Published online Aug 15, 2022. doi: 10.4251/wjgo.v14.i8.1429
Peer-review started: March 8, 2022
First decision: April 17, 2022
Revised: April 30, 2022
Accepted: July 6, 2022
Article in press: July 6, 2022
Published online: August 15, 2022
Processing time: 155 Days and 4 Hours
Neoadjuvant chemoradiotherapy (nCRT) and total rectal mesenteric excision are the main standards of treatment for locally advanced rectal cancer (LARC). Lymph node regression grade (LRG) is an indicator of prognosis and response to preoperative nCRT based on postsurgical metastatic lymph node pathology. Common histopathological findings in metastatic lymph nodes after nCRT include necrosis, hemorrhage, nodular fibrosis, foamy histiocytes, cystic cell reactions, areas of hyalinosis, residual cancer cells, and pools of mucin. A number of LRG systems designed to classify the amount of lymph node regression after nCRT is mainly concerned with the relationship between residual cancer cells and regressive fibrosis and with estimating the number of lymph nodes existing with residual cancer cells. LRG offers significant prognostic information, and in most cases, LRG after nCRT correlates with patient outcomes. In this review, we describe the systematic classification of LRG after nCRT, patient prognosis, the correlation with tumor regression grade, and the typical histopathological findings of lymph nodes. This work may serve as a reference to help predict the clinical complete response and determine lymph node regression in patients based on preservation strategies, allowing for the formulation of more accurate treatment strategies for LARC patients, which has important clinical significance and scientific value.
Core Tip: Studies on lymph node regression grading after neoadjuvant chemoradiotherapy (nCRT) for rectal cancer are limited but serve clinicians for assessing the lymph node response to treatment based on the efficacy of the primary tumor after preoperative nCRT, providing guidance in formulating more accurate surgical or therapeutic strategies for the next stage of patient management and in determining patient prognosis. We discuss its histopathology, prognosis, correlation with tumor regression grading, and clinical applications and prospects.
- Citation: He L, Xiao J, Zheng P, Zhong L, Peng Q. Lymph node regression grading of locally advanced rectal cancer treated with neoadjuvant chemoradiotherapy. World J Gastrointest Oncol 2022; 14(8): 1429-1445
- URL: https://www.wjgnet.com/1948-5204/full/v14/i8/1429.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i8.1429
Neoadjuvant chemoradiotherapy (nCRT) and total rectal mesenteric excision (TME) are the main standards of treatment for locally advanced rectal cancer (LARC)[1-5]. The response of lymph nodes (LNs) to neoadjuvant therapy is reflective of the possibility of regression, similar to the main tumor body. LN regression grade (LRG) is based on postsurgical metastatic LN pathology and is an indicator of the response to preoperative nCRT and patient prognosis[6,7]. The status of tumor-draining LNs (TDLN) has been considered the most significant indicator of prognosis in patients with LARC, and the number of LN metastases is currently the only measure of ypN staging[8-12]. Several studies have demonstrated that nCRT decreases the detection of positive LNs and the total number of positive LNs, thereby affecting the accuracy of the patient's ypN stage[13-16]. In addition, the majority of studies and applications focused on tumor regression have centered on the primary tumor, while the impact of LRG on tumor regression and prognosis has not been fully explored. nCRT treatment based on well-predicted and assessed regression is beneficial for individualized clinical decision making and multidisciplinary diagnosis and treatment.
In the following study, we present the characteristics and histopathological findings of LNs observed as a result of nCRT, summarize the concepts for LRG, introduce some LRG staging systems for rectal cancer, describe the patient prognosis and the relationship with tumor regression grade (TRG), explore the limitations and critical issues, and discuss the clinical impact of LRG on rectal cancer.
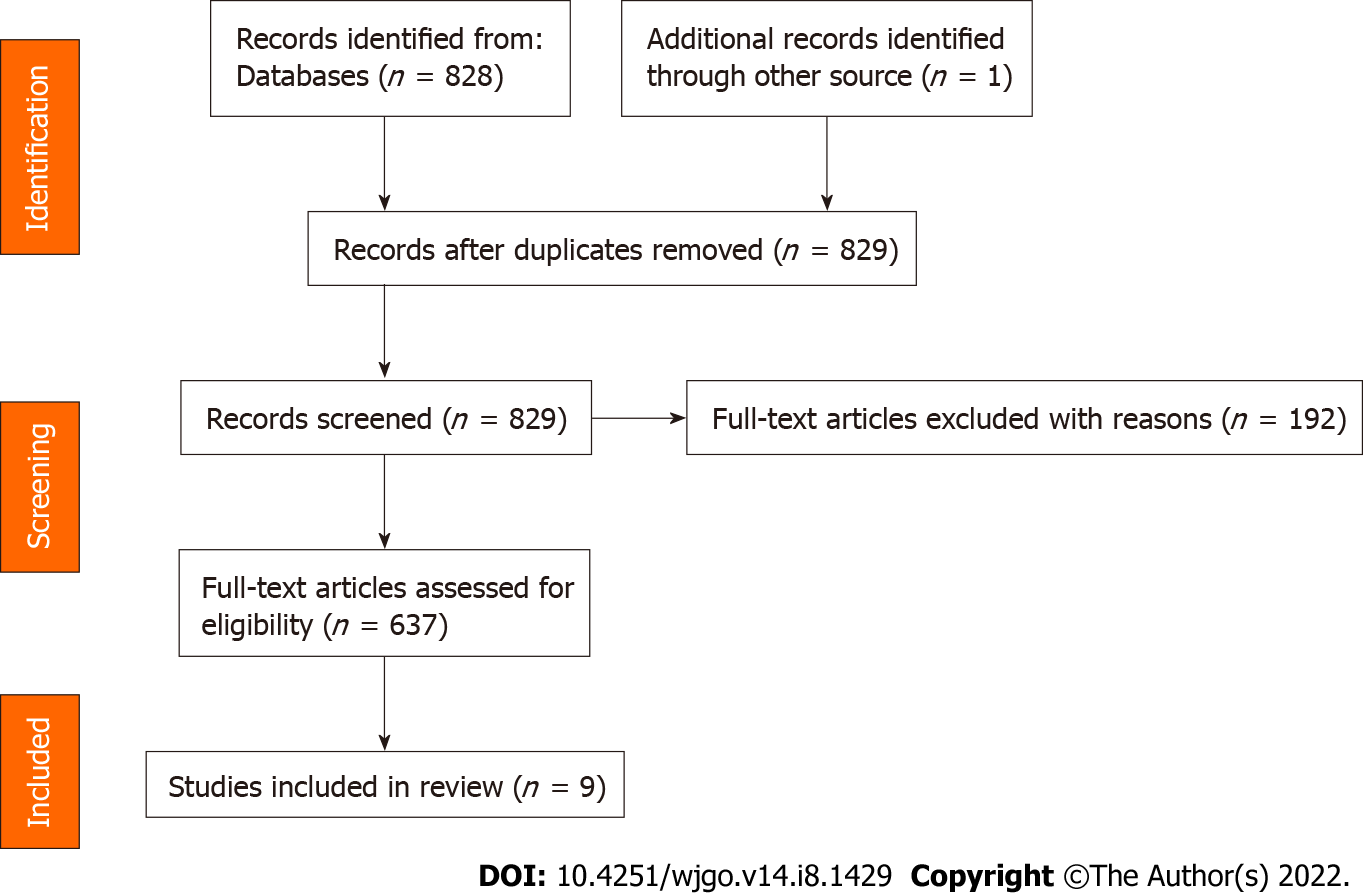
The main purpose of the present review is to identify the latest studies relating to LRG after neoadjuvant radiotherapy in patients with LARC and to compare their main elements. We performed a database search on PubMed and selected papers published in English between January 2000 and January 2022. PubMed was last accessed on 2 February 2022. The following keywords and terms were used. ("rectal OR rectum") AND ("carcinoma OR neoplasm OR malignant OR malignancy OR cancer") AND ("lymph node grade OR LRG OR lymph node grading") AND ("chemoradiotherapy OR therapy OR chemotherapy OR radiotherapy") AND ((2000/1/1[PDAT]: 2022/1/31[PDAT])), to retrieve relevant articles. All articles are in English. Meta-analyses, reviews, and other articles containing nonoriginal data were excluded from our review. All articles retrieved were selected and screened by three independent authors. Related data on the articles were retrieved by a standardized data collection method. A flow chart of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses is shown in Figure 1.
The primary purpose of the pathologic procedure was the macrosurvey of the resected tumor and LN specimens[17]. Operative specimens were detached from the anterior wall with a fixation for 24 h in 40 g/L formaldehyde. External surfaces of the specimen were stained with black ink for the easy identification of surgical margins. Serial sections of the entire tumor and attached mesentery were performed at 3- to 4-mm intervals vertically along the longitudinal axis of the rectum. To assess the LNs around the rectum, the interrectal fat was removed after tumor sampling. All LNs were identified by palpation and removed using scissors and a scalpel, followed by histological examination[18].
Based on the histology, tumor regression after nCRT essentially constitutes subacute to subchronic inflammation that follows the cytotoxic effects occurring weeks before. In the majority of cases, the tumor was removed sometime after completing the final cycle of preoperative chemotherapy[17].
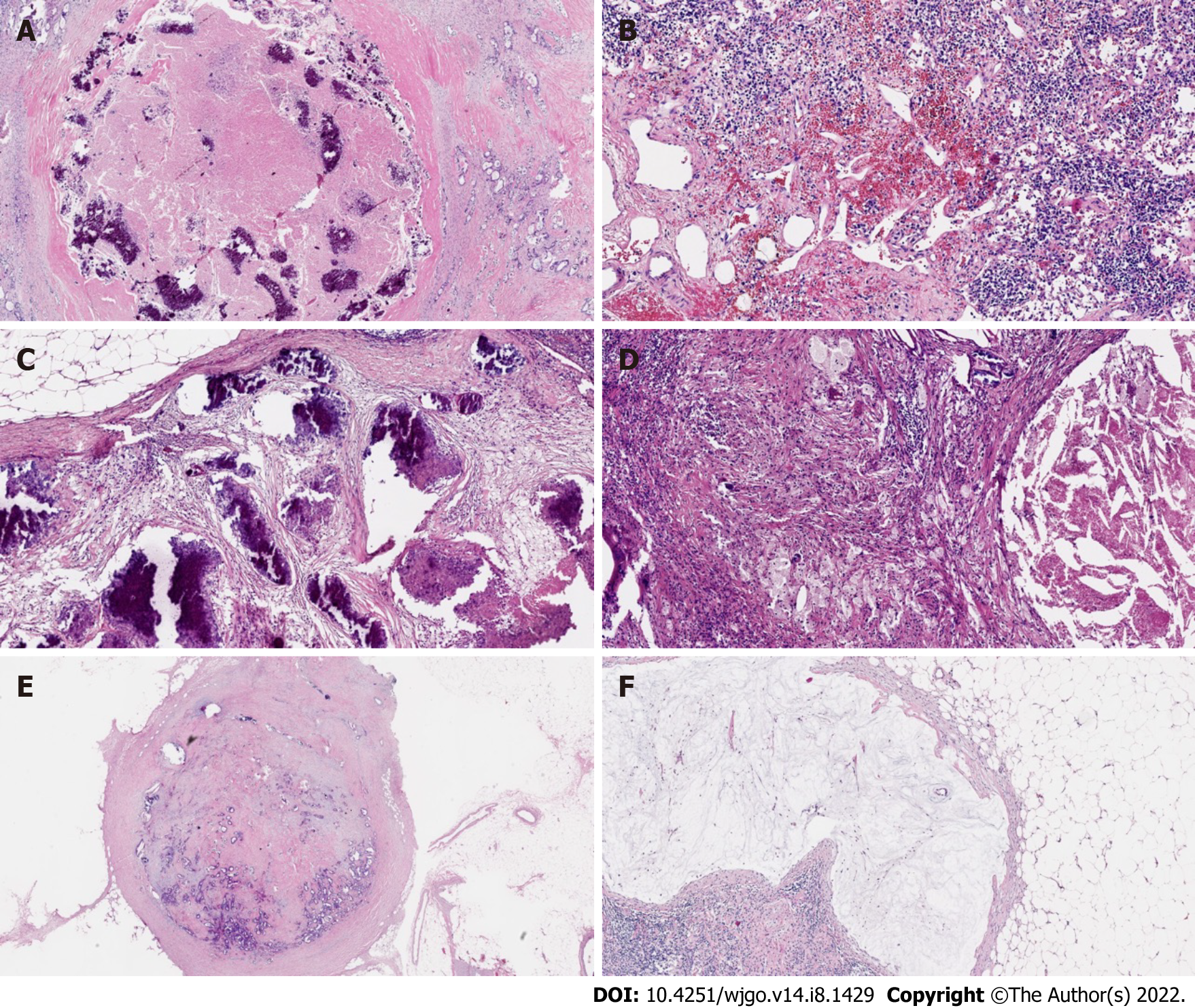
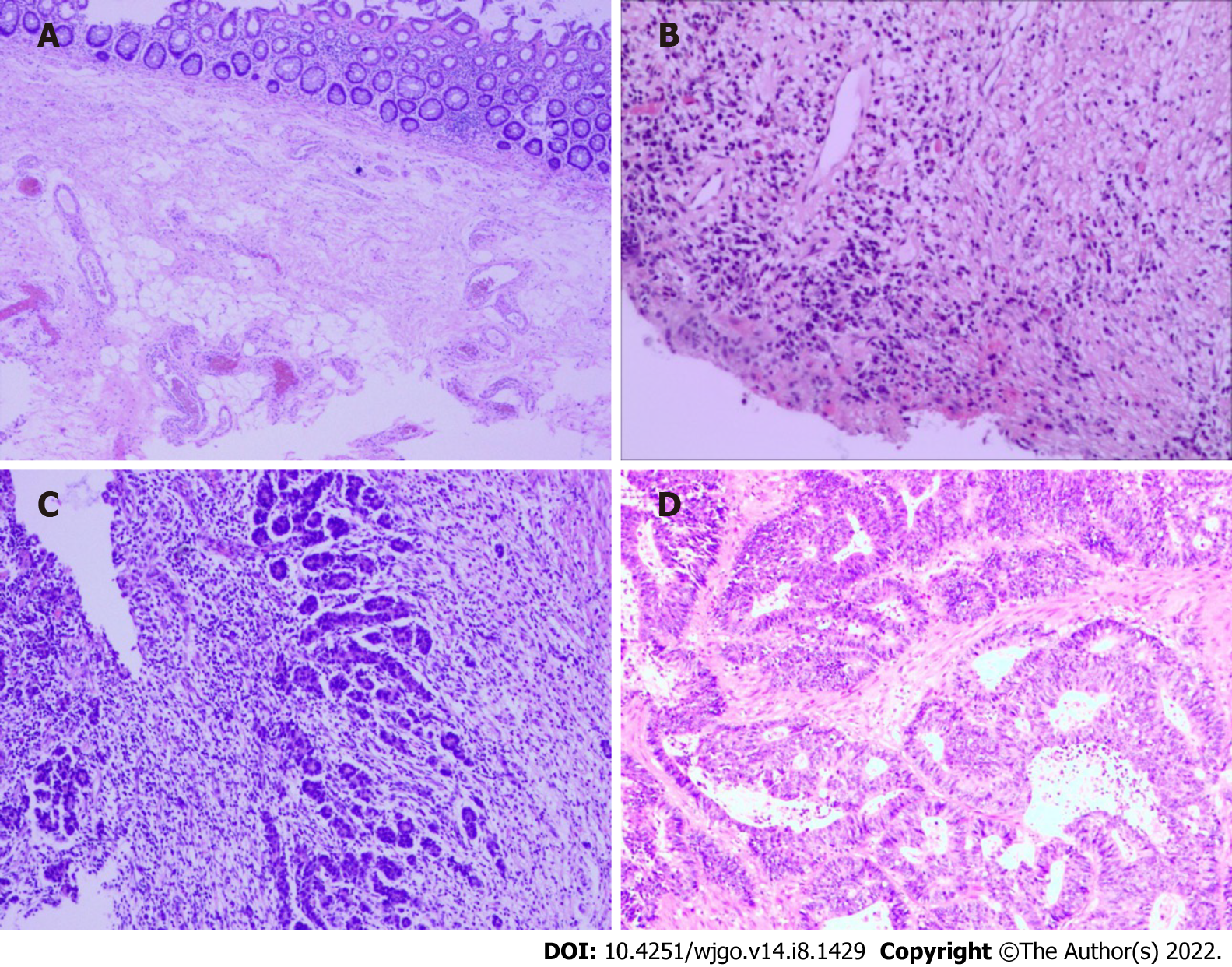
At the cellular level, in the case of complete LN regression, the malignant cells were eradicated through cytotoxic therapy and/or subsequently by the inflammatory response, and the LNs were displaced by fibrous tissue. In contrast, there was a high probability of an abundance of residual tumor cells in the LNs, such as small single cells or tumor cell clusters. Microscopic analysis of metastatic disease was performed on all dissected LNs[19]. The following modes of tumor regression could be observed: Necrosis, hemorrhage, nodular fibrosis, foamy histiocytes, cystic cell reaction, areas of hyalinosis, residual cancer cells, and pools of mucin (Figure 2)[20,21]. Fernández-Aceñero et al[21] analyzed the potential prognostic effects of those response modes, such as cystic cell reaction and mucus pool, on disease-free survival (DFS) and disease-specific survival (DSS) and found no significant correlation between survival and response. In addition, several other LN markers have prognostic significance. For instance, mounting evidence suggests that extracapsular LN involvement is one prognostic contributor to recurrence and poor prognosis in malignancies of the gastrointestinal tract[22,23]. The presence or absence of fibrosis is usually used to differentiate nonmetastatic LNs from metastatic LNs that have completely regressed[19].
However, histopathological assessments have several limitations. First, the number of patients with stage ypN0 disease downgraded to only microscopic LN involvement is difficult to assess. Second, patients receiving nCRT had fewer LNs retrieved than those who underwent only radical surgery. After nCRT, fibrosis in the metastatic LNs is not as pronounced as in the primary tumor. Normal lymphocytes still occupied most LNs, and only fibrosis occurred around metastatic tumor cells. However, the changes in normal lymphocytes after radiotherapy were uncertain, with most showing no response and some fibrosis, making it much more difficult for pathologists to distinguish normal LNs from completely regressed LNs, especially when only a small number of metastatic tumor cells were present. Therefore, only some LRG1 patients were in complete remission after nCRT, while others had normal LNs, so pathologists could not assess whether the small fibrotic tissue lesion was normal LN or a metastatic LN before treatment. Finally, pathologists cannot distinguish patients with fibrosis-free LNs from those with residual ypN0 tumors as complete responders and non-responders. Nevertheless, we ought to recognize that a complete response is not a safe assumption among patients with clinical LN+ on magnetic resonance imaging with no pathological abnormalities. Does the absence of fibrosis among the LN imply that no tumor cells were present before nCRT was performed, or does the presence of fibrosis among the LN imply that tumor cells were once present? These questions should be investigated in future studies.
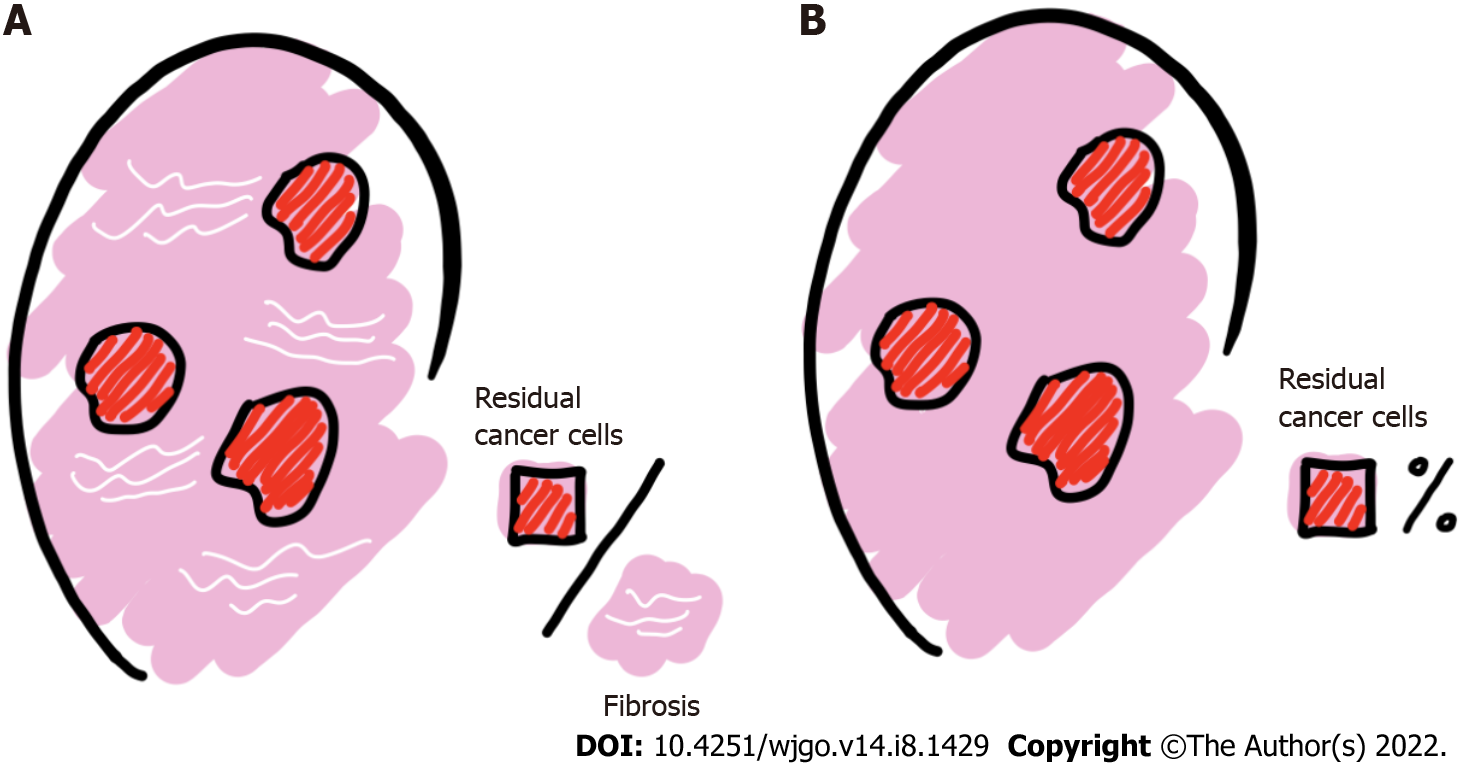
Numerous publications have shown that TRG is significantly relevant to the assessment of patient outcomes[13,24] and is an essential prognostic indicator for patients with LARC[25-27]. LRG, like TRG, is an assessment of local metastatic LN treatment response indicators for nCRT based on postoperative patient histopathology[9,28,29]. When classifying the degree of LN regression, the following two aspects should be assessed: the relationship between residual cancer cells and regressive fibrosis, the basis of which is usually described, and the number of LNs with residual cancer cells, which is usually expressed as a percentage (%) (Figure 3).
Relevant studies have documented that residual tumor cells may still be present in local LNs despite complete regression of the primary disease[30]. In some studies[31], this occurred in up to 17% of cases, especially when a watch-and-wait strategy after nCRT was chosen, likely leading to recurrence and treatment failure. Therefore, pathologic evaluation of LNs in patients undergoing surgery after nCRT can contribute to an accurate determination of the clinical stage of the tumor and the metastatic LN response to nCRT (Table 1).
| Descriptive | Caricato et al[18] | Mirbagheri et al[28] | Beppu et al[32] | Lee et al[34] | Sun et al[35] | Cui et al[38] |
| Negative/normal | LRG1 | LRG0 | - | pLRG0 | LRG0 | LRG0 |
| Absence of histologically identifiable residual cancer and fibrosis extending through the different areas of the lymph node | Normal lymph nodes | - | LN-preserving normal nodal architecture without evidence of cancer cells or fibrosis was scored | Normal lymph node architecture without evidence of regression or cancer cells | Negative lymph node | |
| Complete | LRG2 | LRG1 | LRG3 | pLRG1 | LRG1 | LRG1 |
| Near complete pathologic response (pCR) | 100% fibrosis, no residual cancer | Total regression. No cancer cells, single cells or small groups of cancer | LN with 100% fibrosis | 100% fibrosis | Complete regression with no residual tumor cells | |
| Subtotal | LRG3 | LRG2 | LRG2 | pLRG2 | LRG2 | LRG2 |
| Presence of residual cancer cells with evident fibrosis | 75%-100% fibrosis, 0-25% cancer | Good regression. Residual cancer outgrown by fibrosis | LN with < 25% cancer cells | < 25% remaining cancer cells | Rare residual tumor cells | |
| Partial | LRG4 | LRG3 | LRG1 | pLRG3 | LRG3 | LRG3 |
| Poor response | 50%-75% fibrosis, 25%-50% cancer | Minor regression. Fibrosis outgrown by cancer or no fibrosis with extensive residual cancer | Scattered glandular elements with fibrosis | 25%-50% scattered glandular elements with fibrosis | Fibrosis outgrown by residual tumor cells | |
| No regression | LRG5 | LRG4 | - | pLRG4 | LRG4 | LRG4 |
| Nodal metastasis with absence of regressive changes | 25%-50% fibrosis, 50%-75% cancer | - | LN with > 50% cancer cells | > 50% viable cancer cells | Residual tumor cell outgrown by fibrosis | |
| LRG5 | pLRG5 | LRG5 | LRG5 | |||
| 0-25% fibrosis, 75%-100% cancer | - | Complete replacement with cancer cells | Complete replacement with cancer cells | Absence of regression with no fibrosis |
In 2007, Caricato et al[18] retrospectively analyzed colorectal LNs in 35 patients undergoing preoperative CRT with LARC and reported, for the first time, the tissue effects of preoperative CRT on colorectal LNs and defined the grade of LN regression as follows: LRG1 for the absence of histologically identifiable residual cancer and fibrosis extending through the different areas of the LN; LRG2 for near-complete pathologic response (pCR); LRG3 for the presence of residual cancer cells with evident fibrosis; LRG4 for poor response; and LRG5 for nodal metastasis with the absence of regressive changes. It was also concluded that LRG was significantly correlated with TRG in primary tumors. However, this study had a small sample size, and no follow-up was performed clinically, so the prognosis of patients with LRG was not investigated further.
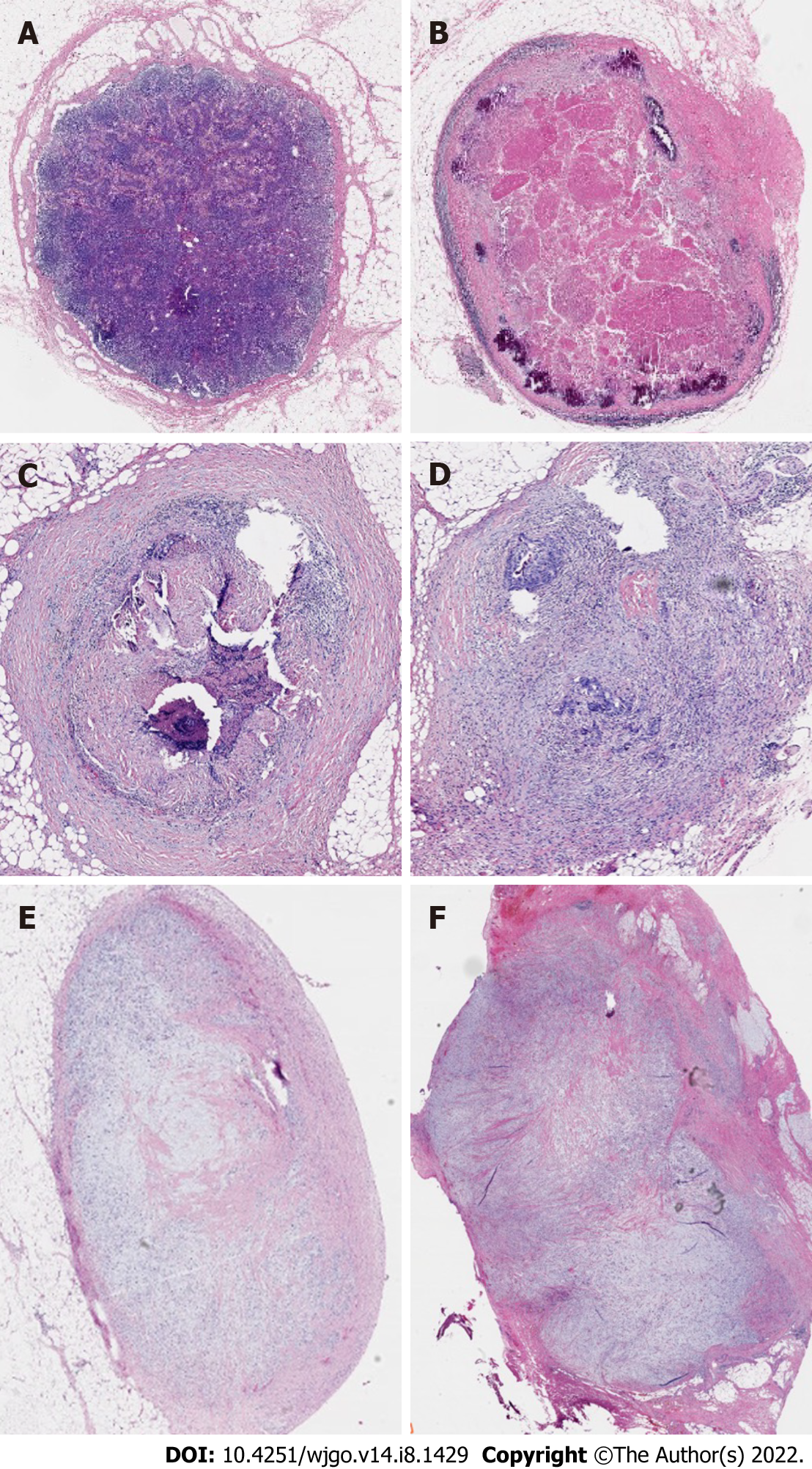
In 2014, Mirbagheri et al[28] retrospectively analyzed clinical data from 190 patients who had LARC and received nCRT and found that LRG, similar to the TRG standard, could be used as an influencing factor for tumor recurrence. They also proposed a TRG-like LRG scoring system as follows for LRG0 for normal LNs; LRG1 for 100% fibrosis, no residual cancer; LRG2 for 75%-100% fibrosis, 0-25% cancer; LRG3 for 50%-75% fibrosis, 25%-50% cancer; LRG4 for 25%-50% fibrosis, 50%-75% cancer; and LRG5 for 0-25% fibrosis, 75%-100% cancer (Figure 4). Their study results indicated that: (1) LVI (P = 0.029), tumors in the middle of the rectum and higher TRG scores were correlated with higher LRG scores; and (2) LN regression was a major factor in the prediction of tumor recurrence, and lower LN regression scores were associated with an enhanced survival curve. Mirbagheri et al[28] also proposed not only the LRG score but, for the first time, LRG maximum (LRG-max) and LRG-sum (LRG-sum). Subsequent analysis of these parameters indicated significant associations with tumor prognosis. Further research has provided additional evidence supporting a significant association between these parameters and tumor prognosis.
LRG-max: Since the number of LNs varies in each specimen and different regression scores may be calculated for different LNs depending on their treatment response, total scores were determined according to the worst score for each patient (specimen). For example, if one specimen contains two LNs whose scores were 2 and 3, the LRG-max would be 3.
LRG-sum: This reflects the overall tumor burden of the specimen for all LNs. For example, if one specimen contains two LNs whose scores were 2 and 3, the LRG-sum would be 5.
In 2015, Beppu et al[32] retrospectively analyzed clinical data from 178 patients suffering from LARC who were treated with nCRT preoperatively, investigated the requirement of chemoradiotherapy for positive LNs that had completely regressed, and proposed the following LRG score set: LRG 1 for minor regression, fibrosis outgrown by cancer or no fibrosis with extensive residual cancer; LRG 2 for good regression, residual cancer outgrown by fibrosis; and LRG 3 for total regression, no cancer cells, single cells or small groups of cancer cells. The results showed that the primary tumor response to chemoradiotherapy was related to a positive nodal response. In contrast, for patients with a TRG of 3, the LRG score was associated with positive node size. The conclusion was also drawn that for the complete regression of positive nodes, the requirements were: (1) Degeneration of the primary tumor, with a TRG of 3; and (2) a diameter of < 6 mm for positive nodes.
The following year, Beppu et al's group performed subgroup analyses with 229 patients receiving preoperative nCRT in T3 rectal cancer and showed that total positive node regression following preoperative chemoradiotherapy is the only factor independently associated with favorable overall survival[33]. Therefore, it was concluded that positive nodes showing complete regression after preoperative chemoradiotherapy could improve the prognosis of rectal cancer patients with positive LNs before treatment.
In 2019, Lee et al[34] evaluated postoperative LNs in 389 patients with rectal cancer treated with nCRT and then received radical resection. Lee defined the degree of regression of metastatic LNs after nCRT according to tumor cell percentage and degree of fibrosis and proposed a system for grading pathological LRG (pLRG) as follows: pLRG0 is a LN with normal nodal architecture, and without evidence of cancer cells or fibrosis, pLRG1 is a LN with 100% fibrosis, pLRG2 is a LN with < 25% cancer cells, pLRG3 has scattered glandular elements with fibrosis, pLRG4 is a LN with > 50% cancer cells, and pLRG5 is a complete replacement with cancer cells. The results showed that: (1) The LRG-sum distribution correlated significantly with the TRG in primary tumors; and (2) In the multivariate analysis, LRG-sum was the factor most related to RFS among the LN-related variables, in addition to ypT staging. According to the findings from this study, LRG was an influential factor for tumor prognosis in patients with rectal cancer following nCRT and surgical resection. It was shown that LRG was associated with a completely regressed primary tumor; accordingly, predicting LN regression based upon completely regressed primary tumors was beneficial, especially in patients considering a nonsurgical approach after nCRT.
In 2020, Sun et al[35] retrospectively analyzed the clinical data of 257 LARC patients receiving nCRT and proposed the following LRG scoring system: LRG 0, normal LN architecture without evidence of regression or cancer cells; LRG 1, 100% fibrosis; LRG 2, < 25% remaining cancer cells; LRG 3, 25–50% scattered glandular elements with fibrosis; LRG 4, > 50% viable cancer cells; and LRG 5, complete replacement with cancer cells. Sun et al[35] suggested that, to some extent, LRG was associated with the primary tumor response. In addition, it may help predict clinical complete remission (the cCR) and determine LN regression in patients based on preservation strategies (e.g., local excision or an approach of "watch and wait"[36,37]. Furthermore, higher LRG scores were correlated with higher TRG, later ypN and ypT staging, and poorer DFS and OS.
In 2020, Cui et al[38] retrospectively analyzed the clinical data of 358 patients with LARC who received nCRT and proposed the following set of LRG scores: LRG0, negative LN; LRG1, complete regression with no residual tumor cells; LRG2, rare residual tumor cells; LRG3, fibrosis outgrown by residual tumor cells; LRG4, residual tumor cell outgrown by fibrosis; and LRG5, absence of regression with no fibrosis. The results showed that in the univariate analysis, the factors that correlated with DFS were ypN, ypT, the number of negative LNs (NLN), LN ratio (LNR), TRG, m-TTRG (modifying ypT stage by combining ypT and TRG), LRG-sum, LRG-max, M-NLRG (modifying ypN stage by combining LNR and LRG-max) and the LRG ratio (average of LRG-sum). M-NLRG and M-TTRG were significantly related to DFS in the multivariate Cox regression analysis. It was concluded that LRG significantly contributes to the prognosis in rectal cancer patients receiving nCRT and can improve the ypTNM staging system. A modified ypTNM staging system combining TRG, LRG-max and LNR could enhance DFS prediction for various subgroups of patients.
The relationship between primary tumors and LRG is still controversial among studies[39,40]. Most of these differences could be accounted for by different treatment plans, varied diagnostic standards for LRG, small sample sizes and patient heterogeneity.
Several studies[18,21,34] have reported that LRG was significantly correlated with TRG in primary tumors. Lee et al[34] evaluated postoperative LNs in 389 patients with rectal cancer treated with nCRT and concluded that LRG-sum distribution correlated significantly with the TRG in primary tumors (P < 0.001). LRG was associated with a completely regressed primary tumor. Accordingly, predicting LN regression based upon completely regressed primary tumors is beneficial, especially for patients considering a nonsurgical approach after nCRT. There are also studies[28,35] that suggest that higher TRG scores are correlated with higher LRG scores. Sun et al[35] retrospectively analyzed the clinical data of 257 LARC patients who were receiving nCRT and found that in the TRG 1, 2 and 3 groups, LRG scores were significantly increased. Higher scores of LRG were also found to be associated with more advanced stages of ypT and ypN. Considering these results, Sun et al[35] suggested that, to some extent, LRG may help predict the clinical complete response (the cCR) and determine LN regression in patients based on preservation strategies (e.g., local excision or an approach of "watch and wait"). Additional studies have suggested that LRG is associated with TRG only under specific conditions, and the study by Beppu et al[32] concluded that: (1) Primary tumor radiosensitivity was associated with positive LNs; and that (2) LRG scores were associated with positive LN size only if the primary tumor had TRG 3 response.
Others[31] have argued that primary tumor TRG does not predict the LN presence of residual lesions. In 2006, Hughes et al[31] examined a total of 211 clinical-stage T3-T4 patients receiving preoperative CRT treatment outcomes and treatment details and concluded that primary tumor pathologic complete response failed to predict the circumrectal LN response, and the extent of the primary tumor response was a predictor of LN response.
Nevertheless, it is significant to note that different diagnostic standards for LRG were used in these previous studies, including the subgrouping of patients, which introduces some heterogeneity. Therefore, no conclusions concerning the association between TRG and LRG can be drawn at this time, and future large-scale research is needed with more homogeneous population groups to clarify this relationship.
Most studies[33,34,41] have suggested that LRG is a factor in the prognosis of rectal cancer patients receiving radical resection after nCRT. The study by Beppu et al[32] concluded that patients with completely regressed LNs typically had the best outcome. Beppu et al’s, Lee et al’s, Cui et al’s subgroup review of 229 patients receiving preoperative nCRT in T3 rectal cancer showed that total positive node regression following preoperative chemoradiotherapy is the only factor independently related to favorable overall survival[32,34,38]. While complete LN regression has been consistently correlated with improved DFS and OS as well as reduced local and distal recurrence risk, the impact of partial and subtotal LN regression [which is expected to be the main advantage of LRG vs TNM and American Joint Committee on Cancer (AJCC) grade] remains poorly understood. Studies from Mirbagheri et al[28] and Sun et al[35] concluded that a higher LRG was correlated with poorer DFS and OS. Mirbagheri et al[28] used multivariate Cox proportional hazard regression analysis and did not find that the LRG score was a factor for mortality, but it was an important predictor of relapse. However, the assumption that patients who had LN complete regression (LRG1) might fare better than LRG0 patients was not adequately tested, considering the small sample size of LRG1 patients. Tominaga et al[41] retrospectively analyzed 421 rectal cancer patients receiving preoperative nCRT, and the results indicated that LRG1 is a significant and independent factor for predicting recurrence-free survival. However, their results indicated that patients with grade 1 LN regression had similar local recurrence rates (LR) and 5-year recurrence-free survival rates as patients with LRG 0. However, in 120 patients with grade 2-5 LN regression, the 5-year recurrence-free survival rate and the LR resembled those of patients with LRG0, and the LR and the 5-year recurrence-free survival rate were poor irrespective of LRG (LR of 8.4%-14.0% and recurrence-free survival rate of 38.1%-61.1%). In addition, a large number of studies[28,34,35] have concluded that LRG-max and/or LRG-sum are significantly associated with prognosis. Lee et al[34] evaluated postoperative LNs in 389 patients with rectal cancer treated with nCRT and then received radical resection. In the multivariate analysis, LRG-sum was the most related contributor to RFS in LN-related variables alongside ypT staging. In 2020, Cui et al[38] suggested that in the univariate analysis, the contributors correlated with DFS were LRG-sum, LRG-max, M-NLRG and the LRG ratio.
However, in 2016, Fernández-Aceñero et al[21] retrospectively analyzed 106 rectal cancer patients receiving treatment at a single institution and concluded that there was no remarkable correlation between any factors or DSS and the LN tumor regression model in terms of prognosis.
In summary, we consider LRG to be an independent predictor of DFS for patients with LARC receiving nCRT and radical surgery. Since LN regression is highly correlated with other significant variables (e.g., LVI and TRG), this characteristic might lose its statistical significance in some computational models, explaining the failure of certain studies to show that LRG has independent prognostic value relative to these other parameters[28].
With the increasing development of comprehensive therapy for rectal cancer, the National Comprehensive Cancer Network has suggested that the therapy criteria for LARC are nCRT and TME[42-46], whose application has brought tremendous prognostic improvement for LARC patients with lower LR[47-50] as well as better anal preservation for patients with low rectal cancer[51,52]. A subset of LARC patients treated with nCRT can achieve complete tumor regression and are thus candidates for nonsurgical treatment[53]. NCRT leads to different degrees of tumor regression, with some patients achieving pCR for the primary tumor[27,54-56]. The LR was low in this patient group, and the tumor-free survival and overall rates were high[27,57,58]. Furthermore, numerous studies have demonstrated that TRG is significantly correlated with patient outcomes[13,24] and is an important prognostic factor for patients with LARC. LRG, like TRG, reflects the response of locally metastatic LNs to nCRT treatment based on postoperative patient histopathology[9,28]. In relevant studies, it is fully documented that residual tumor cells may still be present in local LNs despite the complete regression of primary tumors[30]. Currently, no single histopathological feature of colorectal cancer can reliably predict LN metastasis[59]. Some studies have demonstrated that different responses may exist between primary tumors and mesenteric LNs of the rectum[60]. Despite complete tumor regression, LN involvement may still occur. This was found in up to 17% of cases in some studies[31], especially when a watch-and-wait strategy was chosen after nCRT, likely leading to recurrence and treatment failure. Therefore, the pathologic evaluation of LNs in patients treated with surgery after nCRT could help to accurately determine the clinical staging of tumors and the response of metastatic LNs to nCRT.
The status of TDLN was the most significant factor in the prognosis of patients who have rectal cancer[61-63]. The number of metastatic LNs is currently the only basis for ypN staging, and several studies have demonstrated that nCRT leads to a decrease in the total number of LNs detected and the number of positive LNs[64,65]. Thus, the accuracy of staging ypN can be affected[13,14].
Several studies[66] have shown that current AJCC staging systems cannot accurately evaluate patient prognosis following nCRT because nCRT decreases the tumor stage and leads to varying degrees of treatment response. However, others argue that good prediction and assessment of regression during nCRT treatment and multidisciplinary consultation can allow for more individualized clinical decision making and treatment. The vast majority of studies on tumor response to therapy have focused on the primary tumor, while the effect of LRG on tumor treatment response and prognosis has not yet been fully appreciated.
TRG: The assessment of nCRT treatment regression in clinical practice relies mainly on postsurgical pathological examination results. Tumors were also graded by TRG according to the relative proportions of resident tumor cells in pathological specimens and the degree of fibrosis after treatment. Mandard et al[24] proposed the following: TRG1 for the absence of residual cancer and fibrosis - complete regression; TRG2 for the presence of rare residual cancer; TRG3 for an increase in the number of residual cancer cells but predominantly fibrosis; TRG4 for residual cancer outgrowing fibrosis; and TRG5 for the absence of regressive changes. Dworak et al[25] proposed a TRG staging system in 1997, which classified regression into stages 0 to 4 based on better to worse tumor regression. The seventh edition of the 2010 AJCC Cancer Stage Manual, put forward by the American Joint Committee on Cancer, reads as follows[29,67]: TRG0 for no viable cells present – complete; TRG1 for small groups of cancer cells/moderate-single cells – minimal; TRG2 for residual cancer outgrown by fibrosis; and TRG3 for no tumor-killing or poor/minimal killing, extensive residual cancer (Figure 5). Siddiqui et al[68] showed a strong association between patient prognosis and postoperative TRG grade, and they defined Dworak grades 3 and 4 and Mandard grades 1 and 2 as a better prognosis and Dworak grades 0 to 2 and Mandard grades 3 to 5 as a worse prognosis.
Currently, the AJCC 8th edition staging system, based solely upon the number of positive LNs for ypN staging, still follows the same ypN staging criteria for patients receiving nCRT and those undergoing surgery alone. Of the currently available TNM staging systems, ypN staging is classified according to the absolute number of positive LNs (PLNs). The guideline is based on little evidence and is largely derived from the historic view that evaluating a smaller number of nodes results in understaging[69,70]. In addition, although it has been determined that increases in nodal harvest are related to improved survival, generally accepted staging theories explaining this relationship are unsupported by the evidence, and several authors have suggested that the higher number of LNs may indicate immune competence in individual patients instead of an improved means of detecting metastatic nodes[71,72]. A large population study in the United States showed that less than 50% of patients achieved the recommended number of LNs[73,74]. Thus, there are two main reasons why the AJCC guidelines have been questioned. First, recommendations for staging guidelines and treatment of rectal cancer depend heavily on data collected from colon cancer patients who are thought to be appropriate for rectal cancer[75,76]. Moreover, LNs found in rectal specimens were smaller in number and size than those found in colonic specimens[70,77]. Second, LNs detected after nCRT was significantly decreased[78,79]. Due to the increasing use of preoperative treatment of rectal cancer, pathology reports demonstrating low counts of LNs are increasingly being received by colorectal surgeons.
This ypN staging system only focuses on the numbers of metastatic LNs regardless of the tumor load in LNs following nCRT. The relevant literature suggests that LN regression should also be considered when assessing LN status. The main reasons for this may be twofold. First, the current ypN staging ignores the influence of LN treatment response on prognosis. A similar number of LN-positive patients might have a different number of LN metastases and a different metastatic load before treatment. The degrees of LN metastatic tumor regression following nCRT may reflect the different biological behaviors of tumors in different individuals, leading to different prognoses. Second, a decrease in the detection of positive LNs and the total number of positive LNs following nCRT can result in a bias in ypN staging based on using the number of positive LNs as grouping criteria[80,81].
One meta-analysis[82] demonstrated that patients receiving nCRT had a mean decrease of 3.9 total LNs detected and 0.7 PLN. Patients treated with neoadjuvant radiotherapy had 2.1 fewer total LNs detected. Ceelen et al[83] retrospectively analyzed 4037 patients who have rectal cancer registered in the Belgian Rectal Cancer Registry (Project for Rectal Cancer, PROCARE) between 2006 and 2012 who received nCRT and demonstrated a 12.3% reduction in the total number of detected LNs after short-range radiotherapy and a 31.3% reduction after long-range radiotherapy or long-range simultaneous radiotherapy. For each 1 Gy increase in the radiation dose, the number of detected LNs decreased by 0.21%[84]. Each additional LN detected was related to a 2.7% reduction in the risk of death in patients undergoing surgery alone, a 1.5% reduction in the risk of death in patients with short-range preoperative radiotherapy, and no reduced risk of death in patients with long-range simultaneous preoperative radiotherapy. Data from the publicly available SEER database[85,86] also revealed no significant difference between the two groups in terms of tumor-specific survival rates when the TLN cutoff number was 12, so the criterion of at least 12 LNs may not apply to patients receiving nCRT.
In summary, nCRT can reduce LN retrieval, decrease the N stage, and encourage downstaging of the primary tumor[87] and pN stage migration, leading to staging bias. This bias could affect the ypN staging system and decrease the accuracy in assessing patient prognosis after nCRT for rectal cancer[88,89]. Therefore, the current ypN staging grouping in TNM staging is probably not applicable to patients receiving nCRT.
The evaluation and grading of LN regression are feasible for rectal cancer patients following nCRT by the histopathological examination of specimens excised after treatment. Thus, the implementation of LRG in histopathology reports for rectal cancer patients undergoing neoadjuvant radiotherapy is strongly recommended. LRG may even have more prognostic value than currently used staging systems (e.g., TNM stage), primarily derived from untreated or unspecified tumor data. Suppose an apparently regressing LN also shows evidence of residual tumor. In that case, that LN is designated as a positive LN (ypN+), despite the good prognostic value for LN regression.
Lee et al[34] evaluated postoperative LNs in 389 patients with rectal cancer treated with nCRT followed by radical resection. In the multivariate analysis, LRG-sum was the most related contributor to RFS in LN-related variables alongside ypT staging. In 2020, Cui et al[38] In the univariate analysis, the factors that correlated with DFS were LRG-sum, LRG-max, M-NLRG and the LRG ratio.
However, considering a large number of LRG systems, the main focus of international and interdisciplinary committees should be to determine a consensus that can be applied to LRG reports. Critical concerns such as interobserver variability can also be resolved by individual and institutional training. Efforts should be made by both pathologists and clinicians alike to standardize specimen handling and LRG reporting. Although LRG can be used as a morphologic "biomarker," evidence for clinical trials could not be produced from studies with larger cohorts. The primary purpose of clinical trials should never be to compare different LRG systems but rather to scrutinize the histology and identify a standardized reporting method for LRG, which may further enhance the evidence of the value of LRG for the management of nCRT-treated LARC patients.
Recommendations for the standardized macroscopic and histopathological examination of LNs from rectal cancer excision specimens following nCRT are as follows: We prefer a 5-tier grading system and use the Mirbagheri system[28] in our daily work, which is very similar to the 4-tier modified Dworak TRG system[90]. A reproducible and easy-to-apply grading system for predicting clinical outcomes at a systematic level (comparing adequacy of various therapies) and for the individual patient (assessing their response to treatment, guiding further management, insight into prognosis) are useful. We consider this to be a good option. Based on this concept, additional data from evidence-based studies on the prognostic impact of LRG have confirmed that it is a strong prognostic morphological "biomarker" for guiding clinical decisions, modifying postoperative adjuvant therapy, improving operative strategies and monitoring intensities, and providing potential endpoints and alternative markers of prognosis for research programs and patients within clinical trials, which have yet to be presented.
Moreover, in addition to traditional radiotherapy, chemotherapy and surgery, some new oncological treatment methods have emerged recently, such as Her-2, MSI, and BRAF targeting for rectal cancer or the recently introduced immune checkpoint inhibitors[91]. Although immunotherapy has made considerable advances for a range of cancers, including non-small-cell lung cancer[92], the advances have not yet been extended to most rectal cancer patients[93]. The majority of rectal cancers are microsatellite stable, where immunotherapies targeting cytotoxic T lymphocyte-associated protein 4, programmed death-1 and programmed death-ligand 1 are currently recommended only for patients with high MSI-H[55,94]. Despite this, evidence suggests that it is important for the immune system to combat rectal cancer, as several studies have demonstrated that pretreatment densities of tumor-infiltrating lymphocytes predict better oncologic outcomes[95-97]. Furthermore, increasing numbers of preclinical models demonstrate that current chemotherapy and radiotherapy protocols can activate and synergize the immune system using immunotherapy[98-100]. Nevertheless, there is poor knowledge of the tissue alterations resulting from such emerging therapeutic strategies. Careful histopathological examination of posttreatment tissues and LNs could offer significant insight into the impact of these new agents and resistance mechanisms. Such research is expected to clarify the value of both TRG and LRG and additional detailed histological discoveries equivalent to those reported in the research originally used to introduce TRG into pathology.
In summary, LRG should be recognized as an indicator of the response to nCRT and considered a determinant of prognosis for rectal cancer patients and should be included in pathology reports. With further and more extensive evidence-based validation, LRG may become a strong prognostic morphological "biomarker" that can be used to guide clinical decisions, modify postoperative adjuvant therapy, and improve operative strategies and monitoring radiation intensities, as well as provide potential endpoints and alternative markers of prognosis for research programs and patients in clinical trials.
Throughout the writing of this dissertation, I have received a great deal of support and assistance. I would first like to thank my supervisor, Peng Q, whose expertise was invaluable in formulating the research questions and methodology. Your insightful feedback pushed me to sharpen my thinking and brought my work to a higher level. I would particularly like to acknowledge my teammate/group mate/team members, Xiao J, Zheng P and Zhong L for their wonderful collaboration and patient support. In addition, I would like to thank my parents for their wise counsel and sympathetic ear. You are always there for me. Finally, I could not have completed this dissertation without the support of my friends, who provided stimulating discussions as well as happy distractions to rest my mind outside of my research.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dulskas A, Lithuania; Elkady N, Egypt S-Editor: Yan JP L-Editor: A P-Editor: Yan JP
| 1. | Benson AB 3rd, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fuchs CS, Grem JL, Hunt S, Leong LA, Lin E, Martin MG, May KS, Mulcahy MF, Murphy K, Rohren E, Ryan DP, Saltz L, Sharma S, Shibata D, Skibber JM, Small W Jr, Sofocleous CT, Venook AP, Willett CG, Freedman-Cass DA, Gregory KM. Rectal cancer. J Natl Compr Canc Netw. 2012;10:1528-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (1)] |
| 2. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1340] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 3. | Swedish Rectal Cancer Trial. , Cedermark B, Dahlberg M, Glimelius B, Påhlman L, Rutqvist LE, Wilking N. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med. 1997;336:980-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1848] [Cited by in RCA: 1814] [Article Influence: 64.8] [Reference Citation Analysis (0)] |
| 4. | Aitken RJ. Mesorectal excision for rectal cancer. Br J Surg. 1996;83:214-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS, Bessell E, Griffiths G, Thompson LC, Parmar M. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1109] [Article Influence: 69.3] [Reference Citation Analysis (2)] |
| 6. | Hermanek P, Merkel S, Hohenberger W. Prognosis of rectal carcinoma after multimodal treatment: ypTNM classification and tumor regression grading are essential. Anticancer Res. 2013;33:559-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (2)] |
| 7. | Ryan R, Gibbons D, Hyland JM, Treanor D, White A, Mulcahy HE, O'Donoghue DP, Moriarty M, Fennelly D, Sheahan K. Pathological response following long-course neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Histopathology. 2005;47:141-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 496] [Article Influence: 24.8] [Reference Citation Analysis (1)] |
| 8. | Scabini S, Montecucco F, Nencioni A, Zoppoli G, Sartini M, Rimini E, Massobrio A, De Marini L, Poggi A, Boaretto R, Romairone E, Ballestrero A, Ferrando V. The effect of preoperative chemoradiotherapy on lymph nodes harvested in TME for rectal cancer. World J Surg Oncol. 2013;11:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Morcos B, Baker B, Al Masri M, Haddad H, Hashem S. Lymph node yield in rectal cancer surgery: effect of preoperative chemoradiotherapy. Eur J Surg Oncol. 2010;36:345-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 76] [Article Influence: 5.1] [Reference Citation Analysis (1)] |
| 10. | Scott KW, Grace RH. Detection of lymph node metastases in colorectal carcinoma before and after fat clearance. Br J Surg. 1989;76:1165-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 274] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 11. | Hernanz F, Revuelta S, Redondo C, Madrazo C, Castillo J, Gómez-Fleitas M. Colorectal adenocarcinoma: quality of the assessment of lymph node metastases. Dis Colon Rectum. 1994;37:373-6; discussion 376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 103] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Mukai M, Sato S, Nishida T, Komatsu N, Shiba K, Nakasaki H, Makuuchi H. Selection criteria for high risk and low risk groups of recurrence and metastasis in patients with primary colorectal cancer. Oncol Rep. 2003;10:1753-1758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Amajoyi R, Lee Y, Recio PJ, Kondylis PD. Neoadjuvant therapy for rectal cancer decreases the number of lymph nodes harvested in operative specimens. Am J Surg. 2013;205:289-92; discussion 292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 14. | Persiani R, Biondi A, Gambacorta MA, Bertucci Zoccali M, Vecchio FM, Tufo A, Coco C, Valentini V, Doglietto GB, D'Ugo D. Prognostic implications of the lymph node count after neoadjuvant treatment for rectal cancer. Br J Surg. 2014;101:133-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | La Torre M, Lorenzon L, Pilozzi E, Barucca V, Cavallini M, Ziparo V, Ferri M. Number of harvested lymph nodes is the main prognostic factor in Stage IIa colorectal cancer patients. J Surg Oncol. 2012;106:469-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 16. | Yao YF, Wang L, Liu YQ, Li JY, Gu J. Lymph node distribution and pattern of metastases in the mesorectum following total mesorectal excision using the modified fat clearing technique. J Clin Pathol. 2011;64:1073-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Langer R, Becker K. Tumor regression grading of gastrointestinal cancers after neoadjuvant therapy. Virchows Arch. 2018;472:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 18. | Caricato M, Ausania F, De Dominicis E, Vincenzi B, Rabitti C, Tonini G, Cellini F, Coppola R. Tumor regression in mesorectal lymphnodes after neoadjuvant chemoradiation for rectal cancer. Eur J Surg Oncol. 2007;33:724-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (2)] |
| 19. | Bollschweiler E, Hölscher AH, Metzger R, Besch S, Mönig SP, Baldus SE, Drebber U. Prognostic significance of a new grading system of lymph node morphology after neoadjuvant radiochemotherapy for esophageal cancer. Ann Thorac Surg. 2011;92:2020-2027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Becker K, Mueller JD, Schulmacher C, Ott K, Fink U, Busch R, Böttcher K, Siewert JR, Höfler H. Histomorphology and grading of regression in gastric carcinoma treated with neoadjuvant chemotherapy. Cancer. 2003;98:1521-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 463] [Cited by in RCA: 585] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 21. | Fernández-Aceñero MJ, Granja M, Sastre J, García-Paredes B, Estrada L. Prognostic significance of tumor regression in lymph nodes after neoadjuvant therapy for rectal carcinoma. Virchows Arch. 2016;468:425-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 22. | Wind J, Lagarde SM, Ten Kate FJ, Ubbink DT, Bemelman WA, van Lanschot JJ. A systematic review on the significance of extracapsular lymph node involvement in gastrointestinal malignancies. Eur J Surg Oncol. 2007;33:401-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Metzger R, Bollschweiler E, Drebber U, Mönig SP, Schröder W, Alakus H, Kocher M, Baldus SE, Hölscher AH. Neoadjuvant chemoradiotherapy for esophageal cancer: impact on extracapsular lymph node involvement. World J Gastroenterol. 2010;16:1986-1992. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 25. | Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 1060] [Article Influence: 37.9] [Reference Citation Analysis (1)] |
| 26. | Huh JW, Kim HC, Kim SH, Park YA, Cho YB, Yun SH, Lee WY, Park HC, Choi DH, Park JO, Park YS, Chun HK. Tumor regression grade as a clinically useful outcome predictor in patients with rectal cancer after preoperative chemoradiotherapy. Surgery. 2019;165:579-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 27. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1454] [Article Influence: 96.9] [Reference Citation Analysis (0)] |
| 28. | Mirbagheri N, Kumar B, Deb S, Poh BR, Dark JG, Leow CC, Teoh WM. Lymph node status as a prognostic indicator after preoperative neoadjuvant chemoradiotherapy of rectal cancer. Colorectal Dis. 2014;16:O339-O346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (2)] |
| 29. | Mace AG, Pai RK, Stocchi L, Kalady MF. American Joint Committee on Cancer and College of American Pathologists regression grade: a new prognostic factor in rectal cancer. Dis Colon Rectum. 2015;58:32-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 126] [Article Influence: 12.6] [Reference Citation Analysis (1)] |
| 30. | Hiotis SP, Weber SM, Cohen AM, Minsky BD, Paty PB, Guillem JG, Wagman R, Saltz LB, Wong WD. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. J Am Coll Surg. 2002;194:131-5; discussion 135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 276] [Article Influence: 12.0] [Reference Citation Analysis (1)] |
| 31. | Hughes R, Glynne-Jones R, Grainger J, Richman P, Makris A, Harrison M, Ashford R, Harrison RA, Livingstone JI, McDonald PJ, Meyrick Thomas J, Mitchell IC, Northover JM, Phillips R, Wallace M, Windsor A, Novell JR. Can pathological complete response in the primary tumour following pre-operative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for sterilisation of pelvic lymph nodes, a low risk of local recurrence and the appropriateness of local excision? Int J Colorectal Dis. 2006;21:11-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Beppu N, Kakuno A, Doi H, Kamikonya N, Matsubara N, Tomita N, Yanagi H, Yamanaka N. The impact of the radiation-induced regression of positive nodes on survival in patients with rectal cancer treated with chemoradiotherapy. Surgery. 2017;161:422-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (1)] |
| 33. | Beppu N, Matsubara N, Noda M, Yamano T, Kakuno A, Doi H, Kamikonya N, Yamanaka N, Yanagi H, Tomita N. Pathologic evaluation of the response of mesorectal positive nodes to preoperative chemoradiotherapy in patients with rectal cancer. Surgery. 2015;157:743-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Lee HG, Kim SJ, Park IJ, Hong SM, Lim SB, Lee JB, Yu CS, Kim JC. Effect of Responsiveness of Lymph Nodes to Preoperative Chemoradiotherapy in Patients With Rectal Cancer on Prognosis After Radical Resection. Clin Colorectal Cancer. 2019;18:e191-e199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Sun Y, Wu X, Lin H, Lu X, Huang Y, Chi P. Lymph Node Regression to Neoadjuvant Chemoradiotherapy in Patients with Locally Advanced Rectal Cancer: Prognostic Implication and a Predictive Model. J Gastrointest Surg. 2021;25:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 36. | Minsky BD. Rectal cancer: is 'watch and wait' a safe option for rectal cancer? Nat Rev Gastroenterol Hepatol. 2013;10:698-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 37. | Gani C, Kirschniak A, Zips D. Watchful Waiting after Radiochemotherapy in Rectal Cancer: When Is It Feasible? Visc Med. 2019;35:119-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Cui J, Zhang L, Yang L, Zhu YL, Fang H, Chen B, Ning Y, Zhang HZ. The prognostic significance of the treatment response of regional lymph nodes and the refinement of the current TNM staging system in locally advanced rectal cancer after neoadjuvant chemoradiotherapy. Cancer Med. 2020;9:9373-9384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (1)] |
| 39. | Losi L, Luppi G, Gavioli M, Iachetta F, Bertolini F, D'Amico R, Jovic G, Bertoni F, Falchi AM, Conte PF. Prognostic value of Dworak grade of regression (GR) in patients with rectal carcinoma treated with preoperative radiochemotherapy. Int J Colorectal Dis. 2006;21:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 40. | Mignanelli ED, de Campos-Lobato LF, Stocchi L, Lavery IC, Dietz DW. Downstaging after chemoradiotherapy for locally advanced rectal cancer: is there more (tumor) than meets the eye? Dis Colon Rectum. 2010;53:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Tominaga T, Akiyoshi T, Yamamoto N, Oba K, Nagasaki T, Yamaguchi T, Konishi T, Fukunaga Y, Ueno M. Prognostic value of metastatic lymph node regression grade after neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer. Surgery. 2019;166:1061-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Gurski L, Freedman-Cass DA. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2018;16:874-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 681] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 43. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R; German Rectal Cancer Study Group. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4454] [Article Influence: 212.1] [Reference Citation Analysis (1)] |
| 44. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1190] [Article Influence: 148.8] [Reference Citation Analysis (0)] |
| 45. | Jin C, Deng X, Li Y, He W, Yang X, Liu J. Lymph node ratio is an independent prognostic factor for rectal cancer after neoadjuvant therapy: A meta-analysis. J Evid Based Med. 2018;11:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 46. | Molinari C, Passardi A. Why is neoadjuvant chemoradiation therapy underused for locally advanced rectal cancer? Expert Rev Gastroenterol Hepatol. 2016;10:1317-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 47. | Jwa E, Kim JH, Han S, Park JH, Lim SB, Kim JC, Hong YS, Kim TW, Yu CS. Nomogram to predict ypN status after chemoradiation in patients with locally advanced rectal cancer. Br J Cancer. 2014;111:249-254. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 48. | Avallone A, Aloj L, Pecori B, Caracò C, De Stefano A, Tatangelo F, Silvestro L, Granata V, Bianco F, Romano C, Di Gennaro F, Budillon A, Petrillo A, Muto P, Botti G, Delrio P, Lastoria S. 18F-FDG PET/CT Is an Early Predictor of Pathologic Tumor Response and Survival After Preoperative Radiochemotherapy with Bevacizumab in High-Risk Locally Advanced Rectal Cancer. J Nucl Med. 2019;60:1560-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Landry JC, Feng Y, Prabhu RS, Cohen SJ, Staley CA, Whittington R, Sigurdson ER, Nimeiri H, Verma U, Benson AB. Phase II Trial of Preoperative Radiation With Concurrent Capecitabine, Oxaliplatin, and Bevacizumab Followed by Surgery and Postoperative 5-Fluorouracil, Leucovorin, Oxaliplatin (FOLFOX), and Bevacizumab in Patients With Locally Advanced Rectal Cancer: 5-Year Clinical Outcomes ECOG-ACRIN Cancer Research Group E3204. Oncologist. 2015;20:615-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 50. | Park HJ, Cho S, Kim Y. Patterns of Rectal Cancer Radiotherapy Adopting Evidence-Based Medicine: An Analysis of the National Database from 2005 to 2016. Cancer Res Treat. 2018;50:975-983. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Dayal S, Battersby N, Cecil T. Evolution of Surgical Treatment for Rectal Cancer: a Review. J Gastrointest Surg. 2017;21:1166-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev. 2013;CD006041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 53. | Habr-Gama A, Perez RO. Non-operative management of rectal cancer after neoadjuvant chemoradiation. Br J Surg. 2009;96:125-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 54. | Janjan NA, Khoo VS, Abbruzzese J, Pazdur R, Dubrow R, Cleary KR, Allen PK, Lynch PM, Glober G, Wolff R, Rich TA, Skibber J. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys. 1999;44:1027-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 351] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 55. | Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA Jr. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509-2520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6096] [Cited by in RCA: 7212] [Article Influence: 721.2] [Reference Citation Analysis (0)] |
| 56. | Smith KD, Tan D, Das P, Chang GJ, Kattepogu K, Feig BW, Skibber JM, Rodriguez-Bigas MA. Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg. 2010;251:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, Miccichè F, Ricci R, Morganti AG, Gambacorta MA, Maurizi F, Coco C. The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2005;62:752-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 328] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 58. | Shivnani AT, Small W Jr, Stryker SJ, Kiel KD, Lim S, Halverson AL, Talamonti MS. Preoperative chemoradiation for rectal cancer: results of multimodality management and analysis of prognostic factors. Am J Surg. 2007;193:389-93; discussion 393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 59. | Glasgow SC, Bleier JI, Burgart LJ, Finne CO, Lowry AC. Meta-analysis of histopathological features of primary colorectal cancers that predict lymph node metastases. J Gastrointest Surg. 2012;16:1019-1028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 60. | Aschele C, Cionini L, Lonardi S, Pinto C, Cordio S, Rosati G, Artale S, Tagliagambe A, Ambrosini G, Rosetti P, Bonetti A, Negru ME, Tronconi MC, Luppi G, Silvano G, Corsi DC, Bochicchio AM, Chiaulon G, Gallo M, Boni L. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol. 2011;29:2773-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 544] [Cited by in RCA: 580] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 61. | Kim TH, Chang HJ, Kim DY, Jung KH, Hong YS, Kim SY, Park JW, Oh JH, Lim SB, Choi HS, Jeong SY. Pathologic nodal classification is the most discriminating prognostic factor for disease-free survival in rectal cancer patients treated with preoperative chemoradiotherapy and curative resection. Int J Radiat Oncol Biol Phys. 2010;77:1158-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 62. | Luna-Pérez P, Rodríguez-Ramírez S, Alvarado I, Gutiérrez de la Barrera M, Labastida S. Prognostic significance of retrieved lymph nodes per specimen in resected rectal adenocarcinoma after preoperative chemoradiation therapy. Arch Med Res. 2003;34:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 63. | Benzoni E, Intersimone D, Terrosu G, Bresadola V, Cojutti A, Cerato F, Avellini C. Prognostic value of tumour regression grading and depth of neoplastic infiltration within the perirectal fat after combined neoadjuvant chemo-radiotherapy and surgery for rectal cancer. J Clin Pathol. 2006;59:505-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (1)] |
| 64. | Maschuw K, Kress R, Ramaswamy A, Braun I, Langer P, Gerdes B. Short-term preoperative radiotherapy in rectal cancer patients leads to a reduction of the detectable number of lymph nodes in resection specimens. Langenbecks Arch Surg. 2006;391:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 65. | Sermier A, Gervaz P, Egger JF, Dao M, Allal AS, Bonet M, Morel P. Lymph node retrieval in abdominoperineal surgical specimen is radiation time-dependent. World J Surg Oncol. 2006;4:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 66. | Moon SH, Kim DY, Park JW, Oh JH, Chang HJ, Kim SY, Kim TH, Park HC, Choi DH, Chun HK, Kim JH, Park JH, Yu CS. Can the new American Joint Committee on Cancer staging system predict survival in rectal cancer patients treated with curative surgery following preoperative chemoradiotherapy? Cancer. 2012;118:4961-4968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6447] [Article Influence: 429.8] [Reference Citation Analysis (0)] |
| 68. | Siddiqui MR, Bhoday J, Battersby NJ, Chand M, West NP, Abulafi AM, Tekkis PP, Brown G. Defining response to radiotherapy in rectal cancer using magnetic resonance imaging and histopathological scales. World J Gastroenterol. 2016;22:8414-8434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 69. | Baxter NN. Is lymph node count an ideal quality indicator for cancer care? J Surg Oncol. 2009;99:265-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 70. | Wong JH, Severino R, Honnebier MB, Tom P, Namiki TS. Number of nodes examined and staging accuracy in colorectal carcinoma. J Clin Oncol. 1999;17:2896-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 257] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 71. | Chang GJ, Rodriguez-Bigas MA, Skibber JM, Moyer VA. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst. 2007;99:433-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 778] [Article Influence: 43.2] [Reference Citation Analysis (0)] |
| 72. | Hogan NM, Winter DC. A nodal positivity constant: new perspectives in lymph node evaluation and colorectal cancer. World J Surg. 2013;37:878-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Bilimoria KY, Bentrem DJ, Stewart AK, Talamonti MS, Winchester DP, Russell TR, Ko CY. Lymph node evaluation as a colon cancer quality measure: a national hospital report card. J Natl Cancer Inst. 2008;100:1310-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 140] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 74. | Chou JF, Row D, Gonen M, Liu YH, Schrag D, Weiser MR. Clinical and pathologic factors that predict lymph node yield from surgical specimens in colorectal cancer: a population-based study. Cancer. 2010;116:2560-2570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 75. | Govindarajan A, Gönen M, Weiser MR, Shia J, Temple LK, Guillem JG, Paty PB, Nash GM. Challenging the feasibility and clinical significance of current guidelines on lymph node examination in rectal cancer in the era of neoadjuvant therapy. J Clin Oncol. 2011;29:4568-4573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 76. | Kidner TB, Ozao-Choy JJ, Yoon J, Bilchik AJ. Should quality measures for lymph node dissection in colon cancer be extrapolated to rectal cancer? Am J Surg. 2012;204:843-7; discussion 847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Lee H, Park HC, Park W, Choi DH, Kim YI, Park YS, Park JO, Chun HK, Lee WY, Kim HC, Yun SH, Cho YB, Park YA. Negative impact of pretreatment anemia on local control after neoadjuvant chemoradiotherapy and surgery for rectal cancer. Radiat Oncol J. 2012;30:117-123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Wichmann MW, Müller C, Meyer G, Strauss T, Hornung HM, Lau-Werner U, Angele MK, Schildberg FW. Effect of preoperative radiochemotherapy on lymph node retrieval after resection of rectal cancer. Arch Surg. 2002;137:206-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 79. | Thorn CC, Woodcock NP, Scott N, Verbeke C, Scott SB, Ambrose NS. What factors affect lymph node yield in surgery for rectal cancer? Colorectal Dis. 2004;6:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 80. | Koo T, Song C, Kim JS, Kim K, Chie EK, Kang SB, Lee KW, Kim JH, Jeong SY, Kim TY. Impact of Lymph Node Ratio on Oncologic Outcomes in ypStage III Rectal Cancer Patients Treated with Neoadjuvant Chemoradiotherapy followed by Total Mesorectal Excision, and Postoperative Adjuvant Chemotherapy. PLoS One. 2015;10:e0138728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 81. | Miller ED, Robb BW, Cummings OW, Johnstone PA. The effects of preoperative chemoradiotherapy on lymph node sampling in rectal cancer. Dis Colon Rectum. 2012;55:1002-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 82. | Mechera R, Schuster T, Rosenberg R, Speich B. Lymph node yield after rectal resection in patients treated with neoadjuvant radiation for rectal cancer: A systematic review and meta-analysis. Eur J Cancer. 2017;72:84-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 83. | Ceelen W, Willaert W, Varewyck M, Libbrecht S, Goetghebeur E, Pattyn P; PROCARE. Effect of Neoadjuvant Radiation Dose and Schedule on Nodal Count and Its Prognostic Impact in Stage II-III Rectal Cancer. Ann Surg Oncol. 2016;23:3899-3906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Sprenger T, Rothe H, Langer C, Becker H, Liersch T. Comment on "lymph nodes after preoperative chemoradiotherapy for rectal carcinoma: number, status, and impact on survival". Am J Surg Pathol. 2009;33:1107; author reply 1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 85. | La Torre M, Mazzuca F, Ferri M, Mari FS, Botticelli A, Pilozzi E, Lorenzon L, Osti MF, Marchetti P, Enrici RM, Ziparo V. The importance of lymph node retrieval and lymph node ratio following preoperative chemoradiation of rectal cancer. Colorectal Dis. 2013;15:e382-e388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | McFadden C, McKinley B, Greenwell B, Knuckolls K, Culumovic P, Schammel D, Schammel C, Trocha SD. Differential lymph node retrieval in rectal cancer: associated factors and effect on survival. J Gastrointest Oncol. 2013;4:158-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 87. | Fokas E, Ströbel P, Fietkau R, Ghadimi M, Liersch T, Grabenbauer GG, Hartmann A, Kaufmann M, Sauer R, Graeven U, Hoffmanns H, Raab HR, Hothorn T, Wittekind C, Rödel C; German Rectal Cancer Study Group. Tumor Regression Grading After Preoperative Chemoradiotherapy as a Prognostic Factor and Individual-Level Surrogate for Disease-Free Survival in Rectal Cancer. J Natl Cancer Inst. 2017;109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 88. | McDonald JR, Renehan AG, O'Dwyer ST, Haboubi NY. Lymph node harvest in colon and rectal cancer: Current considerations. World J Gastrointest Surg. 2012;4:9-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 102] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 89. | Park IJ, Yu CS, Lim SB, Yoon YS, Kim CW, Kim TW, Kim JH, Kim JC. Ratio of metastatic lymph nodes is more important for rectal cancer patients treated with preoperative chemoradiotherapy. World J Gastroenterol. 2015;21:3274-3281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | Wittekind C, Tannapfel A. [Regression grading of colorectal carcinoma after preoperative radiochemotherapy. An inventory]. Pathologe. 2003;24:61-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 91. | Moehler M, Delic M, Goepfert K, Aust D, Grabsch HI, Halama N, Heinrich B, Julie C, Lordick F, Lutz MP, Mauer M, Alsina Maqueda M, Schild H, Schimanski CC, Wagner AD, Roth A, Ducreux M. Immunotherapy in gastrointestinal cancer: Recent results, current studies and future perspectives. Eur J Cancer. 2016;59:160-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 92. | Massarelli E, Papadimitrakopoulou V, Welsh J, Tang C, Tsao AS. Immunotherapy in lung cancer. Transl Lung Cancer Res. 2014;3:53-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 35] [Reference Citation Analysis (0)] |
| 93. | Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol. 2014;11:24-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 328] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 94. | Hasan S, Renz P, Wegner RE, Finley G, Raj M, Monga D, McCormick J, Kirichenko A. Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: A National Cancer Database (NCDB) Analysis. Ann Surg. 2020;271:716-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 102] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 95. | Shinto E, Hase K, Hashiguchi Y, Sekizawa A, Ueno H, Shikina A, Kajiwara Y, Kobayashi H, Ishiguro M, Yamamoto J. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol. 2014;21 Suppl 3:S414-S421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 96. | Gooden MJ, de Bock GH, Leffers N, Daemen T, Nijman HW. The prognostic influence of tumour-infiltrating lymphocytes in cancer: a systematic review with meta-analysis. Br J Cancer. 2011;105:93-103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1014] [Cited by in RCA: 989] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 97. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4318] [Cited by in RCA: 4899] [Article Influence: 257.8] [Reference Citation Analysis (0)] |
| 98. | McLaughlin M, Patin EC, Pedersen M, Wilkins A, Dillon MT, Melcher AA, Harrington KJ. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat Rev Cancer. 2020;20:203-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 553] [Article Influence: 110.6] [Reference Citation Analysis (0)] |
| 99. | Guan Y, Kraus SG, Quaney MJ, Daniels MA, Mitchem JB, Teixeiro E. FOLFOX Chemotherapy Ameliorates CD8 T Lymphocyte Exhaustion and Enhances Checkpoint Blockade Efficacy in Colorectal Cancer. Front Oncol. 2020;10:586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 100. | Chen B, Alvarado DM, Iticovici M, Kau NS, Park H, Parikh PJ, Thotala D, Ciorba MA. Interferon-Induced IDO1 Mediates Radiation Resistance and Is a Therapeutic Target in Colorectal Cancer. Cancer Immunol Res. 2020;8:451-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 14.4] [Reference Citation Analysis (0)] |