Published online Nov 15, 2023. doi: 10.4251/wjgo.v15.i11.1988
Peer-review started: August 24, 2023
First decision: September 11, 2023
Revised: September 19, 2023
Accepted: September 27, 2023
Article in press: September 27, 2023
Published online: November 15, 2023
Processing time: 83 Days and 12 Hours
Gastric cancer (GC) is a malignant tumor with a high incidence and mortality rate worldwide for which acute bleeding is a common clinical complication. Gastroscopic hemostasis is an important method for treating acute bleeding in GC; however, its efficacy and safety remain controversial.
To systematically analyze the efficacy and safety of gastroscopic hemostasis for the treatment of acute gastric hemorrhage.
The PUBMED, Web of Science, Wiley Library, EMBASE, Wanfang, CNKI, and VIP databases were searched for studies related to gastroscopic hemostatic treatment for acute GC published through February 20, 2023. The literature was screened according to the inclusion and exclusion criteria, data were extracted, and lite
Six randomized controlled trials and two retrospective analyses were retrieved. Five studies had a low, two had an uncertain, and one had a high risk of bias. Compared with the control group, the hemostatic rate of gastroscopic hemostasis was increased [relative risk (RR) = 1.24; 95% confidence interval (CI): 1.08 to 1.43; P = 0.003]; the rate of rebleeding (RR = 0.27; 95%CI: 0.09 to 0.80; P = 0.02), rate of surgery transfer (RR = 0.16; 95%CI: 0.06 to 0.43; P = 0.0003), serum C-reactive protein level [mean difference (MD) = -5.16; 95%CI: -6.11 to 4.21; P < 0.00001], interleukin-6 level (MD = -6.37; 95%CI: -10.33 to -2.42; P = 0.002), and tumor necrosis factor-α level (MD = -2.29; 95%CI: -4.06 to -0.52; P = 0.01) were decreased; and the quality of life improvement rate was increased (RR = 1.95; 95%C I= 1.41-2.71; P < 0.0001). Begg’s test revealed no significant publication bias.
The efficacy and safety of endoscopic hemostasis were higher than those of the control group, suggesting that it is an effective treatment for acute GC hemorrhage.
Core Tip: This meta-analysis provides a wealth of evidence emphasizing the effectiveness and safety of endoscopic hemostasis for treating acute gastrointestinal bleeding in patients with gastric cancer (GC). Compared with the control group, endoscopic hemostasis effectively controlled acute gastric bleeding in GC while significantly reducing the bleeding and transfer rates, indicating its efficacy at treating patients with acute gastric bleeding in GC. Nevertheless, further high-quality clinical research is required to confirm the safety and efficacy of endoscopic hemostasis in the treatment of acute GC bleeding.
- Citation: Pan HY, Wang XW, He QX, Lu YD, Zhang WY, Jin JW, Lin B. Efficacy and safety of gastroscopic hemostasis in the treatment of acute gastric hemorrhage: A meta-analysis. World J Gastrointest Oncol 2023; 15(11): 1988-1997
- URL: https://www.wjgnet.com/1948-5204/full/v15/i11/1988.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i11.1988
Gastric cancer (GC) is a malignant tumor with one of the highest prevalence and mortality rates among all cancers worldwide. Acute hemorrhage from GC is a serious life-threatening complication[1-3]. GC-related bleeding accounts for an estimated 1%-8% of acute upper gastrointestinal bleeding cases[4,5]. Effective hemostatic treatment is crucial for patients with acute hemorrhage due to GC, as it can reduce the risk of mortality. Emergency gastroscopy can be used for rapid intervention, bleeding assessment, the identification of bleeding sources, and hemostatic treatment[6,7]. Thus, gastroscopic hemostasis is the preferred treatment for acute GC bleeding[8], although little evidence supports the use of endoscopic hemostatic treatment for acute GC bleeding.
The 30 d mortality rate of gastrointestinal bleeding in advanced GC after endoscopic hemostatic treatment is approximately 15.9%-43% higher than that of other causes of gastrointestinal bleeding[9-11]. However, some studies have shown that emergency gastroscopy can improve the detection rate of bleeding causes and aid the assessment of the risk of rebleeding and hemostatic effects[12,13]. The efficacy and safety of endoscopic hemostasis for the treatment of acute bleeding from GC remain controversial. Siau et al[14] reported that early gastroscopy could increase the risk of rebleeding. However, a recent study that included a large number of patients with GC (n = 45) reported a fairly low success rate for endoscopic hemostasis (31%). In the remaining 69% of GC bleeding cases, transarterial embolization was used to save patients after gastroscopy failed[15]. Therefore, this study aimed to systematically evaluate the efficacy and safety of gastroscopic hemostasis in the treatment of acute GC hemorrhage using a meta-analysis to objectively and accurately investigate this question.
The PUBMED, Web of Science, Wiley Library, EMBASE, Wanfang, China National Knowledge Network, and VIP databases were searched for studies published from the inception of each database through February 20, 2023. The reference lists of all retrieved articles were manually searched to identify any other relevant studies.
The search used a combination of subjects and free words. The following English keywords and their Chinese counterparts were used in the search: Gastroscopy, emergency gastroscopy, hemostasis, gastric cancer, GC, acute bleeding, bleeding, and curative effects.
The study inclusion criteria were as follows: (1) Randomized controlled study or retrospective analysis of gastroscopic hemostasis for the treatment of acute GC bleeding; (2) subjects including GC patients with acute gastric bleeding; (3) patients in the experimental group were treated with gastroscopy hemostasis, while patients in the control group were treated with conventional drugs; and (4) outcome indexes were successful hemostasis, rebleeding rate, transfer rate, serum C-reactive protein (CRP), interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and quality of life improvement, including at least one outcome measure.
The study exclusion criteria were as follows: (1) Study subjects included individuals with GC accompanied by acute hemorrhage; (2) experimental group underwent endoscopic hemostasis treatment for managing bleeding, while the control group received conventional medication for hemostasis; (3) publication language not Chinese or English; (4) incomplete or missing data; and (5) duplicate publication.
The literature was searched according to the specified search strategy and screened using the preferred reporting items for systematic reviews and meta-analyses flow chart. The literature was screened separately by two researchers and then crosschecked. In cases of disagreement, a third researcher was invited to participate in the discussions. Two researchers independently extracted the data in accordance with the designated data extraction table, including authors, publication date, country, sample size of the experimental and control groups, patient age, intervention measures, and original outcome data. After the study extraction process, both researchers performed cross-checking and a third researcher ruled out dispute cases.
This randomized controlled study evaluated the quality of the bias risk assessment tools recommended by the Cochrane Handbook and made judgments on the random allocation method, allocation hiding, blinding method, data integrity, selective reporting, and six other items. If all six items were answered “yes,” the study was classified as having a low risk of bias; if all six items were answered “no” or “unclear,” the study was classified as having an uncertain risk of bias; if all items were “no” or “unclear,” the study was classified as having a high risk of bias. The Newcastle-Ottawa Scale (NOS) was used for the retrospective analysis. An NOS score < 5 was classified as a high risk of bias, while a score ≥ 5 was classified as a low risk of bias.
RevMan 5.4 software was used to process the data for the meta-analysis. Risk ratios (RR) and 95% confidence intervals (CI) were used to count the data, and the mean difference (MD) and 95%CI were used for measurement data. The Q test was used for the heterogeneity analysis. Values of P < 0.1 and I2 > 50% indicated interstudy heterogeneity. The random-effects model was used for the meta-analysis in these cases; otherwise, the fixed-effects model was used. Stata 13.0 software was used to perform Begg’s test for the publication bias analysis. Statistical significance was set at P < 0.05.
A total of 1998 papers were preliminarily retrieved from the databases; of them, 253 duplicates were removed using Endnote and NoteExpress, and 1745 papers remained. The title and abstract screening removed 1429 unqualified papers, leaving 316 papers. The full-text review eliminated 273 papers, leaving six randomized controlled trials (RCTs) and two retrospective studies[12,16-22] (Figure 1).
Six RCTs and two retrospective studies were included in this study, including 672 patients (328 in the experimental group, 344 in the control group). General information about the included studies is presented in Table 1. The risk of bias in the included studies was evaluated using the Cochrane Handbook and NOS scale. Five studies had a low risk of bias, two had an uncertain risk of bias, and only one had a high risk of bias (Table 1).
| Ref. | Year of publication | Country | Study type | Experimental group | Control group | Study dates | Risk of bias | Outcomes | ||||||
| Sample size | Age (yr) | Male/female | Intervention methods | Sample size | Age (yr) | Male/female | Intervention methods | |||||||
| Sheibani et al[16] | 2013 | United States | Retrospective analysis | 14 | 57 ± 12 | / | Gastroscopy hemostasis | 18 | 57 ± 12 | / | Drug hemostasis | 2005.1-2012.1 | Low | 1, 2, 3 |
| Zheng[17] | 2017 | China | Retrospective analysis | 30 | 55.1 ± 9.8 | 19/11 | Emergency gastroscopy hemostasis | 47 | 56.2 ± 11.0 | 31/16 | Drug hemostasis | 2011.3-2016.1 | High | 1, 2, 3, 7 |
| Zhang[18] | 2018 | China | RCT | 32 | 55.47 ± 12.31 | 18/14 | Emergency gastroscopy hemostasis | 32 | 56.17 ± 11.62 | 19/13 | Drug hemostasis | 2015.1-2017.3 | Low | 1, 2, 3, 4, 5, 6, 7 |
| Long[19] | 2019 | China | RCT | 36 | 58.95 ± 5.21 | 20/16 | Emergency gastroscopy hemostasis | 36 | 58.75 ± 5.62 | 21/15 | Drug hemostasis | / | Unclear | 1, 2 |
| Qi et al[20] | 2019 | China | RCT | 40 | 56.45 ± 3.23 | / | Emergency gastroscopy hemostasis | 40 | 56.45 ± 3.23 | / | Drug hemostasis | 2017.12-2018.12 | Low | 1 |
| Ren et al[12] | 2021 | China | RCT | 93 | 49 ± 3.01 | 54/38 | Emergency gastroscopy hemostasis | 88 | 47 ± 3.83 | 47/41 | Drug hemostasis | 2018.9-2020.9 | Low | 1, 2 |
| Xiang[21] | 2021 | China | RCT | 34 | 54.45 ± 2.15 | 19/15 | Gastroscopy hemostasis | 34 | 54.63 ± 2.26 | 18/16 | Drug hemostasis | 2019.8-2020.12 | Unclear | 1, 5, 6 |
| Zhang et al[22] | 2021 | China | RCT | 49 | 56.6 ± 4.76 | 31/18 | Gastroscopy hemostasis | 49 | 56.55 ± 4.71 | 29/20 | Drug hemostasis | 2018.10-2019.10 | Low | 3, 4, 5, 6, 7 |
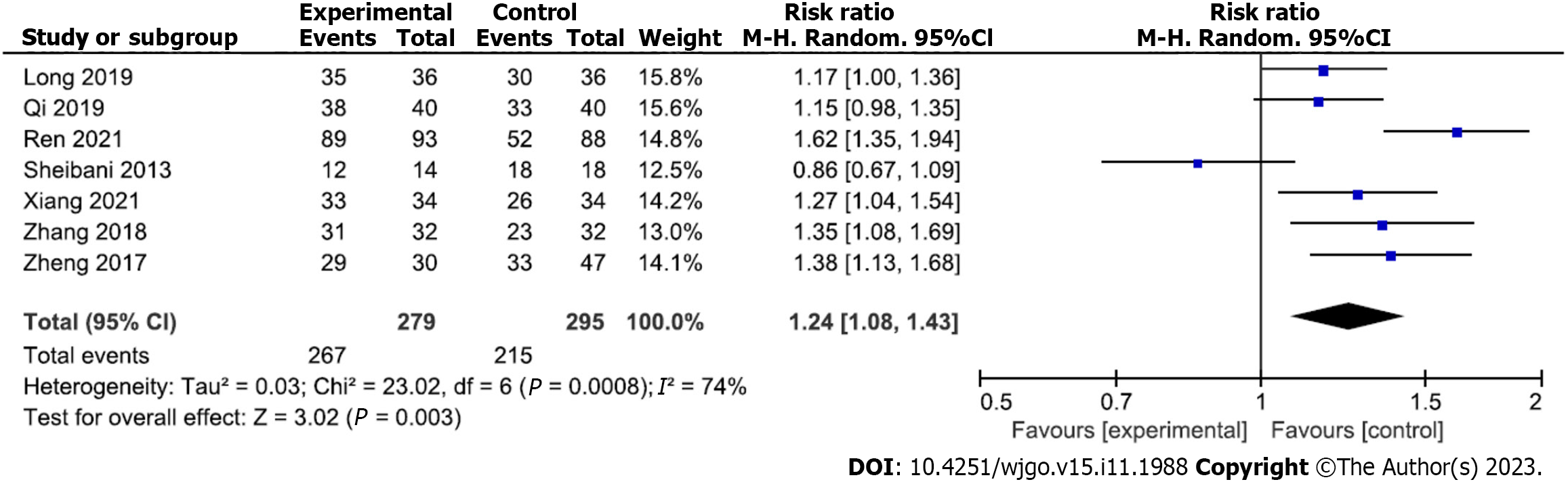
Analysis of hemostasis rate after gastroscopic hemostasis: Analyses of the hemostatic rates of gastroscopic hemostatic treatment were reported in seven studies (267 and 215 patients in the experimental and control groups, respectively). Due to moderate heterogeneity among the included studies (P = 0.0008, I2 = 74%), a random-effects analytical model was adopted. The meta-analysis results showed that the hemostasis rate of the experimental group was significantly higher than that of the control group (RR = 1.24; 95%CI: 1.08 to 1.43; P = 0.003) (Figure 2). Begg’s test found no publication bias among the included studies (P > 0.05).
Analysis of rebleeding rate after gastroscopic hemostasis: Five studies reported analyses of rebleeding rates for gastroscopic hemostatic treatment (207 and 221 patients in the experimental and control groups, respectively). Due to moderate heterogeneity among the included studies (P = 0.004, I2 = 74%), a random-effects model of analysis was adopted. The meta-analysis results showed that the rebleeding rate in the experimental group was significantly lower than that in the control group (RR = 0.27; 95%CI: 0.09 to 0.80; P = 0.02) (Figure 3). Begg’s test found no publication bias among the included studies (P > 0.05).
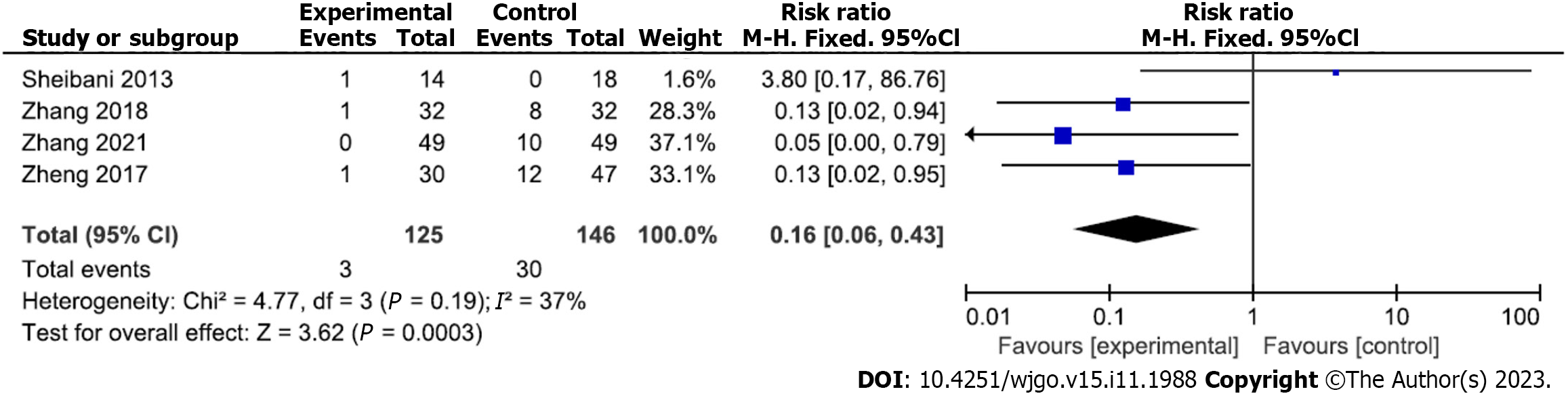
Transfer rate of hemostatic treatment under gastroscopy: Four studies analyzed the transfer rate for gastroscopic hemostatic treatment. A total of 125 and 146 patients were included in the experimental and control groups, respectively. There was no heterogeneity among the included studies (P = 0.19, I2 = 37%); therefore, a fixed-effects analytical model was adopted. The conversion rate of treatment was significantly lower in the experimental than control groups (RR = 0.16; 95%CI: 0.06 to 0.43; P = 0.0003) (Figure 4). Begg’s test found no publication bias among the included studies (P > 0.05).
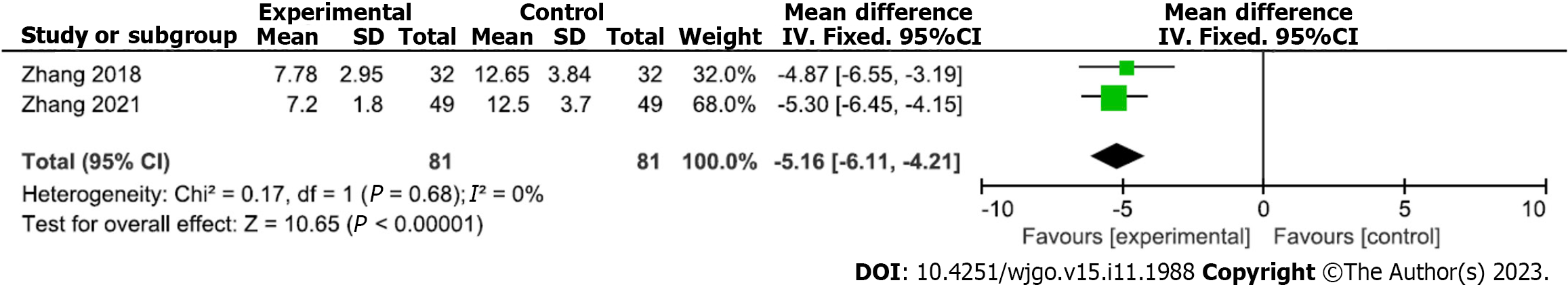
Analysis of serum CRP after gastroscopic hemostasis: Two studies reported analyses of serum CRP levels after gastroscopic hemostatic treatment (81 and 81 patients in the experimental and control groups, respectively). There was no heterogeneity among the included studies (P = 0.68, I2 = 0%); therefore, a fixed-effects analytical model was used. The meta-analysis results showed that mean serum CRP level was significantly lower in the experimental versus control group (MD = -5.16; 95%CI: -6.11 to -4.21; P < 0.00001) (Figure 5). Begg’s test found no publication bias among the included studies (P > 0.05).
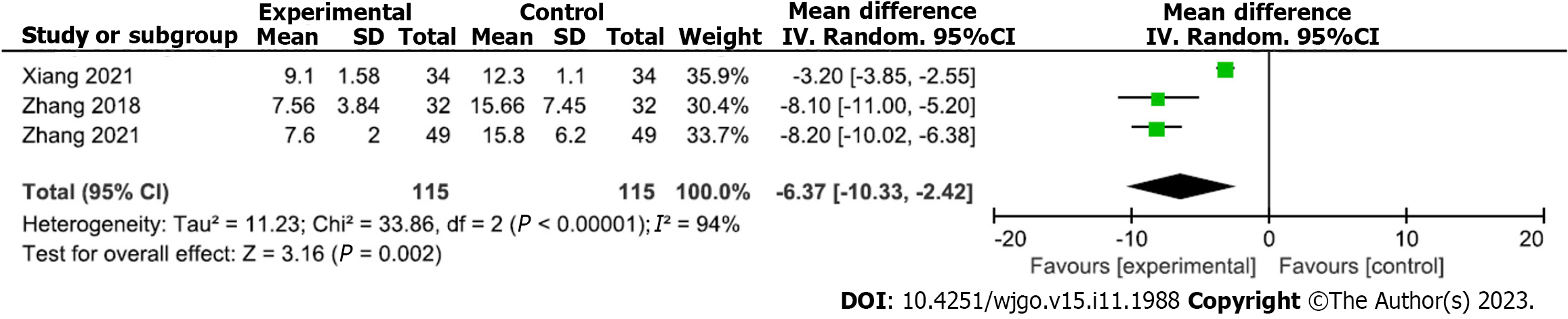
Analysis of serum IL-6 level for hemostatic treatment under gastroscopy: Three studies analyzed serum IL-6 levels after gastroscopic hemostatic treatment (115 and 115 patients in the experimental and control groups, respectively). Heterogeneity was detected among the included studies (P < 0.00001, I2 = 94%); therefore, a random-effects model of analysis was adopted. The meta-analysis results showed that the mean serum IL-6 level after treatment was significantly lower in the experimental versus control group (MD = -6.37; 95%CI: -10.33 to -2.42; P = 0.002) (Figure 6). Begg’s test found no publication bias among the included studies (P > 0.05).
Analysis of serum TNF-α after gastroscopic hemostasis: An analysis of serum TNF-α after gastroscopic hemostasis was reported in three studies (115 and 115 patients in the experimental and control groups, respectively). Heterogeneity existed among the included studies (P < 0.00001, I2 = 98%); therefore, a random-effects analytical model was adopted. The mean serum TNF-α level after treatment was significantly lower in the experimental vs control group (MD = -2.29; 95%CI: -4.06 to 0.52; P = 0.01) (Figure 7). Begg’s test found no publication bias in the included studies (P > 0.05).
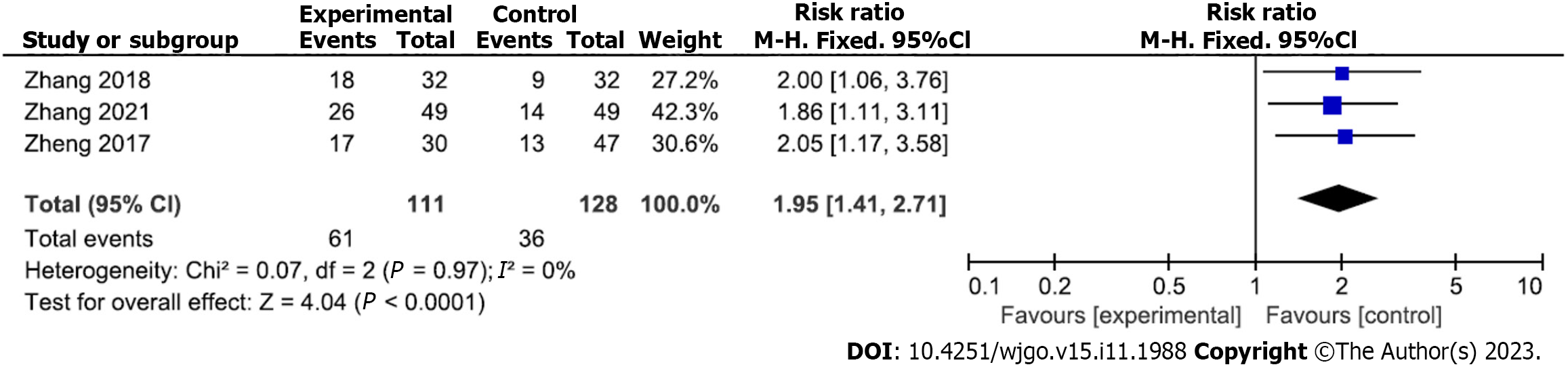
Improvement of quality of life after gastroscopic hemostasis: Three studies analyzed the quality of life improvement rate after gastroscopic hemostasis (111 and 128 patients in the experimental and control groups, respectively). No heterogeneity was noted among the included studies (P = 0.97, I2 = 0%); therefore, a fixed-effects analytical model was adopted. The quality of life improvement rate after treatment was higher in the experimental versus control group (RR = 1.95; 95%CI: 1.41 to 2.71; P < 0.0001) (Figure 8). Begg’s test found no publication bias among the included studies (P > 0.05).
Acute hemorrhage in GC is a common form of gastrointestinal hemorrhage. In response to the high recurrence and malignancy rates of GC[1,6,23-25], great progress has been made in recent years toward improving the diagnostic process and multidisciplinary treatment strategy for resectable GC. However, owing to the high recurrence rate, the patient survival rate is still not ideal[26-28]. Gastroscopic hemostatic treatment can effectively prevent acute bleeding and reduce patient fatality rates, thus aiding the treatment of these patients[12,16]. However, published literature related to the efficacy and safety of endoscopic hemostasis in the treatment of acute GC bleeding is controversial and shows strong differences[14,15]. Therefore, this meta-analysis aimed to evaluate the efficacy and safety of endoscopic hemostasis for the treatment of acute GC-related bleeding by summarizing various studies.
Our results showed that gastroscopic hemostasis could effectively control acute bleeding in GC and that the hemostasis rate was much higher in the treatment vs control group. In addition, the rebleeding and surgical transfer rates were significantly reduced. Thus, endoscopic hemostasis for the treatment of acute bleeding in patients with GC has a relatively high success rate, consistent with previous findings[12,16,29]. In the context of acute bleeding in GC, this meta-analysis particularly emphasizes the elevation of CRP, IL-6, and TNF-α levels[9,12,16,20]. These inflammatory factors play multiple roles in the development of GC. They can promote the proliferation and survival of cancer cells by activating specific signaling pathways such as phosphoinositide 3-kinase/protein kinase B and mitogen-activated protein kinase[30,31]. Moreover, high levels of inflammatory factors promote angiogenesis by providing abundant nutrients to cancer cells[32]. Additionally, the inflammatory microenvironment may locally alter the composition and stiffness of the extracellular matrix locally, thereby facilitating cancer cell invasion and migration[33].
Several studies demonstrated that inflammatory factors play an important role in GC patients with acute bleeding. Serum levels of CRP, IL-6, and TNF-α gradually increase in patients with acute bleeding in GC[18,22,34]. Therefore, when bleeding is controlled, the levels of CRP, IL-6, and TNF-α tend to decrease. Consistent with these findings, our study reached the same conclusion: Serum levels of CRP, IL-6, and TNF-α were significantly lower in the experimental vs control group, indicating that endoscopic hemostasis treatment had better hemostatic control than the control treatment. Moreover, the quality of life of the patients in the endoscopic treatment group improved significantly.
The GC microenvironment is a highly complex biological system that includes tumor cells, immune cells, fibroblasts, various cytokines, and chemical factors[19]. In this environment, immune cells such as tumor-associated macrophages and T cells may further influence the tumor growth dynamics and the risk of bleeding in patients by releasing pro- and anti-inflammatory factors[35]. Some studies found a correlation between high levels of transforming growth factor-β and low levels of interferon-γ with an increased risk of bleeding[9,12]. Although endoscopic hemostasis is widely used to control acute bleeding in GC, the specific molecular mechanisms are not fully understood. In contrast, endoscopic treatment may achieve hemostasis by activating the coagulation cascade, promoting platelet aggregation, and regulating certain inflammatory and coagulation factors[36]. Evidence suggests that these treatment modalities may reduce local inflammatory reactions, thereby improving patient quality of life and prognosis[10,22]. It is worth noting that similar inflammatory and immune responses have been observed in other gastrointestinal cancers such as esophageal and colorectal cancer[37,38]. These observations not only provide valuable perspectives for comparing different types of gastrointestinal cancers, but they also have the potential to reveal common therapeutic strategies for this class of cancer.
The limitations of this study are as follows: (1) Because meta-analyses summarize only published studies, they were limited by the quality of the original studies, and the overall quality of the studies included here was low; (2) this meta-analysis included only published studies, while unpublished studies were not considered; and (3) the number of studies and sample size included here were small. These factors may have affected the accuracy and reliability of the results.
In conclusion, RCTs with larger sample sizes and better quality standards should be conducted to further clarify the safety and effectiveness of gastroscopic hemostasis for the treatment of acute GC bleeding.
Gastric cancer (GC) is a malignant tumor with a high incidence and mortality rate worldwide for which acute bleeding is a common clinical complication.
Gastroscopic hemostasis is an important method for treating acute bleeding in GC; however, its efficacy and safety remain controversial.
This meta-analysis provides a wealth of evidence emphasizing the effectiveness and safety of endoscopic hemostasis for treating acute gastrointestinal bleeding in patients with GC.
Several databases was searched for related to gastroscopic hemostatic treatment for acute GC. The literature was screened according to the inclusion and exclusion criteria, data were extracted, and literature quality was evaluated. The meta-analysis was performed using RevMan software (version 5.3), while Begg’s test for publication bias was performed using Stata 13.0 software.
Compared with the control group, the hemostatic rate of gastroscopic hemostasis was increased [relative risk (RR) = 1.24; 95% confidence interval (CI) = 1.08-1.43; P = 0.003]; the rate of rebleeding (RR = 0.27; 95%CI: 0.09 to 0.80; P = 0.02), rate of surgery transfer (RR = 0.16; 95%CI: 0.06 to 0.43; P = 0.0003), serum C-reactive protein level [mean difference (MD) = -5.16; 95%CI: -6.11 to 4.21; P < 0.00001], interleukin-6 level (MD = -6.37; 95%CI: -10.33 to -2.42; P = 0.002), and tumor necrosis factor-α level (MD=-2.29; 95%CI: -4.06 to -0.52; P = 0.01) were decreased; and the quality of life improvement rate was increased (RR = 1.95; 95%CI: 1.41 to 2.71; P < 0.0001).
The efficacy and safety of endoscopic hemostasis were higher than those of the control group.
Endoscopic hemostasis is an effective treatment for acute GC hemorrhage.
The authors are grateful to the participating researchers for their contributions. We thank the reviewers and editors for their valuable suggestions.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Billeter A, Germany; Choi S, South Korea S-Editor: Qu XL L-Editor: A P-Editor: Zhang XD
| 1. | Zhou XQ, Chang YZ, Shen CY, Han J, Chang RA. Xiaoaiping injection combined with chemotherapy for advanced gastric cancer: An updated systematic review and meta-analysis. Front Pharmacol. 2022;13:1023314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 2. | Yang S, Zou X, Li J, Yang H, Zhang A, Zhu Y, Zhu L, Zhang L. Immunoregulation and clinical significance of neutrophils/NETs-ANGPT2 in tumor microenvironment of gastric cancer. Front Immunol. 2022;13:1010434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 3. | Zhang X, Tsauo J, Tie J, Xue H, Zhao JB, Li JJ, Fang ZT, Guo WH, Li X. Emergent Transjugular Intrahepatic Portosystemic Shunt Creation for Acute Gastric Variceal Bleeding in Patients with Hepatocellular Carcinoma. J Vasc Interv Radiol. 2022;33:702-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 4. | Reitano E, de'Angelis N, Bianchi G, Laera L, Spiliopoulos S, Calbi R, Memeo R, Inchingolo R. Current trends and perspectives in interventional radiology for gastrointestinal cancers. World J Radiol. 2021;13:314-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 5. | Ortega Rodriguez AA, Cohn Reinoso CM, Mateu Esquerda G, de Manuel-Rimbau Muñoz J. Spontaneous acute bleeding within subdural effusion from dural metastasis of gastric cancer: A case report. Neurocirugia (Astur : Engl Ed). 2022;33:340-344. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Zhao YX, Yang Z, Ma LB, Dang JY, Wang HY. Synchronous gastric cancer complicated with chronic myeloid leukemia (multiple primary cancers): A case report. World J Clin Cases. 2022;10:11146-11154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Reference Citation Analysis (0)] |
| 7. | Dai L, Jiang C, Hong D, He X, Zeng X, Li H, Li S, Li D, Wang W. A prospective, open-labeled, and randomized trial for assessing safety and clinical utility of gastric biopsies during emergency gastroscopy for patients with bleeding gastric ulcers. Scand J Gastroenterol. 2023;58:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Khorobrykh TV, Abdulkhakimov NM, Agadzhanov VG, Aghayan DL, Kazaryan AM. Laparoscopic versus open surgery for locally advanced and metastatic gastric cancer complicated with bleeding and/or stenosis: short- and long-term outcomes. World J Surg Oncol. 2022;20:216. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Song IJ, Kim HJ, Lee JA, Park JC, Shin SK, Lee SK, Lee YC, Chung H. Clinical Outcomes of Endoscopic Hemostasis for Bleeding in Patients with Unresectable Advanced Gastric Cancer. J Gastric Cancer. 2017;17:374-383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | Kim YI, Choi IJ, Cho SJ, Lee JY, Kim CG, Kim MJ, Ryu KW, Kim YW, Park YI. Outcome of endoscopic therapy for cancer bleeding in patients with unresectable gastric cancer. J Gastroenterol Hepatol. 2013;28:1489-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Kim YI, Choi IJ. Endoscopic management of tumor bleeding from inoperable gastric cancer. Clin Endosc. 2015;48:121-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 12. | Ren T, Wei J, Han B, Chen X, Zhong J, Lan F. The clinical effect of emergency gastroscopy on upper gastrointestinal hemorrhage patients. Am J Transl Res. 2021;13:3501-3507. [PubMed] [DOI] [Full Text] |
| 13. | Takeda K, Sakayauchi T, Kubozono M, Katagiri Y, Umezawa R, Yamamoto T, Ishikawa Y, Takahashi N, Suzuki Y, Kishida K, Jingu K. Palliative radiotherapy for gastric cancer bleeding: a multi-institutional retrospective study. BMC Palliat Care. 2022;21:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Siau K, Chapman W, Sharma N, Tripathi D, Iqbal T, Bhala N. Management of acute upper gastrointestinal bleeding: an update for the general physician. J R Coll Physicians Edinb. 2017;47:218-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 15. | Koh KH, Kim K, Kwon DH, Chung BS, Sohn JY, Ahn DS, Jeon BJ, Kim SH, Kim IH, Kim SW, Lee SO, Lee ST, Kim DG. The successful endoscopic hemostasis factors in bleeding from advanced gastric cancer. Gastric Cancer. 2013;16:397-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Sheibani S, Kim JJ, Chen B, Park S, Saberi B, Keyashian K, Buxbaum J, Laine L. Natural history of acute upper GI bleeding due to tumours: short-term success and long-term recurrence with or without endoscopic therapy. Aliment Pharmacol Ther. 2013;38:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Zheng C. Clinical efficacy and safety of emergency gastroscopy in the treatment of acute hemorrhage in patients with gastric cancer. Shiyong Aizheng Zazhi. 2017;32:638-640. [DOI] [Full Text] |
| 18. | Zhang JK. Curative effect of emergency gastroscopy on upper gastrointestinal acute hemorrhage caused by gastric cancer. Xiandai Shiyong Yixue. 2018;30:1164-1166. [DOI] [Full Text] |
| 19. | Long P. Clinical effect and safety of emergency gastroscopy in the treatment of acute hemorrhage of gastric cancer. Zhonghua Zhongliu Fangzhi Zazhi. 2019;26:71. |
| 20. | Qi L, Wang C, Wang J. Clinical Effect of Emergency Gastroscopy on Acute Bleeding of Gastric Cancer. Proceedings of Anticancer Research. 2019;3:9-12. |
| 21. | Xiang LQ. Effect analysis of gastroscopic hemostasis in the treatment of advanced gastric cancer with acute bleeding. Dangdai Yiyao Luncong. 2021;19:33-35. [DOI] [Full Text] |
| 22. | Zhang H, Peng B, Zhang CQ, Li ZY, Long C. Clinical efficacy and safety evaluation of emergency gastroscopy in the treatment of acute hemorrhage of gastric cancer. Xiandai Xiaohua Ji Jieru Zhenliao. 2021;26:463-466. |
| 23. | Lee J, Byun HK, Koom WS, Lee YC, Seong J. Efficacy of radiotherapy for gastric bleeding associated with advanced gastric cancer. Radiat Oncol. 2021;16:161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Kamposioras K, Geraghty J, Appleyard J, Dawod M, Papadimitriou K, Lamarca A, Anthoney A. Pancreaticobiliary Malignancies in the Emergency Room: Management of Acute Complications and Oncological Emergencies. J Gastrointest Cancer. 2022;53:1050-1065. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (3)] |
| 25. | Orii T, Karasawa Y, Kitahara H, Yoshimura M, Okumura M. Efficacy of Self-Expandable Metallic Stent Inserted for Refractory Hemorrhage of Duodenal Cancer. Case Rep Gastroenterol. 2016;10:151-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 26. | Huang H, Xu F, Chen Q, Hu H, Qi F, Zhao J. The value of CT-based radiomics nomogram in differential diagnosis of different histological types of gastric cancer. Phys Eng Sci Med. 2022;45:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 27. | Taboada AGM, Lominchar PL, Roman LM, García-Alfonso P, Martin AJM, Rodriguez JAB, Pascual JMA. Advances in neoadjuvant therapy for resectable pancreatic cancer over the past two decades. Ann Hepatobiliary Pancreat Surg. 2021;25:179-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Zhu Z, Gong YB, Xu HM. Neoadjuvant therapy strategies for advanced gastric cancer: Current innovations and future challenges. Chronic Dis Transl Med. 2020;6:147-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Gao DJ, Wang SP, Fu XH, Yin L, Ye X, Yang XW, Zhang YJ, Hu B. Urgent Endoscopy Improves Hemostasis in Patients With Upper Gastrointestinal Bleeding Following Biliary-pancreatic Surgery: A Retrospective Analysis. Surg Laparosc Endosc Percutan Tech. 2021;32:228-235. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Patrad E, Khalighfard S, Amiriani T, Khori V, Alizadeh AM. Molecular mechanisms underlying the action of carcinogens in gastric cancer with a glimpse into targeted therapy. Cell Oncol (Dordr). 2022;45:1073-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 31. | Gao XF, He HQ, Zhu XB, Xie SL, Cao Y. LncRNA SNHG20 promotes tumorigenesis and cancer stemness in glioblastoma via activating PI3K/Akt/mTOR signaling pathway. Neoplasma. 2019;66:532-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Wang Y, Wu H, Deng R. Angiogenesis as a potential treatment strategy for rheumatoid arthritis. Eur J Pharmacol. 2021;910:174500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 64] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 33. | Magdeldin T, López-Dávila V, Pape J, Cameron GW, Emberton M, Loizidou M, Cheema U. Engineering a vascularised 3D in vitro model of cancer progression. Sci Rep. 2017;7:44045. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Navaei-Alipour N, Mastali M, Ferns GA, Saberi-Karimian M, Ghayour-Mobarhan M. The effects of honey on pro- and anti-inflammatory cytokines: A narrative review. Phytother Res. 2021;35:3690-3701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 35. | Choo YW, Kang M, Kim HY, Han J, Kang S, Lee JR, Jeong GJ, Kwon SP, Song SY, Go S, Jung M, Hong J, Kim BS. M1 Macrophage-Derived Nanovesicles Potentiate the Anticancer Efficacy of Immune Checkpoint Inhibitors. ACS Nano. 2018;12:8977-8993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 346] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 36. | Sartim MA, Costa TR, Laure HJ, Espíndola MS, Frantz FG, Sorgi CA, Cintra AC, Arantes EC, Faccioli LH, Rosa JC, Sampaio SV. Moojenactivase, a novel pro-coagulant PIIId metalloprotease isolated from Bothrops moojeni snake venom, activates coagulation factors II and X and induces tissue factor up-regulation in leukocytes. Arch Toxicol. 2016;90:1261-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Men F, Wei L, Liu B, Wu F, Liu J, Guo N, Niu Q. Comparison of the safety of the application of painless gastroscopy and ordinary gastroscopy in chronic hypertension patients combined with early gastric cancer. Oncol Lett. 2018;15:3558-3561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 38. | Xu F, Feng GS, Wang ZJ, Zhang KN. Synchronous double cancers of colonic large cell neuroendocrine carcinoma and gastric squamous-cell carcinoma: a case report and review of literature. Int J Clin Exp Pathol. 2014;7:5177-5180. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |