Published online Dec 15, 2023. doi: 10.4251/wjgo.v15.i12.2138
Peer-review started: September 5, 2023
First decision: September 15, 2023
Revised: October 9, 2023
Accepted: November 8, 2023
Article in press: November 8, 2023
Published online: December 15, 2023
Processing time: 99 Days and 20.6 Hours
Immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) are beneficial to the resumption of anti-tumor immunity response and hold extreme potential as efficient therapies for certain malignancies. However, ICIs with a single target exhibit poor overall response rate in hepatocellular carcinoma (HCC) patients due to the complex pathological mechanisms of HCC.
To investigate the effects of combined TIM-3 and PD-1 blockade on tumor development in an HCC mouse model, aiming to identify more effective immunotherapies and provide more treatment options for HCC patients.
The levels of PD-1 and TIM-3 on CD4+ and CD8+ T cells from tumor tissues, ascites, and matched adjacent tissues from HCC patients were determined with flow cytometry. An HCC xenograft mouse model was established and treated with anti-TIM-3 monoclonal antibody (mAb) and/or anti-PD-1 mAb. Tumor growth in each group was measured. Hematoxylin and eosin staining and immunohistochemical staining were used to evaluate T cell infiltration in tumors. The percentage of CD4+ and CD8+ T cells in tissue samples from mice was tested with flow cytometry. The percentages of PD-1+CD8+, TIM-3+CD8+, and PD-1+TIM-3+ CD8+ T cells was accessed by flow cytometry. The levels of the cytokines including tumor necrosis factor alpha (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-6, and IL-10 in tumor tissues were gauged with enzyme-linked immunosorbent assay kits.
We confirmed that PD-1 and TIM-3 expression was substantially upregulated in CD4+ and CD8+ T cells isolated from tumor tissues and ascites of HCC patients. TIM-3 mAb and PD-1 mAb treatment both reduced tumor volume and weight, while combined blockade had more substantial anti-tumor effects than individual treatment. Then we showed that combined therapy increased T cell infiltration into tumor tissues, and downregulated PD-1 and TIM-3 expression on CD8+ T cells in tumor tissues. Moreover, combined treatment facilitated the production of T cell effector cytokines TNF-α and IFN-γ, and reduced the production of immunosuppressive cytokines IL-10 and IL-6 in tumor tissues. Thus, we implicated that combined blockade could ameliorate T cell exhaustion in HCC mouse model.
Combined TIM-3 and PD-1 blockade restrains HCC development by facilitating CD4+ and CD8+ T cell-mediated antitumor immune responses.
Core Tip: This study investigated the effects of combined blockade of T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) and programmed cell death protein 1 (PD-1) on tumor development in a hepatocellular carcinoma (HCC) mouse model. We showed that combined TIM-3 and PD-1 blockade had more substantial inhibitory effects on tumor growth in mice compared with individual blockade. The combined therapy increased T cell infiltration and ameliorated CD4+ and CD8+ T cell exhaustion in HCC mouse model. Our findings proposed an effective immunotherapy and may provide more treatment options for HCC patients.
- Citation: Zhang XS, Zhou HC, Wei P, Chen L, Ma WH, Ding L, Liang SC, Chen BD. Combined TIM-3 and PD-1 blockade restrains hepatocellular carcinoma development by facilitating CD4+ and CD8+ T cell-mediated antitumor immune responses. World J Gastrointest Oncol 2023; 15(12): 2138-2149
- URL: https://www.wjgnet.com/1948-5204/full/v15/i12/2138.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i12.2138
Hepatocellular carcinoma (HCC) is the fourth most intractable tumor in clinic worldwide[1]. The majority of HCC patients have advanced-stage diagnoses, which are frequently accompanied by distant metastases[2,3]. Surgical resection, chemotherapy and local ablative therapy are three conventional methods used in clinical HCC treatment, but which have limited effects on advanced HCC patients, such as tumor recurrence, drug resistance, and unsatisfactory survival rate and prognosis[4-6]. It’s general known that the aggressive tumor progression is intimately related to immune escape mediated by multiple mechanisms[7,8]. Recently, multiple novel immune therapies, such as blockade of immune checkpoints and adoptive T-cell transfer, have been identified as efficient and safe therapies for malignant tumors with long-term survival and acceptable toxicity, which have attracted more attention and provide novel directions for HCC treatment[9,10]. Therefore, it’s essential to identify more effective immunotherapies and provide more treatment options to achieve satisfactory survival rate and prognosis for HCC patients.
The tumor microenvironment has been frequently studied in cancer research. It’s generally known that tumor-infiltrating lymphocytes (TILs) contribute to anti-tumor immunity and affect the cancer progression of patients[11,12]. Changes in TIL numbers, localization and phenotypes are indispensable indicators of cancer progression and immunotherapy efficacy[13]. Among them, T cells are essential for identifying and destroying tumor cells. The increased T cell infiltration is a positive prognostic indicator in HCC immunotherapy management[14]. Cytotoxic CD8+ T cells can eliminate malignant cancer cells and is an important defense line for anti-tumor immunity[15]. Additionally, there is evidence that CD4+ T cells can eliminate malignant cancer cells[16]. Nevertheless, persistent tumor antigen stimulation can cause T cell exhaustion and loss of function, resulting in tumor immune escape[17].
It is generally recognized that exhausted T cells are accompanied by frequent expression of various co-inhibitory receptors, such as programmed cell death protein 1 (PD-1), T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3)[18]. These receptors can inhibit T cell function by interacting with their specific ligands, which are also called immune checkpoints. It has been previously revealed that blockage of immune checkpoints is beneficial to the resumption of anti-tumor immunity response and holds extreme potential as an efficient method for certain malignancies including advanced melanoma and lung cancer[19,20]. Currently, immune checkpoint inhibitors (ICIs) targeting PD-1 exhibits encouraging outcomes for HCC immunotherapy in clinic[21]. These inhibitors are essentially monoclonal antibodies (mAb) and have received increased attention. However, due to the complex pathological mechanisms of HCC, ICIs with a single target exhibit poor overall response rate in HCC patients[22]. Therefore, combination therapy with more targets may be a better option to achieve better clinical outcomes.
Given the limited studies study about TIM-3 blockade in HCC, our study first characterized the expression of PD-1 and TIM-3 in CD4+ and CD8+ T cells from clinical HCC samples. Then we focused on investigating the effects of combined blockade of TIM-3 and PD-1 on T cell immunological function and cancer development in an HCC mouse model.
We recruited 20 HCC patients (12 males and 8 females; age 63.7 ± 7.3 years) at the General Hospital of Ningxia Medical University between February 2022 and May 2022. Tumor tissues, paired non-tumor adjacent tissues, and malignant ascites were collected during surgery. None of the patients received chemoradiotherapy or immunotherapy before surgery. This study was conducted with the approval of the Ethics Committee of the General Hospital of Ningxia Medical University. All participants provided clinical specimens and signed the informed consent forms (Table 1).
| Patient characteristics | n | |
| Age (yr) | < 60 | 14 |
| ≥ 60 | 6 | |
| Gender | Male | 12 |
| Female | 8 | |
| Pathologic stage | Stage I | 4 |
| Stage II | 12 | |
| Stage III | 4 | |
Healthy male BALB/c nude mice (18-20 g) were obtained from the experimental animal center of Ningxia Medical University. They were maintained in standard laboratory environment with a 12 h light/dark cycle, 22-25 °C temperature, 55%-60% humidity, and free access to food and water. HepG2 cells were obtained from ATCC (Manassas, VA, United States), and cultured in DMEM containing 10% foetal bovine serum and 1% antibiotics at 37 °C in 5% CO2.
Mononuclear cell suspensions from tumor and adjacent tissues were obtained according to previously described method[23,24]. In general, tumor and adjacent tissues were mechanically fragmented and digested in RPMI 1640 with 1 mg/mL collagenase IV, 0.04 mg/mL DNAse I, and 0.4 mg/mL hyaluronidase for 1 h at 37 °C. The obtained mixture was filtered using cell strainers with a 70 μm pore size to acquire cells. Moreover, ascites supernatant was collected using a centrifuge set at 400 × g for 10 min. Afterwards, mononuclear cells in the tissue suspension and ascites were separated and collected by Ficoll density gradient centrifugation with Lymphoprep (Stemcell Technologies, Canada). Cells were then washed and resuspended in RPMI 1640.
The isolated mononuclear cells were stained with fluorescence-conjugated mAb in the dark at 4 °C for 20 min. The fluorescence-conjugated mAb provided by eBioscience (San Diego, United States) were as follows: Fluorescein isothiocyanate (FITC)-conjugated CD4, FITC-conjugated CD8, allophycocyanin-conjugated PD-1, phycoerythrin-conjugated TIM-3, and the corresponding isotype controls. Flow cytometry was conducted with a flow cytometer (BD Biosciences, CA, United States). FlowJo software (Treestar, CA, United States) was used to analyze the data. Antigen expression is represented by the fluorescence intensity on dual-parameter scattergrams.
After acclimatization to the environment for one week, 4 × 105 HepG2 cells were subcutaneously injected into the right flank of mice. These model mice were divided into four treatment groups (n = 8 each group). Mice were administrated with immunoglobulin G (IgG) isotype control (100 μg), anti-TIM-3 mAb (100 μg) or/and anti-PD1 mAb (100 μg) in a volume of 200 μL via intraperitoneal injection twice a week for sustained 4 wk. These mAbs were all purchased from BioXcell (New Hampshire, United States). Tumor volume was calculated every week. Peripheral blood was collected before euthanasia and kept in an anticoagulant tube. Then tumor tissues, spleen, and thymus were completely separated and weight was recorded. All animal experiments in this study were approved by the Ethics Committee of the General Hospital of Ningxia Medical University.
The spleen or thymus index can roughly estimate the strength of immune function. They were calculated by the following equation: Spleen/thymus index (mg/g) = the weight of spleen/thymus (mg)/body weight (g).
Paraffin-embedded tissues were prepared and cut into 4 μm thick slices. Tissue slices were deparaffinized with xylene, dehydrated, and stained with hematoxylin and eosin (HE) solution. After cleaning and sealing with neutral resin, slices were photographed under light microscope.
After deparaffinization with xylene and rehydration, 4 μm paraffin-embedded tissue slices were mixed with sodium citrate solution and heated in a microwave oven, followed by adding 3% H2O2 for 10 min of inactivation. Thereafter, the slices were incubated with primary antibodies against CD4 (ab133616, Abcam) and CD8 (ab217344, Abcam) overnight at 4 °C, and secondary antibody for 1 h. Next, the slices were developed with a diaminobenzidine immunohistochemistry color development kit (Beyotime, Shanghai, China). Finally, positive staining was imaged under a light microscope and analyzed with the ImageJ software (NIHB, Maryland, United States).
Cell supernatants from tumor tissues were obtained according to the method we mentioned above. Moreover, the obtained spleens were softly pulverized by filtering through a 200-gauge steel mesh, and digested in FACS lysis solution. The mixture was centrifuged and washed with phosphate buffered saline. Then, mononuclear cells in tumor and spleen tissue suspensions were obtained by density gradient centrifugation. Peripheral mononuclear cells were isolated with mouse lymphocyte separation medium (Solarbio, Shanghai, China).
Tumor tissues were lysed, and the supernatant was collected by centrifugation. The levels of the cytokines including tumor necrosis factor alpha (TNF-α), interferon-γ (IFN-γ), interleukin (IL)-6, and IL-10 in tumor tissues were gauged with corresponding enzyme-linked immunosorbent assay kits.
Data from at least triplicate independent experiments were presented as mean ± SD and analyzed with SPSS 22.0 software. Student’s t-test or one-way analysis of variance (ANOVA) was used to analyze comparisons among groups. P < 0.05 was considered statistically significant.
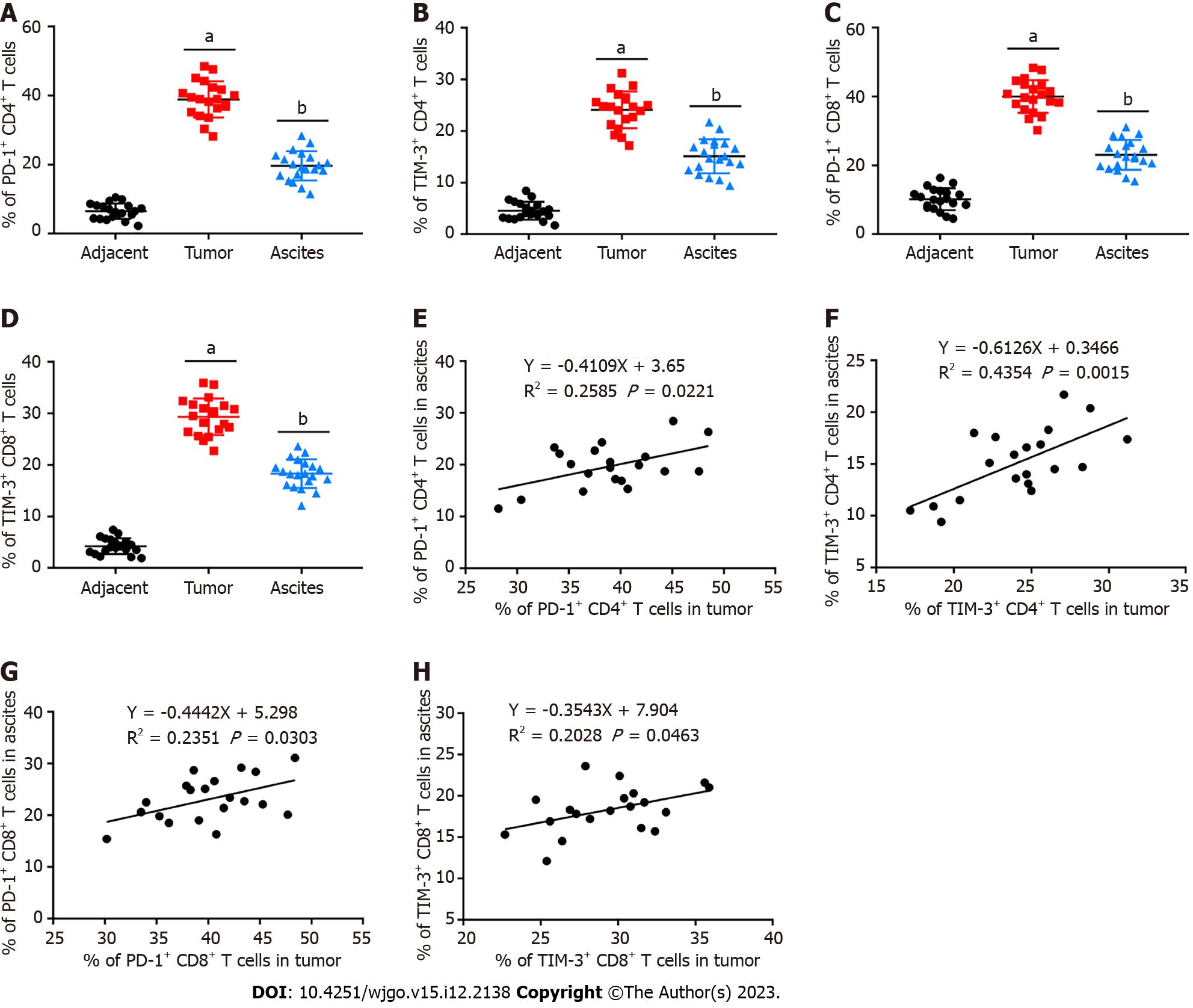
We first determined PD-1 and TIM-3 levels on CD4+ and CD8+ T cells from HCC patients by flow cytometry. As exhibited in Figures 1A-D, compared with matched adjacent tissues, they were both conspicuously upregulated in CD4+ and CD8+ T cells from tumor tissues. Our results also implied that PD-1 and TIM-3 were also highly expressed in conveniently available clinical ascites samples. Afterwards, we observed that their expression on T cells in tumor tissues was positively correlated with that in ascites (Figures 1E-H).
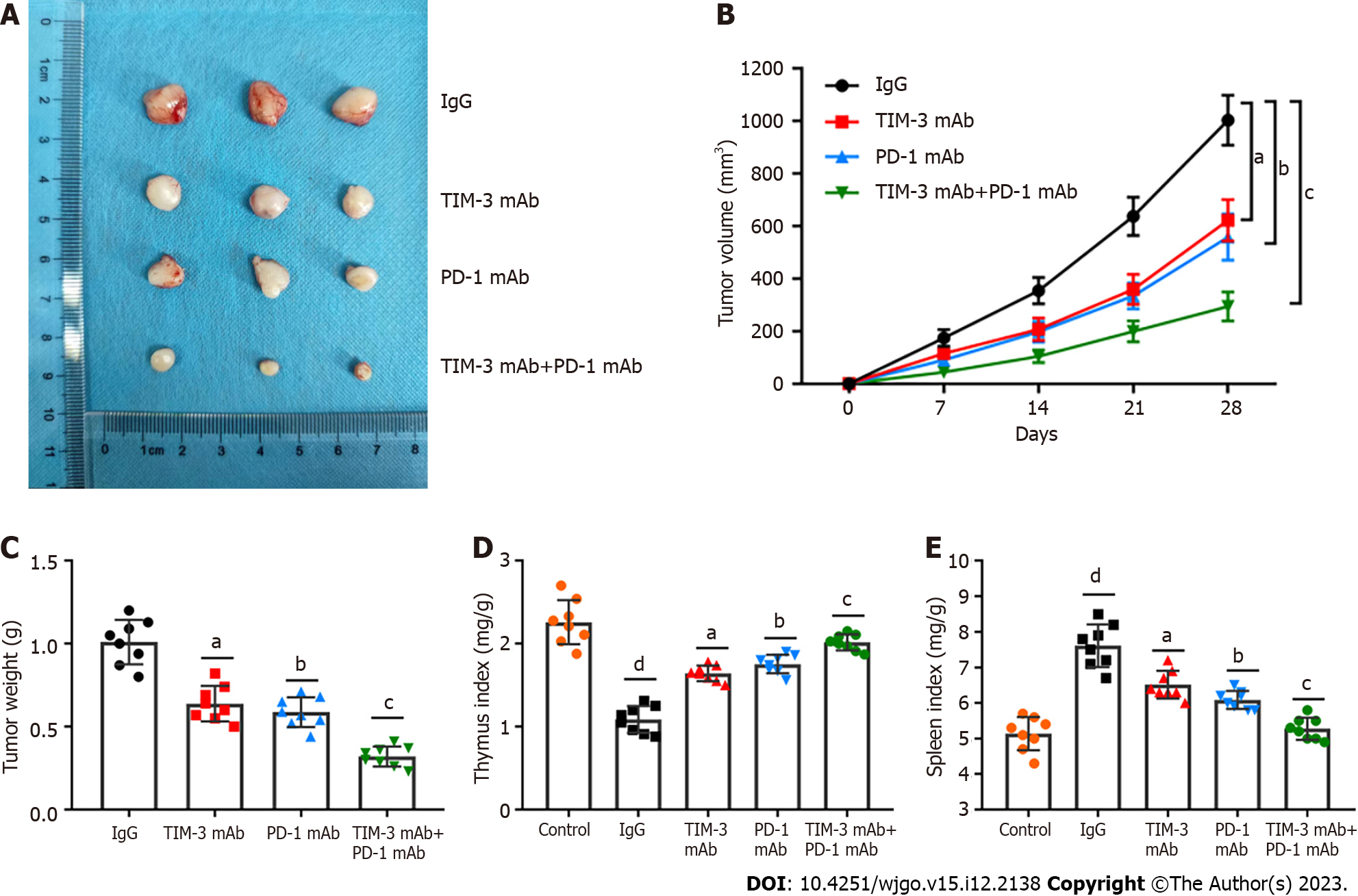
Anti-PD-1 agents have been used in clinic for HCC treatment[25,26]. However, the effects of anti-TIM-3 agents have not been fully clarified. We next determined the anti-tumor activities of anti-TIM-3 mAb, anti-PD-1 mAb and their combination in HCC. A xenograft mouse model of HCC was established. Tumor images were presented in Figure 2A, and it was observed that TIM-3 and PD-1 mAb treatment both reduced tumor volume (Figure 2B) and weight (Figure 2C) compared with IgG treatment. Moreover, combined blockade had more substantial anti-tumor effects than individual treatment. In addition, the spleen index (Figure 2D) was obviously elevated and the thymus index (Figure 2E) was decreased in model mice, indicating the impaired immunity in model mice. However, individual or combination treatment substantially reduced the spleen index and increased the thymus index in mice, while the effects of combination treatment were more significant. Our results illustrated that combined blockade restrained xenograft tumor growth and ameliorated the tumor immunosuppressive microenvironment in mice.
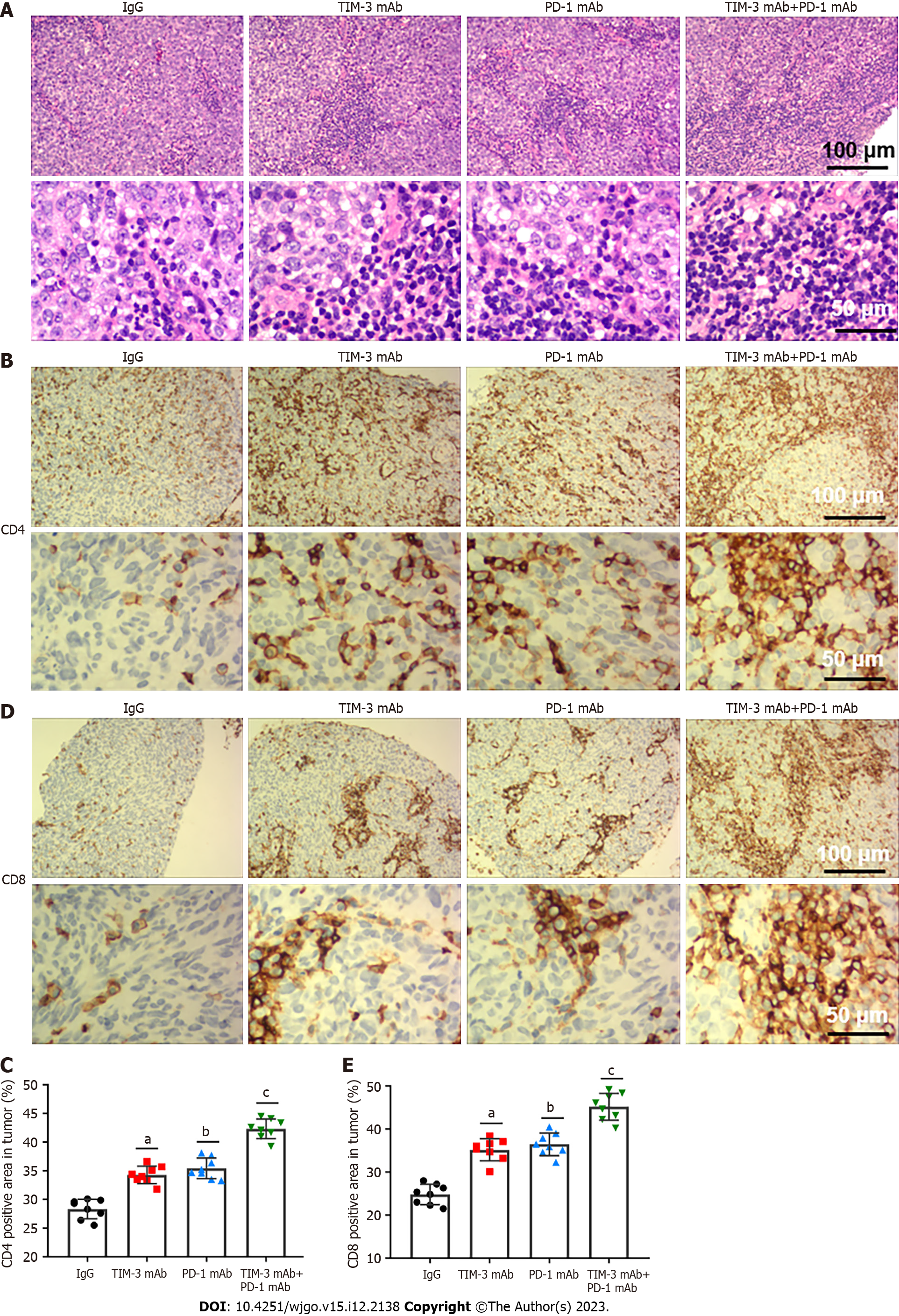
We next investigated the effects of combined blockade on TILs in tumor microenvironment. HE staining illustrated that combined blockade alleviated the apoptotic characteristics of TILs and increased the number of TILs. Particularly, these changes were more obvious in the combination treatment group (Figure 3A). Additionally, immunohistochemical staining indicated that combined blockade improved the number of CD4+ (Figures 3B and C) and CD8+ (Figures 3D and E) T cells in mice. Our results revealed that combined blockade could prominently activate T cell infiltration.
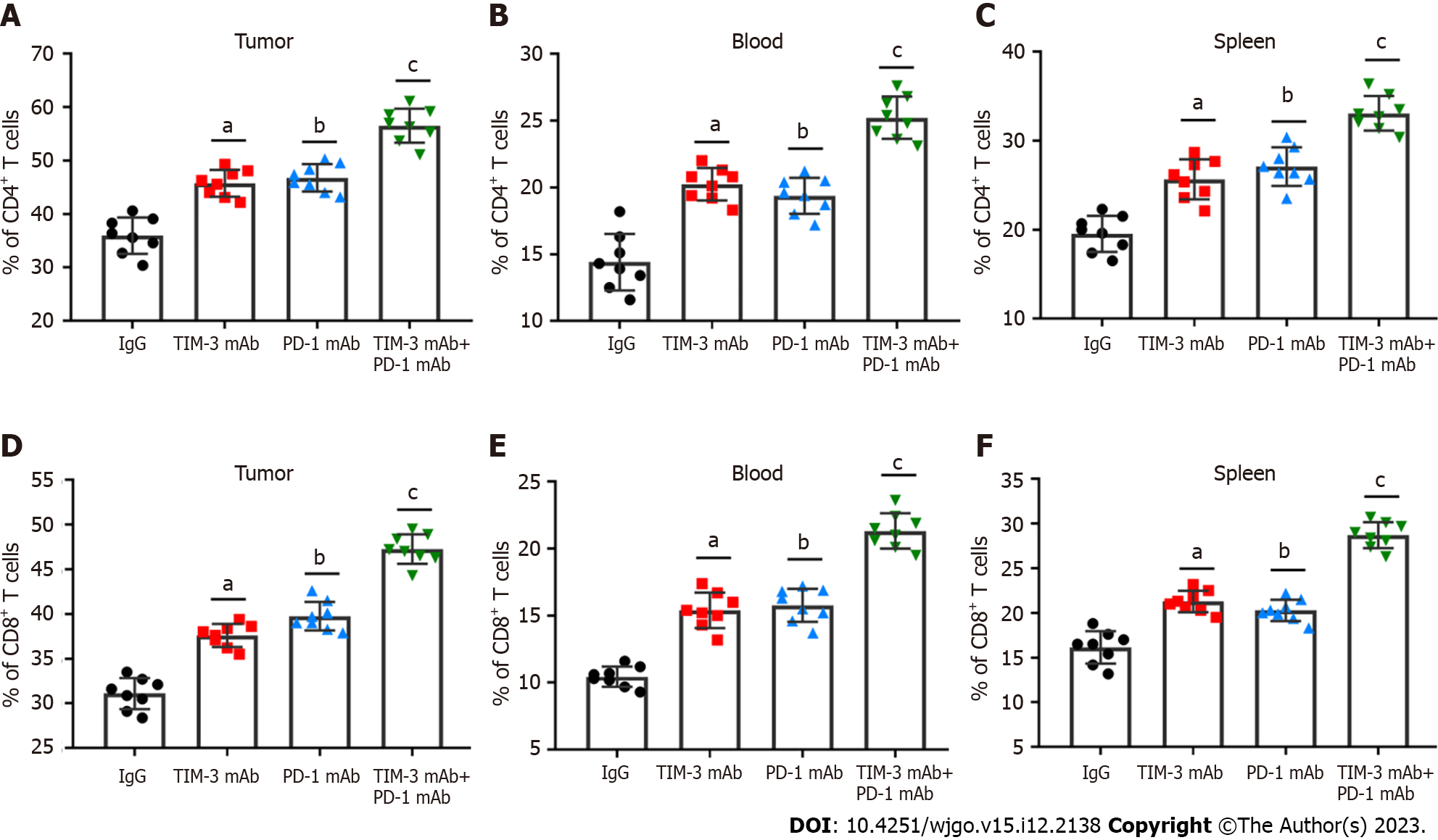
The main immunosuppressive mechanism of cancer cells is intimately related to CD4+ and CD8+ T cells[15,16]. The percentage of CD4+ and CD8+ T cells in tumor tissues, peripheral blood and spleen was then tested with flow cytometry. As expected, TIM-3 and PD-1 blockade prominently increased the percentage of CD4+ (Figures 4A-C) and CD8+ (Figures 4D-F) T cells in all collected samples of mice, especially the combined group, which exhibited more significant alterations. Therefore, we proposed that combined blockade could increase the percentage of CD4+ and CD8+ T cells and improve anti-tumor immune responses.
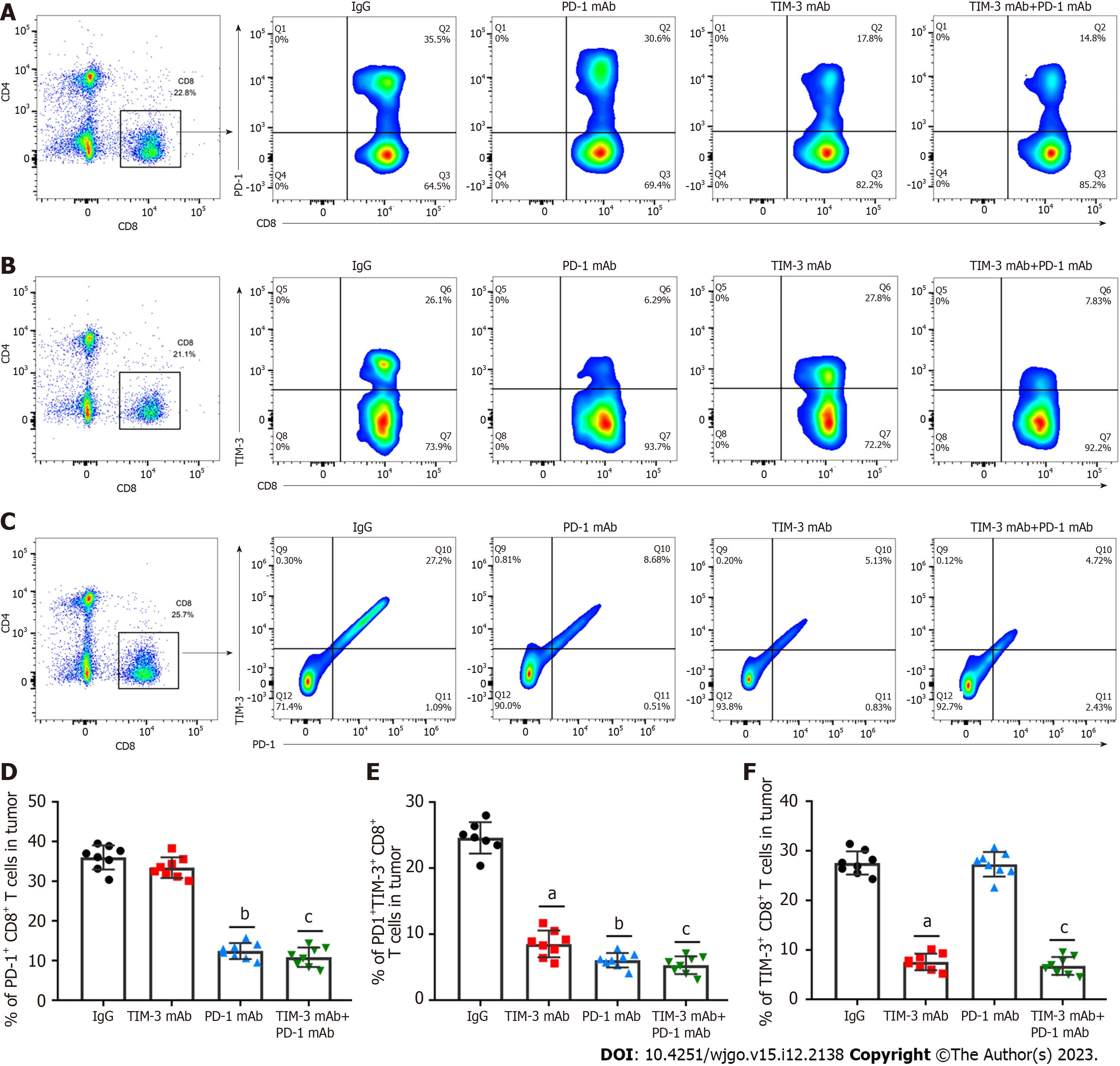
T cell exhaustion is a vital immune escape mechanism in tumor microenvironment, and is accompanied by increased co-inhibitory receptor expression[27,28]. Therefore, we determined the roles of combined blockade in T cell exhaustion phenotypes in a xenograft mouse model. PD-1+CD8+ and TIM-3+CD8+ T cells in tumor tissues were assessed. Our results clearly indicated that PD-1 blockade prominently decreased PD-1+CD8+ T cell levels (Figures 5A and D) compared with IgG treatment, while anti-TIM-3 blockade restrained TIM-3+CD8+ T cell numbers (Figures 5B and E) in tumor tissues. Moreover, combined treatment simultaneously reduced PD-1+CD8+ T cell and TIM-3+CD8+ T cell numbers. Additionally, combined blockade obviously inhibited PD-1+TIM-3+CD8+ T cell numbers (Figures 5C and F). Collectively, our results suggested that combined blockade could ameliorate the exhausted CD8+ T cell phenotype in the immunosuppressive microenvironment of HCC.
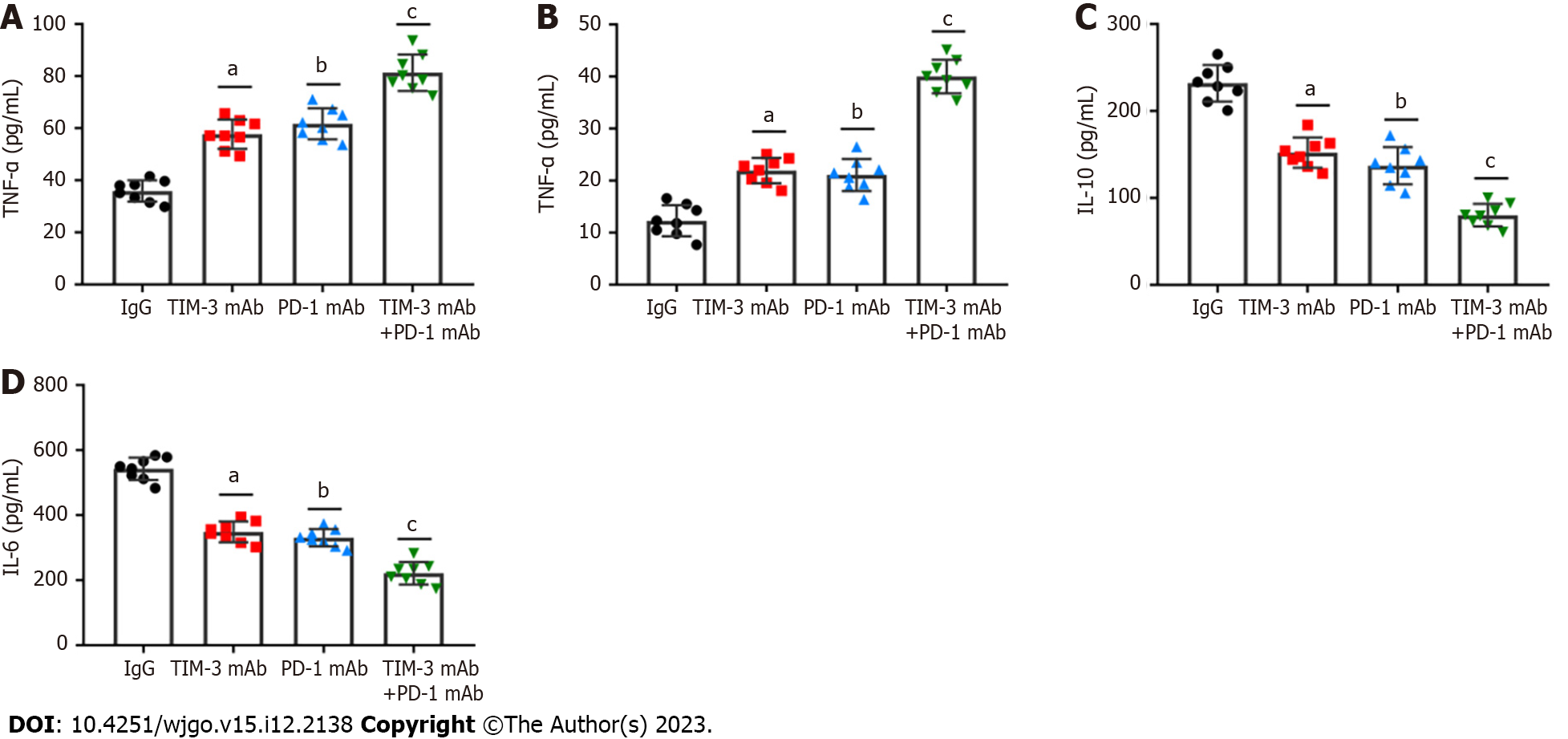
Solvent cytokines provide another crucial distinctive marker for T cell exhaustion. We detected the levels of critical T cell effector cytokines, IFN-γ and TNF-α, and immunosuppressive cytokines, IL-10 and IL-6 in tumor tissues of all groups of mice. It was revealed that the individual blockade of TIM-3 and PD-1 prominently facilitated IFN-γ and TNF-α production (Figures 6A and B), and reduced IL-10 and IL-6 levels (Figures 6C and D). Importantly, such changes were more obvious in the combined group. Thus, the above results highlighted the crucial effect of combined blockade on restoring exhausted CD8+T cell mediated anti-tumor capability.
To date, multiple effective and safe immunotherapies have achieved gratifying survival rate and prognosis for HCC patients. which have attracted more attention and provide novel directions for HCC treatment. It’s generally identified that increased TIL infiltration in tumor microenvironment has consistently been linked to improved outcomes for HCC patients in clinic[14]. The ability of tumor specific effector CD4+ and CD8+ T cells is intimately associated with their numbers, localization, and phenotype[15,16]. T cells are essential for identifying and destroying tumor cells. However, persistent tumor antigen stimulation can cause T cell exhaustion and loss of function, resulting in tumor immune escape[17]. Meanwhile, it has been reported that increased co-inhibitory receptor expression induces T cell exhaustion phenotype, leading to T cell immunodeficiency[18]. Thus, restoring T cell functions through blockade of immune checkpoints continues to be a dominant approach for cancer treatment. To date, advanced development of ICIs has been achieved, and multiple ICIs targeting PD-1 have been applied for HCC patients[25,26]. However, PD-1/PD-L1 is just one of numerous complex elements of anti-tumor immunity, and only blockage of PD-1 has the limitation of unsatisfactory curative effects and drug resistance. Therefore, the combination of multiple ICIs may have additive effects and become a better option to treat advanced HCC.
TIM-3 was identified as a promising immune checkpoint molecule. The therapeutic method of TIM-3 blockade has been studied in multiple human tumor immunotherapies, especially in combination with other immune checkpoints[29]. It has been previously found that multiple co-inhibitory receptors, including PD-1 and TIM-3, are prominently upregulated on CD4+ and CD8+ T cells from tumor tissues of HCC patients[24]. Moreover, TIM-3 and PD-1 positive CD8+ T cells were indicated to be related to adverse outcomes for HCC[23]. However, TIM-3 blockade is relatively poorly studied in HCC. Our study first collected clinical samples from HCC patients, and confirmed that PD-1 and TIM-3 expression was substantially upregulated in CD4+ and CD8+ T cells isolated from tumor tissues and ascites compared with matched adjacent tissues. Ascites are conveniently available clinical samples, and our results support the adoption of ascites as an alternative site for TIM-3 recognition and patient stratification to manage TIM-3 blockers.
A previous meta-analysis revealed that TIM-3 upregulation was linked to adverse prognosis in individuals with various cancers, and they proposed that TIM-3 might be a dominant target[30]. Notably, TIM-3 blockage in combination with radiation could improve anti-tumor efficacy in an HCC mouse model[31]. Our study suggested that individual TIM-3 mAb and PD-1 mAb treatment both reduced tumor volume and weight, while combined blockade had more substantial tumor suppressive effect than individual treatment. In addition, the combination therapy also reduced the spleen index and increased the thymus index in mice, indicating the improvement in the immunosuppressive tumor microenvironment. More importantly, combined blockade prominently upregulated CD4+ and CD8+ T cells and activated cell infiltration into tumor tissues. Additionally, our results showed that combined blockade can simultaneously downregulate PD-1 and TIM-3 expression on CD8+ T cells. Likewise, PD-1+TIM-3+ CD8+ T cell numbers in tumor tissues were obviously reduced by combination therapy. Meanwhile, combined blockade also facilitated IFN-γ and TNF-α production, and reduced the production of IL-10 and IL-6 in tumor tissues. Therefore, we implicated that combined blockade could ameliorate T cell exhaustion in HCC model mice.
Taken together, our study proposed that the combined blockade of TIM-3 and PD-1 had an additive anti-tumor effect on an HCC mouse model compared with individual treatment. Combined therapy improved anti-tumor immune responses in HCC mouse model by increasing CD4+ and CD8+ T cell infiltration, down-regulating the co-inhibitory receptors TIM-3 and PD-1, and ameliorating T cell exhaustion. We hope that our findings will benefit to the clinical treatment of HCC.
Hepatocellular carcinoma (HCC) is the fourth intractable tumors in clinic worldwide. Recently, immune checkpoint inhibitors (ICIs), a novel immune therapy, has been identified as an efficient and safe therapy for malignant tumors, which have attracted more attention and give novel directions for HCC treatment.
ICIs targeting programmed cell death protein 1 (PD-1) and T cell immunoglobulin and mucin domain-containing protein 3 (TIM-3) are benefit to the resume of anti-tumor immunity response and hold extreme potential as efficient therapies for certain malignancies. Given the complex pathological mechanisms of HCC, ICIs with a single target exhibit poor overall response rate in HCC patients. Therefore, it’s of great significance to identify more effective immunotherapies and provide more treatment options to achieve gratifying survival rate and prognosis for HCC patients.
This study investigated the effects of combined TIM-3 and PD-1 blockade on tumor development in an HCC mouse model, aiming to identify more effective immunotherapies and provide more treatment options for HCC patients.
We first detected the levels of PD-1 CD4+, PD-1 CD8+, TIM-3 CD4+, and TIM-3 CD8+ in tumor tissues, ascites, and matched adjacent tissues from HCC patients. Next, we investigated the effects of combined and individual blockage of TIM-3 and PD-1 on an HCC xenograft mouse model. Tumor growth and CD4+ and CD8+ T cell-mediated antitumor immune responses were assessed.
PD-1 and TIM-3 expression was upregulated in CD4+ and CD8+ T cells isolated from tumor tissues and ascites of HCC patients. TIM-3 mAb and PD-1 mAb treatment both reduced tumor volume and weight, while combined blockade had more substantial anti-tumor effects compared with individual treatment. The combined therapy increased T cell infiltration into tumor tissues, and ameliorated CD4+ and CD8+ T cell exhaustion in HCC mouse model.
Combined TIM-3 and PD-1 blockage has more substantial anti-tumor effects compared with individual blockage. Combined TIM-3 and PD-1 blockage restrains HCC development by facilitating CD4+ and CD8+ T cell-mediated antitumor immune responses.
We need to explore the effects of combination of multiple ICIs to provide better options for HCC treatment.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arvanitakis K, Greece; Cuno C, Spain S-Editor: Wang JJ L-Editor: A P-Editor: Yu HG
| 1. | Harris PS, Hansen RM, Gray ME, Massoud OI, McGuire BM, Shoreibah MG. Hepatocellular carcinoma surveillance: An evidence-based approach. World J Gastroenterol. 2019;25:1550-1559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 156] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (4)] |
| 2. | Zhang Y, Liu Z, Ji K, Li X, Wang C, Ren Z, Liu Y, Chen X, Han X, Meng L, Li L, Li Z. Clinical Application Value of Circulating Cell-free DNA in Hepatocellular Carcinoma. Front Mol Biosci. 2021;8:736330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Li S, Wang P, Qiu J, Xie Y, Yin D, Deng K. Comparison of IDEAL-IQ and IVIM-DWI for Differentiating between Alpha Fetoprotein-Negative Hepatocellular Carcinoma and Focal Nodular Hyperplasia. Oncologie. 2022;24:1-12. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 4. | Zhang W, Zhang B, Chen XP. Adjuvant treatment strategy after curative resection for hepatocellular carcinoma. Front Med. 2021;15:155-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 5. | Wang K, Wang C, Jiang H, Zhang Y, Lin W, Mo J, Jin C. Combination of Ablation and Immunotherapy for Hepatocellular Carcinoma: Where We Are and Where to Go. Front Immunol. 2021;12:792781. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 6. | Yan D, Li C, Zhou Y, Yan X, Zhi W, Qian H, Han Y. Exploration of Combinational Therapeutic Strategies for HCC Based on TCGA HCC Database. Oncologie. 2023;24. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 7. | Liu Z, Liu X, Liang J, Liu Y, Hou X, Zhang M, Li Y, Jiang X. Immunotherapy for Hepatocellular Carcinoma: Current Status and Future Prospects. Front Immunol. 2021;12:765101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 8. | Shu X, Wang Q, Wu Q. The Eph/Ephrin System in Hepatocellular Carcinoma: Functional Roles and Potential Therapeutic Targets. Oncologie. 2022;24:427-439. [RCA] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 9. | Jiang Y, Han QJ, Zhang J. Hepatocellular carcinoma: Mechanisms of progression and immunotherapy. World J Gastroenterol. 2019;25:3151-3167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 67] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 10. | Keilson JM, Knochelmann HM, Paulos CM, Kudchadkar RR, Lowe MC. The evolving landscape of immunotherapy in solid tumors. J Surg Oncol. 2021;123:798-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Paijens ST, Vledder A, de Bruyn M, Nijman HW. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell Mol Immunol. 2021;18:842-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 573] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 12. | Yu S, Lv H, Zhang J, Zhang H, Ju W, Jiang Y, Lin L. Heparanase/Syndecan-1 Axis Regulates the Grade of Liver Cancer and Proliferative Ability of Hepatocellular Carcinoma Cells. Oncologie. 2022;24:1-3. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 13. | Maibach F, Sadozai H, Seyed Jafari SM, Hunger RE, Schenk M. Tumor-Infiltrating Lymphocytes and Their Prognostic Value in Cutaneous Melanoma. Front Immunol. 2020;11:2105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 160] [Cited by in RCA: 193] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 14. | Liu YT, Sun ZJ. Turning cold tumors into hot tumors by improving T-cell infiltration. Theranostics. 2021;11:5365-5386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 483] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 15. | Reina-Campos M, Scharping NE, Goldrath AW. CD8(+) T cell metabolism in infection and cancer. Nat Rev Immunol. 2021;21:718-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 372] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 16. | Oh DY, Fong L. Cytotoxic CD4(+) T cells in cancer: Expanding the immune effector toolbox. Immunity. 2021;54:2701-2711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 262] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 17. | Blank CU, Haining WN, Held W, Hogan PG, Kallies A, Lugli E, Lynn RC, Philip M, Rao A, Restifo NP, Schietinger A, Schumacher TN, Schwartzberg PL, Sharpe AH, Speiser DE, Wherry EJ, Youngblood BA, Zehn D. Defining 'T cell exhaustion'. Nat Rev Immunol. 2019;19:665-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 1028] [Article Influence: 171.3] [Reference Citation Analysis (0)] |
| 18. | Ando M, Ito M, Srirat T, Kondo T, Yoshimura A. Memory T cell, exhaustion, and tumor immunity. Immunol Med. 2020;43:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 143] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 19. | Marconcini R, Spagnolo F, Stucci LS, Ribero S, Marra E, Rosa F, Picasso V, Di Guardo L, Cimminiello C, Cavalieri S, Orgiano L, Tanda E, Spano L, Falcone A, Queirolo P; Italian Melanoma Intergroup (IMI). Current status and perspectives in immunotherapy for metastatic melanoma. Oncotarget. 2018;9:12452-12470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | Saigi M, Alburquerque-Bejar JJ, Sanchez-Cespedes M. Determinants of immunological evasion and immunocheckpoint inhibition response in non-small cell lung cancer: the genetic front. Oncogene. 2019;38:5921-5932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 21. | Wang J, Li J, Tang G, Tian Y, Su S, Li Y. Clinical outcomes and influencing factors of PD-1/PD-L1 in hepatocellular carcinoma. Oncol Lett. 2021;21:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Li Q, Han J, Yang Y, Chen Y. PD-1/PD-L1 checkpoint inhibitors in advanced hepatocellular carcinoma immunotherapy. Front Immunol. 2022;13:1070961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 23. | Ma J, Zheng B, Goswami S, Meng L, Zhang D, Cao C, Li T, Zhu F, Ma L, Zhang Z, Zhang S, Duan M, Chen Q, Gao Q, Zhang X. PD1(Hi) CD8(+) T cells correlate with exhausted signature and poor clinical outcome in hepatocellular carcinoma. J Immunother Cancer. 2019;7:331. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 238] [Cited by in RCA: 230] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 24. | Zhou G, Sprengers D, Boor PPC, Doukas M, Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J, Gaspersz M, Dong H, Thielemans K, Pan Q, IJzermans JNM, Bruno MJ, Kwekkeboom J. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology. 2017;153:1107-1119.e10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 322] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 25. | D'Alessio A, Rimassa L, Cortellini A, Pinato DJ. PD-1 Blockade for Hepatocellular Carcinoma: Current Research and Future Prospects. J Hepatocell Carcinoma. 2021;8:887-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Chen Z, Lu X, Koral K. The clinical application of camrelizumab on advanced hepatocellular carcinoma. Expert Rev Gastroenterol Hepatol. 2020;14:1017-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Nagasaki J, Togashi Y. A variety of 'exhausted' T cells in the tumor microenvironment. Int Immunol. 2022;34:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Dolina JS, Van Braeckel-Budimir N, Thomas GD, Salek-Ardakani S. CD8(+) T Cell Exhaustion in Cancer. Front Immunol. 2021;12:715234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 296] [Article Influence: 74.0] [Reference Citation Analysis (0)] |
| 29. | Zhao L, Cheng S, Fan L, Zhang B, Xu S. TIM-3: An update on immunotherapy. Int Immunopharmacol. 2021;99:107933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 30. | Qin S, Dong B, Yi M, Chu Q, Wu K. Prognostic Values of TIM-3 Expression in Patients With Solid Tumors: A Meta-Analysis and Database Evaluation. Front Oncol. 2020;10:1288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 31. | Kim KJ, Lee HW, Seong J. Combination therapy with anti-T-cell immunoglobulin and mucin-domain containing molecule 3 and radiation improves antitumor efficacy in murine hepatocellular carcinoma. J Gastroenterol Hepatol. 2021;36:1357-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |