Published online Aug 15, 2022. doi: 10.4251/wjgo.v14.i8.1499
Peer-review started: April 6, 2022
First decision: May 10, 2022
Revised: May 12, 2022
Accepted: July 11, 2022
Article in press: July 11, 2022
Published online: August 15, 2022
Processing time: 126 Days and 3.7 Hours
Irreversible electroporation (IRE) is a local non-thermal ablative technique which has been suggested as a potential cancer therapy. However, the specific anatomic characteristics of the pancreatic head make it challenging to perform any local ablation in this region. Therefore, the safety and feasibility of IRE in the pancreatic head region should be further explored.
To evaluate the safety of IRE in pancreatic head region including its effects on pancreatic ducts, vessels, and adjacent gastrointestinal organs.
Eight landrace miniature pigs underwent IRE of pancreatic head tissue succe
All pigs tolerated the ablation procedure without serious perioperative complications. Transiently elevated WBC count and amylase were observed at 24 h post-IRE, suggesting an acute pancreatic tissue damage which was confirmed by pathological observations. Vascular endothelial cells and pancreatic duct epi
IRE ablation to the pancreatic head may be safe and feasible without long-term damage to the surrounding vital structures. However, risks of stress injuries in acute phase should be taken into consideration to prevent severe perioperative complications.
Core Tip: This is a basic experimental research paper on irreversible electroporation (IRE) in the pancreatic head region. To examine the feasibility and safety of this technique in Landrace pigs, we designed a series of research experiments. We found that IRE ablation to the pancreatic head may be safe and feasible without long-term damage to the surrounding vital structures. However, risks of stress injuries in acute phase should be taken into consideration to prevent severe perioperative complications.
- Citation: Yan L, Liang B, Feng J, Zhang HY, Chang HS, Liu B, Chen YL. Safety and feasibility of irreversible electroporation for the pancreatic head in a porcine model. World J Gastrointest Oncol 2022; 14(8): 1499-1509
- URL: https://www.wjgnet.com/1948-5204/full/v14/i8/1499.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i8.1499
Irreversible electroporation (IRE) is a novel local ablation technique based on non-thermal damage principle. It mainly causes irreversible perforation of cell membrane through applying instantaneous, high-frequency, and repeated high-voltage pulses to cells, which leads to imbalance of cell homeostasis and induces apoptosis, thus achieving the goal of tumor ablation[1-3]. The risk of thermal damage is significantly reduced due to the non-thermal effect of tumor killing, and there is no heat sink phenomenon that affects the effectiveness of tumor ablation. Therefore, compared with local physical ablations based on the thermal effect such as radiofrequency ablation (RFA), IRE is more suitable for the treatment of locally advanced malignant tumors that cannot be radically resected owing to the invasion of vital vessels and thus has a good application prospect[4,5]. Although the theory of IRE ablation of tumors has been widely accepted, it remains controversial in terms of whether there would be potential damage to tissues and organs adjacent to tumors that are located at special anatomical positions such as pancreatic head cancer[6,7]. Even though the safety of IRE ablation in the pancreas and upper gastrointestinal (GI) tract has been preliminarily validated[8-10], studies on the local and systemic effects of IRE for the pancreatic head of large animals remain limited. Elucidating the short- and long-term effects of IRE on the pancreatic head will be an essential step in demonstrating its safety and feasibility before further implementation in clinical patients. Therefore, our study aimed to investigate the immediate and late complications of IRE on the pancreatic head and evaluate its safety in pancreatic head region including its effects on pancreatic ducts, vessels, and adjacent GI organs.
Eight Landrace miniature pigs weighing approximately 30 kg were selected with no gender restrictions. The pigs were provided by the Experimental Animal Center of the PLA General Hospital, where they were reared under clean experimental and single-cage standard conditions (22 °C, 12 h/12 h light/dark, 60% humidity, ad libitum access to food and water). The experimental procedures were approved by the Institutional Animal Care and Use Committee of PLA General Hospital.
Experimental groups: Eight pigs were randomly divided into four groups (A, B, C, and D), with two pigs per group, corresponding to different observation time points (1 h, day 1, day 7, and day 28 after IRE surgery). The pigs were used to evaluate the effect of IRE (Nanoknife, AngioDynamics, Queensbury, New York, United States) on the pancreatic head and adjacent duodenum to observe the acute and chronic response to IRE ablation of the pancreatic head region. The IRE parameters were set as follows: Fixed pulsed-field intensity of 1500 V/cm, pulse width of 100 μm, frequency of 1 Hz, needle exposure depth of 1 cm, and a preset pulse number of 120. The pancreatic head tissue adjacent to the medial duodenal wall was selected as the target area for ablation.
IRE ablation of the pancreatic head: Animals were fasted for 12 h before the operation. Sedazine II (xylazine hydrochloride injection) + midazolam injection (volume ratio: 1:1) at 0.3 mL/kg was used for anesthesia induction by intramuscular injection. After the induction was successful, the animals were intubated with a video laryngoscope, and isoflurane (0.8%) inhalation at a flow rate of 0.7 L/min combined with intravenous injection of 3-5 mg/kg fentanyl citrate through the ear vein was used for anesthesia maintenance. Rocuronium bromide was administered intravenously at a dose of 1-1.5 mg/kg as a muscle relaxant to prevent severe muscle contraction during electrical pulse generation. Vital signs including blood pressure, heart rate, and temperature were monitored during the operation.
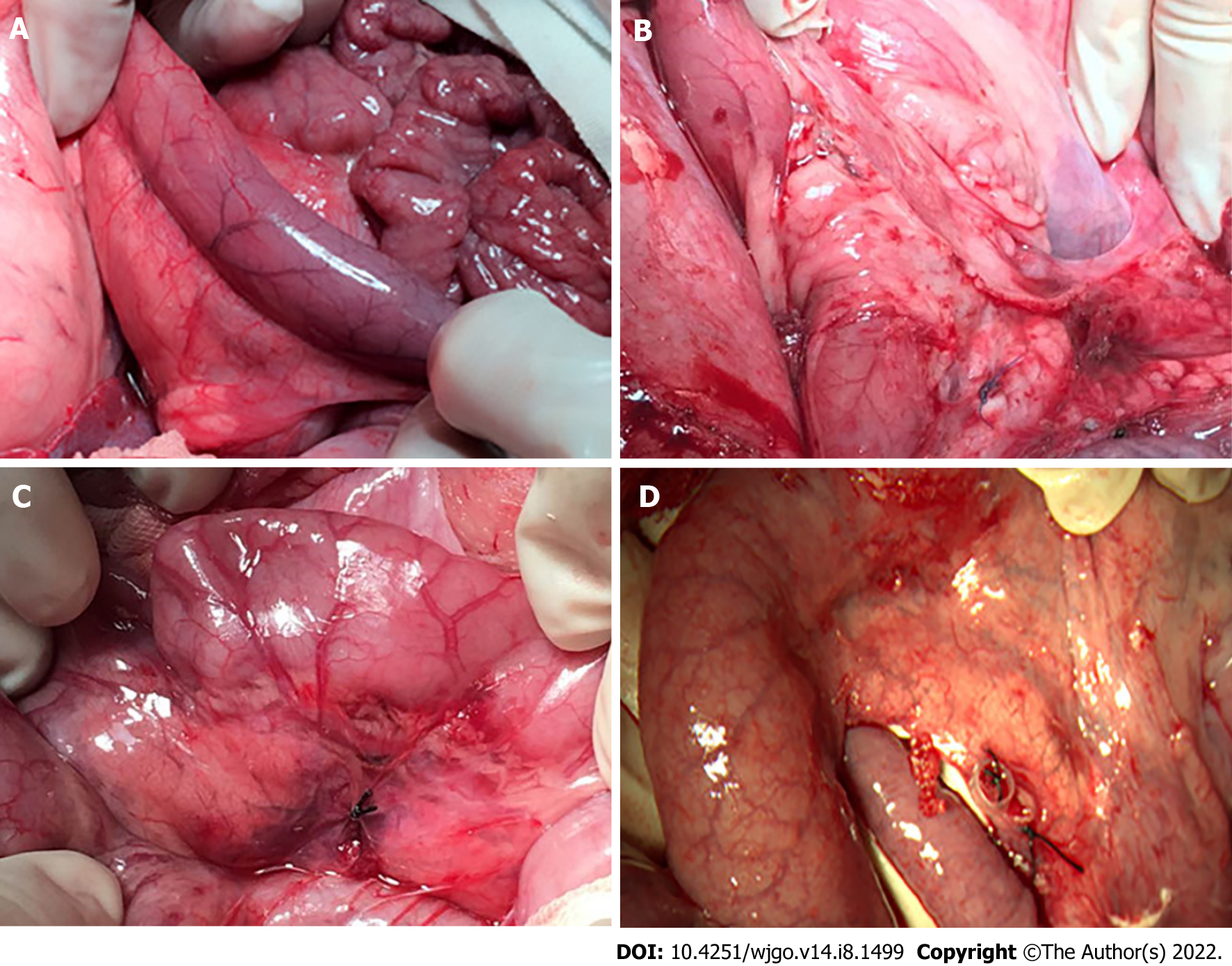
Two 19G IRE probes (AngioDynamics) were used to puncture parallelly into the target area with a distance of 1 cm and puncture depth of 1.5 cm. After completing the probe deployment, 20 trial pulses were applied based on the preset parameters, and the remaining 100 pulses were administered after confirming that there was no voltage overload. Then, the pigs’ response and changes in pancreatic head tissue and the duodenum in the ablation zone were observed and recorded during IRE ablation. After the ablation was completed, the probes were removed and the abdomen was sutured closed layer by layer after observing no abnormality in the pig’s vital signs, and bunarizine hydrochloride injection was used by intramuscular injection (3-5 mg/kg, 1/d) for postoperative analgesia.
General condition of the animals was observed and recorded including activity, feeding, bowel movements, and weight changes. The white blood cell (WBC) count and serum amylase level were measured before surgery and 1 h, 1 d, 3 d, 7 d, 14 d, and 28 d after surgery. Tissue specimens were harvested from pigs in the corresponding groups after 1 h and on days 1, 7, and 28 after IRE. The pigs were euthanized via intravenous injection of 3% nembutal (100 mg/kg), and pathological examinations were conducted on the ablation and non-ablation zones, including hematoxylin and eosin (HE) staining, terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining, and Masson trichrome staining and transmission electron microscopy observation.
SPSS version 22.0 statistical software was used to analyze the experimental results, and measurement data are expressed as the mean ± standard error. The experimental data were subjected to multiple comparisons among groups and the pairwise t-test, and the difference was considered statistically significant at P < 0.05.
All animals were subjected to IRE ablation and survived to the respective experimental endpoints. The animals started to be active 6 h after surgery, but their activity was reduced and they did not consume food. Within 24 h after surgery, the animals gradually increased their activity and had a small amount of food and defecation. Then, at 2 d after surgery, the animals’ activity, food intake, and defecation essentially returned to normal. No significant change in body weight was observed at the preoperative and postoperative time points in each group.
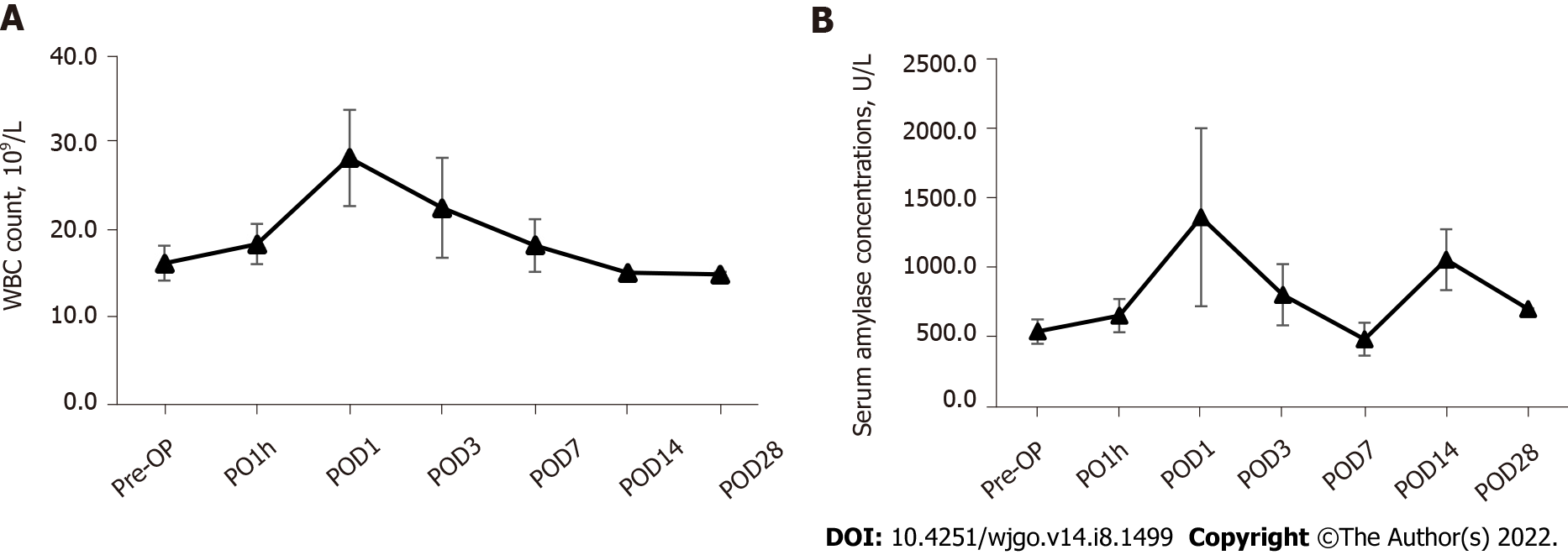
Changes in blood indicators: The results of the laboratory testing showed that the WBC count in the postoperative acute phase of IRE was gradually elevated from the preoperative baseline level (16.2 ± 2.0) × 109/L to the peak (28.2 ± 5.5) × 109/L at 24 h postoperatively and then gradually resolved to normal (Figure 1A). The serum amylase concentration showed a significant increase 1 h after surgery (873.4 ± 118.8 U/L), then reached the highest value at 24 h after surgery (2077.6 ± 637.3 U/L), and essentially returned to normal 3 d after surgery (1383.9 ± 218.8 U/L) (Figure 1B). Statistical comparative analysis showed that the serum amylase concentration at day 1 after surgery were significantly higher (P < 0.05) than that at baseline (700.9 ± 88.1 U/L).
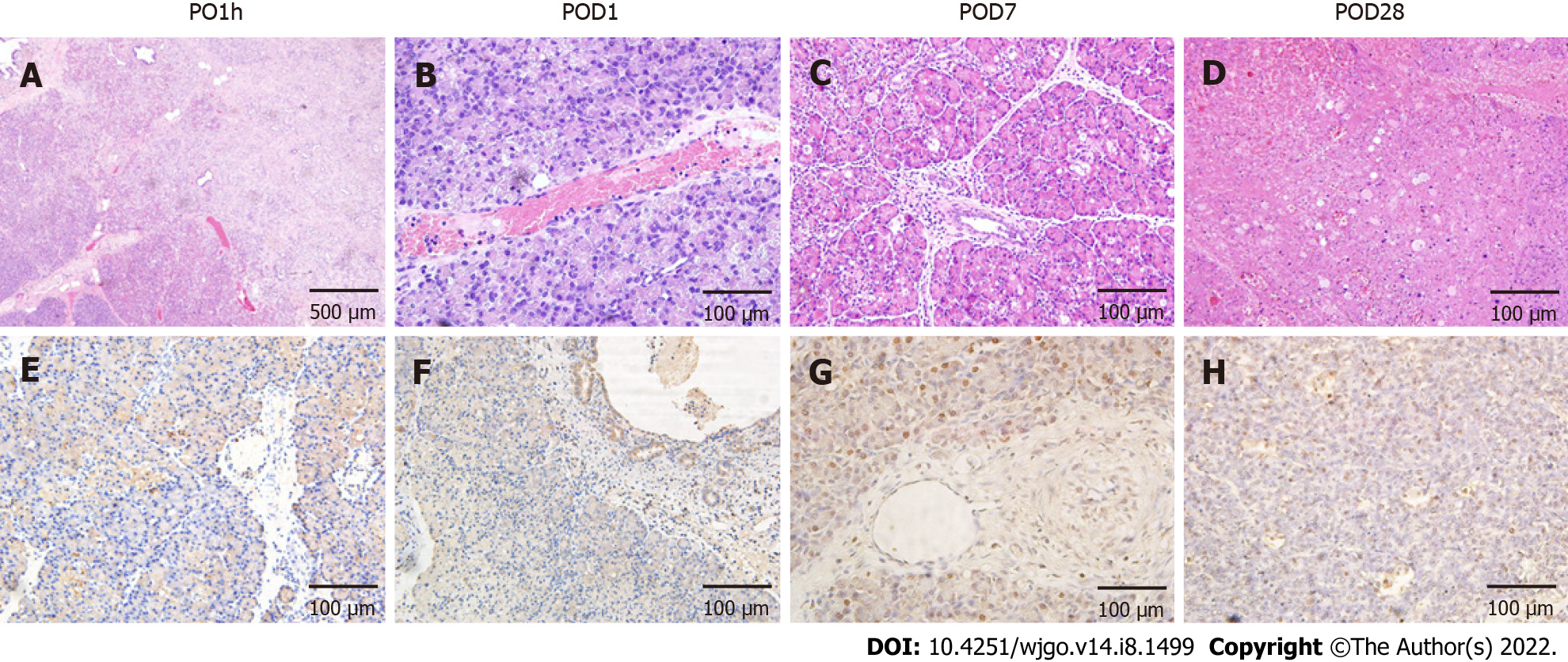
Pathological findings: The pancreatic tissues after IRE ablation showed different pathological changes over time. At 1 h after surgery, the ablation zone showed distinct acute edema and congestion with clear demarcation from the surrounding area (Figure 2). HE staining showed that some of the pancreatic acinar cells were obviously necrotic accompanied by interstitial congestion and edema, and focal hemorrhages were observed locally, but most cells were negative for TUNEL staining (Figure 2A and E). At day 1 after surgery, inflammatory cell infiltration was visible under the microscope, and the pancreatic lobule structure remained intact. A small number of apoptotic cells were seen in TUNEL staining and were mostly concentrated around the probes (Figure 2B and F). At day 7 after surgery, the size of the ablation zone was reduced, and pancreatic tissue edema disappeared. HE staining revealed pancreatic acinar cell atrophy in the ablation zone and increased cell eosinophilia, accompanied by the infiltration of a large number of inflammatory cells and fibrosis (Figure 2C). TUNEL staining revealed that the area centered on the probes in the ablation zone was strongly positive, and apoptotic expression was also seen in pancreatic ductal and vascular endothelial cells (Figure 2G). At day 28 after surgery, severe pancreatic destruction with vacuolation of cells was observed. The positive rate of TUNEL stained cells decreased, while the structure of pancreatic ducts and vessels in the ablation zone was still intact.
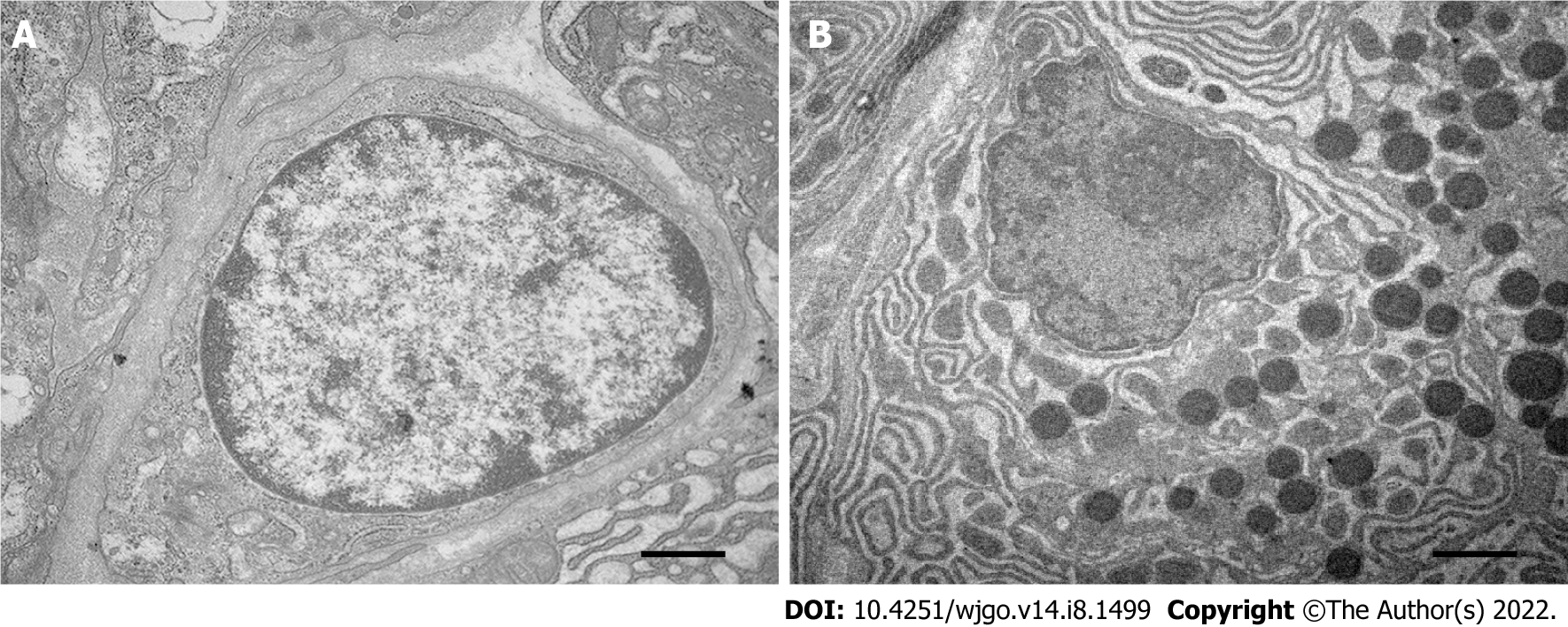
Observation by transmission electron microscopy showed that the pancreatic acinar cells in the ablation zone were atrophied, the nucleoli were broken and disappeared, the chromatin of the cells was highly pyknotic and condensed to the edge, and the endoplasmic reticulum appeared vacuolated (Figure 3).
After IRE, the duodenal segments in the ablation zone showed a gradually deepening color with local congestion and edema as the distance from the probes gradually shortened, and the peristalsis of the corresponding segment slowed down. Postoperative observations at different time points showed that there was no perforation or obstruction in the duodenum, and the edema gradually disappeared. The color of the duodenal serosa in the ablation zone was not significantly different from that of the normal segment (Figure 4). Normally rhythmic peristaltic waves were observed.
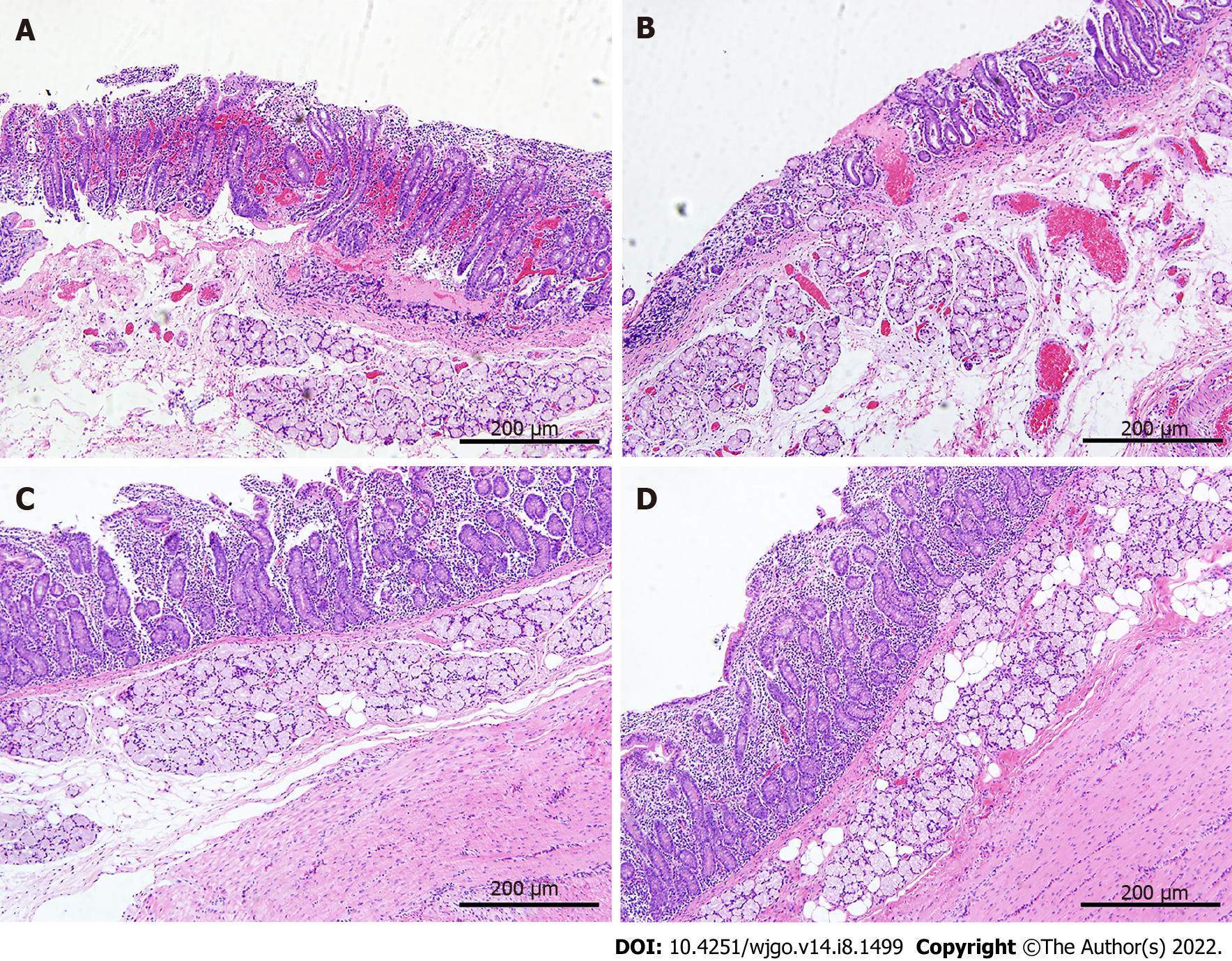
HE staining (Figure 5) revealed that the mucosal structure of the duodenum in the ablation zone was disorganized at 1 h after surgery, with obvious destruction of the villous structure and congestion of the mucosa with localized focal hemorrhage; no significant changes were observed in the manifestation at day 1 after surgery; at day 7 after surgery, dead mucosal epithelial cells were still visible by microscopy and signs of repair could be seen in all layers of the duodenum; at day 28 after surgery, the duodenal structure did not significantly differ from that before surgery.
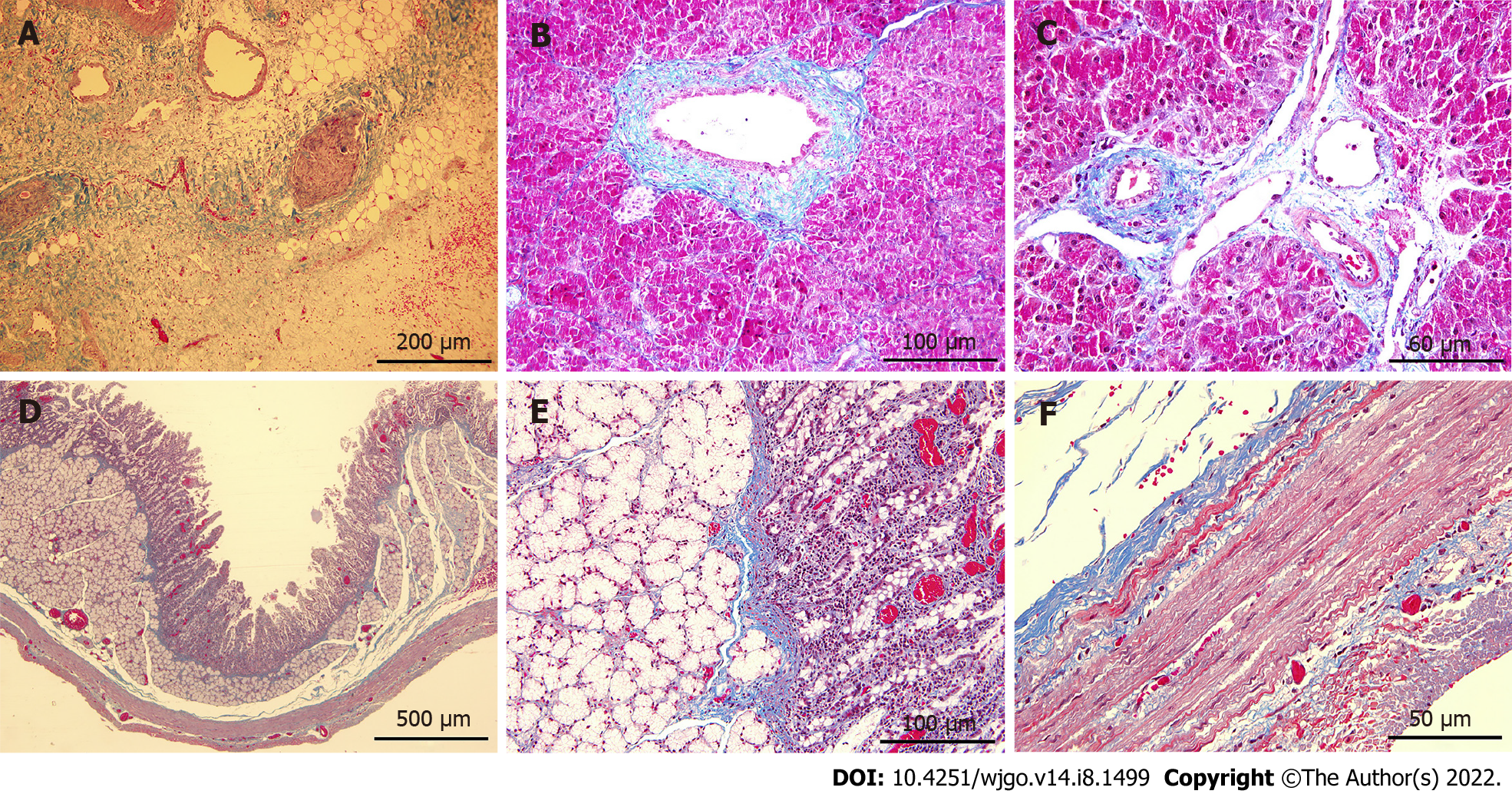
Masson trichrome staining showed proliferation of blue-stained fibrous connective tissue in the ablation zone of the pancreas at day 7 after surgery (Figure 6A), and the vascular and pancreatic duct extracellular matrix structures were intact in the ablation area without loss (Figure 6B and C). Continuous blue-stained collagen fibers was seen between the mucosa, submucosa, muscularis, and serosa, and the structure of each tissue was intact and did not differ considerably from that in the non-ablation zone (Figure 6D-F).
Since IRE was first approved for clinical use, its safety and efficacy have been the focus of scholars both at home and abroad. The pancreatic head/neck region has special anatomical and structural characteristics, surrounded by important vascular structures such as the celiac axis, superior mesenteric artery, and portal vein. The invasion of these important vessels is closely related to the unresectability of pancreatic cancer and restricts the application of traditional physical ablation modalities based on thermal effects in the treatment of locally advanced tumors. Previous studies have shown that traditional physical therapies such as RFA can cause severe complications in treating pancreatic cancer, including GI bleeding, pancreatic fistula, biliary fistulas, pancreatitis, and portal vein thrombosis[11,12]. Among these, GI bleeding and secondary infection caused by biliary and pancreatic fistulas are the most common death-related complications. Therefore, apart from the vessels, the preservation of vital surrounding tissue including the bile duct, pancreatic duct, and duodenum should be taken into primary consideration when IRE is performed. This study provides a novel insight into the short- and long-term effects of IRE on pancreatic head region and adjacent structures. We demonstrated that IRE ablation to the pancreatic head may be safe and feasible without long-term damage to the surrounding vital structures but risks of stress injuries in acute phase should be taken into consideration to prevent severe perioperative complications.
Studies on IRE ablation of hollow organs have been carried out by scholars long before the application of this technique in clinical practice. Phillips et al[13] preliminarily validated the safety of IRE ablation of hollow organs using the small intestine of Sprague Dawley rats as the target organ; however, the differences in anatomical structure and ablation protocols limit the reference significance of this study for the safety assessment of IRE ablation in the pancreatic head. Subsequently, Schoellnast et al[14], Srimathveeravalli et al[15], and Luo et al[16] investigated the feasibility of colorectal IRE ablation using pigs as experimental animals, indicating that it was feasible to use hollow organs of miniature pigs as IRE target organs. This has guiding significance for simulating the application of IRE for tumors from corresponding human organs. However, due to the different target organs and anatomical positions, these studies did not provide meaningful clinical references for assessing the safety of IRE ablation of pancreatic head cancer on adjacent hollow organs. Therefore, the anatomical structure and position as well as the tolerance of the experimental animals to IRE were the main considerations in selecting the experimental subjects. The pancreas of miniature pigs is flat and attached to the inner mesentery of the duodenum in a "herringbone" shape; this anatomical position is similar to that of humans. Therefore, compared with rats, pigs are a relatively more ideal animal model for IRE ablation experiments in the pancreatic head region.
In our experiments, the duodenum in the IRE ablation area gradually deepened in color as the distance from the probes gradually shortened, and the peristaltic rhythm slowed down, indicating that although the duodenum was not a direct target organ for IRE ablation, the tissues within a certain range of the IRE probes were affected by the pulsed electric field, which resulted in an acute stress response. The microscopic changes at day 1 after IRE showed that IRE ablation with conventional parameter settings could cause irreversible tissue death in the mucosa, submucosa, and muscularis of the duodenum. Nevertheless, the microscopic changes at day 7 after surgery showed structural repair of new villi in the small intestine, and the duodenal structures in the ablation zone gradually resolved to normal up to day 28 after surgery, suggesting that the effects of IRE ablation of the pancreatic head on the duodenum may be limited to acute stress injury without long-term effects.
Consistent with reports in the literature, IRE ablation of the pancreatic head did not cause severe duodenal-related injury for the following possible reasons: (1) The IRE effect targets the cell membrane and does not affect the extracellular matrix and other skeletal structures; thus, the structural integrity of the duodenum is preserved, providing the basis for subsequent injury repair[17]; (2) The principle of IRE killing cells is based on inducing apoptosis, thereby causing a mild local inflammatory response, which is conducive to the growth and migration of new cells; (3) The vasoprotective effect of IRE did not significantly affect the blood supply to any layer of the duodenum; (4) The high renewal rate of mucosal epithelial cells in the small intestine allows rapid repair of the damaged duodenum; and (5) A study showed[18] that the pluripotent stem cells of duodenal glands can be induced to differentiate into epithelial cells to form new villous structures in the small intestine, promoting the recovery of duodenal structure and function.
Notably, in the present study, when IRE was used to ablate the head of the pancreas, we found extensive congestive changes in the mucosa and submucosa of the duodenum early after surgery, localized mucosal tissue detachment, and hemorrhagic manifestations; such acute stress changes suggested the risk of stress ulcer bleeding in the GI tract after IRE of tumors in the head of the pancreas. Consistent with the actual clinical situation, it is common for pancreatic head adenocarcinoma to invade the duodenum, and there have been clinical reports on GI bleeding after IRE[5,19]. Therefore, although experimental animal studies have shown that IRE ablation of the pancreatic head does not result in severe long-term complications after ablation, such as duodenal perforation, the reference significance of its acute stress changes for the safety of IRE ablation for pancreatic head cancer in clinical practice still warrants further investigation of the clinical application of this emerging technology in this special region.
Due to the pancreatic head’s special anatomical position and structure, safety has always been the primary consideration in applying physical ablation modalities in this area. Conventional physical ablation modalities kill tumors by causing cell necrosis via thermal effects. Although some studies have reported that they can achieve pain relief and improve survival quality, the high incidence of severe complications dramatically limited their application in treating pancreatic cancer[11,12]. Unlike conventional physical ablations, IRE does not rely on the thermal effect to kill tumors, leading to its acceptance as an ideal ablation modality for pancreatic tumors. Nevertheless, the fragility of the pancreas makes it more vulnerable to any surgical manipulation or local physical ablation compared to other solid organs such as the liver or kidney. Therefore, assessment of the local and systemic effects of IRE ablation in the pancreatic head region remains necessary.
In our study, no abdominal necrosis or exudation was observed in the gross specimen at any time point, suggesting that no significant pancreatic fistula occurred after IRE ablation. The blood test results suggested that IRE can cause an inflammatory response in the first 24 h after surgery, and the WBC count would return to normal 3 d after surgery, indicating that this inflammatory response caused by IRE is only a stress response to this procedure during the acute phase of trauma. Additionally, the trend of the WBC count also suggested that IRE does not increase the risk of perioperative abdominal infection, thus validating the safety of IRE ablation in the head of the pancreas from another perspective. Notably, the trend of postoperative serum amylase also only showed a transient increase in the acute phase, while the long-term serum amylase level suggested that there was no evidence showing that IRE ablation of the pancreatic head could induce chronic pancreatitis. Combined with previous reports in the literature[20,21], we analyzed the reasons why IRE ablation of the pancreatic head did not induce severe pancreatitis, which may be as follows: (1) IRE ablation of the pancreas has a precise and limited scope, and its damage to the pancreatic tissue is limited to a localized area; (2) The ablation using a fine needle probe (19G) is less traumatic to the pancreas and can effectively prevent direct damage to the pancreatic duct; and (3) Unlike other thermal ablation methods such as RFA or cryoablation, IRE does not damage the extracellular matrix, effectively protecting the integrity of the pancreatic duct structure in the ablation zone and avoiding pancreatic fistula.
The histopathological findings corroborated these results. The ablation zone of the pancreatic head showed different changes at different time points during 4 wk after IRE ablation, which was consistent with the findings of Lee et al[8]. Necrosis of pancreatic acinar cells was found 1 h after ablation, suggesting that IRE ablation can cause morphological changes of cells in the ablation zone at an early stage after the procedure. From day 1 to day 28 after ablation, the ablation zone showed a series of pathological changes from massive accumulation of inflammatory cells to gradual regression and from atrophy and death of pancreatic acinar cells to proliferation of fibrous connective tissue, which confirmed that IRE could produce irreversible damage to pancreatic tissues. However, such damage was not coagulation necrosis but apoptosis. This mechanism of IRE was confirmed by results of TUNEL staining. We found that pancreatic ductal and vascular endothelial cells were also positive for TUNEL staining, suggesting that IRE ablation also induced apoptotic effects on cells of ductal structures, such as vessels and pancreatic ducts. Nevertheless, IRE did not damage their structural integrity and function, demonstrating that important ductal structures in the pancreatic head could be preserved while target cells were destroyed.
Although the effectiveness of IRE ablation of the pancreatic head and the safety of vital ductal structures and adjacent organs were validated in this study, it was limited as the study aimed to generate an IRE model in normal pancreatic tissue of pigs, which failed to truly simulate the tumor model that invades the peripheral vessels of the pancreatic head and duodenum. Additionally, owing to the difference between the microenvironment of tumor and that of normal tissues, there is still uncertainty on whether the same results would be obtained if tumor cells are present.
In conclusion, IRE ablation to the pancreatic head may be safe and feasible without long-term damage to the surrounding vital structures. However, risks of stress injuries in acute phase should be brought to our attention. In the future, in vitro studies of IRE ablation on various human pancreatic cancer cell types should be conducted to optimize parameters and techniques of pancreatic IRE ablation in clinical settings, and further studies are needed to investigate the mechanism of tissue repair and regeneration after IRE.
Irreversible electroporation (IRE) is a relatively novel local ablation technique based on the delivery of repeated and high-frequency microsecond- to millisecond-long electrical pulses to a target tissue. It is characterized by non-thermal damage and no heat sink effect, thus able to protect vital anatomic structures such as pancreatic ducts and vessels in close proximity within the targeted organs. These qualities make IRE an attractive alternative for treatment of locally advanced pancreatic cancer. Recently, this novel ablation has been tested successfully on normal and malignant lesions in the prostate, liver, lung, and pancreas in animal models, and even in human subjects; however, the safety and feasibility of IRE for lesions in the pancreatic head are still controversial.
Studies on the local and systemic effects of IRE for pancreatic head of large animals remain limited. Elucidating the short- and long-term effects of IRE on the pancreatic head will be an essential step in demonstrating its safety and feasibility before further implementation in clinical patients. We carried out an animal experiment to examine this procedure.
This study aimed to examine the safety and feasibility of IRE for the pancreatic head in a porcine model.
In total, eight Landrace pigs were randomly divided into four groups, with two pigs per group, corresponding to different observation time points (1 h, day 1, day 7, and day 28 after IRE surgery), and underwent IRE ablation of the pancreatic head successfully. Laboratory testing including white blood cell (WBC) count and serum amylase before IRE with follow-up laboratory analysis and pathological examination at 1, 7, 14, and 28 d postablation were performed.
The effects of IRE on the pancreatic head were characterized by transiently elevated WBC and amylase, and acute damage to targeted area including pancreatic tissue and the duodenum which was confirmed by pathological observations in the early phase after ablation. Vascular endothelial cells and pancreatic duct epithelial cells in ablation zone were also positive for terminal deoxynucleotidyl transferase dUTP nick end labeling staining while the structure was still intact in long-term observation, indicating that the risk of short-term damage should be paid more attention to prevent severe perioperative complications.
IRE ablation to the pancreatic head is safe and feasible without long-term damage to the surrounding vital structures while risks of stress injuries in acute phase should be brought to our attention.
In vitro studies of IRE ablation on various human pancreatic cancer cell types should be conducted to optimize parameters and techniques of pancreatic IRE ablation in clinical settings to keep safe and further studies are needed to investigate the mechanism of tissue repair and regeneration after IRE.
The authors would like to acknowledge the Experimental Animal Center of the PLA General Hospital for providing animal and skillful technical assistance.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lee KG, South Korea; Peng SSF, Taiwan; Thomas G, Australia S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Wu RR
| 1. | Davalos RV, Mir IL, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 891] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 2. | Edd JF, Horowitz L, Davalos RV, Mir LM, Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53:1409-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 336] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 3. | Petrou A, Moris D, Paul Tabet P, David Wensley Richards B, Kourounis G. Ablation of the locally advanced pancreatic cancer: An introduction and brief summary of techniques. J BUON. 2016;21:650-658. [PubMed] |
| 4. | Ruarus A, Vroomen L, Puijk R, Scheffer H, Meijerink M. Locally Advanced Pancreatic Cancer: A Review of Local Ablative Therapies. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Yan L, Chen YL, Su M, Liu T, Xu K, Liang F, Gu WQ, Lu SC. A Single-institution Experience with Open Irreversible Electroporation for Locally Advanced Pancreatic Carcinoma. Chin Med J (Engl). 2016;129:2920-2925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 6. | Månsson C, Nilsson A, Karlson BM. Severe complications with irreversible electroporation of the pancreas in the presence of a metallic stent: a warning of a procedure that never should be performed. Acta Radiol Short Rep. 2014;3:2047981614556409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 7. | Su JJ, Su M, Xu K, Wang PF, Yan L, Lu SC, Gu WQ, Chen YL. Postoperative inflammation as a possible cause of portal vein thrombosis after irreversible electroporation for locally advanced pancreatic cancer. World J Gastroenterol. 2017;23:6003-6006. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Lee EW, Shahrouki P, Peterson S, Tafti BA, Ding PX, Kee ST. Safety of Irreversible Electroporation Ablation of the Pancreas. Pancreas. 2021;50:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 9. | Chan G, Pua U. Irreversible Electroporation of the Pancreas. Semin Intervent Radiol. 2019;36:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Jeon HJ, Choi HS, Keum B, Bang EJ, Lee KW, Kim SH, Yim SY, Lee JM, Kim ES, Seo YS, Jeen YT, Lee HS, Chun HJ, Kim HB, Kim JH. Feasibility and effectiveness of endoscopic irreversible electroporation for the upper gastrointestinal tract: an experimental animal study. Sci Rep. 2021;11:15353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Pezzilli R, Ricci C, Serra C, Casadei R, Monari F, D'Ambra M, Corinaldesi R, Minni F. The problems of radiofrequency ablation as an approach for advanced unresectable ductal pancreatic carcinoma. Cancers (Basel). 2010;2:1419-1431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Pezzilli R, Serra C, Ricci C, Casadei R, Monari F, D'Ambra M, Minni F. Radiofrequency ablation for advanced ductal pancreatic carcinoma: is this approach beneficial for our patients? Pancreas. 2011;40:163-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Phillips MA, Narayan R, Padath T, Rubinsky B. Irreversible electroporation on the small intestine. Br J Cancer. 2012;106:490-495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Schoellnast H, Monette S, Ezell PC, Single G, Maybody M, Weiser MR, Fong Y, Solomon SB. Irreversible electroporation adjacent to the rectum: evaluation of pathological effects in a pig model. Cardiovasc Intervent Radiol. 2013;36:213-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Srimathveeravalli G, Wimmer T, Monette S, Gutta NB, Ezell PC, Maybody M, Weiser MR, Solomon SB. Evaluation of an endorectal electrode for performing focused irreversible electroporation ablations in the Swine rectum. J Vasc Interv Radiol. 2013;24:1249-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Luo X, Liang X, Li J, Shi J, Zhang W, Chai W, Wu J, Guo S, Fang G, Zhou X, Zhang J, Xu K, Zeng J, Niu L. The Effects of Irreversible Electroporation on the Colon in a Porcine Model. PLoS One. 2016;11:e0167275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Wood LSY, Dunn JCY. Irreversible Electroporation for De-epithelialization of Murine Small Intestine. J Surg Res. 2020;256:602-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Dignass AU. Mechanisms and modulation of intestinal epithelial repair. Inflamm Bowel Dis. 2001;7:68-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 181] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Liu S, Qin Z, Xu J, Zeng J, Chen J, Niu L, Xu M. Irreversible electroporation combined with chemotherapy for unresectable pancreatic carcinoma: a prospective cohort study. Onco Targets Ther. 2019;12:1341-1350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Fritz S, Sommer CM, Vollherbst D, Wachter MF, Longerich T, Sachsenmeier M, Knapp J, Radeleff BA, Werner J. Irreversible electroporation of the pancreas is feasible and safe in a porcine survival model. Pancreas. 2015;44:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Zhang Z, Li W, Procissi D, Tyler P, Omary RA, Larson AC. Rapid dramatic alterations to the tumor microstructure in pancreatic cancer following irreversible electroporation ablation. Nanomedicine (Lond). 2014;9:1181-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |