Published online Feb 15, 2023. doi: 10.4251/wjgo.v15.i2.318
Peer-review started: September 22, 2022
First decision: October 21, 2022
Revised: November 4, 2022
Accepted: December 30, 2022
Article in press: December 30, 2022
Published online: February 15, 2023
Processing time: 145 Days and 22.2 Hours
microRNA-627-5p (miR-627-5p) dysregulation has been observed in several cancer types, such as hepatocellular carcinoma, oral squamous cell carcinoma, glioblastoma multiforme, and gastric cancer. The biological function of miR-627-5p in colorectal cancer (CRC) growth and metastasis is yet unclear.
To investigate the effects of miR-627-5p on the malignant biological properties of colorectal malignant tumour cells by targeting Wnt2.
The levels of miR-627-5p in colorectal tumour tissues were assessed in Gene Expression Omnibus datasets. In order to identify Wnt2 transcript expression in CRC tissues, quantitative real-time polymerase chain reaction (qRT-PCR) analysis was used. Luciferase reporter tests were used to explore whether miR-627-5p might potentially target Wnt2. Wnt2 transcript and protein levels were detected in CRC cells with high miR-627-5p expression. To learn more about how miR-627-5p affects CRC development, migration, apoptosis, and invasion, functional experiments were conducted. Cotransfection with the overexpression vector of Wnt2 and miR-627-5p mimics was utilized to verify whether overexpression of Wnt2 could cancel the impact of miR-627-5p in CRC. Western blot and qRT-PCR were conducted to investigate the effects of miR-627-5p on the Wnt/β-catenin signalling pathway.
miR-627-5p was notably decreased in colorectal tumour tissues, while the gene level of Wnt2 was notably upregulated. A dual luciferase reporter assay revealed that miR-627-5p specifically targets the 3’-untranslated regions of Wnt2 and miR-627-5p upregulation markedly reduced the protein and gene expression of Wnt2 in CRC cells. In vitro gain-of-function assays displayed that miR-627-5p overexpression decreased CRC cells’ capabilities to invade, move, and remain viable while increasing apoptosis. Wnt2 overexpression could reverse the suppressive functions of miR-627-5p. Moreover, upregulation of miR-627-5p suppressed the transcript and protein levels of the downstream target factors in the canonical Wnt/β-catenin signalling, such as c-myc, CD44, β-catenin, and cyclinD1.
miR-627-5p acts as a critical inhibitory factor in CRC, possibly by directly targeting Wnt2 and negatively modulating the Wnt/β-catenin signalling, revealing that miR-627-5p could be a possible treatment target for CRC.
Core Tip: It has been well established that miRNAs play vital roles in modulating cancer-related pathways, thereby regulating colorectal cancer (CRC) growth and metastasis. The study comprehensively explored the function of microRNA-627-5p (miR-627-5p), a rarely reported miRNA in CRC. miR-627-5p mimics restrained CRC cells invasion, migration, proliferation, and promoted cell apoptosis, indicated its suppressive effects on CRC development. A dual luciferase reporter test showed miR-627-5p directly binds with the 3’-untranslated region of Wnt2. Furthermore, miR-627-5p prevented the aggressive behaviours of cancer cells via inhibiting the activation of the canonical Wnt signalling. Strategies targeting the miR-627-5p/Wnt2/β-catenin signalling might be a new treatment option for CRC.
- Citation: Zhao DY, Yin TF, Sun XZ, Zhou YC, Wang QQ, Zhou GY, Yao SK. microRNA-627-5p inhibits colorectal cancer cell proliferation, migration and invasion by targeting Wnt2. World J Gastrointest Oncol 2023; 15(2): 318-331
- URL: https://www.wjgnet.com/1948-5204/full/v15/i2/318.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i2.318
Colorectal cancer (CRC) is one of the most prevalent malignant tumours and has high incidence and mortality rates globally, posing a great threat to the health of human beings[1]. Despite great advances in the diagnosis and treatment of CRC over the span of a few decades, CRC is still incurable and often has a poor prognosis, especially in patients with advanced tumours, which necessities clarification of the underlying mechanisms of CRC[2,3]. Generally, a vast number of genetic and molecular changes, such as epigenetic aberrations, genetic alterations, microsatellite instability, and chromosomal instability, have been shown to be correlated with colorectal carcinogenesis and tumour metastasis[4,5]. All of these genomic events can contribute to the activation of important signalling pathways (Wnt/β-catenin, MAPK/PI3K, and SMAD/TGF-β) and the initiation of colorectal tumorigenesis[6]. Since the human genome has been extensively characterized, miRNAs have become a hot spot and have offered fresh understanding of the molecular mechanisms underpinning CRC progression[7].
microRNA (miRNA) is an endogenously derived non-coding RNA sequence composed of 19-25 nucleotides that can modulate the levels of downstream genes through direct binding to their 3’-untranslated regions (3’-UTRs) via cleavage or translational arrest[8]. Many published papers have implicated the role of microRNA-627-5p (miR-627-5p) in controlling the emergence and development of a number of tumours[9-12]. For instance, Wang et al[9] reported that miR-627-5p acted as a inhibitory factor by reducing the expression of Bcl3 in hepatocellular carcinoma. miR-627-5p was also identified to be involved in suppressing the malignant behaviors of glioma cells by binding to the 3’-UTR of NR2C2 and downregulating its expression in glioblastoma multiforme[10]. These studies indicated the tumour inhibitory function of miR-627-5p. Furthermore, Shin et al[11] found miR-627-5p was observably upregulated in gastric cancer tissues than in normal controls, suggesting that miR-627-5p might stimulate gastric cancer progression. In other words, miR-627-5p may play different roles in different cancer types. A growing body of evidence has emphasized that abnormally expressed miRNAs play vital roles in promoting or suppressing the progression from normal to hyperproliferative epithelium, then to precancerous advanced adenoma (AA), and later invasive adenocarcinoma[13-15]. Nevertheless, the functions of miR-627-5p are largely unknown in colorectal carcinogen.
In the present study, TargetScan website speculated that miR-627-5p could be complementary with the 3’-UTR of Wnt2. Wnt2, an evolutionarily conserved secreted-type glycoprotein secreted by the Wnt signalling, performs a crucial role in promoting the malignant progression of gastrointestinal cancers through activation of the Wnt/β-catenin signalling[16-18]. Consequently, the current study’s goal was to investigate how miR-627-5p and Wnt2 contribute to the emergence of CRC, and to assess the relationship between them.
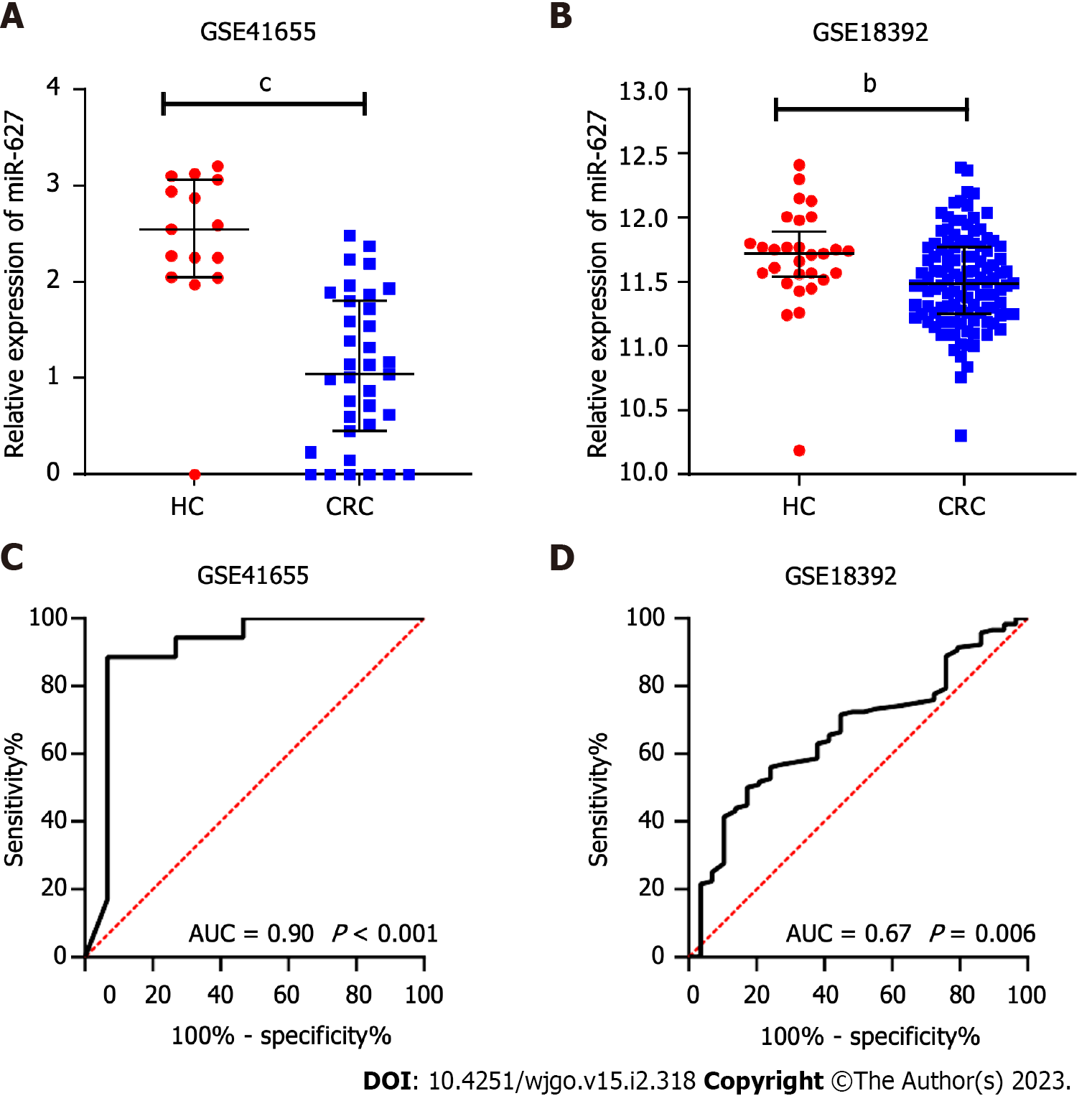
From the Gene Expression Omnibus (GEO) database, the original series matrix files of the miRNAs of patients with colorectal tumours and healthy controls (HCs) were gathered. GSE41655 and GSE18392 were employed to analyze the differential expression of miR-627 between CRC tissues and control tissues. GSE41655 contained 33 tumour tissues and 15 normal control tissues while GSE18392 included 116 tumour tissues and 29 control tissues.
A total of 30 patients with colorectal tumours, 33 AA and 20 HCs aged between 18 and 80 years were employed to explore the tissue levels of Wnt2 in the study. All patients diagnosed by histology as AA and colorectal adenocarcinoma were enrolled as case group. All healthy participants were recruited through advertisements and screened by careful history taking, physical examinations, essential laboratory examinations and colonoscopy. Individuals with negative colonoscopy results were selected as HC group. During the endoscopies, biopsy samples were taken from the rectosigmoid colon in the HCs. The excluding protocol for all subjects were indicated below: (1) Subjects with a history of other major organic diseases, malignant tumours in other organs, and psychiatric disorders; (2) pregnant or lactating female subjects; (3) patients with hereditary CRC or hereditary intestinal polyposis syndromes; (4) participants with a history of radiotherapy, chemotherapy, and major abdominal surgery; and (5) patients who presented with inflammatory or infective diseases. The clinicopathological characteristics of HCs and colorectal neoplasm patients are described in Supplementary Table 1. China-Japan Friendship Hospital’s ethics committee gave the study’s protocol approval under the number 2018-116-K85-1, and all participants gave their written informed permission.
The American Type Culture Collection was used to obtain a colonic epithelial cell line (FHC), human colonic malignant tumor cell lines (HCT116, RKO, and SW480), and a human embryonic kidney cell line (HEK-293T). For the purpose of investigating Wnt2 mRNA expression, FHC and three CRC cell lines were used. Gain of function tests were carried out using HCT116 and SW480 cells. Dual luciferase reporter tests were performed using HEK-293T cells because of their high transfection efficiency[19]. Incubation conditions included 5% CO2 in the air and a minimum relative humidity of 95%, for the RPMI 1640 medium or Dulbecco’s Modified Eagle’s Medium in which cells were placed.
miR-627-5p mimics, mimics negative control oligonucleotides (NC mimics), Wnt2 overexpressing plasmids (pcDNA-Wnt2) and matched negative controls (pcDNA-NC) were designed by GenePharma (Shanghai Province, China). In 6-well culture plates, SW480 and HCT116 cells were plated for 24 h. Following the manufacturer’s instructions, LipofectamineTM 3000 Transfection Reagent (Invitrogen, Carlsbad, CA, United States) was used to transfect a mixture of miR-627-5p mimics or NC mimics (150pmol) with pcDNA-Wnt2 or pcDNA-NC (3 g). In Supplementary Table 2, the oligonucleotide sequences are displayed.
Total RNA that included miRNA from tissues or cells was isolated by the RNAprep Pure Cell Kit (Solarbio, Beijing, China). cDNA was obtained using the Hifair®II 1st Strand cDNA Synthesis Kit (YESEN, Shanghai, China). PCR amplification was conducted in a LineGene 9600 Plus Real-Time PCR system (Bioer Technology, Hangzhou, China) by using Hieff® qPCR SYBR® Green Master Mix (No Rox) (YESEN, Shanghai, China). The comparative threshold method was used to quantify the relative expression of miRNAs and mRNAs. In Supplementary Table 3, the primer sequences used in the study are displayed.
RIPA lysis buffer (Beyotime, Shanghai, China) was used for protein extraction, and the BCA Protein Assay Kit was used to measure the amount of protein (Solarbio, Beijing, China). Total proteins were then subjected to sodium dodecyl sulfate/polyacrylamide gel electrophoresis and electrotransferred to PVDF membranes (Bio-Rad, Hercules, CA, United States). Nonspecific binding sites were blocked using 5% nonfat milk for 2 h and the membranes were incubated at 4 °C overnight with rabbit anti-Wnt2 antibody (1:1000; Affinity, Melbourne, United States), rabbit anti-c-myc antibody (1:1000; Bioss, Beijing, China), rabbit anti-CD44 antibody (1:1000; Affinity, Melbourne, United States), rabbit anti-cyclin D1 antibody (1:1000; Abcam, Cambridge, United Kingdom), and rabbit anti-β-tubulin antibody (1:4000; Proteintech, Chicago, United States). The PVDF membranes were then treated for 1 hour with a goat anti-rabbit secondary antibody that was HRP conjugated (1:5000; Bioss, Beijing, China). Enhanced chemiluminescence reagents (Beyotime, Shanghai, China) were used to visualize the bands. Image J was utilized to quantify the chemiluminescent signals of protein bands using β-tubulin as an internal control.
Cell viability was monitored using cholecystokinin octapeptide (CCK-8) reagents (Solarbio, Beijing, China). After transfection, CRC cells plated in 96-well plates were added to CCK-8 solution and incubated for 1 h. The number of viable cells was determined at a 24 h interval for four consecutive days following the manufacturer’s instructions.
Corning Transwell insert chambers (Corning Incorporated, New York, NY, United States) with a 6.5-µm pore size were used to assess invasive capability. Cancer cells were planted in the upper chamber, cultured with foetal bovine serum (FBS) free medium, and allowed to invade for 72 h. The lower chamber was added to culture medium comprising 10% FBS to attract the invaded cells. The invading cells that broke through the Matrigel were then fixed in paraformaldehyde, stained in crystal violet, and counted in five randomly selected high-power fields.
Homogeneous single cell suspensions were plated in 6-well plates until a single layer formed before being wounded by scraping a straight line with a yellow micropipette tip. The plates were incubated with complete medium after 3 PBS solution washes. All lengthy wounds were captured on camera at 0 and 24 h after the wound.
An Annexin V-fluorescein isothiocyanate/propidium iodide (PI) apoptosis kit (7seabiotech, Shanghai, China) was utilized to detect the proportion of apoptotic cells after transfection. All processes were performed following the manufacturer’s protocols. Flow cytometry (BeckMan, United States) and FlowJoTM (Becton, New York, NY, United States) software were used to determine the cell apoptosis rates. Detailed experimental procedures are described in our previous study[20].
The human Wnt2 3’-UTR comprising the expected complementary site of miR-627-5p (wild type), and its identical sequence with the mutant sequences of specific complementary sites of miR-627-5p (mutant) were inserted into the pmirGLO luciferase vector. HEK-293T cells were cotransfected with pmirGlo-Wnt2 3’-UTR wild type or pmirGLO-Wnt2 3’-UTR mutant and miR-627-5p mimics. The luciferase activity was measured using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, United States).
All data are shown as mean ± SD or median (interquartile range). The Shapiro-wilk test was used to verify the normal distribution. Student’s t test or Wilcoxon rank-sum test were employed to decide significant differences between two groups where appropriate. Spearman correlation analysis was used to calculate the relationship between Wnt2 gene expression and miR-627-5p expression in colorectal neoplasm tissues. All calculations were conducted with IBM SPSS (Chicago, IL, United States) and diagrams were described using GraphPad Prism (La Jolla, CA, United States). A P value of 0.05 or lower was considered significant.
The miR-627 levels were contrasted between the CRC subgroup and the normal subgroup in two GEO datasets, GSE41655 (33 CRCs vs 15 HCs) and GSE18392 (116 CRCs vs 29 HCs), and the findings showed that CRC tissues had noticeably lower levels of miR-627 (Figure 1A and B). The AUCs of miR-627 in the GSE41655 and GSE18392 datasets were 0.90 (P < 0.001) and 0.67 (P = 0.006), respectively, according to receiver operating characteristic analysis (Figure 1C and D).
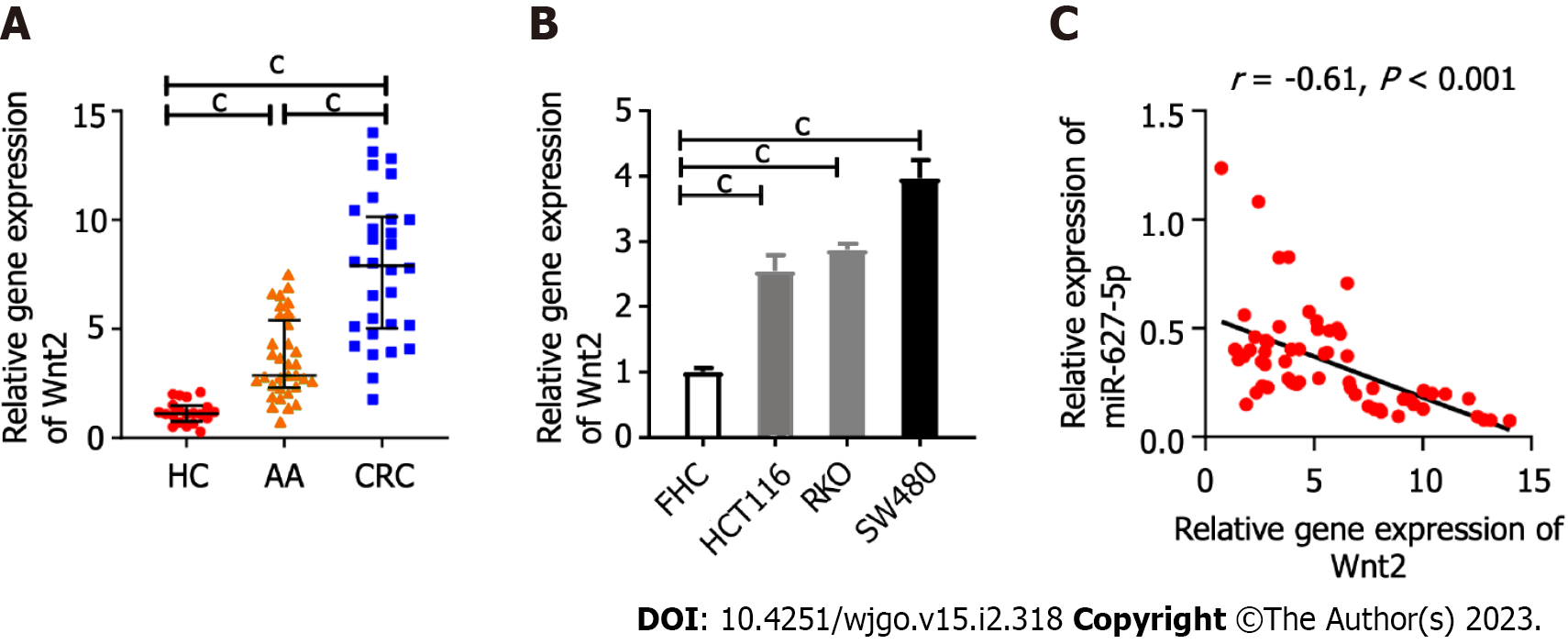
The mRNA levels of Wnt2 in clinical tissues or in the colonic epithelial cells and cancer cells were determined by quantitative real-time polymerase chain reaction (qRT-PCR). The findings indicated Wnt2 expression were sequentially upregulated from HC tissues and AA tissues to CRC tissues (Figure 2A). This observation was supported by the fact that Wnt2 expression in cancer cells was observably higher than it was in epithelial cell line (FHC) cells (Figure 2B). Our previous study examined miR-627-5p expression in the same clinical tissues[20] and we next analyzed the relationship among the expressions of miR-627-5p and Wnt2 in colorectal neoplasm tissues and found an inverse relationship between miR-627-5p and Wnt2 gene levels (r = -0.61, P < 0.001, Figure 2C).
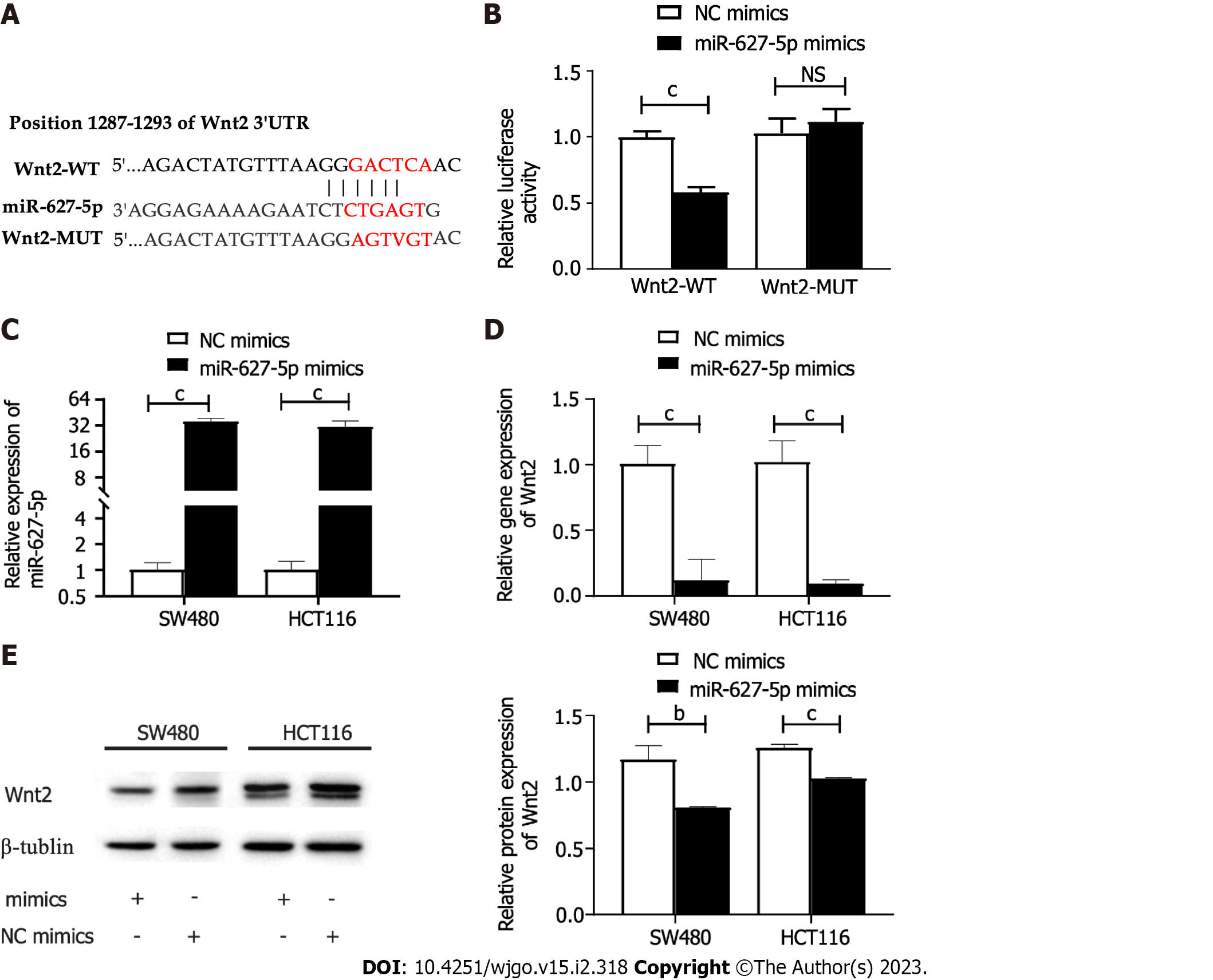
First, the possible miR-627-5p downstream genes were speculatively identified using the TargetScan website. As a result, a sequence located at bases 1287-1293 of the Wnt2 3’-UTR was binding to the seed sequence of miR-627-5p (Figure 3A). To verify that miR-627-5p and Wnt2 have a direct complimentary interaction, we conducted dual luciferase reporter assays. Then, the Wnt2 pmirGLO vector comprising wild type or mutant miR-627-5p target sites was constructed (Figure 3A). Then, the constructed vectors were transfected into 293T cells with the cotransfection of miR-627-5p mimics or scrambled controls. miR-627-5p overexpression reduced the luciferase levels of the reporter vector carrying the wild type sequence of Wnt2 3’-UTR (0.58 ± 0.04 vs 1.00 ± 0.05, P < 0.001), but not those of the reporter vector comprising the mutant target sequence in 293T cells (1.12 ± 0.09 vs 1.03 ± 0.11, P = 0.35, Figure 3B). Moreover, gain-of-function experiments were performed by transfection of miR-627-5p mimics in SW480 and HCT116 cells to verify whether miR-627-5p could influence the expression levels of Wnt2 in CRC cells. RT-PCR analysis demonstrated that miR-627-5p mimics markedly increased miR-627-5p level in SW480 (35.90 ± 3.09 vs 1.02 ± 0.20, P < 0.001) and HCT116 cells (31.30 ± 5.14 vs 1.02 ± 0.23, P < 0.001, Figure 3C). As depicted in Figure 3D-E, upregulation of miR-627-5p directly reduced the transcript expression (SW480, 0.09 ± 0.03 vs 1.01 ± 0.16, P < 0.001; HCT116, 0.02 (0.01-0.31) vs 0.93 (0.91-1.16), P < 0.001) and protein expression (SW480, 0.81 ± 0.01 vs 1.17 ± 0.10, P = 0.004; HCT116, 1.03 ± 0.01 vs 1.26 ± 0.03, P < 0.001) of Wnt2 in SW480 and HCT116 cells. In summary, miR-627-5p functions as a specific complement to Wnt2.
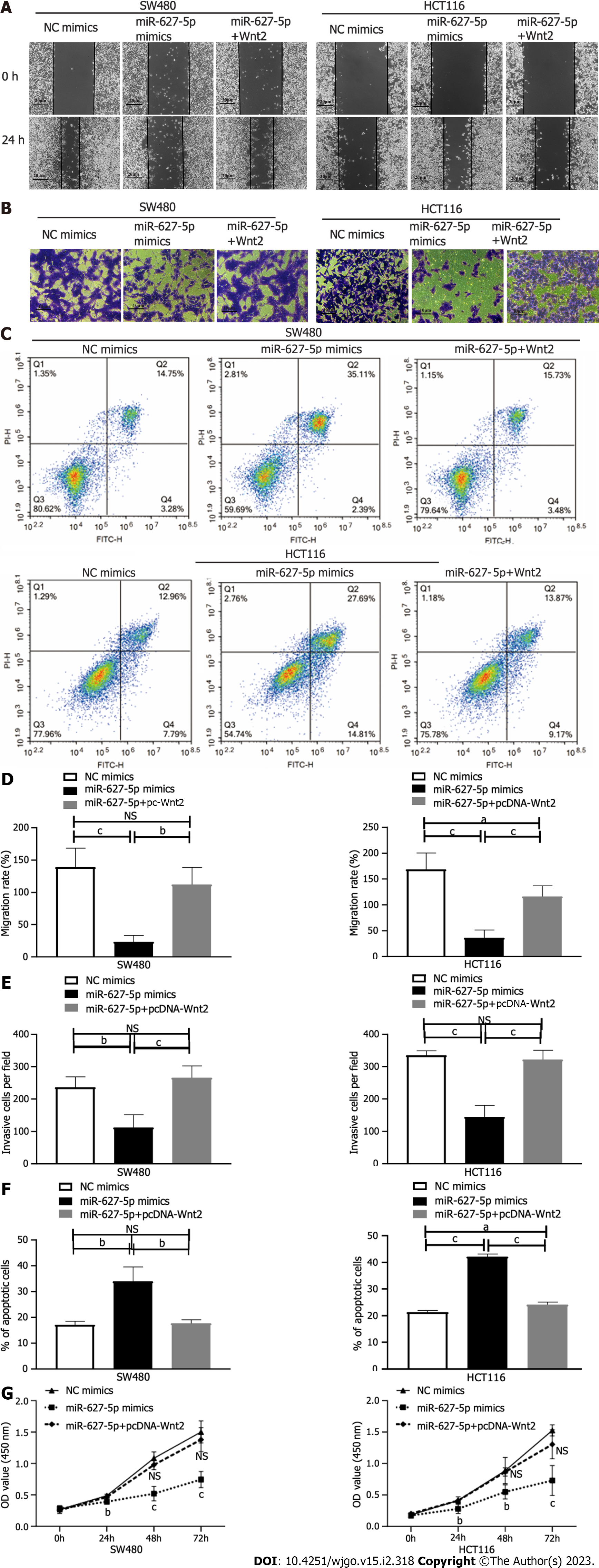
Then, we investigated the biological function of miR-627-5p through gain-of-function tests in SW480 and HCT116 cells. As depicted in Figure 4A and D, wound healing assays showed that miR-627-5p overexpression contributed to a weakened ability of migrating cells (SW480, 23.63% ± 9.62% vs 139.11% ± 29.36%, P < 0.001; HCT116, 36.03% ± 15.15% vs 168.69% ± 31.75%, P < 0.001). Matrigel invasion assays demonstrated that exogenetic upregulation of miR-627-5p markedly blocked cancer cells’ ability to invade (SW480, 112.00 ± 39.77 vs 236.20 ± 33.10, P = 0.001; HCT116, 144.60 ± 35.78 vs 335.20 ± 14.02, P < 0.001; Figure 4B and E). Next, we used flow cytometry analysis to verify whether miR-627-5p overexpression could influence cell apoptosis and the findings showed that miR-627-5p upregulation accelerated cell apoptosis (SW480, 33.91% ± 5.61% vs 17.08% ± 1.40%, P = 0.007; HCT116, 42.15% ± 1.00% vs 21.35% ± 0.61%, P < 0.001, Figure 4C and F). Furthermore, according to CCK-8 experiments, miR-627-5p overexpression attenuated cell growth (Figure 4G). Collectively, miR-627-5p inhibits CRC cells migration, invasion, and proliferation but promotes cell apoptosis.
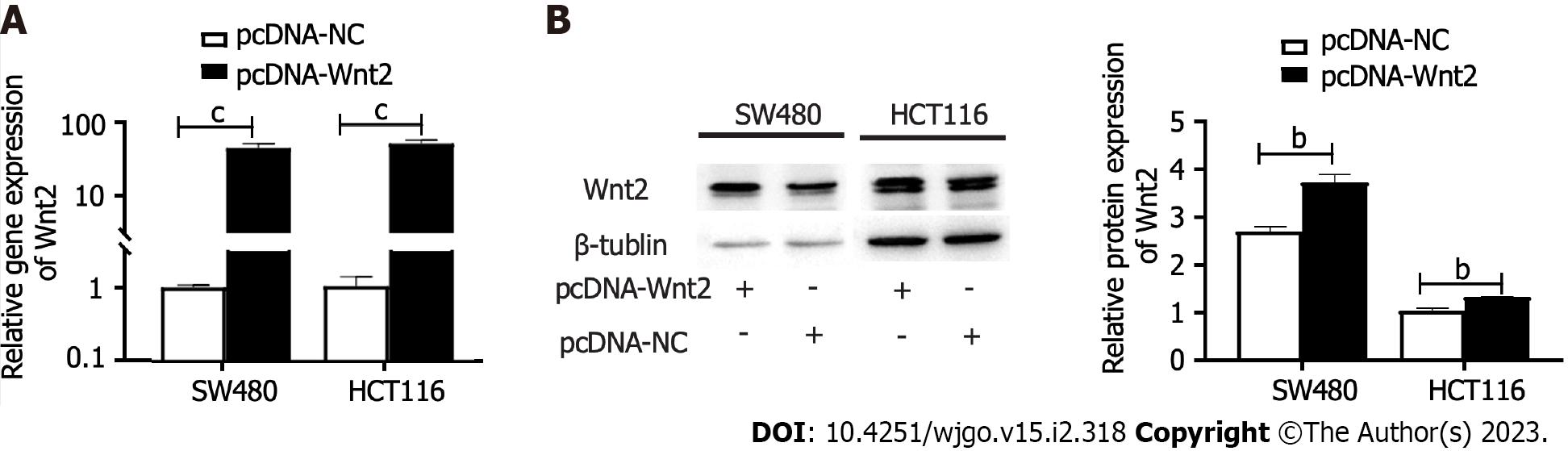
To explore the functional effects of the miR-627-5p/Wnt2 axis in CRC cells, we generated a Wnt2 overexpression vector (pcDNA-Wnt2) and designed rescue assays. As presented in Figure 5, pcDNA-Wnt2 effectively increased the gene [SW480, 44.84 ± 5.98 vs 1.00 ± 0.08, P < 0.001; HCT116, 51.39 (45.06-56.67) vs 1.15 (0.63-1.39), P < 0.001] and protein (SW480, 3.73 ± 0.16 vs 2.70 ± 0.10, P = 0.001; HCT116, 1.32 ± 0.01 vs 1.04 ± 0.05, P = 0.001) expression of Wnt2 in SW480 and HCT116 cells, proving that the construction of this vector was successful. Then, we used it to transfect CRC cells overexpression miR-627-5p. The results of wound healing and Matrigel invasion assays revealed that upregulation of Wnt partially canceled the suppressive functions of miR-627-5p on cell migration [SW480, 102.95% (96.05%-132.64%) vs 22.22% (15.48%-32.49%), P = 0.008; HCT116, 116.23% ± 20.46% vs 36.03% ± 15.15%, P < 0.001] and invasion (SW480, 265.80 ± 36.89 vs 112.00 ± 39.77, P < 0.001; HCT116, 322.00 ± 28.61 vs 144.60 ± 35.78, P < 0.001, Figure 4A, B, D, and E). Besides, flow cytometry analysis demonstrated that cell apoptosis induced by miR-627-5p could be attenuated by Wnt2 overexpression (SW480, 17.69% ± 1.35% vs 33.91% ± 5.61%, P = 0.008; HCT116, 24.17% ± 1.00% vs 42.15% ± 1.00%, P < 0.001, Figure 4C and F). CCK-8 assays demonstrated that the decreased in cell viability caused by miR-627-5p could be attenuated by Wnt2 overexpression (Figure 4G).
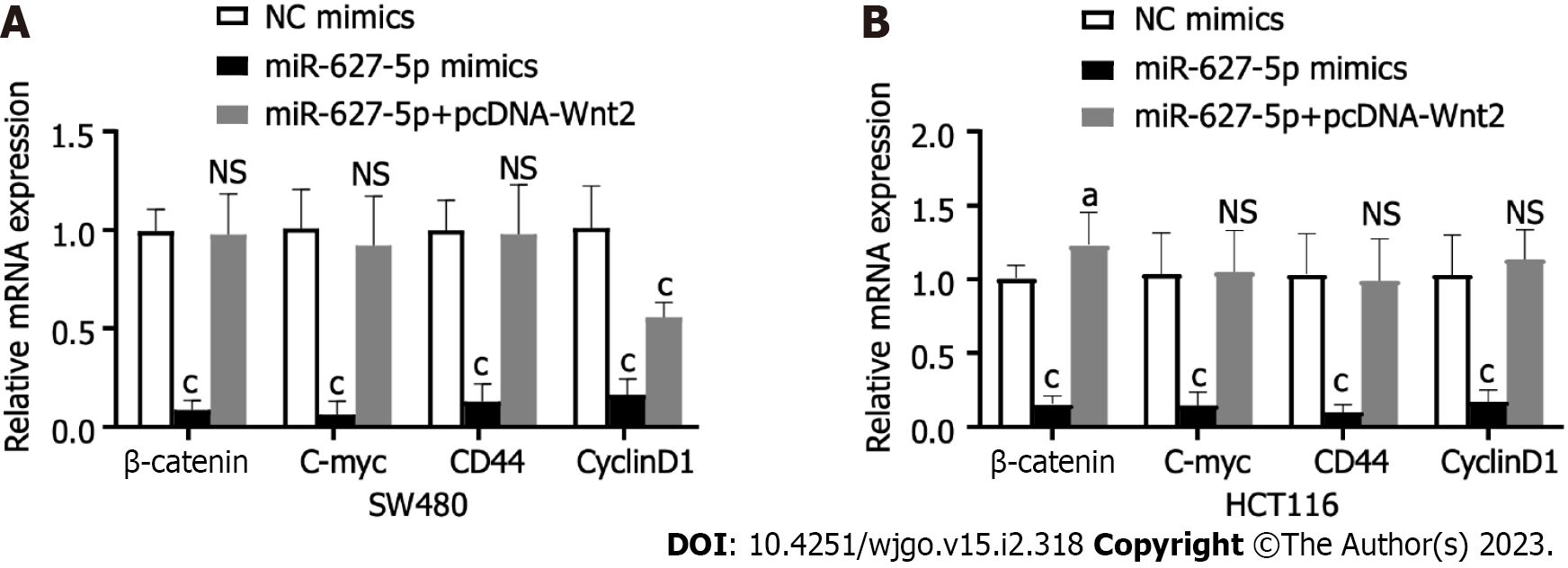
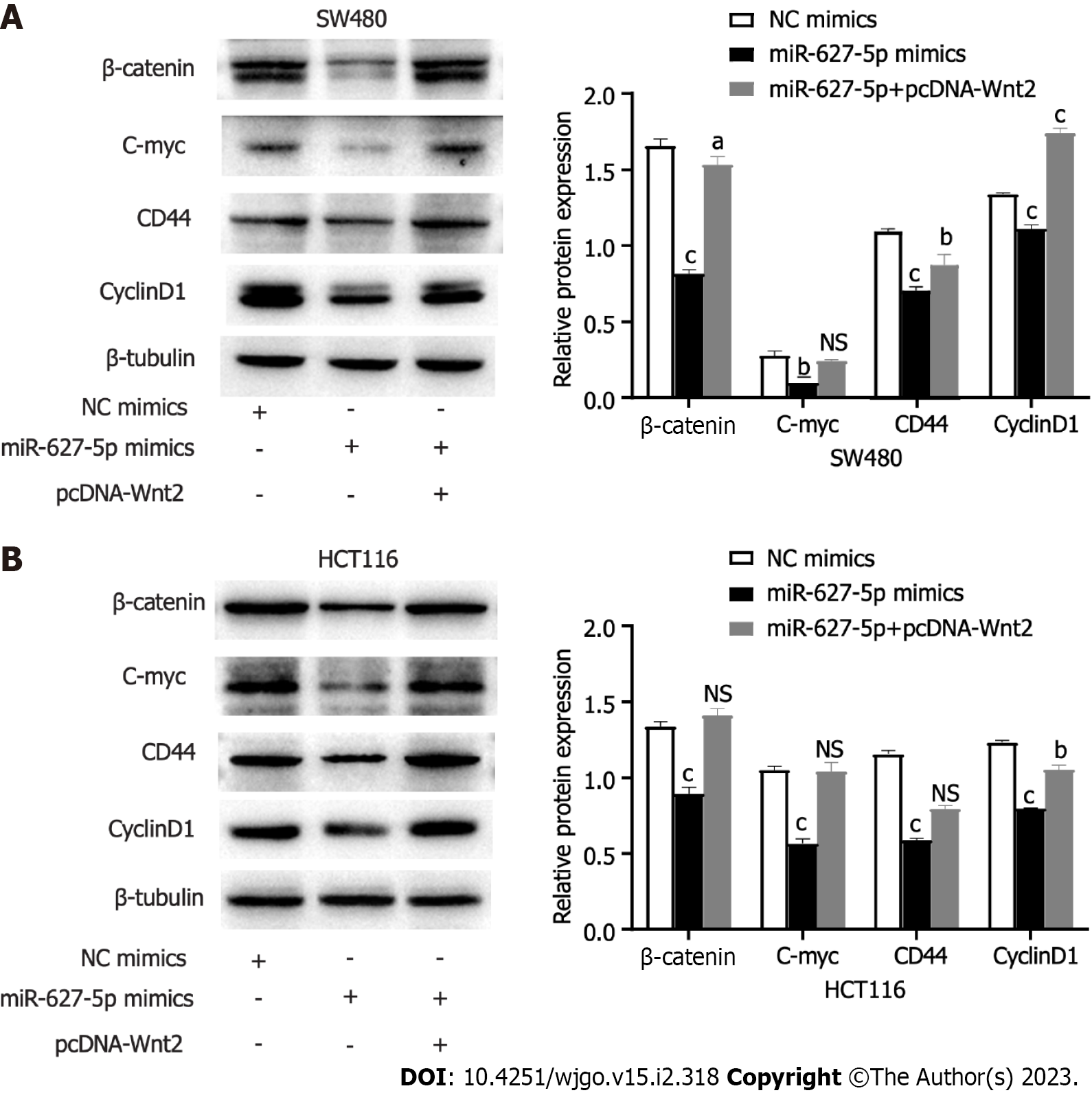
To clarify the signalling pathways influenced by the miR627-5p/Wnt2 axis in CRC cells, we conducted qRT-PCR and western blot analysis to evaluate dysregulated genes in the classical Wnt signalling. In Figures 6 and 7, miR-627-5p overexpression in SW480 and HCT116 cells led to a sharp decline in the transcript and protein levels of β-catenin, c-myc, CD44, and cyclin D1. Next, we monitored the expression of β-catenin, c-myc, CD44, and cyclin D1 by treating miR-627-5p overexpressing CRC cells with pcDNA-Wnt2 or scramble vector. The findings illustrated that the transcript and protein levels of β-catenin, c-myc, CD44, and cyclin D1 were rescued at least partly by the overexpression of Wnt2. Consequently, it was suggested miR-627-5p reduces the gene and protein levels of downstream Wnt/β-catenin signalling components via Wnt2.
In our study, we concentrated on whether miR-627-5p, a rarely reported miRNA in CRC, could exert a suppressive effect on CRC development. First, we selected two GEO datasets to compare the expression of miR-627 in colorectal tumour patients and HCs, and the findings showed the decreased levels of miR-627 in cancer tissues in both GEO datasets. Consistent with our results, a study published in 2013 found the expression of miR-627 were observably downregulated in CRC tissues when compared to those in control tissues[21]. Unfortunately, this study did not distinguish the 5p and 3p forms of miR-627. In our previous study, we collected CRC and AA tissues to assess the expression of miR-627-5p and showed significantly decreased expression in CRC and AA tissues compared to HC tissues. Besides, miR-627-5p was found to be deceased in CRC cell lines in comparison with those in FHC cells[20]. According to these results, miR-627-5p expression was reduced in CRC.
Next, we conducted functional experiments using SW480 and HCT116 cells by transfecting miR-627-5p mimics or NC mimics to clarify the biological function of miR-627-5p in colorectal tumour. According to the findings, miR-627-5p greatly reduced cancer cells’ ability to migrate, invade, and proliferate while also accelerating apoptosis, which was in accordance with past researches in other cancer types. For instance, miR-627-5p is markedly reduced in hepatocellular carcinoma and negatively correlated with the prognosis of cancer patients. miR-627-5p silencing promotes cell multiplication and cell cycle progression of hepatocellular carcinoma cells[9]. In oral squamous cell carcinoma, LINC00958 promotes tumour cell growth, delays apoptosis, and accelerates cell migration and epithelial-mesenchymal transition by suppressing the expression of miR-627-5p[12]. Thus, miR-627-5p is regarded as a tumour suppressor and could serve as a target for the treatment of cancer in the future.
A variety of literature have elucidated that the loss or enhancement of miRNA function is mainly involved in cancer carcinogenesis and progression by targeting the expression of cancer-causing or cancer-suppressing genes[22]. To clarify the key mechanism of miR-627-5p in suppressing CRC growth, we used an online tool to excavate the possible downstream genes of miR-627-5p. We subsequently discovered that miR-627-5p might have a complementary site within the Wnt2 3′-UTR. A series of studies have claimed that Wnt2 contributes to the development of numerous malignant malignancies[17,23-26]. For example, the Wnt2 gene is almost undetectable in the normal gastrointestinal tract but is highly upregulated in precancerous adenomas, primary colorectal tumours and liver metastases[27]. High expression of Wnt2 is implicated as a critical factor in promoting the invasive and metastatic potential of CRC cells[26]. In concordance with past researches, the current study confirmed that Wnt2 mRNA levels were considerably elevated in colorectal neoplasm tissues and inversely related to miR-627-5p levels, suggesting that miR-627-5p might participate in intestinal carcinogenesis by regulating the expression of Wnt2.
To explore whether miR-627-5p is directly complementary to Wnt2, we conducted a series of experiments in vitro. According to the results of luciferase reporter tests, miR-627-5p upregulation inhibited the luciferase level of the reporter vector comprising the wild type sequence of Wnt2 3’-UTR, but no obvious change on the reporter vector comprising the mutation sequence. In addition, ectopic expression of miR-627-5p significantly reduced the gene and protein expression of Wnt2 in CRC cell lines. The above findings suggested that Wnt2 is a specific target of miR-627-5p. However, whether miR-627-5p exerts its tumour inhibitory effect through directly regulating the expression of Wnt2 remains unknown. To answer this question, we conducted rescue experiments and cotransfected miR-672-5p mimics and Wnt2 overexpression plasmids into CRC cells. Rescue experiments showed that the cells’ survival, motility, and invasion were enhanced and the proportion of apoptotic cells were decreased when compared to transfection of miR-627-5p mimics alone. Thus, miR-627-5p decreased CRC cell proliferation, motility, and invasion while promoting death via Wnt2.
As is well-known, Wnt2 is one of the critical ligands that regulates the activity of the Wnt/β-catenin signalling, while aberrant activation of the classical Wnt signalling is a major driver of colorectal carcinogenesis[28,29]. Herein, we hypothesized that miR-627-5p might regulate Wnt2 expression, thereby modulating the Wnt/β-catenin signalling, and exerting its tumour suppressive function on CRC in vitro. Normally, β-catenin, the essential element of the canonical Wnt pathway, is continually eliminated by the destruction complex (AXIN, GSK3β, CK1, and APC) without canonical Wnt ligands. The constant degradation of β-catenin leads to low level of free β-catenin in the cytoplasm and the repression of Wnt target genes. Conversely, the Wnt pathway is activated when canonical Wnt ligands bind to their receptors on the cell surface and subsequently cause the aggregation of the degradation complex, resulting in the accumulation of β-catenin in the cytoplasm. Then, β-catenin gradually migrates to the nucleus, where it serves as a co-activator for T-cell specific factor/lymphoid enhancer-binding factor to activate Wnt target genes such as cyclinD1[30], CD44[31], and c-myc[32], which are identified to be involved in the malignant tendency of cancer cells, including stemness, tumorigenicity, metastasis, and chemoresistance[33-35]. To verify this hypothesis, we investigated how miR-627-5p affected the expression of the Wnt/β-catenin signalling like β-catenin, cyclinD1, c-myc, and CD44. Our current study revealed that upregulation of miR-627-5p could effectively decrease the mRNA and protein expression of β-catenin, cyclinD1, c-myc and CD44, whereas the suppressive effects of miR-627-5p could be partially canceled by Wnt2 overexpression. These results suggested that miR-627-5p/Wnt2 regulates the canonical Wnt pathway in CRC cells.
There are some limitations in the study. First, we performed transient transfection to increase the levels of miRNAs in CRC cell lines. Since miRNAs do not integrate into the cellular genome, the typical effects can only last for several days, and we could not assess the long-term effects of miRNAs. Stable transfection of miRNAs is required to achieve the long-term effects of miR-627-5p on tumour progression. Second, the translocation of cytoplasmic β-catenin to the nucleus is a crucial step in the activation of Wnt signaling. However, the current study only detected alterations in β-catenin in whole cells, not nuclear alterations. Finally, to learn more about the impact of miR-627-5p on the development of tumors in vivo, nude mouse carcinogenesis tests are necessary.
In summary, miR-627-5p could inhibit the malignant tendencies of CRC cells by directly inhibiting Wnt2 expression. The tumour suppressive effects were mainly achieved by inhibiting the activation of the classical Wnt/β-catenin signalling and the levels of its downstream target factors. These findings not only advance our understanding of the pathogenesis of CRC, but also provide evidence for an exploitable therapeutic target for CRC patients.
Population aging has given rise to the incidence rate of colorectal cancer (CRC) worldwide. Better elucidation of the mechanisms underlying the formation and growth of CRC is very helpful for the development of new therapy.
Latest studies have shown that miRNAs generally regulate the expression of oncogenes or tumour suppressor genes and exert integral roles in modulating cancer-related pathways and mediating the formation and progression of CRC. However, whether miR-627-5p is involved in the tumorigenesis of colorectal tumours or not is largely unexplored.
This current study is designed to verify the function of miR-627-5p in colorectal tumorigenesis by targeting Wnt2/β-catenin signalling pathway.
The levels of miR-627-5p and Wnt2 were detected in CRC tissues. Functional experiments, including CCK-8, flow cytometry, Matrigel invasion, and scratch assays, were conducted to elucidate the function of miR-627-5p and wnt2 in colorectal tumour cells. Dual luciferase reporter tests were carried out to investigate how miR-627-5p and Wnt2 interact. The critical signalling pathway modulated by miR-627-5p was further identified.
Wnt2 transcript expression was markedly increased in colorectal tumour tissues and negatively correlated with miR-627-5p level. Upregualtion of miR-627-5p inhibited cancer cells’ abilities to invade growth and migrate by directly restraining Wnt2 expressions. Furthermore, miR-627-5p exerted the suppressive role in CRC via inactivating the Wnt2/β-catenin signalling.
miR-627-5p restrained the malignant biological properties of CRC cells via directly inhibiting Wnt2 expression to modulate the classical Wnt/β-catenin signalling.
miRNA-627-5p/Wnt2/β-catenin may have potential therapeutic application for CRC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shamseldeen AA, Egypt; Toyoshima O, Japan S-Editor: Chen YL L-Editor: A P-Editor: Zhang XD
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55445] [Article Influence: 7920.7] [Reference Citation Analysis (127)] |
| 2. | O'Connell JB, Maggard MA, Ko CY. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2004;96:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1063] [Cited by in RCA: 1158] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 3. | Gellad ZF, Provenzale D. Colorectal cancer: national and international perspective on the burden of disease and public health impact. Gastroenterology. 2010;138:2177-2190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 116] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 4. | Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1570] [Cited by in RCA: 2922] [Article Influence: 487.0] [Reference Citation Analysis (3)] |
| 5. | Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda G, Angelino P, Bot BM, Morris JS, Simon IM, Gerster S, Fessler E, De Sousa E Melo F, Missiaglia E, Ramay H, Barras D, Homicsko K, Maru D, Manyam GC, Broom B, Boige V, Perez-Villamil B, Laderas T, Salazar R, Gray JW, Hanahan D, Tabernero J, Bernards R, Friend SH, Laurent-Puig P, Medema JP, Sadanandam A, Wessels L, Delorenzi M, Kopetz S, Vermeulen L, Tejpar S. The consensus molecular subtypes of colorectal cancer. Nat Med. 2015;21:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3408] [Cited by in RCA: 3498] [Article Influence: 349.8] [Reference Citation Analysis (0)] |
| 6. | Nguyen LH, Goel A, Chung DC. Pathways of Colorectal Carcinogenesis. Gastroenterology. 2020;158:291-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 344] [Cited by in RCA: 298] [Article Influence: 59.6] [Reference Citation Analysis (0)] |
| 7. | Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 520] [Cited by in RCA: 890] [Article Influence: 111.3] [Reference Citation Analysis (1)] |
| 8. | Lau NC, Lim LP, Weinstein EG, Bartel DP. An abundant class of tiny RNAs with probable regulatory roles in Caenorhabditis elegans. Science. 2001;294:858-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2408] [Cited by in RCA: 2409] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 9. | Wang J, Chen T, Wang L, Yao B, Sun L, Chen S, Liu Q. MicroRNA-627-5p inhibits the proliferation of hepatocellular carcinoma cells by targeting BCL3 transcription coactivator. Clin Exp Pharmacol Physiol. 2020;47:485-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Fan Z, Zheng J, Xue Y, Liu X, Wang D, Yang C, Ma J, Liu L, Ruan X, Wang Z, Liu Y. NR2C2-uORF targeting UCA1-miR-627-5p-NR2C2 feedback loop to regulate the malignant behaviors of glioma cells. Cell Death Dis. 2018;9:1165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Shin VY, Ng EK, Chan VW, Kwong A, Chu KM. A three-miRNA signature as promising non-invasive diagnostic marker for gastric cancer. Mol Cancer. 2015;14:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Chen F, Liu M, Yu Y, Sun Y, Li J, Hu W, Wang X, Tong D. LINC00958 regulated miR-627-5p/YBX2 axis to facilitate cell proliferation and migration in oral squamous cell carcinoma. Cancer Biol Ther. 2019;20:1270-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | To KK, Tong CW, Wu M, Cho WC. MicroRNAs in the prognosis and therapy of colorectal cancer: From bench to bedside. World J Gastroenterol. 2018;24:2949-2973. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 116] [Cited by in RCA: 151] [Article Influence: 21.6] [Reference Citation Analysis (2)] |
| 14. | Hur K, Toiyama Y, Takahashi M, Balaguer F, Nagasaka T, Koike J, Hemmi H, Koi M, Boland CR, Goel A. MicroRNA-200c modulates epithelial-to-mesenchymal transition (EMT) in human colorectal cancer metastasis. Gut. 2013;62:1315-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 451] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 15. | Yu Y, Kanwar SS, Patel BB, Oh PS, Nautiyal J, Sarkar FH, Majumdar AP. MicroRNA-21 induces stemness by downregulating transforming growth factor beta receptor 2 (TGFβR2) in colon cancer cells. Carcinogenesis. 2012;33:68-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 16. | Kramer N, Schmöllerl J, Unger C, Nivarthi H, Rudisch A, Unterleuthner D, Scherzer M, Riedl A, Artaker M, Crncec I, Lenhardt D, Schwarz T, Prieler B, Han X, Hengstschläger M, Schüler J, Eferl R, Moriggl R, Sommergruber W, Dolznig H. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene. 2017;36:5460-5472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 17. | Fu L, Zhang C, Zhang LY, Dong SS, Lu LH, Chen J, Dai Y, Li Y, Kong KL, Kwong DL, Guan XY. Wnt2 secreted by tumour fibroblasts promotes tumour progression in oesophageal cancer by activation of the Wnt/β-catenin signalling pathway. Gut. 2011;60:1635-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 100] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 18. | Katoh M. WNT2 and human gastrointestinal cancer (review). Int J Mol Med. 2003;12:811-816. [PubMed] |
| 19. | Backliwal G, Hildinger M, Hasija V, Wurm FM. High-density transfection with HEK-293 cells allows doubling of transient titers and removes need for a priori DNA complex formation with PEI. Biotechnol Bioeng. 2008;99:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 125] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Zhao DY, Zhou L, Yin TF, Zhou YC, Zhou GY, Wang QQ, Yao SK. Circulating miR-627-5p and miR-199a-5p are promising diagnostic biomarkers of colorectal neoplasia. World J Clin Cases. 2022;10:5165-5184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Padi SK, Zhang Q, Rustum YM, Morrison C, Guo B. MicroRNA-627 mediates the epigenetic mechanisms of vitamin D to suppress proliferation of human colorectal cancer cells and growth of xenograft tumors in mice. Gastroenterology. 2013;145:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 22. | Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5833] [Cited by in RCA: 6515] [Article Influence: 383.2] [Reference Citation Analysis (0)] |
| 23. | Yu M, Ting DT, Stott SL, Wittner BS, Ozsolak F, Paul S, Ciciliano JC, Smas ME, Winokur D, Gilman AJ, Ulman MJ, Xega K, Contino G, Alagesan B, Brannigan BW, Milos PM, Ryan DP, Sequist LV, Bardeesy N, Ramaswamy S, Toner M, Maheswaran S, Haber DA. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature. 2012;487:510-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 358] [Cited by in RCA: 393] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 24. | Ricken A, Lochhead P, Kontogiannea M, Farookhi R. Wnt signaling in the ovary: identification and compartmentalized expression of wnt-2, wnt-2b, and frizzled-4 mRNAs. Endocrinology. 2002;143:2741-2749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 100] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Bravo DT, Yang YL, Kuchenbecker K, Hung MS, Xu Z, Jablons DM, You L. Frizzled-8 receptor is activated by the Wnt-2 ligand in non-small cell lung cancer. BMC Cancer. 2013;13:316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 26. | Unterleuthner D, Neuhold P, Schwarz K, Janker L, Neuditschko B, Nivarthi H, Crncec I, Kramer N, Unger C, Hengstschläger M, Eferl R, Moriggl R, Sommergruber W, Gerner C, Dolznig H. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis. 2020;23:159-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 27. | Vider BZ, Zimber A, Chastre E, Prevot S, Gespach C, Estlein D, Wolloch Y, Tronick SR, Gazit A, Yaniv A. Evidence for the involvement of the Wnt 2 gene in human colorectal cancer. Oncogene. 1996;12:153-158. [PubMed] |
| 28. | Jung YS, Jun S, Lee SH, Sharma A, Park JI. Wnt2 complements Wnt/β-catenin signaling in colorectal cancer. Oncotarget. 2015;6:37257-37268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 63] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 29. | Bian J, Dannappel M, Wan C, Firestein R. Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 159] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 30. | Qie S, Diehl JA. Cyclin D1, cancer progression, and opportunities in cancer treatment. J Mol Med (Berl). 2016;94:1313-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 496] [Article Influence: 55.1] [Reference Citation Analysis (0)] |
| 31. | Hassn Mesrati M, Syafruddin SE, Mohtar MA, Syahir A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 233] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 32. | Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479-6483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 590] [Cited by in RCA: 702] [Article Influence: 43.9] [Reference Citation Analysis (0)] |
| 33. | Cselenyi CS, Jernigan KK, Tahinci E, Thorne CA, Lee LA, Lee E. LRP6 transduces a canonical Wnt signal independently of Axin degradation by inhibiting GSK3's phosphorylation of beta-catenin. Proc Natl Acad Sci U S A. 2008;105:8032-8037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 166] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Li VS, Ng SS, Boersema PJ, Low TY, Karthaus WR, Gerlach JP, Mohammed S, Heck AJ, Maurice MM, Mahmoudi T, Clevers H. Wnt signaling through inhibition of β-catenin degradation in an intact Axin1 complex. Cell. 2012;149:1245-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 707] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 35. | Kim SE, Huang H, Zhao M, Zhang X, Zhang A, Semonov MV, MacDonald BT, Garcia Abreu J, Peng L, He X. Wnt stabilization of β-catenin reveals principles for morphogen receptor-scaffold assemblies. Science. 2013;340:867-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 194] [Article Influence: 16.2] [Reference Citation Analysis (0)] |