Published online Apr 15, 2022. doi: 10.4251/wjgo.v14.i4.887
Peer-review started: November 3, 2021
First decision: December 2, 2021
Revised: December 16, 2021
Accepted: March 5, 2022
Article in press: March 5, 2022
Published online: April 15, 2022
Processing time: 163 Days and 2 Hours
Hilar cholangiocarcinoma (HC) is a good adaptation certificate of hepatic arterectomy, and hepatic arterectomy is conductive to the radical resection of cholangiocarcinoma, which simplifies the operation and helps with a combined resection of the peripheral portal tissue. With continuous development of surgical techniques, especially microsurgical technique, vascular invasion is no longer a contraindication to surgery in the past 10 years. However, hepatic artery reconstruction after hepatic arterectomy has been performed to treat liver tumor in many centers with better results, but it is rarely applied in advanced HC.
To determine the prognosis of patients with advanced HC after hepatic artery resection and reconstruction.
A total of 98 patients with HC who underwent radical operation in our hospital were selected for this retrospective analysis. According to whether the patients underwent hepatic artery resection and reconstruction or not, they were divided into reconstruction (n = 40) and control (n = 58) groups. The traumatic indices, surgical resection margin, liver function tests before and after the operation, and surgical complications were compared between the two groups.
Operation time, blood loss, hospital stay, and gastrointestinal function recovery time were higher in the reconstruction group than in the control group (P < 0.05); The R0 resection rates were 90.00% and 72.41% in the reconstruction and control groups, respectively (P < 0.05). Serum alanine aminotransferase was lower in the reconstruction group on day one and three postoperatively, whereas serum aspartate aminotransferase was lower on the third day (P < 0.05). Preoperatively, the Karnofsky performance status scores were similar between the groups (P > 0.05), but was higher in the reconstruction group (P < 0.05) two weeks postoperatively. There was no difference in the complication rate between the two groups (27.50% vs 32.67%, P > 0.05). Two-year survival rate (42.50% vs 39.66%) and two-year survival time (22.0 mo vs 23.0 mo) were similar between the groups (P > 0.05).
Radical surgery combined with reconstruction after hepatic artery resection improves R0 resection rate and reduces postoperative liver injury in advanced HC. However, the operation is difficult and the effect on survival time is not clear.
Core Tip: Through retrospective analysis of 98 patients with hilar cholangiocarcinoma, we confirmed that radical surgery combined with reconstruction after hepatic artery resection can increase the R0 resection rate of advanced hilar cholangiocarcinoma and reduce postoperative liver injury. However, this operation is more difficult, and the impact on survival time is still unclear, and further follow-up studies are still needed.
- Citation: Li YM, Bie ZX, Guo RQ, Li B, Wang CE, Yan F. Effect of hepatic artery resection and reconstruction on the prognosis of patients with advanced hilar cholangiocarcinoma. World J Gastrointest Oncol 2022; 14(4): 887-896
- URL: https://www.wjgnet.com/1948-5204/full/v14/i4/887.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i4.887
Hilar cholangiocarcinoma (HC) is the most common type of cholangiocarcinoma, arising from the epithelium of the bile duct mucosa above the cystic duct opening[1-4]. Operation is the only measure to improve prognosis[5]. However, the procedure can be extremely challenging due to the anatomic parts involved and important adjacent structures[6,7]. Invasion of the hepatic artery corresponds to advanced disease, limiting radical resection and increasing the incidence of postoperative complications, such as abdominal bleeding, infection, and liver failure[8]. In some cases, compromise of the hepatic artery may not be due to true invasion, but due to compression of the artery by the enlarging tumor. Combined vascular resection in advanced HC is proposed by many authors, with notable rates of successful surgical outcomes[9]. How to effectively reduce the incidence of postoperative complications and improve radical cure has become a topic of intense clinical interest. Currently, there is a paucity of research about the impact of hepatic artery resection and reconstruction for advanced HC. If the hepatic artery is invaded, radical excision cannot be performed, and only palliative surgery is offered with the goal of symptom relief and definitive treatment[10,11]. Resection and reconstruction of the affected hepatic artery and portal vein can be combined with resection of the affected lateral liver, to improve surgical outcomes. Our study aimed to determine the effect of hepatic artery resection and reconstruction in patients with advanced HC, and to provide some clinical guidance.
A total of 98 patients with HC who underwent radical operation in our hospital from February 2015 to June 2018 were selected for this retrospective analysis. According to whether the patients underwent hepatic artery resection and reconstruction or not, they were divided into reconstruction (n = 40) and control (n = 58) groups. Inclusion criteria were as follows: (1) Age: 19-75 years; (2) American Society of Anesthesiologists Classification grade I-III; (3) HC diagnosed through abdominal computed tomography, magnetic resonance imaging, or endoscopic retrograde cholangiopancreatography; (4) Bismuth-Corlette type I-IV; (5) Follow up data for at least two years; and (6) Study plan that does not violate the requirements of relevant medical ethics. Exclusion criteria include: (1) Presence of concurrent extrahepatic malignancy; (2) Previous history of hepatobiliary surgery due to other reasons; (3) Serious comorbidities (cerebrovascular accident, acute myocardial infarction, etc.); and (4) Lack of data. The two groups were comparable in their baseline characteristics as shown in Table 1.
| Normal information | Reconstruction group (n = 40) | Regular group (n = 58) | t/χ2 value | P value |
| Age (yr) | 58.6 ± 7.0 | 59.0 ± 6.6 | -0.2877 | 0.77422 |
| Body mass index (kg/m2) | 22.6 ± 1.9 | 22.8 ± 2.3 | -0.4533 | 0.65132 |
| Sex | 1.319 | 0.251 | ||
| Male | 28 (70) | 34 (58.62) | ||
| Female | 12 (30) | 24 (41.38) | ||
| Surgical approach | 1.227 | 0.746 | ||
| Cholangiocarcinoma resection | 11 (27.5) | 22 (37.93) | ||
| Left liver + Caudate lobe | 13 (32.5) | 15 (25.86) | ||
| Right liver + Caudate lobe | 12 (30) | 16 (27.59) | ||
| Other types | 4 (10) | 5 (8.62) | ||
| Bismuth-Corlette type | 1.179 | 0.758 | ||
| I type | 14 (35) | 25 (43.1) | ||
| II type | 16 (40) | 21 (36.21) | ||
| III type | 8 (20) | 8 (13.79) | ||
| IV type | 2 (5) | 4 (6.9) | ||
| Regional lymph node metastasis | 0.764 | 0.382 | ||
| Yes | 18 (45) | 21 (36.21) | ||
| No | 22 (55) | 37 (63.79) | ||
| Adjuvant chemotherapy | 1.696 | 0.193 | ||
| Yes | 35 (87.5) | 55 (94.83) | ||
| No | 5 (12.5) | 3 (5.17) |
The control group underwent routine radical surgery. After general anesthesia, a reverse "L"-shaped incision was made below the right costal margin to explore the tumor, then the hepatic artery and portal vein under the duodenal ligament were separated. The common bile duct was cut open, and the gallbladder was dissociated and pulled upward together with the common bile duct. Nerves and lymph nodes were removed from top down to expose and skeletonize the hepatic artery and portal vein. The location and state of the tumor were evaluated, and invasion of the confluence of the hepatic and bile ducts was carefully determined. Liver lobectomy was performed, the contralateral bile duct was cut open at 0.5 cm of the tumor margin, and the hepatic artery and portal vein on the affected side were resected together with the lesion. Prophylactic antibiotics and hydration were administered as per postoperative protocol.
The reconstruction group underwent combined hepatectomy and hepatic artery reconstruction under general anesthesia. A reverse "L"-shaped incision was made below the right costal margin to explore the tumor, then the hepatic artery and portal vein under the duodenal ligament were separated. Sixteen groups of lymph nodes were examined after the abdominal resection, in order to determine whether there was metastasis to the peritoneum and liver. The bile duct was cut off behind the duodenum, the tumor was dissociated toward the head direction, and the lymph nodes and connective tissue of the hepatic duodenal ligament were completely removed. If the bile duct margin was negative, the dissected portal vein was resected and ligated. The liver was then cut through by a phacoemulsification suction knife. The opposite lateral bile duct was resected and biliary drainage tubes were inserted and fixed. The tumor and affected artery were resected together, and 70 Prolene sutures were used to reconstruct the hepatic artery through interrupted anastomosis. The reconstruction method was based on arterial invasion length, and included reconstruction using a great saphenous vein graft and in situ reconstruction after resection of the involved artery. If there was no evidence of bleeding within 24 h, 4000 IU of low-molecular-weight heparin sodium was administered subcutaneously once daily for 3-5 d. Antiplatelet therapy with clopidogrel was initiated after initiation of oral intake.
The operation time, operation blood loss, length of hospital stay, postoperative gastrointestinal function recovery time, R0 resection rate, serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL), Karnofsky performance status (KPS) score, surgical complications, two-year survival rate and survival time between the two groups were statistically analyzed.
Morning fasting venous blood samples (3 mL) were collected from all the participants and centrifuged at 3000 r/min at room temperature for 5 min to separate the serum. Hitachi 7170 automatic biochemical analyzer was used to measure the serum ALT, AST, and TBIL levels. Alanine aminotransferase was > 40 U/L, AST > 35 U/L, and TBIL > 22 μmol/L, which were considered abnormal[12,13].
The incidence of postoperative complications (including biliary fistula, liver failure, incision infection, urinary tract infection, pulmonary infection, etc.) was recorded in both groups.
Statistical analysis was carried out using SPSS 21.0. Measurement of data, such as operation time, operation blood loss, and length of hospital stay in the two groups were expressed as mean ± SD; t test was used for comparison between the two groups; χ2 test was used to compare categorical variables between the groups. The Kaplan-Meier method was used for survival analysis. P < 0.05 was considered statistically significant.
The operation time, operation blood loss, length of hospital stay, and postoperative gastrointestinal function recovery time in the reconstruction group were higher than those of the control group (P < 0.05, Table 2).
| Group | n | Operation time (min) | Surgical bleeding (mL) | Gastrointestinal function recovery time (h) | Hospital stay (d) |
| Reconstruction group | 40 | 11.36 ± 1.03 | 906.8 ± 155.3 | 73.6 ± 13.0 | 21.61 ± 3.9 |
| Regular group | 58 | 9.71 ± 0.81 | 720.3 ± 160.1 | 64.8 ± 11.0 | 19.3 ± 3.2 |
| t value | 8.863 | 5.737 | 3.612 | 3.210 | |
| P value | 0.000 | 0.000 | 0.000 | 0.002 |
The R0 resection was achieved in 90.00% of the reconstruction group and 72.41% of the control group, with a statistically significant difference (P < 0.05, Table 3).
| Group | n | R0 excision | Non-R0 resection |
| Reconstruction group | 40 | 36 (90.00) | 4 (10.00) |
| Regular group | 58 | 42 (72.41) | 16 (27.59) |
| χ2 | 4.507 | ||
| P value | 0.034 | ||
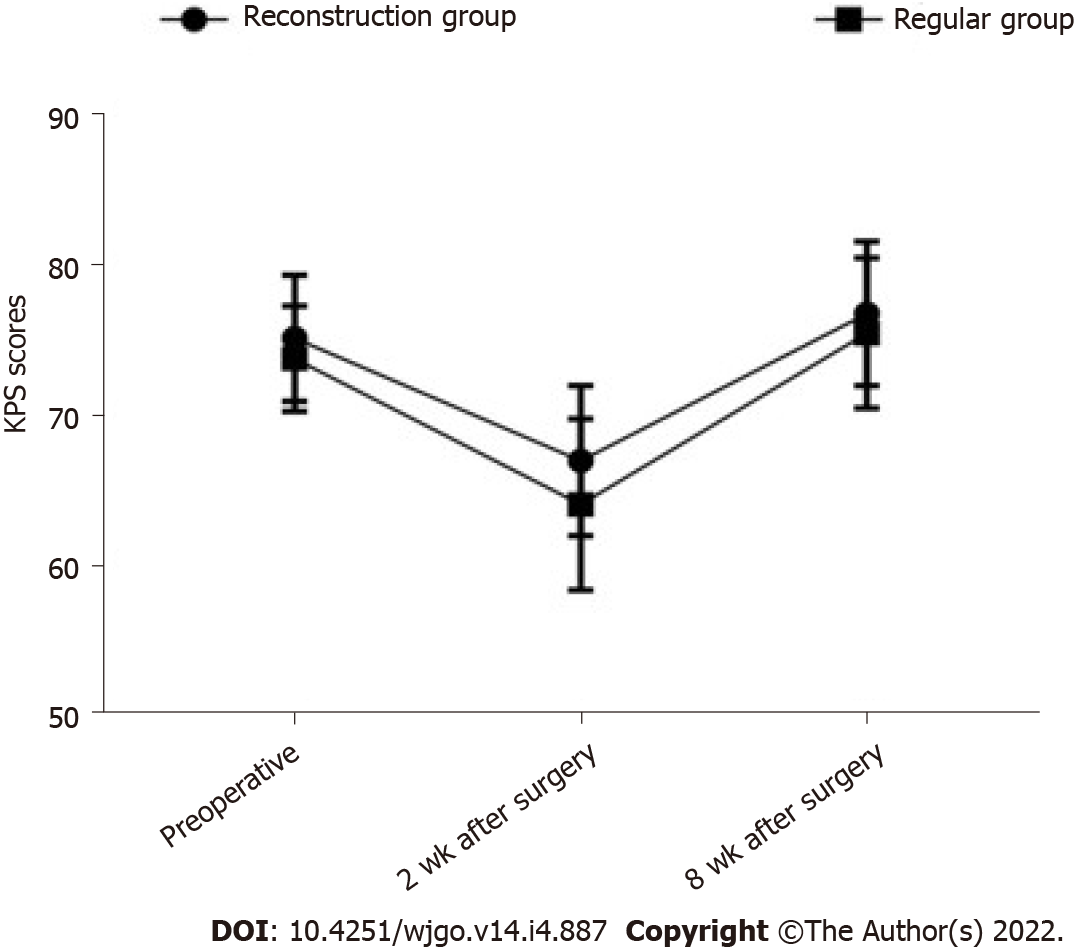
Before the operation, there was no significant difference in the KPS scores between groups (P > 0.05). Two weeks after the surgery, the KPS score of the reconstruction group was significantly higher than that of the control group (P < 0.05), as shown in Table 4 and Figure 1.
| Group | n | Preoperative | 2 wk after surgery | 8 wk after surgery |
| Reconstruction group | 40 | 75.1 ± 4.2 | 66.9 ± 5.0 | 76.7 ± 4.8 |
| Regular group | 58 | 73.7 ± 3.5 | 64.0 ± 5.7 | 75.4 ± 5.0 |
| χ2 | 1.793 | 2.600 | 1.286 | |
| P value | 0.076 | 0.011 | 0.202 |
The liver function indices of the two groups were compared before and after the operation. The results showed that the serum ALT on day one and three postoperatively, was lower in the reconstruction group than in the control group. The same was true for the serum AST three days postoperatively. The differences were statistically significant (P < 0.05), as shown in Table 5.
| Index | Preoperative | 1 d after operation | 3 d after operation |
| ALT (U/L) | |||
| Reconstruction group | 78.9 ± 19.6 | 451.2 ± 88.1 | 357.1 ± 93.0 |
| Regular group | 83.0 ± 20.1 | 489.8 ± 94.7 | 401.2 ± 85.0 |
| t value | -1.003 | -2.040 | -2.429 |
| P value | 0.319 | 0.044 | 0.017 |
| AST (U/L) | |||
| Reconstruction group | 83.5 ± 28.0 | 438.1 ± 93.0 | 395.8 ± 81.7 |
| Regular group | 80.0 ± 24.6 | 470.7 ± 87.2 | 441.5 ± 96.1 |
| t value | 0.654 | -1.770 | -2.456 |
| P value | 0.515 | 0.080 | 0.016 |
| TBIL (μmol/L) | |||
| Reconstruction group | 144.3 ± 35.1 | 122.2 ± 28.0 | 44.1 ± 13.0 |
| Regular group | 138.5 ± 37.3 | 118.0 ± 26.9 | 40.0 ± 12.5 |
| t value | 0.775 | 0.747 | 1.570 |
| P value | 0.440 | 0.457 | 0.120 |
There was no significant difference in the rate of surgical complications between the reconstruction (27.50%) and control (32.67%) groups (P > 0.05, Table 6).
| Group | n | Biliary fistula | Liver failure | Incision infection | Urinary tract infection | Lung infection | Complication rate, n (%) |
| Reconstruction group | 40 | 1 | 1 | 2 | 4 | 3 | 11 (27.50) |
| Regular group | 58 | 3 | 2 | 3 | 5 | 6 | 19 (32.76) |
| χ2 | 0.308 | ||||||
| P value | 0.579 |
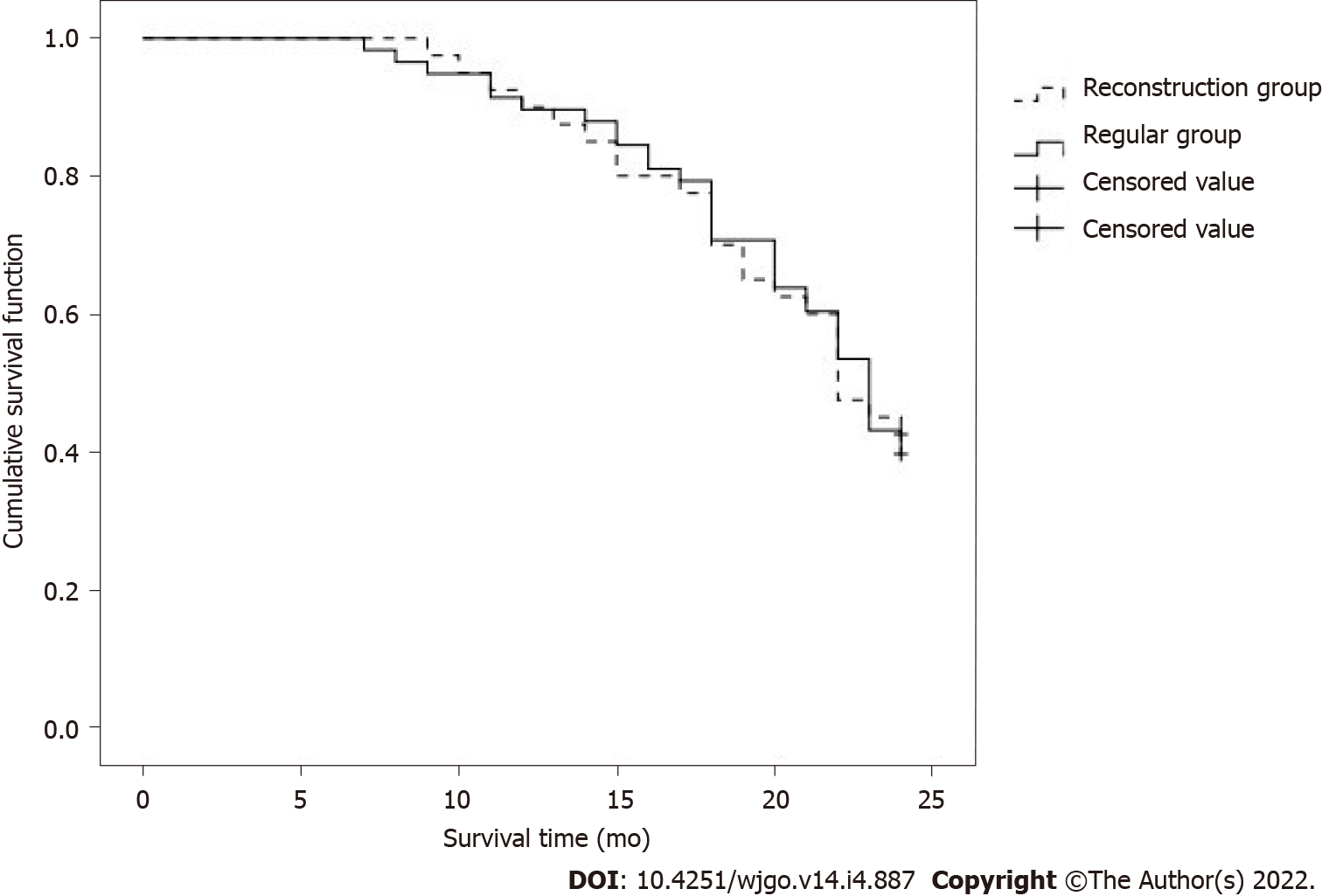
There was no significant difference between the two-year survival rate of 42.50% in the reconstruction group and 39.66% in the control group (P > 0.05), as shown in Table 7.
| Group | n | Survive | Death |
| Reconstruction group | 40 | 17 (42.50) | 23 (57.50) |
| Regular group | 58 | 23 (39.66) | 35 (60.34) |
| χ2 | 0.079 | ||
| P value | 0.778 | ||
There was no significant difference in the two-year survival time between the reconstruction group (22.0 mo) and the control group (23.0 mo) (P > 0.05), as shown in Figure 2.
According to our study, the R0 resection rate in the reconstruction group was higher than in the control group. Hilar cholangiocarcinoma has a higher vascular invasion rate, especially involving the hepatic artery. However, reconstruction after hepatic artery resection can improve the operational resection rate and achieve R0 resection. The combination of portal vein resection and remodeling does not increase the incidence of postoperative complications in HC. The compromise of hepatic artery patency due to tumor invasion, leads to progressive and chronic arterial hypoperfusion of the liver, causing a shift from the normal dual portal vein- and hepatic artery-dependent blood and oxygen supply to single portal vein-dependent supply. After the hepatic artery reconstruction, hepatic arterial blood supply can be fully restored; thus, preventing complications and possibly improving prognosis. Therefore, R0 resection of the tumor is key to a successful negative operational margin.
At present, the hilar vascular structure is relatively complicated, and the biological characteristic of HC is invasive growth. There are still controversies within the academic circles about radical operation of HC combined with hepatic artery resection and reconstruction. By the time HC patients are diagnosed, the liver tissues around the vascular and bile ducts are often invaded. Radical tumor resection is a common method for the treatment of HC. However, the invasion of the hilar blood vessels is a contraindication to surgical resection, making the rate of radical HC resection lower. In recent years, as the vascular anastomosis technology has matured, hilar vascular resection and reconstruction has been applied in the treatment of HC, which has expanded the surgical indications. In this type of surgery, studies have found [11-13] that the degree of tumor differentiation, whether there is lymph node metastasis, and whether there is peripheral nerve invasion are factors that affect the prognosis of HC patients. In addition to the above factors, this study also gives supplementary discussion. Our research results showed that the perioperative indicators of the reconstruction group are better than those of the conventional group, and the recovery of gastrointestinal function after surgery is also better. After the hepatic artery reconstruction, the blood and oxygen supply of the liver cells can be significantly improved. At the same time, it can effectively ensure the normal blood supply of the gastrointestinal tract, and play an important role in the recovery and regeneration of hepatocyte function after surgery, and the prevention and treatment of abnormal gastrointestinal function. Hepatic artery reconstruction is increasingly showing a trend of "precision". In this study, 70 prolene sutures were used to reconstruct the hepatic artery with intermittent anastomosis. The reconstruction method depends on the length of the patient's arterial invasion. Combined with perfect surgical management, surgical safety is well guaranteed. Postoperative complications are mainly related to operation time and surgical trauma. This study found that the reconstruction group’s plan was safe and feasible. Two weeks after the operation, the KPS score in the reconstruction group was higher than in the control group. A higher operational complication rate in the reconstruction group may be due to the advanced stage of the portal cholangiocarcinoma, as indicated by the involvement of the hepatic artery, which limits long-term efficacy of any treatment. The operational treatment of HC is very challenging, especially in advanced cases of vascular invasion[17]. Removal of the portal vein and hepatic artery requires higher surgical skill, relatively longer operation time, and increased blood loss. However, the hepatic artery is a large diameter muscular artery, which provides a good substrate for anastomosis, especially after the branches around the hepatic aorta have already been severed. Anatomic and physiologic variants of the artery need to be taken under consideration prior to determining anastomosis capability. In the right candidates, radical HC resection, combined with hepatic artery resection and reconstruction, can restore the dual blood supply to the liver, as early as possible, can reduce residual cancer tissue, improve resection and cure rate of advanced HC, as well as improve patients’ self-care ability.
Alanine amino transferase and AST can be used to evaluate the extent of hepatocellular injury, with the elevation of ALT being roughly parallel to the degree of cellular injury[18,19]. The vast majority of TBIL in the human body comes from aging red blood cells, which are metabolized by the liver. The TBIL is excreted by the bile ducts and enters the bile ducts at all levels, so it can be elevated upon hepatic or biliary tract injury[10,20]. This study found that postoperatively, the levels of ALT and AST in the reconstruction group were improved compared with those in the control group, and the liver damage in the reconstruction group was less. When patients with hilar cholangiocarcinoma undergo combined hepatic arterial resection, the blood supply of the liver will be affected because most patients with advanced HC have severe jaundice, and the jaundice further affects the blood supply of the Liver’s portal vein. The restoration of liver function is meaningful. The reasons for analyzing the results of this study include liver damage caused by advanced HC patients is different from liver cirrhosis. After the biliary obstruction is relieved, the liver function of advanced HC patients can gradually recover because in the radical operation of HC, the hepatic hilum is used as the cleaning target. Most of the branch connections between the arteries have also been severed. Free hepatoduodenal ligament can affect the hepatic artery collateral circulation in the hilar of the liver. After cutting off, the establishment of collateral circulation becomes difficult, and the hepatic artery becomes ischemic. Prolonged severe liver damage, postoperative liver function indicators (such as ALT and AST) increase, and hepatic artery blood supply are of great significance to the recovery of liver function in patients after radical surgery. The hepatic artery reconstruction implemented in the reconstruction group can increase the blood and oxygen supply of the hepatocytes, while ensuring the blood supply of the bile duct, which has less impact on liver function. Additionally, the reconstruction group first completed arterial resection and reconstruction, opened the blood flow of the hepatic artery, then blocked the portal vein, and completed the portal vein resection and reconstruction to reduce liver ischemic damage.
The application of hepatic artery resection in HC is still controversial. Studies have reported that unfavorable clinicopathological factors in patients with combined hepatic artery resection and reconstruction lead to a higher five-year mortality rate in patients after surgery. Whether reconstruction is necessary is also a dispute. Based on the results of this study, we believe that postoperative reconstruction should be a routine. The following points should be addressed upon reconstruction after hepatic artery resection: (1) The reserved hepatic blood vessels tend to adhere to the tumor. If the tumor cannot be completely removed, vascular reconstruction is required; (2) After resection of the two ends of the blood vessels, the reconstruction tension should be moderate; (3) Portal vein wedge resection and reconstruction can be tried, or alternatively, plastic reconstruction using other vascular branches near the hepatic artery can be attempted; and (4) If blood vessel invasion is detected, but the blood vessel cannot be removed successfully during the operation, it is recommended that the blood vessel be reserved. Because of our short follow up period, the long-term survival after radical operation combined with reconstruction after the hepatic arterectomy was not determined; therefore, more extended follow up is needed in future studies.
In conclusion, radical resection combined with hepatic artery reconstruction for advanced HC can lead to improved R0 resection rate and decrease liver injury, postoperatively. However, the procedure is more difficult, and the effect on survival time remains unclear. Further studies with larger sample sizes and prolonged observation times are needed.
Hilar cholangiocarcinoma (HC) is a good indication for hepatic artery resection, and hepatic artery resection is conducive to radical resection of cholangiocarcinoma. With the continuous development of surgical techniques, especially microsurgery techniques, vascular invasion is no longer a contraindication to surgery. Hepatic artery reconstruction after hepatic artery resection has been used in many centers to treat liver tumors, but it is rarely used in advanced HC.
This study provided treatment strategies for patients with advanced HC.
This study aimed to determine the prognosis of patients with advanced HC after hepatic artery resection and reconstruction.
A total of 98 patients with HC who underwent radical operation in our hospital were selected for retrospective analysis.
The operation time, blood loss, hospitalization time and gastrointestinal function recovery time of the reconstruction group were higher than those of the control group. The R0 resection rates of the reconstruction group and the control group were 90.00% and 72.41%, respectively. In the reconstruction group, serum alanine aminotransferase was lower on the 1st and 3rd day after operation, and serum aspartate aminotransferase was lower on the 3rd day. Although the preoperative Karnovsky performance status score was similar between the groups, it was higher in the reconstruction group 2 wk after the operation. There was no difference in the incidence of complications between the two groups. The 2-year survival rate and 2-year survival time were similar between the groups.
Radical surgery combined with reconstruction after hepatic artery resection improves R0 resection rate and reduces postoperative liver injury in advanced HC. However, the operation is difficult and the effect on survival time is not clear.
Hepatic artery resection may be more widely used in advanced HC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahn KS, South Korea; Kim R, United States; Nathan H, United States S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Giuliante F, Ardito F, Vellone M, Nuzzo G. Liver resections for hilar cholangiocarcinoma. Eur Rev Med Pharmacol Sci. 2010;14:368-370. [PubMed] |
| 2. | Nagino M, Nimura Y, Kamiya J, Kanai M, Uesaka K, Hayakawa N, Yamamoto H, Kondo S, Nishio H. Segmental liver resections for hilar cholangiocarcinoma. Hepatogastroenterology. 1998;45:7-13. [PubMed] |
| 3. | Shimizu H, Kimura F, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Miyazaki M. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg. 2010;251:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Li B, Xiong XZ, Zhou Y, Wu SJ, You Z, Lu J, Cheng NS. Prognostic value of lymphovascular invasion in Bismuth-Corlette type IV hilar cholangiocarcinoma. World J Gastroenterol. 2017;23:6685-6693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Rassam F, Roos E, van Lienden KP, van Hooft JE, Klümpen HJ, van Tienhoven G, Bennink RJ, Engelbrecht MR, Schoorlemmer A, Beuers UHW, Verheij J, Besselink MG, Busch OR, van Gulik TM. Modern work-up and extended resection in perihilar cholangiocarcinoma: the AMC experience. Langenbecks Arch Surg. 2018;403:289-307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 6. | Obulkasim H, Shi X, Wang J, Li J, Dai B, Wu P, Wang S, Wang X, Ding Y. Podoplanin is an important stromal prognostic marker in perihilar cholangiocarcinoma. Oncol Lett. 2018;15:137-146. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Bird NTE, McKenna A, Dodd J, Poston G, Jones R, Malik H. Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg. 2018;105:1408-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Komaya K, Ebata T, Yokoyama Y, Igami T, Sugawara G, Mizuno T, Yamaguchi J, Nagino M. Recurrence after curative-intent resection of perihilar cholangiocarcinoma: analysis of a large cohort with a close postoperative follow-up approach. Surgery. 2018;163:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 9. | Matsumoto T, Kubota K, Aoki T, Shimizu T, Mori S, Kato M, Asato H. A Novel Approach for Hepatic Arterial Reconstruction after Total Pancreatectomy with Common Hepatic Artery Resection Using Inferior Phrenic Artery. Dig Surg. 2019;36:99-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Sugiura T, Okamura Y, Ito T, Yamamoto Y, Ashida R, Ohgi K, Nakagawa M, Uesaka K. Left Hepatectomy with Combined Resection and Reconstruction of Right Hepatic Artery for Bismuth Type I and II Perihilar Cholangiocarcinoma. World J Surg. 2019;43:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Harada N, Yoshizumi T, Uchiyama H, Ikegami T, Itoh S, Takeishi K, Toshima T, Nagao Y, Yoshiya S, Mori M. Impact of middle hepatic artery reconstruction after living donor liver transplantation using the left lobe. Clin Transplant. 2020;34:e13850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Tsai CY, Watanabe N, Ebata T, Mizuno T, Kamei Y, Nagino M. Right hepatectomy for a detoured left hepatic artery in hilar cholangiocarcinoma-report of a rare but rational resection. World J Surg Oncol. 2016;14:288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Dai HS, Bie P, Wang SG, He Y, Li DJ, Tian F, Zhao X, Chen ZY. [Clinical application of combined hepatic artery resection and reconstruction in surgical treatment for hilar cholangiocarcinoma]. Zhonghua Wai Ke Za Zhi. 2018;56:41-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | De Lu C, Huang J, Wu SD, Hua YF, Javed AA, Fang JZ, Wang CN, Ye S. Total Hilar En Bloc Resection with Left Hemihepatectomy and Caudate Lobectomy: a Novel Approach for Treatment of Left-Sided Perihilar Cholangiocarcinoma (with Video). J Gastrointest Surg. 2017;21:1906-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Rhu J, Kim JM, Choi GS, David Kwon CH, Joh JW. Impact of Extra-anatomical Hepatic Artery Reconstruction During Living Donor Liver Transplantation on Biliary Complications and Graft and Patient Survival. Transplantation. 2019;103:1893-1902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Pitt HA, Nakeeb A, Abrams RA, Coleman J, Piantadosi S, Yeo CJ, Lillemore KD, Cameron JL. Perihilar cholangiocarcinoma. Postoperative radiotherapy does not improve survival. Ann Surg. 1995;221:788-97; discussion 797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 212] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 17. | Mizuno T, Ebata T, Yokoyama Y, Igami T, Yamaguchi J, Onoe S, Watanabe N, Kamei Y, Nagino M. Combined Vascular Resection for Locally Advanced Perihilar Cholangiocarcinoma. Ann Surg. 2022;275:382-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 18. | Nanashima A, Imamura N, Hiyoshi M, Yano K, Hamada T, Chiyotanda T, Nagatomo K, Hamada R, Ito H. A successfully resected case of left trisectionectomy with arterio-portal combined resection for advanced cholangiocarcinoma. Int J Surg Case Rep. 2018;53:90-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Li O, Yi W, Yang P, Guo C, Peng C. Relationship between serum MMP-9 level and prognosis after radical resection for Hilar cholangiocarcinoma patients. Acta Cir Bras. 2019;34:e201900409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Yoshikawa J, Kato Y, Shirakata Y, Sugioka A, Uyama I. Transpositional celiac artery graft: Novel graft selection for huge right hepatic artery reconstruction in left-sided hepatectomy for perihilar cholangiocarcinoma. Asian J Surg. 2021;44:562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |