Published online May 15, 2022. doi: 10.4251/wjgo.v14.i5.1002
Peer-review started: November 9, 2021
First decision: December 12, 2021
Revised: December 26, 2021
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 15, 2022
Processing time: 182 Days and 4.7 Hours
Previous studies have suggested that a low albumin-to-alkaline phosphatase ratio (AAPR) is associated with a lower survival rate in patients with various malignancies. However, the relationship between pretreatment AAPR and the prognosis of patients with gastric cancer (GC) remains unclear.
To investigate the prognostic value of AAPR in distant metastatic GC.
A total of 191 patients with distant metastatic cancer from a single institute were enrolled in this study. Pretreatment clinical data, including serum albumin and alkaline phosphatase levels, were collected. A chi-square test or Fisher’s exact test was applied to evaluate the correlations between AAPR and various clinical parameters in GC patients. The Kaplan–Meier method and Cox proportional hazards regression model were used to evaluate the prognostic efficacy of AAPR in metastatic GC patients. A two-sided P value lower than 0.05 was considered statistically significant.
A receiver operating characteristic curve indicated that 0.48 was the optimal threshold value for AAPR. AAPR ≤ 0.48 was significantly associated with bone (P < 0.05) and liver metastasis (P < 0.05). Patients with high levels of AAPR had better survival in terms of overall survival (OS) and progression-free survival (PFS), regardless of the presence of liver/bone metastasis. Pretreatment AAPR was found to be a favorable predictor of OS and PFS based on a multivariate cox regression model. AAPR-M system, constructed based on AAPR and number of metastatic sites, showed superior predictive ability relative to the number of metastatic sites for predicting survival.
Pretreatment AAPR may serve as an independent prognostic factor for predicting PFS and OS in patients with metastatic GC. Furthermore, AAPR may assist clinicians with individualizing treatment.
Core Tip: Previous studies have suggested that a low albumin-to-alkaline phosphatase ratio (AAPR) is associated with inferior survival in patients with various malignancies. However, the relationship between pretreatment AAPR and the prognosis of patients with gastric cancer (GC) remains unclear. In this study, we showed that pretreatment AAPR was a favorable predictor of overall survival (OS) and progression-free survival (PFS) by the multivariate cox regression model with hazard ratios of 0.476 and 0.527, respectively. Pretreatment AAPR may serve as an independent prognostic factor for predicting PFS and OS in patients with metastatic cancer. Furthermore, AAPR may assist clinicians with individualizing treatment.
- Citation: Li YT, Zhou XS, Han XM, Tian J, Qin Y, Zhang T, Liu JL. Pretreatment serum albumin-to-alkaline phosphatase ratio is an independent prognosticator of survival in patients with metastatic gastric cancer. World J Gastrointest Oncol 2022; 14(5): 1002-1013
- URL: https://www.wjgnet.com/1948-5204/full/v14/i5/1002.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i5.1002
Despite the decline in incidence and mortality over the last decade[1], gastric cancer (GC) is still a severe threat to human health, especially in Eastern Asia, including China, Japan, and Korea[2]. Although more effective treatment regimens have been developed for patients with GC, the prognosis of this disease remains poor, especially for those with distant metastasis, and the 5-year overall survival (OS) rate is only 5.3%[3]. Currently, the recognized tumor-node-metastasis staging system does not provide accurate prognostic information and does not aid clinical decision-making for patients with metastatic cancers[4,5]. Therefore, low cost, easy to obtain, and reliable biomarkers are needed to accurately predict survival for patients with metastatic cancers.
Various biomarkers such as serum levels of programmed cell death ligand 1[6], the platelet-to-lymphocyte ratio[7], the neutrophil-to-lymphocyte ratio[8], and serum levels of high-density lipoprotein cholesterol[9], carcinoembryonic antigen, and carbohydrate antigen 19-9[10], are all currently used prognostic indicators for GC in patients. Nevertheless, the predictive powers of these respective markers are not conclusive and need further validation before being integrated into standard clinical practice. Hence, there is still an urgent need to identify precise predictors of survival for GC patients.
The albumin-to-alkaline phosphatase ratio (AAPR), which is calculated as albumin divided by alkaline phosphatase (ALP), has been shown to be closely associated with clinical outcomes in numerous types of cancer, including hepatocellular carcinoma (HCC)[11], cholangiocarcinoma[12], non-small cell lung cancer (NSCLC)[13,14], small cell lung cancer[15], nasopharyngeal carcinoma[16], and pancreatic ductal adenocarcinoma[17]. Recently, AAPR was found to be significantly decreased in patients with resectable GC, and low level AAPR predicted poor prognosis in GC[18]. However, as far as we know, the use of AAPR as a prognostic indicator of survival in metastatic GC patients has not yet been verified. Therefore, in this study, we focused on the association between AAPR and metastatic GC and evaluated its prognostic capability in patients with metastatic GC.
From May 2011 to September 2018, we retrospectively enrolled 191 patients diagnosed with distant metastatic GC at the Cancer Center of the Union Hospital of Huazhong University of Science and Technology (Wuhan, China). The inclusion criteria were as follows: the presence of pathologically proven GC; clinically diagnosed distant metastasis; absence of concurrent malignancies; and availability of pretreatment laboratory tests.
This study was retrospectively designed and in line with the Helsinki Declaration’s principles and followed existing national legislation. A waiver of informed consent was obtained for the study because this was a retrospective study, and anonymous analyses were employed in place to protect patient confidentiality, meaning there was minimal risk to the patients. The study was approved by the Institutional Ethical Board of Wuhan Union Hospital of Tongji Medical College, Huazhong University of Science and Technology.
Clinical data, such as age, sex, smoking status, sites of metastasis, and histopathology, were collected from the hospital medical system. Furthermore, laboratory data, including pretreatment serum levels of albumin and ALP, were collected from the hospital’s laboratory service. The AAPR was calculated by dividing the serum albumin by the serum ALP.
Follow-up was performed by a review of medical records and telephone conversation. The last follow-up date was January 31, 2019. The primary endpoints were OS and progression-free survival (PFS). OS refers to the interval between the dates of diagnosis to the date of death due to any cause or last follow-up. PFS was calculated from the date of diagnosis to the date of disease progression or the date of the last follow-up without evidence of progression.
All statistical analyses were performed using SPSS software version 23.0 (IBM, Chicago, IL, United States). Receiver operating characteristic (ROC) curve analysis was utilized to estimate the optimal cut-off value of AAPR. A chi-square test or Fisher’s exact test was applied to evaluate the correlations between AAPR and various clinical parameters. Propensity score matching was utilized to balance out selection biases. We employed a logistic regression model to estimate propensity scores for all patients. One-to-one nearest-neighbor matching was used between low and high-level AAPR using a 0.1 caliper width. The score-matched pairs were used in the subsequent analyses. Kaplan-Meier (K-M) method was applied to create survival curves using a log-rank test. We employed a Cox proportional hazards model to identify variables that affected the survival of patients with metastatic GC, using univariate and multivariate analyses. A two-sided P value lower than 0.05 was considered statistically significant.
From May 2011 to September 2018, a total of 191 patients with GC were recruited for our study. The demographics and clinical characteristics of the participants are presented in Table 1. The median age was 56 (range: 20-78) years, and 60 (31.4%) patients were older than 60 years. Among the patients, 105 (55.0%) were male and 45 (23.6%) had a history of smoking. The majority (57.1%) of these patients developed metastasis at only one site. There were 57 (29.8%) patients with liver metastasis and 24 (12.6%) patients with bone metastasis. Approximately half of the patients had poorly differentiated carcinoma (40.3%). A total of 146 (76.4%) patients received Taxane- or fluorouracil-based combination chemotherapy as a first-line treatment.
| Characteristics | Before propensity matching | After propensity matching | ||||
| AAPR ≤ 0.48 (n = 86) | AAPR > 0.48 (n = 105) | P value | AAPR ≤ 0.48 (n = 58) | AAPR > 0.48 (n = 58) | P value | |
| Gender | 0.265 | |||||
| Male | 56 | 49 | 0.011 | 32 | 26 | |
| Female | 30 | 56 | 26 | 32 | ||
| Age | ||||||
| ≤ 60 | 59 | 72 | 0.996 | 39 | 40 | 0.842 |
| > 60 | 27 | 33 | 19 | 18 | ||
| Smoking status | ||||||
| No | 61 | 85 | 0.018 | 44 | 48 | 0.359 |
| Yes | 25 | 20 | 14 | 10 | ||
| Number of involved sites | ||||||
| One | 41 | 68 | 0.018 | 30 | 24 | 0.264 |
| Multiple | 45 | 37 | 28 | 34 | ||
| Liver metastasis | ||||||
| No | 48 | 86 | 0.000 | 44 | 44 | 1.000 |
| Yes | 38 | 19 | 14 | 14 | ||
| Bone metastasis | ||||||
| No | 70 | 97 | 0.023 | 50 | 50 | 1.00 |
| Yes | 16 | 8 | 8 | 8 | ||
| Pathology | ||||||
| High differentiated | 3 | 1 | 0.201 | 1 | 0 | 0.334 |
| Moderately differentiated | 3 | 4 | 3 | 2 | ||
| Poorly differentiated | 31 | 46 | 17 | 25 | ||
| Signet ring cell | 13 | 25 | 9 | 13 | ||
| Others | 2 | 3 | 1 | 1 | ||
| Unknown | 34 | 26 | 27 | 17 | ||
| Treatment regimens | ||||||
| Combination chemotherapy | 63 | 83 | 0.520 | 45 | 44 | 0.378 |
| Fluorouracil or taxane alone | 11 | 7 | 7 | 3 | ||
| Radiotherapy | 1 | 2 | 1 | 2 | ||
| Best supportive care | 11 | 13 | 5 | 9 | ||
A ROC curve identified 0.48 as the optimal threshold value of AAPR (Supplementary Figure 1). The distribution of clinical characteristics between the two groups is listed in Table 1. AAPR ≤ 0.48 was significantly associated with bone (P = 0.023) and liver metastasis (P = 0.000). Higher AAPR values were more often observed in female patients, and patients who had metastasis in one site upon diagnosis. The median follow-up period was 8.9 (range, 1–62.13) months, and 41 patients were still alive at the last follow-up session. Fifty-eight pairs of patients were generated using propensity score matching who showed no significant differences (Table 1).
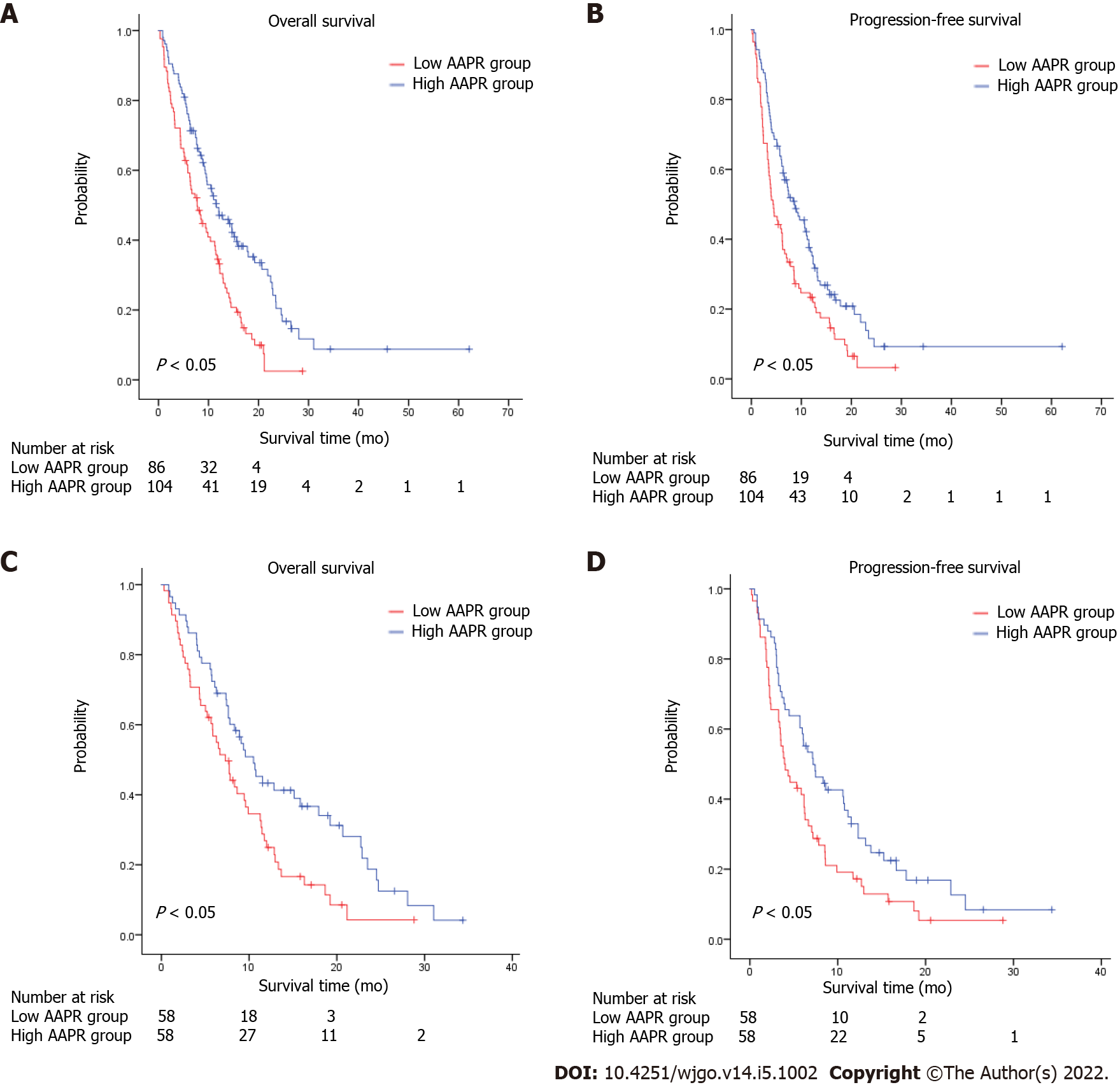
K-M survival analysis of AAPR for OS and PFS was also conducted as a preliminary evaluation of the prognostic capabilities of AAPR. This K-M analysis suggested that high AAPR values were correlated with longer OS [hazard ratio (HR) = 0.536, 95% confidence interval (CI) = 0.385–0.745, P < 0.05] and PFS (HR = 0.611, 95%CI = 0.446–0.837, P < 0.05) (Figure 1A and B). The median OS (mOS) and PFS values of patients with AAPR ≤ 0.48 were 7.73 and 4.37 months, respectively, which were significantly shorter compared with patients in the high AAPR group (> 0.48), which had a median OS and PFS of 11.57 and 8.63 months, respectively. Among propensity-matched pairs of patients, similar results were obtained for OS (HR = 0.634, 95%CI = 0.426–0.944, P < 0.05) and PFS (HR = 0.584, 95%CI = 0.385–0.884, P < 0.05) (Figure 1C and D).
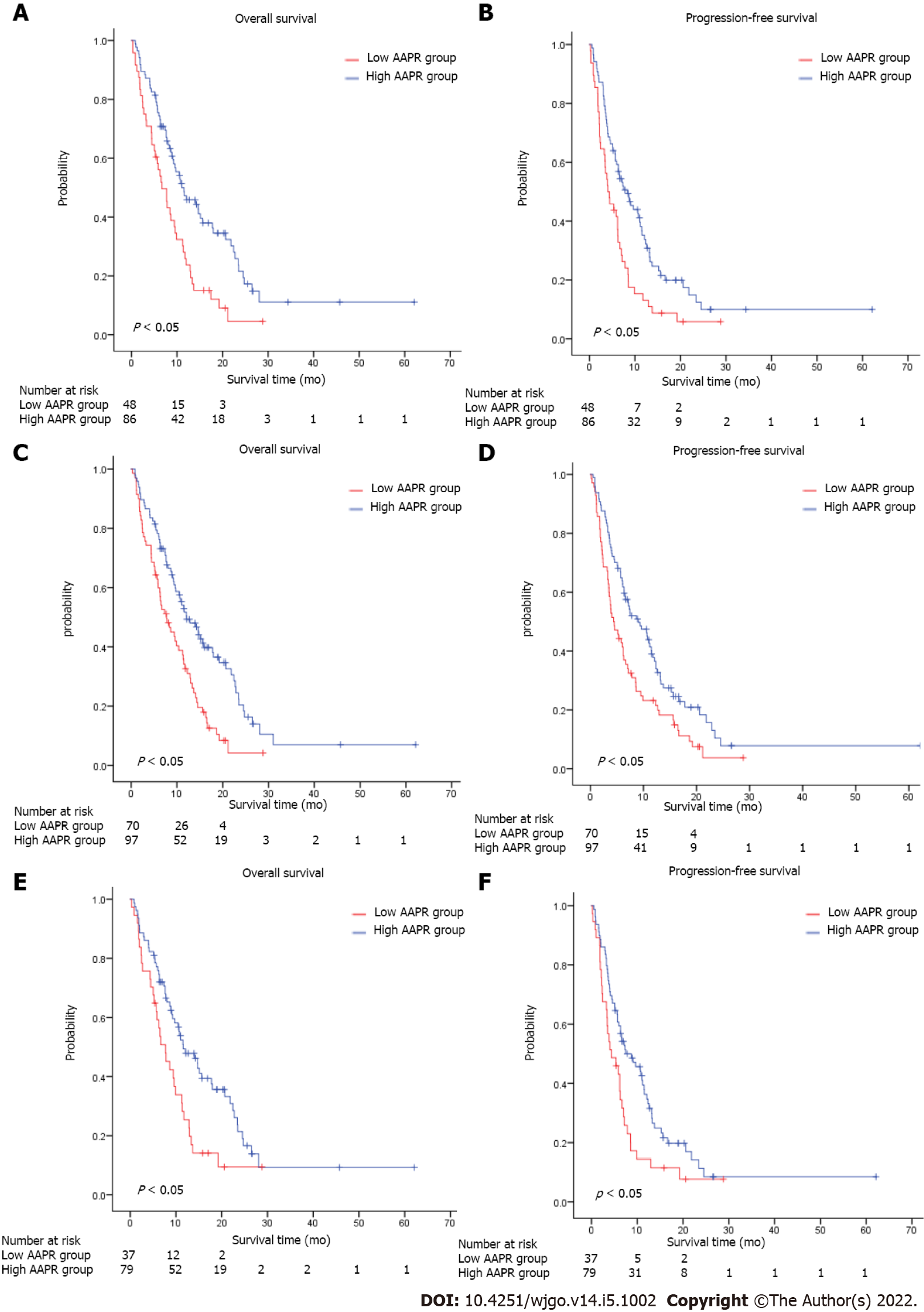
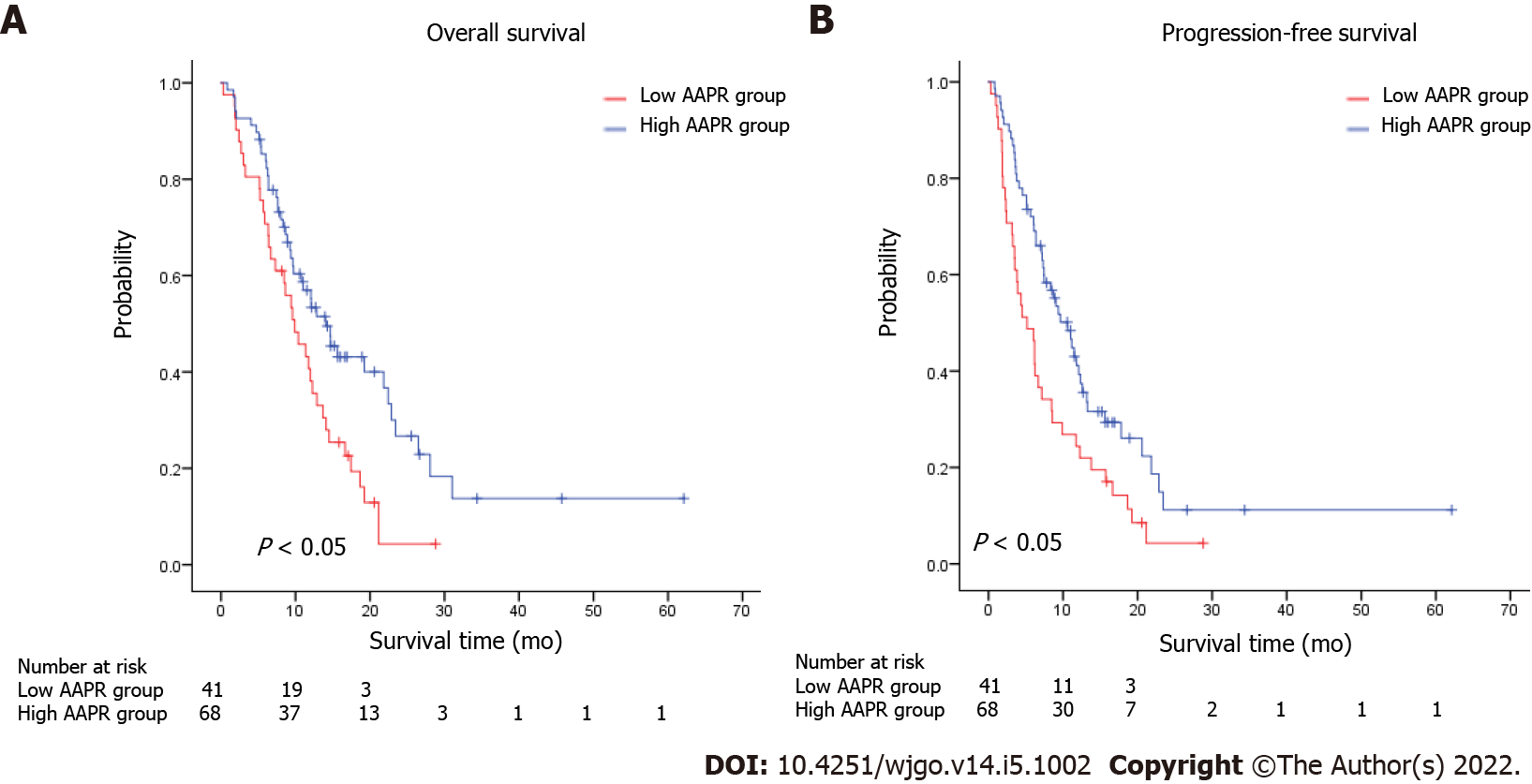
We conducted subgroup analyses to investigate the relationship between AAPR and survival according to the number of sites with metastasis, with or without bone/Liver metastasis. Patients with low AAPR values showed markedly worse OS (HR = 0.512, 95%CI = 0.344–0.763, P < 0.05) and PFS (HR = 0.553, 95%CI = 0.376–0.811, P < 0.05) compared with those with high AAPR values in the subgroup without liver metastasis (Figure 2A and B). Similarly, in the subgroup without bone metastasis, an AAPR ≤ 0.48 was significantly correlated with worse OS (HR = 0.522, 95%CI = 0.366–0.744, P < 0.05) and PFS (HR = 0.607, 95%CI = 0.433–0.850, P < 0.05) (Figure 2C and D). Not surprisingly, patients with high AAPR values had better OS and PFS in the subgroup without liver/bone metastasis (OS: HR = 0.541, 95%CI = 0.347–0.842, P < 0.05; PFS: HR = 0.589, 95%CI = 0.384–0.902, P < 0.05) (Figure 2E and F). In patients with one site of metastasis, AAPR > 0.48 was associated with better survival in terms of OS (HR = 0.540, 95%CI = 0.343–0.849, P < 0.05) and PFS (HR = 0.567, 95%CI = 0.370–0.869, P < 0.05) (Figure 3A and B). Patients receiving fluorouracil or taxane alone as a first-line treatment had a relative short mOS (mOS: 2.40 mo, 95%CI = 1.88–2.92) in the low AAPR group, which was much shorter than in the high AAPR group (mOS: 6.27 mo, 95%CI = 3.27–9.26) (Supplementary Table 1). In contrast, patients who received combination chemotherapy in the low AAPR group had better survival outcomes (mOS: 10.37 mo, 95%CI = 7.40–13.33) (Supplementary Table 1).
Univariate and multivariate analyses were conducted to estimate the predictive value of AAPR. As shown in Tables 2 and 3, univariate analysis demonstrated that high AAPR levels (HR = 0.611, 95%CI = 0.446–0.837, P < 0.05), combined chemotherapy as first-line treatment regimen (HR = 0.448, 95%CI = 0.313–0.639, P < 0.05), and only one metastasis site (HR = 1.484, 95%CI = 1.083–2.034, P < 0.05) were significantly associated with better PFS in patients with metastatic GC. Meanwhile, high AAPR levels (HR = 0.536, 95%CI = 0.385–0.745, P < 0.05), male (HR = 0.705, 95%CI = 0.510–0.975, P < 0.05), only one metastasis site (HR = 1.748, 95%CI = 1.264–2.417, P < 0.05), and combination chemotherapy as first-line treatment regimen (HR = 0.334, 95%CI = 0.232–0.480, P < 0.05) were determined to be favorable prognostic indicators of OS. Subsequent multivariate analyses revealed that AAPR was a significant predictor of both OS (HR = 0.476, 95%CI = 0.328–0.691, P < 0.05) and PFS (HR = 0.527, 95%CI = 0.370–0.751, P < 0.05) in patients with metastatic GC. AAPR > 0.48 was found to be associated with a favorable prognosis in patients with metastatic GC. Combination chemotherapy predicted better OS (HR = 0.269, 95%CI = 0.175–0.411, P < 0.05) and PFS (HR = 0.398, 95%CI = 0.263–0.594, P < 0.05) in patients with metastatic GC. We also determined that the number of metastatic sites involved was also an independent prognostic factor of OS (HR = 1.425, 95%CI = 1.018–1.997, P < 0.05).
| Variables | Univariate analyses | Multivariate analyses | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender (male vs female) | 0.705 (0.510-0.975) | 0.035 | 0.746 (0.508-1.094) | 0.133 |
| Age (> 60 vs ≤ 60) | 1.125 (0.794-1.594) | 0.509 | 0.808 (0.541-1.207) | 0.298 |
| Smoking status (Yes vs No) | 1.451 (0.970-2.173) | 0.070 | 1.364 (0.851-2.188) | 0.197 |
| Number of involved sites (multiple vs one) | 1.748 (1.264-2.417) | 0.001 | 1.425 (1.018-1.997) | 0.038 |
| Liver metastasis (Yes vs No) | 0.950 (0.674-1.339) | 0.731 | 0.758 (0.511-1.124) | 0.167 |
| Bone metastasis (Yes vs No) | 1.319 (0.822-2.115) | 0.251 | 1.395 (0.843-2.307) | 0.195 |
| Treatment regimens (combination chemotherapy vs others) | 0.334 (0.232-0.480) | 0.000 | 0.269 (0.175-0.411) | 0.000 |
| AAPR (> 0.48 vs ≤ 0.48) | 0.536 (0.385-0.745) | 0.000 | 0.476 (0.328-0.691) | 0.000 |
| Variables | Univariate analyses | Multivariate analyses | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender (male vs female) | 0.759 (0.554-1.041) | 0.087 | 0.772 (0.529-1.126) | 0.179 |
| Age (> 60 vs ≤ 60) | 1.162 (0.830-1.626) | 0.382 | 0.955 (0.654-1.395) | 0.813 |
| Smoking status (Yes vs No) | 0.805 (0.550-1.177) | 0.262 | 1.227 (0.781-1.927) | 0.375 |
| Number of involved sites (multiple vs one) | 1.484 (1.083-2.034) | 0.014 | 1.223 (0.880-1.701) | 0.231 |
| Liver metastasis (Yes vs No) | 0.950 (0.674-1.339) | 0.770 | 0.737 (0.506-1.073) | 0.112 |
| Bone metastasis (Yes vs No) | 1.219 (0.762-1.951) | 0.409 | 1.232 (0.754-2.011) | 0.405 |
| Treatment regimens (combination chemotherapy vs others) | 0.448 (0.313-0.639) | 0.000 | 0.398 (0.263-0.594) | 0.000 |
| AAPR (> 0.48 vs ≤ 0.48) | 0.611 (0.446-0.837) | 0.002 | 0.527 (0.370-0.751) | 0.000 |
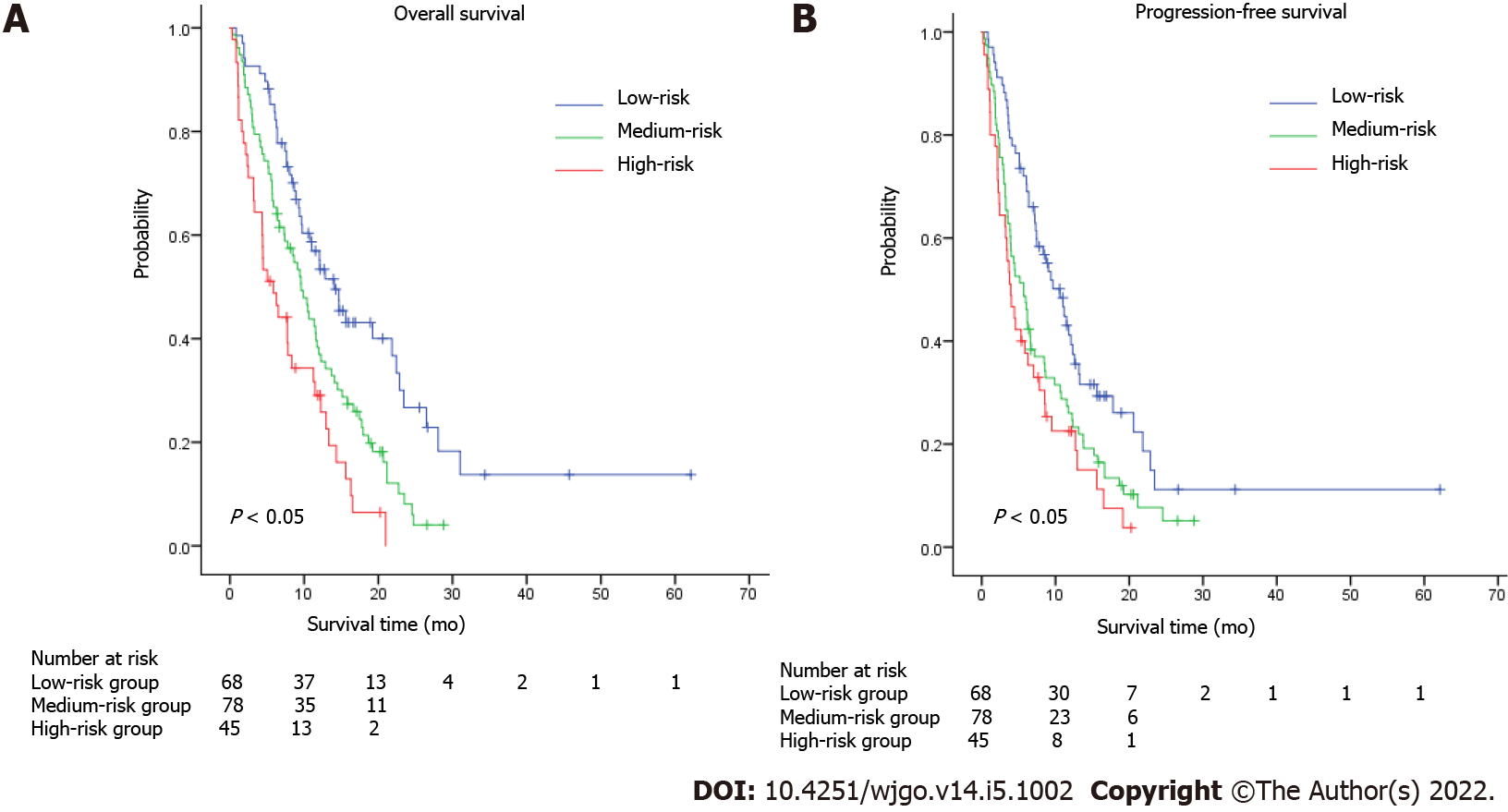
According to a previous study conducted in 2016, several factors were associated with worse prognosis, including age, carcinomatosis, and a larger burden of metastatic disease[19]. In our study, the number of metastatic sites was an independent prognostic factor. A combined model named AAPR-M was constructed based on AAPR and number of metastatic sites aimed at finding more prognostic factors for metastatic GC. We classified the patients into three groups according to this innovative AAPR-M system. Patients with high AAPR levels and only one metastatic site were assigned to a low-risk group, patients with more than one metastatic site and a low AAPR level were assigned to a high-risk group, while the others were grouped into a medium-risk group. Strong association with increased death and progression were documented for patients with high risk according to AAPR-M system. The median OS was 14.03 (95%CI: 10.32–17.75) vs 9.60 (95%CI: 7.59–11.61) vs 5.83 (95%CI: 3.18–8.48) months in the high, medium and low-risk groups, respectively (Figure 4A). Similar results were found in PFS (Figure 4B), suggesting that this AAPR-M system had a very strong predictive ability for survival in patients with metastatic GC.
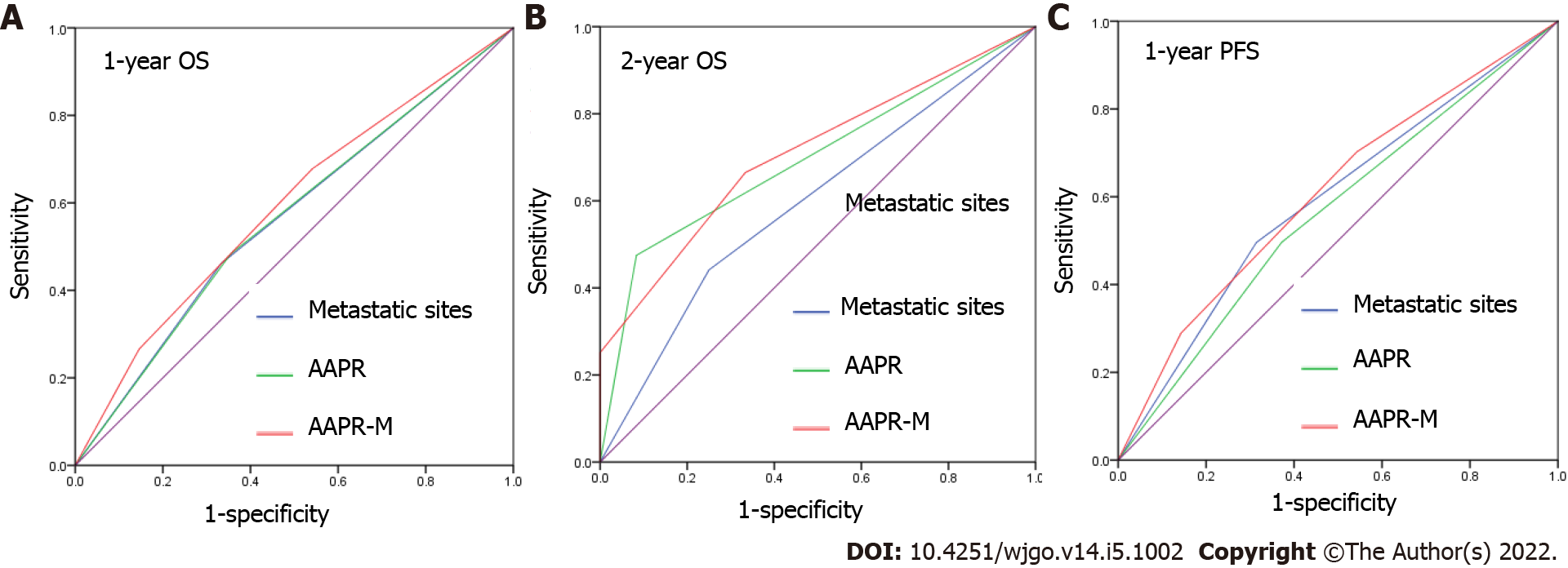
We applied area under the curve (AUC) values to compare the predictive ability between AAPR, the number of metastatic sites, and AAPR-M. AAPR-M showed greater AUC compared with the number of metastatic sites in terms of 1-year OS, 2-year OS, and 1-year PFS (Figure 5). The AAPR-M system had a larger χ2 value relative to the number of metastatic sites for 1-year OS (7.451 vs 6.071), 2-year OS (8.831 vs 1.779), and 1-year PFS (4.239 vs 2.454) prediction in likelihood ratio test analysis. This suggested that AAPR-M was superior to the number of metastatic sites for predicting survival.
This study evaluated the prognostic relevance of AAPR in patients with metastatic GC and, to the best of our knowledge, was the first study to focus on the relationship between the AAPR and prognosis in metastatic GC. This study demonstrated that smaller AAPR values were correlated with inferior clinical outcomes in terms of OS and PFS in patients with metastatic GC. Furthermore, multivariate analysis demonstrated that AAPR was an independent prognostic indicator of metastatic GC.
Albumin is one of the major plasma proteins that indicate an individual’s nutritional status. This protein is involved in maintaining intravascular oncotic pressure, scavenging free radicals, and maintaining steroid hormone hemostasis[20]. Previous studies have demonstrated that hypoalbuminemia is a prognostic indicator in colorectal cancer[21] and glioblastoma multiforme[22]. Notably, low levels of preoperative serum albumin are correlated with poor OS in GC patients after surgery[23], indicating that albumin is a prognostic biomarker for GC.
ALP is a ubiquitous membrane-bound glycoprotein that exist in several mammalian tissues such as liver, bone, and kidney[24]. Serum ALP is closely associated with the presence of liver and bone metastasis in malignant diseases[25-27]. Serum ALP is also an independent predictor of various cancers including breast cancer[28], nasopharyngeal carcinoma[29], prostate cancer[30], and GC[31].
As both albumin and ALP are prognostic indicators of survival in several types of cancer, Chan et al[32] derived the albumin-to-alkaline phosphatase ratio to put these two parameters together, and found that AAPR was a superior prognostic indicator compared with albumin and ALP alone in patients with HCC. Thereafter, the prognostic capability of AAPR has been verified in several types of cancer, and the majority of data indicates that low AAPR values are correlated with poorer survival[11,13,16]. Three meta-analyses were recently conducted and a consistent conclusion was drawn, namely, that cancer patients with higher AAPR levels have better survival than patients with lower levels[33-35]. However, the prognostic significance of pretreatment AAPR in GC remains unclear. We suspected, however, that AAPR would be a promising prognostic indicator in patients with metastatic GC.
Therefore, we used pretreatment AAPR as a predictor of survival in patients with metastatic GC. In our cohort, an AAPR lower than 0.48 was associated with more metastatic sites, as well as the presence of liver and bone metastasis. In accordance with our findings, Li et al[13] demonstrated that elevated AAPR values were more likely to be found in patients with one site of metastasis and advanced NSCLC. A significant correlation was also discovered between patients with liver/bone metastasis and lower AAPR values[13]. Hence, we speculated that high AAPR values at diagnosis may reflect a relatively less aggressive stage of metastatic GC.
Patients with high AAPR values had significantly longer OS and PFS compared to patients with low AAPR values in our study, suggesting that high AAPR values were correlated with favorable survival in metastatic GC. Furthermore, univariate and multivariate cox regression analyses revealed that AAPR was an independent predictor of survival in terms of OS and PFS in patients with metastatic GC. All of these results collectively suggest that AAPR is an excellent predictor of survival in patients with metastatic GC. We demonstrated that patients with low-level AAPR level received fluorouracil or taxane alone as first-line treatment had an mOS of less than 3 months, while patients received combination chemotherapy had an mOS more than 10 months. For patients with rapidly progressing cancers may aggravate in a short time, and loss the chance of treatment. It is particularly important to precisely confirm the gastric patients with advanced malignant disease who are expected to have a poor prognosis. These results gave us a hint that for patients with low AAPR was associated with poor prognosis, and stronger treatment regimens were needed to prolong survival time. Further analysis showed that AAPR-M system may serve as a supplementary strategy to further improve prognostic efficiency for metastatic GC.
However, this study had several limitations. First, this study was retrospective in design, and all of the data was collected from a single institution, which may have introduced bias in the results. Multicenter prospective studies are needed to verify and extend these findings. Second, the cut-off value of AAPR was obtained from a ROC curve in this study. To date, a consensus regarding the optimal threshold has not been reached, and external validation is required. Third, only pretreatment AAPR was adopted to evaluate its prognostic capability in metastatic GC; thus, whether dynamic AAPR is related to the prognosis of GC is unclear. Fourth, our conclusions were restricted by the small sample size, and high quality, large scale, prospective cohort studies are needed to validate these conclusions.
In conclusion, as far as we know, our study demonstrated that pretreatment AAPR is an independent prognostic indicator of both OS and PFS in patients with metastatic GC for the first time. Patients with high levels of pretreatment AAPR showed better survival compared with those with low levels. To verify the prognostic efficacy of AAPR, prospective studies are needed.
Previous studies have suggested that a low albumin-to-alkaline phosphatase ratio (AAPR) is associated with a lower survival rate in patients with various malignancies.
The predictive ability had been established in several malignancies, however, the relationship between pretreatment AAPR and the prognosis of patients with gastric cancer (GC) remains unclear.
To investigate the prognostic value of AAPR in distant metastatic GC.
From May 2011 to September 2018, we retrospectively enrolled 191 patients who were diagnosed with distant metastatic GC.
Patients with high levels of AAPR had better survival in terms of overall survival (OS) and progression-free survival (PFS), regardless of the presence of liver/bone metastasis. Pretreatment AAPR was found to be a favorable predictor of OS and PFS based on a multivariate cox regression model. AAPR-M system, constructed based on AAPR and number of metastatic sites, showed superior predictive ability relative to the number of metastatic sites for predicting survival.
Patients with high levels of pretreatment AAPR showed better survival compared with those with low levels.
Prospective studies are needed to verify the prognostic efficacy of AAPR.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: de Melo FF, Brazil; Mishra TS, India; Silano F, Brazil S-Editor: Wu YXJ L-Editor: A P-Editor: Wu YXJ
| 1. | Stock M, Otto F. Gene deregulation in gastric cancer. Gene. 2005;360:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55821] [Article Influence: 7974.4] [Reference Citation Analysis (132)] |
| 3. | Jim MA, Pinheiro PS, Carreira H, Espey DK, Wiggins CL, Weir HK. Stomach cancer survival in the United States by race and stage (2001-2009): Findings from the CONCORD-2 study. Cancer. 2017;123 Suppl 24:4994-5013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 112] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 4. | Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama W, Toh Y, Nagaie T, Takagi S, Yamamura Y, Yanaoka K, Orita H, Takeuchi M. S-1 plus cisplatin vs S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1422] [Article Influence: 83.6] [Reference Citation Analysis (0)] |
| 5. | Lordick F, Luber B, Lorenzen S, Hegewisch-Becker S, Folprecht G, Wöll E, Decker T, Endlicher E, Röthling N, Schuster T, Keller G, Fend F, Peschel C. Cetuximab plus oxaliplatin/Leucovorin/5-fluorouracil in first-line metastatic gastric cancer: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Br J Cancer. 2010;102:500-505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 6. | Takahashi N, Iwasa S, Sasaki Y, Shoji H, Honma Y, Takashima A, Okita NT, Kato K, Hamaguchi T, Yamada Y. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J Cancer Res Clin Oncol. 2016;142:1727-1738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 7. | Wang J, Qu J, Li Z, Che X, Liu J, Teng Y, Jin B, Zhao M, Liu Y, Qu X. Pretreatment platelet-to-lymphocyte ratio is associated with the response to first-line chemotherapy and survival in patients with metastatic gastric cancer. J Clin Lab Anal. 2018;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Zhang Y, Lu JJ, Du YP, Feng CX, Wang LQ, Chen MB. Prognostic value of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in gastric cancer. Medicine (Baltimore). 2018;97:e0144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Tamura T, Inagawa S, Hisakura K, Enomoto T, Ohkohchi N. Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. J Gastroenterol Hepatol. 2012;27:1635-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Sisik A, Kaya M, Bas G, Basak F, Alimoglu O. CEA and CA 19-9 are still valuable markers for the prognosis of colorectal and gastric cancer patients. Asian Pac J Cancer Prev. 2013;14:4289-4294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 11. | Li Q, Lyu Z, Wang L, Li F, Yang Z, Ren W. Albumin-to-Alkaline Phosphatase Ratio Associates with Good Prognosis of Hepatitis B Virus-Positive HCC Patients. Onco Targets Ther. 2020;13:2377-2384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Zhang F, Lu S, Tian M, Hu K, Chen R, Zhang B, Ren Z, Shi Y, Yin X. Albumin-to-Alkaline Phosphatase Ratio is an Independent Prognostic Indicator in Combined Hepatocellular and Cholangiocarcinoma. J Cancer. 2020;11:5177-5186. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Li D, Yu H, Li W. Albumin-to-alkaline phosphatase ratio at diagnosis predicts survival in patients with metastatic non-small-cell lung cancer. Onco Targets Ther. 2019;12:5241-5249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Zhou S, Jiang W, Wang H, Wei N, Yu Q. Predictive value of pretreatment albumin-to-alkaline phosphatase ratio for overall survival for patients with advanced non-small cell lung cancer. Cancer Med. 2020;9:6268-6280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Li X, Li B, Zeng H, Wang S, Sun X, Yu Y, Wang L, Yu J. Prognostic value of dynamic albumin-to-alkaline phosphatase ratio in limited stage small-cell lung cancer. Future Oncol. 2019;15:995-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Kim JS, Keam B, Heo DS, Han DH, Rhee CS, Kim JH, Jung KC, Wu HG. The Prognostic Value of Albumin-to-Alkaline Phosphatase Ratio before Radical Radiotherapy in Patients with Non-metastatic Nasopharyngeal Carcinoma: A Propensity Score Matching Analysis. Cancer Res Treat. 2019;51:1313-1323. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 17. | Zhang K, Dong S, Jing YH, Gao HF, Chen LY, Hua YQ, Chen H, Chen Z. Albumin-to-alkaline phosphatase ratio serves as a prognostic indicator in unresectable pancreatic ductal adenocarcinoma: a propensity score matching analysis. BMC Cancer. 2020;20:541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 18. | Wang Y, Xiong F, Yang J, Xia T, Jia Z, Shen J, Xu C, Feng J, Lu Y. Decreased albumin-to-alkaline phosphatase ratio predicted poor survival of resectable gastric cancer patients. J Gastrointest Oncol. 2021;12:1338-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 19. | Dixon M, Mahar AL, Helyer LK, Vasilevska-Ristovska J, Law C, Coburn NG. Prognostic factors in metastatic gastric cancer: results of a population-based, retrospective cohort study in Ontario. Gastric Cancer. 2016;19:150-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Esper DH, Harb WA. The cancer cachexia syndrome: a review of metabolic and clinical manifestations. Nutr Clin Pract. 2005;20:369-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 163] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 21. | Heys SD, Walker LG, Deehan DJ, Eremin OE. Serum albumin: a prognostic indicator in patients with colorectal cancer. J R Coll Surg Edinb. 1998;43:163-168. [PubMed] |
| 22. | Schwartzbaum JA, Lal P, Evanoff W, Mamrak S, Yates A, Barnett GH, Goodman J, Fisher JL. Presurgical serum albumin levels predict survival time from glioblastoma multiforme. J Neurooncol. 1999;43:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Liu BZ, Tao L, Chen YZ, Li XZ, Dong YL, Ma YJ, Li SG, Li F, Zhang WJ. Preoperative Body Mass Index, Blood Albumin and Triglycerides Predict Survival for Patients with Gastric Cancer. PLoS One. 2016;11:e0157401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Schoppet M, Shanahan CM. Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int. 2008;73:989-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Han KS, Hong SJ. Serum alkaline phosphatase differentiates prostate-specific antigen flare from early disease progression after docetaxel chemotherapy in castration-resistant prostate cancer with bone metastasis. J Cancer Res Clin Oncol. 2014;140:1769-1776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Kim JM, Kwon CH, Joh JW, Park JB, Ko JS, Lee JH, Kim SJ, Park CK. The effect of alkaline phosphatase and intrahepatic metastases in large hepatocellular carcinoma. World J Surg Oncol. 2013;11:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Zhang L, Gong Z. Clinical Characteristics and Prognostic Factors in Bone Metastases from Lung Cancer. Med Sci Monit. 2017;23:4087-4094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Chen B, Dai D, Tang H, Chen X, Ai X, Huang X, Wei W, Xie X. Pre-treatment serum alkaline phosphatase and lactate dehydrogenase as prognostic factors in triple negative breast cancer. J Cancer. 2016;7:2309-2316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Li G, Gao J, Tao YL, Xu BQ, Tu ZW, Liu ZG, Zeng MS, Xia YF. Increased pretreatment levels of serum LDH and ALP as poor prognostic factors for nasopharyngeal carcinoma. Chin J Cancer. 2012;31:197-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Li D, Lv H, Hao X, Hu B, Song Y. Prognostic value of serum alkaline phosphatase in the survival of prostate cancer: evidence from a meta-analysis. Cancer Manag Res. 2018;10:3125-3139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Namikawa T, Ishida N, Tsuda S, Fujisawa K, Munekage E, Iwabu J, Munekage M, Uemura S, Tsujii S, Tamura T, Yatabe T, Maeda H, Kitagawa H, Kobayashi M, Hanazaki K. Prognostic significance of serum alkaline phosphatase and lactate dehydrogenase levels in patients with unresectable advanced gastric cancer. Gastric Cancer. 2019;22:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Chan AW, Chan SL, Mo FK, Wong GL, Wong VW, Cheung YS, Chan HL, Yeo W, Lai PB, To KF. Albumin-to-alkaline phosphatase ratio: a novel prognostic index for hepatocellular carcinoma. Dis Markers. 2015;2015:564057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 33. | Guo X, Zou Q, Yan J, Zhen X, Gu H. Prognostic effect of pretreatment albumin-to-alkaline phosphatase ratio in human cancers: A meta-analysis. PLoS One. 2020;15:e0237793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Tian G, Li G, Guan L, Yang Y, Li N. Pretreatment albumin-to-alkaline phosphatase ratio as a prognostic indicator in solid cancers: A meta-analysis with trial sequential analysis. Int J Surg. 2020;81:66-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Xie H, Wei L, Tang S, Gan J. Prognostic Value of Pretreatment Albumin-to-Alkaline Phosphatase Ratio in Cancer: A Meta-Analysis. Biomed Res Int. 2020;2020:6661097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |