Published online Sep 15, 2022. doi: 10.4251/wjgo.v14.i9.1727
Peer-review started: March 14, 2022
First decision: April 11, 2022
Revised: April 18, 2022
Accepted: July 31, 2022
Article in press: July 31, 2022
Published online: September 15, 2022
Processing time: 178 Days and 22.7 Hours
In microwave ablation (MWA), although computed tomography (CT) scanning can overcome gas interference, it cannot achieve real-time localization. Therefore, the puncture technique is more important in CT-guided ablation.
To compare the fine needle-assisted puncture (FNP) positioning technique and the conventional puncture (CP) technique for the safety and efficacy of CT-guided MWA in treating hepatocellular carcinoma (HCC).
This retrospective study included 124 patients with 166 tumor nodules from February 2018 and June 2021. Seventy patients received CT-guided MWA under the FNP technique (FNP group), and 54 patients received MWA under the CP technique (CP group). Intergroup comparisons were made regarding local tumor progression (LTP), recurrence-free survival (RFS), overall survival (OS), and complications. The influencing variables of LTP and RFS were analyzed through univariate and multivariate regressions.
The 1-, 2-, and 3-year cumulative incidences of LTP in the FNP group were significantly lower than those in the CP group (7.4%, 12.7%, 21.3% vs 13.7%, 32.9%, 36.4%; P = 0.038). The 1-, 2-, and 3-year RFS rates in the FNP group were significantly higher than those in the CP group (80.6%, 73.3%, 64.0% vs 83.3%, 39.4%, and 32.5%, respectively; P = 0.008). The FNP technique independently predicted LTP and RFS. Minor complications in the FNP group were lower than those in the CP group (P < 0.001). The difference in median OS was insignificant between the FNP and CP groups (P = 0.229).
The FNP technique used in CT-guided MWA may improve outcomes in terms of LTP, RFS, and procedure-related complications for HCC.
Core Tip: This was a retrospective study that compared the fine needle-assisted puncture positioning (FNP) technique and conventional puncture (CP) technique for the safety and efficacy of computed tomography (CT)-guided microwave ablation (MWA) in treating hepatocellular carcinoma (HCC). In total, 124 patients were divided into two groups by the puncture technique. Seventy patients received CT-guided MWA under the FNP technique (FNP group), and 54 patients received MWA under the CP technique (CP group). The FNP technique used in CT-guided MWA may improve outcomes in terms of local tumor progression, recurrence-free survival, and procedure-related complications for HCC.
- Citation: Hao MZ, Hu YB, Chen QZ, Chen ZX, Lin HL. Efficacy and safety of computed tomography-guided microwave ablation with fine needle-assisted puncture positioning technique for hepatocellular carcinoma. World J Gastrointest Oncol 2022; 14(9): 1727-1738
- URL: https://www.wjgnet.com/1948-5204/full/v14/i9/1727.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i9.1727
Apart from being the world’s sixth most frequently diagnosed malignancy, hepatocellular carcinoma (HCC) is also the third primary reason for cancer-associated mortality on a global scale. In 2018, about 841080 new incidences and 781631 mortalities were caused by HCC[1]. For nearly 20 years since the 1990s, HCC has been managed by percutaneous radiofrequency ablation (RFA) and microwave ablation (MWA). The development of ablation technology is of extensive value in the treatment of HCC[2]. It is considered the third major treatment for HCC, following surgical resection and liver transplantation[3].
Tumors located ≤ 5 mm from large vessels, gallbladder, gastrointestinal tract, diaphragm, or liver capsule have been defined as high-risk locations (HRLs), which are contraindicated for RFA treatment[4,5]. The RFA of tumors lying close to large vessels with at least 3 mm in diameter is often incomplete due to the heat sink effect[6]. Compared with RFA, MWA can shorten the ablation time, increase the local temperature faster, reduce the heat sink effect of adjacent vessels, and simultaneously use multiple therapeutic probes[7]. Due to these advantages, MWA is more attractive in the ablation of HCC[8].
Ultrasound (US) and computed tomography (CT) are the most commonly applied image guidance methods for MWA. Due to the influence of the gas, the subphrenic area is one of the most difficult places for ultrasound guidance[9]. A CT scan can compensate for this shortcoming. CT-guided punc
Although a CT-guided stereotactic navigation system can provide accurate puncture path planning, the equipment is still not popularized due to economic reasons[11,12]. The fine needle-assisted puncture (FNP) positioning technique is to insert a separate fine needle (21G) near the tumor nodule as the positioning and marking of the microwave antenna insertion to improve the success rate of microwave needle puncture. Although Wu et al[13] recently confirmed that the FNP technique is a safe and effective puncture auxiliary technique for CT-guided biopsy or MWA of small tumor nodules near the dia
The current work investigated the efficacy and safety of CT-guided MWA for HCC under the FNP and CP techniques.
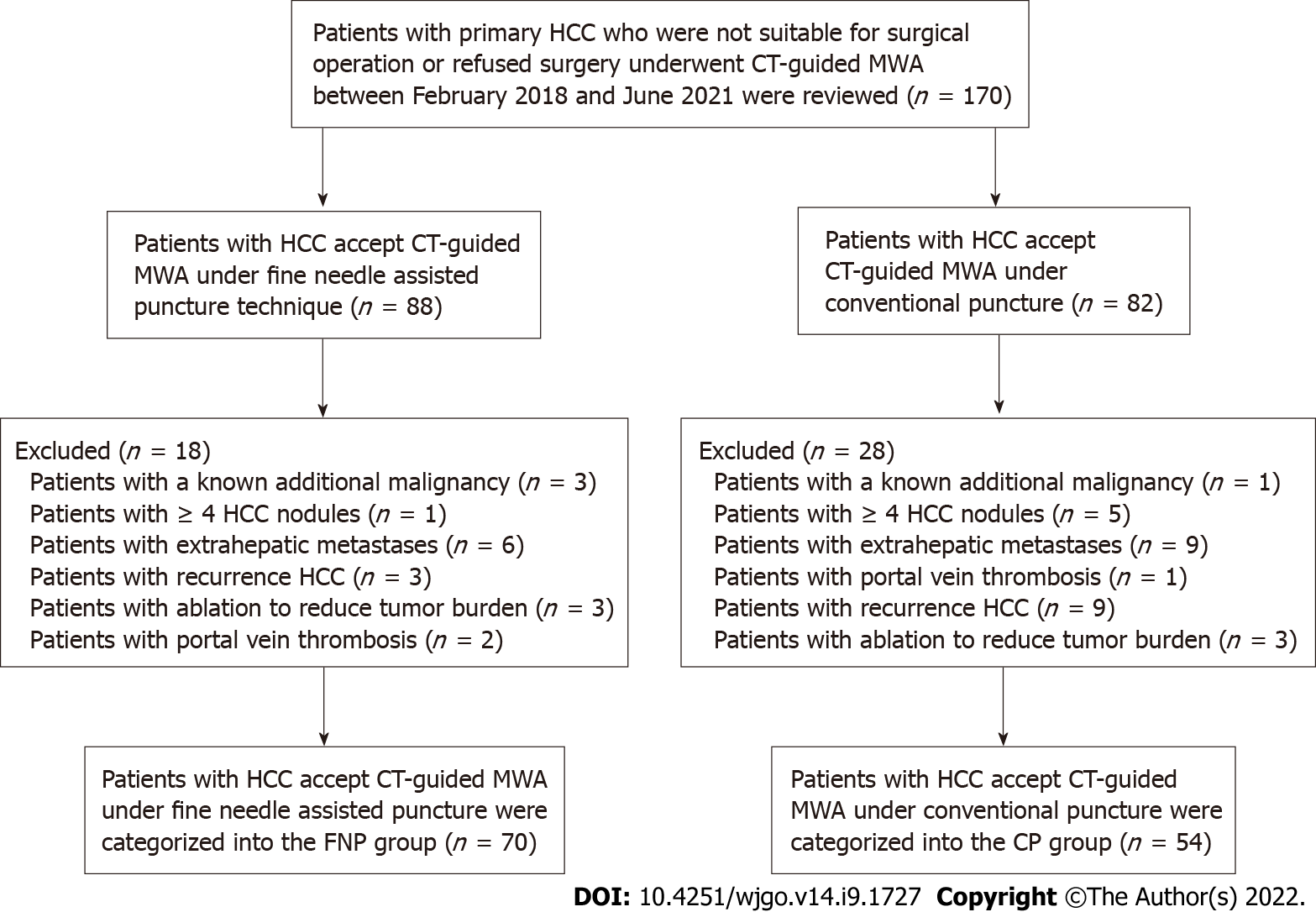
We retrospectively analyzed 170 patients with consecutive primary HCC, who received CT-guided MWA or transarterial chemoembolization (TACE) combined with MWA at our hospital from February 2018 to June 2021. These patients were either inappropriate for or rejected surgery. The diagnosis of HCC was verified by imaging and serum alpha-fetoprotein (AFP) assay or hepatic needle biopsy as per the Chinese guidelines for the primary liver cancer diagnosis and treatment (2017 edition).
The inclusion criteria were: Child-Pugh class A or B, single tumor with the largest diameter ≤ 5 cm before MWA, 2-3 tumors with the largest diameter ≤ 5 cm, Eastern Cooperative Oncology Group performance status (ECOG PS) 0-1, and patients with platelet count > 50 × 109/L. The exclusion criteria were: Patients with a known additional malignancy that is progressing concurrently; patients with portal vein thrombosis or extrahepatic metastases; patients with ≥ 4 HCC nodules; patients with ablation to reduce tumor burden; and recurrent HCC, except for recurrence after resection.
Figure 1 shows 4 patients with a known additional malignancy that is progressing concurrently, 6 patients with ≥ 4 HCC nodules, 15 patients with extrahepatic metastases, 3 patients with portal vein thrombosis, 6 patients with ablation to reduce tumor burden, and 12 patients with recurrent HCC after treatment other than surgery. For 17 patients who had intrahepatic distal recurrence (IDR) and 4 patients with local tumor progression (LTP) who underwent MWA twice or more, the second or subsequent MWA procedure was excluded from the study to avoid statistical bias caused by the repetition of patient data twice.
Thus, 124 patients with 166 nodules were incorporated in the current study. Patients were divided into two groups according to the microwave needle puncture methods. Seventy patients received CT-guided MWA under the FNP technique and were categorized into the FNP group, whereas 54 patients received CT-guided MWA supported with a conventional puncture and were categorized into the CP group. The operators of all TACE and MWA procedures belonged to the same attending physician team, and the chief operator in the CT-guided MWA procedures of both groups had at least 15 years of experience in RFA. The choice of puncture method was not based on the tumor size, tumor number, and tumor location. The corresponding author and the assistant performed CT-guided MWA under the FNP technique, whereas another chief operator and assistant performed CT-guided MWA under the CP technique.
Study protocol approval was obtained from the corresponding ethics committee (2018-022-02). The experimental procedures conformed to the principles of the Declaration of Helsinki. Before the retrospective study, each patient provided written informed consent.
MWA procedure: MTC-3 C MWA equipment (Yigao Microwave System Engineering Co. Ltd., Nanjing, Jiangsu Province, China) was used at 2450 MHz (± 10%) in a continuous wave mode and 5-120 W ± 30% power output. The MWA antenna was 1.8 mm in diameter with a surface coating. Before MWA, the tumor size, number, site, and relationship with important structures were evaluated by contrast-enhanced magnetic resonance imaging (MRI) and/or helical CT scan. A multidisciplinary team comprising a liver surgeon, radiologist, sonographer, oncologist, and interventional radiologist created the treatment plans for patients with HCC.
Percutaneous CT-guided MWA was conducted on an inpatient basis under local anesthesia and analgesics. The patient was awake during the MWA procedures. Before performing MWA, each tumor’s antenna layout, power output setup, and emission duration were meticulously planned. A single MWA antenna was used in the nodules ≤ 1.7 cm; if > 1.7 cm, then a double-needle was used, keeping a space of 2.0 cm between the two needles. The ablation margin was kept between 5 and 10 mm at 60 and 70 W for 5 to 10 min. After treatment, the needle was gradually withdrawn with a parallel needle tract ablation. For tumors under the liver capsule attached to the diaphragm, intestinal tube, or gallbladder, saline was injected between the target lesion and the adjacent organs, a process called hydrodissection to protect them from possible heat damage if a safe distance could not be maintained. Adjuvant hydrodissection techniques were performed on 2 patients.
A CT scan was acquired immediately after treatment and again 24 h after operation to evaluate the margin of ablation and complications, such as bleeding and pneumothorax. Nearly 2 mo post-operation, treatment response was evaluated through contrast-enhanced MRI or helical CT examination. Complete ablation was verified based on the absence of enhanced areas. In the case of incomplete ablation, a second ablation was considered. An ablation failure was indicated if complete ablation was not achieved even after two ablations, and other treatment methods were applied.
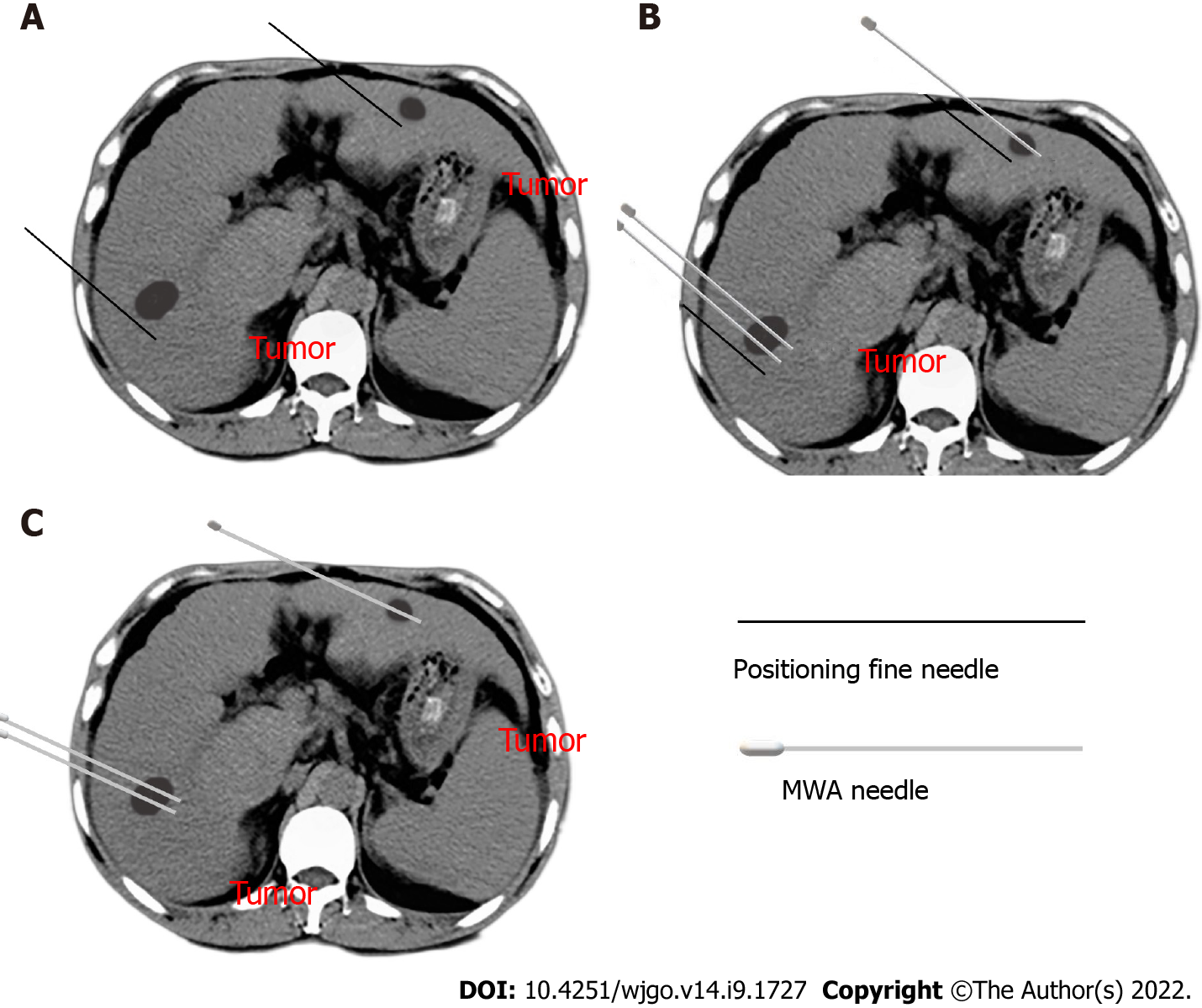
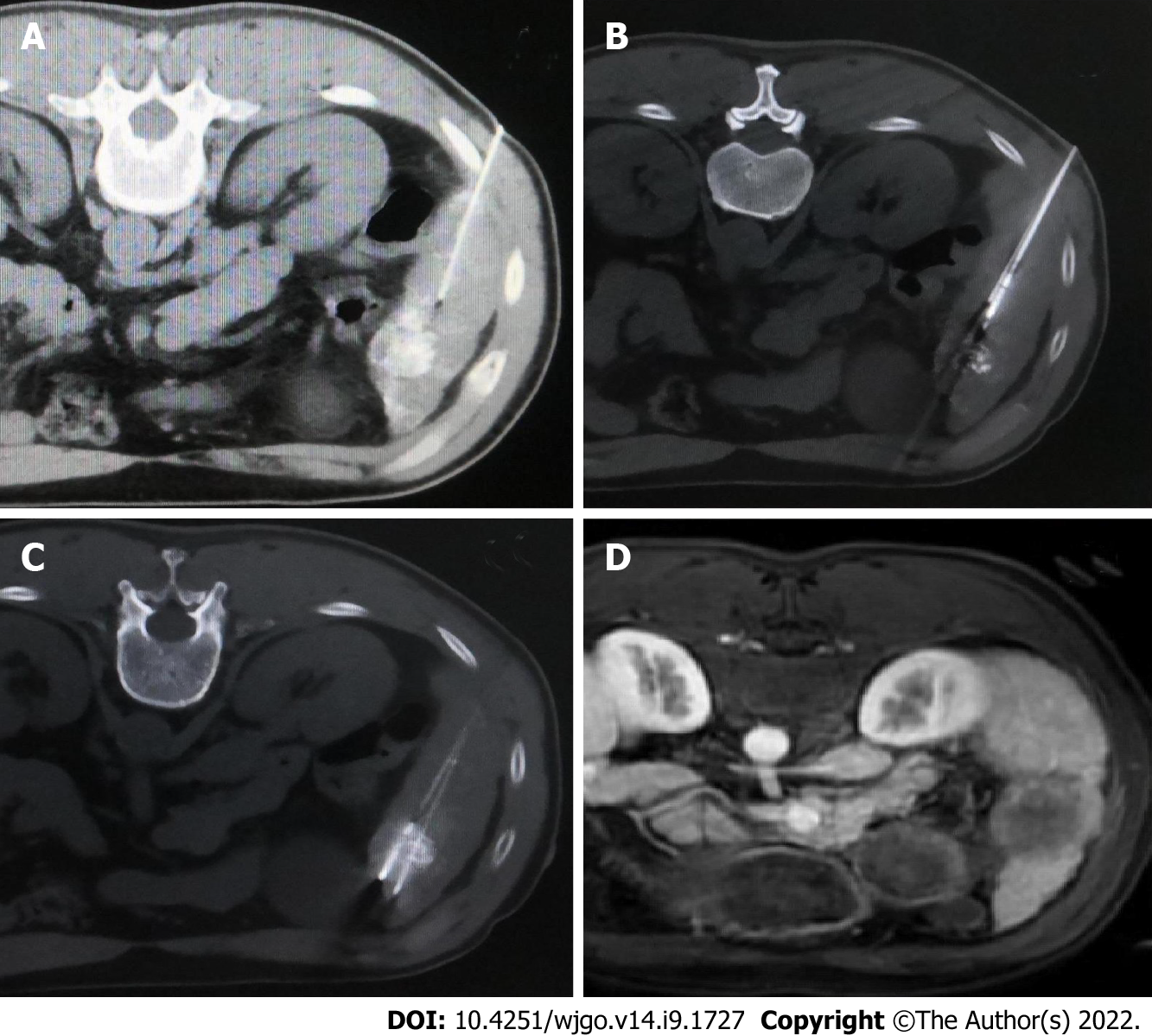
The MWA puncture technique: Before the operation, the ablation needle electrode was selected based on the size and location of the tumor, and the puncture angle and depth were set under CT guidance. The percutaneous transhepatic puncture was performed by free hands following a detailed procedure. In the CP group, the needle path had to pass through the normal liver tissue at > 1.5 cm, avoiding large blood vessels and bile ducts. CT scanning was repeated at half the depth of needle insertion to observe the relationship between the electrode needle or tumor and the surrounding tissue structure. Next, the puncture angle was adjusted, and the needle was inserted gradually inside the tumor and approximately 0.5 to 1 cm beyond the tumor margin. In the FNP group, a 21-gauge fine needle of 15 cm in length was inserted near the tumor nodule before ablation needle puncture as a marker (Figure 2A). Following that, CT scanning was used to determine the angle and direction of the MWA electrode needle puncture based on fine-needle marking. Subsequently, the microwave electrode needle was step by step gradually inserted inside the tumor and approximately 0.5 to 1 cm beyond the tumor margin, and each step was confirmed by a CT scan (Figure 2B). Afterward, the fine needle was pulled out after the electrode needle was consistent with the plan confirmed by CT scanning (Figure 2C). Figure 3 shows a sequence of images of a patient with an HCC nodule in segment 5 treated with CT-guided MWA by the FNP technique after TACE.
Assessment of the outcome: The patients were followed up 2 mo after MWA and then every 3 to 6 mo. Follow-up included general, physical, imaging, and laboratory examinations such as biochemistry and tumor marker levels. LTP was indicated when following thorough tumor ablation, any new lesions connected to ablation focus were seen at the focus rim[14]. IDR was defined as the appearance of any new lesions distant from the ablation zone (excluding extrahepatic metastasis)[15,16]. Recurrent-free survival (RFS) refers to the duration between the first ablation and the final follow-up or the tumor recurrence including LTP and IDR, whereas overall survival (OS) refers to the duration between the time of diagnosis and the time of death or date of the last follow-up. Under the Society of Interventional Radiology Classification System, we classified the complications into major and minor types[17]. In addition, we monitored the hospital stay of patients after treatment completion. Follow-up was continued through December 25, 2021, and the median follow-up time was 22.6 mo (range: 6.0-43.4 mo).
Student’s t-test was used to assess the mean difference, whereas the χ2 test was adopted for frequency distribution comparison. The LTP, RFS, and OS were tested using the Kaplan–Meier technique and log-rank tests. The multifactor influences on LTP and RFS were examined through Cox regression analysis. Differences were regarded as significant when the two-tailed P < 0.05. SPSS v24 was used for data processing and analysis.
The patient information and tumor characteristics are detailed in Table 1. Between the two groups, there were insignificant differences in age, sex, ECOG PS, AFP, tumor number, tumor size, tumor location, and the application rate of combined TACE therapy before MWA. At the end of the follow-up, no significant difference was observed in the detection rate of LTP between the two groups (P = 0.063). The liver function of all patients was Child-Pugh class A before MWA. A total of 91 patients were treated with TACE combined with MWA in 124 patients. MWA was conducted approximately 4 wk after TACE. No patient achieved complete remission before ablation after TACE as per the Response Evaluation Criteria in Solid Tumors criteria. There was an insignificant difference in post-MWA hospital stay between both groups (P = 0.130).
| Variables | FNP group, n = 70 | CP group, n = 54 | P value |
| Age (yr)1 | 58.6 ± 1.7 | 59.4 ± 1.4 | 0.728 |
| Sex (M/F) | 60/10 | 48/6 | 0.601 |
| ECOG PS | 0.200 | ||
| 0 | 22 | 23 | |
| 1 | 48 | 31 | |
| AFP (ng/mL) | 0.530 | ||
| < 400 | 61 | 49 | |
| ≥ 400 | 9 | 5 | |
| No. of nodules in each patient | 0.871 | ||
| 1 | 47 | 37 | |
| 2-3 | 23 | 17 | |
| Total no. of nodules | 93 | 73 | |
| Tumor size before MWA (cm) | 0.922 | ||
| < 3 | 50 | 39 | |
| 3-5 | 20 | 15 | |
| Mean tumor diameter (cm) pre-MWA | 2.3 ± 0.1 | 2.2 ± 0.2 | 0.711 |
| Proportion of TACE prior to MWA | 50 (71.4%) | 41 (75.9%) | 0.574 |
| Post-MWA hospital stay (d)1 | 3.3 ± 0.3 | 3.9 ± 0.2 | 0.130 |
| Tumor location | |||
| HRL | 49 | 34 | 0.409 |
| Liver subcapsular region | 30 | 16 | |
| Diaphragmatic surface | 7 | 4 | |
| Adjacent to large vessel | 8 | 7 | |
| Adjacent to gallbladder | 3 | 4 | |
| Adjacent to gastrointestinal tract | 1 | 3 | |
| LRL | 21 | 20 | |
| LTP | 11 (15.7%) | 16 (29.6%) | 0.063 |
One patient in the FNP group and 2 patients in the CP group were detected to have residual tumor by MRI scan 2 mo post-MWA, which was completely ablated by MWA again. The one-stage ablation success rate was 98.6% in the FNP group and 96.3% in the CP group (P = 0.820). During the follow-up, LTP was detected in 11 (15.7%) patients in the FNP group and 16 (29.6%) patients in the CP group. In the last follow-up, 6 patients were dead, 5 were lost to the follow-up, and 113 were alive.
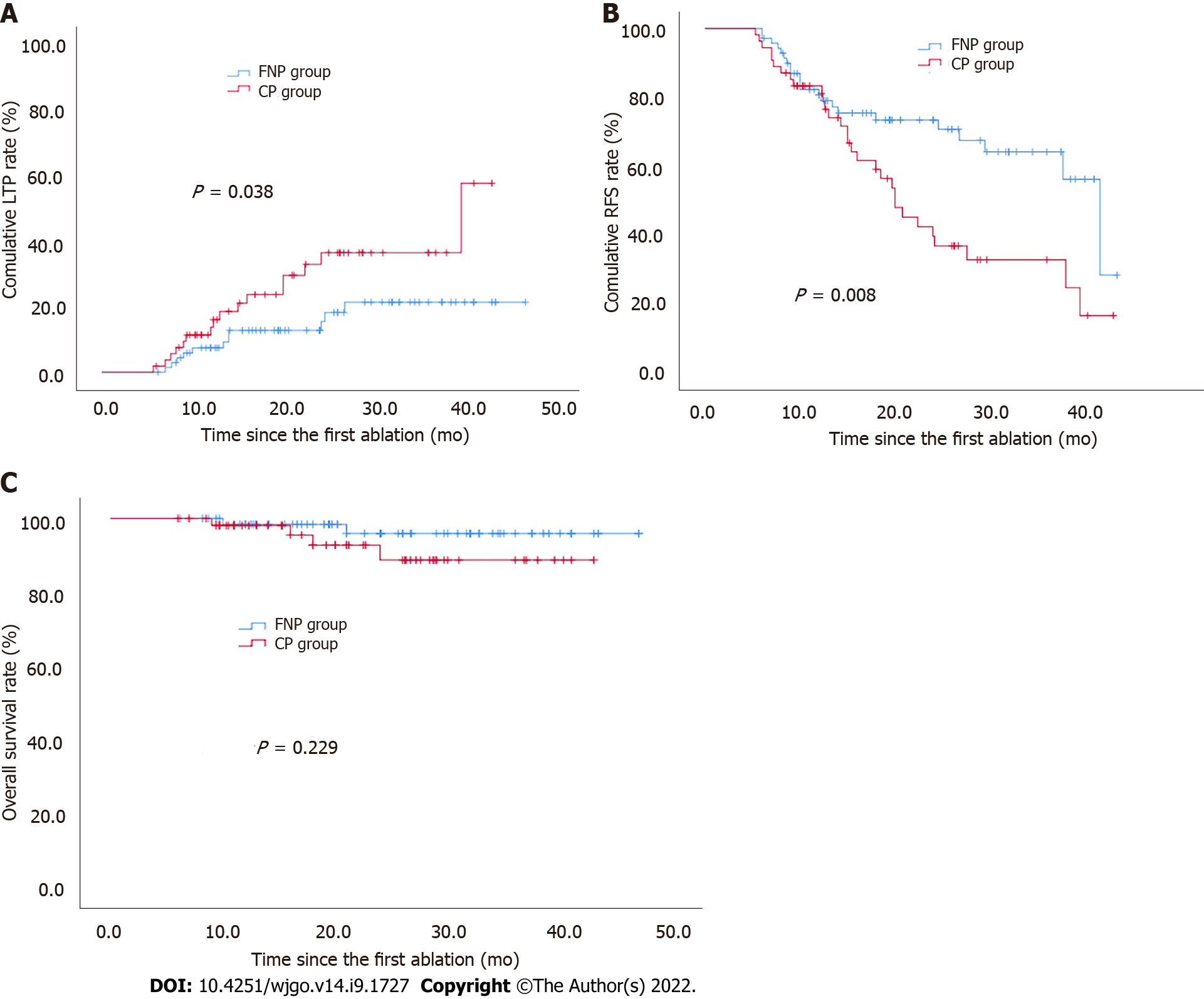
The 1-, 2-, and 3-year cumulative incidences of LTP in the FNP group were significantly lower than those in the CP group (7.4%, 12.7%, 21.3% vs 13.7%, 32.9%, 36.4%, respectively; P = 0.038 via log-rank test, Figure 4A).
The 1-, 2-, and 3-year RFS rates in the FNP group were significantly higher than those in the CP group (80.6%, 73.3%, 64.0% vs 83.3%, 39.4%, and 32.5%, respectively; P = 0.008 via log-rank test, Figure 4B).
The 1- and 2-year OS rates were 98.4% and 96.0% in the FNP group and 98.1% and 88.8% in the CP group, with a median OS of 45.8 mo [95% confidence interval (CI): 44.1-47.4] and 40.2 mo (95%CI: 37.6-42.8) (P = 0.229 via log-rank test, Figure 4C).
According to univariate analysis, ECOG PS, tumor number, and puncture method were significantly related to LTP. According to multivariate analysis, tumor number (≥ 2) was independently related to a poor LTP and superior ECOG PS, and the FNP technique was independently related to a good LTP (Table 2).
| Factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | 0.706 (0.166, 2.966) | 0.637 | ||
| Age (yr): > 60; 60 | 1.041 (0.486, 2.229) | 0.918 | ||
| ECOG PS: 0; 1 | 3.169 (1.197, 8.392) | 0.020 | 2.979 (1.108, 8.014) | 0.031 |
| Tumor number: 1; 2-3 | 3.370 (1.561, 7.277) | 0.002 | 3.008 (1.383, 6.546) | 0.005 |
| AFP (ng/mL): < 400; ≥ 400 | 0.601 (0.142, 2.538) | 0.488 | ||
| Tumor size (cm): < 3; 3-5 | 1.649 (0.754, 3.603) | 0.210 | ||
| Auxiliary TACE pre-MWA: Yes; No | 1.232 (0.497, 3.055) | 0.653 | ||
| Tumor location: HRL; LRL | 1.523 (0.937, 2.476) | 0.090 | ||
| Puncture method: FNP technique; CP technique | 2.205 (1.021, 4.761) | 0.044 | 2.596 (1.197, 5.631) | 0.016 |
Univariate analysis indicated that ECOG PS, tumor number, and puncture method were significantly related to RFS. Multivariate analysis indicated that tumor number (≥ 2) was independently associated with a poor RFS, and the FNP technique was independently associated with a good RFS (Table 3).
| Factors | Univariate analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Sex | 0.547 (0.170, 1.764) | 0.313 | ||
| Age (yr): > 60; 60 | 1.021 (0.590, 1.767) | 0.942 | ||
| ECOG PS: 0; 1 | 1.831 (1.008, 3.325) | 0.047 | 1.609 (0.878, 2.948) | 0.124 |
| Tumor number: 1; 2-3 | 3.692 (2.112, 6.456) | < 0.001 | 3.910 (2.195, 6.966) | < 0.001 |
| AFP (ng/mL): < 400; ≥ 400 | 0.606 (0.218, 1.682) | 0.336 | ||
| Tumor size (cm): < 3; 3-5 | 1.491 (0.841, 2.642) | 0.171 | ||
| Auxiliary TACE pre-MWA: Yes; No | 0.947 (0.504, 1.780) | 0.867 | ||
| Tumor location: HRL; LRL | 1.060 (0.789, 1.424) | 0.699 | ||
| Puncture method: FNP technique; CP technique | 2.078 (1.196, 3.612) | 0.009 | 2.484 (1.415, 4.359) | 0.002 |
In the subgroup analysis stratified by tumor number, the median time to LTP in the FNP group was significantly longer than that in the CP group for patients with a single tumor (44.9 ± 1.2 vs 33.7 ± 2.4 mo, respectively; P = 0.005 via log-rank test).
Furthermore, in the subgroup analysis stratified by tumor number, the median RFS time in the FNP group was considerably higher than that in the CP group for patients with a single tumor (37.3 ± 1.8 vs 28.5 ± 2.5 mo, respectively; P = 0.013 via log-rank test).
No deaths were directly related to the early complications of MWA. Table 4 shows the frequency of complications in all patients. The FNP group had two cases of major complications. One patient diagnosed with bacteremia recovered with anti-infective therapy, whereas another patient developed massive pneumothorax and recovered by thoracic drainage. The CP group had 4 cases of massive pneumothorax recovered by thoracic drainage. The intergroup differences were insignificant regarding major complications (P = 0.454). The CP group exhibited more minor complications, including postoperative pain and fever compared to those in the FNP group (P < 0.001).
| Type of reactions | FNP group, n = 70 | CP group, n = 54 | χ2 | P value |
| Major complications | 0.561 | 0.454 | ||
| Bacteremia | 1 | 0 | ||
| Pneumothorax | 1 | 4 | ||
| Total | 2 | 4 | ||
| Minor complications | 12.345 | < 0.001 | ||
| Postoperative pain | 15 | 11 | ||
| Postoperative fever | 2 | 7 | ||
| Self-limiting pneumothorax | 2 | 1 | ||
| Self-limiting pleural effusion | 3 | 0 | ||
| Transient elevation of aminotransferase | 18 | 27 | ||
| Bleeding at the probe-inserting point | 1 | 2 | ||
| Total | 41 | 48 |
According to the outcomes of this study, although no significant difference was observed in 1- and 2-year OS rates and median OS between the FNP and CP groups, the FNP technique may improve outcomes in terms of LTP, RFS, and procedure-related complications for HCC treated with CT-guided MWA. The FNP technique was independently associated with good LTP and RFS. In the subgroup analysis, the FNP technique may improve the median time to LTP and median RFS time for patients with a single HCC nodule. The results of this study are clinically important considering that the FNP technique has better efficacy and safety in CT-guided MWA compared to the CP technique.
Image guidance techniques play a critical role in MWA. Although no reports are available that compare the efficacies of US-guided and CT-guided MWA, several studies have demonstrated that both US-guided and CT-guided RFA are similar in terms of LTP and complete ablation[18,19]. In US-guided ablation, there are blind spots and vaporization interferences[20]; these disadvantages can be overcome by CT. However, it is a non–real-time image guidance technology. Repeated CT scans caused by unskilled puncture techniques can significantly increase radiation exposure. Thus, it has higher requirements for puncture technology under CT guidance.
A recent study reported that the FNP technique used in biopsy or MWA for small nodules near the diaphragm offered an improved puncture success rate and a low reduced radiation dose[13]. In this method, a fine needle puncture is made, which reduces the radiation exposure because the biopsy needle or MWA electrode needle can be inserted easily in the subsequent process. Repeated puncture in ablation procedure may lead to complications of needle bleeding, tumor implantation, and pneumothorax, etc[4,21]. Moreover, electrode needle placement is associated with complications such as bleeding, vascular injuries, and pneumothorax[22]. We used the FNP technique in CT-guided MWA procedures to treat HCC without limiting tumors near the diaphragm. The fine needle inserted near the tumor nodule could fix the liver and thoracoabdominal wall. The fine needle, liver, thoracoabdominal wall, and diaphragm are moved as a whole unit during breathing. The fine needle inserted near the tumor nodule can be used as a sign for subsequent electrode needle insertion and also help judge the patient's respiratory movement and reduce the error of subsequent puncture and puncture times. Due to the artifact of the microwave electrode needle in CT scanning, it is difficult to judge whether the position of the end of the electrode needle is consistent with the pre-designed position. The fine needle without artifact in CT scanning as a mark is helpful for the proper placement of the MWA electrode needle. The primary reason for the better performance of the FNP than CP in CT-guided MWA may be the reduction of puncture times and the appropriate MWA electrode needle placement. Univariate and multivariate analyses showed that the FNP technique was significantly related to good RFS and LTP, which further supported the advantages of the FNP technique.
Tumor number, tumor size, and performance status are important factors affecting tumor recurrence and survival[23-26]. In this study, tumor number (≥ 2) was independently associated with a poor LTP and RFS, as in previous studies. Nevertheless, tumor size was not an independent prognostic factor for LTP and RFS, and it may be attributed to the application of adjuvant TACE before MWA in 73.4% of patients. According to a meta-analysis, TACE + MWA contributed to prominently higher rates of local control and objective remission[27,28]. TACE + MWA achieved better efficacy than TACE or MWA monotherapy for managing HCC of 3 to 5 cm or even > 5 cm in size[29,30]. In this study, no significant difference was observed in the proportion of TACE before MWA between the two groups, which does not affect the primary results of the study.
This study had a few limitations, including the possibility of bias due to the retrospective analysis of a single-center small sample. Another limitation is the lack of comparison between FNP and CT-guided stereotactic navigation systems. Prospective multicenter studies must be conducted in the future to gain further insight.
The FNP technique used in CT-guided MWA in the current study may improve outcomes in terms of LTP, RFS, and procedure-related complications for HCC. The FNP technique was independently associated with good LTP and RFS. The results of this study have important clinical value in CT-guided MWA for HCC with the FNP technique.
Due to the influence of the gas, the subphrenic area is one of the most difficult places for ultrasound guidance. A computed tomography (CT) scan can compensate for this shortcoming. CT-guided ablation is a commonly used ablation image-guided method in our department.
CT-guided puncture does not allow for real-time positioning and the microwave electrode needle will produce artifacts in CT scanning, which is different from our previous application of radiofrequency ablation.
To compare fine needle-assisted puncture (FNP) positioning technique and conventional puncture technique for the safety and efficacy of CT-guided microwave ablation (MWA) in treating hepatocellular carcinoma (HCC).
The efficacy and safety were compared between the patients received CT-guided MWA under FNP technique and patients received MWA under conventional puncture technique.
The 1-, 2-, and 3-year cumulative incidences of local tumor progression (LTP) in the FNP group were significantly lower than those in the conventional puncture (CP) technique (CP group). The 1-, 2-, and 3-year RFS rates in the FNP group were significantly higher than those in the CP group. The FNP technique independently predicted LTP and recurrence-free survival (RFS). The minor complications in the FNP group were lower those in the CP group.
The FNP technique used in CT-guided MWA may improve outcomes in terms of LTP, RFS, and procedure-related complications for HCC.
Prospective multicenter randomized controlled studies must be conducted in the future to obtain further insights.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Anti-Cancer Association of China, No. M166300908s.
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Giacomelli L, Italy; Nakano M, Japan; Nakano M, Japan; Raissi D, United States S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
| 1. | Erratum: Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2020;70:313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 453] [Article Influence: 90.6] [Reference Citation Analysis (1)] |
| 2. | Zhu F, Rhim H. Thermal ablation for hepatocellular carcinoma: what's new in 2019. Chin Clin Oncol. 2019;8:58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 3. | Lee MW, Raman SS, Asvadi NH, Siripongsakun S, Hicks RM, Chen J, Worakitsitisatorn A, McWilliams J, Tong MJ, Finn RS, Agopian VG, Busuttil RW, Lu DSK. Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: A 10-year intention-to-treat analysis. Hepatology. 2017;65:1979-1990. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 4. | Wu MC, Tang ZY, Ye SL, Fan J, Qin SK, Yang JM, Chen MS, Chen MH, Lv MD, Ma KS, Wu YL, Chen Y, Qian GJ, Lu SC, Zheng JS, Sun WB, Zou YH, Liang HM, Huang ZY, Han XW, Jing X, Pan HM, Jiang TA, Liang P, Ren ZG, Zhang YJ; Chinese Society of Liver Cancer; Chinese Society of Clinical Oncology; Liver Cancer Group; Chinese Society of Hepatology. Expert consensus on local ablation therapies for primary liver cancer. Chin Clin Oncol. 2012;1:11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Foltz G. Image-guided percutaneous ablation of hepatic malignancies. Semin Intervent Radiol. 2014;31:180-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD 3rd, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT Jr, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN; International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology,; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 895] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 7. | Li X, Zhang L, Fan W, Zhao M, Wang L, Tang T, Jiang H, Zhang J, Liu Y. Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: results in ex vivo porcine livers. Int J Hyperthermia. 2011;27:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 355] [Article Influence: 59.2] [Reference Citation Analysis (1)] |
| 9. | Koda M, Ueki M, Maeda Y, Mimura K, Okamoto K, Matsunaga Y, Kawakami M, Hosho K, Murawaki Y. Percutaneous sonographically guided radiofrequency ablation with artificial pleural effusion for hepatocellular carcinoma located under the diaphragm. AJR Am J Roentgenol. 2004;183:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010;33:11-17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 195] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 11. | Bale R, Widmann G. Navigated CT-guided interventions. Minim Invasive Ther Allied Technol. 2007;16:196-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Engstrand J, Toporek G, Harbut P, Jonas E, Nilsson H, Freedman J. Stereotactic CT-Guided Percutaneous Microwave Ablation of Liver Tumors With the Use of High-Frequency Jet Ventilation: An Accuracy and Procedural Safety Study. AJR Am J Roentgenol. 2017;208:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Wu Q, Cao B, Zheng Y, Liang B, Liu M, Wang L, Zhang J, Meng L, Luo S, He X, Zhang Z. Feasibility and safety of fine positioning needle-mediated breathing control in CT-guided percutaneous puncture of small lung/liver nodules adjacent to diaphragm. Sci Rep. 2021;11:3411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Shady W, Petre EN, Do KG, Gonen M, Yarmohammadi H, Brown KT, Kemeny NE, D'Angelica M, Kingham PT, Solomon SB, Sofocleous CT. Percutaneous Microwave versus Radiofrequency Ablation of Colorectal Liver Metastases: Ablation with Clear Margins (A0) Provides the Best Local Tumor Control. J Vasc Interv Radiol. 2018;29:268-275.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 200] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 15. | Ishikawa T, Higuchi K, Kubota T, Seki K, Honma T, Yoshida T, Kamimura T. Prevention of intrahepatic distant recurrence by transcatheter arterial infusion chemotherapy with platinum agents for stage I/II hepatocellular carcinoma. Cancer. 2011;117:4018-4025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Chen HY, Lu SN, Hung CH, Wang JH, Chen CH, Yen YH, Kuo YH, Kee KM. Predicting outcomes for recurrent hepatocellular carcinoma within Milan criteria after complete radiofrequency ablation. PLoS One. 2020;15:e0242113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D; Society of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009;20:S189-S191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 198] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 18. | Yuan C, Yuan Z, Cui X, Gao W, Zhao P, He N, Cui S, Wang Y, Zhang Y, Li W, Zheng J. Efficacy of ultrasound-, computed tomography-, and magnetic resonance imaging-guided radiofrequency ablation for hepatocellular carcinoma. J Cancer Res Ther. 2019;15:784-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Lee LH, Hwang JI, Cheng YC, Wu CY, Lee SW, Yang SS, Yeh HZ, Chang CS, Lee TY. Comparable Outcomes of Ultrasound versus Computed Tomography in the Guidance of Radiofrequency Ablation for Hepatocellular Carcinoma. PLoS One. 2017;12:e0169655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Fahey BJ, Nelson RC, Hsu SJ, Bradway DP, Dumont DM, Trahey GE. In vivo guidance and assessment of liver radio-frequency ablation with acoustic radiation force elastography. Ultrasound Med Biol. 2008;34:1590-1603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Kim JW, Shin SS, Heo SH, Hong JH, Lim HS, Seon HJ, Hur YH, Park CH, Jeong YY, Kang HK. Ultrasound-Guided Percutaneous Radiofrequency Ablation of Liver Tumors: How We Do It Safely and Completely. Korean J Radiol. 2015;16:1226-1239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Mendiratta-Lala M, Brook OR, Midkiff BD, Brennan DD, Thornton E, Faintuch S, Sheiman RG, Goldberg SN. Quality initiatives: strategies for anticipating and reducing complications and treatment failures in hepatic radiofrequency ablation. Radiographics. 2010;30:1107-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Li Z, Wang C, Si G, Zhou X, Li Y, Li J, Jiao D, Han X. Image-guided microwave ablation of hepatocellular carcinoma (≤5.0 cm): is MR guidance more effective than CT guidance? BMC Cancer. 2021;21:366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Liu B, Long J, Wang W, Huang T, Xie X, Chen S, Huang G, Jiang C, Ye J, Long H, Kuang M. Predictive factors of treatment outcomes after percutaneous ablation of hepatocellular carcinoma in the caudate lobe: a retrospective study. BMC Cancer. 2019;19:699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Yang Y, Chen Y, Zhang X, Xin Y, Wang Y, Li X, Fan Q, Zhou X, Ye F. Predictors and patterns of recurrence after radiofrequency ablation for hepatocellular carcinoma within up-to-seven criteria: A multicenter retrospective study. Eur J Radiol. 2021;138:109623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Ni JY, Sun HL, Chen YT, Luo JH, Chen D, Jiang XY, Xu LF. Prognostic factors for survival after transarterial chemoembolization combined with microwave ablation for hepatocellular carcinoma. World J Gastroenterol. 2014;20:17483-17490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Wang L, Ke Q, Lin N, Huang Q, Zeng Y, Liu J. The efficacy of transarterial chemoembolization combined with microwave ablation for unresectable hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. 2019;36:1288-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | Yang WZ, Jiang N, Huang N, Huang JY, Zheng QB, Shen Q. Combined therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation for small hepatocellular carcinoma. World J Gastroenterol. 2009;15:748-752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Zaitoun MMA, Elsayed SB, Zaitoun NA, Soliman RK, Elmokadem AH, Farag AA, Amer M, Hendi AM, Mahmoud NEM, Salah El Deen D, Alsowey AM, Shahin S, Basha MAA. Combined therapy with conventional trans-arterial chemoembolization (cTACE) and microwave ablation (MWA) for hepatocellular carcinoma >3-<5 cm. Int J Hyperthermia. 2021;38:248-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 30. | Si ZM, Wang GZ, Qian S, Qu XD, Yan ZP, Liu R, Wang JH. Combination Therapies in the Management of Large (≥ 5 cm) Hepatocellular Carcinoma: Microwave Ablation Immediately Followed by Transarterial Chemoembolization. J Vasc Interv Radiol. 2016;27:1577-1583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |