Published online Jul 15, 2022. doi: 10.4251/wjgo.v14.i7.1356
Peer-review started: January 23, 2022
First decision: April 17, 2022
Revised: April 30, 2022
Accepted: June 21, 2022
Article in press: June 21, 2022
Published online: July 15, 2022
Processing time: 170 Days and 15.8 Hours
Signet ring cell carcinoma (SRCC) is a specific type of mucinous secretory adenocarcinoma, which contains abundant mucus in the cytoplasm and pushes the nucleus to one side of the cell membrane, forming a round or oval, and the nuclear deviations give the cells a signet ring-like appearance. SRCC often originates in the gastrointestinal tract, especially in the stomach. However, primary SRCC of the extrahepatic bile duct is extremely rare. Therefore, little is known about its epidemiology, treatment, and prognosis.
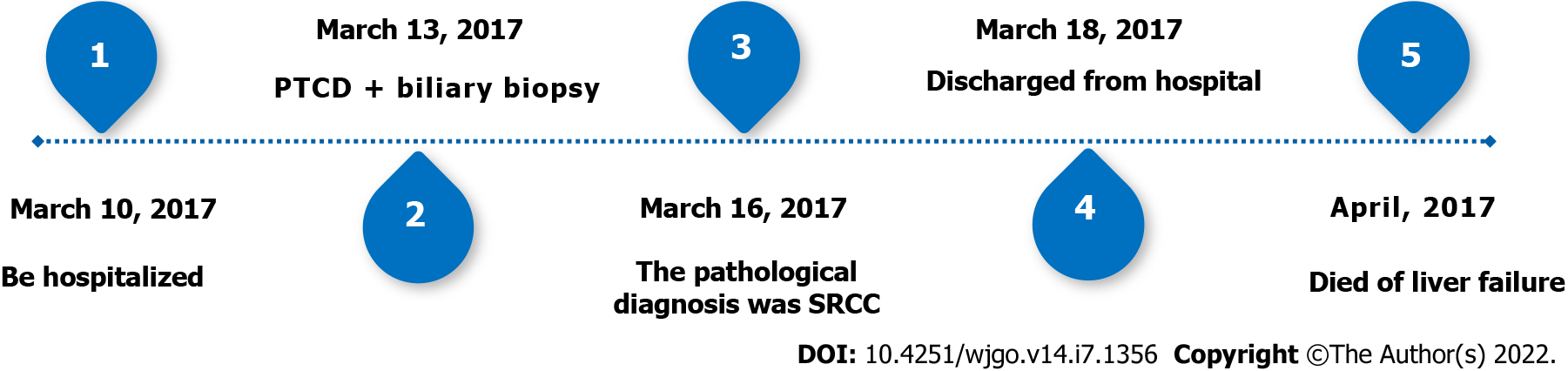
An 82-year-old female was admitted with abdominal pain, jaundice, and skin pruritus for 2 mo. She had no specific family history. Physical examination presented normal vital signs, icteric sclera, visible jaundice, and mild tenderness in the right upper abdominal quadrant. Tumor-related cell markers were within normal values. Contrast-enhanced computed tomography revealed a thickened wall of the common bile duct, strengthened with intrahepatic bile duct dilation and multiple round-like lesions in the liver. In addition, the lymph nodes in the hepatic hilum area, the pancreatic head area, and around the abdominal aorta were enlarged. Thus, a preoperative diagnosis of cholangiocarcinoma was established. To alleviate jaundice and prolong the overall survival, percutaneous transhepatic cholangiopancreatic drainage (PTCD) was performed. During the operation, segmental stenosis of the extrahepatic bile duct and a vine-like expansion of the intrahepatic bile duct was observed. Furthermore, a biliary biopsy was performed under fluoroscopy to determine the nature and origin of the lesion. The pathological diagnosis of the biopsy was SRCC. Finally, a diagnosis of primary SRCC of extrahepatic bile duct with distant lymph node metastasis and multiple liver metastases was made based on the radiographic, PTCD, and pathological characteristics. The tumor was diagnosed as T3N1M1 stage IV. Despite our aggressive approach, the patient died of liver failure after 1 mo.
This is the only case report on primary SRCC of the extrahepatic bile duct with distant organ metastasis to date.
Core Tip: We report a case where an 82-year-old female was admitted with abdominal pain, jaundice, and skin pruritus for 2 mo. The radiological diagnosis was a cholangiocarcinoma. To alleviate jaundice and prolong the overall survival, percutaneous transhepatic cholangiopancreatic drainage (PTCD) was performed. During the operation, in order to determine the nature and origin of the lesion, a biliary biopsy was performed under fluoroscopy. Finally, a diagnosis of primary signet ring cell carcinoma of extrahepatic bile duct with distant lymph node metastasis and multiple liver metastases was made based on the radiographic, PTCD, and pathological characteristics. However, despite active treatment, the disease progressed rapidly, and the patient died after 1 mo due to liver failure.
- Citation: Xie CB, Wu Y, Li F, Zhao KF, Shi RS, Huang Q, Ao J, Ke D. Primary signet-ring cell carcinoma of the extrahepatic bile duct: A case report. World J Gastrointest Oncol 2022; 14(7): 1356-1362
- URL: https://www.wjgnet.com/1948-5204/full/v14/i7/1356.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i7.1356
Cholangiocarcinoma (CCA) is a common malignant tumor of the biliary system, and 90%-95% of the pathologic types of CCA are adenocarcinomas[1]. Signet ring cell carcinoma (SRCC) is a subtype of poorly differentiated adenocarcinoma with strong invasion and poor prognosis[2]. Although it can occur in various organs, including the stomach, colon, esophagus, bladder, prostate, pancreas, and breast[2], it mainly arises in the stomach[3], where > 96% of SRCCs occur[4]. However, the occurrence of the extrahepatic bile duct is extremely rare.
Previously, only a few studies have reported cases of primary SRCC of the extrahepatic bile duct due to the rarity this disease . Herein, we report that a case of primary SRCC of the extrahepatic bile duct diagnosed via a biopsy of the biliary tree. Additionally, we conducted a literature review to describe the epidemiology and explore the treatment and prognosis of the disease.
Abdominal pain, jaundice, and skin pruritus for 2 mo.
The patient was admitted with abdominal pain, jaundice, and skin pruritus for 2 mo.
The patient was previously healthy and had no specific medical history.
She had no specific family history.
Physical examination presented normal vital signs, icteric sclera, visible jaundice, and mild tenderness in the upper right abdominal quadrant.
Tumor-related cell markers were as follows: carbohydrate antigen 19-9, > 2 044 U/mL (reference range: < 35 U/mL); cancer antigen 125, 146 U/mL (reference range: < 35 U/mL); human chorionic gonadotropin, 23.38 IU/L (reference range: < 5 IU/L); alpha-fetoprotein and carcinoembryonic antigen (CEA), within normal range. Laboratory tests: total bilirubin, 446 μmol/L (reference range: 5-21 μmol/L); direct bilirubin, 232.9 μmol/L (reference range: 0-3.4 μmol/L); indirect bilirubin, 213.1 μmol/L (reference range: 1.7-13.6 μmol/L).
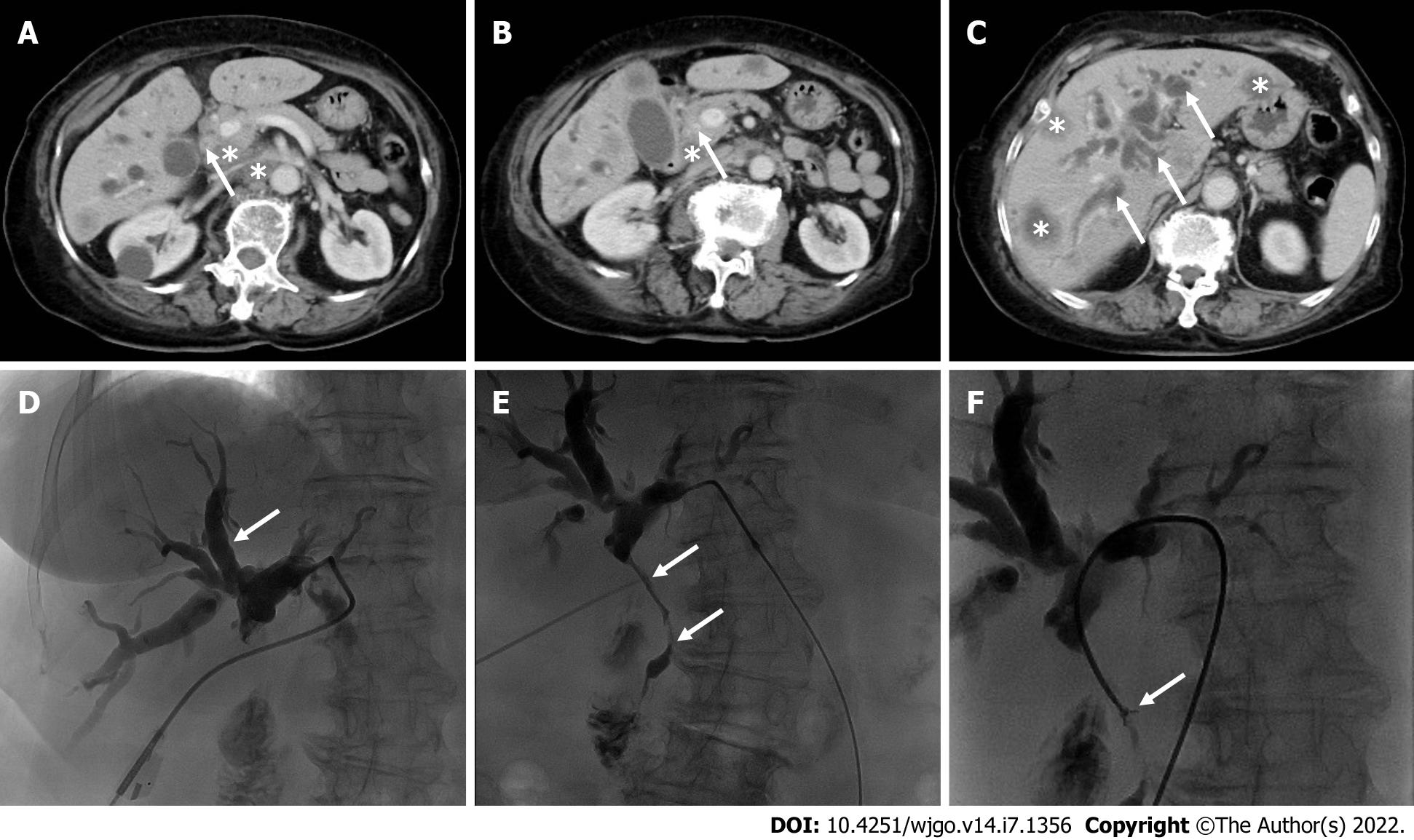
Contrast-enhanced computed tomography revealed that the wall of the common bile duct was thickened and strengthened (Figure 1A and B, arrows) with intrahepatic bile duct dilation (Figure 1C, arrows), and numerous hypodense lesions in the liver showed slight annular enhancement (Figure 1C, asterisks). In addition, the lymph nodes in the hepatic hilum area, the pancreatic head area, and around the abdominal aorta were enlarged (diameter 1.5-2.5 cm) with mild enhancement (Figure 1A and B, asterisks).
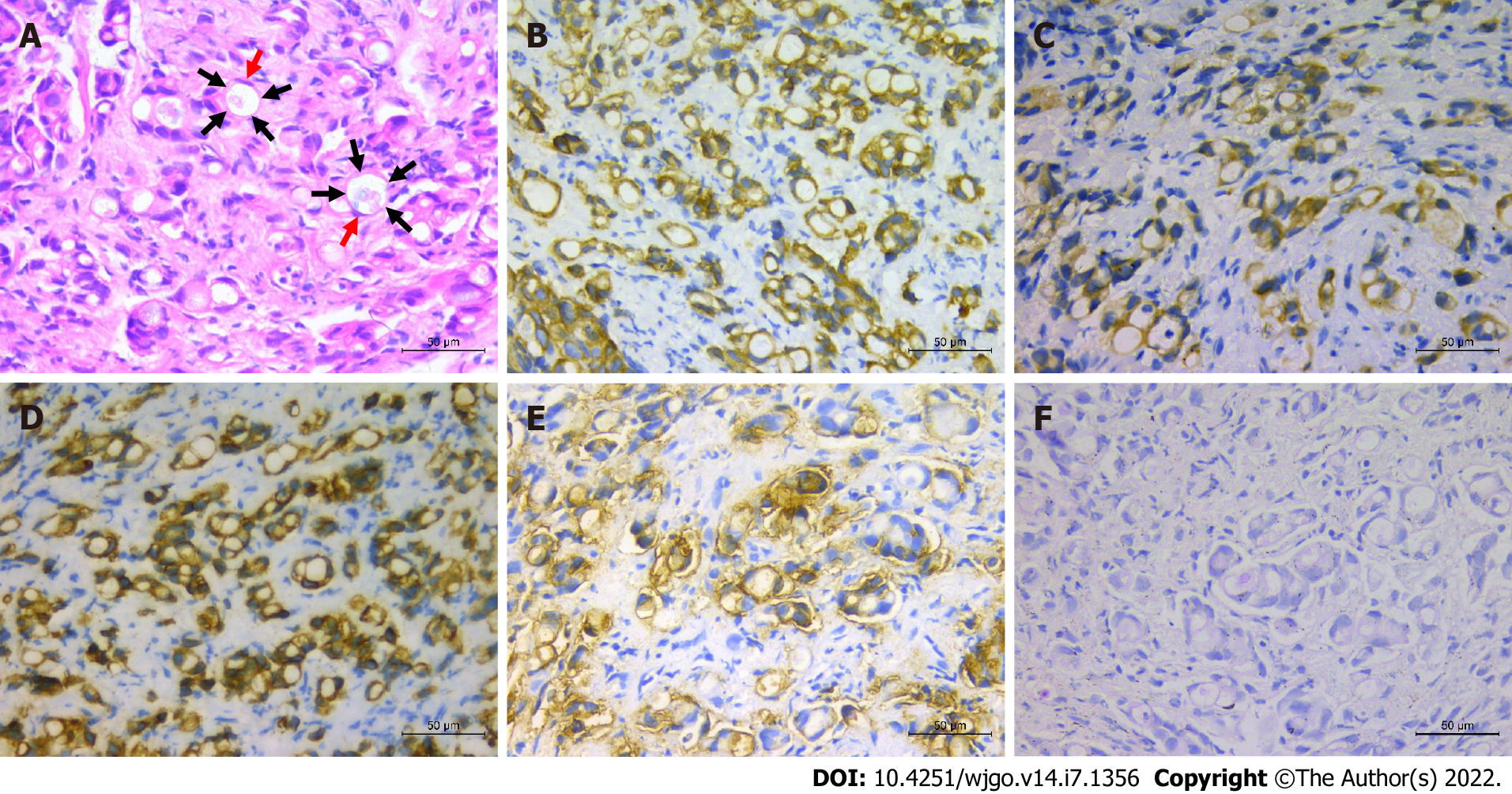
To alleviate jaundice and prolong the overall survival, percutaneous transhepatic cholangiopancreatic drainage (PTCD) was performed. During the operation, segmental stenosis of the extrahepatic bile duct (Figure 1E, arrows) and a vine-like expansion of the intrahepatic bile duct (Figure 1D, arrows) were observed. To determine the nature and origin of the lesion, a biliary biopsy was performed under fluoroscopy (Figure 1F, arrows). Hematoxylin and eosin (H&E) staining revealed abundant mucin in the cytoplasm pushed the nuclei aside, giving the cells the characteristic signet ring morphology (Figure 2A). The pathological diagnosis of the biopsy was SRCC. Immunohistochemistry (IHC) showed the following indicators: CK19 (++), CAM5.2 (+), CK7 (++), CK broad-spectrum (++), CEA (++), Ki-67 (10%), CK20 (-), CDX2 (-), glypican-3 (-), hepatocyte (-), vimentin (-), and special staining for PAS (+) (Figure 2B-F). The IHC of biopsy materials showed that the tumor cells were positive for CK19 (Figure 2B) and CK7 (Figure 2D) but negative for both CK20 and CDX2, suggesting the biliary tract origin of carcinoma. In addition, the gastroscopy and colonoscopy of the common primary site of SRCC did not show any abnormality.
Finally, a diagnosis of primary SRCC of extrahepatic bile duct with distant lymph node metastasis and multiple liver metastases was made based on the radiographic, PTCD, and pathological characteristics. The tumor was diagnosed as T3N1M1 stage IV according to the 8th edition of the American Joint Committee on Cancer (AJCC) cancer staging system[5]. Further imaging, such as positron emission tomography-computed tomography (PET-CT), was essential to determine any other sites of distant metastasis. Nonetheless, due to rapidly deteriorating condition in a short period, the patient died before the PET-CT was completed.
This patient was treated with S-adenosylmethionine (intravenous infusion, 1.5 g once a day) to protect the liver and relieve jaundice. PTCD was performed simultaneously to alleviate obstructive jaundice.
The patient died after 1 mo due to liver failure. The timeline can be seen in Figure 3.
Herein, we present a rare case of primary SRCC of extrahepatic bile duct with distant lymph node metastasis and multiple intrahepatic metastases. To the best of our knowledge, this is the second case confirmed by direct forceps biopsy under fluoroscopy and the first case of primary SRCC of the extrahepatic bile duct with distant organ metastasis. Distant organ metastasis is a critical factor influencing prognosis. Therefore, our case had a worse prognosis compared to those reported previously. Moreover, surgical resection was not a reasonable treatment due to the patient’s old age and poor liver function; hence, she received only palliative treatment, including liver protection and PTCD. However, despite active treatment, the disease progressed rapidly, and the patient died after 1 mo due to liver failure. Therefore, we concluded that primary SRCC of the extrahepatic bile duct is not prone to distant organ metastasis, and if accompanied by distant organ metastasis, it grows rapidly and has a strong invasion and poor prognosis.
Primary SRCC of the extrahepatic bile duct is an extremely rare subtype of bile duct adenocarcinoma of unknown origin. Presently, there are two theories regarding its origin. One is that the tumors may arise from ectopic gastric mucosa, while the other is that SRCCs may develop from gastric-type epithelial metaplasia[6]. In our case, no ectopic gastric mucosa and epithelial metaplasia were detected in the biliary biopsy. Thus, the origin of primary SRCC in the extrahepatic bile duct needs to be evaluated in future studies[14].
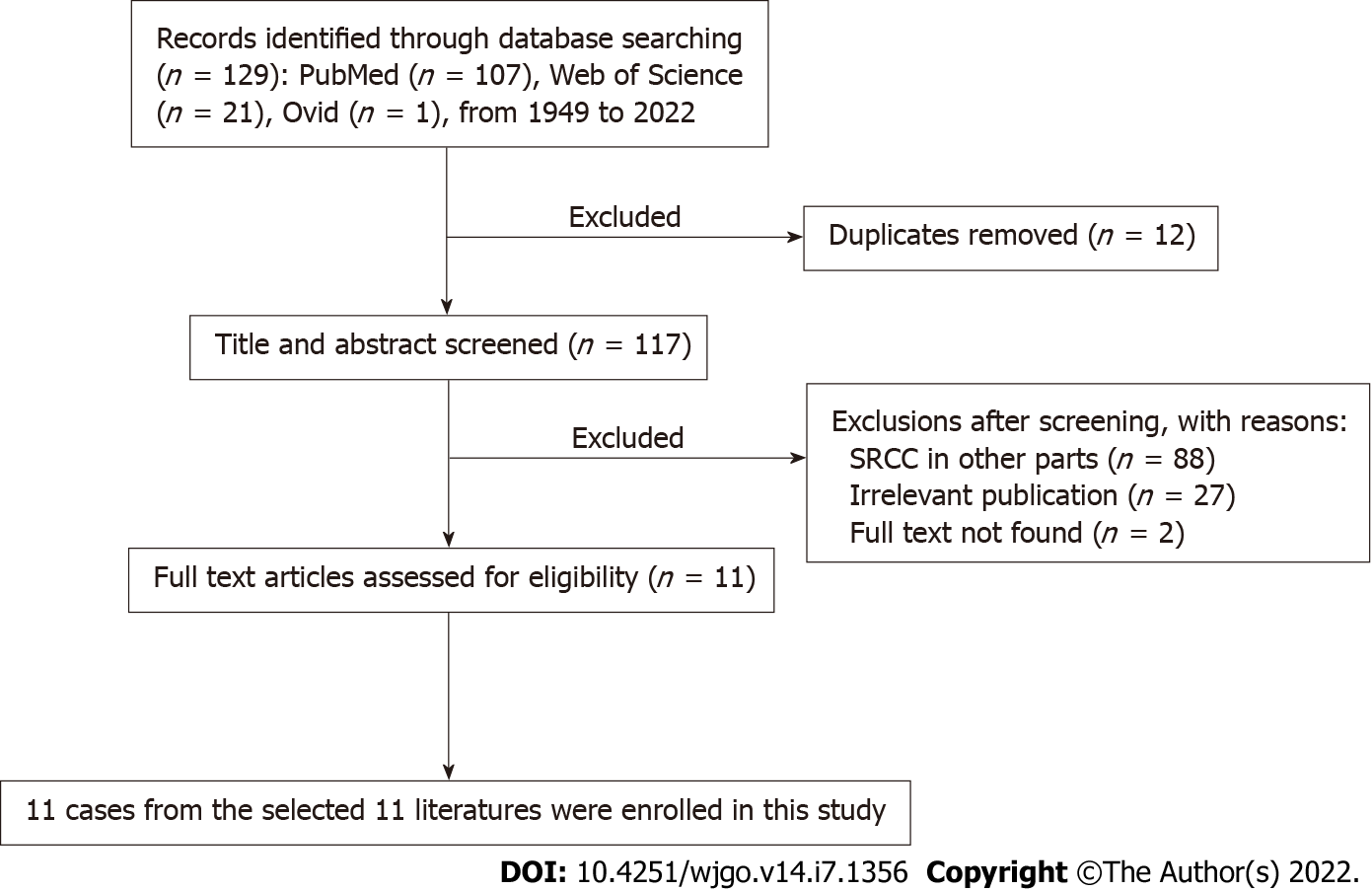
For the literature review, relevant articles in English were retrieved from the PubMed, Ovid database, and Web of Science from 1949 to January 10, 2022. The keywords used for the search were “signet ring cell cholangiocarcinoma” OR “signet ring cell carcinoma of bile duct”. These words were used individually or with the Boolean operator “AND”. A total of 129 articles were analyzed from 1949 to 2022. The flow chart of the literature screening process is illustrated in Figure 4. Finally, 11 cases were included in this meta-analysis. The following data were collected: the name of the first author, year of publication, patient’s age, sex, location, TNM staging, treatment, and follow-up results (Table 1).
| Ref. | Age | Sex | Location | TNM1 | Treatment | Outcome |
| Lee et al[7], 2010 | 55 | M | Distal | T3N1M0 | Resection; Chemoradiation | Alive at 24 mo |
| Ogata et al[8], 2010 | 42 | F | Distal | T4N1M0 | Resection | Alive at 6 mo |
| Somer et al[9], 2012 | 66 | F | Perihilar | T3N0M0 | Resection | No described |
| Kita et al[10], 2014 | 73 | F | Distal | T3N0M0 | Resection; Gemcitabine/cisplatin | Alive at 12 mo |
| Kwon et al[11], 2014 | 63 | M | Distal | T3N0M0 | Resection | Dead at 15 mo |
| Hua et al[12], 2015 | 52 | M | Distal | T4N1M0 | Resection | Dead at 6 mo |
| Chedid et al[13], 2015 | 66 | F | Perihilar | T4N0M0 | Resection | Dead at 15 mo |
| Zhang et al[14], 2018 | 32 | F | Distal | T3N1M0 | Resection | Dead at 5 mo |
| Welsh et al[15], 2018 | 55 | F | Distal | T3N1M0 | Chemoradiation | Dead at 4 mo |
| Hameed et al[16], 2019 | 72 | F | Distal | T1N0M0 | Resection | No described |
| Ghoddoosi et al[17], 2021 | 68 | F | Perihilar | T1N0M0 | Gemcitabine/cisplatin | Alive at 9 mo |
| Present case | 82 | F | Distal | T3N1M1 | PTCD | Dead at 1 mo |
In the current study, we included 3 males[7,10,12] and 8 females[8,9,11,13-17] with an average age of 58.5 years (range: 32-73 years). This phenomenon suggested that primary SRCC of the extrahepatic bile duct occurs in elderly patients, which was similar to previous reports[9]. However, due to the small number of patients, the correlation between the incidence of primary SRCC of the extrahepatic bile duct and gender needs to be investigated further. The analysis of 11 patients revealed that primary SRCC of the extrahepatic bile duct occurred in the distal bile duct in 8 cases[7,8,10-12,14-16] and the perihilar bile duct in only 3 cases[9,13,17]. This showed that primary SRCC of the extrahepatic bile duct occurs in the distal bile duct compared to the perihilar bile duct. The mechanism of occurrence may be that the distal bile duct is prone to the ectopic gastric mucosa and gastric-type epithelial metaplasia; however, it needs to be evaluated further[14]. According to the 8th edition of the AJCC cancer staging system, 9 cases[7-15] had an obvious invasion of the extrahepatic bile duct, of which 3[8,12,13] showed infiltration of the adjacent tissue structures. Peritoneum and retroperitoneal lymph node metastasis were observed in 5 cases[7,8,12,14,15] without distant metastasis. This phenomenon indicated that primary SRCC of the extrahepatic bile duct mainly grows along the wall, often with lymph node metastasis, but distant organ metastasis is extremely rare. The treatment for primary SRCC of extrahepatic bile duct includes resection, such as pancreaticoduodenectomy or resection of the biliary tree, followed by radiotherapy and chemotherapy. Also, integrative treatments that combine two or three have been applied[7-17]. 7/11 cases[8-10,12-14,16] received only surgical resection, 1/11 case[17] received chemotherapy alone, 1/11 case[11] received surgical resection followed by chemotherapy, 1/11 case[15] received chemoradiotherapy, and 1/11 case[7] received combined treatment of surgical resection, radiotherapy, and chemotherapy. Currently, surgical treatment is the gold standard for patients with cancer without distant metastasis. However, no standardized protocol and guidelines for treating primary SRCC of the extrahepatic bile duct are currently available because of the limited number of cases and studies. Yang et al[2] proposed that the primary SRCC location can be used as an independent prognostic factor of survival and that compared to stomach SRCC, the primary gallbladder, the ampulla of Vater, and pancreatic SRCCs have a worse prognosis. Therefore, an optimal treatment strategy is essential. Based on the results of this study, active surgical treatment may improve the prognosis in the event of surgical conditions in patients. Nonetheless, cases with poor prognosis even after radical resection are apparent. However, a standard recommendation of whether to perform adjuvant radiotherapy or chemotherapy cannot be established because of the small number of patients who received radiotherapy or chemotherapy in this study. Thus, to improve the survival and quality of life of patients, a multidisciplinary treatment such as concomitant use of chemotherapy is necessary.
Overall, primary SRCC of the extrahepatic bile duct is extremely rare, and cases with distant organ metastases have never been reported. Currently, surgical treatment is the gold standard for patients with primary SRCC of the extrahepatic bile duct without distant metastasis. However, aggressive multidisciplinary treatment is essential when surgical resection is not feasible or metastasis is observed.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kitagawa Y, Japan; Singh I, United States S-Editor: Yan JP L-Editor: Filipodia P-Editor: Yan JP
| 1. | Brindley PJ, Bachini M, Ilyas SI, Khan SA, Loukas A, Sirica AE, Teh BT, Wongkham S, Gores GJ. Cholangiocarcinoma. Nat Rev Dis Primers. 2021;7:65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 468] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 2. | Yang M, Yang Y, Chen J, Stella GM, Um SW, Tandon YK, Liu H. A case report of primary signet ring cell carcinoma of the lung: imaging study and literature review. Transl Lung Cancer Res. 2021;10:3840-3849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | el-Zimaity HM, Itani K, Graham DY. Early diagnosis of signet ring cell carcinoma of the stomach: role of the Genta stain. J Clin Pathol. 1997;50:867-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Wang S, Li J, You J, Zhou Y. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the gallbladder. BMC Gastroenterol. 2021;21:248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Amin M. AJCC Cancer Staging Manual. 8th ed. American Joint Committee on Cancer; 2017. [DOI] [Full Text] |
| 6. | Hoedemaeker PJ. Heterotopic gastric mucosa in the duodenum. Digestion. 1970;3:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 44] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Lee EY, Kim C, Kim MJ, Park JY, Park SW, Song SY, Chung JB, Kim H, Bang S. Signet ring cell carcinoma of the extrahepatic bile- duct. Gut Liver. 2010;4:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Ogata S, Kimura A, Hatsuse K, Yamamoto J, Shimazaki H, Nakanishi K, Kawai T. Poorly differentiated adenocarcinoma with signet-ring cell carcinoma of the extrahepatic bile duct in a 42-year-old Japanese female: a case report. Acta Med Okayama. 2010;64:63-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 9. | Somer L, Andrejić B, Milošević P. Origin and pathological characteristics of Klatskin tumor: a case report and literature review. Pol J Pathol. 2012;63:65-70. [PubMed] |
| 10. | Kita E, Tsujimoto A, Nakamura K, Sudo K, Hara T, Kainuma O, Yamamoto H, Itami M, Yamaguchi Y. Signet ring cell carcinoma of the extrahepatic bile duct diagnosed by preoperative biopsy: a case report. Case Rep Gastroenterol. 2014;8:353-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Kwon HJ, Yoon GS, Kwon YC, Kim SG, Jeong JY. Signet-ring cell carcinoma of the distal common bile duct: report of a case. Korean J Pathol. 2014;48:315-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Hua R, Zhang JF, Liu W, Huo YM, Sun YW. Signet-ring cell carcinoma coexisting with adenocarcinoma arising in a choledochal cyst: report of a case. Surg Today. 2015;45:1049-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Chedid MF, Lucas ET, Cerski CT, Lopes MF, Amaral OB, Chedid AD. Signet-ring cell hilar cholangiocarcinoma: case report. Arq Bras Cir Dig. 2015;28:148-149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Zhang C, Zhou J, Kou K, Liu S, We F, Wang G. Occurrence of signet-ring cell carcinoma with cholangiocarcinoma 25 years after choledochal cyst excision: A case report. Medicine (Baltimore). 2018;97:e9956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Welsh JL, Jaber O, Ivanovic M, Johlin FC, El Abiad RG, Clamon GH, Smith MC, Chan CHF. Rapidly Progressing Primary Extrahepatic Bile Duct Signet-Ring Cell Carcinoma in a Caucasian Woman. J Gastrointest Cancer. 2018;49:63-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Hameed N, Stephen M. Signet Ring Cell Carcinoma of the Extrahepatic Bile Duct: A Case Report. Am J Clin Pathol. 2019;152:S68-S69. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Ghoddoosi M, Hormati A, Ouladdameshghi D, Ahmadpour S. Signet ring cell hilar cholangiocarcinoma diagnosed via direct transpapillary cold forceps biopsy under fluoroscopy. Gastroenterol Hepatol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |