Published online Jan 15, 2023. doi: 10.4251/wjgo.v15.i1.112
Peer-review started: September 23, 2022
First decision: November 16, 2022
Revised: November 23, 2022
Accepted: December 21, 2022
Article in press: December 21, 2022
Published online: January 15, 2023
Processing time: 109 Days and 5.4 Hours
Peritoneal metastasis (PM) after primary surgery for colorectal cancer (CRC) has the worst prognosis. Prediction and early detection of metachronous PM (m-PM) have an important role in improving postoperative prognosis of CRC. However, commonly used imaging methods have limited sensitivity to detect PM early. We aimed to establish a nomogram model to evaluate the individual probability of m-PM to facilitate early interventions for high-risk patients.
To establish and validate a nomogram model for predicting the occurrence of m-PM in CRC within 3 years after surgery.
We used the clinical data of 878 patients at the Second Hospital of Jilin University, between January 1, 2014 and January 31, 2019. The patients were randomly divided into training and validation cohorts at a ratio of 2:1. The least absolute shrinkage and selection operator (LASSO) regression was performed to identify the variables with nonzero coefficients to predict the risk of m-PM. Multivariate logistic regression was used to verify the selected variables and to develop the predictive nomogram model. Harrell’s concordance index, receiver operating characteristic curve, Brier score, and decision curve analysis (DCA) were used to evaluate discrimination, distinctiveness, validity, and clinical utility of this nomogram model. The model was verified internally using bootstrapping method and verified externally using validation cohort.
LASSO regression analysis identified six potential risk factors with nonzero coefficients. Multivariate logistic regression confirmed the risk factors to be indep
We have established and validated a nomogram model to predict m-PM in patients undergoing curative surgery, which shows good discrimination and high accuracy.
Core Tip: The prediction and early detection of metachronous peritoneal metastasis remain a difficult task in clinical practice. Conventional imaging modalities have limited sensitivity for detecting peritoneal nodules < 5 mm in diameter. Second-look surgery may be an alternative means for early detection of PM; however, its invasive nature and surgical complications mean that this approach should only be applied to high-risk patients. The present study aimed to develop a nomogram to help surgeons screen out high-risk patients and select appropriate individualized follow-up and treatment strategies.
- Citation: Ban B, Shang A, Shi J. Development and validation of a nomogram for predicting metachronous peritoneal metastasis in colorectal cancer: A retrospective study. World J Gastrointest Oncol 2023; 15(1): 112-127
- URL: https://www.wjgnet.com/1948-5204/full/v15/i1/112.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v15.i1.112
Colorectal cancer (CRC) ranks third among global cancers in terms of its mortality with more than 850000 deaths annually and metastasis is one of the most common causes for death in CRC[1]. After the liver, the peritoneum is the second most common metastatic site for CRC spread[2,3]. Approximately 10%-25% of patients with CRC develop peritoneal metastasis (PM) after initial diagnosis[4]. Compared with other CRC metastatic sites, PM is associated with poorer progression-free survival and overall survival[5,6]. As a result, in the 8th edition of the tumor, node, metastasis staging system published by the American Joint Committee on Cancer, PM was classified as M1c since it has a worse prognosis when compared with patients with one distant organ metastasis (M1a) and those with more than one distant organ metastasis (M1b)[7]. PM of CRC can be divided into synchronous and metachronous PM[8]. In m-PM, peritoneal recurrence occurs after primary surgery[9,10]. The prediction and early detection of m-PM have an important role in improving postoperative prognosis of CRC, because surgeons are more likely to achieve complete cytoreduction (CCR-0) in patients with lower peritoneal cancer index (PCI) values[11]. Cytoreduction surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) and systemic treatment have been widely applied in the treatment of early m-PM from CRC, and have markedly improved the oncological outcomes[12,13].
However, conventional imaging modalities have limited sensitivity for detecting peritoneal nodules < 5 mm in diameter, making it difficult to detect PM early[14]. Second-look surgery may be an alternative means for early detection of PM. However, its invasive nature and surgical complications mean that this approach should only be applied to high-risk patients[14,15]. Previous research has demonstrated that several clinicopathological factors including T4 tumor, mucinous adenocarcinoma and signet-ring cell carcinoma are closely related to m-PM[16]. However, few studies have reported the genetic alterations of m-PM. A reliable and integrated predictive model is needed to evaluate the risk of developing m-PM and improve the management of high-risk patients. A nomogram is a simple and practical scoring system that is mainly used for predicting risk and evaluating prognosis, with good clinical application[17]. The present study aimed to develop a nomogram to help surgeons screen out high-risk patients and select appropriate individualized follow-up and treatment strategies.
The study was approved by the Ethics Committee of the Second Hospital of Jilin University and carried out in line with the Declaration of Helsinki. Patients were carefully selected, and finally, 878 patients with CRC undergoing curative-intent resection were considered to be eligible between January 1, 2014 and January 31, 2019, at the Colorectal Center of Jilin University. The inclusion criteria were as follows: Primary CRC confirmed by colonoscopy and biopsy; American Society of Anesthesiologists Grades I-III; no signs of distant metastasis based on imaging examinations as well as intraoperative exploration; no history of other malignancy; patients undergoing curative-intent resection; and patients undergoing tumor kirsten rat sarcoma viral oncogene homolog (KRAS), neuroblastoma RAS viral oncogene homolog (NRAS) and v-raf murine sarcoma viral oncogene homolog B (BRAF) mutation testing as well as microsatellite instability (MSI) analysis. Patients were excluded if they underwent emergency surgery, had synchronous peritoneal metastasis (s-PM) before resection, rectal cancer below the peritoneum, were treated with neoadjuvant chemotherapy or radiotherapy, or had incomplete follow-up data. At present, there is no international consensus concerning the defining time points of s-PM and m-PM[10]. The most common method is to adopt initial surgery as the cutoff point for distinguishing between s-PM and m-PM[9,10,18], which was adopted in this study.
The preoperative clinical stage was determined by physical examination, chest-abdominal computed tomography (CT), and colonoscopy. The postoperative examination follow-up protocol included an evaluation of serum tumor marker every 3 mo, thoracoabdominal CT every 6 mo, and colonoscopy every 12 mo. The occurrence of m-PM within 3 years after curative surgery was defined as the target event for this predictive model. m-PM was diagnosed by laparoscopic exploration or imaging examination. In the present study, follow-up was terminated if the patients were diagnosed with m-PM and remaining patients were followed up for 3 years. All follow-up was completed on January 31, 2022.
A total of 23 potential risk factors for m-PM were evaluated. These included: Gender; age at the time of surgery (≥ 60 years or < 60 years); body mass index (≥ 25 kg/m2 or < 25 kg/m2); preoperative ascites; surgical method (laparoscopic or open); number of examined lymph nodes (≥ 12 or < 12); anastomotic leakage; tumor site (right colon, left colon, or rectum); tumor size (≥ 5 cm or < 5 cm); tumor type (ulcer type, uplift type, or infiltrating type); differentiation (well/moderate or poor/undifferentiated); histological type (adenocarcinoma, mucinous adenocarcinoma, or signet-ring cell carcinoma); pathological T stage; pathological N stage; neural invasion; vascular invasion; preoperative serum tumor marker [carcinoembryonic antigen, carbohydrate antigen 125 (CA125) and carbohydrate antigen 19-9], KRAS, NRAS or BRAF mutation, and MSI status.
All tumors were grouped according to their primary location as right colon (from cecum to transverse colon), left colon (from splenic flexure to sigmoid colon), and rectum. The tumor type was classified into: ulcer type (tumor grew deep into the intestinal wall and infiltrated around it, forming a crater-like ulcer with a raised edge); uplift type (tumor grew into the lumen of the intestine); and infiltrating type (tumor grew invasively within the wall of the intestinal tract, causing stiffness of the intestinal wall). The MSI analysis was performed using the five Bethesda instability markers BAT-25, BAT-26, D5S346, D2S123, and D17S250. Tumors expressing more than one instability marker were classified as high-frequency (MSI-H), those expressing only one instability marker were classified as low frequency (MSI-L), and those which did not express any markers were classified as stable (MSS). The MSI-L and MSS cases were included in the same group (MSI-L/MSS) because no significant difference in treatment outcomes was observed between the two variables in previous studies[19].
All data were analyzed using SPSS version 26.0 and R version 4.0.3. The categorical variables were presented as numbers and percentages. The patients were randomly divided into training and validation cohorts at a ratio of 2:1 using a random split-sample method. The ranked data were analyzed using the Mann-Whitney U test. The χ2 and Fisher’s exact tests were used to compare the categorical variables. Least absolute shrinkage and selection operator (LASSO) regression was performed to identify the variables with nonzero coefficients to predict the risk of m-PM[17,20]. Based on the results of the LASSO regression, multivariate logistic regression was used to verify the selected variables and to develop the predictive nomogram model. For all statistical tests, P < 0.05 was deemed statistically significant.
Various tests were performed to assess the performance of the developed nomogram. The discriminative performance of the model was evaluated using C-index and AUC. The C-index and AUC range from 0 to 1, and a higher value indicates that the model has a higher differentiation performance[21]. The Brier score was used to determine the predictive validity of the model. A Brier score < 0.25 indicates that the model can correctly predict the occurrence of the target event. When the score of the model is between 0 and 0.25, the closer the score is to 0, the better the model performance[22]. The model was verified internally using a bootstrapping method with 1000 resamples. A calibration plot was used to evaluate the consistency between actual and predicted probability. The performance of the model was validated externally using a validation cohort. Decision curve analysis (DCA) was used to evaluate the clinical utility of the model based on net benefits at different threshold probabilities[23].
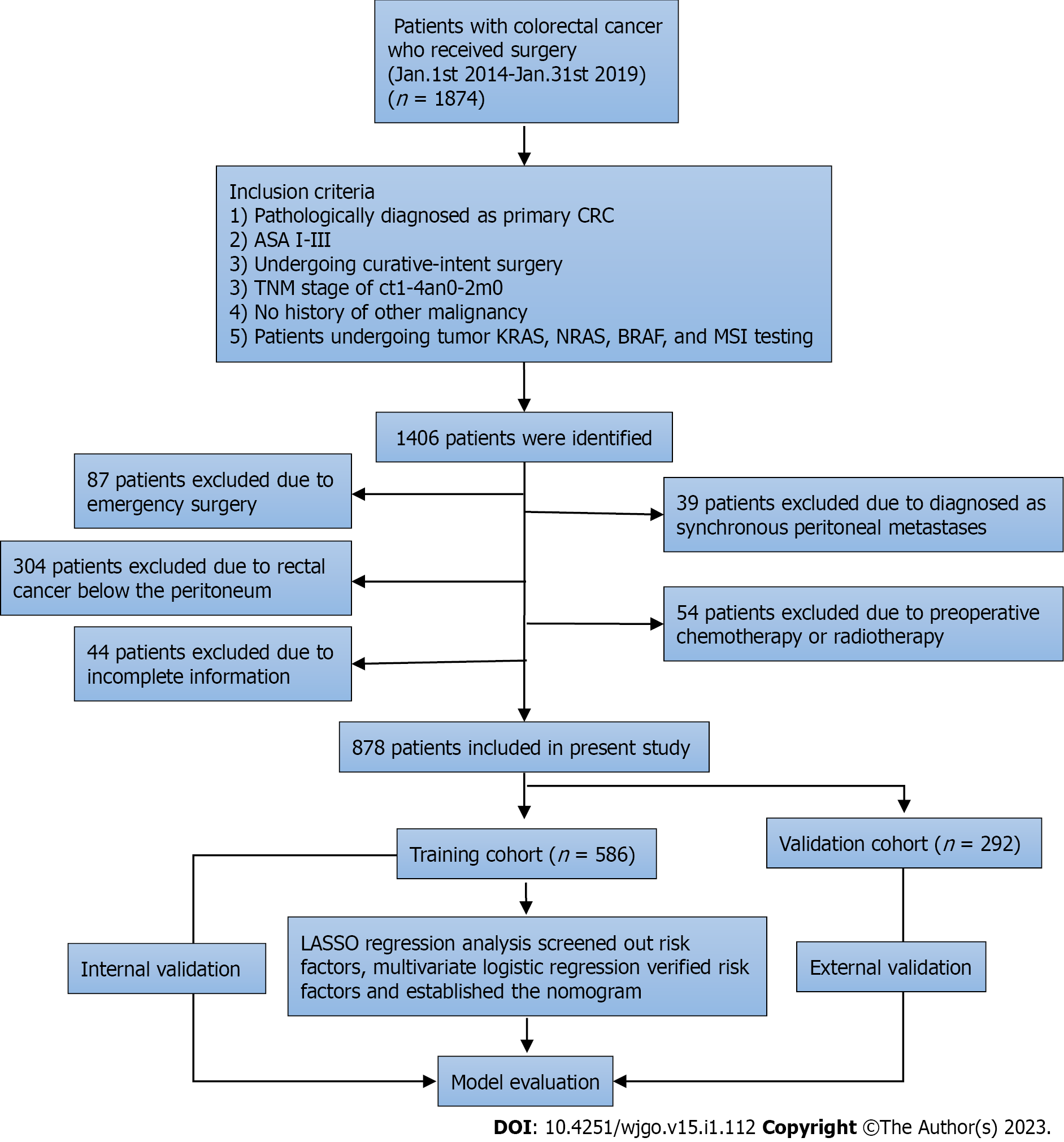
A total of 1874 patients underwent surgery for CRC between January 1, 2014, and January 31, 2019, at the Second Affiliated Hospital of Jilin University. A total of 1406 patients met the inclusion criteria for further analysis. From this cohort, 528 patients were found to be ineligible for the study because they underwent emergency surgery (n = 87), had rectal cancer below the peritoneum (n = 304), s-PM (n = 39), received preoperative chemotherapy or radiotherapy (n = 54), or had incomplete clinical data (n = 44) (Figure 1). Finally, 878 patients were enrolled in the study. The eligible patients were randomly assigned to the validation (n = 586) and training (n = 292) cohorts. The 3-year cumulative incidence of m-PM was 11.1% (65/586) in the training cohort and 9.9% (29/292) in the validation cohort. However, the difference was not significant (P = 0.600). Correlations between various clinicopathological factors in the two cohorts are summarized in Table 1. Patients’ characteristics did not show any significant difference between the two cohorts.
| Variables | Training cohort (n = 586) | Validation cohort (n = 292) | P value |
| Gender | 0.777 | ||
| Male | 321 (54.8) | 157 (53.8) | |
| Female | 265 (45.2) | 135 (46.2) | |
| Age (yr) | 0.924 | ||
| ≥ 60 | 293 (50.0) | 147 (50.3) | |
| < 60 | 293 (50.0) | 145 (49.7) | |
| BMI (kg/m2) | 0.683 | ||
| ≥ 25 | 154 (26.3) | 73 (25.0) | |
| < 25 | 432 (73.7) | 219 (75.0) | |
| Preoperative ascites | 0.794 | ||
| Yes | 80 (13.7) | 38 (13.0) | |
| No | 506 (86.3) | 254 (87.0) | |
| Operation mode | 0.228 | ||
| Open | 183 (31.2) | 103 (35.3) | |
| Laparoscopic | 403 (68.8) | 189 (64.7) | |
| Anastomotic leakage | 0.585 | ||
| Yes | 44 (7.5) | 25 (8.6) | |
| No | 542 (92.5) | 267 (91.4) | |
| Tumor site | 0.767 | ||
| Right colon | 160 (27.3) | 80 (27.4) | |
| Left colon | 176 (30.0) | 94 (32.2) | |
| Rectum | 250 (42.7) | 118 (40.4) | |
| Tumor size (cm) | 0.223 | ||
| ≥ 5 | 254 (43.3) | 114 (39.0) | |
| < 5 | 332 (56.7) | 178 (61.0) | |
| Tumor type | 0.819 | ||
| Ulcer | 395 (67.4) | 202 (69.2) | |
| Uplift | 152 (25.9) | 70 (24.0) | |
| Infiltrating | 39 (6.7) | 20 (6.8) | |
| Differentiation | 0.448 | ||
| Well/moderate | 519 (88.6) | 253 (86.6) | |
| Poor/undifferentiated | 67 (11.4) | 39 (13.4) | |
| Histology | 0.707 | ||
| Adenocarcinoma | 497 (84.8) | 245 (83.9) | |
| Mucinous | 69 (11.8) | 39 (13.4) | |
| Signet-ring | 20 (3.4) | 8 (2.7) | |
| T stage | 0.650 | ||
| 1 | 54 (9.2) | 21 (7.2) | |
| 2 | 137 (23.4) | 64 (21.9) | |
| 3 | 272 (46.4) | 146 (50.0) | |
| 4 | 123 (22.0) | 61 (20.9) | |
| N stage | 0.976 | ||
| 0 | 263 (44.9) | 129 (44.2) | |
| 1 | 168 (28.7) | 84 (28.8) | |
| 2 | 155 (26.4) | 79 (27.1) | |
| Examined lymph nodes | 0.147 | ||
| ≥ 12 | 509 (86.9) | 243 (83.2) | |
| < 12 | 77 (13.1) | 49 (16.8) | |
| Nerve invasion | 0.985 | ||
| Yes | 185 (31.6) | 92 (31.5) | |
| No | 401 (68.4) | 200 (68.5) | |
| Vascular invasion | 0.680 | ||
| Yes | 213 (36.3) | 102 (34.9) | |
| No | 373 (63.7) | 190 (65.1) | |
| CEA | 0.535 | ||
| Normal | 344 (58.7) | 165 (56.5) | |
| Elevated | 242 (41.3) | 127 (43.5) | |
| CA125 | 0.778 | ||
| Normal | 349 (59.6) | 171 (58.6) | |
| Elevated | 237 (40.4) | 121 (41.4) | |
| CA199 | 0.781 | ||
| Normal | 490 (83.6) | 242 (82.9) | |
| Elevated | 96 (16.4) | 50 (17.1) | |
| KRAS mutation | 0.971 | ||
| Wild | 370 (63.1) | 184 (63.0) | |
| Mutation | 216 (36.9) | 108 (37.0) | |
| NRAS mutation | 0.392 | ||
| Wild | 561 (95.7) | 283 (96.9) | |
| Mutation | 25 (4.3) | 9 (3.1) | |
| BRAF mutation | 0.783 | ||
| Wild | 514 (87.7) | 258 (88.4) | |
| Mutation | 72 (12.3) | 34 (11.6) | |
| MSI status | 0.612 | ||
| MSS/MSI-L | 509 (86.9) | 250 (85.6) | |
| MSI-H | 77 (13.1) | 42 (14.4) | |
| Peritoneal metastasis | 0.600 | ||
| Yes | 521 (88.9) | 263 (90.1) | |
| No | 65 (11.1) | 29 (9.9) |
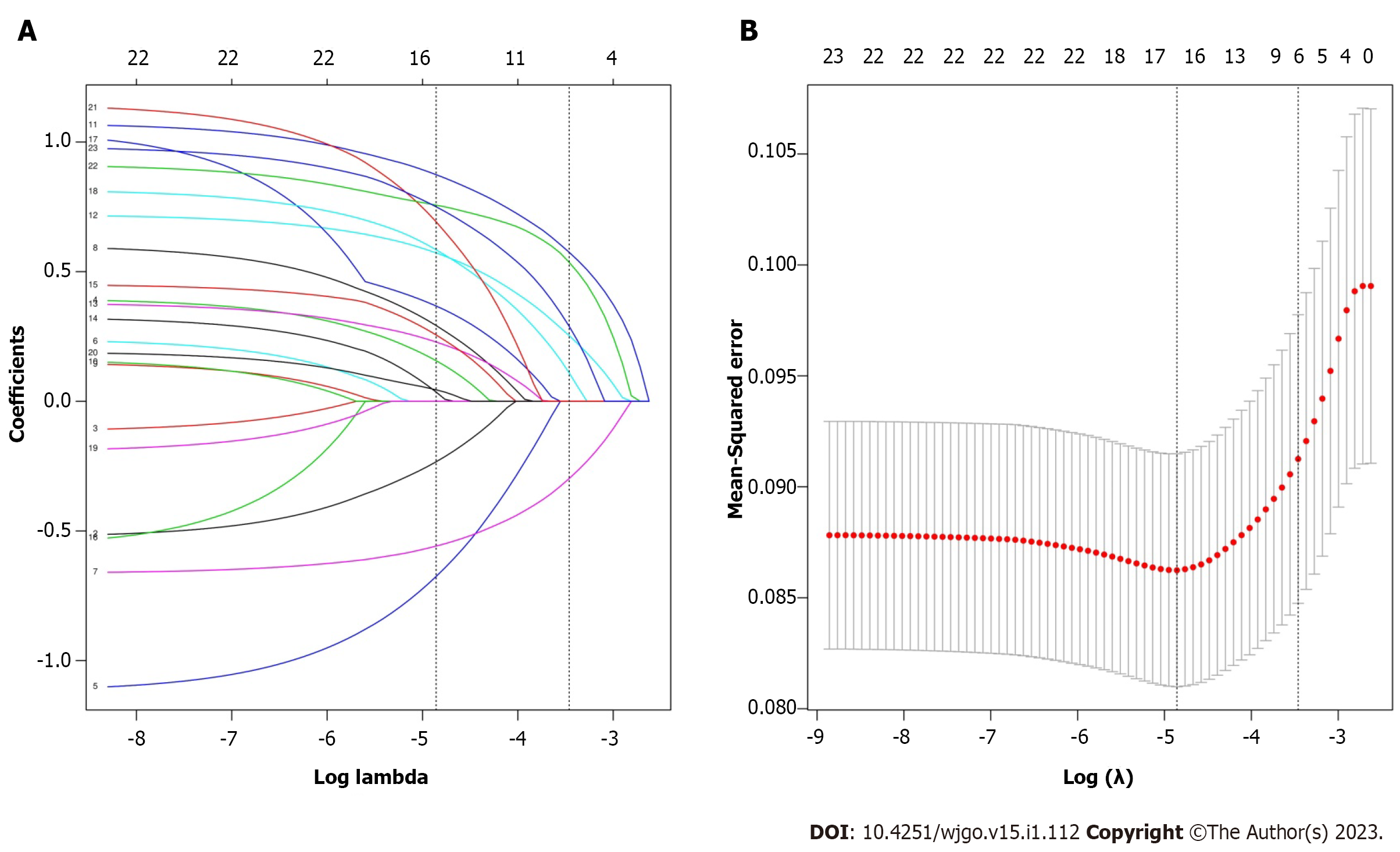
Among the 23 variables, six potential risk factors with nonzero coefficients were identified by LASSO regression analysis, including tumor site, histological type, pathological T stage, CA125, BRAF mutation, and MSI status (Figure 2). Multivariate logistic regression identified right colon cancer, pT4, histological type of mucinous adenocarcinoma and signet-ring cell carcinoma, elevated CA125, BRAF mutation, and MSI-H as independent risk factors for m-PM (Table 2).
| Risk factors | Multivariate logistic regression | ||
| OR | 95%CI | P value | |
| Tumor site | |||
| Right colon | 1 | ||
| Left colon | 0.461 | 0.231-0.917 | 0.027 |
| Rectum | 0.250 | 0.120-0.520 | < 0.001 |
| Histology | |||
| Adenocarcinoma | 1 | ||
| Mucinous | 2.993 | 1.441-6.220 | 0.003 |
| Signet-ring | 6.453 | 2.122-19.625 | 0.001 |
| T stage | |||
| T1 | 1 | ||
| T2 | 1.822 | 0.364-9.103 | 0.465 |
| T3 | 2.284 | 0.498-10.484 | 0.288 |
| T4 | 6.871 | 1.487-31.736 | 0.014 |
| CA125 | |||
| Normal | 1 | ||
| Elevated | 2.176 | 1.211-3.912 | 0.009 |
| BRAF mutation | |||
| Wild | 1 | ||
| Mutation | 2.586 | 1.277-5.236 | 0.008 |
| MSI status | |||
| MSS/MSI-L | 1 | ||
| MSI-H | 2.547 | 1.245-5.212 | 0.010 |
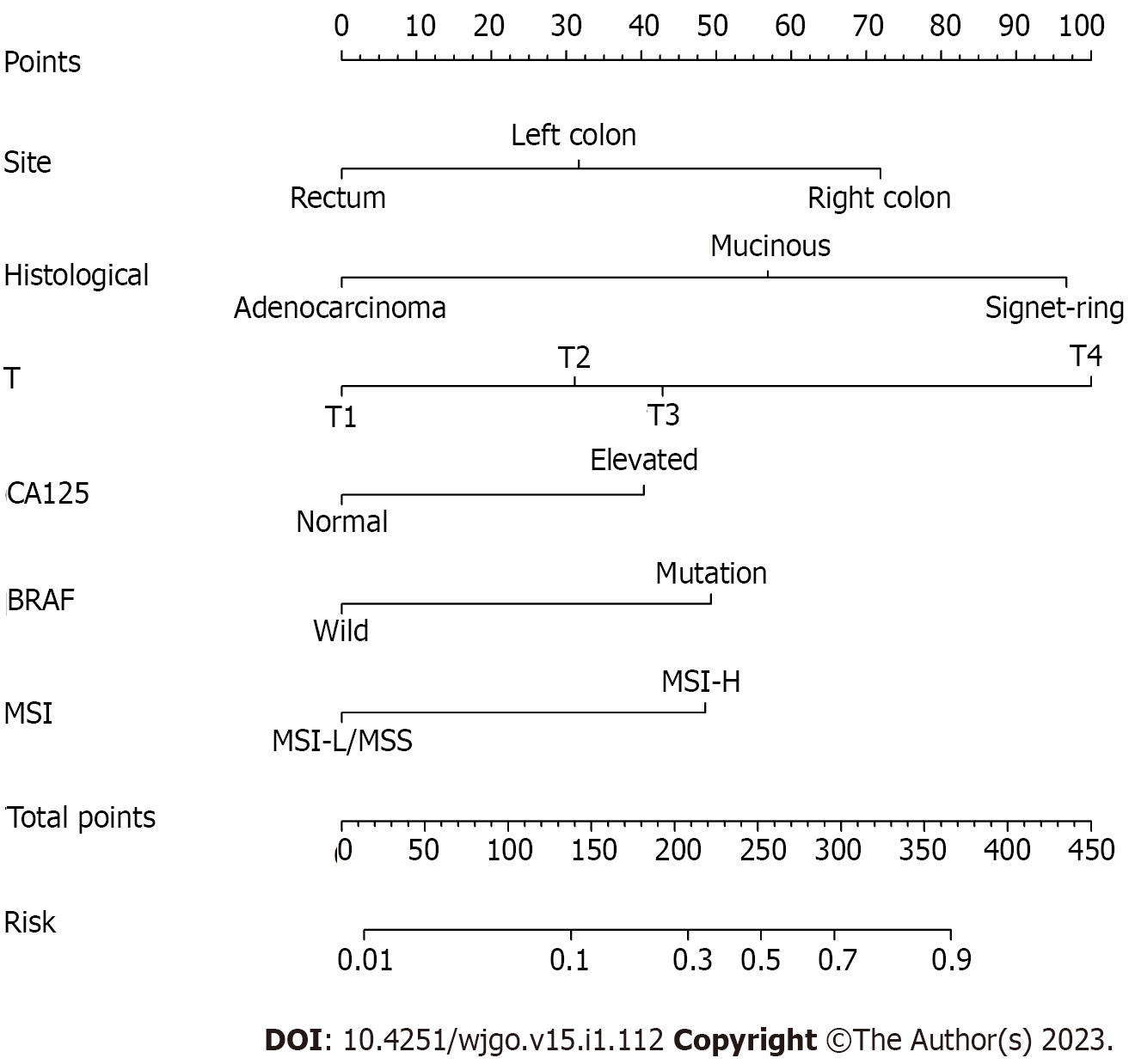
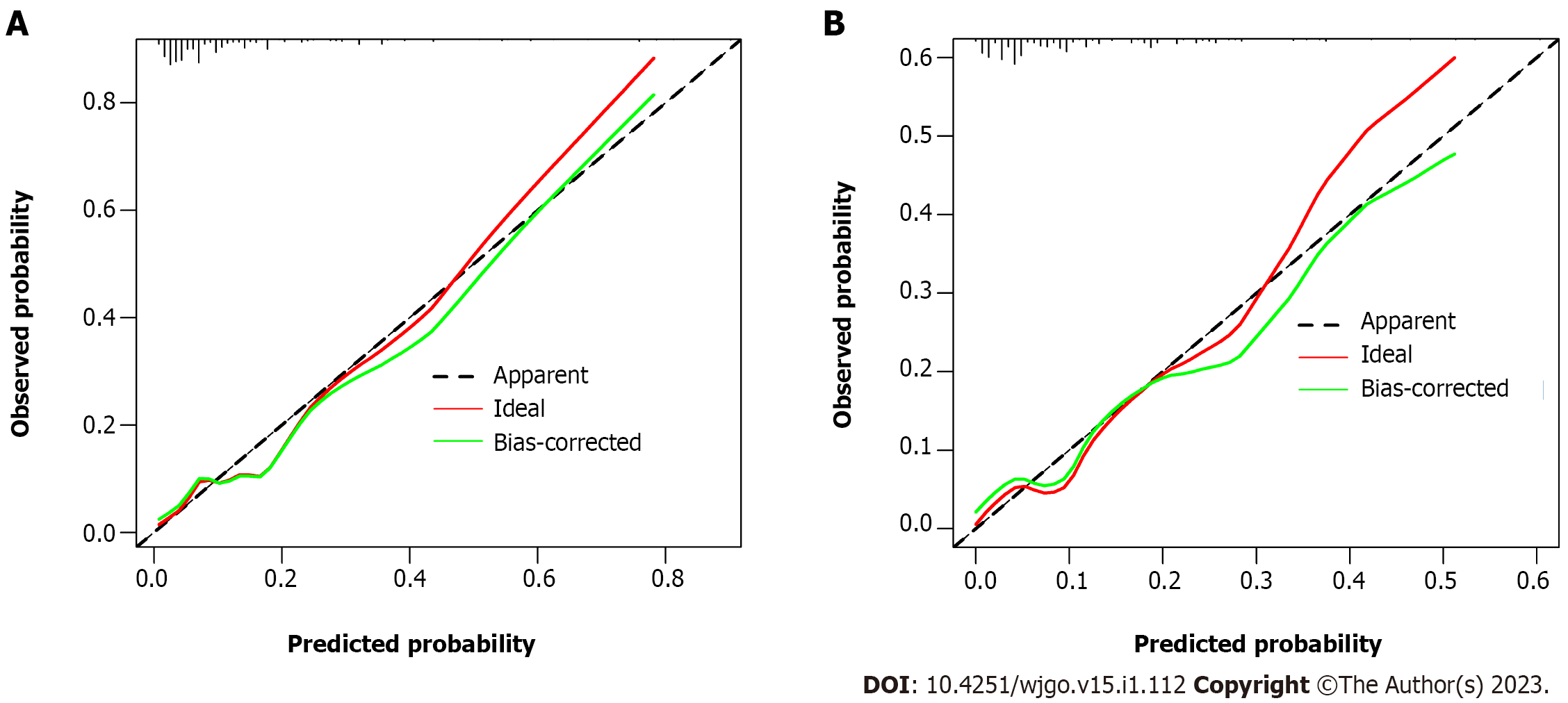
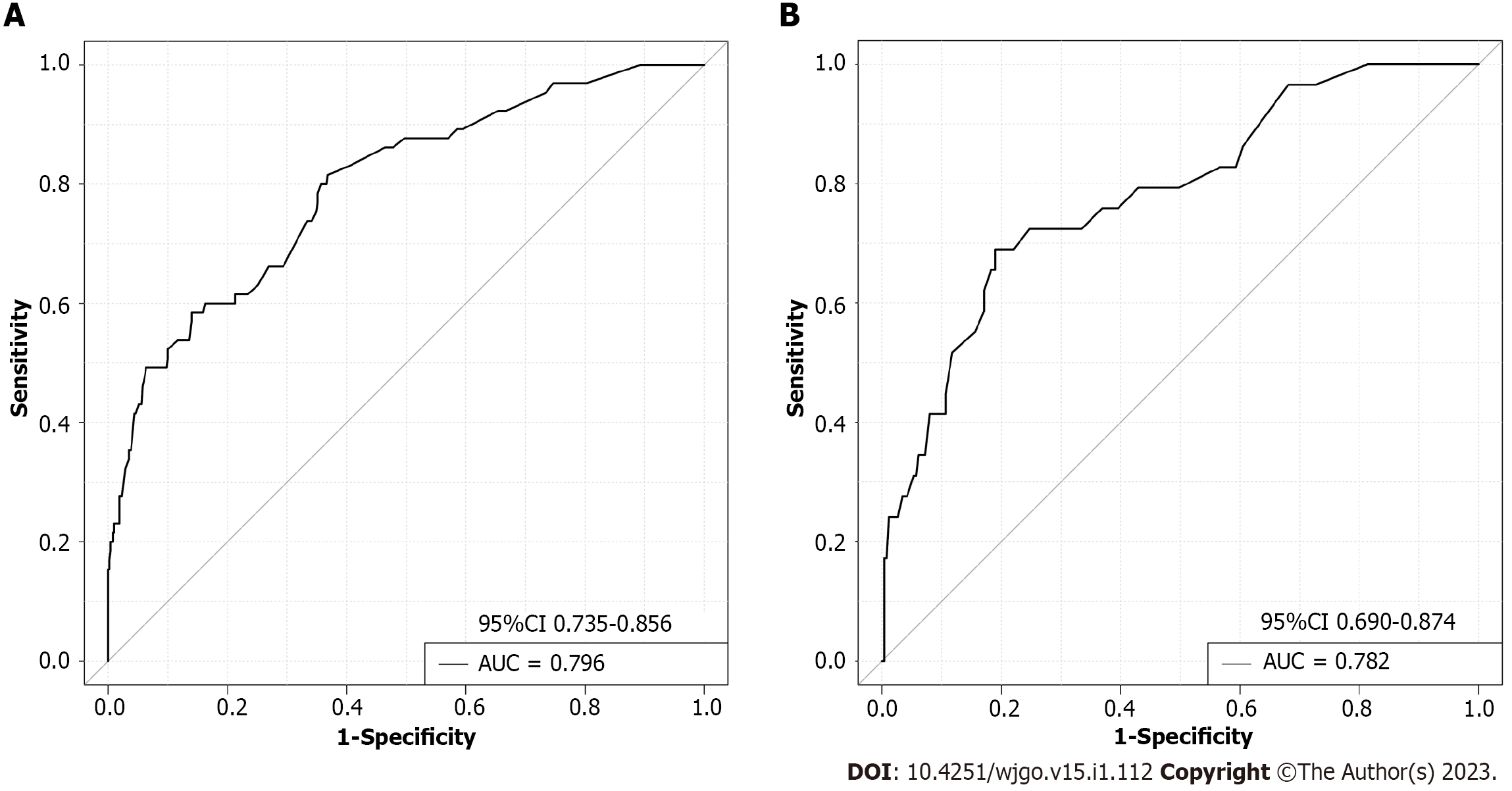
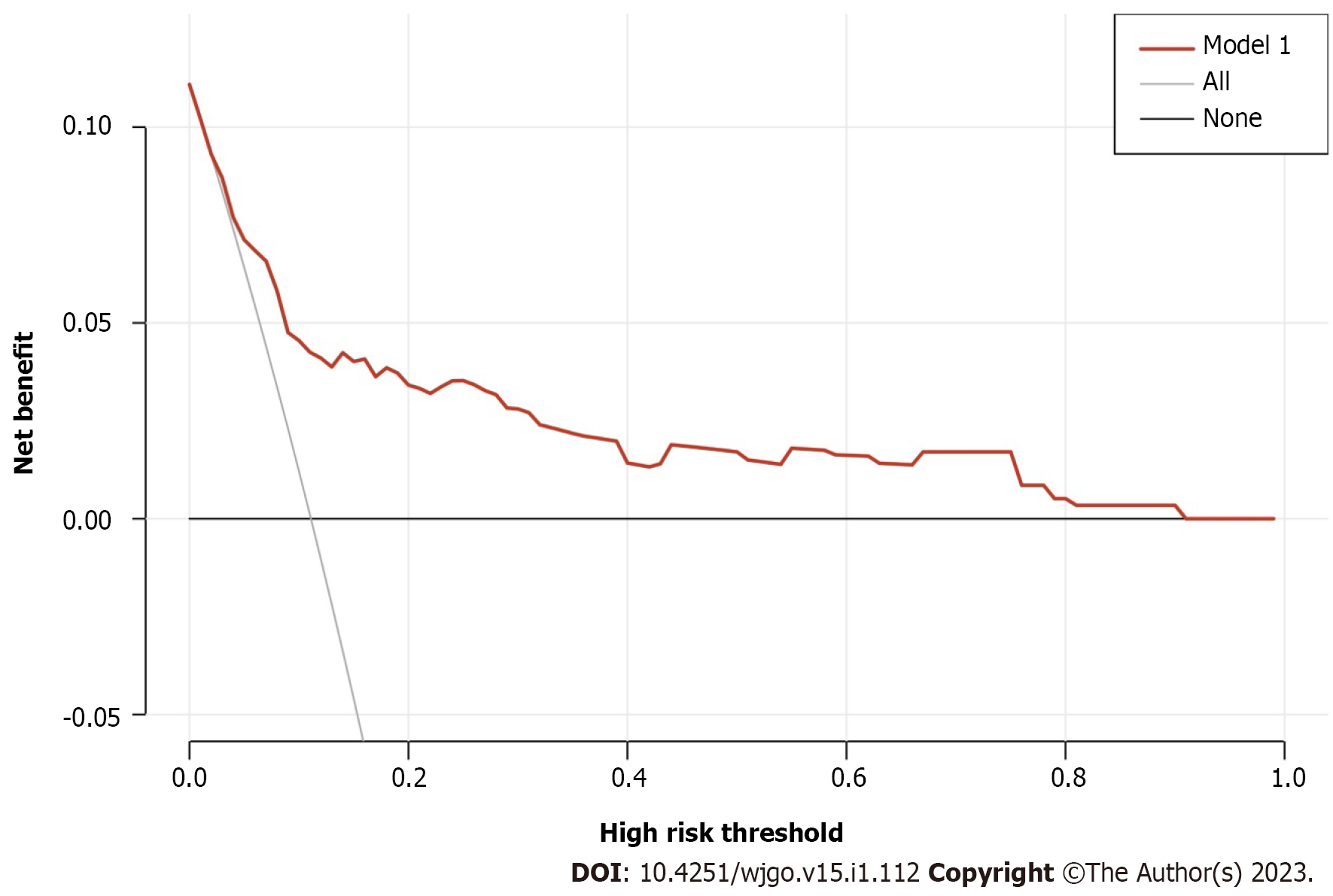
The m-PM predictive nomogram for CRC patients developed based on the multivariate regression analysis is illustrated in Figure 3. The scores for the tumor site were rectal cancer = 0, left colon cancer = 32, and right colon cancer = 72. For the histological subtype, the scores were adenocarcinoma = 0, mucinous adenocarcinoma = 57, and signet-ring = 97. For pathological T stage, the scores were T1 = 0, T2 = 31, T3 = 43, and T4 = 100. For CA125, the scores were normal level = 0 and elevated level = 40. For BRAF mutation, the scores were wild type = 0 and mutation = 49. For MSI status, the scores were MSI-L/MSS = 0 and MSI-H = 49. We evaluated the scores of all patients and used the receiver operating characteristic curve and Youden index to identify the optimum cutoff value of this model. This cutoff value was 168. All patients were divided into two subgroups: Low-risk group (total score ≤ 168) and high-risk group (total score > 168) (Table 3). Most of the patients (712 cases, 81.1%) were classified into the low-risk group. The percentage of patients developing m-PM in this subgroup was 5.6%. Using this simple grouping mothed, our nomogram model can achieve a high negative predictive rate (94.4%). The calibration curve showed good consistency between the predicted and actual observed outcomes since the bias-corrected curve was close to the ideal curve (Figure 4). The model achieved a good predictive accuracy on both the training and validation datasets. The C-index, AUC and Brier scores were 0.796, 0.796 (95%CI 0.735-0.856) and 0.081, and 0.782, 0.782 (95%CI 0.690-0.874) and 0.089 for the training cohort, respectively (Figure 5). DCA showed that when the threshold probability was between 0.01 and 0.90, using this model to predict m-PM achieved a net clinical benefit (Figure 6).
| m-PM | P value | ||
| No | Yes | ||
| Low-risk group | 672 (94.4) | 40 (5.6) | < 0.001 |
| High-risk group | 112 (67.5) | 54 (32.5) | |
PM is traditionally considered as an end-stage disease in CRC. Although nomograms, statistical models and other risk prediction systems have been widely used for predicting the risk of recurrence in clinical practice, to our knowledge, few studies have constructed predictive models based on the risk factors for developing m-PM. One Swedish group conducted two studies to build a model for predicting m-PM in CRC patients[24,25]. These two studies had a large simple size and showed good internal validity. However, limitations including the use of registry-based data and enrolling patients undergoing R2 resection may limit the wider applicability of their model. Pedrazzani et al[26] conducted an international multicenter study to predict the risk of m-PM. Using easily available clinical and pathological variables, their scoring model achieved good predictive value. In this study, we used LASSO regression analysis to assess the impact of 23 clinical variables on the risk of developing m-PM following CRC surgery. This method can optimize the performance of the model by reducing the influence of multicollinearity between variables and selection bias[27]. Among the 23 clinical variables, six risk factors were screened out by LASSO regression analysis. Multiple logistic regressions further confirmed that right colon cancer, pT4, histological types of mucinous adenocarcinoma and signet-ring cell carcinoma, elevated CA125, BRAF mutation, and MSI-H were independent risk factors for m-PM in CRC. Based on the results of the above two regression analyses, we established a predictive nomogram model to evaluate the risk of m-PM in individuals. The final nomogram model showed good discrimination accuracy, calibration, and reliability in both training and validation cohorts.
The highest scoring variable observed in this predictive model was the T4 stage, which has been widely considered as an independent risk factor for PM in previous studies[28,29]. The “seed and soil” hypothesis is often used to explain the process involved in peritoneal dissemination for CRC[30]. According to this hypothesis, the intraperitoneal free cancer cells which shed from the primary tumor are likened to “seed” and the favorable environment for the proliferation of the cancer cells are likened to “soil”. T4 tumors invade the serosa of the bowel and hence facilitate detachment and implantation of cancer cells into the peritoneum[31].
Consistent with previous studies, we found that patients with right-sided colon cancer have a higher risk of developing m-PM[32,33]. Compared with left-sided tumors, right-sided tumors tend to be asymptomatic until advanced. Therefore, these patients tend to present with advanced tumors that have already invaded the serosal layer, which facilitates dissemination into the peritoneal cavity[34]. Moreover, differences in embryonic origin between right-sided and left-sided colon cancers may also contribute to this discrepancy[35]. Right-sided tumors tend to be more aggressive as they are more likely to present with hypermethylated phenotypes, BRAF mutated expression profiles, and MSI-H status[35,36].
The tumor-associated glycoprotein antigen CA125 is expressed in mesothelial cells of the peritoneum, epithelium of the oviduct, endocervix, and endometrium. This antigen is a reliable biomarker for monitoring the development of ovarian and gastrointestinal cancers[37,38]. Previous studies demonstrated its accuracy, sensitivity, and specificity in diagnosing peritoneal dissemination of gastric cancer[39,40]. Huang et al[38] retrospectively analyzed the clinical data of 853 patients and found that CA125 was a reliable clinical markers in the diagnosis of PM for CRC patients. In this study, patients with elevated preoperative serum CA125 Levels were more likely to develop m-PM within 3 years than those with normal levels. We found that the mucinous and signet-ring carcinomas, aggressive subtypes of adenocarcinoma, were also significantly associated with PM, which was in agreement with previous studies[41].
The expression of specific oncogenes and binding proteins may facilitate the detachment of tumor cells from the primary site and subsequent implantation and proliferation of CRC cells in the peritoneal cavity[12]. Therefore, an in-depth analysis of the genetic and molecular mechanisms is essential to evaluate the risk of developing m-PM. The RAS-RAF-MAPK pathway regulates the signal transduction involved in the growth, proliferation, and differentiation for cell[42]. Mutations in upstream genes regulating this pathway, such as BRAF, may result in continuous abnormal activation of the downstream signal pathway[43]. BRAF mutation occurs in about 12% of CRC patients[44]. Approximately 90% of these mutations result in a V600E substitution[42]. The BRAF V600E mutation has been identified as a biomarker for poor prognosis in CRC patients in several clinical studies[45,46]. This mutation is also significantly associated with PM, and this metastatic pattern may contribute to poor survival[47,48]. Moreover, BRAF mutation occurs more frequently in right-sided tumors and the mucinous/signet ring cell histology subtypes, which may further explain why tumors with those characteristics are more likely to spread in the peritoneum[49,50]. MSI is caused by mutations of the DNA mismatch repair genes, which lead to functional defects in the repair of repetitive sequencing (microsatellite) errors during DNA replication[51]. MSI-H is detected in about 15% of CRC patients[52]. CRC with MSI-H is inclined to present in younger patients, with a predominance in proximal colon[53]. Pathologically, MSI-H is associated with a Crohn’s-disease-like lymphocytic reaction, tumor-infiltrating lymphocytes, and mucinous/signet ring cell histology subtypes[53,54]. From a molecular biology point of view, several studies demonstrated the coexistence of MSI-H and BRAF mutation[55,56]. MSI-H has good and bad clinicopathological features, which may explain the different prognostic implications of MSI-H in different stages of CRC. In nonmetastatic CRC, MSI-H was associated with a good prognosis[57,58]. However, in metastatic CRC, patients with MSI-H had a poor prognosis[59,60]. Consistent with our study, Kim et al[61] demonstrated that MSI-H was significantly associated with more frequent PM than MSI-L/MSS. In the present study, BRAF mutation and MSI-H were identified as significant risk factors for m-PM by LASSO and multivariate analyses, and incorporated in this nomogram. Both factors scored 49 points, effectively predicting the probability of m-PM.
Currently, multidetector-row CT remains the primary means to monitor the occurrence of PM after curative surgery in clinical practice. However, CT has limited accuracy to detect PM and underestimates the extent of disease[62]. Whole-body diffusion-weighted magnetic resonance imaging (WB-DWI/MRI) is reported to have a high sensitivity in detecting PM for CRC, and outperform CT in evaluating both cancer distribution and lesion size[63,64]. Several studies have demonstrated good diagnostic performance for positron emission tomography/CT (PET/CT) in detecting peritoneal metastases[65,66]. However, these imaging methods are relatively expensive and time-consuming and unsuitable for general screening. In future clinical practice, our nomogram could be used to identify high-risk patients (total score > 168) that would benefit from further screening with these aggressive imaging modalities. Then, if a positive result is suspected on the targeted examinations, we could perform second-look surgery using laparoscope to evaluate the extent of disease and obtain pathological evidence. Finally, if m-PM is diagnosed, surgeons are supposed to estimate the PCI score and decide whether aggressive treatment including CRS plus HIPEC should be performed in targeted patient.
This study had some limitations. First, the training and validation cohorts were obtained retrospectively from a single center. Therefore, the nomogram requires further validation in multicenter prospective clinical studies. Second, the diagnosis of m-PM was mainly based on postoperative imaging such as CT. This could have delayed diagnosis because of the limited sensitivity of CT in detecting small peritoneal nodules. However, we believe this limitation was minor because the main purpose of this study was to identify risk factors affecting m-PM within the follow-up period, and establish a predictive model for early detection in future clinical practice. Third, because of the limited follow-up time of the included patients in this study, we could only assess the risk of developing m-PM within 3 years after surgery. Although the typical chronological span of m-PM occurrence was covered, further research is still recommended to investigate the risk factors for m-PM at different times points after primary surgery.
We have established and validated a nomogram model to predict m-PM in patients undergoing curative CRC surgery. The model showed good discrimination and high calibration in the training and validation cohorts. Our proposed model could be used clinically to help surgeons identify CRC patients at risk of developing m-PM post-surgery and take timely and effective interventions to improve prognosis of these patients. This may provide a reference for future clinical practice.
The prediction and early detection of metachronous peritoneal metastasis (m-PM) remain a difficult task in clinical practice. Few studies have reported the genetic alterations of m-PM.
To explore risk factors in patients with m-PM after curative-intent colorectal cancer (CRC) surgery.
To establish and validate a nomogram model for predicting the occurrence of m-PM in CRC within 3 years after surgery.
We used the clinical data of 878 patients at the Second Hospital of Jilin University, between January 1, 2014 and January 31, 2019. The patients were randomly divided into training and validation cohorts at a ratio of 2:1. All data were analyzed using SPSS version 26.0 and R version 4.0.3.
The 3-year cumulative incidence of m-PM was 11.1% (65/586) in the training cohort and 9.9% (29/292) in the validation cohort. Least absolute shrinkage and selection operator regression analysis and multiple logistic regressions identified that right colon cancer, pT4, histological types of mucinous adenocarcinoma and signet-ring cell carcinoma, elevated carbohydrate antigen 125 (CA125), v-raf murine sarcoma viral oncogene homolog B (BRAF) mutation, and microsatellite instability-high-frequency (MSI-H) were independent risk factors for m-PM in CRC. These six predictors could be used to establish a nomogram for predicting m-PM. The nomogram model showed good discrimination accuracy, calibration, and reliability in both training and validation cohorts.
The nomogram model based on six predictors (right colon cancer, pT4, and histological types of mucinous adenocarcinoma and signet-ring cell carcinoma, elevated CA125, BRAF mutation, and MSI-H) showed good discrimination and high accuracy.
The nomogram requires further validation in multicenter prospective clinical studies.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Amornyotin S, Thailand; Sahin TT, Turkey S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Biller LH, Schrag D. Diagnosis and Treatment of Metastatic Colorectal Cancer: A Review. JAMA. 2021;325:669-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 1418] [Article Influence: 354.5] [Reference Citation Analysis (0)] |
| 2. | van Gestel YR, de Hingh IH, van Herk-Sukel MP, van Erning FN, Beerepoot LV, Wijsman JH, Slooter GD, Rutten HJ, Creemers GJ, Lemmens VE. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol. 2014;38:448-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 3. | van der Geest LG, Lam-Boer J, Koopman M, Verhoef C, Elferink MA, de Wilt JH. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis. 2015;32:457-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 374] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 4. | Sluiter N, de Cuba E, Kwakman R, Kazemier G, Meijer G, Te Velde EA. Adhesion molecules in peritoneal dissemination: function, prognostic relevance and therapeutic options. Clin Exp Metastasis. 2016;33:401-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 5. | Lau JW, Chang HS, Lee KY, Gwee YX, Lee WQ, Chong CS. Survival outcomes following primary tumor resection for patients with incurable metastatic colorectal carcinoma: Experience from a single institution. J Dig Dis. 2018;19:550-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Arakawa K, Kawai K, Ishihara S, Hata K, Nozawa H, Oba K, Sugihara K, Watanabe T. Prognostic Significance of Peritoneal Metastasis in Stage IV Colorectal Cancer Patients With R0 Resection: A Multicenter, Retrospective Study. Dis Colon Rectum. 2017;60:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Yang Z, Li Y, Qin X, Lv Z, Wang H, Wu D, Yuan Z. Development and Validation of a Prognostic Nomogram for Colorectal Cancer Patients With Synchronous Peritoneal Metastasis. Front Oncol. 2021;11:615321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Enblad M, Graf W, Birgisson H. Risk factors for appendiceal and colorectal peritoneal metastases. Eur J Surg Oncol. 2018;44:997-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Dietz MV, van Kooten JP, Said I, Brandt-Kerkhof ARM, Verhoef C, Bremers AJA, de Wilt JHW, de Reuver PR, Madsen EVE. Survival Outcomes After Cytoreductive Surgery with Hyperthermic Intraperitoneal Chemotherapy in Patients with Synchronous Versus Metachronous Onset of Peritoneal Metastases of Colorectal Carcinoma. Ann Surg Oncol. 2022;29:6566-6576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Hentzen JEKR, Rovers KP, Kuipers H, van der Plas WY, Been LB, Hoogwater FJH, van Ginkel RJ, Hemmer PHJ, van Dam GM, de Hingh IHJT, Kruijff S. Impact of Synchronous Versus Metachronous Onset of Colorectal Peritoneal Metastases on Survival Outcomes After Cytoreductive Surgery (CRS) with Hyperthermic Intraperitoneal Chemotherapy (HIPEC): A Multicenter, Retrospective, Observational Study. Ann Surg Oncol. 2019;26:2210-2221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Zhang Y, Qin X, Chen W, Liu D, Luo J, Wang H. Risk factors for developing peritoneal metastases after curative surgery for colorectal cancer: A systematic review and meta-analysis. Colorectal Dis. 2021;23:2846-2858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Sánchez-Hidalgo JM, Rodríguez-Ortiz L, Arjona-Sánchez Á, Rufián-Peña S, Casado-Adam Á, Cosano-Álvarez A, Briceño-Delgado J. Colorectal peritoneal metastases: Optimal management review. World J Gastroenterol. 2019;25:3484-3502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 57] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 13. | Solaini L, D'Acapito F, Passardi A, Framarini M, Tauceri F, Di Pietrantonio D, Frassineti GL, Casadei Gardini A, Cucchetti A, Cavaliere D, Ercolani G. Cytoreduction plus hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis in colorectal cancer patients: a single-center cohort study. World J Surg Oncol. 2019;17:58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Elias D, Honoré C, Dumont F, Ducreux M, Boige V, Malka D, Burtin P, Dromain C, Goéré D. Results of systematic second-look surgery plus HIPEC in asymptomatic patients presenting a high risk of developing colorectal peritoneal carcinomatosis. Ann Surg. 2011;254:289-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 167] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 15. | Goéré D, Glehen O, Quenet F, Guilloit JM, Bereder JM, Lorimier G, Thibaudeau E, Ghouti L, Pinto A, Tuech JJ, Kianmanesh R, Carretier M, Marchal F, Arvieux C, Brigand C, Meeus P, Rat P, Durand-Fontanier S, Mariani P, Lakkis Z, Loi V, Pirro N, Sabbagh C, Texier M, Elias D; BIG-RENAPE group. Second-look surgery plus hyperthermic intraperitoneal chemotherapy versus surveillance in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP-PRODIGE 15): a randomised, phase 3 study. Lancet Oncol. 2020;21:1147-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 146] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 16. | Lurvink RJ, Bakkers C, Rijken A, van Erning FN, Nienhuijs SW, Burger JW, Creemers GJ, Verhoef C, Lemmens VE, De Hingh IH. Increase in the incidence of synchronous and metachronous peritoneal metastases in patients with colorectal cancer: A nationwide study. Eur J Surg Oncol. 2021;47:1026-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Hao M, Li H, Wang K, Liu Y, Liang X, Ding L. Predicting metachronous liver metastasis in patients with colorectal cancer: development and assessment of a new nomogram. World J Surg Oncol. 2022;20:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Nagata H, Ishihara S, Hata K, Murono K, Kaneko M, Yasuda K, Otani K, Nishikawa T, Tanaka T, Kiyomatsu T, Kawai K, Nozawa H, Watanabe T. Survival and Prognostic Factors for Metachronous Peritoneal Metastasis in Patients with Colon Cancer. Ann Surg Oncol. 2017;24:1269-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1346] [Article Influence: 67.3] [Reference Citation Analysis (1)] |
| 20. | Wen J, Pan T, Yuan YC, Huang QS, Shen J. Nomogram to predict postoperative infectious complications after surgery for colorectal cancer: a retrospective cohort study in China. World J Surg Oncol. 2021;19:204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Pencina MJ, D'Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109-2123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1028] [Cited by in RCA: 1172] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 22. | Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3195] [Cited by in RCA: 3332] [Article Influence: 222.1] [Reference Citation Analysis (1)] |
| 23. | Li R, Xue M, Ma Z, Qu C, Wang K, Zhang Y, Yue W, Zhang H, Tian H. Construction and validation of a nomogram for predicting prolonged air leak after minimally invasive pulmonary resection. World J Surg Oncol. 2022;20:249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Segelman J, Akre O, Gustafsson UO, Bottai M, Martling A. Individualized prediction of risk of metachronous peritoneal carcinomatosis from colorectal cancer. Colorectal Dis. 2014;16:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Segelman J, Akre O, Gustafsson UO, Bottai M, Martling A. External validation of models predicting the individual risk of metachronous peritoneal carcinomatosis from colon and rectal cancer. Colorectal Dis. 2016;18:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 26. | Pedrazzani C, Turri G, Marrelli D, Kim HJ, Park EJ, Spolverato G, Foppa C, Spinelli A, Pucciarelli S, Baik SH, Choi GS. Prediction of Metachronous Peritoneal Metastases After Radical Surgery for Colon Cancer: A Scoring System Obtained from an International Multicenter Cohort. Ann Surg Oncol. 2022;29:7896-7906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | McEligot AJ, Poynor V, Sharma R, Panangadan A. Logistic LASSO Regression for Dietary Intakes and Breast Cancer. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 180] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 28. | Bastiaenen VP, Aalbers AGJ, Arjona-Sánchez A, Bellato V, van der Bilt JDW, D'Hoore AD, Espinosa-Redondo E, Klaver CEL, Nagtegaal ID, van Ramshorst B, van Santvoort HC, Sica GS, Snaebjornsson P, Wasmann KATGM, de Wilt JHW, Wolthuis AM, Tanis PJ. Risk of metachronous peritoneal metastases in patients with pT4a versus pT4b colon cancer: An international multicentre cohort study. Eur J Surg Oncol. 2021;47:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 29. | Kim BC, Bae JH, Park SM, Won DY, Lee IK. Is ascites CEA a risk factor for peritoneal carcinomatosis in colorectal cancer? Int J Colorectal Dis. 2020;35:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Akhtar M, Haider A, Rashid S, Al-Nabet ADMH. Paget's "Seed and Soil" Theory of Cancer Metastasis: An Idea Whose Time has Come. Adv Anat Pathol. 2019;26:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 182] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 31. | Mo S, Dai W, Xiang W, Li Q, Wang R, Cai G. Predictive factors of synchronous colorectal peritoneal metastases: Development of a nomogram and study of its utilities using decision curve analysis. Int J Surg. 2018;54:149-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 32. | de Boer NL, Rovers K, Burger JWA, Madsen EVE, Brandt-Kerkhof ARM, Kok NFM, de Wilt JHW, de Reuver PH, Bos A, de Hingh IHJT, Verhoef C. A population-based study on the prognostic impact of primary tumor sidedness in patients with peritoneal metastases from colon cancer. Cancer Med. 2020;9:5851-5859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Shida D, Inoue M, Tanabe T, Moritani K, Tsukamoto S, Yamauchi S, Sugihara K, Kanemitsu Y. Prognostic impact of primary tumor location in Stage III colorectal cancer-right-sided colon versus left-sided colon versus rectum: a nationwide multicenter retrospective study. J Gastroenterol. 2020;55:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 34. | Le VH, Thornblade L, Ituarte PHG, Lai LL, Melstrom KA. Metachronous peritoneal metastases following curative resection for colon cancer: Understanding risk factors and patterns of recurrence. J Surg Oncol. 2021;123:622-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Price TJ, Beeke C, Ullah S, Padbury R, Maddern G, Roder D, Townsend AR, Moore J, Roy A, Tomita Y, Karapetis C. Does the primary site of colorectal cancer impact outcomes for patients with metastatic disease? Cancer. 2015;121:830-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 36. | Hsu YL, Lin CC, Jiang JK, Lin HH, Lan YT, Wang HS, Yang SH, Chen WS, Lin TC, Lin JK, Lin PC, Chang SC. Clinicopathological and molecular differences in colorectal cancer according to location. Int J Biol Markers. 2019;34:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 37. | Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene. 2010;29:2893-2904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 38. | Huang CJ, Jiang JK, Chang SC, Lin JK, Yang SH. Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine (Baltimore). 2016;95:e5177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Huang C, Liu Z, Xiao L, Xia Y, Huang J, Luo H, Zong Z, Zhu Z. Clinical Significance of Serum CA125, CA19-9, CA72-4, and Fibrinogen-to-Lymphocyte Ratio in Gastric Cancer With Peritoneal Dissemination. Front Oncol. 2019;9:1159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 40. | Yang C, Yang Y, Huang X, Li H, Cheng H, Tong S, Zheng Y. A Nomogram Based on Clinicopathologic Features and Preoperative Hematology Parameters to Predict Occult Peritoneal Metastasis of Gastric Cancer: A Single-Center Retrospective Study. Dis Markers. 2020;2020:1418978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 41. | Kermanshahi TR, Magge D, Choudry H, Ramalingam L, Zhu B, Pingpank J, Ahrendt S, Holtzman M, Zeh H, Bartlett D, Zureikat A, Pai RK. Mucinous and Signet Ring Cell Differentiation Affect Patterns of Metastasis in Colorectal Carcinoma and Influence Survival. Int J Surg Pathol. 2017;25:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 42. | Afrăsânie VA, Marinca MV, Alexa-Stratulat T, Gafton B, Păduraru M, Adavidoaiei AM, Miron L, Rusu C. KRAS, NRAS, BRAF, HER2 and microsatellite instability in metastatic colorectal cancer - practical implications for the clinician. Radiol Oncol. 2019;53:265-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 43. | Wan XB, Wang AQ, Cao J, Dong ZC, Li N, Yang S, Sun MM, Li Z, Luo SX. Relationships among KRAS mutation status, expression of RAS pathway signaling molecules, and clinicopathological features and prognosis of patients with colorectal cancer. World J Gastroenterol. 2019;25:808-823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 44. | Grothey A, Fakih M, Tabernero J. Management of BRAF-mutant metastatic colorectal cancer: a review of treatment options and evidence-based guidelines. Ann Oncol. 2021;32:959-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 132] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 45. | Price TJ, Hardingham JE, Lee CK, Weickhardt A, Townsend AR, Wrin JW, Chua A, Shivasami A, Cummins MM, Murone C, Tebbutt NC. Impact of KRAS and BRAF Gene Mutation Status on Outcomes From the Phase III AGITG MAX Trial of Capecitabine Alone or in Combination With Bevacizumab and Mitomycin in Advanced Colorectal Cancer. J Clin Oncol. 2011;29:2675-2682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 46. | Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D, Kaplan R, Quirke P, Seymour MT, Richman SD, Meijer GA, Ylstra B, Heideman DA, de Haan AF, Punt CJ, Koopman M. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res. 2014;20:5322-5330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 580] [Article Influence: 52.7] [Reference Citation Analysis (0)] |
| 47. | Prasanna T, Karapetis CS, Roder D, Tie J, Padbury R, Price T, Wong R, Shapiro J, Nott L, Lee M, Chua YJ, Craft P, Piantadosi C, Sorich M, Gibbs P, Yip D. The survival outcome of patients with metastatic colorectal cancer based on the site of metastases and the impact of molecular markers and site of primary cancer on metastatic pattern. Acta Oncol. 2018;57:1438-1444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 48. | Lipsyc M, Yaeger R. Impact of somatic mutations on patterns of metastasis in colorectal cancer. J Gastrointest Oncol. 2015;6:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 49. | Yokota T, Ura T, Shibata N, Takahari D, Shitara K, Nomura M, Kondo C, Mizota A, Utsunomiya S, Muro K, Yatabe Y. BRAF mutation is a powerful prognostic factor in advanced and recurrent colorectal cancer. Br J Cancer. 2011;104:856-862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 319] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 50. | Yalcin S, Onguru O. BRAF mutation in colorectal carcinomas with signet ring cell component. Cancer Biol Med. 2017;14:287-292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Sun BL. Current Microsatellite Instability Testing in Management of Colorectal Cancer. Clin Colorectal Cancer. 2021;20:e12-e20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 52. | Lochhead P, Kuchiba A, Imamura Y, Liao X, Yamauchi M, Nishihara R, Qian ZR, Morikawa T, Shen J, Meyerhardt JA, Fuchs CS, Ogino S. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J Natl Cancer Inst. 2013;105:1151-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 349] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 53. | Gelsomino F, Barbolini M, Spallanzani A, Pugliese G, Cascinu S. The evolving role of microsatellite instability in colorectal cancer: A review. Cancer Treat Rev. 2016;51:19-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 205] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 54. | Fujiyoshi K, Yamamoto G, Takenoya T, Takahashi A, Arai Y, Yamada M, Kakuta M, Yamaguchi K, Akagi Y, Nishimura Y, Sakamoto H, Akagi K. Metastatic Pattern of Stage IV Colorectal Cancer with High-Frequency Microsatellite Instability as a Prognostic Factor. Anticancer Res. 2017;37:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 55. | Goldstein J, Tran B, Ensor J, Gibbs P, Wong HL, Wong SF, Vilar E, Tie J, Broaddus R, Kopetz S, Desai J, Overman MJ. Multicenter retrospective analysis of metastatic colorectal cancer (CRC) with high-level microsatellite instability (MSI-H). Ann Oncol. 2014;25:1032-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 216] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 56. | Aasebø KØ, Dragomir A, Sundström M, Mezheyeuski A, Edqvist PH, Eide GE, Ponten F, Pfeiffer P, Glimelius B, Sorbye H. Consequences of a high incidence of microsatellite instability and BRAF-mutated tumors: A population-based cohort of metastatic colorectal cancer patients. Cancer Med. 2019;8:3623-3635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 44] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 57. | Ooki A, Akagi K, Yatsuoka T, Asayama M, Hara H, Takahashi A, Kakuta M, Nishimura Y, Yamaguchi K. Combined microsatellite instability and BRAF gene status as biomarkers for adjuvant chemotherapy in stage III colorectal cancer. J Surg Oncol. 2014;110:982-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Klingbiel D, Saridaki Z, Roth AD, Bosman FT, Delorenzi M, Tejpar S. Prognosis of stage II and III colon cancer treated with adjuvant 5-fluorouracil or FOLFIRI in relation to microsatellite status: results of the PETACC-3 trial. Ann Oncol. 2015;26:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 59. | Tran B, Kopetz S, Tie J, Gibbs P, Jiang ZQ, Lieu CH, Agarwal A, Maru DM, Sieber O, Desai J. Impact of BRAF mutation and microsatellite instability on the pattern of metastatic spread and prognosis in metastatic colorectal cancer. Cancer. 2011;117:4623-4632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 596] [Article Influence: 42.6] [Reference Citation Analysis (0)] |
| 60. | Smith CG, Fisher D, Claes B, Maughan TS, Idziaszczyk S, Peuteman G, Harris R, James MD, Meade A, Jasani B, Adams RA, Kenny S, Kaplan R, Lambrechts D, Cheadle JP. Somatic profiling of the epidermal growth factor receptor pathway in tumors from patients with advanced colorectal cancer treated with chemotherapy ± cetuximab. Clin Cancer Res. 2013;19:4104-4113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 61. | Kim CG, Ahn JB, Jung M, Beom SH, Kim C, Kim JH, Heo SJ, Park HS, Kim NK, Min BS, Kim H, Koom WS, Shin SJ. Effects of microsatellite instability on recurrence patterns and outcomes in colorectal cancers. Br J Cancer. 2016;115:25-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 125] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 62. | Dohan A, Hoeffel C, Soyer P, Jannot AS, Valette PJ, Thivolet A, Passot G, Glehen O, Rousset P. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br J Surg. 2017;104:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 63. | Dresen RC, De Vuysere S, De Keyzer F, Van Cutsem E, Prenen H, Vanslembrouck R, De Hertogh G, Wolthuis A, D'Hoore A, Vandecaveye V. Whole-body diffusion-weighted MRI for operability assessment in patients with colorectal cancer and peritoneal metastases. Cancer Imaging. 2019;19:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 73] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 64. | Zhang H, Dai W, Fu C, Yan X, Stemmer A, Tong T, Cai G. Diagnostic value of whole-body MRI with diffusion-weighted sequence for detection of peritoneal metastases in colorectal malignancy. Cancer Biol Med. 2018;15:165-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 65. | van 't Sant I, Engbersen MP, Bhairosing PA, Lambregts DMJ, Beets-Tan RGH, van Driel WJ, Aalbers AGJ, Kok NFM, Lahaye MJ. Diagnostic performance of imaging for the detection of peritoneal metastases: a meta-analysis. Eur Radiol. 2020;30:3101-3112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 97] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 66. | Honma Y, Terauchi T, Tateishi U, Kano D, Nagashima K, Shoji H, Iwasa S, Takashima A, Kato K, Hamaguchi T, Boku N, Shimada Y, Yamada Y. Imaging peritoneal metastasis of gastric cancer with (18)F-fluorothymidine positron emission tomography/computed tomography: a proof-of-concept study. Br J Radiol. 2018;91:20180259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |