Published online Dec 15, 2023. doi: 10.4251/wjgo.v15.i12.2111
Peer-review started: September 10, 2023
First decision: October 13, 2023
Revised: October 18, 2023
Accepted: October 30, 2023
Article in press: October 30, 2023
Published online: December 15, 2023
Processing time: 95 Days and 0.2 Hours
Gastric subepithelial tumors (SETs) may harbor potential malignancy. Although it is well recognized that large SETs should be resected, the precise treatment strategy remains controversial. Compared to surgical resection, endoscopic resec
To evaluate the safety and efficacy of endoscopic full-thickness resection (EFTR) for the treatment of gastric cardia SETs.
We retrospectively reviewed data from all patients with SETs originating from the muscularis propria layer in the gastric cardia that were treated by EFTR or submucosal tunneling ER (STER) at Zhongshan Hospital Fudan University between November 2014 and May 2022. Baseline characteristics and clinical outcomes, including procedure times and complications rates, were compared between groups of patients receiving EFTR and STER.
A total of 171 tumors were successfully removed [71 (41.5%) tumors in the EFTR and 100 (58.5%) tumors in the STER group]. Gastrointestinal stromal tumors (GISTs) were the most common SET. The en bloc resection rate was 100% in the EFTR group vs 97.0% in STER group (P > 0.05). Overall, the EFTR group had a higher complete resection rate than the STER group (98.6% vs 91.0%, P < 0.05). The procedure time was also shorter in the EFTR group (44.63 ± 28.66 min vs 53.36 ± 27.34, P < 0.05). The most common major complication in both groups was electrocoagulation syndrome. There was no significant difference in total complications between the two groups (21.1% vs 22.0%, P = 0.89).
EFTR of gastric cardia SETs is a very promising method to facilitate complete resection with similar complications and reduced operative times compared to STER. In cases of suspected GISTs or an unclear diagnosis, EFTR should be recommended to ensure complete resection.
Core Tip: Efficacy of endoscopic full-thickness resection (EFTR) is safe and effective in the treatment of cardiac subepithelial tumors. Compared with submucosal tunneling endoscopic resection, EFTR can better completely resect subepithelial tumors and provide a better pathological diagnosis. When lesions with a high index of suspicion for gastrointestinal stromal tumors are found or there is an unclear diagnosis, EFTR should be recommended to ensure complete resection.
- Citation: Xu EP, Qi ZP, Li B, Ren Z, Cai MY, Cai SL, Lyv ZT, Chen ZH, Liu JY, Shi Q, Zhong YS. The efficacy of full-thickness endoscopic resection of subepithelial tumors in the gastric cardia. World J Gastrointest Oncol 2023; 15(12): 2111-2119
- URL: https://www.wjgnet.com/1948-5204/full/v15/i12/2111.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i12.2111
Gastric subepithelial tumors (SETs) are rare, accounting for less than 2% of all gastric tumors[1]. This group is comprised of different pathologies, commonly correlating with their location. Benign SETs are more frequently found in the cardia than in other locations[2].
With technological advancements, laparoscopic wedge resection is currently considered the best option for the treatment of gastric SETs[3]; however, it is difficult to resect SETs locating in the cardiaand is associated with several complications, including leak, stenosis, and reflux[4,5]. Due to their typically benign nature and these postoperative risks, surgical resection of small, benign SETs is typically not mandatory.
Since the advent of submucosal tunneling endoscopic resection (ER) (STER), it has been widely used for SETs resection in the esophagus and cardia, and has achieved positive results[6-8]. The American Gastroenterological Association Clinical Practice Guidelines recommend using ER techniques to remove SETs[9]; however, in clinical application, damage to the tunneled surface mucosa may occur. This may be due to a large tumor and/or a lesion located in the deep layer of the muscularis propria (MP) (or even outside the cavity) or if the operating space in the tunnel is small, or the tunnel cannot be established at the tumor site. Additionally, submucosal fibrosis can lead to technical difficulty and tunnel establishment failure can occur.
Endoscopic full-thickness resection (EFTR) is a new surgical method of repairing the gastric wall after full-thickness resection so that endoscopic surgery is no longer limited by the depth of the tumor and submucosal fibrosis[10]. In 2009, EFTR without laparoscopic assistance for the treatment of gastrointestinal SET was first proposed. Since then, EFTR has been developed and widely applied clinically. Li et al[11] reported using dental floss and a hemoclip for assisted EFTR for SETs in the gastric fundus and demonstrated advantages in reducing surgical time and occurrence of post-endoscopic submucosal dissection (ESD) electrocoagulation syndrome (PEECS). Many studies have also proven that EFTR is safe and effective in the treatment of gastric SETs[12,13]; however, data regarding the clinical outcomes of EFTR for gastric cardia SETs are limited. Therefore, we evaluated the clinical outcomes of gastric cardia SETs treated by EFTR resection at our institution.
We collected and reviewed data from patients with SETs originating from the MP layer in the gastric cardia, which were treated by EFTR or STER at Zhongshan Hospital Fudan University (Shanghai, China) between November 2014 and May 2022. The inclusion criteria were: (1) Proven diagnosis of SET by gastroscopy, endoscopic ultrasonography (EUS), and computed tomography (CT); (2) eligibility for endoscopic treatment; (3) lesion located in the cardia; and (4) final application of STER or EFTR. The exclusion criteria were: (1) Patients disagreement regarding resection; (2) malignant tumors with metastasis; and (3) coagulation disorders.
Informed patient consent was obtained from all patients. The study and procedures were conducted in accordance with the ethical standards of the Helsinki Declaration of 1975. This study was approved by the Institutional Review Board of Zhongshan Hospital (reference number: B2020-265).
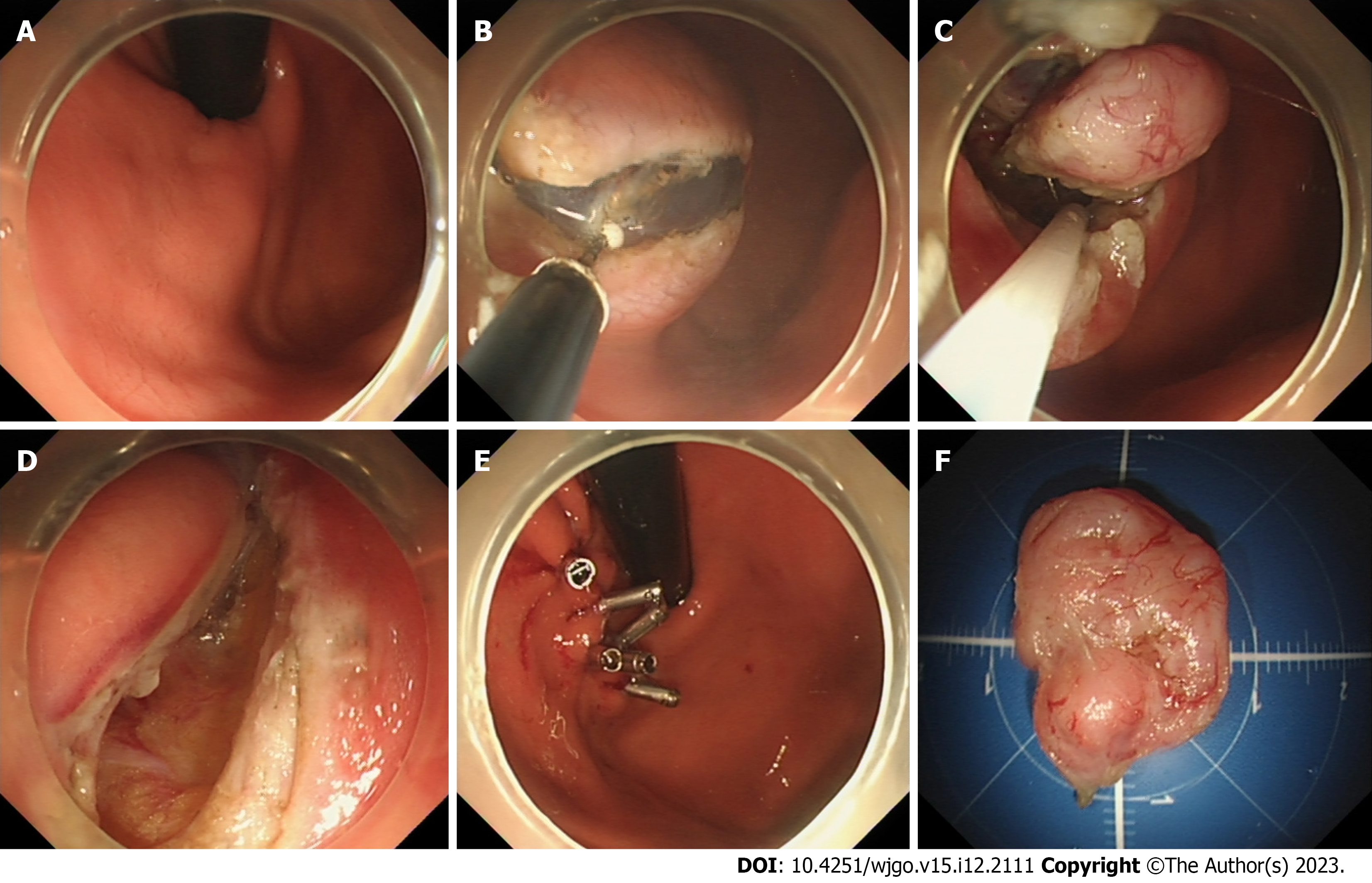
EUS and/or CT were used to characterize the lesions in terms of size and other features prior to EFTR or STER. All patients were airway intubated under intravenous anesthesia, while vital signs were monitored. The ER method was selected based on the tumor characteristics. If the tumor deviates to the esophageal side, STER is used, and if the tumor deviates to the stomach side, EFTR is used. Some cases underwent EFTR while others underwent STER according to the patient’s preference, after being informed of the merits and disadvantages of each technique. Description of the specific resection procedures can be found in the literature previously published by our center (Figure 1)[7,10].
The surgical operator was categorized as a trainee with experience of 25 EFTR and STER procedures per year or as an expert with experience of > 25 procedures per year[11]. All were certified EFTR endoscopists. En bloc resection was characterized as the complete removal of a tumor without fragmentation. Complete resection was characterized as the en bloc removal of a lesion with the tumor extracted in a single piece and the capsule remaining intact[7]. Postoperative bleeding was characterized as hematemesis or melena within 14 d after completion of EFTR or STER. Hydrothorax was excess fluid in the pleural space, as confirmed by chest X-ray. Pneumoperitoneum was diagnosed by the presence of gas in the peritoneal cavity, observable on either X-ray or CT scan. Minor cases of pneumoperitoneum and minor hydro
Data were initially collected from medical records in our hospital. If patients have been discharged from our hospital, we make an effort to gather outcome information by contacting the patient or a family member by telephone. The minimum follow-up duration is 12 mo.
SPSS 21.0 software (IBM Corp, Armonk, NY, United States) was used for analysis. We compared categorical variables with the chi-square or Fisher’s exact test. The Student’s t-test or analysis of variance was used to compare continuous variables. Statistical analysis of independent risk factors for long operative times was assessed using a combination of univariate and multivariate analyses. A two-tailed P value < 0.05 was considered statistically significant.
A total of 171 patients with SETs in the cardia were included in the study. Seventy-one (41.5%) patients underwent EFTR, while 100 (58.5%) received STER treatment. The clinical characteristics of these patients are presented in Table 1. The mean age of the EFTR and STER group was 51.32 ± 12.44 (median: 52; range: 43–61 years) and 50.29 ± 12.19 years (median: 51; range: 41–60 years), respectively. The average tumor size of the EFTR and STER group was 2.16 ± 1.81 cm (median: 1.5; range: 1.0-3.0 cm) and 2.09 ± 1.38 cm (median: 1.5; range: 1.2–2.5 cm), respectively. Six (8.5%) and 5 (5.0%) tumors showed extraluminal growth in the EFTR and STER groups. 59 (83.1%) patients underwent EFTR by expert surgeons, and 85 (85%) patients underwent EFTR by trainee surgeons. Patient characteristics and clinical data relating to tumors and procedures were similar between the EFTR and STER groups.
| Patients | EFTR | STER | P value |
| Age (yr) | 0.59 | ||
| mean ± SD | 51.32 ± 12.44 | 50.29 ± 12.19 | |
| Median (range) | 52.00 (43.00-61.00) | 51.00 (41.00-59.75) | |
| Sex | 0.01 | ||
| Male | 24 (33.8%) | 53 (53.0%) | |
| Female | 47 (66.2%) | 47 (47.0%) | |
| Lesion characteristics | |||
| Size (cm) | 0.78 | ||
| mean ± SD | 2.16 ± 1.81 | 2.09 ± 1.38 | |
| Median (range) | 1.50 (1.00-3.00) | 1.50 (1.20-2.50) | |
| Extraluminal growth | 0.37 | ||
| Yes | 6 (8.5%) | 5 (5.0%) | |
| No | 65 (91.5%) | 95 (95.0%) | |
| Operator level | 0.74 | ||
| Experts | 59 (83.1%) | 85 (85.0%) | |
| Trainees | 12 (16.9%) | 15 (15.0%) |
The primary surgery-related outcomes from the two groups of patients are described in Table 2. All gastric cardia SETs were resected by ER. The en bloc resection rate was 100% in the EFTR group. There was no significant difference between the groups. EFTR had a higher completed resection rate than STER (98.6% vs 91.0%, P < 0.05). The procedure time was also shorter in the EFTR group (44.63 ± 28.66 min vs 53.36 ± 27.34, P < 0.05). In the EFTR group, there were 28 (39.4%) gastrointestinal stromal tumors (GISTs) and 43 (60.6%) leiomyomas. In the STER group, there were 7 (7%) GISTs, 88 (88%) leiomyomas, 3 (3%) lipomas, and 2 (2%) cysts. Metallic clips and endoloop was applied in 29 (40.8%) EFTR patients and 2 (2%) STER patients. There was no significant difference in total complications between two groups (21.1% vs 22.0%, P = 0.89). The most common complication in both groups was electrocoagulation syndrome. All complications were managed successfully by endoscopic methods and conservative treatment.
| Outcomes | EFTR | STER | P value |
| En bloc resection | 71 (100%) | 97 (97.0%) | 0.14 |
| Complete resection | 70 (98.6%) | 91 (91.0%) | 0.04 |
| Procedure time (min) | 44.63 ± 28.66 | 53.36 ± 27.34 | 0.04 |
| Procedure-related characteristics | |||
| Suturing methods | 0.01 | ||
| Metallic clips | 38 (53.5%) | 98 (98.0%) | |
| Metallic clips with endoloop | 29 (40.8%) | 2 (2.0%) | |
| Stent | 4 (5.6%) | 0 (0%) | |
| Histopathology | 0.01 | ||
| GIST | 28 (39.4%) | 7 (7.0%) | |
| Leiomyoma | 43 (60.6%) | 88 (88.0%) | |
| Others | 0 (0%) | 5 (5.0%) | |
| Complications | 15 (21.1%) | 21 (22.0%) | 0.89 |
| Pneumoperitoneum | 0 (0%) | 0 (0%) | |
| Hydrothorax | |||
| Minor hydrothorax | 3 (4.2%) | 4 (4.0%) | |
| Major hydrothorax | 0 (0%) | 0 (0%) | |
| PEECS | 10 (14.1%) | 18 (18.0%) | |
| Delayed bleeding | 2 (2.8%) | 0 (0%) | |
| Delayed perforation | 0 (0%) | 0 (0.0%) | |
| Follow-up | |||
| Recurrence | 0 (0%) | 0 (0%) | |
| Metastasis | 0 (0%) | 0 (0%) |
Univariate and multivariate analyses showed that larger tumor size (> 2 cm) and extraluminal growth were significant risk factors for long procedure times (Table 3).
| Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P value | OR (95%CI) | P value | |
| Age (yr) | 0.37 | |||
| < 50 | 1 (reference) | |||
| ≥ 50 | 1.88 (0.48-7.46) | |||
| Sex | 0.41 | |||
| Male | 1 (reference) | |||
| Female | 1.76 (0.46-6.68) | |||
| Size (cm) | 0.01 | |||
| < 2.0 | 1 (reference) | 1 (reference) | 0.02 | |
| ≥ 2.0 | 6.58 (1.60-27.09) | 3.75 (1.21-11.58) | ||
| Extraluminal growth | 0.048 | 0.04 | ||
| No | 1 (reference) | 1 (reference) | ||
| Yes | 7.83 (1.02-60.03) | 7.64 (1.15-50.83) | ||
| Histopathology | 0.42 | |||
| Others | 1 (reference) | |||
| Leiomyoma | 1.85 (0.42-8.17) | |||
| Metallic clips with endoloop | 0.05 | |||
| No | 1 (reference) | |||
| Yes | 3.68 (0.98-13.91) | |||
| Operator level | 0.19 | |||
| Trainees | 1 (reference) | |||
| Experts | 0.31 (0.05-1.76) | |||
Of the 171 patients with gastric cardiac SETs, seven cases were lost to follow-up. The remaining 164 cases were followed for more than 12 mo. The median follow-up was 28 (range: 16-52.5) mo. All patients did not develop local recurrence or distant metastasis during follow-up.
The present work was the first time that the efficacy of EFTR for gastric cardia SETs was studied. Current methods to remove gastric SETs include surgical and ER. ER has several advantages over surgical approaches, such as being minimally invasive and incurring a shorter hospital stay[14,15]. The STER procedure was derived from peroral endoscopic myotomy and was initially reported in 2012[6]. A series of subsequent studies have reported that, compared to ESD, STER has benefits for the removal of SETs, such as maintaining the integrity of the mucosa, faster wound healing, and reduced risks of complications including perforation, extraluminal infection, and esophageal stenosis[16-18]. STER has also been reported as a successful treatment option for SETs located in the cardia or esophagogastric junction[19,20]. The advantages of shorter operative and hospitalization times and reduced cost are described[21].
STER requires the establishment of adequate operating space beyond the tumor in the tunnel. The tumor is then pushed into the distal portion of the submucosal tunnel during resection, separating the tumor from the deep MP and increasing the safety of the operation[22,23]. Performing the operation for SETs in the cardia is more complicated than in other parts owing to its specific anatomic characteristics[24]. There is a significant change in the angle of this gastric muscle layer. Therefore, the formation of the tunnel beyond the tumor requires a greater degree of curvature of the anterior part of the endoscope. Additionally, the gastric cavity is relatively narrow, which greatly increases the difficulty of resection[25,26]. Meanwhile, the blood supply at the cardia and gastric fundus is abundant. Thus, the risk of intraoperative bleeding is high, which also increases the difficulty of the operation[26]. In addition, in the lesser curvature or the anterior aspect of the cardia, when the tumor is located in the deep layer of the MP (or even growing extraluminally), and/or where a submucosal tunnel cannot be established, EFTR is needed.
EFTR, as a technical extension of ESD, offers distinct advantages, particularly when dealing with SETs deeply embedded within the MP layer or exhibiting extraluminal growth patterns. The characteristics of EFTR make it a very suitable form of treatment for SETS[12]. Our center previously reported the successful application of EFTR in 26 gastric SETs without laparoscopic assistance. No gastric bleeding, sign of peritonitis, or abdominal infections/abscesses occurred after EFTR[10]. In addition, we have improved the EFTR procedure by incorporating dental floss and a hemoclip, which facilitate countertraction. This improvement enables better visualization of the submucosal layer, resulting in a reduced incidence of adverse events in the gastric fundus[11].
Similar to the data from our previous studies, EFTR in the gastric cardia had a 100% en bloc and 98.6% complete resection rate. The complete resection rate was higher than STER (98.6% vs 91.0%, P < 0.05). Complete ER of SETs is the key to ensuring successful operation and avoiding recurrence[26]. Upper gastrointestinal SETs are composed primarily of leiomyomas and GISTs[27]. GISTs have greater potential for malignancy and should be completely resected. An irregular shape and larger size have been shown to be the risk factors for STERs having piecemeal resection[28]. Most esophageal SETs are regular while the majority of cardial SETs are irregular and lobulated[8], which makes it more difficult for STERs to be completely resected. Additionally, tumor resection by STER is limited by the diameter of the tunnel. Therefore, oversized tumors cannot be completely resected. Due to the anatomy, the tunnel has a turn at the cardia, which can easily lead to compression of the tumor and tumor rupture. Conversely, EFTR allows full-thickness excision of the complete gastrointestinal wall without a diameter limit and the risk for poor resection margins and residual tumor which increases the accuracy of histopathology measurement to direct future therapy[29]. In a meta-analysis including 952 G-SETs treated with EFTR, it was suggested that EFTR was a highly effective therapeutic option for removing deep G-SETs, with an R0 resection achieved in 99.3% of cases[30]. Therefore, for lesions that are highly suspicious for GIST or lesions that are not clearly diagnosed, EFTR should be recommended to ensure completed resection.
In our study, EFTR had a shorter procedure time than STER (44.63 ± 28.66 vs 53.36 ± 27.34 min, P < 0.05). Chen revealed that STER was relatively difficult and time consuming when used to resect gastric SETs because of limited space in the established submucosal tunnel[7]. They found that an irregular shape and large size were also risk factors for procedures requiring a long operative time. Therefore, irregular shaped and larger sized SETs were more suitable for EFTR. Additionally, compared to STER, EFTR required the creation of a tunnel. Also, there was no risk of the tumor being too large to rupture in the tunnel, which kept the tumor intact.
The key of the EFTR procedure is the successful closure of the wall defect after resection to prevent peritonitis and the need for surgical intervention[10]. Although a growing body of evidence has demonstrated that gastrointestinal defects after ER can be effectively managed by endoscopy, the closure of large gastrointestinal defects is still technically demanding for most endoscopists[31,32]. Several clips can close small defects. When the diameter of the defect is larger than the width of the open clip, and before applying metallic clips, the defect can first be reduced by air suction using the “suction-clip-suture” method[10]. If the defect is too large to be closed only by clips, a few new techniques have been used in the stomach, such as nylon loop suturing and the over-the-scope clip[33]. In our study, 53.5% patients underwent closure of the defect with clips. 40.8% patients had defect closure using metallic clips with an endoloop. Four patients with large defects had covered, retrievable self-expandable metallic stents used to close the defect. As we our previous study showed, no leakage occured[33].
There are also having several limitations. The study was retrospective and was conducted at a single institution. Thus, prospective study will be needed to further verify our views. Additionally, gastric EFTR was first performed our center and center has many experienced surgeons in the field. It may be difficult for other hospitals with less experience to carry out the procedure.
In conclusion, EFTR was demonstrated to be a very promising method with which to facilitate complete resection and reduce operation time compared to STER in gastric cardia SETs. GISTs were the most common type of SETs in the cardia. When lesions with a high index of suspicion for GIST are found or there is an unclear diagnosis, EFTR should be recommended to ensure complete resection. EFTR by experienced surgeons was shown to be the better option in cases of gastric cardia SETs > 2 cm or with extraluminal growth.
The endoscopic full-thickness resection (EFTR) of cardiac subepithelial tumors (SETs) is still difficult.
To evaluate the safety and efficacy of EFTR for the treatment of gastric cardia SETs.
The objective was the comparison of treatment outcomes between EFTR and submucosal tunnel endoscopic resection (STER), and the factors of difficult of EFTR.
We retrospectively analyzed the data of all patients with SET originating from the muscularis propria of the gastric cardia who underwent EFTR or STER from November 2014 to May 2022 at Zhongshan Hospital Fudan University.
Gastrointestinal stromal tumors were the most common SET. The EFTR group had a higher complete resection rate than the STER group (98.6% vs 91.0%, P < 0.05) and the procedure time was also shorter in the EFTR group (44.63 ± 28.66 min vs 53.36 ± 27.34, P < 0.05). There was no significant difference in total complications between the two groups (21.1% vs 22.0%, P = 0.89).
Compared to STER, EFTR for gastric cardia SETs is also safe and effective.
For patients with suspected cardia gastrointestinal stromal tumor, EFTR can be used to achieve better complete resection. Of course, subsequent prospective studies should be conducted for verification this opinion.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Langner C, Austria; Milone M, Italy S-Editor: Lin C L-Editor: A P-Editor: Xu ZH
| 1. | Cheng HL, Lee WJ, Lai IR, Yuan RH, Yu SC. Laparoscopic wedge resection of benign gastric tumor. Hepatogastroenterology. 1999;46:2100-2104. [PubMed] |
| 2. | Lee HH, Hur H, Jung H, Jeon HM, Park CH, Song KY. Analysis of 151 consecutive gastric submucosal tumors according to tumor location. J Surg Oncol. 2011;104:72-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Akahoshi K, Oya M, Koga T, Shiratsuchi Y. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24:2806-2817. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 180] [Cited by in RCA: 237] [Article Influence: 33.9] [Reference Citation Analysis (9)] |
| 4. | Ko SY, Lee JS, Kim JJ, Park SM. Higher incidence of gastroesophageal reflux disease after gastric wedge resections of gastric submucosal tumors located close to the gastroesophageal junction. Ann Surg Treat Res. 2014;86:289-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Kim SG, Eom BW, Yoon H, Kook MC, Kim YW, Ryu KW. Necessity of Individualized Approach for Gastric Subepithelial Tumor Considering Pathologic Discrepancy and Surgical Difficulty Depending on the Gastric Location. J Clin Med. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 6. | Xu MD, Cai MY, Zhou PH, Qin XY, Zhong YS, Chen WF, Hu JW, Zhang YQ, Ma LL, Qin WZ, Yao LQ. Submucosal tunneling endoscopic resection: a new technique for treating upper GI submucosal tumors originating from the muscularis propria layer (with videos). Gastrointest Endosc 2012; 75: 195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 237] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 7. | Chen T, Zhou PH, Chu Y, Zhang YQ, Chen WF, Ji Y, Yao LQ, Xu MD. Long-term Outcomes of Submucosal Tunneling Endoscopic Resection for Upper Gastrointestinal Submucosal Tumors. Ann Surg 2017; 265: 363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 8. | Du C, Chai N, Linghu E, Gao Y, Li Z, Li L, Zhai Y, Lu Z, Meng J, Tang P. Treatment of cardial submucosal tumors originating from the muscularis propria layer: submucosal tunneling endoscopic resection versus endoscopic submucosal excavation. Surg Endosc 2018; 32: 4543-4551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Sharzehi K, Sethi A, Savides T. AGA Clinical Practice Update on Management of Subepithelial Lesions Encountered During Routine Endoscopy: Expert Review. Clin Gastroenterol Hepatol. 2022;20:2435-2443.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 64] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 10. | Zhou PH, Yao LQ, Qin XY, Cai MY, Xu MD, Zhong YS, Chen WF, Zhang YQ, Qin WZ, Hu JW, Liu JZ. Endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors originated from the muscularis propria. Surg Endosc. 2011;25:2926-2931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 11. | Li B, Shi Q, Qi ZP, Yao LQ, Xu MD, Lv ZT, Yalikong A, Cai SL, Sun D, Zhou PH, Zhong YS. The efficacy of dental floss and a hemoclip as a traction method for the endoscopic full-thickness resection of submucosal tumors in the gastric fundus. Surg Endosc. 2019;33:3864-3873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Cai MY, Martin Carreras-Presas F, Zhou PH. Endoscopic full-thickness resection for gastrointestinal submucosal tumors. Dig Endosc. 2018;30 Suppl 1:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 13. | Ni L, Liu X, Wu A, Yu C, Zou C, Xu G, Wang C, Gao X. Endoscopic full-thickness resection with clip- and snare-assisted traction for gastric submucosal tumours in the fundus: A single-centre case series. Oncol Lett. 2023;25:151. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Nishida T, Kawai N, Yamaguchi S, Nishida Y. Submucosal tumors: comprehensive guide for the diagnosis and therapy of gastrointestinal submucosal tumors. Dig Endosc. 2013;25:479-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 15. | Kim SY, Kim KO. Management of gastric subepithelial tumors: The role of endoscopy. World J Gastrointest Endosc. 2016;8:418-424. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Song S, Feng M, Zhou H, Liu M, Sun M. Submucosal Tunneling Endoscopic Resection for Large and Irregular Submucosal Tumors Originating from Muscularis Propria Layer in Upper Gastrointestinal Tract. J Laparoendosc Adv Surg Tech A. 2018;28:1364-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Ye LP, Zhang Y, Mao XL, Zhu LH, Zhou X, Chen JY. Submucosal tunneling endoscopic resection for small upper gastrointestinal subepithelial tumors originating from the muscularis propria layer. Surg Endosc. 2014;28:524-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 110] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 18. | Gong W, Xiong Y, Zhi F, Liu S, Wang A, Jiang B. Preliminary experience of endoscopic submucosal tunnel dissection for upper gastrointestinal submucosal tumors. Endoscopy. 2012;44:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 19. | Chen H, Xu Z, Huo J, Liu D. Submucosal tunneling endoscopic resection for simultaneous esophageal and cardia submucosal tumors originating from the muscularis propria layer (with video). Dig Endosc. 2015;27:155-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Liu BR, Song JT, Kong LJ, Pei FH, Wang XH, Du YJ. Tunneling endoscopic muscularis dissection for subepithelial tumors originating from the muscularis propria of the esophagus and gastric cardia. Surg Endosc. 2013;27:4354-4359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 21. | Li QY, Meng Y, Xu YY, Zhang Q, Cai JQ, Zheng HX, Qing HT, Huang SL, Han ZL, Li AM, Huang Y, Zhang YL, Zhi FC, Cai RJ, Li Y, Gong W, Liu SD. Comparison of endoscopic submucosal tunneling dissection and thoracoscopic enucleation for the treatment of esophageal submucosal tumors. Gastrointest Endosc. 2017;86:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 22. | Werner YB, Rösch T. POEM and Submucosal Tunneling. Curr Treat Options Gastroenterol. 2016;14:163-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Zhang Q, Cai JQ, Xiang L, Wang Z, de Liu S, Bai Y. Modified submucosal tunneling endoscopic resection for submucosal tumors in the esophagus and gastric fundus near the cardia. Endoscopy. 2017;49:784-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Wang S, Shen L. Efficacy of Endoscopic Submucosal Excavation for Gastrointestinal Stromal Tumors in the Cardia. Surg Laparosc Endosc Percutan Tech. 2016;26:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Jang YS, Lee BE, Kim GH, Park DY, Jeon HK, Baek DH, Kim DU, Song GA. Factors Associated With Outcomes in Endoscopic Submucosal Dissection of Gastric Cardia Tumors: A Retrospective Observational Study. Medicine (Baltimore). 2015;94:e1201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Wang S, Luo H, Shen L. Clinical Efficacy of Single-Channel Gastroscopy, Double-Channel Gastroscopy, and Double Gastroscopy for Submucosal Tumors in the Cardia and Gastric Fundus. J Gastrointest Surg. 2020;24:1307-1313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Hong JB, Choi CW, Kim HW, Kang DH, Park SB, Kim SJ, Kim DJ. Endoscopic resection using band ligation for esophageal SMT in less than 10 mm. World J Gastroenterol. 2015;21:2982-2987. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Mao XL, Ye LP, Zheng HH, Zhou XB, Zhu LH, Zhang Y. Submucosal tunneling endoscopic resection using methylene-blue guidance for cardial subepithelial tumors originating from the muscularis propria layer. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Li J, Tang J, Lua GW, Chen J, Shi X, Liu F, Li Z. Safety and efficacy of endoscopic submucosal dissection of large (≥3 cm) subepithelial tumors located in the cardia. Surg Endosc. 2017;31:5183-5191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Granata A, Martino A, Ligresti D, Tuzzolino F, Lombardi G, Traina M. Exposed endoscopic full-thickness resection without laparoscopic assistance for gastric submucosal tumors: A systematic review and pooled analysis. Dig Liver Dis. 2022;54:729-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 31. | Yamamoto Y, Uedo N, Abe N, Mori H, Ikeda H, Kanzaki H, Hirasawa K, Yoshida N, Goto O, Morita S, Zhou P. Current status and feasibility of endoscopic full-thickness resection in Japan: Results of a questionnaire survey. Dig Endosc. 2018;30 Suppl 1:2-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Zhang Y, Peng JB, Mao XL, Zheng HH, Zhou SK, Zhu LH, Ye LP. Endoscopic resection of large (≥ 4 cm) upper gastrointestinal subepithelial tumors originating from the muscularis propria layer: a single-center study of 101 cases (with video). Surg Endosc. 2021;35:1442-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Zhang Y, Yao L, Xu M, Berzin TM, Li Q, Chen W, Hu J, Wang Y, Cai M, Qin W, Xu J, Huang Y, Zhou P. Treatment of leakage via metallic stents placements after endoscopic full-thickness resection for esophageal and gastroesophageal junction submucosal tumors. Scand J Gastroenterol. 2017;52:76-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |