Published online Apr 15, 2022. doi: 10.4251/wjgo.v14.i4.872
Peer-review started: September 7, 2021
First decision: December 4, 2021
Revised: December 30, 2021
Accepted: March 14, 2022
Article in press: March 14, 2022
Published online: April 15, 2022
Processing time: 219 Days and 19 Hours
The phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin (PI3K/Akt/mTOR) signalling pathway is crucial for cell survival, differentiation, apoptosis and metabolism. Xihuang pills (XHP) are a traditional Chinese preparation with antitumour properties. They inhibit the growth of breast cancer, glioma, and other tumours by regulating the PI3K/Akt/mTOR signalling pathway. However, the effects and mechanisms of action of XHP in hepatocellular carcinoma (HCC) remain unclear. Regulation of the PI3K/Akt/mTOR signalling pathway effectively inhibits the progression of HCC. However, no study has focused on the XHP-associated PI3K/Akt/mTOR signalling pathway. Therefore, we hypothesized that XHP might play a role in inhibiting HCC through the PI3K/Akt/mTOR signalling pathway.
To confirm the effect of XHP on HCC and the possible mechanisms involved.
The chemical constituents and active components of XHP were analysed using ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry (UPLC-Q-TOF-MS). Cell-based experiments and in vivo xenograft tumour experiments were utilized to evaluate the effect of XHP on HCC tumorigenesis. First, SMMC-7721 cells were incubated with different concentrations of XHP (0, 0.3125, 0.625, 1.25, and 2.5 mg/mL) for 12 h, 24 h and 48 h. Cell viability was assessed using the CCK-8 assay, followed by an assessment of cell migration using a wound healing assay. Second, the effect of XHP on the apoptosis of SMMC-7721 cells was evaluated. SMMC-7721 cells were stained with fluorescein isothiocyanate and annexin V/propidium iodide. The number of apoptotic cells and cell cycle distribution were measured using flow cytometry. The cleaved protein and mRNA expression levels of caspase-3 and caspase-9 were detected using Western blotting and quantitative reverse-transcription polymerase chain reaction (RT-qPCR), respectively. Third, Western blotting and RT–qPCR were performed to confirm the effects of XHP on the protein and mRNA expression of components of the PI3K/Akt/mTOR signalling pathway. Finally, the effects of XHP on the tumorigenesis of subcutaneous hepatocellular tumours in nude mice were assessed.
The following 12 compounds were identified in XHP using high-resolution mass spectrometry: Valine, 4-gingerol, myrrhone, ricinoleic acid, glycocholic acid, curzerenone, 11-keto-β-boswellic acid, oleic acid, germacrone, 3-acetyl-9,11-dehydro-β-boswellic acid, 5β-androstane-3,17-dione, and 3-acetyl-11-keto-β-boswellic acid. The cell viability assay results showed that treatment with 0.625 mg/mL XHP extract decreased HCC cell viability after 12 h, and the effects were dose- and time-dependent. The results of the cell scratch assay showed that the migration of HCC cells was significantly inhibited in a time-dependent manner by the administration of XHP extract (0.625 mg/mL). Moreover, XHP significantly inhibited cell migration and resulted in cell cycle arrest and apoptosis. Furthermore, XHP downregulated the PI3K/Akt/mTOR signalling pathway, which activated apoptosis executioner proteins (e.g., caspase-9 and caspase-3). The inhibitory effects of XHP on HCC cell growth were determined in vivo by analysing the tumour xenograft volumes and weights.
XHP inhibited HCC cell growth and migration by stimulating apoptosis via the downregulation of the PI3K/Akt/mTOR signalling pathway, followed by the activation of caspase-9 and caspase-3. Our findings clarified that the antitumour effects of XHP on HCC cells are mediated by the PI3K/Akt/mTOR signalling pathway, revealing that XHP may be a potential complementary therapy for HCC.
Core Tip: The study revealed that Xihuang pills (XHP) increases caspase-9 and caspase-3 activities by inhibiting the phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin signalling pathway and induces apoptosis and cell cycle arrest. Consequently, our study indicated that XHP inhibits the growth, migration, and proliferation of hepatocellular carcinoma (HCC) cells. Our study provides a better understanding of the antitumour effects of XHP and reveals the underlying mechanism. The findings of this study suggest that XHP might serve as a supplementary medicine in HCC treatment.
- Citation: Teng YJ, Deng Z, Ouyang ZG, Zhou Q, Mei S, Fan XX, Wu YR, Long HP, Fang LY, Yin DL, Zhang BY, Guo YM, Zhu WH, Huang Z, Zheng P, Ning DM, Tian XF. Xihuang pills induce apoptosis in hepatocellular carcinoma by suppressing phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin pathway. World J Gastrointest Oncol 2022; 14(4): 872-886
- URL: https://www.wjgnet.com/1948-5204/full/v14/i4/872.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i4.872
Hepatocellular carcinoma (HCC) is a primary liver cancer with a poor prognosis, and limited treatments are available for patients with advanced HCC[1]. The absence of specific clinical signs and symptoms makes the early diagnosis of HCC even more difficult[2]. Although diagnostic and therapeutic methods have improved in recent years, the efficacy of HCC treatments is only 30%–40%[3]. In addition, the toxicity and side effects associated with conventional treatments remain a clinical challenge that demands a prompt solution. Therefore, the development of effective antitumour drugs with reduced toxicity is needed. The carcinogenesis of HCC is regulated by several signalling pathways, and the phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin (PI3K/Akt/mTOR) pathway is one of the most important pathways[4].
The PI3K/Akt/mTOR signalling pathway is crucial for cell survival, differentiation, apoptosis and metabolism[5-7]. PI3K is a member of the lipid kinase family[8], and its activation initiates the expression of the second messenger phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3 induces the activation of multiple protein kinases, such as Akt. Activated Akt then facilitates cell differentiation, proliferation, metabolism, apoptosis and angiogenesis via the upregulation of several downstream effectors, including mTOR, B-cell lymphoma 2 (Bcl-2) family proteins, glycogen synthase kinase 3, S6 protein kinase, and caspase-9[3]. Activated caspase-9 induces the function of caspase-3 zymogen and induces apoptosis through proteolysis[9]. As a highly conserved mechanism of programmed cell death, apoptosis maintains tissue homeostasis. A reduction in apoptosis can induce the occurrence of tumours and promote their development[10]. PI3K/Akt/mTOR signalling, an apoptosis-related pathway[11], is often abnormally activated in HCC[4]. Therefore, apoptosis may be induced by suppressing the PI3K/Akt/mTOR pathway, which inhibits the proliferation of HCC cells.
Xihuang pills (XHP), a traditional Chinese antitumour prescription, are composed of four Chinese herbs, namely, Bos taurus domesticus Gmelin, Boswellia carteri Birdwood, Moschus berezovskii Flerov and Commiphora myrrha (Nees) Engl[12]. XHP exerts antitumour effects, reduces side effects, and improves the quality of life and survival rate of patients receiving tumour therapy[12]. Clinical studies have shown that XHP combined with chemotherapy effectively enhances the tumour response in patients with breast cancer, and reduces the toxicity and side effects of chemotherapy[13]. Several studies have confirmed that the antitumour activity of XHP depends on the PI3K-Akt-mTOR signalling pathway. Li et al[14] reported that XHP promotes apoptosis of Treg cells through the PI3K/Akt/AP-1 signalling pathway, improves the immunosuppressive state of the tumour microenvironment, and inhibits tumour growth. Fu et al[15] reported that XHP enhances the antitumour effect of temozolomide on glioblastoma-transplanted tumours through the Akt/mTOR pathway. According to Shao et al[16], XHP regulates the apoptosis of U-8MG glioblastoma cells through the ROS-mediated Akt/mTOR/FOXO1 pathway.
Collectively, the antitumour effect of XHP is associated with suppression of the PI3K/Akt/mTOR signalling pathway. Regulation of the PI3K/Akt/mTOR signalling pathway effectively inhibits the progression of HCC[4]. A growing number of studies have documented the antitumour effects of XHP on breast cancer, lung cancer, colon cancer, glioma, etc. However, few studies have been performed on HCC. No research has focused on the XHP-associated PI3K/Akt/mTOR signalling pathway. Therefore, we hypothesized that XHP might play a role in inhibiting HCC through the PI3K/Akt/mTOR signalling pathway. Our results confirm that XHP induces apoptosis and inhibits proliferation, both in vivo and in vitro. Moreover, this inhibitory effect depends on the PI3K/Akt/mTOR signalling pathway. Thus, this research revealed a potential antitumour effect of XHP on HCC cells.
XHP was purchased from Tong Ren Tang Technologies Co., Ltd. (Beijing, China, Lot number: 17043278). All study parameters fulfilled the requirements of standard quality. For extraction, XHP was soaked in 6 mL of double distilled water for 24 h, followed by ultrasonic dissolution. The precipitates were collected via centrifugation at 3000 rpm for 5 min and resuspended in 6 mL of dimethyl sulfoxide (DMSO; Sigma–Aldrich, Shanghai, China) using an ultrasonic dissolver. Another centrifugation step was performed, and the precipitates were collected. An XHP extract was then prepared using the obtained precipitates and stored at 4 °C for future use. According to the pharmacological dosage regulations of XHP, the daily dose of XHP for adults is 6 g/d, and the body weight of each mice is about 20 g. The dose of XHP was determined by dose extrapolation based on dose-body surface area normalization, this dosage was converted to 78 mg/kg[17].
The compounds present in XHP were analysed using high-resolution mass spectrometry (MS). XHP (3 g) was ground into a powder and extracted with 10 mL of 100% methanol, followed by ultrasonic extraction for 45 min. The supernatant was collected by centrifugation at 8000 rpm for 5 min and then filtered with a 0.22 μm microporous filter membrane. The filtrate was collected and measured using a UPLC-Q-TOF-MS (1290 UPLC-6540, Agilent Technologies Inc., United States) system. An Agilent ZORBAX Eclipse Plus C18 column (100 mm × 3.0 mm, 1.8 μm) was utilized for chromatographic separation. The mobile phase consisted of acetonitrile (A) and 0.1% formic acid, which were prepared as solutions with different gradients: 0–10 min containing 5%–15% A; 10–15 min containing 15%–20% A; 15–25 min containing 25%–45% A; and 25–40 min containing 45%–80% A. The flow rate of the mobile phase was 0.4 mL/min, and the injection volume was 1 μL/sample. Parameters for the MS system were fixed according to the manufacturer’s recommendation. In detail, the ionization mode, electrospray ionization and accurate mass data correction were performed using electrospray ionization-L Low Concentration Tuning Mix (G1969-85000). Positive and negative ion switching and the MRE scan model were used, and the mass range was set from 100 m/z to 1700 m/z. The electrospray capillary voltage was 4.0 kV. The sheath gas temperature and drying gas temperature were set to 350 °C. The drying gas (nitrogen) flow rate was 6.8 L/min. After the first mass analysis in full scan mode, a secondary MS scan was conducted in data-dependent mode, and collision-induced dissociation (CID) was used to fragment the first three strong peaks. Subsequently, the secondary scan data were obtained from 50 to 1000 m/z with fragmentation voltages of 10, 20, and 30 kV.
The SMMC-7721 HCC cell line was purchased from the Beinac Biotechnology Research Institute (Beijing, China) and was not contaminated, as determined by short tandem repeat (STR) identification. SMMC-7721 cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco, New York, United States) supplemented with 10% heat-inactivated foetal bovine serum (Gibco, New York, United States) and 1% (v/v) penicillin–streptomycin (Gibco, New York, United States). Cells were cultured in a humidified atmosphere containing 5% CO2 at 37 °C. When cells reached 80% confluence, they were treated with different concentrations of the XHP extract (0, 0.3125, 0.625, 1.25, and 2.5 mg/mL) for various durations (0 h, 6 h, 12 h, 24 h, and 48 h).
Cell Counting Kit-8 (CCK-8, Gibco, New York, United States) was used to measure cell viability. Briefly, 100 μL of suspended SMMC-7721 cells (1 × 105 cells/mL) in logarithmic growth phase were inoculated in 96-well plates. Subsequently, cells were treated with different concentrations of XHP extract (0, 0.625, 1.25, and 2.5 mg/mL) for different durations. Cells treated with the same volume of 0.1% DMSO and cultured for the same time were considered the control group. After an incubation for 0 h, 6 h, 12 h, 24 h, or 48 h, the absorbance was measured using a microplate reader (Awareness Stat Fax ®2600, Qingdao, China) at 450 nm. Cell viability was determined by comparing treated cells to controls. All assays were repeated at least three times.
SMMC-7721 cells were inoculated into 12-well plates after digestion. After spreading cells over the bottom of the plate, a pipette tip (1 mL) was used to create scratch wounds in the cell layer, ensuring that all wounds had a consistent width. The cell culture media were then aspirated, and cell debris created by the scratch were removed by rinsing the plate three times with phosphate-buffered saline (PBS). Then, serum-free culture medium was added to the plate, and an inverted fluorescence microscope (Olympus, type IX71) was used for imaging. The culture plate was placed in a cell incubator (Thermo) for 48 h to allow cell migration. The plate was removed from the incubator every 12 h for imaging. The assay results were analysed according to the collected imaging data.
Apoptosis was detected using the Annexin V-APC Apoptosis Detection Kit (KeyGEN, Nanjing, Jiangsu, China) according to the manufacturer’s instructions. Briefly, cells were digested with 0.25% trypsin (Beyotime Biotechnology Co., Ltd., Shanghai, China) after washes with PBS, followed by centrifugation at 3000 rpm for 5 min. The collected cells were washed with PBS twice and suspended in 500 μL of binding buffer. Then, 5 μL of fluorescein isothiocyanate-labelled annexin V-APC were added and evenly mixed with 5 μL of propidium iodide (PI) at room temperature for 15 min in the dark. Subsequently, a flow cytometer (Beckman Coulter, Brea, CA, United States) was used to quantify the apoptotic cells.
The DNA content and cell cycle distribution were measured using a FACSCalibur flow cytometer (Beckman Coulter, Brea, CA, United States). SMMC-7721 cells were inoculated in 6-well plates at a density of 5 × 105 cells/well. After a 24 h incubation, cells were treated with or without XHP extract for 48 h. Subsequently, the cells were fixed with 75% ethanol at 4 °C overnight, followed by washes with PBS. The cells were incubated at room temperature for 15–30 min in the dark and then analysed using flow cytometry.
Mouse tumour tissue specimens were obtained and lysed in RIPA lysis buffer (20 mmol/L HEPES, 1 mmol/L EDTA, 1% Triton X-100, 2 mmol/L EGTA, 150 mmol/L NaCl, 20 mmol/L phosphoglycerol, and 10% protease glycerol). The suspension was then homogenized and subsequently centrifuged at 12000 rpm for 15 min at 4 °C. A BCA detection kit (Beyotime, Shanghai, China) was used to determine the protein concentration. Polyacrylamide gel electrophoresis was used for protein separation Then, the proteins were transferred to a polyvinylidene fluoride membrane with a transfer device in cold buffer. A freshly prepared 5% skim milk solution was utilized for blocking at room temperature for 2 h. Afterwards, specific primary antibodies were incubated with the membrane at 4 °C overnight. The membrane was then washed with Tris-buffered saline supplemented with 0.1% Tween-20 (TBST) and incubated with peroxidase-conjugated secondary antibodies. Visualization was performed using the Super Sight West Pico Blotting kit (Pierce, Massachusetts, United States). The following primary antibodies were used: Cleaved caspase-3 (1:4000), cleaved caspase-9 (1:2000), Akt (1:2000), mTOR (1:2000), P-PI3K (1:1000), P-AKT (1:2000), and P-mTOR (1:2000), which were obtained from Abcam plc. (Cambridge, England). Antibodies against PI3K (1:5000) and β-actin (1:5000) were obtained from Proteintech Group, Inc. (Chicago, United States). Trypsin and PBS were purchased from HyClone Company (Logan, Utah, United States), and DMSO was obtained from Sigma (Germany).
Total RNA was extracted from SMMC7721 cells using TRIzol reagent (Takara) according to the manufacturer’s instructions. Reverse transcription was then performed using reverse transcriptase to produce cDNA templates. The quantitative reverse-transcription polymerase chain reaction (RT–qPCR) conditions were set up as follows: 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 30 s. The following primers were used: F-TGGCAACAGAATTTGAGTCCT and R-ACCATCTTCTCACTTGGCAT for caspase 3, F-AAGCCAACCCTAGAAAACCTTACCC and R-AGCACCGACATCACCAAATCCTC for caspase-9, F-TGCGTCTACTAAAATGCATGG and R-AACTGAAGGTTAATGGGTCA for PI3K, F-AGCCCTGGACTACCTGCACTCG, R-CTGTGATCTTAATGTGCCCGTCCT for AKT, and F-CCAAAGGCAACAAGCGATCCCGAA and R-CTCCAAGTTCCACACCGTCCA for mTOR.
All laboratory animals were carefully monitored, and the animal experiments were reviewed and approved by the Ethical Review Committee of Experimental Animal Welfare at Central South University. Additionally, experiments were performed according to the European Community guidelines for laboratory animal use and care. All animals were housed under specific pathogen-free conditions. Subcutaneous xenograft tumours were established by subcutaneously injecting SMMC7721 cells (1 × 107 cells/mouse) (Shanghai SLAC Laboratory Animal Co., Ltd., Shanghai, China) into 5-week-old male BALB/c nude mice. Mouse weights and tumour volumes were measured every other day using the formula for an ellipsoid (length × width2 × 0.5). When the tumour volume reached approximately 100 mm3, mice were randomly classified into two groups (n = 5 mice per group). Mice in the control group received oral administration of distilled water daily, whereas mice in the XHP group received XHP extract (78 mg/kg body weight/day) by oral gavage. After 2 wk, mice were sacrificed to investigate the effect of XHP on subcutaneous xenograft tumours.
All statistical calculations were performed using GraphPad Prism 7 software (GraphPad Software Company, United States). Data are presented as the means ± SD. One-way analysis of variance (ANOVA) followed by the least significant difference test were conducted to analyse the differences between two groups. A P < 0.05 was considered statistically significant.
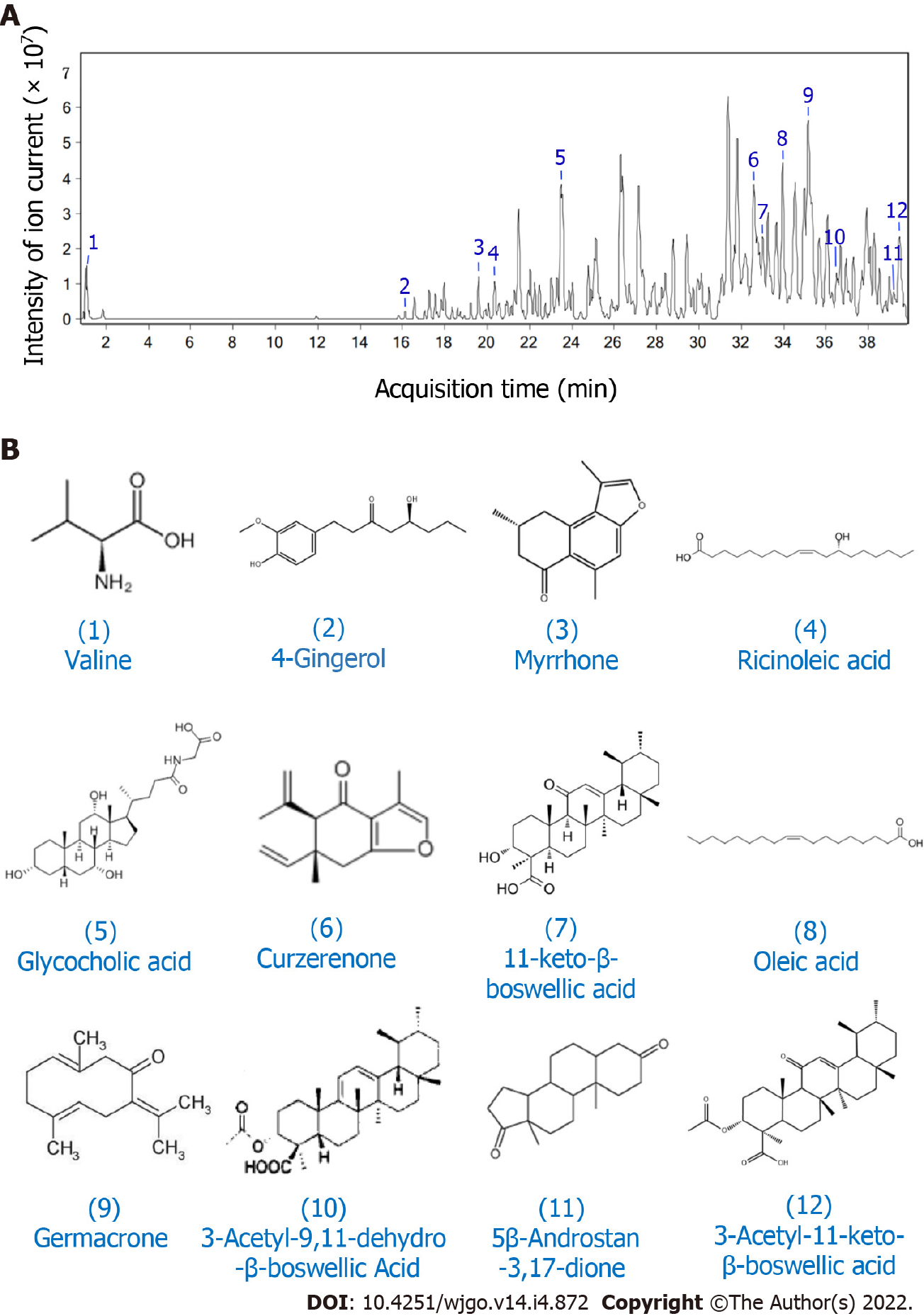
The main components of XHP responsible for its inhibitory effects on HCC were analysed using high-resolution MS. Ion flow diagrams were extracted, and the molecular formulas of the compounds were compared with the information in the literature and database for identification (Figure 1). The following 12 compounds were identified: Valine, 4-gingerol, myrrhone, ricinoleic acid, glycocholic acid, curzerenone, 11-keto-β-boswellic acid, oleic acid, germacrone, 3-acetyl-9,11-dehydro-β-boswellic acid, 5β-androstane-3,17-dione, and 3-acetyl-11-keto-β-boswellic acid (Table 1).
| Number | RT (min) | Mass | Molecular formula | Name |
| 1 | 1.088 | 117.0778 | C5H11NO2 | Valine |
| 2 | 16.110 | 267.1588 | C15H22O4 | 4-Gingerol |
| 3 | 19.579 | 228.1131 | C15H16O2 | Myrrhone |
| 4 | 20.357 | 321.2403 | C18H34O3 | Ricinoleic acid |
| 5 | 23.486 | 465.3060 | C26H43NO6 | Glycocholic acid |
| 6 | 32.635 | 231.1366 | C15H18O2 | Curzerenone |
| 7 | 33.057 | 470.3377 | C30H46O4 | 11-keto-β-boswellic acid |
| 8 | 33.977 | 282.4610 | C18H34O2 | Oleic acid |
| 9 | 35.078 | 218.1674 | C15H22O | Germacrone |
| 10 | 36.543 | 496.3517 | C32H48O4 | 3-Acetyl-9,11-dehydro-β-boswellic Acid |
| 11 | 38.816 | 288.2084 | C19H28O2 | 5β-androstane-3,17-dione |
| 12 | 39.500 | 512.3485 | C32H48O5 | 3-Acetyl-11-keto-β-boswellic acid |
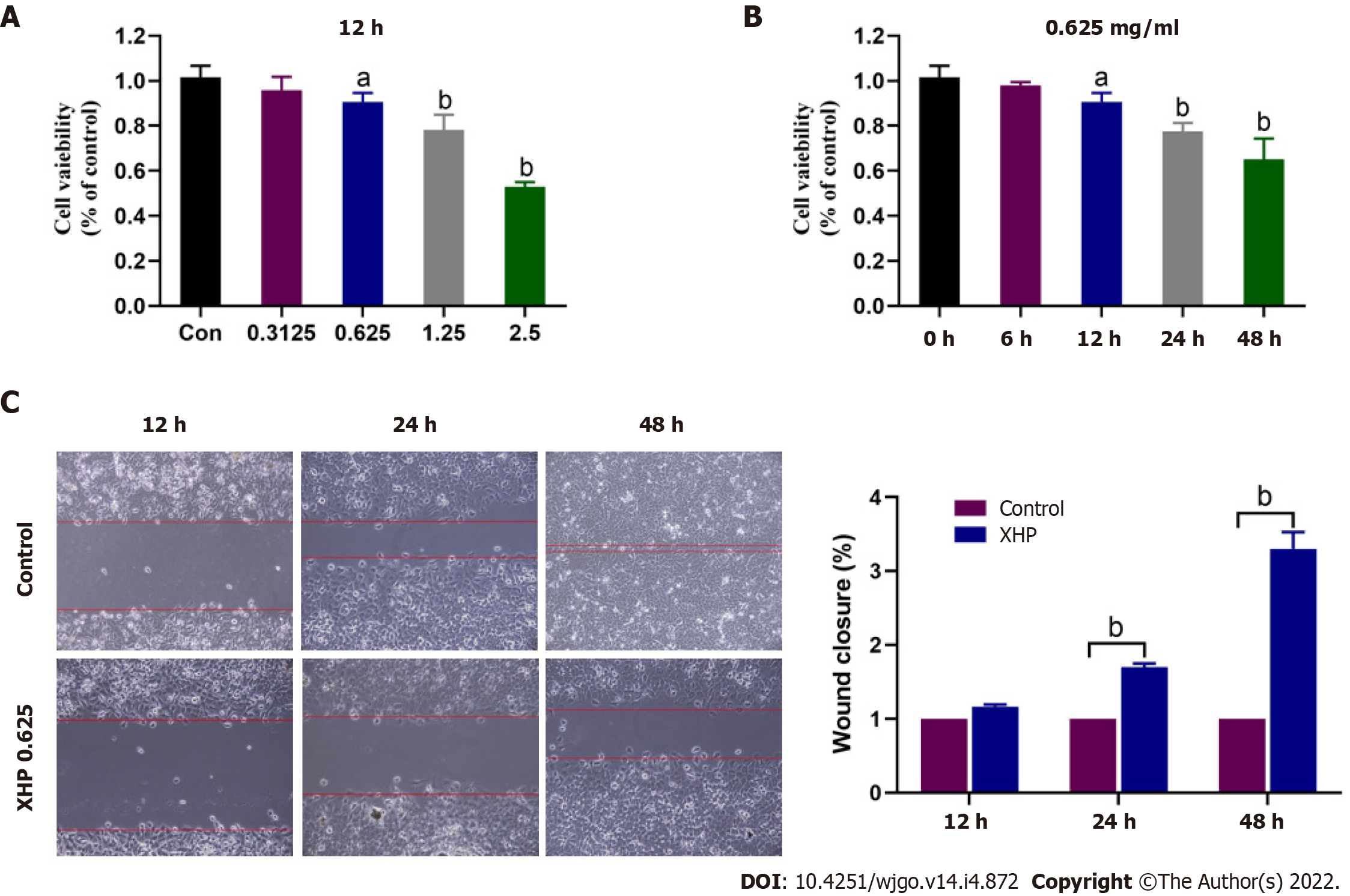
The effects of XHP extract on HCC cell viability were investigated. Cells were separated into five groups and treated with different concentrations of the XHP extract according to their human equivalent doses (0, 0.3125, 0.625, 1.25, and 2.5 mg/mL), and cell viability was determined using the CCK-8 assay at 12, 24, and 48 h posttreatment. The lowest concentration at which the XHP extract exerted an antitumour effect was 0.625 mg/mL (Figure 2A) at 12 h post-treatment (Figure 2B). The XHP extract inhibited SMMC-7721 cell viability in a dose- and time-dependent manner (Figure 2A and B). Treatment with the effective XHP extract dose, i.e., 0.625 mg/mL, significantly inhibited the migration of SMMC-7721 cells in a time-dependent manner (Figure 2C), as determined using a cell scratch assay.
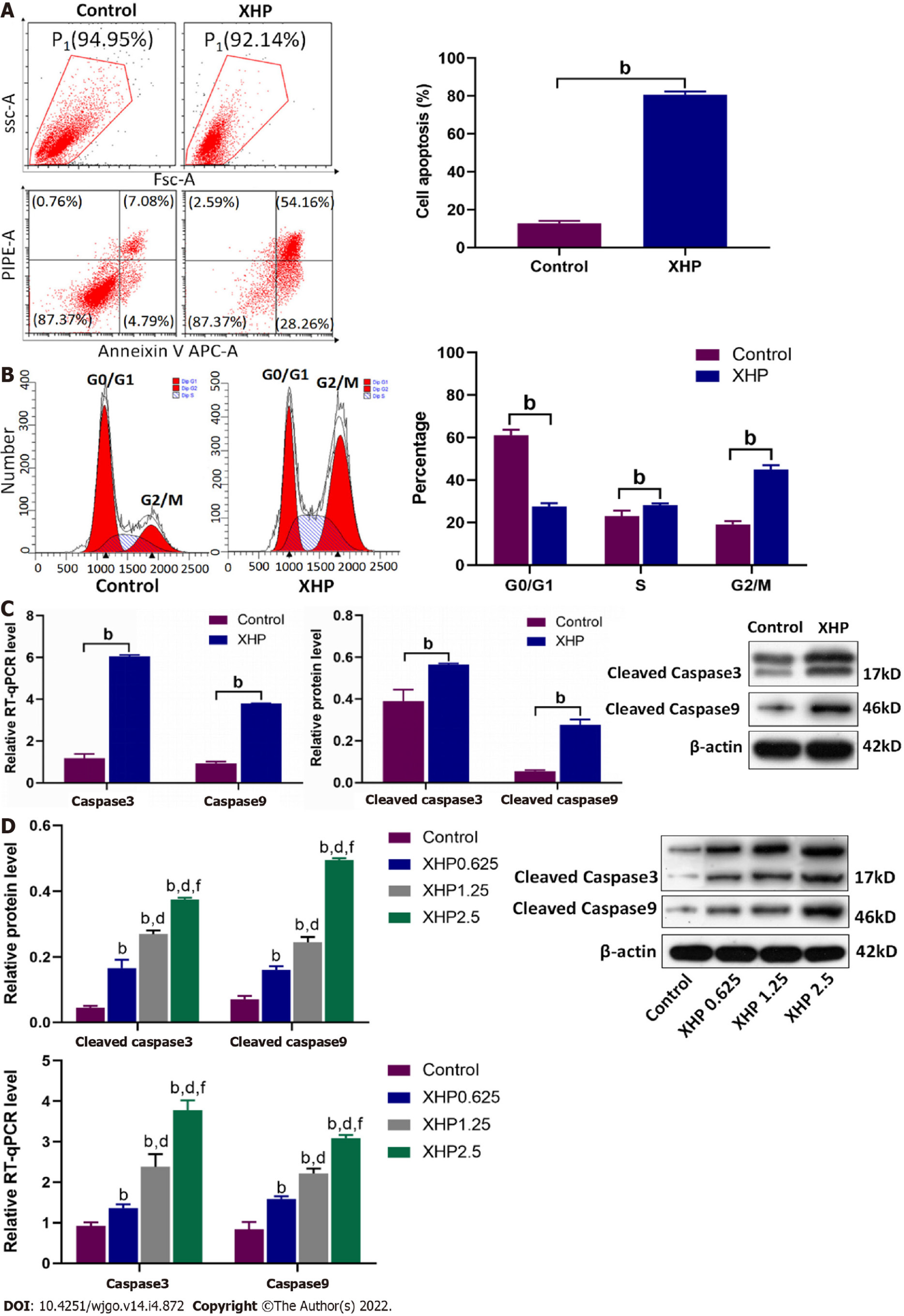
Apoptosis plays an important role in anti-tumour therapy. Therefore, annexin V and PI staining were performed to confirm the apoptosis-inducing effect of the XHP extract on SMMC-7721 cells. As shown in Figure 3A, the proportion of apoptotic cells among XHP-treated SMMC-7721 cells was significantly increased, ranging from 11.87% to 82.42%. Moreover, flow cytometry showed that the proportion of SMMC-7721 cells in G2/M phase was substantially increased, from 18.83% to 42.66% (Figure 3B), indicating cell cycle arrest in SMMC-7721 cells after XHP extract treatment. Subsequently, the expression levels of apoptosis-related proteins caspase-3 and caspase-9 were determined. Similar to the results of the flow cytometry analysis, in vivo experiments revealed that the protein expression levels of cleaved caspase-3 and cleaved caspase-9 were significantly increased in the subcutaneous xenograft tumours from nude mice. The mRNA expression levels of caspase-3 and caspase-9 also showed similar trends. (Figure 3C). These results were confirmed by in vitro experiments. After treatment with different concentrations of the XHP extract, mRNA expression, as well as protein levels of caspase-3 and caspase-9, were increased in SMMC-7721 cells in a dose-dependent manner (Figure 3D). These results were consistent with the data obtained from the cell viability and migration assays. Collectively, our results indicated that XHP extract promotes HCC cell apoptosis in a dose-dependent manner.
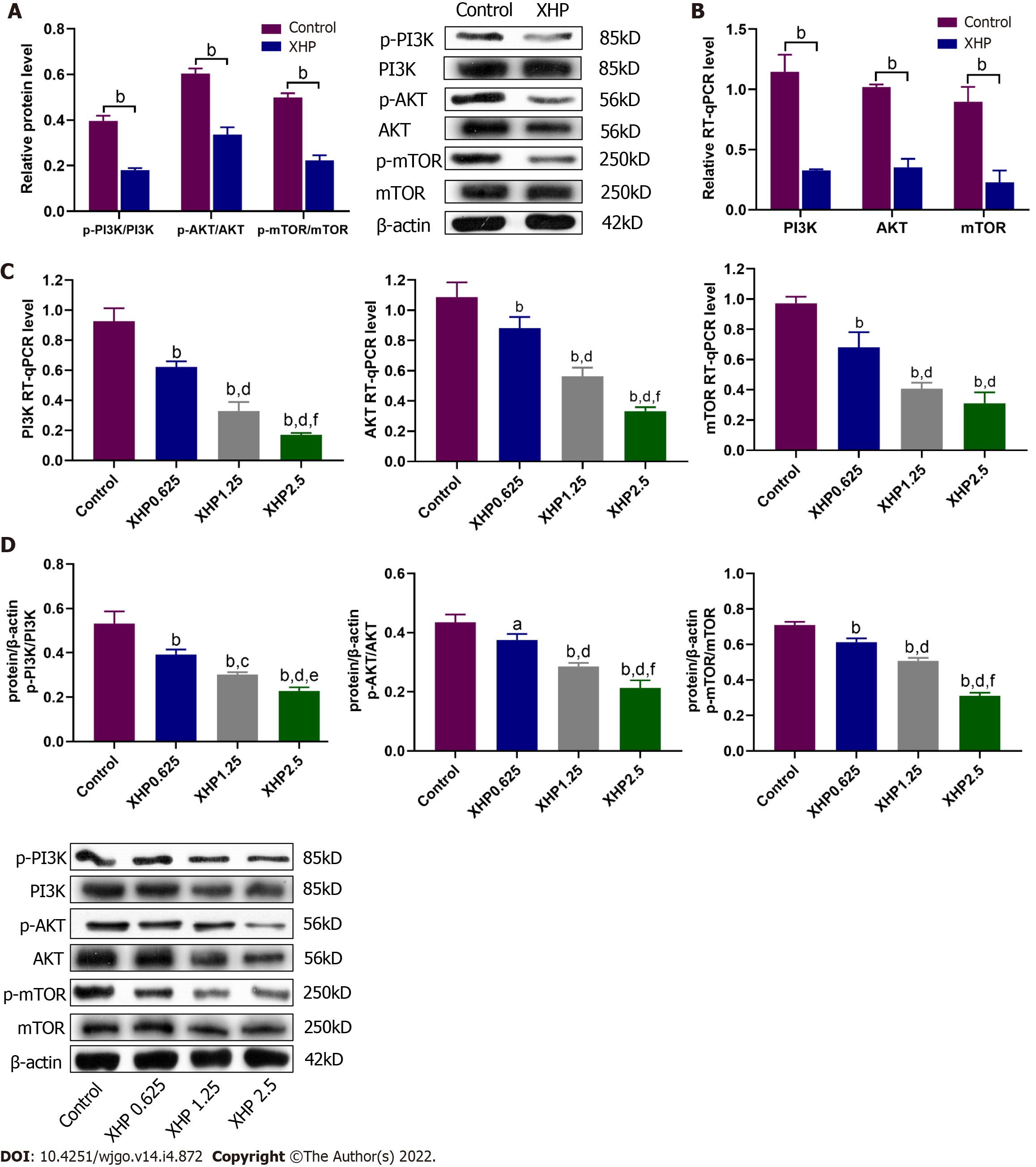
The PI3K/Akt/mTOR signalling pathway plays significant roles in regulating the cell cycle, apoptosis, and proliferation of HCC. The protein and mRNA expression levels of PI3K, Akt and mTOR were detected to clarify the mechanism involving the PI3K/Akt/mTOR signalling pathway in XHP-induced apoptosis and migration. In vivo experiments showed that the ratios of phosphorylated PI3K, Akt, and mTOR to the total protein were noticeably reduced after treatment with the proper concentration of XHP (i.e., 78 mg/kg) (Figure 4A) in the subcutaneous xenograft HCC mouse model. RT–qPCR analysis further confirmed the inhibitory effects of XHP on PI3K, Akt, and mTOR mRNA expression levels (Figure 4B). Moreover, in vitro cell experiments showed that XHP extract inhibited the phosphorylation and mRNA expression levels of components of the PI3K/Akt/mTOR signalling pathway in a dose-dependent manner (Figure 4C and D). The data described above indicate that XHP extract inhibits the PI3K/Akt/mTOR signalling pathway in a dose-dependent manner.
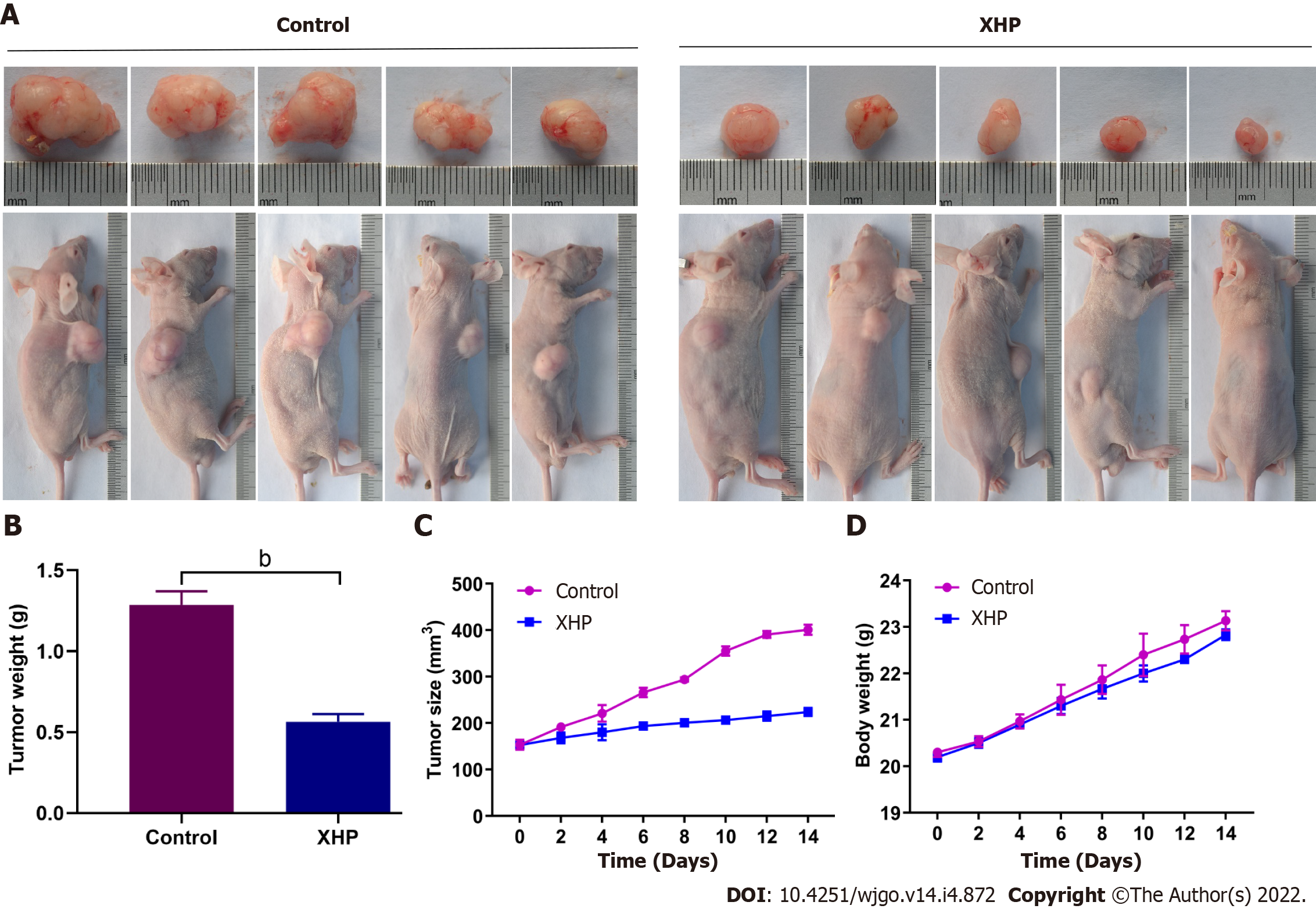
A xenograft tumour model was established using male BALB/c nude mice to confirm the antitumour effect of XHP in vivo. As shown in Figure 5A, the subcutaneous xenograft tumours were significantly reduced after XHP treatment compared to the control group. This result was further confirmed by calculating the volume (Figure 5B) and weight (Figure 5C). However, the overall body weight of the nude mice did not decrease. A significant difference in body weight was not observed between the two groups (Figure 5D). The in vivo experiment revealed the protective effect of XHP on preventing the progression of HCC tumorigenesis in the established mouse model.
HCC is an aggressive tumour characterized by a high degree of proliferation and invasion. Considering the limitations of current treatment methods, more effective treatments with reduced toxicity must be established. Notably, traditional Chinese medicine and its prepared compounds may serve as supplementary treatments for HCC[16]. XHP is an effective antitumour drug included in the traditional Chinese medicine system. Studies have shown that XHP inhibits the proliferation and metastasis of tumour cells[12]. However, the specific effects and mechanisms of XHP in HCC progression have not been discussed. In this study, we showed that XHP exerts an inhibitory effect on HCC cells by in vivo and in vitro experiments. Moreover, we identified that XHP reduces the viability and migration of tumour cells via the PI3K/Akt/mTOR signalling pathway. This pathway is involved in tumour cell apoptosis of HCC. Thus, the results of our study confirmed the antitumour properties of XHP in HCC, as well as the potential mechanism.
Abnormal activation of the PI3K/Akt/mTOR signalling pathway is a crucial factor promoting HCC development. It induces HCC cell proliferation and cell cycle arrest by inhibiting apoptosis[18-20]. The combination of growth factors and receptors in tumour cells activates PI3K, increasing cell viability, proliferation, and migration[21,22]. PI3K increases the viability of tumour cells in HCC by regulating apoptosis[23-25]. Moreover, Akt serves as a downstream target and mediates the antiapoptotic effects of PI3K, with Akt regulating the sequential steps of apoptotic signalling[26]. Akt activates many downstream proteins, including mTOR, Bcl-2-associated agonist of cell death (Bad), and GSK3[27,28]. In terms of cell cycle regulation, Akt expression suppresses the activity of GSK3β, reduces the expression of cyclin D1 and promotes the expression of Rb, thereby promoting cell cycle progression[29]. Caspase-3 is the primary executioner of apoptosis. It specifically lyses poly (ADP-ribose) polymerase and other substrates, leading to DNA fragmentation and eventual apoptosis[30]. Thus, this mechanism plays an important role in the inhibition of cancer cell invasion and metastasis[31]. The caspase-3 zymogen is regulated by caspase-9[19]. Activation of PI3K may inhibit caspase 3 activity and DNA fragmentation in several cell types. Therefore, PI3K is essential for maintaining cell viability. Furthermore, Bcl-2 has an important role in caspase-3-mediated apoptosis[32]. mTOR, a downstream target of Akt, phosphorylates Bad (an apoptotic molecule) and induces the expression of antiapoptotic Bcl-2 family proteins. Moreover, it inhibits the release of cytochrome c from the mitochondria, thereby inhibiting the activation of caspase-9 and caspase-3 and increasing cell viability[19,23,33]. Thus, the PI3K/Akt/mTOR signalling pathway regulates apoptosis by modulating caspase-9 and caspase-3 activity.
In this study, the high resolution MS was used to analyze the compound composition of XHP. Twelve compounds in XHP were identified, which were valine, 4-gingerol, myrrhone, ricinoleic acid, glycocholic acid, curzerenone, 11-keto-β-boswellic acid, oleic acid, germacrone, 3-acetyl-9,11-dehydro-β-boswellic acid, 5β-androstane-3,17-dione, and 3-acetyl-11-keto-β-boswellic acid. Among them, Curcuzederone, 11-keto-α-Boswellic Acid, Oleic Acid and 3-acetyl-11-keto-beta-Boswellic Acid have been reported to have good anticancer activity. They inhibit tumor growth by promoting apoptosis of cancer cells[34-38]. We speculate that the anti-tumor effect of XHP may be through these active ingredients, and we will further clarify the role of the active ingredients in future studies.
Activation of apoptosis is one of the crucial strategies for anti-tumour therapy[10,39-41]. The results of this study revealed that XHP effectively suppressed the growth of HCC cells at a concentration of 0.625 mg/mL in a dose- and time-dependent manner. The scratch assay revealed that XHP treatment also inhibited HCC cell migration in a time-dependent manner. Apoptosis experiments further confirmed that XHP treatment arrested the cell cycle in G2/M phase, facilitating the apoptosis of HCC cells. Based on these results, XHP exerts an inhibitory effect on the growth and migration of tumour cells by inducing apoptosis.
We further investigated the effects of XHP on the PI3K/Akt/mTOR pathway. XHP treatment inhibited the PI3K/Akt/mTOR pathway both in vivo and in vitro in a dose-dependent manner. Moreover, the activities of cleaved caspase-9 and cleaved caspase-3 were increased in response to XHP treatment. Therefore, we hypothesized that XHP may cause cell cycle arrest via the PI3K/Akt/mTOR signalling pathway and regulates the expression of the apoptosis executioner proteins cleaved caspase-9 and cleaved caspase-3, thereby promoting apoptosis in HCC and inhibiting the growth and migration of tumour cells. In addition, an in vivo experiment in nude mice further confirmed the inhibitory effects of XHP on HCC cells, as evidenced by decreases in tumour volume and weight. However, the effect of XHP on the mitochondrial apoptotic pathway, such as autophagy, was not detected in this study. The exact step that XHP modulated in the PI3K/Akt/mTOR pathway is unclear. Therefore, further research is needed to obtain a comprehensive interpretation of the XHP-regulated antitumour effect.
In conclusion, XHP increases cleaved caspase-9 and cleaved caspase-3 activities by inhibiting the PI3K/Akt/mTOR signalling pathway and induces apoptosis and cell cycle arrest. Consequently, XHP inhibits the growth, migration, and proliferation of HCC cells. Our study provides a better understanding of the antitumour effects of XHP and reveals the underlying mechanism. The findings of this study suggest that XHP may serve as a supplementary medicine in HCC treatment.
Xihuang pills (XHP) are a traditional Chinese preparation with antitumour properties. They inhibit the growth of breast cancer, glioma, and other tumours by regulating the phosphoinositide 3-kinase/protein kinase-B/mechanistic target of rapamycin (PI3K/Akt/mTOR) signalling pathway. However, the effects and mechanisms of action of XHP in hepatocellular carcinoma (HCC) remain unclear. Regulation of the PI3K/Akt/mTOR signalling pathway effectively inhibits the progression of HCC.
We hypothesized that XHP might play a role in inhibiting HCC through the PI3K/Akt/mTOR signalling pathway.
To confirm the effect of XHP on HCC and the possible mechanisms involved.
The chemical constituents and active components of XHP were analysed using ultra-performance liquid chromatography-quadrupole time of flight mass spectrometry (MS) (UPLC-Q-TOF-MS). First, cell-based experiments and in vivo xenograft tumour experiments were utilized to evaluate the effect of XHP on HCC tumorigenesis. Cell viability was assessed using the CCK-8 assay, followed by an assessment of cell migration using a wound healing assay. Second, the effect of XHP on the apoptosis of SMMC-7721 cells was evaluated. Third, Western blotting and reverse-transcription polymerase chain reaction were performed to confirm the effects of XHP on the protein and mRNA expression of components of the PI3K/Akt/mTOR signalling pathway. Finally, the effects of XHP on the tumorigenesis of subcutaneous hepatocellular tumours in nude mice were assessed.
The 12 compounds were identified in XHP by high-resolution MS. The cell viability assay results showed that treatment with 0.625 mg/mL XHP extract decreased HCC cell viability after 12 h. Moreover, XHP significantly inhibited cell migration and resulted in cell cycle arrest and apoptosis. Furthermore, XHP downregulated the PI3K/Akt/mTOR signalling pathway, which activated apoptosis executioner proteins (e.g., caspase-9 and caspase-3). The inhibitory effects of XHP on HCC cell growth were determined in vivo by analysing the tumour xenograft volumes and weights.
XHP inhibited HCC cell growth and migration by stimulating apoptosis via the downregulation of the PI3K/Akt/mTOR signalling pathway, followed by the activation of caspase-9 and caspase-3. Our findings clarified that the antitumour effects of XHP on HCC cells are mediated by the PI3K/Akt/mTOR signalling pathway.
Our findings revealed that XHP may be a potential complementary therapy for HCC.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hassaan NA, Egypt; Prasetyo EP, Indonesia S-Editor: Fan JR L-Editor: A P-Editor: Guo X
| 1. | Dasgupta P, Henshaw C, Youlden DR, Clark PJ, Aitken JF, Baade PD. Global Trends in Incidence Rates of Primary Adult Liver Cancers: A Systematic Review and Meta-Analysis. Front Oncol. 2020;10:171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 2. | Ko KL, Mak LY, Cheung KS, Yuen MF. Hepatocellular carcinoma: recent advances and emerging medical therapies. F1000Res. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 36] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 3. | Rahmani F, Ziaeemehr A, Shahidsales S, Gharib M, Khazaei M, Ferns GA, Ryzhikov M, Avan A, Hassanian SM. Role of regulatory miRNAs of the PI3K/AKT/mTOR signaling in the pathogenesis of hepatocellular carcinoma. J Cell Physiol. 2020;235:4146-4152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 4. | Zhou Q, Lui VW, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011;7:1149-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Brotelle T, Bay JO. [PI3K-AKT-mTOR pathway: Description, therapeutic development, resistance, predictive/prognostic biomarkers and therapeutic applications for cancer]. Bull Cancer. 2016;103:18-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Fresno Vara JA, Casado E, de Castro J, Cejas P, Belda-Iniesta C, González-Barón M. PI3K/Akt signalling pathway and cancer. Cancer Treat Rev. 2004;30:193-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1407] [Cited by in RCA: 1787] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 7. | Polivka J Jr, Janku F. Molecular targets for cancer therapy in the PI3K/AKT/mTOR pathway. Pharmacol Ther. 2014;142:164-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 613] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 8. | Janku F. Phosphoinositide 3-kinase (PI3K) pathway inhibitors in solid tumors: From laboratory to patients. Cancer Treat Rev. 2017;59:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 174] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 9. | Zhang Y, Xie C, Li A, Liu X, Xing Y, Shen J, Huo Z, Zhou S, Xie Y, Cao W, Ma Y, Xu R, Cai S, Tang X, Ma D. PKI-587 enhances chemosensitivity of oxaliplatin in hepatocellular carcinoma through suppressing DNA damage repair pathway (NHEJ and HR) and PI3K/AKT/mTOR pathway. Am J Transl Res. 2019;11:5134-5149. [PubMed] |
| 10. | Pistritto G, Trisciuoglio D, Ceci C, Garufi A, D'Orazi G. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY). 2016;8:603-619. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 607] [Cited by in RCA: 1116] [Article Influence: 139.5] [Reference Citation Analysis (0)] |
| 11. | Hong SW, Jung KH, Lee HS, Choi MJ, Son MK, Zheng HM, Hong SS. SB365 inhibits angiogenesis and induces apoptosis of hepatocellular carcinoma through modulation of PI3K/Akt/mTOR signaling pathway. Cancer Sci. 2012;103:1929-1937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Guo Q, Xu X, He S, Yuan Y, Chen S, Hua B. Xi huang pills enhance the tumor treatment efficacy when combined with chemotherapy: A meta-analysis and systematic review. J Cancer Res Ther. 2018;14:S1012-S1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Mao D, Feng L, Huang S, Zhang S, Peng W. Meta-Analysis of Xihuang Pill Efficacy When Combined with Chemotherapy for Treatment of Breast Cancer. Evid Based Complement Alternat Med. 2019;2019:3502460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Li XY, Su L, Jiang YM, Gao WB, Xu CW, Zeng CQ, Song J, Xu Y, Weng WC, Liang WB. The Antitumor Effect of Xihuang Pill on Treg Cells Decreased in Tumor Microenvironment of 4T1 Breast Tumor-Bearing Mice by PI3K/AKT~AP-1 Signaling Pathway. Evid Based Complement Alternat Med. 2018;2018:6714829. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Fu J, Zhu SH, Xu HB, Xu YQ, Wang X, Wang J, Kong PS. Xihuang pill potentiates the anti-tumor effects of temozolomide in glioblastoma xenografts through the Akt/mTOR-dependent pathway. J Ethnopharmacol. 2020;261:113071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Shao M, He Z, Yin Z, Ma P, Xiao Q, Song Y, Huang Z, Ma Y, Qiu Y, Zhao A, Zhou T, Wang Q. Xihuang Pill Induces Apoptosis of Human Glioblastoma U-87 MG Cells via Targeting ROS-Mediated Akt/mTOR/FOXO1 Pathway. Evid Based Complement Alternat Med. 2018;2018:6049498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Nair A, Morsy MA, Jacob S. Dose translation between laboratory animals and human in preclinical and clinical phases of drug development. Drug Dev Res. 2018;79:373-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 272] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 18. | Golob-Schwarzl N, Krassnig S, Toeglhofer AM, Park YN, Gogg-Kamerer M, Vierlinger K, Schröder F, Rhee H, Schicho R, Fickert P, Haybaeck J. New liver cancer biomarkers: PI3K/AKT/mTOR pathway members and eukaryotic translation initiation factors. Eur J Cancer. 2017;83:56-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 19. | Wu T, Dong X, Yu D, Shen Z, Yu J, Yan S. Natural product pectolinarigenin inhibits proliferation, induces apoptosis, and causes G2/M phase arrest of HCC via PI3K/AKT/mTOR/ERK signaling pathway. Onco Targets Ther. 2018;11:8633-8642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Wu Y, Zhang Y, Qin X, Geng H, Zuo D, Zhao Q. PI3K/AKT/mTOR pathway-related long non-coding RNAs: roles and mechanisms in hepatocellular carcinoma. Pharmacol Res. 2020;160:105195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 100] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 21. | Goncalves MD, Hopkins BD, Cantley LC. Phosphatidylinositol 3-Kinase, Growth Disorders, and Cancer. N Engl J Med. 2018;379:2052-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 223] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 22. | Jiang S, Wang Q, Feng M, Li J, Guan Z, An D, Dong M, Peng Y, Kuerban K, Ye L. C2-ceramide enhances sorafenib-induced caspase-dependent apoptosis via PI3K/AKT/mTOR and Erk signaling pathways in HCC cells. Appl Microbiol Biotechnol. 2017;101:1535-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Khemlina G, Ikeda S, Kurzrock R. The biology of Hepatocellular carcinoma: implications for genomic and immune therapies. Mol Cancer. 2017;16:149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 320] [Article Influence: 40.0] [Reference Citation Analysis (1)] |
| 24. | Song L, Luo Y, Li S, Hong M, Wang Q, Chi X, Yang C. ISL Induces Apoptosis and Autophagy in Hepatocellular Carcinoma via Downregulation of PI3K/AKT/mTOR Pathway in vivo and in vitro. Drug Des Devel Ther. 2020;14:4363-4376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Xue S, Zhou Y, Zhang J, Xiang Z, Liu Y, Miao T, Liu G, Liu B, Liu X, Shen L, Zhang Z, Li M, Miao Q. Anemoside B4 exerts anti-cancer effect by inducing apoptosis and autophagy through inhibiton of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am J Transl Res. 2019;11:2580-2589. [PubMed] |
| 26. | Lee YI, Kang-Park S, Do SI, Lee YI. The hepatitis B virus-X protein activates a phosphatidylinositol 3-kinase-dependent survival signaling cascade. J Biol Chem. 2001;276:16969-16977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 153] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 27. | Aoki M, Fujishita T. Oncogenic Roles of the PI3K/AKT/mTOR Axis. Curr Top Microbiol Immunol. 2017;407:153-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 163] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 28. | Coutte L, Dreyer C, Sablin MP, Faivre S, Raymond E. [PI3K-AKT-mTOR pathway and cancer]. Bull Cancer. 2012;99:173-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 29. | Li A, Zhang R, Zhang Y, Liu X, Wang R, Liu J, Xie Y, Cao W, Xu R, Ma Y, Cai W, Wu B, Cai S, Tang X. BEZ235 increases sorafenib inhibition of hepatocellular carcinoma cells by suppressing the PI3K/AKT/mTOR pathway. Am J Transl Res. 2019;11:5573-5585. [PubMed] |
| 30. | Crowley LC, Waterhouse NJ. Detecting Cleaved Caspase-3 in Apoptotic Cells by Flow Cytometry. Cold Spring Harb Protoc. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 31. | Zhou M, Liu X, Li Z, Huang Q, Li F, Li CY. Caspase-3 regulates the migration, invasion and metastasis of colon cancer cells. Int J Cancer. 2018;143:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 32. | Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 553] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 33. | Li TT, Zhu D, Mou T, Guo Z, Pu JL, Chen QS, Wei XF, Wu ZJ. IL-37 induces autophagy in hepatocellular carcinoma cells by inhibiting the PI3K/AKT/mTOR pathway. Mol Immunol. 2017;87:132-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 34. | Al-Amin M, Eltayeb NM, Khairuddean M, Salhimi SM. Bioactive chemical constituents from Curcuma caesia Roxb. rhizomes and inhibitory effect of curcuzederone on the migration of triple-negative breast cancer cell line MDA-MB-231. Nat Prod Res. 2021;35:3166-3170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Schmiech M, Ulrich J, Lang SJ, Büchele B, Paetz C, St-Gelais A, Syrovets T, Simmet T. 11-Keto-α-Boswellic Acid, a Novel Triterpenoid from Boswellia spp. with Chemotaxonomic Potential and Antitumor Activity against Triple-Negative Breast Cancer Cells. Molecules. 2021;26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Jung S, Lee S, Lee H, Yoon J, Lee EK. Oleic acid-embedded nanoliposome as a selective tumoricidal agent. Colloids Surf B Biointerfaces. 2016;146:585-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Mizushina Y, Takeuchi T, Sugawara F, Yoshida H. Anti-cancer targeting telomerase inhibitors: β-rubromycin and oleic acid. Mini Rev Med Chem. 2012;12:1135-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Riaz A, Rasul A, Kanwal N, Hussain G, Shah MA, Sarfraz I, Ishfaq R, Batool R, Rukhsar F, Adem Ş. Germacrone: A Potent Secondary Metabolite with Therapeutic Potential in Metabolic Diseases, Cancer and Viral Infections. Curr Drug Metab. 2020;21:1079-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 39. | Goldar S, Khaniani MS, Derakhshan SM, Baradaran B. Molecular mechanisms of apoptosis and roles in cancer development and treatment. Asian Pac J Cancer Prev. 2015;16:2129-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 400] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 40. | Lin Y, Chen Y, Wang S, Ma J, Peng Y, Yuan X, Lv B, Chen W, Wei Y. Plumbagin induces autophagy and apoptosis of SMMC-7721 cells in vitro and in vivo. J Cell Biochem. 2019;120:9820-9830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 41. | Wang K. Autophagy and apoptosis in liver injury. Cell Cycle. 2015;14:1631-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 299] [Article Influence: 33.2] [Reference Citation Analysis (0)] |