Published online May 15, 2022. doi: 10.4251/wjgo.v14.i5.1037
Peer-review started: December 20, 2021
First decision: February 21, 2022
Revised: April 8, 2022
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: May 15, 2022
Processing time: 140 Days and 23 Hours
Biliary strictures after liver transplantation (LT) remain clinically arduous and challenging situations, and endoscopic retrograde cholangiopancreatography (ERCP) has been considered as the gold standard for the management of biliary strictures after LT. Nevertheless, in the treatment of biliary strictures after LT with ERCP, many studies show that there is a large variation in diagnostic accuracy and therapeutic success rate. Digital single-operator peroral cholangioscopy (DSOC) is considered a valuable diagnostic modality for indeterminate biliary strictures.
To evaluate DSOC in addition to ERCP for management of biliary strictures after LT.
Nineteen patients with duct-to-duct biliary reconstruction who underwent ERCP for suspected biliary complications between March 2019 and March 2020 at Beijing Chaoyang Hospital, Capital Medical University, were consecutively enrolled in this observational study. After evaluating bile ducts using fluoro
Twenty-one biliary strictures were found in a total of 19 patients, among which anastomotic strictures were evident in 18 (94.7%) patients, while non-anastomotic strictures in 2 (10.5%), and space-occupying lesions in 1 (5.3%). Stones were found in 11 (57.9%) and loose sutures in 8 (42.1%). A benefit of cholangioscopy was seen in 15 (78.9%) patients. Cholangioscopy was crucial for selective guidewire placement prior to planned intervention in 4 patients. It was instrumental in identifying biliary stone and/or loose sutures in 9 patients in whom ERCP failed. It also provided a direct vision for laser lithotripsy. A space-occupying lesion in the bile duct was diagnosed by cholangioscopy in one patient. Patients with biliary stricture after LT displayed four types: (A) mild inflammatory change (n = 9); (B) acute inflammatory change edema, ulceration, and sloughing (n = 3); (C) chronic inflammatory change; and (D) acute suppurative change. Complications were seen in three patients with post-interventional cholangitis and another three with hyperamylasemia.
DSOC can provide important diagnostic information, helping plan and perform interventional procedures in LT-related biliary strictures.
Core Tip: Biliary strictures represent a leading cause of morbidity and mortality in liver transplant recipients. To date, endoscopic retrograde cholangiopancreatography remains the gold standard for diagnosing and treating such complications. The present study examined the benefit of complementary digital single-operator cholangioscopy. Our results are encouraging and demonstrate strong evidence for a diagnostic and therapeutic advantage of additional cholangioscopy for the management of biliary disorders following liver transplantation.
- Citation: Yu JF, Zhang DL, Wang YB, Hao JY. Digital single-operator cholangioscopy for biliary stricture after cadaveric liver transplantation. World J Gastrointest Oncol 2022; 14(5): 1037-1049
- URL: https://www.wjgnet.com/1948-5204/full/v14/i5/1037.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i5.1037
Liver transplantation (LT) has become a standard of care in patients with end-stage liver disease[1]. Despite improvements in surgical techniques, graft preservation technology, immunosuppressive therapy, and close follow-up[2], a biliary stricture is still the most common adverse event (AE) after LT, occurring in 5 % to 19 % of patients[3-6]. Biliary strictures include a wide array of biliary abnormalities that have different anatomical locations, clinical presentation, and pathogenesis. Biliary strictures after LT can be either anastomotic (AS) or non-anastomotic (NAS) based on the morphology and location of stenosis observed during imaging procedures[7,8]. AS account for approximately 80% of the post LT biliary strictures, are usually isolated, localized within 5 mm to the anastomosis site, and formed over short ductal lengths[9]. NAS account for the remaining 10% to 25%, are found more than 5 mm proximal to the anastomosis[10]; which can occur in both the extrahepatic or intrahepatic ducts and often develop at multiple sites and over greater lengths[10-12]. The first-line approach to resolving biliary strictures involves endoscopic retrograde cholangiopancreatography (ERCP), with stenosis dilatation and placement of multiple plastic stents, and fully covered self-expandable metallic stents[13-16]. Currently, ERCP represents the gold standard for the diagnosis and treatment of biliary strictures after LT[17]. The success rate of endoscopic therapy of the bile duct is 80%-100% in cases of LT[13,14], but successful long-term outcomes of endoscopic management of biliary anastomotic strictures after liver transplantation are 36.9%-100%[11,18,19]. Because not all strictures can be correctly diagnosed and treated with ERCP alternative methods are needed.
In 2015, digital single-operator cholangioscopy (DSOC), a high-resolution cholangioscopy (SpyGlass DSTM), was introduced by Boston Scientific (Boston Scientific Corp.), enabling high-definition imaging of bile ducts. DSOC provides detailed imaging of the biliary tree, assisting both with diagnosis and treatment through biopsy under direct vision, lithotripsy of difficult stones, retrieval of migrated stents, foreign body removal, and guidewire placement[20]. Therefore, since its development, DSOC has gained increasing attention in the field of management of biliary strictures after LT[21].
A few case reports and small case series analyzing the role of single-operator cholangioscopy (SOC) for management of biliary strictures after LT suggest that this approach is safe and feasible and can identify distinct features of anastomotic strictures[18-24]. This additional information may help guide effective treatment and predict patient outcomes. However, further studies are needed to fully evaluate the benefits of SOC in this respect, while for DSOC, to the best of our knowledge, there is little data available on its effect on the management of biliary strictures in LT recipients.
As it is likely that DSOC will benefit endoscopic management of biliary strictures after LT, by providing important high-resolution information of the bile duct, we decided to undertake an observational study of its use. Therefore, this study aimed to examine the role of complementary DSOC using the SpyGlass DS system during ERCP for the management of biliary strictures following LT.
This retrospective, observational study was performed at the Beijing ChaoYang Hospital, Capital Medical University, China. The study was performed in accordance with the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of ChaoYang Hospital (Beijing, China). All patients signed written informed consent for surgery. The statistical methods of this study were reviewed by Dr. Li-Rong Liang from the Department of Clinical Epidemiology, Beijing Chao-Yang Hospital, Capital Medical University. Patients with LT and duct-to-duct biliary anastomosis who presented with clinical or biochemical signs of biliary strictures and/or suspected biliary complications based upon imaging and/or histology between February 2019 and March 2020 were included in the study.
Inclusion criteria: (1) Patients after LT with clinical manifestations or biochemical changes of biliary stricture from February 2019 to March 2020; and (2) Imaging examinations by B-ultrasound, computed tomography, or magnetic resonance cholangiopancreatography (MRCP) suggested biliary stricture. Exclusion criteria: (1) Severe changes in the anatomical structure of the upper digestive tract; (2) Patients with severe coagulopathy; (3) Patients with severe cardiopulmonary insufficiency; and (4) Patients who cannot tolerate anesthesia.
All patients underwent transabdominal ultrasound. In the case of inconclusive findings on transabdominal ultrasound (common bile duct cannot be shown due to excessive gastrointestinal gas) and the absence of clinically evident cholangitis, MRCP was performed before ERCP. All patients received ERCP performed using a large diameter channel duodenoscope (TJF-260V, Olympus Corp., Tokyo, Japan). If a plastic biliary stent was previously placed in the patient, it was removed before cannulation. Cannulation of the bile duct was guidewire-assisted (0.035 inches, Hydra JagwireTM, Boston Scientific Corp) using a cannulating sphincterotome (Autotome RX, Boston Scientific Corp). If necessary, biliary sphincterotomy was performed. During the procedures, patients received conscious sedation with propofol and sufentanil.
ERCP was followed by cholangioscopy during the same procedure. Cholangioscopy was carried out using a single operator cholangioscopy device (SpyGlass DS; Boston Scientific Corp.) that was pushed along the guidewire through the working channel of the duodenoscope into the bile duct. The guidewire was then removed, and cholangioscopy was conducted under visual guidance. A biopsy was performed in case of unrecognized bile duct mucosal lesions. After the intervention, patients remained hospitalized for at least 3 d.
The interventions were performed by two highly experienced investigators with a yearly case volume of more than 200 endoscopic biliary interventions. Procedure-related complications were evaluated according to the American Society for Gastrointestinal Endoscopy guidelines[10].
Standard antibiotic prophylaxis included intravenous cefoxitin (New Asia Pharmaceutical Co. Ltd) at least 6 h before the procedure and up to 3 d thereafter. During ERCP/cholangioscopy, bile was collected for microbial analysis and for antibiotic susceptibility testing.
All patients were maintained on a calcineurin inhibitor (Ciclosporin A, Novartis Pharma Stein AG) alone or in combination with either an mTOR inhibitor (rapamycin, Kerry Centre) or mycophenolate mofetil (Roche).
Strictures were determined as an abrupt narrowing of the bile duct with a delayed outflow of contrast media through the stricture. Bile strictures were fluoroscopically subdivided into AS at the site of biliary anastomosis and NAS affecting donor bile ducts that were proximal to the biliary anastomosis. Dilatation was determined as an abrupt increase of the diameter of the bile duct, leading to a bag or column appearance of the bile duct. Bile duct stones were determined as intraluminal filling defects of contrast media, which were rounded or cloud-like, free-moving, and could be pushed by endoscopic instruments.
Strictures were determined as above and were visible as an abrupt substantial narrowing of bile ducts compared with distal and proximal segments of the bile duct. Stones were determined as free-moving, hard, foreign bodies in the bile duct or soft floccule stuck to the wall of the bile duct. A loose suture was determined as wire floating in the bile duct near the anastomosis. A neoplasm was determined as a quasi-circular lesion protruding into the lumen of the bile duct, which was connected with the wall of the bile duct.
The biliary stricture could be characterized into 4 types (Type A, B, C, and D) based on the cholangioscopic appearance of the mucosa at the anastomotic site and donor bile duct.
The main observation indicators were the success rate of ERCP intubation, the success rate of DSOC auxiliary guide wire passing through the stenosis, the correct rate of ERCP and DSOC to diagnose the nature of stenosis, routine blood analysis 2h and 24h postoperatively, serum total bilirubin, serum direct bilirubin, serum amylase.
ERCP was intubated through the duodenal papilla, and if the guidewire failed to enter the intrahepatic bile duct through the stenosis, it was judged as a failure of ERCP.
Statistical analysis was conducted using SPSS 24 (IBM Corp., Armonk, NY, United States). All data are presented as absolute and relative frequencies or reported as mean ± SD. Categorical variables were compared using Fisher’s exact test. P values < 0.05 were considered statistically significant.
Over the course of our study, 19 patients (12 males and 7 females), with a median age of 50.3 ± 8.9, underwent ERCP followed by cholangioscopy. Procedures were carried out at a median of 13.7 ± 8.2 mo after LT. 9 of the 19 patients underwent ERCP and had plastic stent placement in the common bile duct within three months prior to this study, while the remaining 10 received the procedure for the first time after LT. The patients’ clinical and demographic data are shown in Table 1.
| Patient No. | Age (yr) | Sex | Indication for LT | Post-LT time (mo) | Stent placement status | Number of previous ERCPs |
| 1 | 42 | M | Hepatitis B liver cirrhosis | 25 | Y | 3 |
| 2 | 55 | M | Alcoholic liver cirrhosis | 6 | Y | 1 |
| 3 | 55 | M | Alcoholic liver cirrhosis | 9 | Y | 1 |
| 4 | 52 | M | Hepatitis B liver cirrhosis | 7 | Y | 1 |
| 5 | 47 | M | Cryptogenic liver cirrhosis | 11 | N | 0 |
| 6 | 63 | F | Hepatitis C liver cirrhosis | 6 | N | 0 |
| 7 | 52 | F | Primary biliary cholangitis | 17 | Y | 2 |
| 8 | 52 | F | Hepatitis B liver cirrhosis | 36 | Y | 3 |
| 9 | 44 | M | Alcoholic liver cirrhosis | 26 | N | 0 |
| 10 | 37 | F | Drug-induced liver injury, acute liver failure | 2 | N | 0 |
| 11 | 37 | F | Drug-induced liver injury, acute liver failure | 4 | Y | 1 |
| 12 | 54 | M | Alcoholic liver cirrhosis | 4 | N | 1 |
| 13 | 42 | M | Hepatitis B liver cirrhosis, acute liver failure | 30 | N | 0 |
| 14 | 54 | M | Hepatocellular carcinoma/hepatitis B | 17 | Y | 2 |
| 15 | 72 | F | Cryptogenic liver cirrhosis | 20 | N | 0 |
| 16 | 41 | F | Primary biliary cholangitis | 21 | N | 0 |
| 17 | 59 | M | Cryptogenic liver cirrhosis | 13 | N | 0 |
| 18 | 49 | M | Alcoholic liver cirrhosis | 15 | N | 0 |
| 19 | 49 | M | Hepatitis B liver cirrhosis | 17 | Y | 0 |
During ERCP, AS were observed in 12 patients, NAS in 7, and stones in 3 (Table 2). Observation with cholangioscopy revealed AS in 18 patients, NAS in 2, stones in 11, loose suture in 7, and a space-occupying lesion in one (Table 2).
| Patient No. | Finding of ERCP | Findings of DSOC | Biliary stricture classification by DSOC | Endoscopic intervention |
| 1 | NAS | NAS; AS; stone; suture | Type B | Extraction of stones; MSP |
| 2 | NAS | AS; stone; suture | Type C | Extraction of stones; MSP |
| 3 | NAS | AS; stone; suture | Type D | MSP; ENBD |
| 4 | NAS | AS; stone | Type B | CAGP; balloon dilation; extraction of stones; MSP |
| 5 | AS | AS; stone; suture | Type A | CAGP; laser lithotripsy; balloon dilation; extraction of stones; MSP |
| 6 | NAS | Space-occupying lesions | Biopsy | |
| 7 | AS | AS; stone; suture | Type B | Extraction of stones; MSP |
| 8 | AS | AS; stone; suture | Type B | Extraction of stones; MSP |
| 9 | AS, stone | AS; stone | Type A | Balloon dilation; laser lithotripsy; extraction of stones; ENBD |
| 10 | AS | AS; stone; suture | Type B | Balloon dilation; SSP |
| 11 | AS | AS; suture | Type B | Balloon dilation; MSP |
| 12 | AS | AS | Type B | Balloon dilation; MSP |
| 13 | AS, stone | AS; stone | Type A | Extraction of stones; ENBD |
| 14 | NAS; stone | AS; stone | Type B | Extraction of stones; MSP |
| 15 | AS | AS | Type A | CAGP; bougienage; SSP |
| 16 | AS | AS | Type A | CAGP; bougienage; SSP |
| 17 | AS | AS | Type A | Balloon dilation; SSP |
| 18 | AS | AS | Type C | ENBD |
| 19 | NAS | NAS; AS | Type B | MSP |
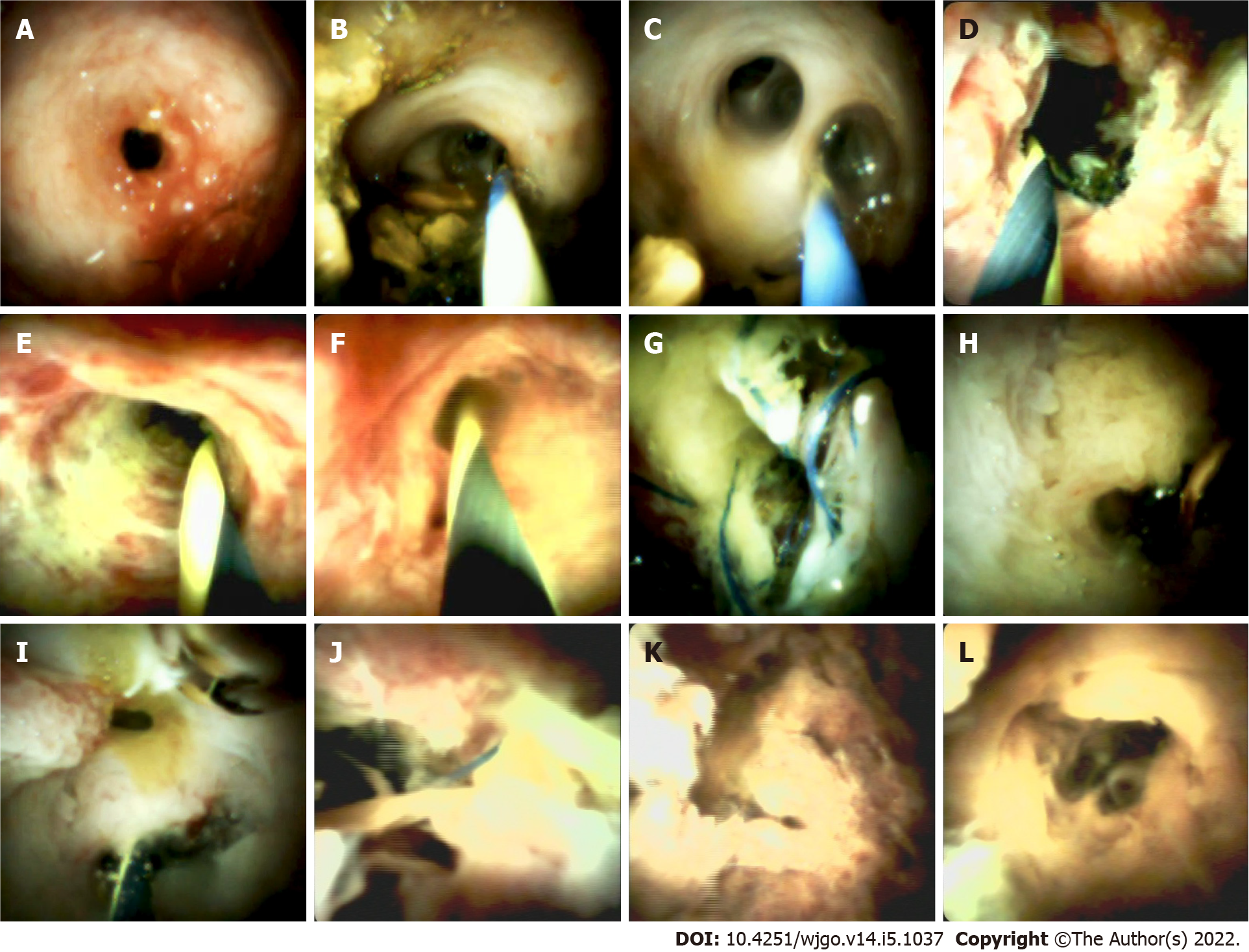
The biliary stricture could be characterized into 4 types. Type A (Figure 1) was found in 6 patients, which showed mild inflammatory changes, including fibrotic stenosis with mild erythema at the anastomotic site, pale smooth mucosa of the donor hepatobiliary duct, dimly visible branching of the submucosal vessels, and circular or elliptic opening of the intrahepatic bile duct. Type B (Figure 1) was found in 9 patients, which showed acute inflammatory changes, including anastomotic stenosis with hyperemia, edema, or polypoid growth tissues. In addition, the donor bile duct might show hyperemia, edema, clear submucosal vessels, and other manifestations of acute inflammation and even ulceration. This type was often associated with the presence of stones and sludge. Type C (Figure 1) was found in 2 patients, showing chronic inflammatory changes in anastomotic and donor hepatobiliary ducts. The mucosa of the anastomotic site and the donor hepatobiliary duct were thickened and pale, the surface was granular or villous, submucosa vessels had become thinner or disappeared, and the form of the intrahepatic bile duct opening had changed. When combined with acute inflammation, the mucosa could have also shown signs of hyperemia, edema, or other acute inflammatory manifestations. Type D (Figure 1) was found in one patient, which showed suppurative changes of the anastomotic site and donor hepatobiliary ducts. The mucosa of the anastomotic site and donor bile duct was greyish-yellow, the lumen of the bile duct was filled with pus and looked dirty, and submucosal vessels appeared.
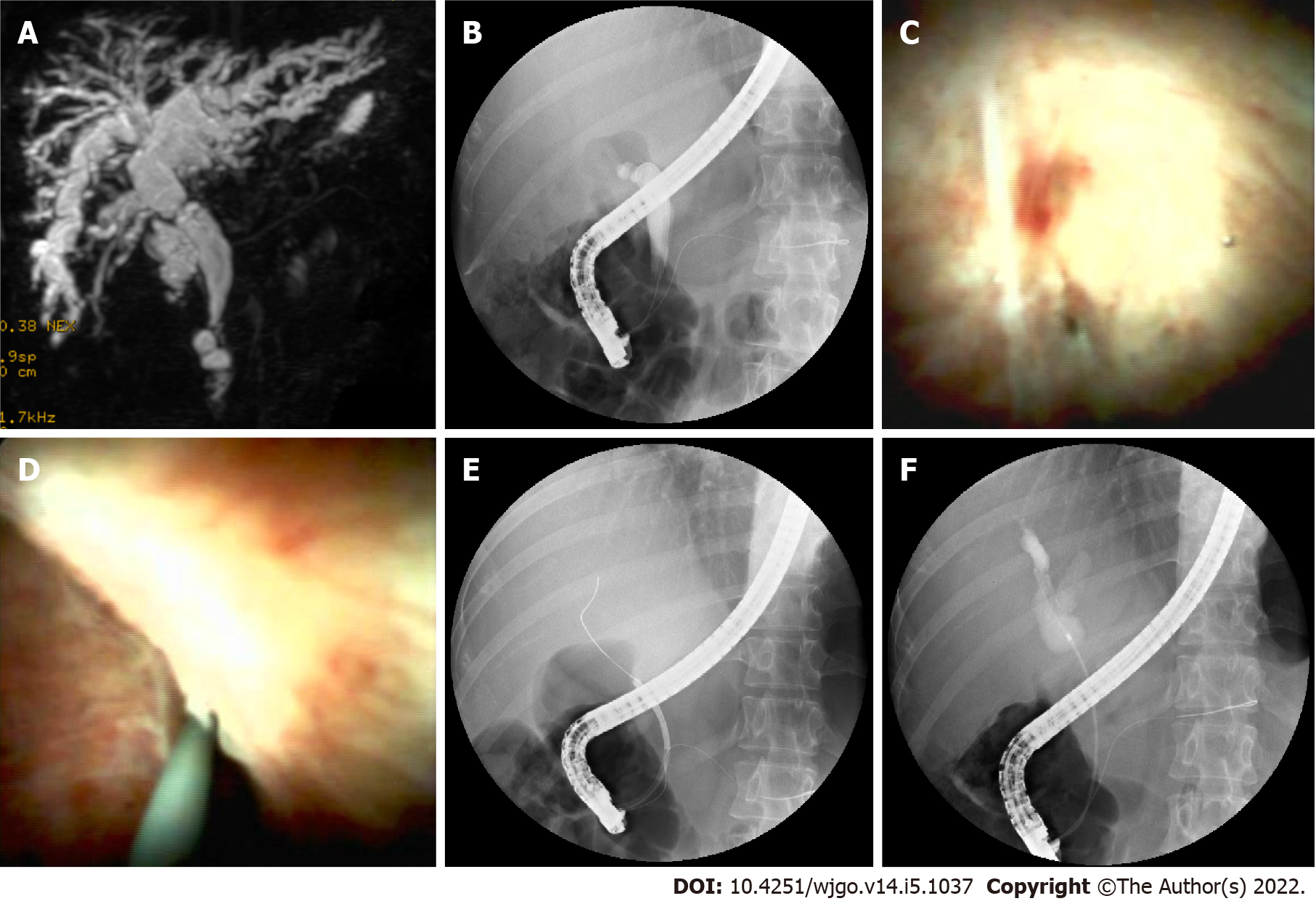
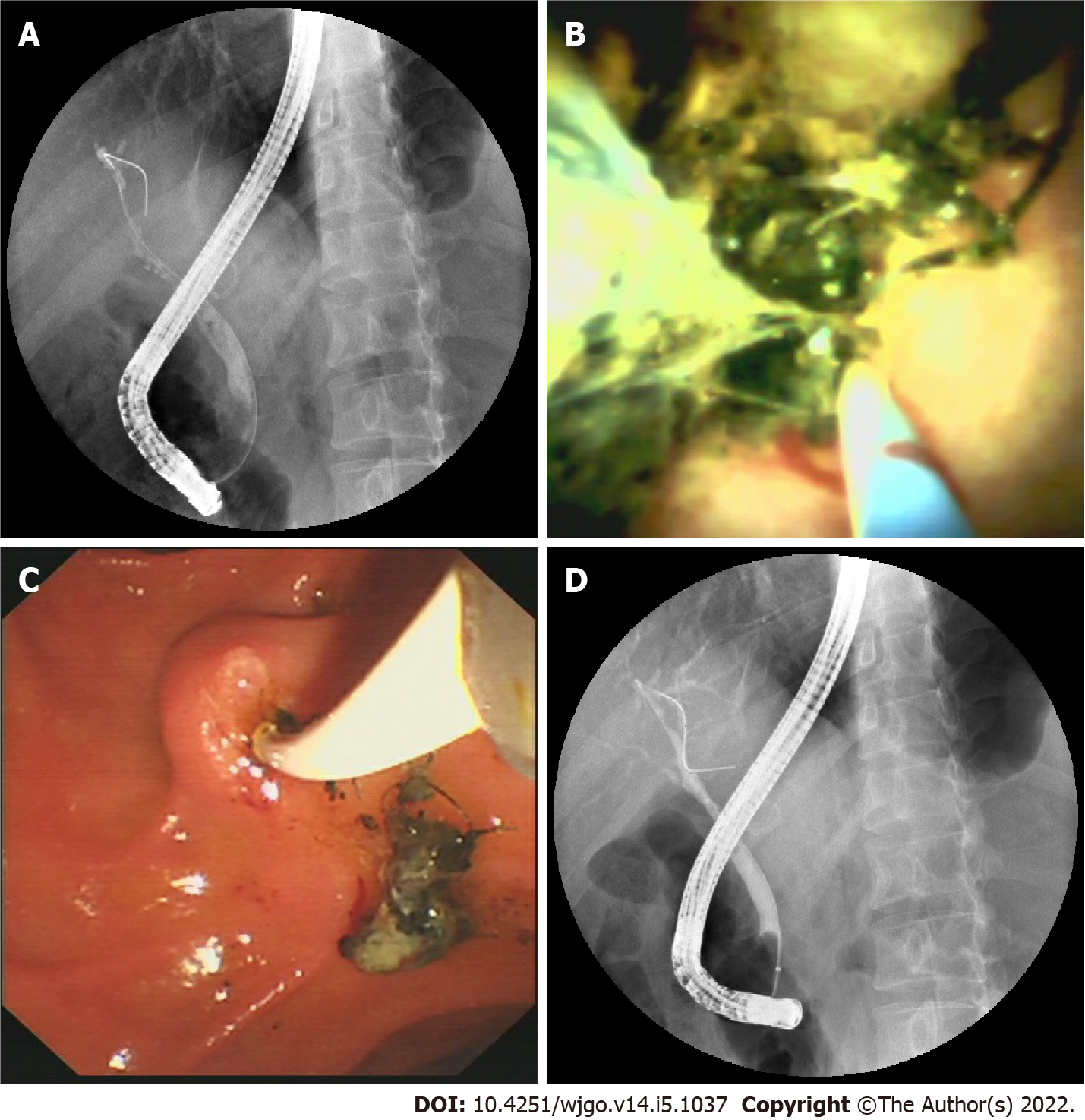
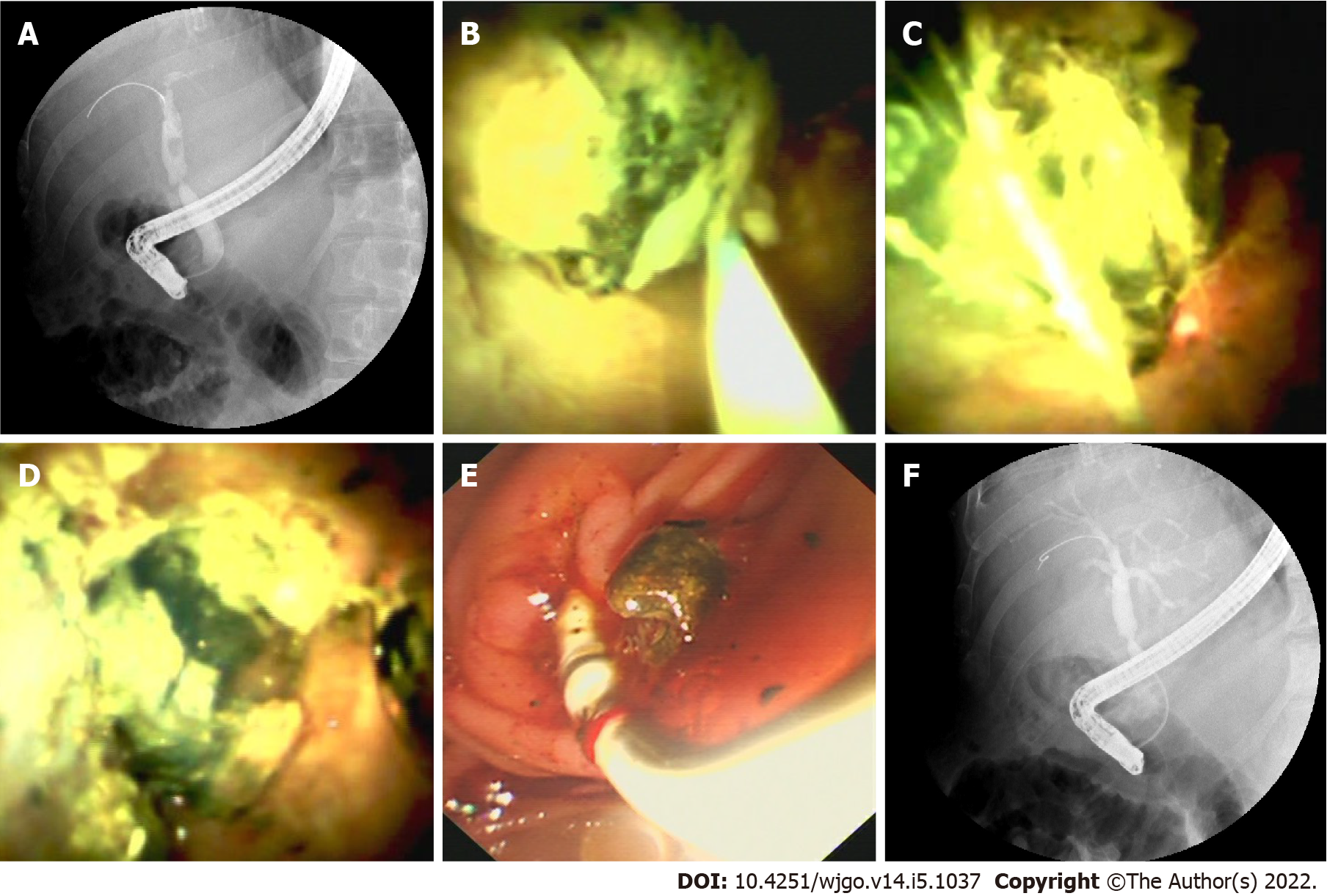
Cannulation was successful in 14 of the 18 patients attempted during ERCP. Selective guidewire placement was achieved during DSOC under direct vision in the remaining 4 patients that had failed during ERCP (Figure 2). Furthermore, cholangioscopy successfully identified stones and sludge in 8 more patients (P = 0.005) that ERCP missed. It also successfully detected loose sutures in 8 patients (P = 0.008) that ERCP failed to detect (Figure 3). Four patients diagnosed with NAS by ERCP were later determined to be AS by choledochoscopy, with 2 type A, 1 type B, and 1 type C. The findings of ERCP and cholangioscopy, as well as endoscopic intervention, are summarized in Table 2.
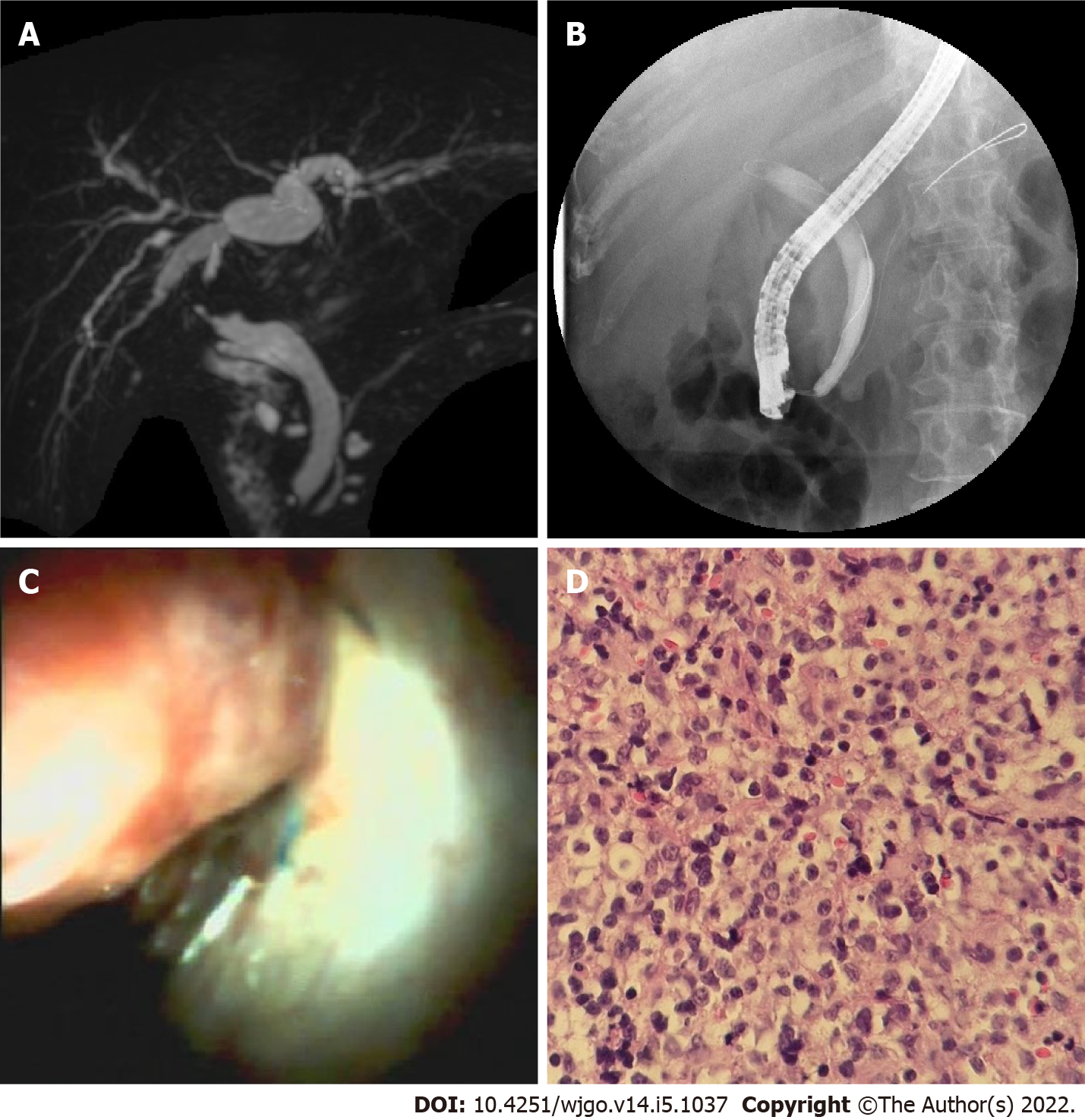
A total of 8 biopsies were obtained. Studies of the histology of 7 anastomotic stricture samples, including 3 type A, 3 type B, and 1 type C, demonstrated fibrous hyperplasia with mixed infiltration of lymphocytes, plasmacytes, and granulocytes, as well as granulation tissue and scars. A neoplasm with a red surface (Figure 4) in the donor bile tract was observed in one patient. Histology of the biopsy revealed a large number of infiltrating lymphocytes with uniform, diffuse distribution, and obvious atypia. Liver-localized post-transplantation lymphoproliferative disease (LL-PTLD) was confirmed by immunohistochemistry in this patient.
In patients with biliary strictures, a total of 7 balloon dilatations, 2 bougienage of a tight stricture, 9 extractions of stones, 10 multiple plastic stent placement, 4 endoscopic nasobiliary drainage, 4 single plastic stent placement, and 2 Laser lithotripsies under direct vision were performed (Figure 5).
No serious adverse events occurred in any of the cases. However, mild complications were observed in 6/19 (31.6%), in which 3 were documented as post-ERCP cholangitis, and 3 were hyperamylasemia (15.7%). All cases of DSOC-related complications had a mild clinical course and were treated successfully with conservative therapeutic approaches.
The aim of this study was to evaluate whether the use of DSOC added any benefits for patients undergoing ERCP for the management of biliary strictures after LT. The results from 19 patients showed that during ERCP, AS was observed in 12 patients, NAS in 7, and stones in 3. However, DSOC revealed AS in 18 patients, NAS in 2, stones in 11, loose suture in 7, and a space-occupying lesion in one patient. The DSOC also meant that AS could be characterized into 4 types (A to D) based on the cholangioscopic appearance of the donor bile duct mucosa. Therefore, these results suggest that DSOC can provide important diagnostic information for patients with suspected biliary strictures after LT.
The results of this study agree with those of previous studies that have shown SOC can identify biliary strictures in patients after LT[21-24]. Our results also suggest DSOC provided a more accurate diagnosis of biliary stenosis than ERCP. Similar to Hüsing-Kabar et al[23] who found a benefit of cholangioscopy in 46.2% of patients, our study potentially showed an even greater benefit, in 15 (78.9%) patients. Initially, seven patients were diagnosed with NAS by ERCP, but of these, only two were confirmed with choledochoscopy. Among the five remaining patients, four were confirmed with AS, including 2 type B cases, 1 type C case, and 1 type D case; and one patient was diagnosed with LL-PTLD according to histology. These five patients all presented with NAS-like imaging in ERCP, possibly due to a large number of stones and sludge adhered to the donor’s bile duct wall, which made the angiography images resemble multi-segment bile duct stenosis. The biliary strictures resolved after the extraction of stones and sludge. However, it is not known whether the NAS-like imaging resulting from mural calculi above the stenosis is a misleading phenomenon or actually an early manifestation of NAS, and further study is needed to investigate this. In our study, one case of NAS diagnosed by ERCP was found to be a neoplasm in the bile tract under direct vision of cholangioscopy, which was later confirmed as LL-PTLD based on histology. Our finding indicated that AS and NAS are not the only etiologies of biliary stenosis after LT. Thus, the use of DSOC in our study may have provided an additional advantage.
A previous study by Balderramo et al[22] divided AS into two patterns according to cholangioscopy finding of anastomosis: (A) the presence of mild erythema and scarring of the AS; and (B) the presence of severe edema and erythema plus ulceration with sloughing at the AS. Based on cholangioscopy imaging of the anastomosis and donor bile duct, we divided them into four types. It should be noted that one patient in type C and one patient in type D underwent second liver transplantation for chronic rejection within 6 mo, which indicated that patients with type C or D might have a poor prognosis after treatment. Further research is needed to confirm whether this classification method has guiding significance for treatment and prognosis.
In our study, cholangioscopy was superior to ERCP in the detection of stones and sludge, discovered in 11(61.1%) patients by DSOC, while only 3 (16.7%) by ERCP. This may be because these tiny stones and sludge were kept close to the wall of the bile duct and were difficult to discern by ERCP. The presence of bile stones, including sludge and casts formation, is a common biliary complication after LT, with a reported incidence of 5% to 10%[25]. Early diagnosis and treatment of stones are crucial for patient and graft survival. Therefore, DSOC could be more helpful than ERCP in post LT care.
In addition, loose sutures at the anastomotic site, one of the causes of calculus formation, were found with DSOC but not ERCP in 7 (41.2%) patients in our study. In 1897, Homans reported the first case of migration of silk sutures into the common bile duct and the formation of gallstones[26]. Since then, many cases of bile duct stone formation around sutures have been reported[27,28]. The stone can form over the nidus of the introduced unabsorbable suture material when cholesterol and/or pigment aggregate around it. Thus, bile duct anastomosis with absorbable sutures may help to reduce stone formation. However, there is no consensus on the use of suitable suture material for anastomosis of bile ducts in LT. Although DSOC provides a great tool to locate the loose sutures at the anastomotic site, currently, there is no suitable device to remove these sutures. We have attempted to remove the loose sutures with balloons but only achieved the removal of sutures with a small amount of tissue in two patients.
Passing the stricture with a guidewire is a fundamental prerequisite for the technical success of endoscopic stricture management. In LT patients, the strictures are often very tight and twisted due to the presence of dense fibrotic tissue and the hypertrophic transplanted liver, rendering this procedure challenging. The incidence of failed guidewire passage through the stricture is between 16%-38%[11,29]. While ERCP can only determine the location, length, and morphology of the coronary plane, by contrast, choledochoscopy can distinguish the mucosal manifestations of the bile duct, the presence or absence of attachments, as well as the morphology of the horizontal plane. It is plausible that DSOC may facilitate the passage of a guidewire through the more challenging strictures under direct visualization in LT patients[21,30]. A study by Woo et al[21] revealed poor performance of cholangioscopy-assisted guidewire placement in 60% of cases. However, Hüsing-Kabar et al[23] reported that they steered the guidewire over the stricture successfully under direct vision in all patients for whom conventional cannulation failed. In our study, cholangioscopy-assisted guidewire placement was performed successfully in four patients for whom the procedure failed previously in ERCP. The low success rate of guidewire placement in Woo et al[21] study may be due to the fact that their study was performed in patients receiving living donor LT, which involved special and sometimes complex anatomy of bile ducts and required complicated bile duct anastomosis[20]. In contrast, all patients included in our study underwent whole cadaveric LT.
We successfully performed laser lithotripsy with DSOC in one patient. The calculi with bile duct stricture after LT are most commonly located above the anastomosis, and AS makes it difficult to extract the stones. We broke the stone with the laser under direct visualization and then extracted the rubble successfully with a balloon into the duodenum. This approach avoids the risk of cholangitis resulting from long-term stent implantation and stone stimulation. We believed it was a good choice for the complex stone treatment.
In our study, post-DSOC cholangitis occurred in 15.8% (3/19) of the patients, which was higher than that was reported with ERCP alone (0.5%-3.0%)[31]. Sethi et al[32] reported that cholangioscopy increased the risk of post-ERCP cholangitis in a retrospective study. LT recipients are more likely to develop post-ERCP cholangitis during choledochoscopy due to immunosuppressive medications, water injection during choledochoscopy, and incomplete biliary drainage. Therefore, proper evaluation of selected indications to identify patients who may benefit most for such procedures and attention to detail at peri-procedure, such as antibiotic prophylaxis and appropriate water injection pressure and speed, are crucial for the prevention of post-ERCP cholangitis. Furthermore, microbial analysis of bile collected during bile duct interventions should be regularly performed to guide the treatments in case of post-ERCP septic complications.
Some limitations of this study should be noted. First, this study is retrospective, and the number of analyzed patients was small, making it necessary to treat statistical comparisons with caution. Second, this was a single-center study, and the procedures were performed by physicians with ample experience in the management of biliary complications after LT. Thus, these results may not be applicable to all centers. Finally, we did not include patients who underwent living donor LT or recipients of transplants from donors after cardiac death, who have a higher incidence of AS vs recipients of cadaveric donors.
In conclusion, DSOC is feasible and safe in LT recipients with biliary strictures and offers useful diagnostic information in addition to ERCP. These results suggest that cholangioscopy is superior to ERCP in diagnosing and classifying biliary strictures after LT, diagnosing biliary stones and sludge, and optimizing treatment in the patients concerned. Therefore, we recommend performing DSOC concurrently with the first ERCP procedure in LT recipients with strictures who require choledochoscopy-assisted guidewire placement or need laser lithotripsy under direct visualization.
Liver transplantation (LT) has become a standard of care in patients with end-stage liver disease. Biliary strictures after LT can be either anastomotic or non-anastomotic based on the morphology and location of stenosis observed during imaging procedures. The first-line approach to resolving biliary strictures involves endoscopic retrograde cholangiopancreatography (ERCP), with stenosis dilatation and placement of multiple plastic stents, and fully covered self-expandable metallic stents.
Biliary strictures after LT remain clinically arduous and challenging situations, and ERCP has been considered as the gold standard for the management of biliary strictures after LT. Nevertheless, in the treatment of biliary strictures after LT with ERCP, many studies show that there is a large variation in diagnostic accuracy and therapeutic success rate. Digital single-operator peroral cholangioscopy (DSOC) is considered a valuable diagnostic modality for indeterminate biliary strictures.
This study aimed to evaluate DSOC in addition to ERCP for management of biliary strictures after LT.
Total 19 patients with duct-to-duct biliary reconstruction who underwent ERCP for suspected biliary complications were consecutively enrolled in this observational study. After evaluating bile ducts using fluoroscopy, cholangioscopy using a modern digital single-operator cholangioscopy system was performed during the same procedure with patients under conscious sedation. Biliary strictures after LT were classified according to the manifestations of choledochoscopic strictures and the manifestations of transplanted hepatobiliary ducts.
Twenty-one biliary strictures were found in a total of 19 patients, among which anastomotic strictures were evident in 18 (94.7%) patients, while non-anastomotic strictures in 2 (10.5%), and space-occupying lesions in 1 (5.3%). Stones were found in 11 (57.9%) and loose sutures in 8 (42.1%). A benefit of cholangioscopy was seen in 15 (78.9%) patients. It was instrumental in identifying biliary stone and/or loose sutures in 9 patients in whom ERCP failed. It also provided a direct vision for laser lithotripsy.
The present study examined the benefit of complementary DSOC. DSOC can provide important diagnostic information, helping plan and perform interventional procedures in LT-related biliary strictures. Our results are encouraging and demonstrate strong evidence for a diagnostic and therapeutic advantage of additional cholangioscopy for the management of biliary disorders following liver transplantation.
This study was retrospective, and prospective multicenter trials should be performed. Patients with living donor LT should also be investigated.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Microscopy
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Incorvaia L, Italy; Kitagawa K, Japan; Tsou YK, Taiwan S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Halliday N, Westbrook RH. Liver transplantation: need, indications, patient selection and pre-transplant care. Br J Hosp Med (Lond). 2017;78:252-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Charlton MR. Roadmap for improving patient and graft survival in the next 10 years. Liver Transpl. 2016;22:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 247] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Kochhar G, Parungao JM, Hanouneh IA, Parsi MA. Biliary complications following liver transplantation. World J Gastroenterol. 2013;19:2841-2846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 186] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (1)] |
| 5. | Nemes B, Gámán G, Doros A. Biliary complications after liver transplantation. Expert Rev Gastroenterol Hepatol. 2015;9:447-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 6. | Suárez F, Otero A, Solla M, Arnal F, Lorenzo MJ, Marini M, Vázquez-Iglesias JL, Gómez M. Biliary complications after liver transplantation from maastricht category-2 non-heart-beating donors. Transplantation. 2008;85:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Rao HB, Prakash A, Sudhindran S, Venu RP. Biliary strictures complicating living donor liver transplantation: Problems, novel insights and solutions. World J Gastroenterol. 2018;24:2061-2072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 8. | Thethy S, Thomson BNj, Pleass H, Wigmore SJ, Madhavan K, Akyol M, Forsythe JL, James Garden O. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 173] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 9. | Balderramo D, Navasa M, Cardenas A. Current management of biliary complications after liver transplantation: emphasis on endoscopic therapy. Gastroenterol Hepatol. 2011;34:107-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 10. | ASGE Standards of Practice Committee; Chandrasekhara V, Khashab MA, Muthusamy VR, Acosta RD, Agrawal D, Bruining DH, Eloubeidi MA, Fanelli RD, Faulx AL, Gurudu SR, Kothari S, Lightdale JR, Qumseya BJ, Shaukat A, Wang A, Wani SB, Yang J, DeWitt JM. Adverse events associated with ERCP. Gastrointest Endosc. 2017;85:32-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 536] [Article Influence: 67.0] [Reference Citation Analysis (0)] |
| 11. | Hsieh TH, Mekeel KL, Crowell MD, Nguyen CC, Das A, Aqel BA, Carey EJ, Byrne TJ, Vargas HE, Douglas DD, Mulligan DC, Harrison ME. Endoscopic treatment of anastomotic biliary strictures after living donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc. 2013;77:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 12. | Chang JH, Lee IS, Choi JY, Yoon SK, Kim DG, You YK, Chun HJ, Lee DK, Choi MG, Chung IS. Biliary Stricture after Adult Right-Lobe Living-Donor Liver Transplantation with Duct-to-Duct Anastomosis: Long-Term Outcome and Its Related Factors after Endoscopic Treatment. Gut Liver. 2010;4:226-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Seehofer D, Eurich D, Veltzke-Schlieker W, Neuhaus P. Biliary complications after liver transplantation: old problems and new challenges. Am J Transplant. 2013;13:253-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Tringali A, Barbaro F, Pizzicannella M, Boškoski I, Familiari P, Perri V, Gigante G, Onder G, Hassan C, Lionetti R, Ettorre GM, Costamagna G. Endoscopic management with multiple plastic stents of anastomotic biliary stricture following liver transplantation: long-term results. Endoscopy. 2016;48:546-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Nacif LS, Bernardo WM, Bernardo L, Andraus W, Torres L, Chaib E, D'Albuquerque LC, Maluf-Filho F. Endoscopic treatment of post-liver transplantation anastomotic biliary stricture: systematic review and meta-analysis. Arq Gastroenterol. 2014;51:240-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Landi F, de'Angelis N, Sepulveda A, Martínez-Pérez A, Sobhani I, Laurent A, Soubrane O. Endoscopic treatment of anastomotic biliary stricture after adult deceased donor liver transplantation with multiple plastic stents vs self-expandable metal stents: a systematic review and meta-analysis. Transpl Int. 2018;31:131-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 17. | Hüsing A, Cicinnati VR, Beckebaum S, Wilms C, Schmidt HH, Kabar I. Endoscopic ultrasound: valuable tool for diagnosis of biliary complications in liver transplant recipients? Surg Endosc. 2015;29:1433-1438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Ranjan P, Bansal RK, Mehta N, Lalwani S, Kumaran V, Sachdeva MK, Kumar M, Nundy S. Endoscopic management of post-liver transplant billiary complications: A prospective study from tertiary centre in India. Indian J Gastroenterol. 2016;35:48-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Kim TH, Lee SK, Han JH, Park DH, Lee SS, Seo DW, Kim MH, Song GW, Ha TY, Kim KH, Hwang S, Lee SG. The role of endoscopic retrograde cholangiography for biliary stricture after adult living donor liver transplantation: technical aspect and outcome. Scand J Gastroenterol. 2011;46:188-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Karagyozov P, Boeva I, Tishkov I. Role of digital single-operator cholangioscopy in the diagnosis and treatment of biliary disorders. World J Gastrointest Endosc. 2019;11:31-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Woo YS, Lee JK, Noh DH, Park JK, Lee KH, Lee KT. SpyGlass cholangioscopy-assisted guidewire placement for post-LDLT biliary strictures: a case series. Surg Endosc. 2016;30:3897-3903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Balderramo D, Sendino O, Miquel R, de Miguel CR, Bordas JM, Martinez-Palli G, Leoz ML, Rimola A, Navasa M, Llach J, Cardenas A. Prospective evaluation of single-operator peroral cholangioscopy in liver transplant recipients requiring an evaluation of the biliary tract. Liver Transpl. 2013;19:199-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Hüsing-Kabar A, Heinzow HS, Schmidt HH, Stenger C, Gerth HU, Pohlen M, Thölking G, Wilms C, Kabar I. Single-operator cholangioscopy for biliary complications in liver transplant recipients. World J Gastroenterol. 2017;23:4064-4071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Franzini T, Moura R, Rodela G, Andraus W, Herman P, D'Albuquerque L, de Moura E. A novel approach in benign biliary stricture - balloon dilation combined with cholangioscopy-guided steroid injection. Endoscopy. 2015;47 Suppl 1:E571-E572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Ayoub WS, Esquivel CO, Martin P. Biliary complications following liver transplantation. Dig Dis Sci. 2010;55:1540-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 26. | Homans J. VIII. Gall-Stones formed around Silk Sutures Twenty Months after Recovery from Cholecystotomy. Ann Surg. 1897;26:114-116. [PubMed] |
| 27. | Beardsley C, Lim J, Gananadha S. Nonabsorbable suture material in the biliary tract. J Gastrointest Surg. 2012;16:2182-2183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 28. | Li Q, Tao L, Wu X, Mou L, Sun X, Zhou J. Bile duct stone formation around a Prolene suture after cholangioenterostomy. Pak J Med Sci. 2016;32:263-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Tsujino T, Isayama H, Sugawara Y, Sasaki T, Kogure H, Nakai Y, Yamamoto N, Sasahira N, Yamashiki N, Tada M, Yoshida H, Kokudo N, Kawabe T, Makuuchi M, Omata M. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol. 2006;101:2230-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Parsi MA, Guardino J, Vargo JJ. Peroral cholangioscopy-guided stricture therapy in living donor liver transplantation. Liver Transpl. 2009;15:263-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 31. | Dumonceau JM, Kapral C, Aabakken L, Papanikolaou IS, Tringali A, Vanbiervliet G, Beyna T, Dinis-Ribeiro M, Hritz I, Mariani A, Paspatis G, Radaelli F, Lakhtakia S, Veitch AM, van Hooft JE. ERCP-related adverse events: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2020;52:127-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 502] [Article Influence: 100.4] [Reference Citation Analysis (1)] |
| 32. | Sethi A, Chen YK, Austin GL, Brown WR, Brauer BC, Fukami NN, Khan AH, Shah RJ. ERCP with cholangiopancreatoscopy may be associated with higher rates of complications than ERCP alone: a single-center experience. Gastrointest Endosc. 2011;73:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |