Published online May 15, 2022. doi: 10.4251/wjgo.v14.i5.1027
Peer-review started: December 10, 2021
First decision: January 12, 2022
Revised: January 24, 2022
Accepted: April 24, 2022
Article in press: April 24, 2022
Published online: May 15, 2022
Processing time: 150 Days and 19.3 Hours
Accurate target volume delineation is the premise for the implementation of precise radiotherapy. Inadequate target volume delineation may diminish tumor control or increase toxicity. Although several clinical target volume (CTV) delineation guidelines for rectal cancer have been published in recent years, significant interobserver variation (IOV) in CTV delineation still exists among radiation oncologists. However, proper education may serve as a bridge that connects complex guidelines with clinical practice.
To examine whether an education program could improve the accuracy and consistency of preoperative radiotherapy CTV delineation for rectal cancer.
The study consisted of a baseline target volume delineation, a 150-min education intervention, and a follow-up evaluation. A 42-year-old man diagnosed with stage IIIC (T3N2bM0) rectal adenocarcinoma was selected for target volume delineation. CTVs obtained before and after the program were compared. Dice similarity coefficient (DSC), inclusiveness index (IncI), conformal index (CI), and relative volume difference [ΔV (%)] were analyzed to quantitatively evaluate the disparities between the participants’ delineation and the standard CTV. Maximum volume ratio (MVR) and coefficient of variation (CV) were calculated to assess the IOV. Qualitative analysis included four common controversies in CTV delineation concerning the upper boundary of the target volume, external iliac area, groin area, and ischiorectal fossa.
Of the 18 radiation oncologists from 10 provinces in China, 13 completed two sets of CTVs. In quantitative analysis, the average CTV volume decreased from 809.82 cm3 to 705.21 cm3 (P = 0.001) after the education program. Regarding the indices for geometric comparison, the mean DSC, IncI, and CI increased significantly, while ΔV (%) decreased remarkably, indicating improved agreement between participants’ delineation and the standard CTV. Moreover, an 11.80% reduction in MVR and 18.19% reduction in CV were noted, demonstrating a smaller IOV in delineation after the education program. Regarding qualitative analysis, the greatest variations in baseline were observed at the external iliac area and ischiorectal fossa; 61.54% (8/13) and 53.85% (7/13) of the participants unnecessarily delineated the external iliac area and the ischiorectal fossa, respectively. However, the education program reduced these variations.
Wide variations in CTV delineation for rectal cancer are present among radiation oncologists in mainland China. A well-structured education program could improve delineation accuracy and reduce IOVs.
Core Tip: Accurate clinical target volume (CTV) delineation is essential to ensure appropriate tumor control while minimizing the exposure of surrounding normal tissues. However, a large degree of variation in CTV delineation for rectal cancer still exists, despite the availability of several CTV delineation guidelines. Our study aimed to evaluate the impact of an education program on CTV delineation for rectal cancer. The results first confirmed the wide variations in CTV delineation for rectal cancer among radiation oncologists from mainland China and proved that a well-structured education program could improve the accuracy and consistency of delineation.
- Citation: Zhang YZ, Zhu XG, Song MX, Yao KN, Li S, Geng JH, Wang HZ, Li YH, Cai Y, Wang WH. Improving the accuracy and consistency of clinical target volume delineation for rectal cancer by an education program. World J Gastrointest Oncol 2022; 14(5): 1027-1036
- URL: https://www.wjgnet.com/1948-5204/full/v14/i5/1027.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i5.1027
Colorectal cancer is one of the most common cancers in China, with morbidity and mortality occupying the fifth place among all malignant tumors[1]. Due to occult symptoms, most rectal cancer patients would have progressed to locally advanced stages (cT3-4/N+) at diagnosis, which are associated with high risks of both locoregional recurrence and distant metastasis. Neoadjuvant chemoradiotherapy has become one of the standard treatment strategies for locally advanced rectal cancer, with the ability to increase resectability and the chance of sphincter preservation, as well as improve local control[2,3]. Compared with conventional three-dimensional conformal radiation therapy, intensity-modulated radiation therapy (IMRT) can yield superior plans with respect to target coverage, homogeneity, and conformality, while lowering the dose to adjacent critical organs-at-risk[4]. However, accurate target volume delineation is the premise for the implementation of IMRT. An omission of the target volume may decrease tumor control rate, whereas inappropriate expansion of the irradiation area would result in added normal tissue damage.
Nevertheless, defining a radiation field requires a combination of knowledge from multiple disciplines, including oncology, anatomy, imaging, radiophysics, and radiobiology. Differences in the personal theoretical understanding and clinical experience of radiation oncologists may lead to inaccurate and inconsistent target volume delineation. Although several clinical target volume (CTV) delineation guidelines for rectal cancer[5-7] have been published in recent years, significant interobserver variations (IOV) still exist in target volume delineation among radiation oncologists[8-10]. However, proper education may serve as a bridge that connects complex guidelines with clinical practice. Given the importance of accurate target volume delineation and the fact that no study concerning educational interventions within the target volume delineation field for rectal cancer is available, we conducted this study to examine the variations in preoperative radiotherapy CTV for rectal cancer among Chinese radiation oncologists and assess the short-term effects of an education program on target volume delineation.
The study consisted of a baseline CTV delineation, a 150-min education intervention, and a follow-up evaluation. The study protocol was approved by the Beijing Cancer Hospital Research Ethics Committee. A total of 18 radiation oncologists from 18 tertiary hospitals in 10 provinces located in north, south, central, and northeast China participated in the education program. Their median age was 37 (range, 31-49) years, and the ratio of men to women was 1.25:1. Regarding their educational background, 72.22% of the participants (13/18) had a master’s degree or above. As for their professional title, 33.33% (6/18) were attending physicians; the remaining 66.67% (12/18) were associate chief physicians. The median number of rectal cancer target volumes that they had delineated before the program was 38 (range, 6-300).
A 42-year-old male patient diagnosed with stage IIIC (T3N2bM0) rectal adenocarcinoma according to the seventh American Joint Committee on Cancer/Union for International Cancer Control TNM staging system[11] was selected for target volume delineation. The tumor was located 4 cm from the anal verge and extended cranially for 4 cm with mesorectal nodes and left internal iliac node metastases. The anal canal was not infiltrated. This patient was selected for clarifying several important issues in delineation and avoiding divergence at the same time. He underwent contrast-enhanced computed tomography (CT) and magnetic resonance imaging (MRI) simulations with 5 mm slice thickness from the L2-L3 junction to the proximal femur, in the supine position with a full bladder and an empty rectum. The simulation images were transferred to a Pinnacle 9.10 Treatment Planning System (Elekta, Sweden). The patient’s medical history, physical examination, colonoscopy results, and a full set of pelvic MRI images were introduced through PowerPoint software. All the radiation oncologists participating in the education program were required to independently delineate a CTV on the CT-MRI fusion images based on their previous clinical experience. The window width and the window level used for contouring were 400 Hounsfield units (HU) and 40 HU, respectively.
Subsequently, a 150-min education program on CTV delineation for rectal cancer was conducted. The program consisted of four parts. First, a lecture on the lymphatic drainage mode and the postoperative recurrence pattern of rectal cancer was given. Second, the 2006 version of the definition and delineation of the CTV for rectal cancer[5], the 2009 version of the Radiation Therapy Oncology Group contouring atlas[6], and the 2016 version of the international consensus guidelines on CTV delineation[7] were introduced. Third, a standard CTV (CTV-ref) based on the 2016 version of the CTV delineation guidelines was displayed, and the anatomical boundaries of each lymphatic drainage area were explained in detail. The standard CTV was contoured by an expert who has been engaged in rectal cancer radiotherapy for more than 20 years in our center and was determined through discussion by the entire department; the CTV included the mesorectal and presacral regions, obturator and internal iliac lymph node drainage areas, and 2 cm margins from the cephalic and caudal extents of the primary lesion in the rectum. Fourth, real-time feedback on each participant’s delineation deficiencies was conducted, and a question-and-answer period was provided for further clarification. Then, after the training session, the participants were asked to contour a CTV again on the same CT-MRI fusion images.
Quantitative evaluation of the target volume parameters: The volumes delineated by each participant before and after the education program were imported to a single Pinnacle 9.10 Treatment Planning System (Elekta, Sweden) for analysis. First, the average volumes and lengths of the CTVs were compared. Then, taking the standard CTV contoured by the expert as a reference (Vref), the two sets of CTVs delineated by the participants (Vstu) were compared with Vref for geometric comparison analysis. The indices used for comparison included the Dice similarity coefficient (DSC) index[12], inclusiveness index (IncI)[13], concordance index (CI)[14], and relative volume difference [ΔV (%)][13]. These indices were calculated for measuring the participants’ delineation accuracy relative to the standard contour. The definitions and formulas of the above indices are listed in Table 1. The DSC, IncI, and CI can vary between 0 and 1, where 0 means there is a complete disagreement between the Vstu and Vref, and 1 indicates perfect agreement. A ΔV (%) of 0 means that the Vstu is exactly the same as the Vref, and the higher the value, the greater the difference between the two volumes.
| Indices | Definition | Formula | |
| Indices for geometric comparison analysis | Dice similarity coefficient index | Intersection of Vstu and Vref divided by their average | 2 (Vstu ∩ Vref)/(Vstu + Vref) |
| Inclusiveness index | Intersection of Vstu and Vref divided by Vstu | (Vref ∩ Vstu)/Vstu | |
| Concordance index | Intersection of Vstu and Vref divided by their union | (Vref ∩ Vstu)/(Vref ∪ Vstu) | |
| Relative volume difference | Difference between Vstu and Vref divided by Vref and multiplied by 100 | (Vstu-Vref)/Vref × 100 | |
| Indices for interobserver variation | Maximum volume ratio | Ratio of the maximum volume to minimum volume contoured by the participants | Vmax/Vmin |
| Coefficient of variation | Standard deviation of the volumes contoured by the participants multiplied by 100 and divided by the mean value | SD × 100/mean |
Evaluation of IOV: The indices used for evaluating the IOV were the maximum volume ratio (MVR) and coefficient of variation (CV). The MVR expresses the greatest extent of the difference between the volumes, and the CV expresses the dispersion of volumes around the mean (see definitions and formulas in Table 1), with larger values representing greater variability and lower values suggesting higher consistency among the participants[15]. The MVR and CV were calculated to assess the impact of the education program on IOV.
Qualitative analysis of areas of variability: Qualitative analysis included the following four common controversies in the delineation of preoperative radiotherapy CTV for rectal cancer: (1) Should the upper boundary of the target volume start from the bifurcation of the abdominal aorta, the bifurcation of the common iliac artery or the superior border of the first sacral vertebrae? (2) Whether the external iliac area should be included; (3) Whether the groin area should be included; and (4) Whether the ischiorectal fossa should be included.
Continuous data were expressed as mean ± SD, and their normality of distribution was tested using the Shapiro-Wilk test. Comparisons were made using paired t-test when both groups of data had normal distribution, whereas the Wilcoxon singed-rank test was used when any group of data deviated from the normal distribution. Categorical variables were expressed as numbers (n) and percentages (%) and compared using the χ2 test or Fisher’s exact test. All the analyses were performed using SPSS for Windows (version 22.0, IBM Corp., Armonk, NY, United States). P values < 0.05 were considered statistically significant.
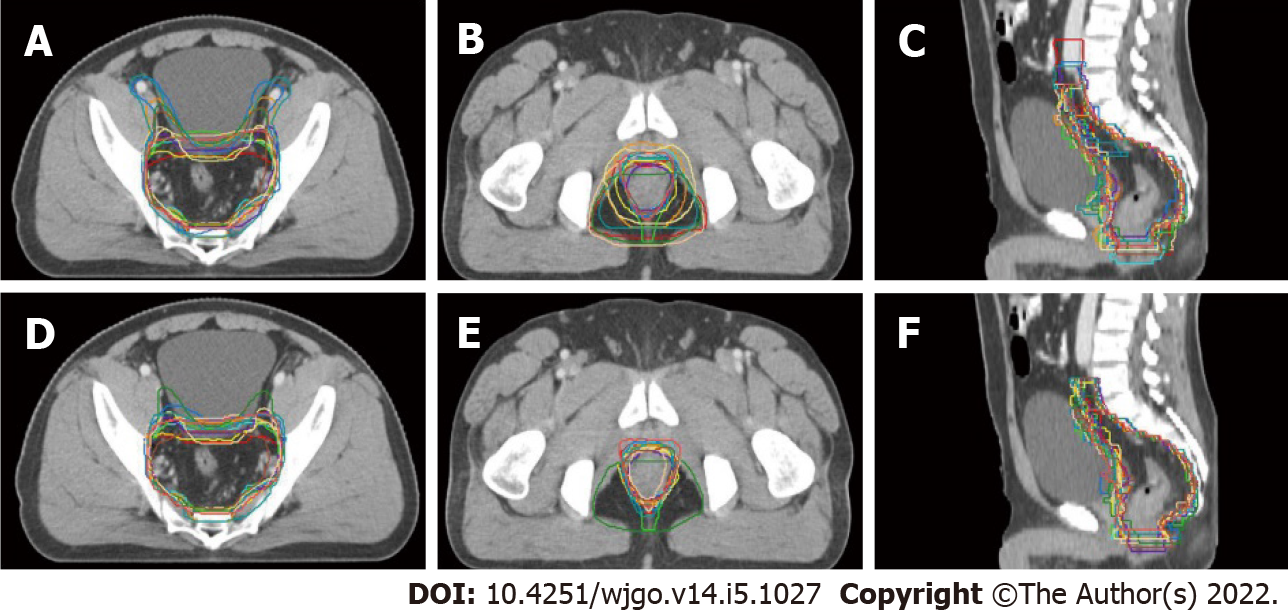
Although all the 18 radiation oncologists participating in the education program were asked to delineate two sets of CTVs, only 14 completed the baseline target volume delineation, and 13 submitted two sets of CTVs that could be used for analysis. Figure 1 displays the transverse and sagittal planes of the CTVs delineated by the 13 participants before and after the training sessions.
Table 2 shows the results of the quantitative analysis of the target volume parameters. After the education program, the average volume of the delineated CTVs decreased significantly from 809.82 ± 141.17 cm3 to 705.21 ± 100.53 cm3 (P = 0.001). However, no remarkable difference was observed in the average length of the delineated CTVs (18.19 ± 1.01 cm vs 17.77 ± 0.60 cm, P = 0.175). Regarding the indices for geometric comparison, the mean DSC, IncI, and CI increased significantly, while the ΔV (%) decreased remarkably, P values were 0.009, 0.002, 0.011, and 0.002, respectively.
| Indices | Before the education program | After the education program | t/Z value | P value |
| Volume (cm3) | 809.82 ± 141.17 (624.69-1112.79) | 705.21 ± 100.53 (603.97-949.53) | -3.180 | 0.001 |
| Length (cm) | 18.19 ± 1.01 (16.50-20.00) | 17.77 ± 0.60 (17.00-19.00) | 1.442 | 0.175 |
| DSC | 0.78 ± 0.06 (0.68-0.87) | 0.84 ± 0.04 (0.71-0.88) | -2.621 | 0.009 |
| IncI | 0.69 ± 0.10 (0.57-0.83) | 0.79 ± 0.08 (0.58-0.87) | -3.926 | 0.002 |
| CI | 0.65 ± 0.08 (0.52-0.77) | 0.73 ± 0.06 (0.56-0.78) | -2.551 | 0.011 |
| ΔV (%) | 30.79 ± 10.65 (17.33-47.65) | 21.43 ± 7.80 (12.93-41.70) | 3.926 | 0.002 |
The results of the comparison analysis for IOV are displayed in Table 3. The mean MVR decreased by 11.80% from 1.78 to 1.57, and the mean CV decreased by 18.19% from 17.43 to 14.26, demonstrating a smaller IOV in delineation after the education program.
| Vmax (cm3) | Vmin (cm3) | Mean (cm3) | SD (cm3) | MVR | CV | |
| Before the education program | 1112.79 | 624.69 | 809.82 | 141.17 | 1.78 | 17.43 |
| After the education program | 949.53 | 603.97 | 705.21 | 100.53 | 1.57 | 14.26 |
| Decrease ratio | 14.67% | 3.32% | 12.92% | 28.79% | 11.80% | 18.19% |
Table 4 shows the qualitative assessment of target volume variations before and after the education program. The greatest variations in the CTVs were observed at the external iliac area and the ischiorectal fossa; 61.54% of the participants (8/13) delineated the external iliac area and 53.85% of the participants (7/13) delineated the ischiorectal fossa unnecessarily at the baseline. However, after the education program, the proportion significantly decreased. Regarding the upper boundary, eight CTVs started from the bifurcation of the common iliac artery, three started from the bifurcation of the abdominal aorta or above, and two started from the superior border of the first sacral vertebrae at the baseline. After the education program, 12 CTVs started from the bifurcation of the common iliac artery, and one started from the superior border of the first sacral vertebrae. However, the difference was not statistically significant. The inguinal area was consistently excluded from the CTVs regardless of the education program.
| Parameters | Before the education program | After the education program | P value | ||
| Yes | No | Yes | No | ||
| CTV start from the bifurcation of the common iliac artery | 8 (61.54%) | 5 (38.46%) | 12 (92.31%) | 1 (7.69%) | 0.16 |
| Delineate external iliac area | 8 (61.54%) | 5 (38.46%) | 1 (7.69%) | 12 (92.31%) | 0.01 |
| Delineate inguinal area | 0 (0%) | 13 (100%) | 0 (0.00%) | 13 (100%) | NA |
| Delineate ischiorectal fossa | 7 (53.85%) | 6 (46.15%) | 1 (7.69%) | 12 (92.31%) | 0.03 |
This study confirmed the presence of wide variations in preoperative CTV contouring for rectal cancer among radiation oncologists from mainland China and indicated that a well-structured education program could improve delineation accuracy and reduce IOVs. To our knowledge, this is the first study to evaluate the impact of an education program on CTV delineation for rectal cancer.
The participants in this study represented the levels of major tertiary hospitals in China; all of them were attending physicians or above, and 72.22% had a master’s or doctor’s degree. They all had experience in rectal cancer radiotherapy, and half of them had delineated more than 30 cases of rectal cancer previously. However, our data showed a 1.8-fold variation in CTVs (range, 624.69-1112.79 cm3) at baseline. After the education program, the delineation accuracy of the participants relative to the standard contour improved remarkably and the IOV decreased. Besides, we found a statistically significant reduction in the average volume of the delineated CTVs. Qualitative analysis indicated that the larger CTV at baseline was associated with an inaccurate higher superior border as well as an inappropriate inclusion of the external iliac region and the ischiorectal fossa.
The 2009 version of the guidelines clearly stated that the most cephalad aspect of the CTV should be where the common iliac vessels bifurcate into the external/internal iliac vessels[6]. The 2016 version of the guidelines generally agreed on this point except for cases with T3N0 and circumferential resection margin (-) disease[7]. An approximate bony landmark is the sacral promontory, which is commonly used as the upper border of radiation fields in traditional two-dimensional radiotherapy. However, occasionally, these two anatomical locations are not equal; under that situation, the correct choice should be where the common iliac vessels bifurcate. Our study revealed that two participants still used the bony landmark as the upper border, and three participants mistakenly increased the CTV’s upper border to the bifurcation of the abdominal aorta at baseline. The external iliac region does not belong to the regional lymph nodes of rectal cancer. Elective irradiation of the external iliac region is only recommended for patients with positive obturator lymph nodes or T4b disease with anterior organ invasion[7]. The case in our study had clinical stage T3 without obturator lymph node metastasis; thus, the external iliac region was unnecessary to be included. Nevertheless, 61.54% of the participants (8/13) delineated this area at baseline. The variation in the delineation of the ischiorectal fossa may be related to the alteration in the guidelines recommendation. The 2006 version of the guidelines suggested the inclusion of the inferior pelvic subsite in the irradiated volume when the tumor is located within 6 cm from the anal margin[5]. However, currently, it is believed that inferior pelvic recurrences are more related to tumor spillage during inadequate surgical procedures[7]. Besides, irradiation of the ischiorectal fossa could increase the rate of perineal wound complications after abdominoperineal resection[16]. Therefore, the 2016 version of the guidelines suggested that ischiorectal fossa irradiation can be omitted unless the primary tumor directly invades this area or the external anal sphincter[7]. The case in our study was a low rectal cancer without ischiorectal fossa or external anal sphincter infiltration. Yet 53.85% of the participants (7/13) delineated this area at baseline. However, following the education program, delineation accuracy was improved for the above areas. These qualitative findings, which have not been demonstrated in previous studies, are notable and instructive for the clinical practice of radiation oncologists.
Why was the variability so large among the radiation oncologists? The reasons could be multifactorial. First, radiation oncologists might be unsure about which areas to delineate. Second, they might be unfamiliar with the anatomical borders of each lymphatic drainage area. Third, their knowledge might not have been updated following the publication of new guidelines. Fourth, there is incoherence between knowledge and practice. Considering the vast area of mainland China, the real variations among different levels of medical institutions may be much larger. The geometric inaccuracy in target volume delineation has proved to have a significant impact on dosimetric coverage of CTV, which probably affects the clinical outcomes[15]. Further, major multi-institutional clinical trials also require consistent delineation of the target area to ensure the accuracy of results in the correlation analysis among various dosimetric data and clinical outcomes. A key question is whether any effective measures could be adopted to reduce these variations.
Literature regarding interventions to reduce IOV in target volume delineation included the importation of additional imaging into the radiotherapy planning system, the implementation of auto-contouring systems, the introduction of standardized guidelines or protocols, and specific teaching interventions. The advances in imaging modalities can help us better distinguish the boundaries between tumors and normal tissues. The use of registered positron emission tomography scans improved gross target volume (GTV) contouring in lung cancer[17] and rectal cancer[18]. Registered MRI scans decreased IOV in target volume delineation for prostate cancer[19] and avoided inadvertent geographical misses during postoperative radiotherapy treatment planning for brain tumors[20]. However, these improvements were more associated with GTV rather than CTV. The implementation of auto-contour systems increased contouring accuracy and saved work time[13,21]. Nevertheless, even with the aid of a computer-assistant system, an accurate target volume delineation still requires the radiation oncologist’s own knowledge and judgement.
Nijkamp et al[9] found that a reduction of delineation variation in early-stage rectal cancer was achieved by establishing national consensus guidelines. The study of Fuller et al[8] revealed that including a visual atlas in addition to written instructions can improve conformance to a reference expert’s contours and reduce IOV. However, substantial residual variability still exists in rectal target volume delineation after atlas use[8]. One possible explanation is that the guidelines themselves are complex, which require considerable study and repeated practice before profound understanding and proficient application, especially for non-native English speakers[10]. After the residency program, most clinicians gain knowledge through reading literature or attending academic conferences. However, simply reading literature by themselves is not very effective, and general academic conferences do not include the skills of target volume delineation. Therefore, it is necessary to develop continuing education programs, such as the one in this study, to train clinicians on how to transform the guidelines into clinical practice.
When performing an education program, a simply didactic lecture is not sufficient; hands-on practical sessions and interactive communication are essential. Dewas et al’s[22] study revealed that didactic teaching did not significantly improve lung cancer delineation. The experience of Davis et al[23] suggested that a combination of didactic and interactive learning was more effective in changing clinicians’ practice than didactic sessions alone. Our specially designed education program organically integrated theoretical knowledge, clinical practice, and real-time feedback, provided two chances for target area delineation, and achieved favorable teaching effects in a relatively short period. This education program generated a positive response and has been incorporated into the national continuing education programs.
This study had several limitations, including the small sample size and only a single case for contouring. Furthermore, the long-term outcomes were not assessed; thus, it is unclear whether the education program is associated with lasting effects. Further studies need to include more participants and rule out possible selection biases resulting from a single patient and anatomic differences by tumor locations. Moreover, a prolonged follow-up period is needed to investigate the long-term effects of the education program.
Wide variations in the delineation of CTV for rectal cancer were present among radiation oncologists from mainland China. Inappropriate inclusion of the external iliac area and ischiorectal fossa were the two main issues in the CTV contouring. A well-structured education program could improve delineation accuracy and reduce IOVs. It is feasible to incorporate such a program into the continuing education programs for radiation oncologists.
Accurate target volume delineation is essential for precise radiotherapy. Inappropriate target volume may reduce local control or bring more normal tissue damage. However, defining a radiation field is not easy since it requires an integration of knowledge from multiple disciplines and rich clinical experience.
Previous studies have proved that wide variations in clinical target volume (CTV) delineation for rectal cancer were present among radiation oncologists despite the availability of several guidelines. Thus, how to improve the delineation accuracy and consistency has emerged as a key question in the era of precise radiotherapy. However, no study regarding the current situation of CTV delineation for rectal cancer is available in China, and there is also a lack of study on the impact of educational interventions on rectal cancer target delineation.
To examine the interobserver variation (IOV) in CTV delineation for rectal cancer among radiation oncologists in mainland China and evaluate whether an education program could improve the accuracy and consistency of delineation.
The study consisted of a baseline CTV delineation, a 150-min education intervention, and a follow-up CTV delineation. CTVs contoured by the participants before and after the program were obtained and compared. Quantitative evaluation included the indices for measuring the delineation accuracy of the participants relative to the standard contour and the indices for assessing IOV. Qualitative analysis included four common problems in CTV delineation.
Eighteen radiation oncologists from 10 provinces in China attended the education program and 13 of them completed two sets of CTVs. After the education program, a statistically significant reduction in the average volume of the delineated CTVs was detected (P = 0.001). The agreement between the participants’ delineation and the standard CTV improved remarkably and the IOV decreased. Qualitative analysis indicated that 61.54% of the participants (8/13) delineated the external iliac area, and 53.85% of the participants (7/13) delineated the ischiorectal fossa unnecessarily at the baseline, and the proportions reduced significantly after the program.
Our study first confirmed the wide variations in CTV delineation for rectal cancer among radiation oncologists from mainland China and proved that education interventions could improve the accuracy and consistency of delineation.
Further studies need to recruit more participants and include more cases for target volume delineation. Besides, the long-term effects of the education program also need to be investigated.
The authors would like to thank the radiation oncologists who participated in the study.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bustamante-Lopez LA, Brazil; Kang MK, South Korea; Paun VP, Romania; Sano W, Japan S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13206] [Article Influence: 1467.3] [Reference Citation Analysis (3)] |
| 2. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv22-iv40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1112] [Cited by in RCA: 1194] [Article Influence: 149.3] [Reference Citation Analysis (0)] |
| 3. | National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. Version 1. 2021. [cited 22 December 2020]. Available from: https://www.poijaya.org/wp-content/uploads/2021/08/rectal.pdf. |
| 4. | Mok H, Crane CH, Palmer MB, Briere TM, Beddar S, Delclos ME, Krishnan S, Das P. Intensity modulated radiation therapy (IMRT): differences in target volumes and improvement in clinically relevant doses to small bowel in rectal carcinoma. Radiat Oncol. 2011;6:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Roels S, Duthoy W, Haustermans K, Penninckx F, Vandecaveye V, Boterberg T, De Neve W. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65:1129-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Myerson RJ, Garofalo MC, El Naqa I, Abrams RA, Apte A, Bosch WR, Das P, Gunderson LL, Hong TS, Kim JJ, Willett CG, Kachnic LA. Elective clinical target volumes for conformal therapy in anorectal cancer: a radiation therapy oncology group consensus panel contouring atlas. Int J Radiat Oncol Biol Phys. 2009;74:824-830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 7. | Valentini V, Gambacorta MA, Barbaro B, Chiloiro G, Coco C, Das P, Fanfani F, Joye I, Kachnic L, Maingon P, Marijnen C, Ngan S, Haustermans K. International consensus guidelines on Clinical Target Volume delineation in rectal cancer. Radiother Oncol. 2016;120:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 161] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 8. | Fuller CD, Nijkamp J, Duppen JC, Rasch CR, Thomas CR Jr, Wang SJ, Okunieff P, Jones WE 3rd, Baseman D, Patel S, Demandante CG, Harris AM, Smith BD, Katz AW, McGann C, Harper JL, Chang DT, Smalley S, Marshall DT, Goodman KA, Papanikolaou N, Kachnic LA; Radiation Oncology Committee of the Southwest Oncology Group. Prospective randomized double-blind pilot study of site-specific consensus atlas implementation for rectal cancer target volume delineation in the cooperative group setting. Int J Radiat Oncol Biol Phys. 2011;79:481-489. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Nijkamp J, de Haas-Kock DF, Beukema JC, Neelis KJ, Woutersen D, Ceha H, Rozema T, Slot A, Vos-Westerman H, Intven M, Spruit PH, van der Linden Y, Geijsen D, Verschueren K, van Herk MB, Marijnen CA. Target volume delineation variation in radiotherapy for early stage rectal cancer in the Netherlands. Radiother Oncol. 2012;102:14-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Franco P, Arcadipane F, Trino E, Gallio E, Martini S, Iorio GC, Piva C, Moretto F, Ruo Redda MG, Verna R, Tseroni V, Bona C, Pozzi G, Fiandra C, Ragona R, Bertetto O, Ricardi U. Variability of clinical target volume delineation for rectal cancer patients planned for neoadjuvant radiotherapy with the aid of the platform Anatom-e. Clin Transl Radiat Oncol. 2018;11:33-39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 11. | Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471-1474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5537] [Cited by in RCA: 6457] [Article Influence: 430.5] [Reference Citation Analysis (0)] |
| 12. | Zou KH, Warfield SK, Bharatha A, Tempany CM, Kaus MR, Haker SJ, Wells WM 3rd, Jolesz FA, Kikinis R. Statistical validation of image segmentation quality based on a spatial overlap index. Acad Radiol. 2004;11:178-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 1068] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 13. | La Macchia M, Fellin F, Amichetti M, Cianchetti M, Gianolini S, Paola V, Lomax AJ, Widesott L. Systematic evaluation of three different commercial software solutions for automatic segmentation for adaptive therapy in head-and-neck, prostate and pleural cancer. Radiat Oncol. 2012;7:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 128] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 14. | Konert T, Vogel WV, Everitt S, MacManus MP, Thorwarth D, Fidarova E, Paez D, Sonke JJ, Hanna GG. Multiple training interventions significantly improve reproducibility of PET/CT-based lung cancer radiotherapy target volume delineation using an IAEA study protocol. Radiother Oncol. 2016;121:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Peng YL, Chen L, Shen GZ, Li YN, Yao JJ, Xiao WW, Yang L, Zhou S, Li JX, Cheng WQ, Guan Y, Xia HQ, Liu S, Zhao C, Deng XW. Interobserver variations in the delineation of target volumes and organs at risk and their impact on dose distribution in intensity-modulated radiation therapy for nasopharyngeal carcinoma. Oral Oncol. 2018;82:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Bullard KM, Trudel JL, Baxter NN, Rothenberger DA. Primary perineal wound closure after preoperative radiotherapy and abdominoperineal resection has a high incidence of wound failure. Dis Colon Rectum. 2005;48:438-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 272] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 17. | Morarji K, Fowler A, Vinod SK, Ho Shon I, Laurence JM. Impact of FDG-PET on lung cancer delineation for radiotherapy. J Med Imaging Radiat Oncol. 2012;56:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Whaley JT, Fernandes AT, Sackmann R, Plastaras JP, Teo BK, Grover S, Perini RF, Metz JM, Pryma DA, Apisarnthanarax S. Clinical utility of integrated positron emission tomography/computed tomography imaging in the clinical management and radiation treatment planning of locally advanced rectal cancer. Pract Radiat Oncol. 2014;4:226-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 19. | Villeirs GM, Van Vaerenbergh K, Vakaet L, Bral S, Claus F, De Neve WJ, Verstraete KL, De Meerleer GO. Interobserver delineation variation using CT vs combined CT + MRI in intensity-modulated radiotherapy for prostate cancer. Strahlenther Onkol. 2005;181:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Datta NR, David R, Gupta RK, Lal P. Implications of contrast-enhanced CT-based and MRI-based target volume delineations in radiotherapy treatment planning for brain tumors. J Cancer Res Ther. 2008;4:9-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Ma CY, Zhou JY, Xu XT, Guo J, Han MF, Gao YZ, Du H, Stahl JN, Maltz JS. Deep learning-based auto-segmentation of clinical target volumes for radiotherapy treatment of cervical cancer. J Appl Clin Med Phys. 2022;23:e13470. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 22. | Dewas S, Bibault JE, Blanchard P, Vautravers-Dewas C, Pointreau Y, Denis F, Brauner M, Giraud P. Delineation in thoracic oncology: a prospective study of the effect of training on contour variability and dosimetric consequences. Radiat Oncol. 2011;6:118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 23. | Davis D, O'Brien MA, Freemantle N, Wolf FM, Mazmanian P, Taylor-Vaisey A. Impact of formal continuing medical education: do conferences, workshops, rounds, and other traditional continuing education activities change physician behavior or health care outcomes? JAMA. 1999;282:867-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1549] [Cited by in RCA: 1463] [Article Influence: 56.3] [Reference Citation Analysis (0)] |