Published online Dec 15, 2023. doi: 10.4251/wjgo.v15.i12.2197
Peer-review started: June 21, 2023
First decision: September 6, 2023
Revised: September 22, 2023
Accepted: October 30, 2023
Article in press: October 30, 2023
Published online: December 15, 2023
Processing time: 176 Days and 1.6 Hours
The frequency and content of follow-up strategies remain controversial for co
To compare intensive and conventional follow-up strategies for the prognosis of non-metastatic CRC treated with curative intent using a meta-analysis.
PubMed, Embase, and the Cochrane Library databases were systematically searched for potentially eligible randomized controlled trials (RCTs) from inception until April 2023. The Cochrane risk of bias was used to assess the methodological quality of the included studies. The hazard ratio, relative risk, and 95% confidence interval were used to calculate survival and categorical data, and pooled analyses were performed using the random-effects model. Additional exploratory analyses were performed for sensitivity, subgroups, and publication bias.
Eighteen RCTs involving 8533 patients with CRC were selected for the final analysis. Intensive follow-up may be superior to conventional follow-up in improving overall survival, but this difference was not statistically significant. Moreover, intensive follow-up was associated with an increased incidence of salvage surgery compared to conventional follow-up. In addition, there was no significant difference in the risk of recurrence between intensive and conventional follow-up strategies, whereas intensive follow-up was associated with a reduced risk of interval recurrence compared to conventional follow-up. Finally, the effects of intensive and conventional follow-up strategies differed when stratified by tumor location and follow-up duration.
Intensive follow-up may have a beneficial effect on the overall survival of patients with non-metastatic CRC treated with curative intent.
Core Tip: This systematic review and meta-analysis aimed to determine the effects of intensive vs conventional follow-up strategies on the prognosis of patients with colorectal cancer (CRC) treated with curative intent by examining randomized controlled trials (RCTs). This study found that an intensive follow-up strategy might have beneficial effects on overall survival. Moreover, an intensive follow-up strategy was associated with an increased incidence of salvage surgery and a reduced risk of interval survival. Further large-scale RCTs should assess the effects of intensive follow-up with a specific frequency and content for non-metastatic CRC treated with curative intent.
- Citation: Cui LL, Cui SQ, Qu Z, Ren ZQ. Intensive follow-up vs conventional follow-up for patients with non-metastatic colorectal cancer treated with curative intent: A meta-analysis. World J Gastrointest Oncol 2023; 15(12): 2197-2211
- URL: https://www.wjgnet.com/1948-5204/full/v15/i12/2197.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i12.2197
Colorectal cancer (CRC) is the third most frequently diagnosed cancer, which accounted for more than 1.9 million cases and 900000 cancer-related deaths worldwide in 2000, thereby causing a great public health burden[1,2]. The incidence and prognosis of CRC have improved because of the use of population-based screening programs and understanding the necessity of a healthy lifestyle. Early diagnosis and treatment are significantly related to CRC prognosis[3]. The 5-year survival rate is 90% for stage I-II CRC and is reduces to 14% for stage IV CRC[4]. The standard treatment for early-stage CRC is curative surgery, and tumor node metastasis is an important predictor of early-stage CRC prognosis and other prognostic factors, including tumor location and clinicopathological results[5-7]. Nevertheless, 10%-20% of patients develop recurrent disease, and an additional follow-up strategy should be applied to improve CRC prognosis.
Curative surgery aims for the early detection of treatable recurrence and improving CRC prognosis. Generally, there is a long follow-up duration for patients with CRC treated through curative surgery. However, the frequency and content of follow-ups remain controversial for CRC, and scheduled follow-ups have limited value[8-10]. A prior meta-analysis found that the use of intensive follow-up strategies could improve overall survival compared to conventional follow-up strategies. However, the pooled analyses did not yield a conclusive solution[11]. Moreover, stratified analyses based on studies and patient characteristics were not performed. Therefore, this systematic review and meta-analysis was conducted to determine the effects of intensive vs conventional follow-up strategies on the prognosis of patients with CRC treated with curative intent. The study chose randomized controlled trials (RCTs) for its data.
This study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines[12]. RCTs comparing the effects of intensive and conventional follow-up strategies for non-metastatic CRC treated with curative intent were eligible for our study, and the publication language was restricted to English. We systematically searched PubMed, Embase, and the Cochrane library for eligible trials throughout April 2023, and we used the following search terms: (“colorectal neoplasms”) AND (“recurrence” OR “metastasis” OR “survival analysis” OR “mortality“ OR ”prognosis“) AND (“follow up“ OR “episode of care” OR “surveillance”) AND (“randomized controlled trials”). Trials that had already been completed but had not yet been published were also searched on the ClinicalTrials. gov website (NIH, United States). Manual searches were also performed on the reference lists of the relevant reviews to identify any new trials that met the inclusion criteria.
Two reviewers independently conducted the literature search and trial screening, and conflicts between the reviewers were resolved via discussions. Studies were included if they met the following criteria: (1) Patients: All patients with non-metastatic CRC who were treated with curative intent surgery; (2) Intervention: Intensive follow-up strategy; (3) Control: Conventional follow-up strategy; (4) Outcome: The study should report at least one outcome of overall survival, cancer-specific survival, relapse-free survival, salvage surgery, recurrence, and interval recurrences; and (5) Study design: All included studies had to have an RCT design.
The following data were independently collected from the included trials: First author’s name, publication year, region, sample size, mean age, proportion of males, tumor stage (Dukes’ stage A/B/C), tumor location (colon cancer/rectal cancer), treatments (curative intent surgery and subsequent adjuvant treatments), intervention, control, follow-up, and reported outcomes (overall survival, cancer-specific survival, relapse-free survival, salvage surgery, recurrence, and interval recurrences). The Cochrane risk of bias was used to assess methodological quality, including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases[13]. Each item was defined as having a low, high, or an unclear risk of bias. Two reviewers independently performed the abstracted data and methodological quality assessments, and a third reviewer who referred to the original article settled inconsistent results.
The effects of intensive vs conventional follow-up strategies on survival and categorical data were assigned as hazard ratios (HR), relative risks (RR), and 95% confidence intervals (CI), and pooled analyses were performed using the random-effects model because it considers the underlying variations across the included trials[14,15]. Heterogeneity among the included trials was evaluated using I2 and Q statistics, and significant heterogeneity was defined as I2 > 50% or P < 0.10[16,17]. The stability of the pooled analyses were determined using sensitivity analysis through the sequential removal of a single trial[18]. Subgroup analyses of the investigated outcomes were performed based on sample size, mean age, proportion of males, tumor location, and follow-up duration, and the differences between subgroups were assessed using the interaction t-test, which assumes that the data distribution was normal[19]. Moreover, the ratio of HR (RHR) to RR (RRR) between the subgroups was assessed among patients without specific characteristics[20]. Funnel plots, Egger’s test, and Begg’s test were used to assess potential publication bias[21,22]. All reported P values for the pooled analyses were 2-sided, and the inspection level was 0.05. Statistical analyses were performed using the STATA software (version 10.0; Stata Corporation, College Station, TX, United States).
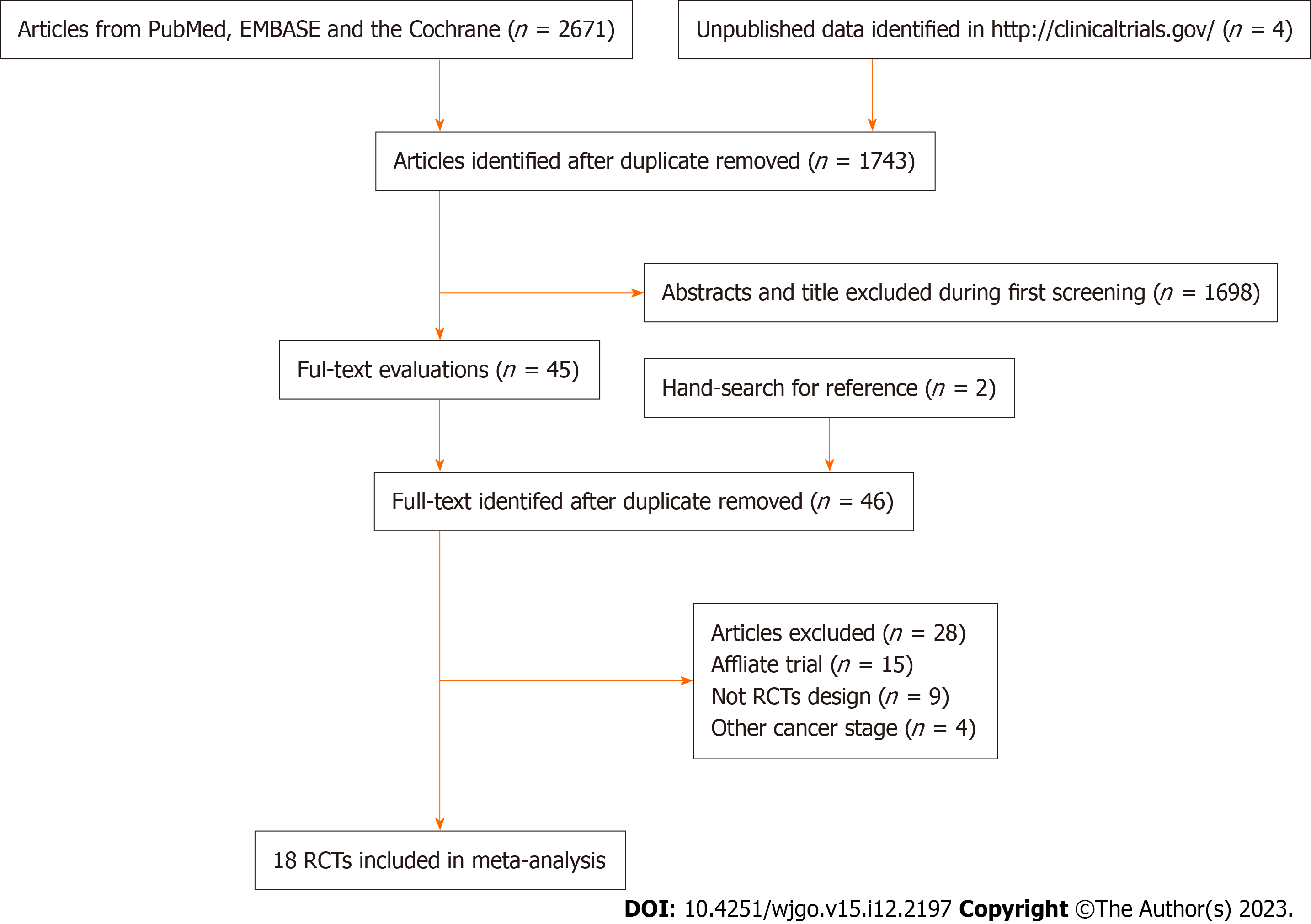
A total of 2671 articles were identified from the initial electronic search, and 1743 studies were retained after duplicate articles were removed. Subsequently, 1698 studies were excluded because they reported irrelevant topics, and the remaining 45 studies were retrieved for full-text evaluation. Reviewing the reference lists yielded two potentially eligible studies, and 46 articles were subjected to detailed evaluation. After this, 28 studies were excluded because they reported the same population (n = 15), did not have an RCT design (n = 9), or included cancers at other stages (n = 4). The remaining 18 RCTs were included in the final meta-analysis[23-40]. Details of the study selection process are shown in Figure 1.
Table 1 summarizes the baseline characteristics of the identified trials and patients involved. A total of 8533 patients with CRC were included from 18 RCTs, and the sample sizes ranged from 106 to 2509. Seventeen of the included trials were performed in Western countries, including Australia and European countries, whereas the remaining one trial was conducted in China. The follow-up duration ranged from 1.0-10.0 years. Details of the methodological quality of the included trials are listed in Table 2. Most of the included trials were of moderate to high quality, and three were of low quality.
| Ref. | Region | Sample size | Age (yr) | Male (%) | Stage (A/B/C) | Tumor location (C/R) | Treatments | Intervention | Control | Follow-up duration |
| Mäkelä et al[23], 1995 | Finland | 106 | 66.0 | 49.1 | (A/B/C) 28/48/30 | 75/31 | Radical resection denotes surgical removal of all macroscopic tumor tissue with microscopically evaluated clearance of the surgical margins | Flexible sigmoidoscopy with video imaging every 3 mo, colonoscopy at 3 mo, then annually. They also had ultrasound of the liver and primary site at 6 mo, then annually | Rigid sigmoidoscopy and barium enema annually | 5.0 yr |
| Ohlsson et al[24], 1995 | Sweden | 107 | 65.6 | 47.7 | (A/B/C) 19/47/41 | 71/36 | Resection with curative intent and early postoperative colonoscopy | Performed at each visit were clinical exam, rigid proctosigmoidoscopy, CEA, alkaline phosphatase, gamma-glutaryl transferase, faecal haemoglobin, and CXR. Examination of anastomosis was performed at 9, 21, and 42 mo. Colonoscopy was performed at 3, 15, 30, and 60 mo. CT of the pelvis was performed at 3, 6, 12, 18, and 24 mo | Written instructions recommending that they leave faecal samples with the district nurse for examination every 3 mo during the first 2 yr then once a year. They contact the surgical department if they had any symptoms | 5.5-8.8 yr |
| Kjeldsen et al[25], 1997 | Denmark | 597 | < 76.0 | 54.6 | (A/B/C) 138/293/166 | 314/283 | Radical primary surgery and no residual neoplasia was detected by complete colonoscopy or incomplete colonoscopy plus double-contrast barium enema, chest radiograph, histological examination of all resection margins in surgical specimens, biopsy of lesions, and inspection and palpation of the liver during surgery | Examinations at 6, 12, 18, 30, 36, 48, 60, 120, 150, and 180 mo after radical surgery (medical history, clinical examination, digital rectal examination, gynaecological examination, Haemoccult-II test, colonoscopy, CXR, haemoglobin level, erythrocyte sedimentation rate, and liver enzymes) | Examinations at 60, 120, and 180 mo (medical history, clinical examination, digital rectal examination, gynaecological examination, Haemoccult-II test, colonoscopy, CXR, haemoglobin level, erythrocyte sedimentation rate, and liver enzymes) | 5.0-10.0 yr |
| Pietra et al[26], 1998 | Italy | 207 | 63.3 | 53.6 | (A/B/C) 0/122/85 | 139/68 | Curative resection defined as one in which no macroscopic tumor remained at the end of the operation and in which histopathologic examination of the operative specimen showed no tumor at the lines of resection | Examinations at 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 54, and 60 mo, then annually thereafter. There was clinical examination, ultrasound, CEA, and CXR at each visit. Annual CT of the liver and colonoscopy were performed | Examinations at 6 and 12 mo, then annually. At each visit, clinical examination, CEA, and ultrasound were performed. They had annual CXR, yearly colonoscopy, and CT scan | 5.0 yr |
| Schoemaker et al[27], 1998 | Australia | 325 | 68.0 | 63.7 | (A/B/C) 71/153/101 | 238/87 | Curative resection | Yearly CXR, CT of the liver, and colonoscopy | Clinical grounds or after screening test abnormality, and at 5 yr of follow-up, to exclude a reservoir of undetected recurrences | 5.0 yr |
| Secco et al[28], 2002 | Italy | 337 | 65.1 | 48.4 | (A or B/C) 201/136 | NA | Putative curative surgery alone, which defined as macriscopic excision of the primary tumour, peritumoral tissues and palpable locoregional lymph nodes | Clinic visits and serum CEA, abdomen/pelvic US scans, and CXR. Participants with rectal carcinoma had rigid sigmoidoscopy and CXR | Minimal follow-up programme performed by physicians | 4.0-5.1 yr |
| Rodríguez-Moranta et al[29], 2006 | Spain | 259 | 68.0 | 62.2 | (II/III) 157/102 | 194/65 | Curative resection, complete colon study was achieved with colonoscopy to determine the presence of synchronous lesions. If colonoscopy of the entire bowel could not be performed before resection, a postoperative colonoscopy was warranted | Seen with history, examination, and bloods (including CEA) at 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, 39, 42, 45, 48, 51, 54, 57, and 60 mo; US/CT at 6, 12, 18, 24, 30, 36, 42, 48, and 56 mo; CXR and colonoscopy at 12, 24, 36, 48, and 56 mo | Seen with history, examination, and bloods (including CEA) at 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36, 39, 42, 45, 48, 51, 54, 57, and 60 mo | 4.0 yr |
| Wattchow et al[30], 2006 | Australia | 203 | NA | 53.6 | (A/B/C) 47/96/60 | 203/0 | Curative surgery and completion of postsurgical chemotherapy | Every 3 mo for the first 2 yr postoperatively, then every 6 mo for the next 3 yr | Asking a list of set questions about symptoms, physical examination, annual faecal occult blood testing, and colonoscopy every 3 yr | 2.0 yr |
| Sobhani et al[31], 2008 | France | 130 | 60.1 | NA | IV: 17 | 75/55 | Curative surgery, compliance with adjuvant chemotherapy, and the absence of disease progression and/or missed synchronous metastases were checked | PET performed at 9 and 15 mo and conventional follow-up | Conventional follow-up | 2.0 yr |
| Wang et al[32], 2009 | China | 326 | 54.5 | 54.3 | (A/B/C) 100/133/93 | 171/155 | Curative surgery, which was defined as one in which no macroscopic tumor remained at the end of the operation and in which histopathologic examination of the operative specimen demonstrated no tumor at the margins of resection | Colonoscopy at each visit | Colonoscopy at 6 mo, 30 mo, and 60 mo from randomisation | 5.3-6.5 yr |
| Strand et al[33], 2011 | Sweden | 110 | 68.0 | 53.6 | (I/II/III/IV) 26/40/36/8 | 0/110 | Curative surgery, all patients had a first postoperative visit with the surgeon for information on histology and adjuvant therapy. Consecutive patients were asked to participate at various postoperative controls starting after the adjuvant chemotherapy was terminated | Surgeon-led follow-up | Nurse-led follow-up | 5.0 yr |
| Augestad et al[34], 2013 | Norway | 110 | 65.4 | 59.1 | (A/B/C) 24/55/32 | 110/0 | Surgery and received postsurgical adjuvant chemotherapy | Surgeon follow-up | GP follow-up | 2.0 yr |
| Primrose et al[35], 2014 | United Kingdom | 1202 | 69.2 | 61.2 | (A/B/C) 254/553/354 | 811/359 | Curative surgery, and adjuvant treatment if indicated, with no evidence of residual disease on investigation | CEA testing every 3 mo for 2 yr, then every 6 mo for 3 yr with a single CT scan of the chest/abdomen/pelvis if requested at study entry by clinician; CT scan of the chest/ abdomen/pelvis every 6 mo for 2 yr, then annually for 3 yr, plus colonoscopy at 2 yr; CEA and CT follow-up: Both blood and imaging as above, plus colonoscopy at 2 yr | No scheduled follow-up except a single CT scan of the chest/ abdomen/pelvis if requested at study entry by a clinician | 3.4 yr |
| Treasure et al[36], 2014 | United Kingdom | 216 | 63.0 | 59.3 | (A/B/C) 10/95/101 | NA | Curative resection for adenocarcinoma of the colon or rectum and who were fit and willing to adhere to the postoperative monitoring routine | CEA rise triggered the “second-look” surgery, with intention to remove any recurrence discovered | Conventional follow-up | 2.0 yr |
| Rosati et al[37], 2016 | Italy | 1228 | 63.9 | 60.7 | (B/C) 617/611 | 933/295 | Curative intent, with adjuvant radio-chemotherapy if indicated | 4, 8, 12, 16, 20, 24, 30, 36, 42, 48, and 60 monthly office visits and history and clinical examination, FBC, CEA, and CA 19-9; colonoscopy and CXR at 12, 24, 36, 48, and 60 mo; liver US at 4, 8, 12, 16, 24, 36, 48, and 60 mo; for rectal participants, pelvic CT at 4, 12, 24, and 48 mo | 4, 8, 12, 16, 20, 24, 30, 42, 48, and 60 monthly office visits, including history, examination, and CEA; colonoscopy at 12 and 48 mo; liver US at 4 and 16 mo; rectal cancer participants in addition had rectoscopy at 4 mo, CXR at 12 mo, and liver US at 8 and 16 mo. A single pelvic CT was allowed if a radiation oncologist required it as baseline following adjuvant treatment | 5.2 yr |
| Wille-Jørgensen et al[38], 2018 | Denmark and Uruguay | 2509 | 64.9 | 55.0 | (II/III) 1352/1157 | 884/1625 | Curative intent, with adjuvant treatment if indicated, a colon and rectum free of neoplasia verified by perioperative barium enema or a colonoscopy within 3 mo after surgery | Multislice contrast-enhanced CT of the thorax and abdomen and CEA at 6, 12, 18, 24, and 36 mo after surgery | Multislice contrast-enhanced CT of the thorax and abdomen and CEA at 12 and 36 mo after surgery | 3.0 yr |
| Rahr et al[39], 2019 | Denmark | 196 | 70.0 | 63.8 | (I/II/III/IV) 47/66/49/16 | 140/56 | Elective surgery for verified or suspected CRC were screened by a study nurse for cardiopulmonary comorbidity at the preoperative visit | Routine follow-up with one extra medical visit and additional visits to the Cardiology and Respiratory Medicine Clinics 1 and 3 mo postoperatively | Routine follow-up | 1.0 yr |
| Monteil et al[40], 2021 | France | 365 | 65.0 | 54.8 | (I/II/III/IV) 2/176/185/2 | 290/75 | Curative surgery, with adjuvant treatment if indicated | PET/CT and conventional follow-up every 3 mo | CEA, liver echography, and alternated between lung radiography and CT scans | 3.0 yr |
| Ref. | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
| Mäkelä et al[23], 1995 | Unclear | Unclear | Low risk | Unclear | Low risk | Unclear | Unclear |
| Ohlsson et al[24], 1995 | Unclear | Unclear | Low risk | Unclear | Low risk | Unclear | Unclear |
| Kjeldsen et al[25], 1997 | Unclear | Unclear | Unclear | High risk | Low risk | Unclear | Unclear |
| Pietra et al[26], 1998 | Unclear | Unclear | Low risk | Unclear | High risk | Unclear | Unclear |
| Schoemaker et al[27], 1998 | Low risk | High risk | Low risk | Low risk | Low risk | Unclear | Unclear |
| Secco et al[28], 2002 | Unclear | Unclear | Unclear | Low risk | Unclear | Unclear | Unclear |
| Rodríguez-Moranta et al[29], 2006 | Low risk | Low risk | Unclear | Unclear | Low risk | Unclear | Unclear |
| Wattchow et al[30], 2006 | Low risk | Low risk | Low risk | Low risk | Low risk | Unclear | Unclear |
| Sobhani et al[31], 2008 | Unclear | Unclear | Low risk | Low risk | Low risk | Unclear | Unclear |
| Wang et al[32], 2009 | Unclear | Unclear | High risk | High risk | Unclear | Unclear | Unclear |
| Strand et al[33], 2011 | Unclear | Unclear | Low risk | Low risk | Unclear | Unclear | Unclear |
| Augestad et al[34], 2013 | Unclear | Low risk | Low risk | Low risk | Low risk | Unclear | Unclear |
| Primrose et al[35], 2014 | Low risk | Low risk | Unclear | Low risk | Low risk | Low risk | Unclear |
| Treasure et al[36], 2014 | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk |
| Rosati et al[37], 2016 | Unclear | Unclear | Unclear | Unclear | Low risk | Unclear | Unclear |
| Wille-Jørgensen et al[38], 2018 | Low risk | Low risk | Low risk | Low risk | Unclear | Low risk | Low risk |
| Rahr et al[39], 2019 | Low risk | Unclear | Unclear | Unclear | Low risk | Unclear | Unclear |
| Monteil et al[40], 2021 | Unclear | Unclear | Unclear | Low risk | Low risk | Unclear | Unclear |
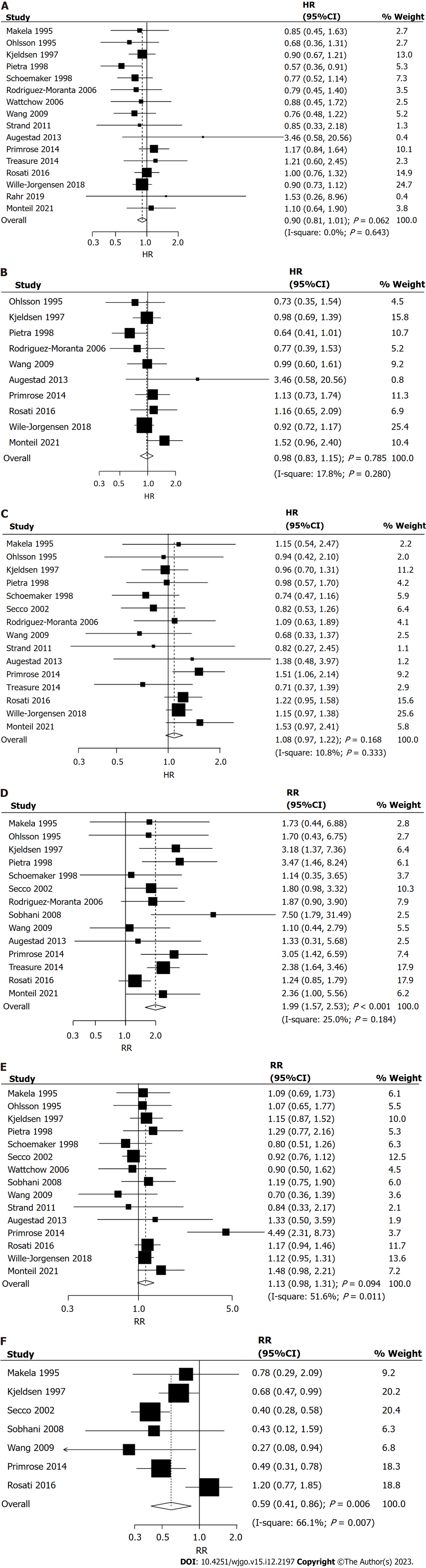
Sixteen trials reported the effects of intensive vs conventional follow-up strategies on overall survival. There was no significant difference between intensive and conventional follow-up strategies for the improvement of overall survival (HR = 0.90; 95%CI: 0.81-1.01; P = 0.062; Figure 2A), and no evidence of heterogeneity was observed across the included trials (I2 = 0.0%; P = 0.643). Sensitivity analysis indicated that an intensive follow-up strategy might be associated with an improvement in overall survival compared to a conventional follow-up strategy (Supplementary material). Subgroup analyses found that intensive follow-up was superior to conventional follow-up in overall survival if the sample size was < 500, proportion of males was < 60.0%, and follow-up duration was ≥ 5.0 years (Table 3). There were no significant differences between subgroups when stratified by sample size (RHR = 1.19; 95%CI: 0.95-1.48; P = 0.135), mean age (RHR = 1.07; 95%CI: 0.84-1.35; P = 0.584), proportion of males (RHR = 1.11; 95%CI: 0.89-1.39; P = 0.339), tumor location (RHR = 1.07; 95%CI: 0.84-1.36; P = 0.584), and follow-up (RHR = 0.86; 95%CI: 0.69-1.06; P = 0.163). No significant publication bias for overall survival was observed (P value for Egger’s test: 0.753; P value for Begg’s test: 0.558; Supplementary material).
| Outcomes | Factors | Subgroups | No. of studies | HR or RR and 95%CI | P value | I2(%) | P value for I2 | Interaction P value | RHR or RRR with 95%CI |
| Overall survival | Sample size | ≥ 500 | 4 | 0.96 (0.84-1.10) | 0.579 | 0.0 | 0.581 | 0.135 | 1.19 (0.95-1.48) |
| < 500 | 12 | 0.81 (0.68-0.97) | 0.019 | 0.0 | 0.693 | ||||
| Mean age (yr) | ≥ 65.0 | 9 | 0.94 (0.78-1.14) | 0.538 | 0.0 | 0.563 | 0.584 | 1.07 (0.84-1.35) | |
| < 65.0 | 6 | 0.88 (0.77-1.02) | 0.082 | 7.7 | 0.367 | ||||
| Male (%) | ≥ 60.0 | 5 | 0.97 (0.81-1.16) | 0.758 | 0.0 | 0.503 | 0.339 | 1.11 (0.89-1.39) | |
| < 60.0 | 11 | 0.87 (0.76-0.99) | 0.035 | 0.0 | 0.620 | ||||
| Tumor location (C/R) | ≥ 70.0 | 8 | 0.93 (0.78-1.11) | 0.429 | 0.0 | 0.742 | 0.584 | 1.07 (0.84-1.36) | |
| < 70.0 | 7 | 0.87 (0.74-1.02) | 0.082 | 16.9 | 0.301 | ||||
| Follow-up (yr) | ≥ 5.0 | 8 | 0.84 (0.72-0.97) | 0.017 | 0.0 | 0.635 | 0.163 | 0.86 (0.69-1.06) | |
| < 5.0 | 8 | 0.98 (0.84-1.15) | 0.837 | 0.0 | 0.658 | ||||
| Cancer-specific survival | Sample size | ≥ 500 | 4 | 0.99 (0.83-1.17) | 0.866 | 0.0 | 0.804 | 0.837 | 1.04 (0.70-1.54) |
| < 500 | 6 | 0.95 (0.67-1.36) | 0.782 | 49.4 | 0.079 | ||||
| Mean age (yr) | ≥ 65.0 | 5 | 1.12 (0.80-1.57) | 0.515 | 29.3 | 0.226 | 0.281 | 1.23 (0.84-1.79) | |
| < 65.0 | 5 | 0.91 (0.77-1.08) | 0.276 | 0.0 | 0.510 | ||||
| Male (%) | ≥ 60.0 | 3 | 1.05 (0.77-1.43) | 0.750 | 0.0 | 0.603 | 0.645 | 1.09 (0.75-1.60) | |
| < 60.0 | 7 | 0.96 (0.77-1.20) | 0.732 | 37.6 | 0.142 | ||||
| Tumor location (C/R) | ≥ 70.0 | 4 | 1.23 (0.84-1.81) | 0.281 | 23.9 | 0.268 | 0.155 | 1.35 (0.89-2.05) | |
| < 70.0 | 6 | 0.91 (0.78-1.07) | 0.254 | 0.0 | 0.560 | ||||
| Follow-up (yr) | ≥ 5.0 | 5 | 0.89 (0.72-1.10) | 0.288 | 0.0 | 0.464 | 0.245 | 0.82 (0.58-1.15) | |
| < 5.0 | 5 | 1.09 (0.83-1.42) | 0.552 | 35.9 | 0.182 | ||||
| Relapse-free survival | Sample size | ≥ 500 | 4 | 1.18 (1.02-1.36) | 0.025 | 18.8 | 0.296 | 0.063 | 1.24 (0.99-1.56) |
| < 500 | 11 | 0.95 (0.80-1.14) | 0.589 | 0.0 | 0.583 | ||||
| Mean age (yr) | ≥ 65.0 | 9 | 1.10 (0.89-1.36) | 0.388 | 23.5 | 0.234 | 0.885 | 1.02 (0.79-1.31) | |
| < 65.0 | 6 | 1.08 (0.95-1.23) | 0.220 | 3.6 | 0.394 | ||||
| Male (%) | ≥ 60.0 | 4 | 1.14 (0.87-1.50) | 0.340 | 51.1 | 0.105 | 0.633 | 1.08 (0.80-1.45) | |
| < 60.0 | 11 | 1.06 (0.94-1.20) | 0.364 | 0.0 | 0.569 | ||||
| Tumor location (C/R) | ≥ 70.0 | 6 | 1.15 (0.94-1.40) | 0.171 | 9.5 | 0.355 | 0.778 | 1.04 (0.81-1.32) | |
| < 70.0 | 7 | 1.11 (0.96-1.28) | 0.159 | 6.2 | 0.380 | ||||
| Follow-up (yr) | ≥ 5.0 | 8 | 1.01 (0.86-1.18) | 0.917 | 0.0 | 0.597 | 0.265 | 0.87 (0.68-1.11) | |
| < 5.0 | 7 | 1.16 (0.96-1.39) | 0.120 | 27.9 | 0.215 | ||||
| Salvage surgery | Sample size | ≥ 500 | 3 | 2.12 (1.05-4.29) | 0.036 | 71.7 | 0.029 | 0.990 | 1.00 (0.48-2.11) |
| < 500 | 11 | 2.11 (1.67-2.66) | < 0.001 | 0.0 | 0.567 | ||||
| Mean age (yr) | ≥ 65.0 | 8 | 1.95 (1.42-2.69) | < 0.001 | 0.0 | 0.910 | 0.675 | 0.89 (0.50-1.56) | |
| < 65.0 | 6 | 2.20 (1.38-3.50) | 0.001 | 65.8 | 0.012 | ||||
| Male (%) | ≥ 60.0 | 4 | 1.64 (1.06-2.53) | 0.026 | 38.1 | 0.183 | 0.256 | 0.75 (0.45-1.23) | |
| < 60.0 | 9 | 2.19 (1.72-2.80) | < 0.001 | 0.0 | 0.726 | ||||
| Tumor location (C/R) | ≥ 70.0 | 6 | 1.44 (1.08-1.91) | 0.013 | 0.0 | 0.759 | 0.022 | 0.54 (0.31-0.92) | |
| < 70.0 | 6 | 2.69 (1.71-4.24) | < 0.001 | 24.0 | 0.254 | ||||
| Follow-up (yr) | ≥ 5.0 | 7 | 1.69 (1.15-2.48) | 0.007 | 28.2 | 0.213 | 0.189 | 0.73 (0.46-1.16) | |
| < 5.0 | 7 | 2.30 (1.79-2.97) | < 0.001 | 0.0 | 0.591 | ||||
| Recurrence | Sample size | ≥ 500 | 4 | 1.38 (1.00-1.89) | 0.048 | 82.1 | 0.001 | 0.075 | 1.37 (0.97-1.93) |
| < 500 | 11 | 1.01 (0.89-1.15) | 0.891 | 0.0 | 0.585 | ||||
| Mean age (yr) | ≥ 65.0 | 8 | 1.23 (0.87-1.73) | 0.238 | 75.4 | < 0.001 | 0.645 | 1.09 (0.76-1.56) | |
| < 65.0 | 6 | 1.13 (1.01-1.26) | 0.027 | 0.0 | 0.808 | ||||
| Male (%) | ≥ 60.0 | 3 | 1.54 (0.71-3.30) | 0.273 | 89.7 | < 0.001 | 0.357 | 1.44 (0.66-3.12) | |
| < 60.0 | 11 | 1.07 (0.97-1.18) | 0.185 | 0.0 | 0.618 | ||||
| Tumor location (C/R) | ≥ 70.0 | 6 | 1.13 (0.96-1.32) | 0.130 | 0.0 | 0.461 | 0.583 | 0.92 (0.68-1.24) | |
| < 70.0 | 8 | 1.23 (0.95-1.59) | 0.116 | 64.3 | 0.006 | ||||
| Follow-up (yr) | ≥ 5.0 | 8 | 1.09 (0.95-1.25) | 0.223 | 0.0 | 0.715 | 0.317 | 0.85 (0.62-1.17) | |
| < 5.0 | 7 | 1.28 (0.97-1.71) | 0.085 | 76.3 | < 0.001 | ||||
| Interval recurrence | Sample size | ≥ 500 | 3 | 0.74 (0.45-1.20) | 0.221 | 74.8 | 0.019 | 0.060 | 1.76 (0.98-3.18) |
| < 500 | 4 | 0.42 (0.30-0.58) | < 0.001 | 0.0 | 0.557 | ||||
| Mean age (yr) | ≥ 65.0 | 3 | 0.45 (0.34-0.60) | < 0.001 | 0.0 | 0.423 | 0.173 | 0.65 (0.35-1.21) | |
| < 65.0 | 4 | 0.69 (0.40-1.19) | 0.182 | 62.0 | 0.048 | ||||
| Male (%) | ≥ 60.0 | 2 | 0.77 (0.32-1.85) | 0.558 | 86.9 | 0.006 | 0.424 | 1.48 (0.57-3.87) | |
| < 60.0 | 4 | 0.52 (0.35-0.77) | 0.001 | 47.6 | 0.126 | ||||
| Tumor location (C/R) | ≥ 70.0 | 2 | 1.12 (0.75-1.67) | 0.586 | 0.0 | 0.435 | 0.007 | 1.96 (1.21-3.20) | |
| < 70.0 | 4 | 0.57 (0.43-0.75) | < 0.001 | 0.0 | 0.412 | ||||
| Follow-up (yr) | ≥ 5.0 | 4 | 0.76 (0.47-1.23) | 0.265 | 57.1 | 0.072 | 0.044 | 1.77 (1.02-3.07) | |
| < 5.0 | 3 | 0.43 (0.33-0.57) | < 0.001 | 0.0 | 0.795 |
Ten trials reported the effects of intensive vs conventional follow-up strategies on cancer-specific survival. No significant difference between intensive and conventional follow-up strategies was observed for improvement in cancer-specific survival (HR = 0.98; 95%CI: 0.83-1.15; P = 0.785; Figure 2B), and unimportant heterogeneity was detected across the included trials (I2 = 17.8%; P = 0.280). Sensitivity analysis indicated that the pooled analyses were stable and not altered by the sequential removal of a single trial (Supplementary material). The results of the subgroup analyses were consistent with those of the overall analysis in all subgroups (Table 3). Moreover, the differences between subgroups were not statistically significant when stratified by sample size (RHR = 1.04; 95%CI: 0.70-1.54; P = 0.837), mean age (RHR = 1.23; 95%CI: 0.84-1.79; P = 0.281), proportion of males (RHR = 1.09; 95%CI: 0.75-1.60; P = 0.645), tumor location (RHR = 1.35; 95%CI: 0.89-2.05; P = 0.155), and follow-up (RHR = 0.82; 95%CI: 0.58-1.15; P = 0.245). There was no significant publication bias for cancer-specific survival (P value for Egger’s test: 0.492; P value for Begg’s test: 0.858; Supplementary material).
Fifteen trials reported the effects of intensive vs conventional follow-up strategies on relapse-free survival. There was no significant difference between intensive and conventional follow-up strategies for improvement in relapse-free survival (HR = 1.08; 95%CI: 0.97-1.22; P = 0.168; Figure 2C), and non-significant heterogeneity was observed among the included trials (I2 = 10.8%; P = 0.333). Sensitivity analysis revealed that intensive follow-up may be associated with poor relapse-free survival after excluding the trial performed by Schoemaker et al[27] (Supplementary material). Subgroup analyses indicated that an intensive follow-up strategy was associated with poor relapse-free survival when the sample size was ≥ 500 (Table 3). Furthermore, there were no significant differences between subgroups when stratified by sample size (RHR = 1.24; 95%CI: 0.99-1.56; P = 0.063), mean age (RHR = 1.02; 95%CI: 0.79-1.31; P = 0.885), proportion of males (RHR = 1.08; 95%CI: 0.80-1.45; P = 0.633), tumor location (RHR = 1.04; 95%CI: 0.81-1.32; P = 0.778), and follow-up (RHR = 0.87; 95%CI: 0.68-1.11; P = 0.265). No significant publication bias was observed for relapse-free survival (P value for Egger’s test: 0.189; P value for Begg’s test: 0.621; Supplementary material).
Fourteen trials reported the effects of intensive vs conventional follow-up strategies on the incidence of salvage surgery. We noted that intensive follow-up significantly increased the risk of salvage surgery compared to a conventional follow-up strategy (RR = 1.99; 95%CI: 1.57-2.53; P < 0.001; Figure 2D), and unimportant heterogeneity was detected among the included trials (I2 = 25.0%; P = 0.184). The pooled analyses for the incidence of salvage surgery were robust and not altered by any specific trial (Supplementary material). The results of the subgroup analyses were consistent with those of the overall analysis, and significant differences between the intensive and conventional follow-up strategies were observed in all subgroups (Table 3). We noted that intensive vs conventional follow-up strategies on salvage surgery in tumor location [colon/rectal ratio (C/R)] ≥ 70.0% was lower than tumor location (C/R) < 70.0% (RRR = 0.54; 95%CI: 0.31-0.92; P = 0.022). There was no significant publication bias for salvage surgery (P value for Egger’s test: 0.419; P value for Begg’s test: 1.000; Supplementary material).
Fifteen trials reported the effects of intensive vs conventional follow-up strategies on the risk of recurrence. We noted that the intensive follow-up strategy had no significant effect on the risk of recurrence (RR = 1.13; 95%CI: 0.98-1.31; P = 0.094; Figure 2E), and significant heterogeneity was observed across the included trials (I2 = 51.6%; P = 0.011). Sensitivity analysis indicated that the intensive follow-up strategy was associated with an elevated risk of recurrence when the trial conducted by Secco et al[28] was excluded (Supplementary material). Subgroup analyses suggested that the intensive follow-up strategy was associated with an increased risk of recurrence when the sample size was ≥ 500 and the mean age was < 65.0 years (Table 3). Moreover, the differences between subgroups were not statistically significant when stratified by sample size (RRR = 1.37; 95%CI: 0.970-1.93; P = 0.075), mean age (RRR = 1.09; 95%CI: 0.76-1.56; P = 0.645), proportion of males (RRR = 1.44; 95%CI: 0.66-3.12; P = 0.357), tumor location (RRR = 0.92; 95%CI: 0.68-1.24; P = 0.583), and follow-up (RRR = 0.85; 95%CI: 0.62-1.17; P = 0.317). No significant publication bias was observed for recurrence (P value for Egger’s test: 0.492; P value for Begg’s test: 0.843; Supplementary material).
Seven trials reported the effects of intensive vs conventional follow-up strategies on the risk of interval recurrence. We noted that intensive follow-up significantly reduced the risk of interval recurrence compared to conventional follow-up (RR = 0.59; 95%CI: 0.41-0.86; P = 0.006; Figure 2F), and significant heterogeneity was observed among the included trials (I2 = 66.1%; P = 0.007). The sensitivity analysis indicated that the pooled analyses were not altered when a particular trial was excluded (Supplementary material). Subgroup analyses found that intensive vs conventional follow-up strategies were associated with a lower risk of interval recurrence if the sample size was < 500, mean age was ≥ 65.0, proportion of males was < 60.0, tumor location (C/R) was < 70.0%, and follow-up duration was < 5.0 years (Table 3). Moreover, the effects of intensive vs conventional follow-up strategies on the risk of interval recurrence in the subgroups of tumor location (C/R) ≥ 70.0% (RRR = 1.96; 95%CI: 1.21-3.20; P = 0.007) and follow-up ≥ 5.0 years (RRR = 1.77; 95%CI: 1.02-3.07; P = 0.044) were greater than the corresponding subgroups. There was no significant publication bias for interval recurrence (P value for Egger’s test: 0.790; P value for Begg’s test: 1.000; Supplementary material).
Numerous studies have addressed the effects of intensive vs conventional follow-up strategies on the prognosis of patients with non-metastatic CRC treated with curative intent. However, the study results are controversial. This comprehensive quantitative meta-analysis identified 8533 patients with CRC from 18 RCTs, and the patients had a broad range of characteristics. We noted that the intensive follow-up strategy was not associated with overall survival, cancer-specific survival, relapse-free survival, or recurrence compared to the conventional follow-up strategy. Moreover, intensive follow-up significantly increased the incidence of salvage surgery and reduced the risk of interval recurrence compared to conventional follow-up. Finally, the effects of intensive and conventional follow-up strategies differed when stratified by tumor location and follow-up duration.
Several systematic reviews and meta-analyses have compared the effects of intensive treatment with those of conventional follow-up strategies on the prognosis of patients with non-metastatic CRC treated with curative intent[11,41]. The results of a meta-analysis conducted by Zhao et al[11] were consistent with those of a Cochrane review, and the investigated outcomes were similar. A Cochrane review found that using an intensive follow-up strategy did not affect survival outcomes but could increase the incidence of salvage surgeries[41]. Although the analysis in this study was comprehensive, stratified analyses were performed only through the intervention protocol and according to the study or patient characteristics. Therefore, this study was conducted to compare the effects of intensive vs conventional follow-up strategies on the prognosis of non-metastatic CRC treated with curative intent by examining published RCTs.
The summary result did not reveal significant differences between intensive and conventional follow-up strategies for improving overall survival. However, this pooled analysis was not stable, and the sensitivity analysis revealed a potentially beneficial role of intensive follow-up on overall survival. A potential reason for this could be that recurrent cases can be detected early and further curative procedures can be applied among patients who receive an intensive follow-up strategy, which could improve the prognosis of CRC after curative surgery. Moreover, patients in the intensive follow-up group showed an increased frequency of clinic visits, tests, and examinations, which could improve CRC prognosis[41]. Furthermore, subgroup analyses found that the beneficial effects of intensive follow-up strategies were mainly relevant when the sample size was < 500, proportion of males was < 60.0%, and follow-up duration was ≥ 5.0, which could be explained by the fact that patients with rectal cancer need longer follow-up durations owing to the delayed liver and lung recurrences[42]. Finally, intensive follow-up might be superior to conventional follow-up among women because the difference in lifestyle and compliance among women was better than that among men.
There were no significant differences between the intensive and conventional follow-up strategies in improving cancer-specific survival and relapse-free survival. These results were consistent with those of prior meta-analyses[11,41]. However, subgroup analyses found that intensive follow-up was associated with poor relapse-free survival when the sample size was ≥ 500 patients. The potential reason for this could be the large sample size with sufficient power to detect potential differences, and that residual cancer could be detected through a more thorough follow-up[43]. Similar to a previous meta-analysis, we noted that intensive follow-up significantly increased the incidence of salvage surgery, which could be explained by the early detection of recurrent cases, and salvage surgery was performed for patients with recurring issues.
Although there was no significant difference in the risk of recurrence between groups, intensive follow-up significantly reduced the risk of interval recurrence. Moreover, intensive follow-up was associated with an increased risk of recurrence when the sample size was ≥ 500 and the mean age was < 65.0 years. A potential reason for the risk of recurrence could be that the recurrent cases were consistent and could be affected by the colon/rectal cancer ratio[42]. Moreover, most recurrent cases occurred within 36 mo, and the mean age of the patients was significantly related to the tumor stage[44]. Interval recurrence was defined as symptomatic recurrence, and recurrent presentation in asymptomatic cases was observed when using an intensive follow-up strategy.
This study has several limitations. First, the disease status and treatments across the included trials were different, which could affect the prognosis of CRC after curative surgery. Second, the follow-up protocol differed among the included trials, and the frequency and content of examination could affect the prognosis of CRC. Third, there was substantial heterogeneity for recurrence and interval recurrence, which was not fully explained using sensitivity and subgroup analyses. Finally, there are inherent limitations of meta-analyses on published articles, including inevitable publication bias and restricted detailed analyses.
This study found that an intensive follow-up strategy might have beneficial effects on the overall survival of patients with CRC. Moreover, an intensive follow-up strategy was associated with an increased incidence of salvage surgery and a reduced risk of interval survival. Further large-scale studies should be performed to explore suitable follow-up plans after CRC surgery.
Colorectal cancer (CRC) is the third most frequently diagnosed cancer, and the prognosis of CRC at early stage was relative better. The frequency and content of follow-up strategies play an important role on the prognosis of CRC, and intensive follow-up may improve the prognosis of CRC.
Assess the effects of intensive with conventional follow-up strategies for CRC patients after curative intention using a meta-analysis.
This study aimed to compare the overall survival, cancer-specific survival, relapse-free survival, salvage surgery, recurrence, and interval recurrences between intensive and conventional follow-up strategies for non-metastatic CRC treated with curative intent.
The eligible trials were identified from PubMed, Embase, and the Cochrane Library databases from inception until April 2023. All of pooled analyses were calculated using the random-effects model, which considering the underlying varies across included trials.
We noted intensive follow-up play a beneficial effects in improving overall survival, and interval recurrence as compared with conventional follow-up. Moreover, the incidence of salvage surgery was significantly increased for patients received intensive follow-up.
This study found intensive follow-up was superior than conventional follow-up for CRC patients after curative intention, which should introduce in clinical practice.
The results of this study based on randomized controlled trials, and the evidence level for pooled conclusions was high.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kobayashi S, Japan; Moshref L, Saudi Arabia S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH
| 1. | World Health Organization. International Agency for Research on Cancer. Global Cancer Observatory. [cited 10 June 2023]. Available from: https://gco.aws-csu.iarc.who.int/. |
| 2. | GBD 2017 Colorectal Cancer Collaborators. The global, regional, and national burden of colorectal cancer and its attributable risk factors in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. 2019;4:913-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 279] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 3. | National Cancer Institute. SEER Cancer Statistics Review, 1975–2016. [cited 10 June 2023]. Available from: https://seer.cancer.gov/archive/csr/1975_2016/. |
| 4. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15468] [Article Influence: 2578.0] [Reference Citation Analysis (2)] |
| 5. | Sakin A, Sahin S, Sakin A, Karatas F, Sengul Samanci N, Yasar N, Arici S, Demir C, Geredeli C, Dikker O, Cihan S. Mean platelet volume and platelet distribution width correlates with prognosis of early colon cancer. J BUON. 2020;25:227-239. [PubMed] |
| 6. | Baqar AR, Wilkins S, Staples M, Angus Lee CH, Oliva K, McMurrick P. The role of preoperative CEA in the management of colorectal cancer: A cohort study from two cancer centres. Int J Surg. 2019;64:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 7. | Skancke M, Arnott SM, Amdur RL, Siegel RS, Obias VJ, Umapathi BA. Lymphovascular Invasion and Perineural Invasion Negatively Impact Overall Survival for Stage II Adenocarcinoma of the Colon. Dis Colon Rectum. 2019;62:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 67] [Article Influence: 11.2] [Reference Citation Analysis (1)] |
| 8. | Figueredo A, Rumble RB, Maroun J, Earle CC, Cummings B, McLeod R, Zuraw L, Zwaal C; Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario's Program in Evidence-based Care. Follow-up of patients with curatively resected colorectal cancer: a practice guideline. BMC Cancer. 2003;3:26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 261] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Verberne CJ, Zhan Z, van den Heuvel E, Grossmann I, Doornbos PM, Havenga K, Manusama E, Klaase J, van der Mijle HC, Lamme B, Bosscha K, Baas P, van Ooijen B, Nieuwenhuijzen G, Marinelli A, van der Zaag E, Wasowicz D, de Bock GH, Wiggers T. Intensified follow-up in colorectal cancer patients using frequent Carcino-Embryonic Antigen (CEA) measurements and CEA-triggered imaging: Results of the randomized "CEAwatch" trial. Eur J Surg Oncol. 2015;41:1188-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Jones RP, McWhirter D, Fretwell VL, McAvoy A, Hardman JG. Clinical follow-up does not improve survival after resection of stage I-III colorectal cancer: A cohort study. Int J Surg. 2015;17:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Zhao Y, Yi C, Zhang Y, Fang F, Faramand A. Intensive follow-up strategies after radical surgery for nonmetastatic colorectal cancer: A systematic review and meta-analysis of randomized controlled trials. PLoS One. 2019;14:e0220533. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement on the quality of published systematic review and meta-analyses. PLoS One. 2013;8:e83138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 541] [Cited by in RCA: 608] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 13. | Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. [cited 10 June 2023]. Available from: http://handbook-5-1.cochrane.org/. |
| 14. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26739] [Cited by in RCA: 30381] [Article Influence: 779.0] [Reference Citation Analysis (0)] |
| 15. | Ades A, Lu G, Higgins J. The interpretation of random-effects metaanalysis in decision models. Med Decis Mak. 2005;25:646-654. [RCA] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 656] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 16. | Deeks J, Higgins JPT, Altman D. Analyzing data and undertaking meta-analyses. In: Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1. Oxford: The Cochrane Collaboration; 2008. |
| 17. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46419] [Article Influence: 2110.0] [Reference Citation Analysis (3)] |
| 18. | Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999;8. |
| 19. | Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ. 2003;326:219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2009] [Cited by in RCA: 2383] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 20. | Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378:1297-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 626] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 21. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34245] [Cited by in RCA: 40425] [Article Influence: 1443.8] [Reference Citation Analysis (2)] |
| 22. | Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088-1101. [PubMed] |
| 23. | Mäkelä JT, Laitinen SO, Kairaluoma MI. Five-year follow-up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Arch Surg. 1995;130:1062-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 227] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow-up after curative surgery for colorectal carcinoma. Randomized comparison with no follow-up. Dis Colon Rectum. 1995;38:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 215] [Article Influence: 7.2] [Reference Citation Analysis (1)] |
| 25. | Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. A prospective randomized study of follow-up after radical surgery for colorectal cancer. Br J Surg. 1997;84:666-669. [PubMed] |
| 26. | Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis Colon Rectum. 1998;41:1127-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 206] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 27. | Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998;114:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 253] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 28. | Secco GB, Fardelli R, Gianquinto D, Bonfante P, Baldi E, Ravera G, Derchi L, Ferraris R. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur J Surg Oncol. 2002;28:418-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 153] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Rodríguez-Moranta F, Saló J, Arcusa A, Boadas J, Piñol V, Bessa X, Batiste-Alentorn E, Lacy AM, Delgado S, Maurel J, Piqué JM, Castells A. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 30. | Wattchow DA, Weller DP, Esterman A, Pilotto LS, McGorm K, Hammett Z, Platell C, Silagy C. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br J Cancer. 2006;94:1116-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 31. | Sobhani I, Tiret E, Lebtahi R, Aparicio T, Itti E, Montravers F, Vaylet C, Rougier P, André T, Gornet JM, Cherqui D, Delbaldo C, Panis Y, Talbot JN, Meignan M, Le Guludec D. Early detection of recurrence by 18FDG-PET in the follow-up of patients with colorectal cancer. Br J Cancer. 2008;98:875-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Wang T, Cui Y, Huang WS, Deng YH, Gong W, Li CJ, Wang JP. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointest Endosc. 2009;69:609-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 33. | Strand E, Nygren I, Bergkvist L, Smedh K. Nurse or surgeon follow-up after rectal cancer: a randomized trial. Colorectal Dis. 2011;13:999-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Augestad KM, Norum J, Dehof S, Aspevik R, Ringberg U, Nestvold T, Vonen B, Skrøvseth SO, Lindsetmo RO. Cost-effectiveness and quality of life in surgeon versus general practitioner-organised colon cancer surveillance: a randomised controlled trial. BMJ Open. 2013;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 35. | Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, George S, Mant D; FACS Trial Investigators. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 345] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 36. | Treasure T, Monson K, Fiorentino F, Russell C. The CEA Second-Look Trial: a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer. BMJ Open. 2014;4:e004385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Rosati G, Ambrosini G, Barni S, Andreoni B, Corradini G, Luchena G, Daniele B, Gaion F, Oliverio G, Duro M, Martignoni G, Pinna N, Sozzi P, Pancera G, Solina G, Pavia G, Pignata S, Johnson F, Labianca R, Apolone G, Zaniboni A, Monteforte M, Negri E, Torri V, Mosconi P, Fossati R; GILDA working group. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann Oncol. 2016;27:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 99] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 38. | Wille-Jørgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Petersen SH, Renehan AG, Horváth-Puhó E, Påhlman L, Sørensen HT; COLOFOL Study Group. Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients With Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial. JAMA. 2018;319:2095-2103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 165] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 39. | Rahr HB, Streym S, Kryh-Jensen CG, Hougaard HT, Knudsen AS, Kristensen SH, Ejlersen E. Screening and systematic follow-up for cardiopulmonary comorbidity in elective surgery for colorectal cancer: a randomised feasibility study. World J Surg Oncol. 2019;17:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Monteil J, Le Brun-Ly V, Cachin F, Zasadny X, Seitz JF, Mundler O, Selvy M, Smith D, Rullier E, Lavau-Denes S, Lades G, Labrunie A, Lecaille C, Valli N, Leobon S, Terrebonne E, Deluche E, Tubiana-Mathieu N. Comparison of 18FDG-PET/CT and conventional follow-up methods in colorectal cancer: A randomised prospective study. Dig Liver Dis. 2021;53:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2019;9:CD002200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 55] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 42. | Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, Mukoyama S, Yasuda S, Tajima T, Makuuchi H, Murayama C. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology. 2003;50:1362-1366. [PubMed] |
| 43. | Morris EJ, Sandin F, Lambert PC, Bray F, Klint A, Linklater K, Robinson D, Påhlman L, Holmberg L, Møller H. A population-based comparison of the survival of patients with colorectal cancer in England, Norway and Sweden between 1996 and 2004. Gut. 2011;60:1087-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 44. | Ryuk JP, Choi GS, Park JS, Kim HJ, Park SY, Yoon GS, Jun SH, Kwon YC. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Ann Surg Treat Res. 2014;86:143-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |