Published online Dec 15, 2023. doi: 10.4251/wjgo.v15.i12.2064
Peer-review started: July 6, 2023
First decision: October 9, 2023
Revised: October 17, 2023
Accepted: November 10, 2023
Article in press: November 10, 2023
Published online: December 15, 2023
Processing time: 160 Days and 15.4 Hours
Members of the transient receptor potential (TRP) protein family shape oncogenic development, but the specific relevance of TRP-related genes in hepatocellular carcinoma (HCC) has yet to be defined.
To investigate the role of TRP genes in HCC, their association with HCC deve
HCC patient gene expression and clinical data were downloaded from The Cancer Genome Atlas database, and univariate and least absolute shrinkage and selection operator Cox regression models were employed to explore the TRP-related risk spectrum. Based on these analyses, clinically relevant TRP family genes were selected, and the association between the key TRP canonical type 1 (TRPC1) gene and HCC patient prognosis was evaluated.
In total, 28 TRP family genes were screened for clinical relevance, with multiva
These three TRP genes help determine HCC patient prognosis, providing insight into tumor immune status and immunological composition. These findings will help design combination therapies including immunotherapeutic and anti-TRP agents.
Core Tip: The most common form of primary liver cancer is hepatocellular carcinoma (HCC). People with chronic liver conditions, such as cirrhosis caused by hepatitis B or hepatitis C infection, are most likely to develop HCC. Although the predictive value of transient receptor potential (TRP)-related genes in HCC is unknown, TRP family gene proteins influence tumor progression. Our current study assessed the family-related TRP factors to establish the prognosis and treatment plan for HCC.
- Citation: Mei XC, Chen Q, Zuo S. Transient receptor potential-related risk model predicts prognosis of hepatocellular carcinoma patients. World J Gastrointest Oncol 2023; 15(12): 2064-2076
- URL: https://www.wjgnet.com/1948-5204/full/v15/i12/2064.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i12.2064
Liver cancer is the sixth most prevalent cancer globally, with 840000 diagnoses and 780000 deaths annually[1-3]. Rising incidence rates and poor survival rates for hepatocellular carcinoma (HCC) patients in China[4,5] have made it a leading type of primary liver malignancy[6,7]. As HCC tumors exhibit a prolonged latency period and are generally not symptomatic during the early stages of disease, many patients already present with advanced or metastatic disease upon initial diagnosis[8-10]. Thus, the identification of new biomarkers capable of guiding the prognostic evaluation of HCC patients is critical to improve clinical outcomes for this deadly disease.
Ion channels play essential roles in the maintenance of cellular physiology and responsivity, and their dysregulation is common in cancers and other diseases. Proteins in the transient receptor potential (TRP) channel family function as non-selective cationic channels on the cell surface involved in regulating calcium homeostasis and signaling[11-13]. The regulation of calcium stores is directly associated with how tumor cells, including HCC cells, proliferate[14], migrate, invade[15], and tolerate drug treatment[16]. TRP family genes have previously been implicated in the pathogenesis of diseases and cancers, including breast[17], pancreatic[18], ovarian[19], and prostate malignancies[20].
HCC has a high infiltration of immune cells, and immune responses and immune checkpoints are co-stimulators or co-suppressors required for an immune response to take place in HCC tumor cells. HCC is comprised of multiple cells, including tumor cells and non-tumor cells, with non-tumor cells containing regulatory factors, such as cytokines, growth factors, and certain hormones, which make up the tumor microenvironment (TME). Most immune cells, including macrophages, are critical for initiating tumor immune responses, such as immune surveillance, immune self-stabilization, and immune regulation. Wu et al[21] demonstrated important roles for TRP family gene members in pan-cancer analyses, indicating an intricate association between these genes and the TME. Takahashi et al[16] additionally demonstrated the ability of nuclear factor erythroid 2-related factor 2 to promote the upregulation of TRP cation channel, subfamily A, member 1 (TRPA1), thus promoting oxidative stress within tumor cells, and such efforts to target TRPA1 may represent a viable means of treating certain cancers.
At present, systematic bioinformatics-based studies of TRP family genes are lacking in the HCC research field. Accordingly, the present study was designed to comprehensively explore the prognostic relevance of TRP family genes in HCC by utilizing data from public databases, with further analyses aimed at the exploration of biological processes through which these genes may impact HCC patient prognosis to provide an effective foundation for the individualized treatment of HCC patients.
The Cancer Genome Atlas (TCGA), https://portal.gdc.cancer.gov/, a project developed by the National Cancer Institute and the National Human Genome Research Institute, provides transcriptomic and clinical data on patients with many different cancer types. In the present study, we queried TCGA-HCC patient data and used TCGAbiolinks to download the quantitative gene expression data and clinical information of HCC patients in TCGA database. From these data, the expression data of the 28 TRP family genes were extracted for subsequent analyses.
Gene expression data of HCC patients (370 cases) and related clinical information were downloaded from TCGA website for further analyses. All statistical analyses were performed using R. Logistic regression and receiver operating characteristic (ROC) methods were used to analyze the relationship between clinicopathological features and TRP family genes. The Kaplan-Meier method and Cox regression analysis were used to determine the clinicopathologic characteristics associated with overall survival (OS) of patients in TCGA database. Critical values for TRP family gene expression were based on median expression values. Kaplan-Meier curves were used to estimate the effect of 28 TRP family genes on OS in HCC patients. Based on the optimal segregation results, patients were categorized into TRP family gene low expression and TRP family gene high expression groups. P < 0.05 were considered statistically significant.
The STRING database (https://string-db.org/) enables the analysis of complex interactions among proteins, and Cytoscape is an open-source bioinformatics tool for creating and visualizing molecular interaction networks. A confidence level of 0.4 was used to construct protein-protein interaction (PPI) network maps for the identified differential genes of the TRP family. Cytoscape software was then used to visualize and construct the interaction network map, and the key gene modules in the network map were screened using the Molecular Complex Detection (MCODE) plug-in, which identifies the key gene modules in the network. The following settings were used in MCODE: Degree cutoff of 2, node score cutoff of 0.2, K-core of 2, and maximum depth of 10024.
To examine the prognosis of patients with HCC in TCGA dataset, a Cox proportional hazards regression model was constructed. Initially, HCC patient prognosis-related TRP family genes were screened through one-way Cox regression analyses with a significance threshold of P < 0.05 using the survival package in R. A least absolute shrinkage and selection operator (LASSO) regression analysis was then implemented using the glmnet package in R to eliminate any overfitted genes included within this model. A prognostic nomogram was further generated based on a multifactorial Cox proportional risk regression analysis.
Patients with HCC were separated into two groups according to whether they were above or below the median risk score value (high risk and low risk) in an effort to clarify the prognostic relevance of this risk score. Patients’ OS was then analyzed with Kaplan-Meier curves. Moreover, the sensitivity and specificity of column plots were estimated based on the area under the ROC curve (AUC), with an AUC > 0.06 considered indicative of good predictive validity.
The rms package in R was utilized to generate a nomogram incorporating clinical characteristics and risk score values related to patient outcomes in an effort to establish a tool capable of predicting HCC patient prognosis. The accuracy and predictive performance of this model were assessed using calibration curves.
The cell-type identification by estimating relative subsets of RNA transcripts (CIBERSORT) anti-convolution algorithm is a machine learning method based on linear support vector regression to assess the percentage of 22 immune cells in a tissue or cell. We used the CIBERSORT deconvolution method to simulate the transcriptionally characterized substrates of 22 immune cells including B cells, plasma cells, T cells, natural killer (NK) cells, monocytes, macrophages, dendritic cells (DC), mast cells, eosinophils, and neutrophils. The immune cell infiltration of the TRP family gene-expressing HCC patient samples was compared to normal samples to investigate the relationship between TRP family genes and the infiltration of 22 immune cell types in HCC patients in the high- and low-risk groups.
The Huh7, HepG2, MHCC-97H, and LM3 HCC cell lines, as well as the control immortalized LO2 hepatic epithelial cell line, were cultured in Dulbecco’s Modified Eagle Medium (Hyclone, Logan, UT, United States) containing 10 g/L fetal bovine serum (Gibco, Waltham, MA, United States) and 1 g/L penicillin/streptomycin in a 37 ℃ 5% CO2 incubator. A Mycoprobe Mycoplasma Detection Kit (R&D Systems, Minneapolis, MN, United States) was used to confirm that cells were mycoplasma-free.
HCC cells with good cell growth were collected and lysed using radioimmunoprecipitation assay buffer, and protein concentration was determined using a bicinchoninic acid protein assay kit. Proteins were separated using sodium dodecyl sulfate polyacrylamide gel electrophoresis and then transferred to polyvinylidene difluoride membranes. The membranes were blocked in blocking buffer for 1 h and then incubated overnight with the following primary antibodies: Anti-TRP canonical type 1 (TRPC1) (ab192091; Abcam, Cambridge, MA, United States) and anti-GAPDH (10494-1-AP; Proteintech, Rosemont, IL, United States). Then the membranes were incubated with secondary antibodies. The protein bands were visualized using enhanced chemical reagents and analyzed with ImageJ software (V1.8.0.0).
Following treatment with actinomycin D (1 g/mL) for the appropriate intervals, the RNAiso Plus Kit (Cat# 108-95-2; Takara Bio, Beijing, China) was used to extract total cellular RNA. The RT Reagent Kit (Cat# RR047A, Takara Bio; Cat# KR211-02, TIANGEN Biotech, Beijing, China) was used to prepare the cDNA, and quantitative PCR (qPCR) reactions were performed with the FastStart Universal SYBR Green Master Mix (Cat# 04194194001, Roche, Basel, Switzerland; Cat# FP411-02, TIANGEN Biotech) and the Applied Biosystems 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA, United States). The following primers were used: TRPC1 forward, 5’-AAACGGGGATTATTAGTAG-3’; TRPC1 reverse, 5’-TATCTTCTTACAGGTGCT-3’; GAPDH forward, 5’-GGAGCGAGATCCCTCCAAAAT-3’; and GAPDH reverse, 5’-GGCTGTTGTCATACTTCTCATGG-3’.
Tissue sections were deparaffinized using xylene, rehydrated using an ethanol gradient, and incubated for 20 min in sodium citrate buffer (pH 6.0) at 95 ℃, followed by treatment for 20 min each with 3% hydrogen peroxide and 10% goat serum in phosphate-buffered saline (PBS) + 0.2% Tween. Sections were probed overnight at 4 ℃ with an appropriate primary antibody (ab110837; Abcam) in 5 g/L goat serum, washed with PBS, incubated for 40 min at room temperature with secondary antibodies, and developed using 3,3-diaminobenzidine tetrahydrochloride. All tissue samples were obtained from patients under the approval of the Ethical Review Committee of the Affiliated Hospital of Guizhou Medical University (Research Ethics Committee, 2021039; Guizhou, China), with patients providing written informed consent (Supplementary Table 1). Two pathologists independently scored all tissue and immunohistochemistry (IHC) sections based on the staining intensity (0, negative; 1, weak; 2, moderate; and 3, strong) and positive staining area (0, 10%; 1, 10%-25%; 2, 26%-50%; 3, 50%-75%; and 4, 75%-100%). These scores were multiplied to yield a final score, with high expression indicated by a score ≥ 6.
R 3.6.2 was used for the statistical analyses, with differences in prognosis between low- and high-risk groups being assessed via the Kaplan-Meier method. P < 0.05 was the significance threshold.
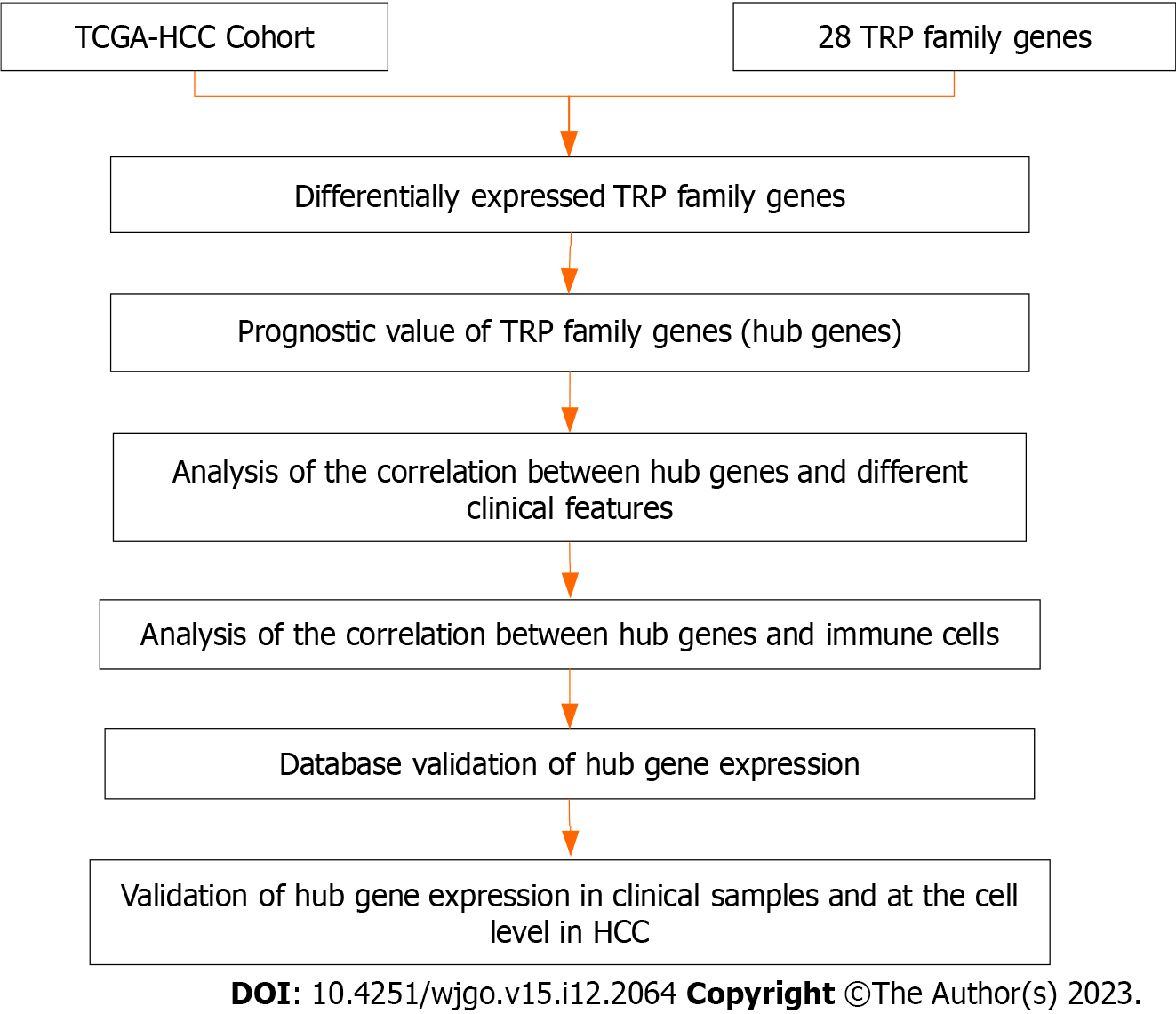
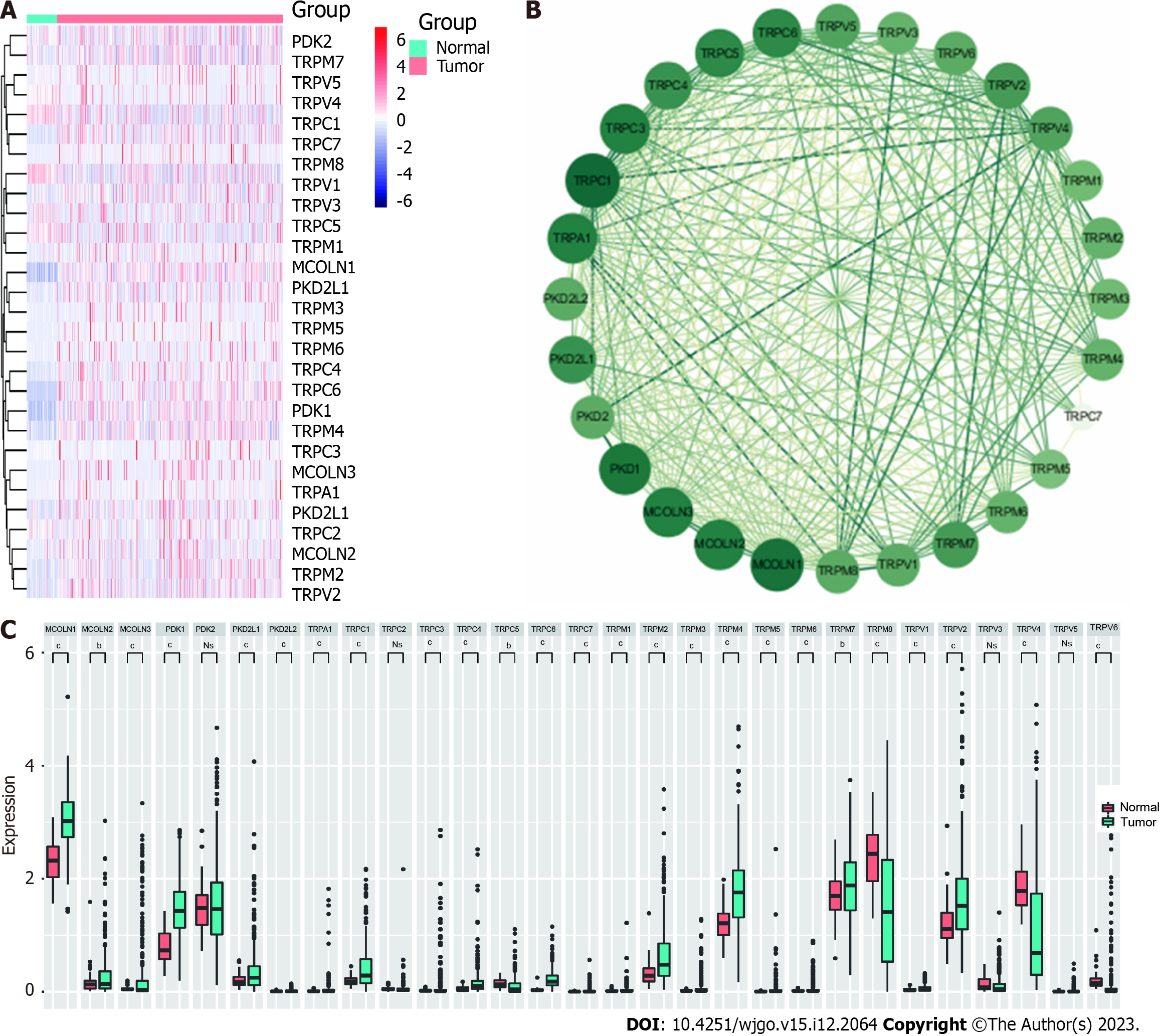
A flow chart outlining the present study process is provided in Figure 1. In total, 28 TRP family genes were retrieved from the literature and TCGA database (Supplementary Table 1). The limma package in R was used to analyze TCGA-HCC patient RNA sequencing data with the goal of assessing the expression of these TRP genes in 374 HCC samples and 50 normal control tissue samples (Figure 2A). Among the 28 TRP family genes, only polycystin-2, TRP cation channel subfamily C member 2, TRP vanilloid-3, and TRP vanilloid-6 were not differentially expressed in HCC tumor tissues and adjacent tissues, but 24 TRP family genes were differentially expressed in cancer tissues and adjacent paracancerous tissues of HCC patients (Figure 2B). A PPI network was then constructed to assess interactions among these genes (Figure 2C), and additional details regarding the roles and scores of these genes are provided in Supplementary Table 2. These results highlighted the important roles that TRP family genes may play as regulators of key processes in HCC patients.
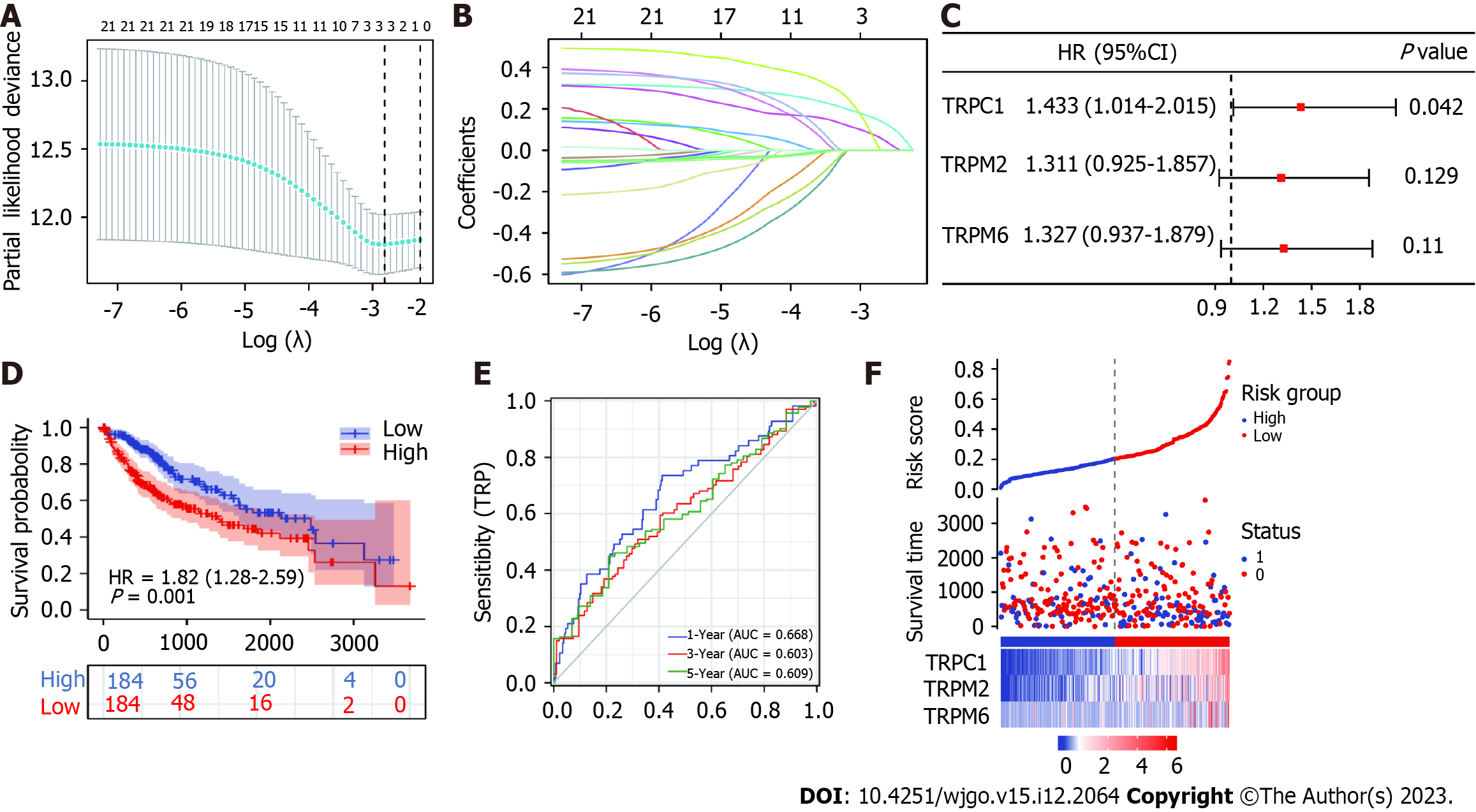
To identify prognosis-related TRP family-related genes, we performed Cox regression modeling using the LASSO algorithm on the 24 differentially expressed genes between HCC patients and normal healthy controls. Using the best λ parameter in the 10-fold validation, three TRP family-related genes, namely, TRPC1, TRP cation channel subfamily M member 2 (TRPM2), and TRP cation channel subfamily M member 2 (TRPM6), were selected (Figure 3A and B) As expected, univariate Cox analysis showed that some of the 24 genes were associated with OS in HCC patients, and multifactorial regression analysis indicated that TRPC1 was one of the independent prognostic factors in HCC patients (Figure 3C). A risk score was computed using the following model: Risk score = (TRPC1 × 0.156495) + (TRPM2 × 0.075215) + (TRPM6 × 0.058265). Median risk score values were then used to stratify patients into low and high-risk groups, with Kaplan-Meier curves revealing a shorter OS among high-risk patients compared to low-risk individuals (P = 0.001; Figure 3D). ROC curve analyses demonstrated the accuracy of this risk scoring model (Figure 3E), and risk score distributions for these HCC patients together with their survival status are presented in Figure 3F. These results suggested that TRPC1 may serve as a valuable prognostic marker in individuals diagnosed with HCC either alone or in a 3-TRP gene signature.
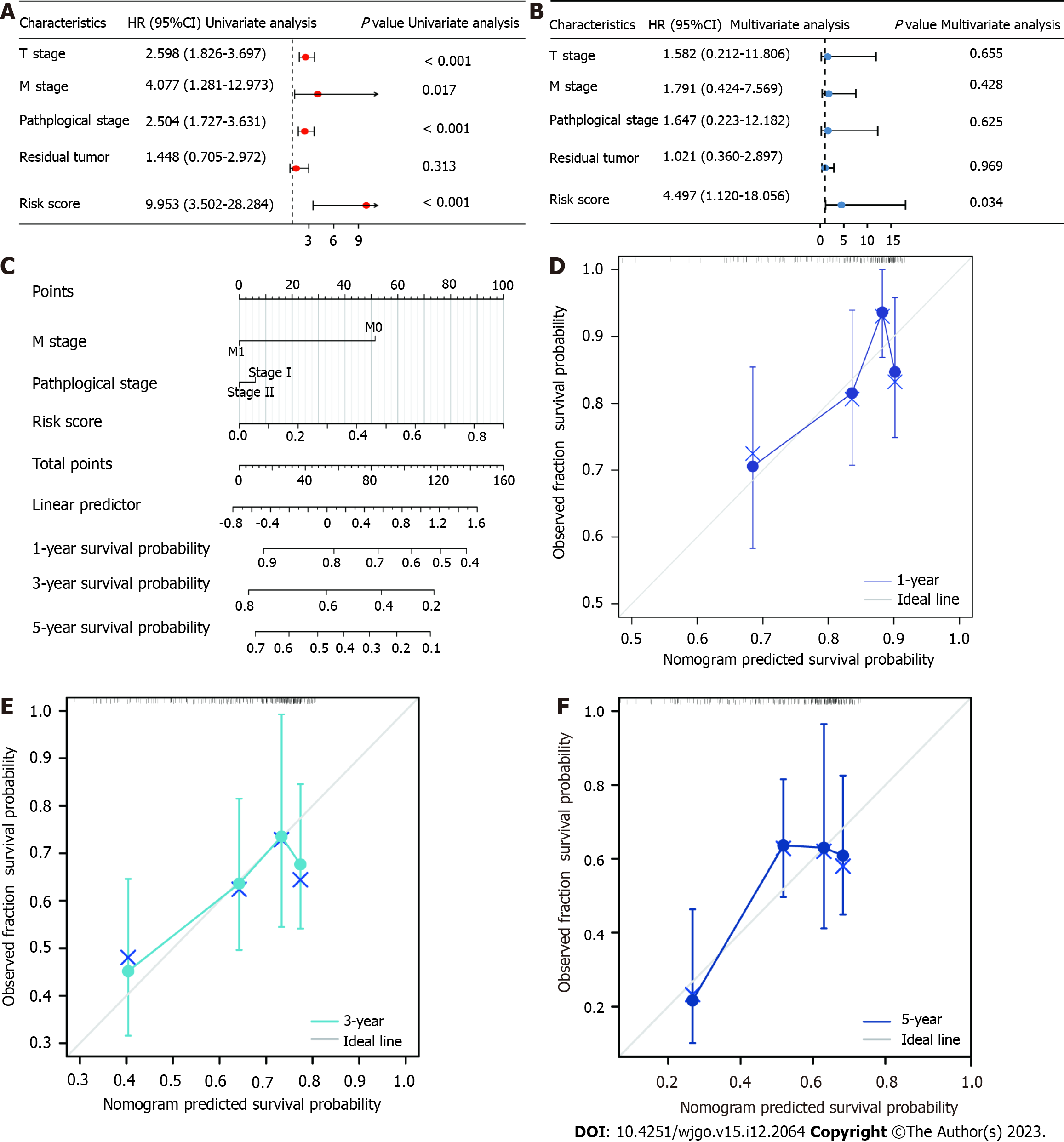
The ability of the developed 3-TRP gene signature to serve as a predictor of HCC patient OS independent of other clinical characteristics (T stage, M stage, pathological grade, residual tumor, and risk score) was examined through univariate and multivariate Cox regression approaches. Factors significantly associated with patient OS in the univariate analyses included T stage, M stage, pathological grade, and risk score values (P < 0.05) (Figure 4A), while only risk score values remained independently associated with OS in multivariate analyses of TCGA patient cohort (Figure 4B). These results suggested that the developed risk scoring model can be utilized as a tool to independently predict HCC patient disease outcomes. A nomogram was additionally constructed with the rms package in R, which incorporated this 3-TRP gene signature-based risk score and other clinical characteristics to gauge survival odds (Figure 4C). In this model, higher scores were indicative of a poorer prognosis. Calibration curves revealed good consistency of actual patient 1-, 3-, and 5-year OS with that predicted by this nomogram (Figure 4D-F). Therefore, this predictive model is a valuable tool for the evaluation of HCC patient long-term prognosis.
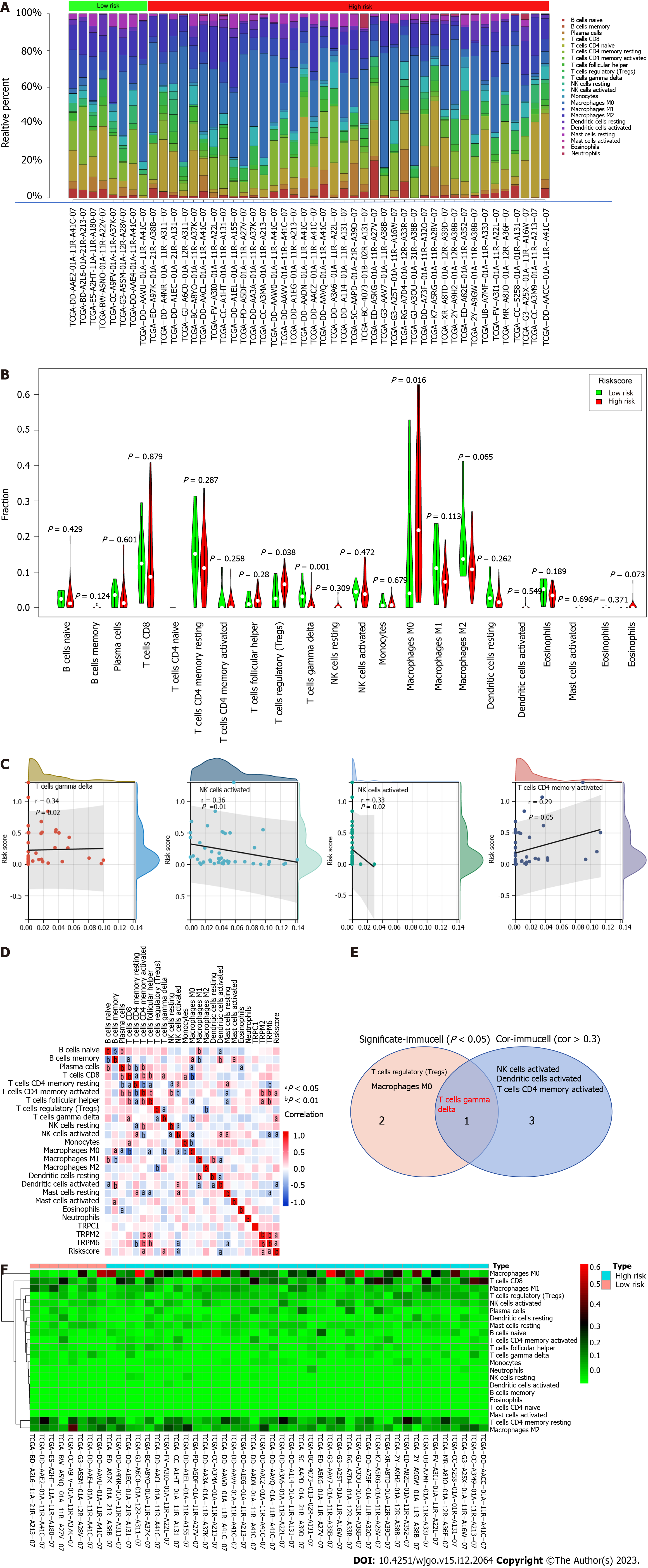
To fully explore immune cell distributions and their relationships with established risk groups in individuals with HCC, the CIBERSORT algorithm was employed to approximate tumor infiltration of 22 different immune cell types and the relationship between such infiltration and TRP family genes in TCGA HCC patient cohort and corresponding control samples. The degree of immune cell infiltration was higher in the high-risk group than in the low-risk group, especially in γ-δ T cells and M0 macrophages (Figure 5A and B). We further analyzed the correlation of risk scores with the degree of immune cell infiltration. Core TRP family genes were closely correlated with the infiltration of important immune cell types including T cells, NK cells, DCs, and memory T cells (|cor > 0.3|) (Figure 5C and D). γ-δ T cells (P = 0.02, r = 0.34) and activated CD4 memory T cells (P = 0.05, r = 0.29) were positively correlated with the risk scores, whereas activated NK cells (P = 0.01, r = 0.36) and activated DCs (P = 0.02, r = 0.33) were negatively correlated with the risk scores (Figure 5C and D). Of these cell types, only T cells exhibited both a significant correlation with TRP family genes and differential infiltration levels between sample types (Figure 5E). Thus, these data indicated that core TRP family genes are closely related to immune cell infiltration, with individuals in the TRP-based high-risk group exhibiting upregulation of most immune-related cell activities (Figure 5F).
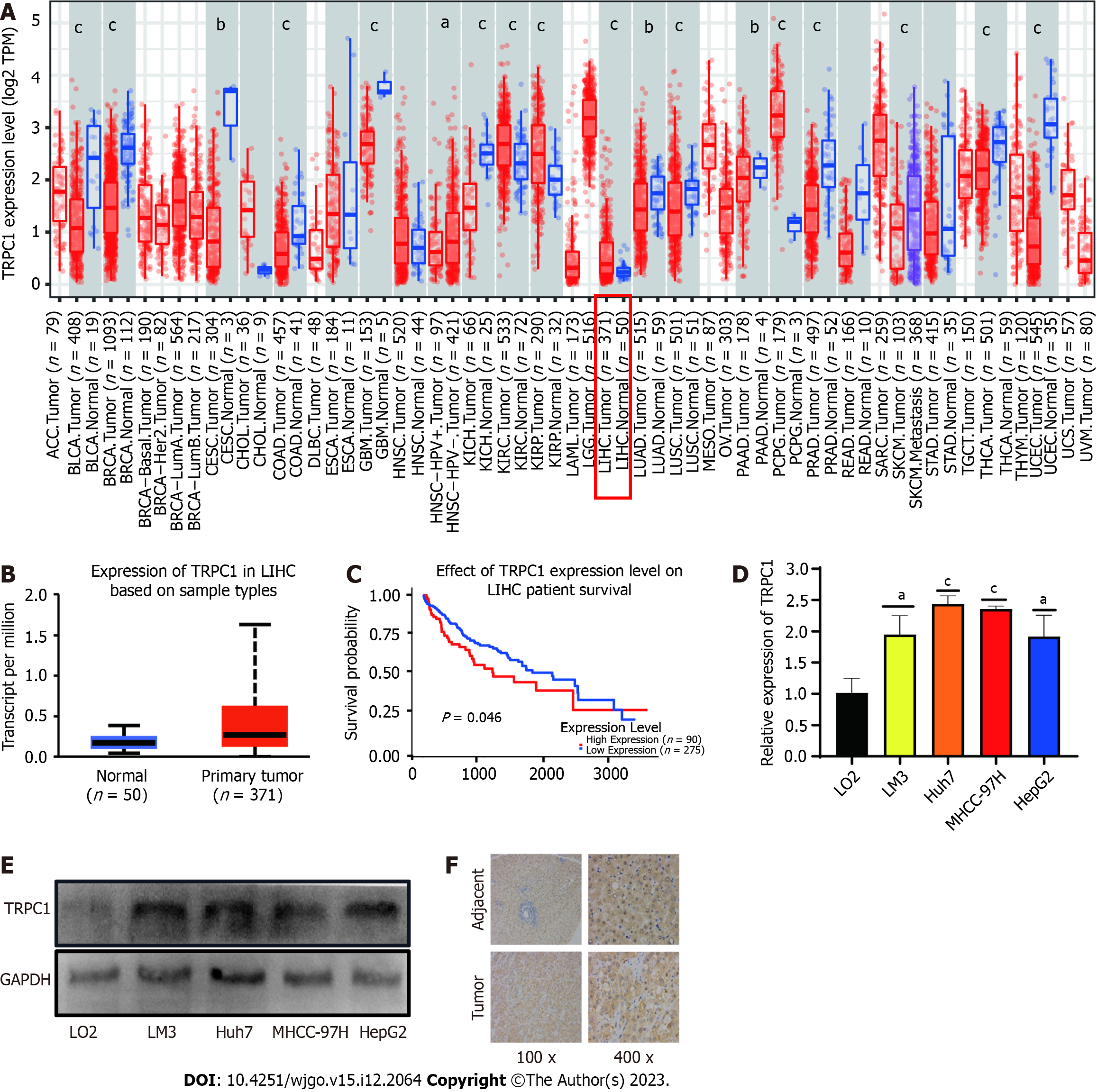
While TRPC1, TRPM2, and TRPM6 were identified as core TRP family genes potentially associated with HCC pathogenesis, no significant differences in HCC patient prognosis were observed as a function of TRPM2 or TRPM6 expression. As such, TRPC1 expression and prognostic significance were further explored in greater detail. Initial pan-cancer analyses highlighted high levels of TRPC1 expression in most tumor types relative to corresponding normal tissue controls, including HCC (Figure 6A). Consistently, significantly higher levels of TRPC1 expression were detected in liver cancer samples relative to paired paracancerous tissues (Figure 6B), and the HCC patients exhibiting higher TRPC1 expression levels also had a poorer prognosis (Figure 6C). To independently confirm these results, qPCR and western blot analyses were conducted using the LM3, Huh7, MHCC-97H, and HepG2 HCC cell lines, as well as immortalized liver epithelial cells. In line with patient data, TRPC1 expression at the mRNA and protein levels was increased in HCC cells compared to epithelial controls (Figure 6D and E). Additional tissue samples were also collected from liver cancer patients at the Guizhou Medical University Affiliated Hospital and used for IHC staining, confirming that TRPC1 expression was upregulated in HCC patient tumors relative to adjacent healthy tissue (Figure 6F). In summary, HCC patients who have elevated TRPC1 expression have poorer prognostic outcomes.
Unlike many other gene families, TRP family members have been largely overlooked in oncological studies despite their strong associations with the onset and progression of other disease types[22-24]. Here, TRP family gene expression profiles were analyzed for the first time in HCC, underscoring potentially important roles for these genes as mediators of oncogenesis. Moreover, TRPC1 expression was experimentally confirmed to be dysregulated in HCC, suggesting that this protein may be a viable target for pharmaceutical intervention.
TCGA database is widely utilized throughout the world to study patterns of gene expression across many tumor types[25,26]. In the present study, 28 TRP family genes were selected, and their expression levels were analyzed in TCGA-HCC cohort. The majority of these genes were upregulated in HCC, and multivariate Cox regression analysis demonstrated that three of these genes (TRPC1, TRPM2, and TRPM6) were related to patient OS. These genes were then incorporated into a LASSO regression analysis-based predictive model. The TME is closely related to tumor progression in HCC and many other malignancies[27-29], and TRP channels facilitate communication between cells. These ion channels may thus influence immune cell activity in the TME, potentially facilitating tumor immune evasion[30-32]. Correlation analyses indicated that TRP family gene expression was closely correlated with the intratumoral infiltration of many immune cell types. Survival analyses further highlighted TRPC1 as being closely tied to HCC patient outcomes, prompting further analysis of this gene as a core TRP gene in subsequent analyses.
TRP channels function as critical non-selective cation channels related to the onset of a range of tumor types[33,34]. TRPC1 was herein identified as the most relevant member of this gene family in HCC. In colorectal cancer, TRPC1 has been reported to be upregulated, and TRPC1 knockdown suppresses the in vivo growth of tumors, highlighting its functional importance in this form of cancer[35]. To the best of our knowledge, the present study was the first to assess the expression of TRPC1 in HCC based on TCGA database. The present results provided clear evidence in support of pronounced TRPC1 upregulation in HCC as determined through both qPCR and western blot analyses of tumors and paracancerous samples. IHC staining yielded similar results, supporting the identification of TRPC1 as a candidate oncogene in the development of HCC.
While these results offer a valuable new foundation for studies focused on the association between TRPC1 and HCC, these findings are subject to certain limitations. First, only TCGA database was analyzed in the present study, potentially introducing some degree of sample bias into the results. Additional studies that include more HCC patient samples and additional clinical parameters will be conducted in the future to enhance the accuracy of these findings. Second, experimental evidence explaining the mechanistic role of TRPC1 as a regulator of HCC progression is currently lacking and warrants further study. Although these results offered new perspectives regarding the role of TRP family genes in HCC, more work is necessary to clarify the underlying mechanisms and the potential value of TRPC1 as a target for pharmacological intervention.
In summary, the present comprehensive study of the part of TRP-related genes led to the successful establishment of a TRP-related gene signature associated with HCC patient outcomes. Moreover, TRPC1 was identified as a potential oncogenic driver and candidate target for therapeutic intervention in this cancer type.
The most typical form of primary liver cancer is hepatocellular carcinoma (HCC). People with chronic liver conditions, such as cirrhosis caused by hepatitis B or hepatitis C infection, are most likely to develop HCC. Although the predictive value of TRP-related genes in HCC is unknown, transient receptor potential (TRP) family gene proteins influence tumor progression. Our current study aimed to assess the family-related TRP factors to establish the prognosis and treatment plan for HCC. We downloaded the mRNA expression profiles and corresponding clinical information for the HCC patients from the cancer genome atlas (TCGA) database. Univariate and least absolute contraction and selection operator (LASSO) Cox regression models were used to construct the TRP risk spectrum, infer the clinically significant TRP family core genes, and examine the correlation between the core gene TRP canonical type 1 (TRPC1) and the expression and prognosis of HCC. Our findings propose that the predictive characteristics of the 3-TRP gene discussed in this study are not only effective for prognosis prediction but also related to the tumor's immune status and the infiltration of various immune cells in the tumor microenvironment. These results may provide significant clinical indications for HCC patients to propose a new combination therapy consisting of targeted anti-TRP treatment and immunotherapy.
To investigate the role of TRP genes in HCC, their association with HCC development and treatment was examined.
To investigate the role of TRP genes in HCC, their association with HCC development and treatment was examined.
HCC patient gene expression and clinical data were downloaded from The Cancer Genome Atlas database, and univariate and LASSO Cox regression models were employed to explore the TRP-related risk spectrum. Based on these analyses, clinically relevant TRP family genes were selected, and the association between the key TRPC1 gene and HCC patient prognosis was evaluated.
In total, 28 TRP family genes were screened for clinical relevance, with multivariate analyses ultimately revealing three of these genes (TRPC1, TRP cation channel subfamily M member 2, and TRP cation channel subfamily M member 6) to be significantly associated with HCC patient prognosis (P < 0.05). These genes were utilized to establish a TRP-related risk model. Patients were separated into low and high-risk groups based on the expression of these genes, and high-risk patients exhibited a significantly poorer prognosis (P = 0.001). Functional analyses highlighted pronounced differences in the immune status of patients in these two groups and associated enriched immune pathways. TRPC1 was identified as a candidate gene in this family worthy of further study, with HCC patients expressing higher TRPC1 levels exhibiting poorer survival outcomes. Consistently, quantitative, immunohistochemistry, and western blot analyses revealed increased TRPC1 expression in HCC.
These three TRP genes help determine HCC patient prognosis, providing insight into tumor immune status and immunological composition. These findings will help design combination therapies including immunotherapeutic and anti-TRP agents.
In the future, we will focus on in-depth studies on the mechanism of how TRPC1 regulates the development of HCC.
We would like to thank all our colleagues in the Department of Hepatobiliary Surgery and Organ Transplantation of the Affiliated Hospital of Guizhou Medical University for their help.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elshimi E, Egypt; Hori T, Japan; Moldogazieva NT, Russia S-Editor: Qu XL L-Editor: Filipodia P-Editor: Zhao S
| 1. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11936] [Article Influence: 2984.0] [Reference Citation Analysis (4)] |
| 2. | Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F. Cancer statistics for the year 2020: An overview. Int J Cancer. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2411] [Cited by in RCA: 2968] [Article Influence: 742.0] [Reference Citation Analysis (7)] |
| 3. | Lee S, Kang TW, Song KD, Lee MW, Rhim H, Lim HK, Kim SY, Sinn DH, Kim JM, Kim K, Ha SY. Effect of Microvascular Invasion Risk on Early Recurrence of Hepatocellular Carcinoma After Surgery and Radiofrequency Ablation. Ann Surg. 2021;273:564-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 217] [Article Influence: 54.3] [Reference Citation Analysis (0)] |
| 4. | Chidambaranathan-Reghupaty S, Fisher PB, Sarkar D. Hepatocellular carcinoma (HCC): Epidemiology, etiology and molecular classification. Adv Cancer Res. 2021;149:1-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 557] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 5. | Kim E, Viatour P. Hepatocellular carcinoma: old friends and new tricks. Exp Mol Med. 2020;52:1898-1907. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 6. | Lin YL, Li Y. Study on the hepatocellular carcinoma model with metastasis. Genes Dis. 2020;7:336-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 7. | Liu J, Geng W, Sun H, Liu C, Huang F, Cao J, Xia L, Zhao H, Zhai J, Li Q, Zhang X, Kuang M, Shen S, Xia Q, Wong VW, Yu J. Integrative metabolomic characterisation identifies altered portal vein serum metabolome contributing to human hepatocellular carcinoma. Gut. 2022;71:1203-1213. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 8. | Wang J, Li CD, Sun L. Recent Advances in Molecular Mechanisms of the NKG2D Pathway in Hepatocellular Carcinoma. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Gentile D, Donadon M, Lleo A, Aghemo A, Roncalli M, di Tommaso L, Torzilli G. Surgical Treatment of Hepatocholangiocarcinoma: A Systematic Review. Liver Cancer. 2020;9:15-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 10. | Hashimoto A, Sarker D, Reebye V, Jarvis S, Sodergren MH, Kossenkov A, Sanseviero E, Raulf N, Vasara J, Andrikakou P, Meyer T, Huang KW, Plummer R, Chee CE, Spalding D, Pai M, Khan S, Pinato DJ, Sharma R, Basu B, Palmer D, Ma YT, Evans J, Habib R, Martirosyan A, Elasri N, Reynaud A, Rossi JJ, Cobbold M, Habib NA, Gabrilovich DI. Upregulation of C/EBPα Inhibits Suppressive Activity of Myeloid Cells and Potentiates Antitumor Response in Mice and Patients with Cancer. Clin Cancer Res. 2021;27:5961-5978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 11. | Zhao Y, McVeigh BM, Moiseenkova-Bell VY. Structural Pharmacology of TRP Channels. J Mol Biol. 2021;433:166914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 12. | Zhong T, Zhang W, Guo H, Pan X, Chen X, He Q, Yang B, Ding L. The regulatory and modulatory roles of TRP family channels in malignant tumors and relevant therapeutic strategies. Acta Pharm Sin B. 2022;12:1761-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 73] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Diver MM, Lin King JV, Julius D, Cheng Y. Sensory TRP Channels in Three Dimensions. Annu Rev Biochem. 2022;91:629-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 14. | Farfariello V, Gordienko DV, Mesilmany L, Touil Y, Germain E, Fliniaux I, Desruelles E, Gkika D, Roudbaraki M, Shapovalov G, Noyer L, Lebas M, Allart L, Zienthal-Gelus N, Iamshanova O, Bonardi F, Figeac M, Laine W, Kluza J, Marchetti P, Quesnel B, Metzger D, Bernard D, Parys JB, Lemonnier L, Prevarskaya N. TRPC3 shapes the ER-mitochondria Ca(2+) transfer characterizing tumour-promoting senescence. Nat Commun. 2022;13:956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 49] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 15. | Xing Y, Wei X, Liu Y, Wang MM, Sui Z, Wang X, Zhu W, Wu M, Lu C, Fei YH, Jiang Y, Zhang Y, Wang Y, Guo F, Cao JL, Qi J, Wang W. Autophagy inhibition mediated by MCOLN1/TRPML1 suppresses cancer metastasis via regulating a ROS-driven TP53/p53 pathway. Autophagy. 2022;18:1932-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 99] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 16. | Takahashi N, Chen HY, Harris IS, Stover DG, Selfors LM, Bronson RT, Deraedt T, Cichowski K, Welm AL, Mori Y, Mills GB, Brugge JS. Cancer Cells Co-opt the Neuronal Redox-Sensing Channel TRPA1 to Promote Oxidative-Stress Tolerance. Cancer Cell. 2018;33:985-1003.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 200] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 17. | Saldías MP, Maureira D, Orellana-Serradell O, Silva I, Lavanderos B, Cruz P, Torres C, Cáceres M, Cerda O. TRP Channels Interactome as a Novel Therapeutic Target in Breast Cancer. Front Oncol. 2021;11:621614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 18. | Mesquita G, Prevarskaya N, Schwab A, Lehen'kyi V. Role of the TRP Channels in Pancreatic Ductal Adenocarcinoma Development and Progression. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Wang X, Li G, Zhang Y, Li L, Qiu L, Qian Z, Zhou S, Wang X, Li Q, Zhang H. Pan-Cancer Analysis Reveals Genomic and Clinical Characteristics of TRPV Channel-Related Genes. Front Oncol. 2022;12:813100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 20. | Günther T, Konrad M, Stopper L, Kunert JP, Fischer S, Beck R, Casini A, Wester HJ. Optimization of the Pharmacokinetic Profile of [(99m)Tc]Tc-N(4)-Bombesin Derivatives by Modification of the Pharmacophoric Gln-Trp Sequence. Pharmaceuticals (Basel). 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 21. | Wu G, He M, Yin X, Wang W, Zhou J, Ren K, Chen X, Xue Q. The Pan-Cancer Landscape of Crosstalk Between TRP Family and Tumour Microenvironment Relevant to Prognosis and Immunotherapy Response. Front Immunol. 2022;13:837665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Fang C, Xu H, Liu Y, Huang C, Wang X, Zhang Z, Xu Y, Yuan L, Zhang A, Shao A, Lou M. TRP Family Genes Are Differently Expressed and Correlated with Immune Response in Glioma. Brain Sci. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 23. | Jimenez I, Prado Y, Marchant F, Otero C, Eltit F, Cabello-Verrugio C, Cerda O, Simon F. TRPM Channels in Human Diseases. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 24. | Pan F, Wang K, Zheng M, Ren Y, Hao W, Yan J. A TRP Family Based Signature for Prognosis Prediction in Head and Neck Squamous Cell Carcinoma. J Oncol. 2022;2022:8757656. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Poore GD, Kopylova E, Zhu Q, Carpenter C, Fraraccio S, Wandro S, Kosciolek T, Janssen S, Metcalf J, Song SJ, Kanbar J, Miller-Montgomery S, Heaton R, Mckay R, Patel SP, Swafford AD, Knight R. Microbiome analyses of blood and tissues suggest cancer diagnostic approach. Nature. 2020;579:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 718] [Article Influence: 143.6] [Reference Citation Analysis (0)] |
| 26. | Kuzu OF, Noory MA, Robertson GP. The Role of Cholesterol in Cancer. Cancer Res. 2016;76:2063-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 504] [Article Influence: 56.0] [Reference Citation Analysis (0)] |
| 27. | Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 664] [Cited by in RCA: 5018] [Article Influence: 1672.7] [Reference Citation Analysis (0)] |
| 28. | Peng S, Xiao F, Chen M, Gao H. Tumor-Microenvironment-Responsive Nanomedicine for Enhanced Cancer Immunotherapy. Adv Sci (Weinh). 2022;9:e2103836. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 221] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 29. | Downs-Canner SM, Meier J, Vincent BG, Serody JS. B Cell Function in the Tumor Microenvironment. Annu Rev Immunol. 2022;40:169-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 167] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 30. | Kwiatkowska I, Hermanowicz JM, Przybyszewska-Podstawka A, Pawlak D. Not Only Immune Escape-The Confusing Role of the TRP Metabolic Pathway in Carcinogenesis. Cancers (Basel). 2021;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Parenti A, De Logu F, Geppetti P, Benemei S. What is the evidence for the role of TRP channels in inflammatory and immune cells? Br J Pharmacol. 2016;173:953-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 32. | Partida-Sanchez S, Desai BN, Schwab A, Zierler S. Editorial: TRP Channels in Inflammation and Immunity. Front Immunol. 2021;12:684172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 33. | Bertin S, Raz E. Transient Receptor Potential (TRP) channels in T cells. Semin Immunopathol. 2016;38:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 34. | Huang T, Xu T, Wang Y, Zhou Y, Yu D, Wang Z, He L, Chen Z, Zhang Y, Davidson D, Dai Y, Hang C, Liu X, Yan C. Cannabidiol inhibits human glioma by induction of lethal mitophagy through activating TRPV4. Autophagy. 2021;17:3592-3606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 129] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 35. | Sun Y, Ye C, Tian W, Ye W, Gao YY, Feng YD, Zhang HN, Ma GY, Wang SJ, Cao W, Li XQ. TRPC1 promotes the genesis and progression of colorectal cancer via activating CaM-mediated PI3K/AKT signaling axis. Oncogenesis. 2021;10:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |