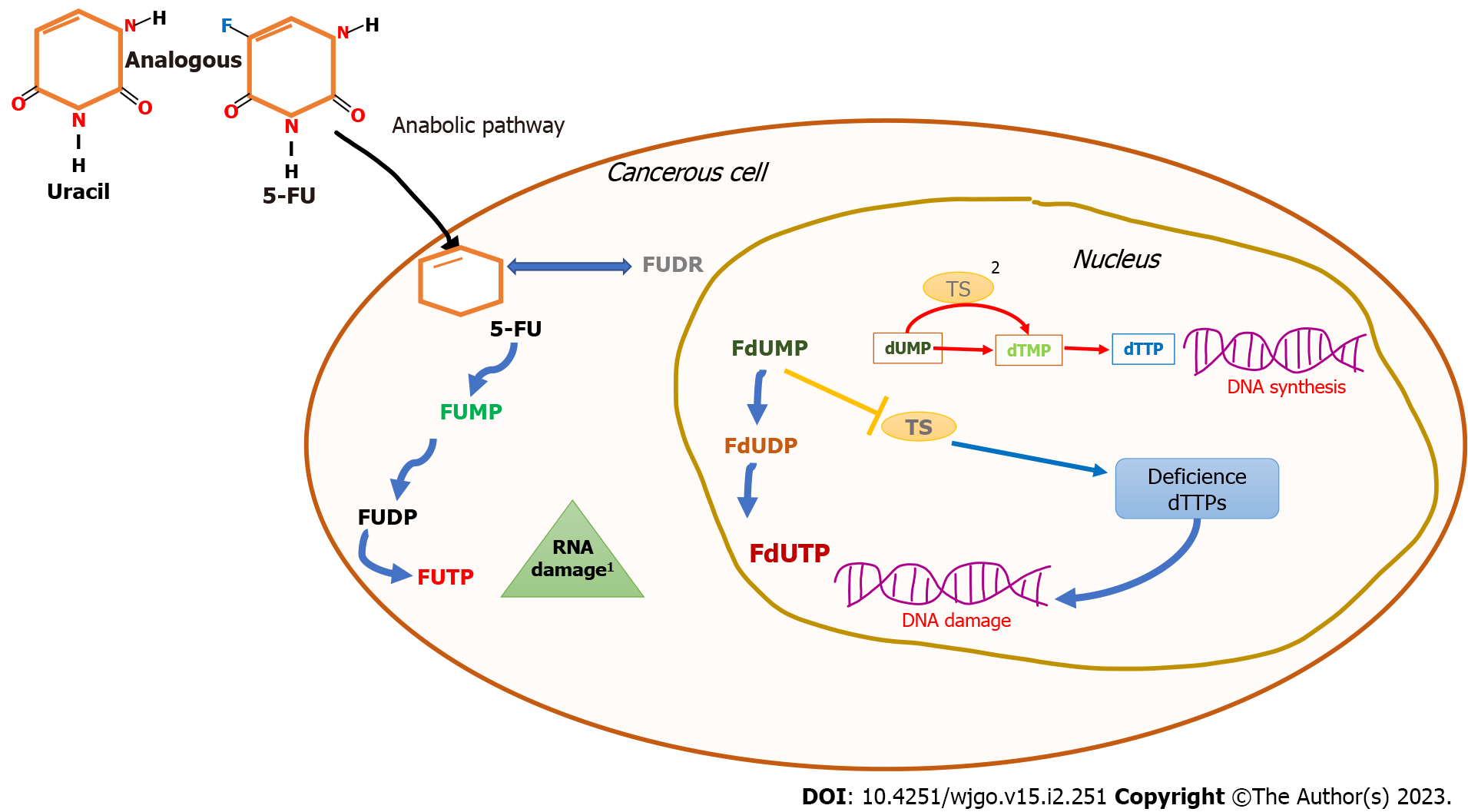
Figure 1 5-Fluorouracil mechanism of action.
The 5-fluorouracil structure is analogous to that of the nucleotide uracil; its ability to disrupt standard RNA processing and function is mediated by three primary metabolites: fluorodeoxyuridine monophosphate, fluorodeoxyuridine diphosphate, and fluorouridine triphosphate. 1: 5-fluorouracil inhibits thymidylate synthase activity by fluorodeoxyuridine monophosphate metabolite binding, blocking the typical substrate deoxyuridine monophosphate that inhibits deoxythymidine monophosphate synthesis leading to deoxythymidine triphosphate imbalance. The consequent result is DNA damage due to a deficiency in its synthesis and its repair; 2: DNA replication and repair are regulated by deoxyuridine monophosphate transition to deoxythymidine monophosphate. This step is coordinated by thymidylate synthase; FUMP: Fluorodeoxyuridine monophosphate; FUDP: Fluorodeoxyuridine diphosphate; FUTP: Fluorouridine triphosphate; dUMP: Deoxyuridine monophosphate; dTMP: Deoxythymidine monophosphate; FUDR: Fluorodeoxyuridine; FdUMP: Fluorodeoxyuridine monophosphate; dTTP: Deoxythymidine triphosphate; FdUDP: Fluorodeoxyuridine diphosphate; FdUTP: Fluorodeoxyuridine triphosphate; 5-FU: 5-Fluorouracil; TS: Thymidylate synthase.
- Citation: Olguin JE, Mendoza-Rodriguez MG, Sanchez-Barrera CA, Terrazas LI. Is the combination of immunotherapy with conventional chemotherapy the key to increase the efficacy of colorectal cancer treatment? World J Gastrointest Oncol 2023; 15(2): 251-267
- URL: https://www.wjgnet.com/1948-5204/full/v15/i2/251.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i2.251