Published online Jun 15, 2022. doi: 10.4251/wjgo.v14.i6.1148
Peer-review started: December 29, 2021
First decision: March 13, 2022
Revised: April 11, 2022
Accepted: May 22, 2022
Article in press: May 22, 2022
Published online: June 15, 2022
Processing time: 162 Days and 21.6 Hours
Contemporary treatment of stage II/III rectal cancer combines chemotherapy, chemoradiation, and surgery, though the sequence of surgery with neoadjuvant treatments and benefits of minimally-invasive surgery (MIS) is debated.
To describe patterns of surgical approach for stage II/III rectal cancer in relation to neoadjuvant therapies.
A retrospective cohort was created using the National Cancer Database. Primary outcome was rate of sphincter-sparing surgery after neoadjuvant therapy. Secondary outcomes were surgical approach (open, laparoscopic, or robotic), surgical quality (R0 resection and 12+ lymph nodes), and overall survival.
A total of 38927 patients with clinical stage II or III rectal adenocarcinoma underwent surgical resection from 2010-2016. Clinical stage II patients had neoadjuvant chemoradiation less frequently compared to stage III (75.8% vs 84.7%, P < 0.001), but had similar rates of total neoadjuvant therapy (TNT) (27.0% vs 27.2%, P = 0.697). Overall rates of total mesorectal excision without sphincter preservation were similar between clinical stage II and III (30.0% vs 30.3%) and similar if preoperative treatment was chemoradiation (31.3%) or TNT (30.2%). Over the study period, proportion of cases approached laparoscopically increased from 24.9% to 32.5% and robotically 5.6% to 30.7% (P < 0.001). This cohort showed improved survival for MIS approaches compared to open surgery (laparoscopy HR 0.85, 95%CI 0.78-0.93, and robotic HR 0.82, 95%CI 0.73-0.92).
Sphincter preservation rates are similar across stage II and III rectal cancer, regardless of delivery of preoperative chemotherapy, chemoradiation, or both. At a national level, there is a shift to predominantly MIS approaches for rectal cancer, regardless of whether sphincter sparing procedure is performed.
Core Tip: At a population level, there have been increases in neoadjuvant treatment and minimally-invasive surgical (MIS) approaches for stage II and III rectal cancer. These shifts have are not associated with changes in rates of permanent ostomy which remain about 30%. In contrast to prior trials, this ‘real-world’ cohort showed an association with higher quality surgical resection and improved survival with MIS.
- Citation: Soriano C, Bahnson HT, Kaplan JA, Lin B, Moonka R, Pham HT, Kennecke HF, Simianu V. Contemporary, national patterns of surgery after preoperative therapy for stage II/III rectal adenocarcinoma. World J Gastrointest Oncol 2022; 14(6): 1148-1161
- URL: https://www.wjgnet.com/1948-5204/full/v14/i6/1148.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i6.1148
The management of rectal cancer has evolved, with emphasis on optimizing oncological outcomes and minimizing operative morbidity. Treatment of locally advanced rectal cancer typically involves multimodality therapies and total mesorectal excision (TME)[1,2]. Neoadjuvant therapy using chemotherapy and/or radiotherapy has several advantages, such as locoregional control and improved overall survival, compared to surgery alone[3-5]. Additionally, the administration of chemoradiation combined with induction or consolidation chemotherapy, known as total neoadjuvant therapy (TNT), has gained popularity due to increased treatment compliance without compromise of pathologic complete response or complete resection rates[6-8].
Despite advances in multimodality treatment paradigms, the optimal sequence of surgery in relation to chemotherapy and radiation remains unknown. Recent trials have assessed pre-operative treatment regimens and improved rates of organ preservation, disease free survival, and pathological complete response rates in patients with high risk, locally advanced rectal cancer[9-11]. Several factors, including anatomic considerations, tumor features, and functional symptoms, can influence decision-making, and treatment is typically individualized. Due to the complexity of rectal cancer care, variation has been described, with differences in curative resection rates, postoperative morbidity and mortality, and long-term oncologic outcomes among both surgeons and hospitals[12]. Furthermore, practices of how surgery is sequenced with other modalities, especially in the era of minimally invasive surgery (MIS), is not well described.
Therefore, the aim of this study was to characterize surgical resection of locally advanced rectal adenocarcinoma in the setting of multimodal therapy at the national level, with a focus on describing patterns of surgery in sequence with neoadjuvant treatment delivery and shift in surgical approach trends over time. We hypothesized that there would be increases in the delivery of neoadjuvant chemotherapy and chemoradiation, performance of sphincter-sparing resections, and use of minimally invasive surgical approaches.
This study was determined to be exempt from human subjects review by the Benaroya Research Institute Institutional Review Board.
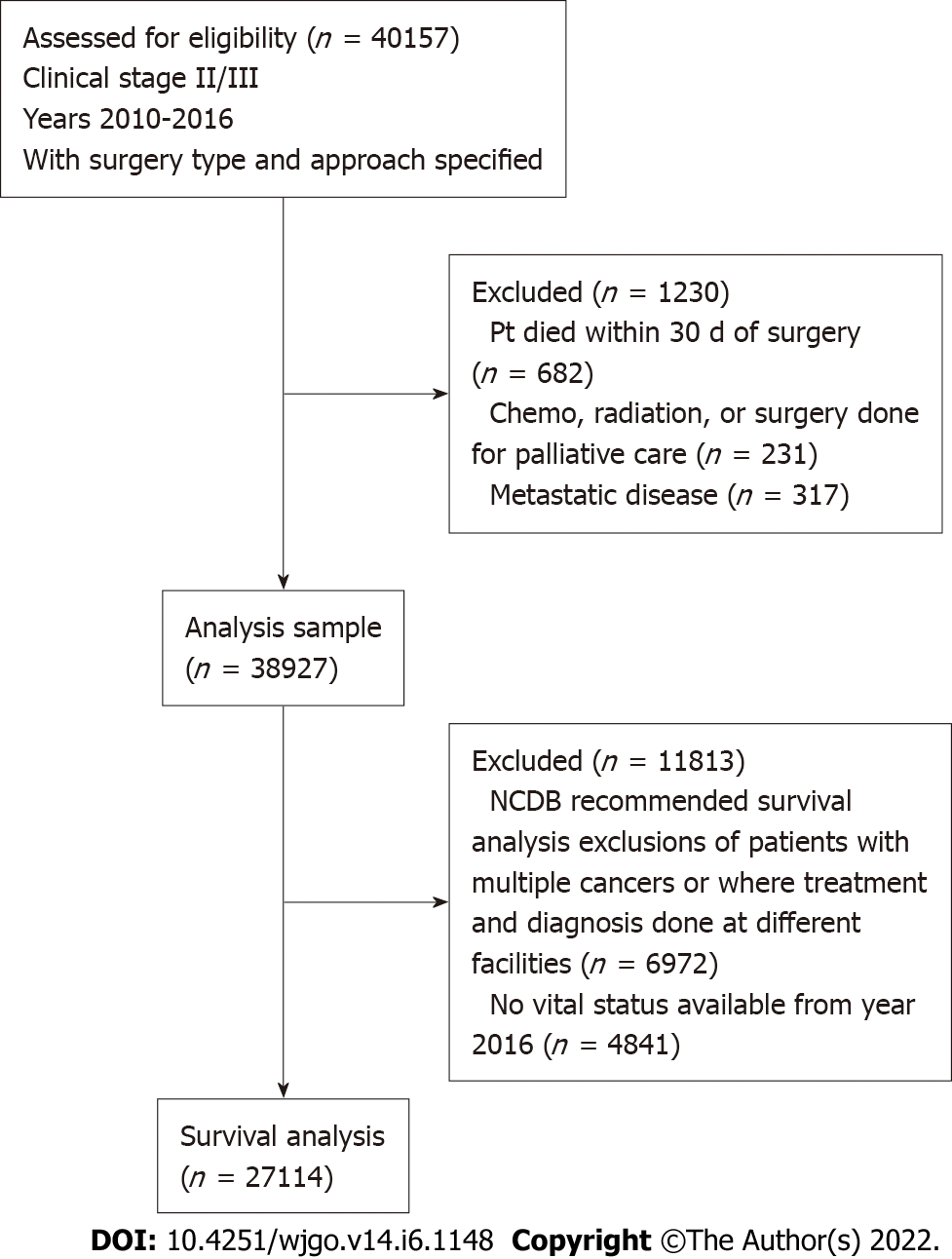
A retrospective cohort of patients with clinical stage II and stage III rectal adenocarcinoma who underwent surgical resection between 2010 and 2016 was created using the National Cancer Database (NCDB). The NCDB is a validated national cancer registry of the American College of Surgeons and American Cancer Society, collected from more than 1500 Commission on Cancer-accredited facilities. Stage was defined according to the seventh edition of the American Joint Committee on Cancer’s clinical group. The cohort was based on clinical stage, rather than pathologic stage, as treatment delivery is established once staging workup is complete. Patients with a diagnosis of multiple cancers and undergoing palliative surgery were excluded (Figure 1).
To describe patterns of surgical care delivery, the primary outcome was proportion of patients receiving local excision or TME with or without sphincter preservation. The frequency of sphincter preservation was characterized by surgery alone or in sequence with chemotherapy or radiation therapy. Using NCDB definitions, local excision was defined as conventional trans-anal excision or trans-anal endoscopic microsurgery. TME with sphincter preservation was defined as any rectal resection that included anastomosis [low anterior resection (LAR) and total proctocolectomy and pouch-anal anastomosis]. TME without sphincter preservation was defined as any rectal resection without anastomosis [abdominoperineal resection (APR), LAR with colostomy, and total proctocolectomy with ileostomy]. Surgical approach to TME was subcategorized into open, laparoscopic, and robotic. Conversion to open from laparoscopy and robotics was also reported, but these cases were included in their intended approach categories. Chemotherapy delivery was defined as single or multi-agent systemic administration before or after surgery. TNT was defined as delivery of both multiagent chemotherapy and radiation therapy prior to surgical date.
Secondary outcomes that were assessed include pathologic stage, quality of surgical resection, and overall survival. Quality of surgical resection included proportion of cases with negative margins, total lymph node harvest and proportion of cases with 12+ lymph nodes harvested. To explore potential variation in care delivery, patient factors (age, sex, insurance status, comorbidities) and location of care (facility information, geographic area) were described and used as covariates in the survival analysis. Comorbidities were defined using the Charlson-Deyo comorbidity index.
Categorical and continuous variables based on clinical interest were compared with chi-square and Kruskal Wallis tests, respectively. While the hypothesis did not focus on differences in treatment based on rectal cancer stage, stage-specific data are provided in supplemental text (Supplementary Table 1). Because of the expected uptake of MIS over time, we described trends in surgical approach by year. Test for trend of surgical approach were done with Chi-squared test. Univariate- and multivariate-adjusted overall survival analyses were performed using cox proportional hazards model on a subset of the analysis sample, excluding patients with multiple cancers or where treatment and diagnosis were done at different facilities, as per NCDB recommendations. The final survival model was adjusted for age, sex, race, insurance, rurality, geography, facility type, pathologic stage, cancer grade, preoperative radiation, chemotherapy type and sequence, surgery type (LE, TME with or without sphincter preservation), intent of surgical approach (open, laparoscopic, robotic), resection margin status and 12+ lymph nodes resected status. Kaplan Meier survival curves stratified by TME with and without sphincter preservation are shown, by intent of surgical approach (open, laparoscopic, robotic). Statistical significance was determined by P < 0.05. Survival and patient characteristics tables were run with Mayo Clinic’s SAS macros[13] on SAS version 9.4 and JMP Pro Version 15 was also used for graphics and data analysis.
From 2010-2016, a total of 38,927 patients underwent resection of stage II/III rectal cancer (mean age 60.9 ± 12.7 years, and 61% male). Baseline patient and facility characteristics are outlined in Table 1. Sphincter was not preserved in 30.2% (n = 11748). Patients with clinical stage III disease represented 55% of the cohort, and stage distribution was similar whether TME with sphincter preservation (55.5%) or not (54.9%) was performed. It was rare to undergo local excision after initially presenting with clinical stage II (5.2%) or clinical stage III (2.5%) rectal cancer.
| Local excision (n = 1442) | TME with sphincter preservation (n = 25737) | TME without sphincter preservation (n = 11748) | Total (n = 38927) | P value | |
| Age at diagnosis | < 0.0011 | ||||
| mean ± SD | 66.2 ± 14.13 | 60.3 ± 12.47 | 61.6 ± 12.72 | 60.9 ± 12.67 | |
| Sex | < 0.0012 | ||||
| Male | 787 (54.6%) | 15810 (61.4%) | 7251 (61.7%) | 23848 (61.3%) | |
| Charleson Comorbidity Score | < 0.0012 | ||||
| 0 | 1057 (73.3%) | 19828 (77.0%) | 8828 (75.1%) | 29713 (76.3%) | |
| 1 | 278 (19.3%) | 4486 (17.4%) | 2206 (18.8%) | 6970 (17.9%) | |
| 2+ | 107 (7.4%) | 1423 (5.5%) | 714 (6.1%) | 2244 (5.8%) | |
| Race3 | < 0.0012 | ||||
| Black | 185 (12.8%) | 2006 (7.8%) | 1108 (9.4%) | 3299 (8.5%) | |
| Other | 61 (4.2%) | 1501 (5.8%) | 562 (4.8%) | 2124 (5.5%) | |
| White | 1183 (82.0%) | 22054 (85.7%) | 10012 (85.2%) | 33249 (85.4%) | |
| Insurance status3 | < 0.0012 | ||||
| Medicare/medicaid/other government | 852 (59.1%) | 11308 (43.9%) | 5825 (49.6%) | 17985 (46.2%) | |
| Not insured | 38 (2.6%) | 1019 (4.0%) | 663 (5.6%) | 1720 (4.4%) | |
| Private insurance/managed care | 518 (35.9%) | 13117 (51.0%) | 5066 (43.1%) | 18701 (48.0%) | |
| Living location3 | < 0.0012 | ||||
| Metropolitan | 1164 (83.2%) | 20618 (82.2%) | 9142 (79.4%) | 30924 (81.4%) | |
| Rural | 30 (2.1%) | 548 (2.2%) | 295 (2.6%) | 873 (2.3%) | |
| Urban | 205 (14.7%) | 3916 (15.6%) | 2083 (18.1%) | 6204 (16.3%) | |
| Facility type3 | < 0.0012 | ||||
| Academic/research program | 540 (37.4%) | 9852 (38.3%) | 4536 (38.6%) | 14928 (38.3%) | |
| Community cancer program | 95 (6.6%) | 1453 (5.6%) | 711 (6.1%) | 2259 (5.8%) | |
| Comprehensive community cancer program | 562 (39.0%) | 9593 (37.3%) | 4538 (38.6%) | 14693 (37.7%) | |
| Integrated network cancer program | 193 (13.4%) | 3689 (14.3%) | 1451 (12.4%) | 5333 (13.7%) | |
| Facility geographic region3 | < 0.0012 | ||||
| Midwest | 343 (24.7%) | 6883 (28.0%) | 3528 (31.4%) | 10754 (28.9%) | |
| Northeast | 313 (22.5%) | 5040 (20.5%) | 2027 (18.0%) | 7380 (19.8%) | |
| South | 533 (38.3%) | 8522 (34.7%) | 3983 (35.4%) | 13038 (35.0%) | |
| West | 201 (14.5%) | 4142 (16.8%) | 1698 (15.1%) | 6041 (16.2%) |
Patients with clinical stage II disease more frequently had no radiation (16.8% vs 8.7%, P < 0.001) or no chemotherapy (14.9% vs 5.9%, P < 0.001) compared to clinical stage III patients (Supplementary Table 1). Clinical stage II patients less frequently had neoadjuvant chemoradiation (75.2%, vs 84.1% P < 0.001), but had similar rates of TNT (27.0% vs 27.2%, respectively, P = 0.697) compared to clinical stage III. Overall rates of TME without sphincter preservation were similar between clinical stage II and III, 30.0% vs 30.3%, respectively, and similar if preoperative treatment was neoadjuvant chemoradiation (31.3%, n = 9762 TME without sphincter preservation out of n = 31160 that received neoadjuvant chemoradiation) or TNT (30.2%, n = 1302 TME without sphincter preservation out of n = 4302 that received TNT).
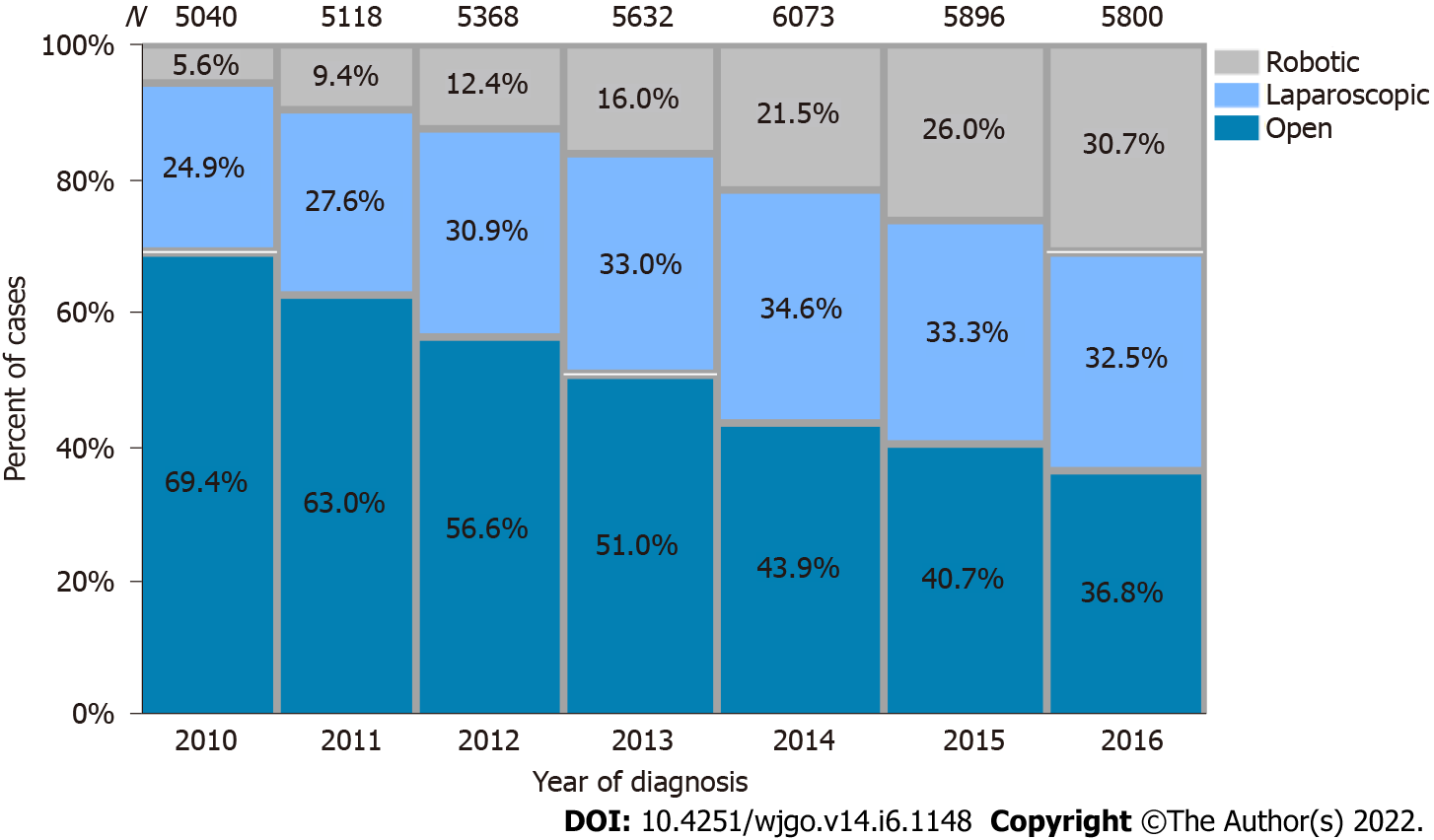
Rates of open resection in the cohort were approximately 50%, but over the period of the study decreased from 69.4% in 2010 to 36.8% in 2016. There were concomitant rises in laparoscopic resection from 24.9% to 32.5% and robotic resection 5.6% to 30.7% (P < 0.001) (Figure 2). Open approach was used for 60% of TME without sphincter preservation compared to 47% of TME with sphincter preservation (P < 0.001).
The distribution of surgical approach is described in Table 2. Conversion to an open operation was lower with robotic approach (6.9%) compared to laparoscopy (14.5%). This was maintained regardless of whether sphincter sparing procedure was performed (conversion rate 15% laparoscopic, 6.9% robotic) or not (conversion rate 16.4% laparoscopic, 7.1% robotic), or whether TNT (conversion rate 15.6% laparoscopic, 6.5% robotic) was delivered.
| Open (n = 19830) | Laparoscopic (n = 12144) | Robotic (n = 6953) | Total (n = 38927) | P value | |
| Clinical stage | < 0.0011 | ||||
| II | 9286 (46.8%) | 5477 (45.1%) | 2906 (41.8%) | 17669 (45.4%) | |
| III | 10544 (53.2%) | 6667 (54.9%) | 4047 (58.2%) | 21258 (54.6%) | |
| Pathological stage | < 0.0011 | ||||
| 0 | 508 (3.2%) | 323 (3.4%) | 222 (3.9%) | 1053 (3.4%) | |
| 1 | 3801 (23.8%) | 2736 (28.7%) | 1669 (29.6%) | 8206 (26.3%) | |
| 2 | 5416 (33.9%) | 2941 (30.8%) | 1669 (29.6%) | 10026 (32.2%) | |
| 3 | 6107 (38.2%) | 3480 (36.5%) | 2044 (36.3%) | 11631 (37.3%) | |
| 4 | 152 (1.0%) | 57 (0.6%) | 29 (0.5%) | 238 (0.8%) | |
| Chemotherapy sequence | < 0.0011 | ||||
| No chemotherapy | 2031 (10.2%) | 1397 (11.5%) | 459 (6.6%) | 3887 (10.0%) | |
| Chemotherapy after surgery | 1864 (9.4%) | 1210 (10.0%) | 421 (6.1%) | 3495 (9.0%) | |
| Chemotherapy before and after surgery | 5435 (27.4%) | 3691 (30.4%) | 2256 (32.4%) | 11382 (29.2%) | |
| Chemotherapy before surgery | 10481 (52.9%) | 5840 (48.1%) | 3810 (54.8%) | 20131 (51.7%) | |
| Radiation sequence | < 0.0011 | ||||
| No radiation | 2449 (12.3%) | 1713 (14.1%) | 651 (9.4%) | 4813 (12.4%) | |
| Radiation after surgery | 1470 (7.4%) | 911 (7.5%) | 315 (4.5%) | 2696 (6.9%) | |
| Radiation before surgery | 15911 (80.2%) | 9515 (78.4%) | 5987 (86.1%) | 31418 (80.7%) | |
| Total neoadjuvant therapy | 2194 (28.1%) | 1262 (25.1%) | 846 (28.0%) | 4302 (27.1%) | < 0.0011 |
| Surgery type | < 0.0011 | ||||
| TME with sphincter preservation | 12118 (61.1%) | 8633 (71.1%) | 4986 (71.7%) | 25737 (66.1%) | |
| TME without sphincter preservation | 7061 (35.6%) | 2760 (22.7%) | 1927 (27.7%) | 11748 (30.2%) | |
| Conversion to open | 0 (0.0%) | 1760 (14.5%) | 480 (6.9%) | 2240 (11.7%) | < 0.0011 |
| Residual tumor | < 0.0011 | ||||
| R0 | 18012 (91.9%) | 11174 (93.6%) | 6568 (95.1%) | 35754 (93.0%) | |
| R1 | 806 (4.1%) | 413 (3.5%) | 193 (2.8%) | 1412 (3.7%) | |
| R2 | 782 (4.0%) | 352 (2.9%) | 148 (2.1%) | 1282 (3.3%) | |
| Number of lymph nodes examined (mean ± SD) | 14.7 ± 9.7 | 14.8 ± 9.8 | 15.7 ± 9.0 | 14.9 ± 9.6 | < 0.0011 |
| 12 or more lymph nodes examined | 13198 (67.1%) | 8148 (67.7%) | 5088 (73.6%) | 26434 (68.4%) | < 0.0011 |
R0 resection was obtained 94.8% of patients who underwent TME with sphincter preservation, and 90.3% of patients who underwent TME without sphincter preservation (P < 0.001). Twelve or more lymph nodes were examined more frequently in TME with sphincter preservation (71.6%) than without sphincter preservation (68.4%). Rates of R0 resection and 12+ lymph nodes harvested were both lower with open, compared to minimally invasive, approaches.
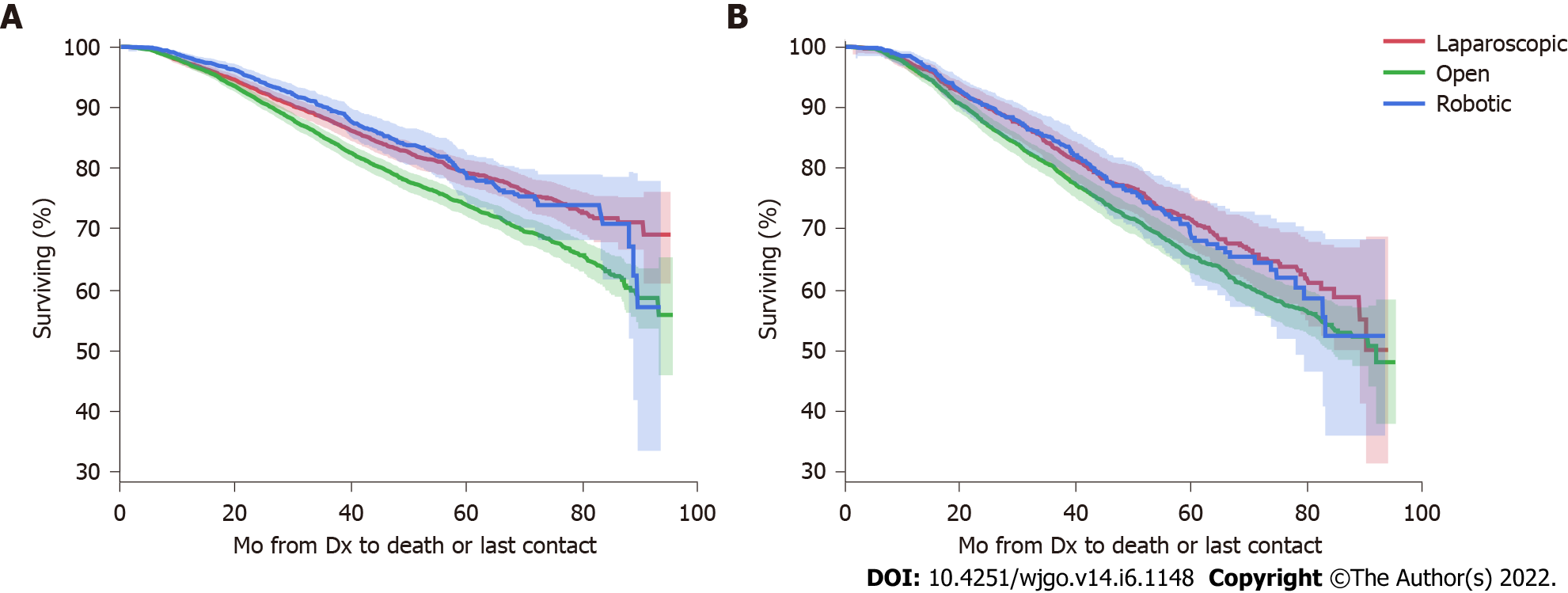
Table 3 summarizes factors impacting overall survival in this cohort. After adjustment, TME without sphincter preservation was associated with worse survival HR 1.30 (95%CI 1.20-1.40) compared to sphincter preservation. Interestingly, this cohort showed improved survival for minimally invasive approaches compared to open surgery (laparoscopy HR 0.85, 95%CI 0.78-0.93, and robotic HR 0.82, 95%CI 0.73-0.92). This improved survival in cases approached minimally invasively was sustained after stratification into TME with and without sphincter preservation (Figure 3).
| Variable | n | Events | 5-yr survival% (95%CI) | Cox univariate HR (95%CI) | Cox univariate score P value | Cox multivariate HR (95%CI) | Cox multivariate likelihood ratio P value (n = 15618) |
| Age at diagnosis | 27114 | 5281 (19%) | 73.3 (72.6, 74.0) | 1.03 (1.03, 1.04) | < 0.0001 | 1.02 (1.02, 1.02) | < 0.0001 |
| Sex | < 0.0001 | < 0.0001 | |||||
| Female | 10502 | 1869 (18%) | 75.5 (74.4, 76.6) | ||||
| Male | 16612 | 3412 (21%) | 71.9 (70.9, 72.8) | 1.19 (1.13, 1.26) | 1.23 (1.14, 1.32) | ||
| Charleson comorbidity score | < 0.0001 | < 0.0001 | |||||
| 0 | 20949 | 3656 (17%) | 75.7 (74.9, 76.5) | ||||
| 1 | 4792 | 1202 (25%) | 67.4 (65.6, 69.1) | 1.43 (1.34, 1.53) | 1.25 (1.14, 1.36) | ||
| 2+ | 1373 | 423 (31%) | 58.5 (55.0, 62.0) | 2.00 (1.81, 2.22) | 1.59 (1.38, 1.82) | ||
| Race | < 0.0001 | 0.3372 | |||||
| Black | 2307 | 508 (22%) | 69.7 (67.1, 72.2) | 1.18 (1.08, 1.29) | 1.04 (0.92, 1.19) | ||
| Other | 1542 | 246 (16%) | 76.6 (73.6, 79.5) | 0.85 (0.74, 0.96) | 1.01 (0.85, 1.20) | ||
| White | 23091 | 4498 (19%) | 73.4 (72.7, 74.2) | ||||
| Insurance status | < 0.0001 | < 0.0001 | |||||
| Insurance status unknown | 382 | 64 (17%) | 71.8 (64.8, 78.8) | 1.46 (1.14, 1.88) | 0.93 (0.62, 1.39) | ||
| Medicare/medicaid/other government | 11607 | 3039 (26%) | 64.8 (63.6, 66.0) | 2.12 (2.01, 2.25) | 1.33 (1.22, 1.46) | ||
| Not insured | 1357 | 294 (22%) | 71.1 (67.9, 74.2) | 1.61 (1.42, 1.82) | 1.14 (0.97, 1.33) | ||
| Private insurance/managed care | 13768 | 1884 (14%) | 80.8 (79.9, 81.7) | ||||
| Living location | 0.0407 | 0.3867 | |||||
| Metropolitan | 21521 | 4134 (19%) | 73.7 (72.9, 74.5) | ||||
| Rural | 611 | 117 (19%) | 72.9 (68.2, 77.6) | 1.01 (0.84, 1.22) | 0.85 (0.67, 1.08) | ||
| Urban | 4344 | 900 (21%) | 71.7 (69.9, 73.5) | 1.10 (1.02, 1.18) | 0.98 (0.89, 1.08) | ||
| Facility type | < 0.0001 | 0.5531 | |||||
| Academic/research program | 10235 | 1850 (18%) | 75.4 (74.2, 76.5) | ||||
| Community cancer program | 1624 | 384 (24%) | 67.4 (64.4, 70.5) | 1.37 (1.22, 1.53) | 1.00 (0.86, 1.17) | ||
| Comprehensive community cancer program | 10158 | 2097 (21%) | 71.6 (70.4, 72.8) | 1.17 (1.10, 1.25) | 1.06 (0.98, 1.15) | ||
| Integrated network cancer program | 3719 | 761 (20%) | 72.2 (70.2, 74.1) | 1.17 (1.07, 1.27) | 1.04 (0.93, 1.16) | ||
| Facility geographic region | 0.0008 | 0.0971 | |||||
| Midwest | 7378 | 1455 (20%) | 73.6 (72.2, 74.9) | 0.91 (0.85, 0.97) | 0.926 (0.846, 1.014) | ||
| Northeast | 5058 | 967 (19%) | 74.1 (72.4, 75.7) | 0.87 (0.80, 0.94) | 0.912 (0.820, 1.014) | ||
| South | 9090 | 1885 (21%) | 71.2 (69.9, 72.4) | ||||
| West | 4210 | 785 (19%) | 74.0 (72.2, 75.8) | 0.89 (0.82, 0.97) | 0.878 (0.783, 0.986) | ||
| Pathological stage | < 0.0001 | < 0.0001 | |||||
| 0 | 782 | 66 (8%) | 89.2 (86.4, 92.1) | ||||
| 1 | 5631 | 604 (11%) | 84.8 (83.5, 86.1) | 1.277 (0.990, 1.646) | 1.11 (0.84, 1.46) | ||
| 2 | 6861 | 1399 (20%) | 72.1 (70.6, 73.5) | 2.48 (1.94, 3.17) | 1.97 (1.50, 2.57) | ||
| 3 | 8247 | 2338 (28%) | 61.6 (60.2, 63.1) | 3.74 (2.93, 4.77) | 3.32 (2.55, 4.33) | ||
| 4 | 136 | 82 (60%) | 25.1 (15.6, 34.6) | 10.93 (7.90, 15.11) | 8.70 (5.97, 12.67) | ||
| Chemotherapy (multi or single agent) | < 0.0001 | 0.001 | |||||
| Multiagent chemotherapy | 10043 | 1616 (16%) | 77.9 (76.8, 79.0) | ||||
| Single-agent chemotherapy | 12445 | 2467 (20%) | 72.6 (71.5, 73.6) | 1.31 (1.23, 1.39) | 1.14 (1.06, 1.24) | ||
| Chemotherapy sequence | < 0.0001 | < 0.0001 | |||||
| Chemotherapy after surgery | 2387 | 543 (23%) | 71.7 (69.4, 74.0) | 1.12 (1.02, 1.23) | 0.89 (0.75, 1.06) | ||
| Chemotherapy before surgery | 14351 | 2849 (20%) | 72.6 (71.6, 73.6) | ||||
| Chemotherapy before and after surgery | 8128 | 1134 (14%) | 79.7 (78.5, 80.9) | 0.67 (0.63, 0.72) | 0.73 (0.67, 0.79) | ||
| Radiation sequence | < 0.0001 | 0.3489 | |||||
| Radiation after surgery | 1934 | 463 (24%) | 70.5 (68.0, 73.1) | 1.30 (1.18, 1.43) | 0.92 (0.77, 1.10) | ||
| Radiation before surgery | 22529 | 4054 (18%) | 75.0 (74.2, 75.7) | ||||
| Surgery type | < 0.0001 | < 0.0001 | |||||
| Local excision | 953 | 249 (26%) | 65.0 (61.0, 69.1) | 1.62 (1.42, 1.84) | 1.26 (0.94, 1.68) | ||
| TME with sphincter preservation | 18237 | 3107 (17%) | 76.4 (75.6, 77.2) | ||||
| TME without sphincter preservation | 7924 | 1925 (24%) | 67.5 (66.1, 68.8) | 1.44 (1.36, 1.53) | 1.30 (1.20, 1.40) | ||
| Surgical approach | < 0.0001 | < 0.0001 | |||||
| Laparoscopic | 8510 | 1400 (16%) | 76.8 (75.5, 78.0) | 0.77 (0.72, 0.82) | 0.85 (0.78, 0.93) | ||
| Open | 14207 | 3300 (23%) | 70.7 (69.8, 71.7) | ||||
| Robotic | 4397 | 581 (13%) | 75.7 (73.5, 77.8) | 0.72 (0.66, 0.79) | 0.82 (0.73, 0.92) | ||
| Tumor grade | < 0.0001 | < 0.0001 | |||||
| Other (ND/UNK/NA/high grade dysplasia) | 3918 | 594 (15%) | 77.3 (75.5, 79.2) | 0.87 (0.80, 0.95) | 0.99 (0.88, 1.11) | ||
| Poor/undifferentiated | 3023 | 1004 (33%) | 58.9 (56.7, 61.1) | 1.97 (1.84, 2.11) | 1.67 (1.52, 1.83) | ||
| Well/moderate differentiation | 20173 | 3683 (18%) | 74.8 (74.0, 75.6) | ||||
| Residual tumor | < 0.0001 | < 0.0001 | |||||
| R0 | 24991 | 4432 (18%) | 75.5 (74.8, 76.2) | ||||
| R1 | 932 | 414 (44%) | 44.5 (40.4, 48.6) | 3.03 (2.74, 3.36) | 2.23 (1.96, 2.54) | ||
| R2 | 869 | 347 (40%) | 46.5 (42.2, 50.9) | 2.76 (2.47, 3.08) | 1.99 (1.71, 2.30) | ||
| 12 or more lymph nodes examined | < 0.0001 | < 0.0001 | |||||
| No | 8705 | 1919 (22%) | 70.9 (69.6, 72.1) | 1.19 (1.12, 1.25) | 1.26 (1.17, 1.36) | ||
| Yes | 18176 | 3317 (18%) | 74.5 (73.6, 75.4) |
This contemporary, nationwide cohort study identified an expected shift towards a minimally-invasive surgical approach for stage II/III rectal cancer with high quality surgical outcomes. Most of the patients are getting neoadjuvant radiation, but only a small fraction receives TNT. Neoadjuvant treatment at the population level does not seem to affect sphincter-sparing rates. Interestingly, this cohort also showed improved survival in cases approached minimally invasively - a finding that is at odds with prior, high-quality randomized control trials, but may reflect important differences between the randomized control trial population and surgeon and patient selection that occur in broader practice.
Contemporary treatment for rectal cancer is multidisciplinary. The most common neoadjuvant regimen utilizes chemoradiotherapy, which has been shown to lower the recurrence rate and is associated with less toxicity than post-operative radiation, with no difference in overall survival[14]. Additionally, neoadjuvant therapy may promote tumor shrinkage and affect sphincter-sparing rates. Still, despite recommendations in national guidelines describing neoadjuvant treatment for locally advanced rectal cancer or nodal disease[15,16], variation in radiation delivery is seen[17,18]. Midura et al[19] identified that factors such as hospital volume and facility type affected delivery of neoadjuvant therapy, including decreased use of neoadjuvant therapy for higher stage rectal cancer at lower-volume, community cancer centers. Furthermore, total neoadjuvant therapy has been increasingly promoted, in which studies have reported local disease control and decreased recurrence rates[20]. A majority of patients in our cohort underwent some type of neoadjuvant treatment, and sphincter-sparing rates were similar in patients with stage II or stage III disease. A prior meta-analysis supports the approximate rate of permanent colostomy to be approximately 30%[21]. It is important to note that certain clinical features, such as tumor distance from the anal verge or patients’ prior continence status, which might influence the decision for a non-sphincter sparing operation, are not available in this dataset. Most decisions about sphincter preservation happen before surgery, and rates of low tumors and incontinence rates are not expected to have meaningfully changed during this time period.
The equivalence of minimally-invasive and open approaches for rectal cancer surgery continues to be debated. Laparoscopy and robotic-assisted colorectal surgery have enabled decreased length of hospital stay, better analgesia, and improved visibility and ergonomics, specifically in the pelvis[22-24]. However, adoption of MIS for rectal cancer has been controversial, as both the Z6051 and ALaCaRT trials were unable to establish non-inferiority of pathological outcomes for minimally invasive vs open resection in patients with rectal cancer[25,26]. Follow-up of these trials found no significant difference in survival between approaches, with Z6051 showing 2-year disease free survival (DFS) of 79.5% in the laparoscopic group and 83.5% in the open group and ALaCaRT showing 2-year DFS of 94% in the laparoscopic group and 93% in the open group[27,28]. Finally, the ROLARR trial found no significant difference in conversion to open laparotomy between conventional laparoscopy vs robotic-assisted surgery, and concluded no short term benefit of robotic surgery over laparoscopy[29]. Our findings of improved survival with minimally invasive approaches, even after adjustment for pathological stage, neoadjuvant treatment, and patient/center features, are at odds with these prior, high-quality studies. However, the NCDB has a wider, national representation, and the findings herein may reflect patient- and approach- selection in broader practice, including training, resources, and institutional factors that impact approach outside of randomized trial patients. For example, it is unclear if the improved resection margins and lymph node harvest in the laparoscopic and robotic subgroups are due to the approaches themselves or the cases that lent themselves to be approached minimally invasively (or the surgeons choosing a minimally-invasive approach in these cases). Additionally, our findings are limited by the absence of information regarding local recurrence rate. However, it is notable that this effect of surgical approach on survival in this national cohort was maintained even after adjustment for multiple confounders or when stratifying the analysis by the subgroups with and without sphincter preservation.
Local excision operations in the setting of stage II/III are controversial and deserve special mention in this cohort. Patients with stage II/III who underwent transanal local excision make up a minority of operations and are not the standard treatment because of the inability to evaluate mesorectal lymph nodes. Still, several studies have shown the feasibility of this approach in the setting of neoadjuvant treatment[30-33]. In select patients showing tumor response to short course radiotherapy or chemotherapy, high rates of organ preservation can be achieved. Therefore, patients and their surgeons may opt for this approach if facing a decision about permanent colostomy or if they are poor surgical candidates for the standard TME. Further randomized studies to better assess the feasibility of this approach, and long term follow up for meaningful oncologic outcomes are underway[34].
This study is further limited by the inability to address the magnitude of treatment response and the impact of treatment response on decisions for sphincter preservation and surgical approach. For instance, we were unable to assess clinical complete responders, which occurs as frequently as 20%-30%[20], and would not be included unless they underwent resection and pathology confirmed no residual tumor. Patients may avoid resection if they have a complete clinical response but would need an APR, so there is bias in this study such that APR surgery only occurred in those patients that likely did not have good response and still needed resection. This presumably also impacts overall survival estimates. Finally, there is a lack of data available regarding local staging studies that could lead to misclassification of clinical stage. For instance, it has been reported that magnetic resonance imaging, which has become the standard of care, can over-stage rectal cancer as high as 30%[35-37]. Misclassification of stage could result in undertreatment or overtreatment, and that cannot be determined using this dataset. Despite these limitations, this study provides important information regarding treatment delivery patterns.
At a national level, minimally invasive surgery has become the predominant approach for rectal cancer. Sphincter preservation rates, when patients undergo surgical resection, do not vary with delivery of neoadjuvant treatment. In this broad national cohort, both open surgery and non-sphincter sparing operations were associated with worse overall survival for patients with stage II/III rectal adenocarcinoma.
It is not well described whether the contemporary, multi-disciplinary approaches to stage II/III rectal cancer are resulting in meaningful changes in sphincter preservation, surgical quality, or overall survival.
While we push to individualize treatment decisions, it is important to recognize whether contemporary patterns to increase minimally-invasive surgery (MIS) and neoadjuvant treatment offer meaningful change the expected outcome of locally advanced rectal cancer.
Describe broad uptake in sphincter preservation, minimally-invasive approaches to rectal cancer, and the associated surgical outcomes of resection margins, lymph node harvest, and overall survival.
Retrospective 'real-world' cohort of National Cancer Database (NCDB) sites, limited to stage II/III surgically treated rectal cancer.
Neither stage nor neoadjuvant treatment made a meaningful impact on rates of permanent colostomy, which was about 30% across all subgroups. From 2010 to 2016, there was a broad shift to MIS (laparoscopic and robotic) approaches to rectal cancer. These MIS approaches were associated with more frequent negative margins, better lymph node harvest, and improved overall survival after adjustment.
There has been a shift to MIS approaches to locally advanced rectal cancer. Sphincter preservation rates remain similar in contemporary years, despite increasing neoadjuvant therapy. In recent years, more cases at NCDB sites are done MIS, which associate with better surgical quality and improved overall survival in this study.
The findings of improved surgical quality and overall survival in this cohort are in contrast to randomized trial data that preceded this study. This may highlight the difference between randomized patients are 'real-world' practices or call into question the need for more contemporary, and pragmatic, trials for locally advanced rectal cancer surgery.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: American Society of Colon and Rectal Surgeons; American College of Surgeons; Society of Surgeons of the Alimentary Tract; SWOG Cancer Research Network.
Specialty type: Surgery
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Chen SY, China; Han JG, China; Lee TG, South Korea A-Editor: Liu X, China S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Kapiteijn E, Marijnen CA, Nagtegaal ID, Putter H, Steup WH, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van Krieken JH, Leer JW, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3104] [Cited by in RCA: 3119] [Article Influence: 130.0] [Reference Citation Analysis (0)] |
| 2. | van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Påhlman L, Glimelius B, van de Velde CJ; Dutch Colorectal Cancer Group. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12:575-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1348] [Article Influence: 96.3] [Reference Citation Analysis (0)] |
| 3. | Gastrointestinal Tumor Study Group. Prolongation of the disease-free interval in surgically treated rectal carcinoma. N Engl J Med. 1985;312:1465-1472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 995] [Cited by in RCA: 913] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 4. | Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23:5644-5650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 564] [Cited by in RCA: 587] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 5. | Schou JV, Larsen FO, Rasch L, Linnemann D, Langhoff J, Høgdall E, Nielsen DL, Vistisen K, Fromm A, Jensen BV. Induction chemotherapy with capecitabine and oxaliplatin followed by chemoradiotherapy before total mesorectal excision in patients with locally advanced rectal cancer. Ann Oncol. 2012;23:2627-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 6. | Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, Tebbutt N, Hill M, Ross PJ, Massey A, Oates J. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 7. | Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C, Safont MJ, Salud A, Vera R, Massuti B, Escudero P, Alonso V, Bosch C, Martin M, Minsky BD. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial†. Ann Oncol. 2015;26:1722-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 8. | Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Yaeger R, Stadler ZK, Seier K, Gonen M, Segal NH, Reidy DL, Varghese A, Shia J, Vakiani E, Wu AJ, Crane CH, Gollub MJ, Garcia-Aguilar J, Saltz LB, Weiser MR. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018;4:e180071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 445] [Article Influence: 63.6] [Reference Citation Analysis (1)] |
| 9. | Fukuokaya W, Kimura T, Urabe F, Kimura S, Tashiro K, Tsuzuki S, Koike Y, Sasaki H, Miki K, Egawa S. Reply to Cengiz Beyan and Esin Beyan. Mean platelet volume may not be a significant prognostic factor in patients with metastatic castration-resistant prostate cancer. Int J Clin Oncol 97(2), 3-4, 2020. Int J Clin Oncol. 2020;25:2177-2178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Conroy T, Bosset JF, Etienne PL, Rio E, François É, Mesgouez-Nebout N, Vendrely V, Artignan X, Bouché O, Gargot D, Boige V, Bonichon-Lamichhane N, Louvet C, Morand C, de la Fouchardière C, Lamfichekh N, Juzyna B, Jouffroy-Zeller C, Rullier E, Marchal F, Gourgou S, Castan F, Borg C; Unicancer Gastrointestinal Group and Partenariat de Recherche en Oncologie Digestive (PRODIGE) Group. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:702-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 737] [Article Influence: 184.3] [Reference Citation Analysis (0)] |
| 11. | Bahadoer RR, Dijkstra EA, van Etten B, Marijnen CAM, Putter H, Kranenbarg EM, Roodvoets AGH, Nagtegaal ID, Beets-Tan RGH, Blomqvist LK, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; RAPIDO collaborative investigators. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22:29-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 962] [Article Influence: 240.5] [Reference Citation Analysis (0)] |
| 12. | Dietz DW; Consortium for Optimizing Surgical Treatment of Rectal Cancer (OSTRiCh). Multidisciplinary management of rectal cancer: the OSTRICH. J Gastrointest Surg. 2013;17:1863-1868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 13. | Novotny PJ, Tan AD, Foster NR, Sloan JA. SAS® Tools for Cost Effective and High Quality Clinical Trial Reporting. SAS Global Forum 2012. |
| 14. | Sauer R, Liersch T, Merkel S, Fietkau R, Hohenberger W, Hess C, Becker H, Raab HR, Villanueva MT, Witzigmann H, Wittekind C, Beissbarth T, Rödel C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30:1926-1933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1487] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 15. | You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, Paquette IM, Steele SR, Feingold DL; On Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer. Dis Colon Rectum. 2020;63:1191-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 224] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 16. | National Comprehensive Cancer Network. Rectal Cancer (Version 1.2021). (cited 10 May, 2021). Available from: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. |
| 17. | Fitzgerald TL, Zervos E, Wong JH. Patterns of Pelvic Radiotherapy in Patients with Stage II/III Rectal Cancer. J Cancer Epidemiol. 2013;2013:408460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Morris EJA, Finan PJ, Spencer K, Geh I, Crellin A, Quirke P, Thomas JD, Lawton S, Adams R, Sebag-Montefiore D. Wide Variation in the Use of Radiotherapy in the Management of Surgically Treated Rectal Cancer Across the English National Health Service. Clin Oncol (R Coll Radiol). 2016;28:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Midura EF, Jung AD, Daly MC, Hanseman DJ, Davis BR, Shah SA, Paquette IM. Cancer Center Volume and Type Impact Stage-Specific Utilization of Neoadjuvant Therapy in Rectal Cancer. Dig Dis Sci. 2017;62:1906-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Kasi A, Abbasi S, Handa S, Al-Rajabi R, Saeed A, Baranda J, Sun W. Total Neoadjuvant Therapy vs Standard Therapy in Locally Advanced Rectal Cancer: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3:e2030097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 149] [Cited by in RCA: 247] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 21. | Gerard JP, Rostom Y, Gal J, Benchimol D, Ortholan C, Aschele C, Levi JM. Can we increase the chance of sphincter saving surgery in rectal cancer with neoadjuvant treatments: lessons from a systematic review of recent randomized trials. Crit Rev Oncol Hematol. 2012;81:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 22. | Schootman M, Hendren S, Ratnapradipa K, Stringer L, Davidson NO. Adoption of Robotic Technology for Treating Colorectal Cancer. Dis Colon Rectum. 2016;59:1011-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Damle A, Damle RN, Flahive JM, Schlussel AT, Davids JS, Sturrock PR, Maykel JA, Alavi K. Diffusion of technology: Trends in robotic-assisted colorectal surgery. Am J Surg. 2017;214:820-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 24. | Lee GI, Lee MR, Green I, Allaf M, Marohn MR. Surgeons' physical discomfort and symptoms during robotic surgery: a comprehensive ergonomic survey study. Surg Endosc. 2017;31:1697-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 25. | Fleshman J, Branda M, Sargent DJ, Boller AM, George V, Abbas M, Peters WR Jr, Maun D, Chang G, Herline A, Fichera A, Mutch M, Wexner S, Whiteford M, Marks J, Birnbaum E, Margolin D, Larson D, Marcello P, Posner M, Read T, Monson J, Wren SM, Pisters PW, Nelson H. Effect of Laparoscopic-Assisted Resection vs Open Resection of Stage II or III Rectal Cancer on Pathologic Outcomes: The ACOSOG Z6051 Randomized Clinical Trial. JAMA. 2015;314:1346-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 755] [Cited by in RCA: 830] [Article Influence: 83.0] [Reference Citation Analysis (0)] |
| 26. | Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J; ALaCaRT Investigators. Effect of Laparoscopic-Assisted Resection vs Open Resection on Pathological Outcomes in Rectal Cancer: The ALaCaRT Randomized Clinical Trial. JAMA. 2015;314:1356-1363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 768] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 27. | Fleshman J, Branda ME, Sargent DJ, Boller AM, George VV, Abbas MA, Peters WR Jr, Maun DC, Chang GJ, Herline A, Fichera A, Mutch MG, Wexner SD, Whiteford MH, Marks J, Birnbaum E, Margolin DA, Larson DW, Marcello PW, Posner MC, Read TE, Monson JRT, Wren SM, Pisters PWT, Nelson H. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann Surg. 2019;269:589-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 274] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 28. | Stevenson ARL, Solomon MJ, Brown CSB, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Wilson K, Hague W, Simes J; Australasian Gastro-Intestinal Trials Group (AGITG) ALaCaRT investigators. Disease-free Survival and Local Recurrence After Laparoscopic-assisted Resection or Open Resection for Rectal Cancer: The Australasian Laparoscopic Cancer of the Rectum Randomized Clinical Trial. Ann Surg. 2019;269:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 203] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 29. | Jayne D, Pigazzi A, Marshall H, Croft J, Corrigan N, Copeland J, Quirke P, West N, Rautio T, Thomassen N, Tilney H, Gudgeon M, Bianchi PP, Edlin R, Hulme C, Brown J. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA. 2017;318:1569-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 921] [Article Influence: 115.1] [Reference Citation Analysis (0)] |
| 30. | Borschitz T, Wachtlin D, Möhler M, Schmidberger H, Junginger T. Neoadjuvant chemoradiation and local excision for T2-3 rectal cancer. Ann Surg Oncol. 2008;15:712-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 31. | Verseveld M, de Graaf EJ, Verhoef C, van Meerten E, Punt CJ, de Hingh IH, Nagtegaal ID, Nuyttens JJ, Marijnen CA, de Wilt JH; CARTS Study Group. Chemoradiation therapy for rectal cancer in the distal rectum followed by organ-sparing transanal endoscopic microsurgery (CARTS study). Br J Surg. 2015;102:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 138] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 32. | Bach SP, Gilbert A, Brock K, Korsgen S, Geh I, Hill J, Gill T, Hainsworth P, Tutton MG, Khan J, Robinson J, Steward M, Cunningham C, Levy B, Beveridge A, Handley K, Kaur M, Marchevsky N, Magill L, Russell A, Quirke P, West NP, Sebag-Montefiore D; TREC collaborators. Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): a randomised, open-label feasibility study. Lancet Gastroenterol Hepatol. 2021;6:92-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 122] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 33. | Kennecke HF, Brown CJ, Loree JM, Moloo H, Jonker DJ, Raval M, et al CCTG CO. 28 primary endpoint analysis: Neoadjuvant chemotherapy, excision and observation for early rectal cancer, the NEO trial. J Clin Oncol. 2021;39:3508. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 34. | Rombouts AJM, Al-Najami I, Abbott NL, Appelt A, Baatrup G, Bach S, Bhangu A, Garm Spindler KL, Gray R, Handley K, Kaur M, Kerkhof E, Kronborg CJ, Magill L, Marijnen CAM, Nagtegaal ID, Nyvang L, Peters FP, Pfeiffer P, Punt C, Quirke P, Sebag-Montefiore D, Teo M, West N, de Wilt JHW; for STAR-TREC Collaborative Group. Can we Save the rectum by watchful waiting or TransAnal microsurgery following (chemo) Radiotherapy versus Total mesorectal excision for early REctal Cancer (STAR-TREC study)? BMJ Open. 2017;7:e019474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 35. | Beets-Tan RG. MRI in rectal cancer: the T stage and circumferential resection margin. Colorectal Dis. 2003;5:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | MERCURY Study Group. Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study. Radiology. 2007;243:132-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 330] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 37. | Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G; MERCURY study group. Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study. Ann Surg. 2011;253:711-719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 465] [Article Influence: 33.2] [Reference Citation Analysis (0)] |