Published online Dec 15, 2022. doi: 10.4251/wjgo.v14.i12.2367
Peer-review started: August 1, 2022
First decision: August 21, 2022
Revised: August 29, 2022
Accepted: November 16, 2022
Article in press: November 16, 2022
Published online: December 15, 2022
Processing time: 132 Days and 20.5 Hours
Liver cancer is a malignant tumor with high morbidity and mortality. Transcatheter arterial chemoembolization (TACE) is the main method for surgically unresectable liver cancer. In recent years, drug-loaded microspheres have been gradually applied in TACE technology. There are some controversies about the therapeutic effects of drug-loaded microspheres TACE (D-TACE) and traditional TACE.
To explore the short-term efficacy of D-TACE and traditional TACE in the treatment of advanced liver cancer.
The clinical data of 73 patients with advanced liver cancer admitted to the First and Sixth Medical Centers of Chinese PLA General Hospital from January 2017 to October 2019 were retrospectively analyzed. Among them, 15 patients were treated with D-TACE, and 58 patients were treated with traditional TACE. Clinical baseline characteristics, perioperative laboratory indices, postoperative adverse reactions and postoperative complications were compared between the two groups.
There was no statistical difference between the two groups for the postoperative response: The highest postoperative body temperature of the drug-loaded microsphere group was 38.0 ± 0.9℃ and the postoperative highest body temperature of the traditional TACE group was 38.3 ± 0.7℃ (t = -1.414, P = 0.162). For the 24 h postoperative nausea and vomiting after surgery in terms of scoring and postoperative pain scores, the traditional TACE group was higher than the drug-loaded microsphere group (χ2 = 14.33, P = 0.014; χ2 = 32.967, P = 0.000) and the two groups had significant statistical differences. The disease control rate at 3 mo after treatment in the drug-loaded microsphere group was 60% and the disease control rate at 3 mo after treatment in the traditional TACE group was 75.9% (χ2 = 4.091, P = 0.252). There was no statistical difference between the two groups of data. During the follow-up period, the number of interventional treatments received was once in the drug-loaded microsphere group and the traditional TACE group received an average of 1.48 treatments (χ2 = 10.444 P = 0.005). There was a statistical difference between the two groups.
Compared with traditional TACE, D-TACE may have some advantages in the treatment of advanced hepatocellular carcinoma with a large tumor load in the short term, but the long-term clinical efficacy needs additional follow-up studies. In addition, compared with the traditional group, the patients in the drug-loaded microsphere group had better subjective tolerance and could reduce the number of interventional treatments. Therefore, D-TACE is worthy of clinical promotion.
Core Tip: Hepatocellular carcinoma with a very high mortality rate is insidious and about 80 per cent of patients have no chance of surgery when diagnosed. Transcatheter arterial chemoembolization (TACE) is recommended as a first-line treatment. Traditional TACE uses iodized oil and gelatin sponge as the main embolization materials, and the chemotherapeutic drugs are mixed with iodized oil and injected into the tumor feeding artery to achieve the dual role of embolization and chemotherapy. Some scholars believe that liver cancer cells are not sensitive to chemotherapy drugs, so the role of chemotherapy drugs in TACE treatment is controversial. In recent years, drug-loaded microspheres have been gradually applied in TACE technology, which can significantly improve the killing effect of drugs on tumor tissues, and significantly reduce the systemic drug concentration, thereby reducing the side effects of chemotherapy drugs. In this study, the effects of using the same size of embolization particles and drug-eluting beads during TACE were compared. To investigate the effect and systemic response of chemotherapy drugs in TACE under the new local drug delivery mode.
- Citation: Ye T, Shao SH, Ji K, Yao SL. Evaluation of short-term effects of drug-loaded microspheres and traditional transcatheter arterial chemoembolization in the treatment of advanced liver cancer. World J Gastrointest Oncol 2022; 14(12): 2367-2379
- URL: https://www.wjgnet.com/1948-5204/full/v14/i12/2367.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i12.2367
The prevalence of liver cancer ranks fourth among malignant tumors and its fatality rate ranks second among all tumors[1]. Surgical resection, liver transplantation and ablation are the main treatment methods for early liver cancer. However, due to the insidious onset of liver cancer, approximately 80% of patients have no chance of surgery when they are diagnosed[2]. For such patients, transcatheter arterial chemoembolization (TACE) is recommended as the first-line treatment[3-6]. Traditional TACE (conventional TACE, cTACE) uses iodized oil and gelatin sponges as the main embolization materials and injects chemotherapeutic drugs and iodized oil into the tumor feeding artery to achieve a dual effect of embolization and chemotherapy. Previously, some researchers thought that liver cancer cells were not sensitive to chemotherapeutic drugs, so the role of chemotherapeutic drugs in cTACE treatment was controversial[7].
In recent years, drug-eluting beads (DEBs) have been gradually applied in TACE technology. A DEB is a nonabsorbable embolic microsphere loaded with chemotherapeutic drugs that immediately causes embolization after reaching the tumor tissue. At the same time, local high concentrations due to the continuous release of chemotherapeutic drugs significantly improves the killing effect of drugs on tumor tissue and significantly reduces the systemic drug concentration, thereby reducing the side effects of chemotherapeutic drugs[7,8]. In addition, D-TACE is easier to standardize than cTACE[9].
There are some previous clinical studies comparing D-TACE and cTACE. Some studies have shown that D-TACE can have better therapeutic effects[10-14], but other studies found no significant difference in the therapeutic effect between the two groups[15-19]. For these studies, we found that there were some differences in the selection and use of the cTACE embolization agent. When comparing the embolization effect between the two groups, the curative effect difference caused by embolization agents and technical reasons cannot be excluded. Based on the above reasons, this study compared the effect of using the same size of embolization particles and DEB during TACE. We investigated the effect and systemic response of chemotherapy drugs in TACE under the new local drug delivery mode.
We retrospectively analyzed the clinical data of 73 patients with advanced liver cancer treated with D-TACE and traditional TACE at the First Medical Center and the Sixth Medical Center of the PLA General Hospital from January 2017 to October 2019. All patients signed informed consent before surgery, were informed of the risk of surgery, and voluntarily chose D-TACE or traditional TACE treatment. Patients' inclusion criteria: (1) Comply with the diagnostic criteria of primary liver cancer in the diagnostic and therapeutic norms of primary liver cancer (2019 edition), and the primary lesion cannot be removed by surgery; (2) Child–Pugh A or B liver function classification; (3) The Eastern Cooperative Oncology Group (ECOG) score was 0–2; (4) The patient's expected survival time was more than 3 mo; (5) The Barcelona stage of liver cancer was B and C; (6) In patients with massive hepatocellular carcinoma (HCC), the tumors accounted for less than 70% of the liver mass; and (7) The portal vein trunk was not completely blocked. Exclusion criteria: (1) A combination of active hepatitis [hepatitis B e antigen (HBeAg) positive + male, alanine transaminase (ALT) ≥ 30 IU/L/female ALT ≥ 19 IU/L or HBeAg negative + hepatitis B virus (HBV) DNA ≥ 1 × 105 cps/mL + male ALT ≥ 30 IU/L/female ALT ≥ 19 IU/L] or a severe infection that cannot be treated simultaneously; (2) Tumors have extensive distant metastasis; (3) Severe heart and kidney dysfunction; (4) Peripheral white blood cells < 3.0 × 109 L and platelets < 50 × 109/L, which cannot be corrected by oral drugs, injection of injections, transfusion of white blood cells or platelets; (5) With other malignant tumors; (6) Other treatments (ablation, radiotherapy, targeted therapy, etc.) during the follow-up period after TACE; and (7) Missing or incomplete information during the follow-up period.
Preoperative laboratory examination included routine blood, urine, stool, liver and kidney function, coagulation, alpha-fetoprotein, and eight preoperative items (HBV five, hepatitis C, syphilis, human immunodeficiency viruses antibody). Imaging examination included abdominal dynamic enhanced magnetic resonance imaging (MRI) or computed tomography (CT), which was completed within one month before TACE. In addition, head and lung CT and electrocardiogram examinations were performed to screen for extrahepatic metastasis and to examine the cardiac function.
Treatment methods included D-TACE and traditional TACE. All patients underwent the modified Seldinger technique, percutaneous femoral artery puncture, superior mesenteric artery, abdominal artery or common hepatic artery angiography. Before embolization, combined with abdominal enhanced MRI or CT, the corresponding arteries (including subphrenic artery, left gastric artery, intercostal artery, adrenal artery, lumbar artery, etc.) were explored according to the location of lesions that may have collateral circulation, and a cone beam CT (CBCT) examination was performed if necessary. After defining the responsible vessels, the microcatheter superselective technique was used to superselect the target vessels. TACE group: Pirarubicin (50 mg), oxaliplatin/cisplatin (100/50 mg), and fluorouracil (750 mg) were used as chemotherapy drugs. Embolization materials were 100-300 µm CalliSpheres drug-loaded microspheres, chemotherapy drug loading, and 1-1.2 times of a nonionic contrast agent, left to stand for 5 min before use. Traditional TACE group: Conventional TACE group: chemotherapy drugs with pirarubicin/epirubicin 50 mg, oxaliplatin/cisplatin 100/50 mg, fluorouracil (750 mg perfusion), embolization materials with iodized oil, polyethylene embolization particles 100-300 µm (polyvinyl alcohol, PVA), and a gelatin sponge. During embolization, the chemotherapeutic drugs (pirarubicin and platinum) were mixed evenly with the iodized oil to prepare an emulsion before use. When the lesion could not be over selected, the embolization agent was iodized oil or iodized oil plus chemotherapy (because the ischemic effect caused by iodized oil is transient). The endpoint of embolization is to slow down the blood flow in the target artery, and the use of the contrast agent shows that the blood vessels are “dead branches”, which can be considered to have achieved the endpoint of embolization. After the operation, the catheter and sheath were removed, the puncture site was compressed for 10 min and then pressurized for 6-10 h. The color of the punctured lower limbs and the pulsation of the dorsal pedis artery were observed.
The common complications after postoperative treatment include fever, nausea, vomiting, liver pain, abnormal liver function, bone marrow suppression, infection, etc. After the operation, symptomatic treatments, such as gastric protection and acid suppression, antiemetics, liver protection, pain relief, and anti-infection, are routinely given[20,21]. Routine blood examination, liver and kidney function and other indicators were reviewed before discharge and discharge was only arranged after the indicators were close to normal, and the patients were asked to attend one month after discharge.
The clinical follow-up data were obtained by admission review, telephone or letter follow-up. The follow-up data were independently evaluated by two experienced physicians above the specialist level, and consensus was reached after consultation when there was disagreement. The imaging data were mainly abdominal enhanced CT or MRI at 1 and 3 mo after the operation. The modified solid tumor efficacy evaluation criteria were used to evaluate the efficacy: All target lesions without enhancement in the arterial phase were considered complete remission (CR); the total diameter of the target lesion in the arterial phase was reduced by ≥ 30% for partial remission (PR); the disease was stable (SD) when the total diameter of the lesion was reduced by less than 30% or increased by less than 20%; when the total diameter of the target lesion in the arterial phase increased by ≥ 20% or new lesions developed was defined as disease progression. The objective response of the tumor was equal to the sum of CR and PR. Disease control equals the sum of CR, PR and SD.
EmpowerStats software was used for data analysis. Measurement data with an approximate normal distribution are expressed as mean ± SD, and a t-test was used for comparisons between groups. Measurement data with a nonnormal distribution are expressed as the mean (SD), median and P25, P75 values. Rank sum tests were used for comparisons between groups. Count data are expressed as the number of cases and percentage. χ2 tests were used for comparisons between groups. P < 0.05 indicated that the difference was statistically significant. Age, tumor size and body temperature were the measurement data that conformed to or approximately conformed to the normal distribution. Sex, liver cirrhosis, portal vein tumor thrombus, ECOG physical status score, Child–Pugh score, number of tumors, Barcelona stage, HBV infection, postoperative 24 h postoperative nausea and vomiting (PONV) score, postoperative pain score, number of treatments, and 1 and 3 mo of follow-up were recorded.
The objective response rate was count data. The changes in aspartate aminotransferase, alanine aminotransferase, total bilirubin, serum albumin, white blood cells, red blood cells, platelets, and alpha-fetoprotein before and after surgery were nonnormally distributed measurement data.
The general information of patients is shown in Table 1. There were no statistical differences between the drug-loaded microsphere group and the conventional TACE group in terms of age, gender, presence of cirrhosis, presence of portal vein cancer thrombosis, ECOG physical status score, Child-Pugh score, preoperative methemoglobin, tumor number and Barcelona stage. There were statistical differences between the two groups in terms of preoperative tumor size (longest diameter) and whether they were infected with HBV, with the preoperative tumor size of 102.7 ± 44.4 mm in the drug-loaded microsphere group being larger than that of 75.0 ± 34.1 mm in the conventional TACE group; in terms of HBV infection, there were 6 cases in the drug-loaded microsphere group (40.0% of the whole group) and 48 cases in the conventional TACE group (82.8% of the whole group ). The HBV infection rate was higher in the conventional TACE group.
| Influencing factors | D-TACE (15 cases) | C-TACE (58 cases) | t/χ2 value | P value |
| Age (yr) | 57.3 ± 11.5 | 59.9 ± 10.7 | -0.8361 | 0.406 |
| The longest tumor size before surgery (mm) | 102.7 ± 44.4 | 75.0 ± 34.1 | 2.6261 | 0.011 |
| Gender | 1.907 | 0.167 | ||
| Female | 1 (6.7) | 13 (22.4) | ||
| Male | 14 (93.3) | 45 (77.6) | ||
| Whether infected with HBV | 11.317 | 0.0008 | ||
| No | 9 (60.0) | 10 (17.2) | ||
| Yes | 6 (40.0) | 48 (82.8) | ||
| Whether there is liver cirrhosis | 3.137 | 0.069 | ||
| No | 9 (60.0) | 18 (31.0) | ||
| Yes | 6 (40.0) | 40 (69.0) | ||
| Is there a portal vein tumor thrombus | 0.602 | 0.438 | ||
| No | 13 (86.7) | 45 (77.6) | ||
| Yes | 2 (13.3) | 13 (22.4) | ||
| ECOG stamina score | 3.769 | 0.152 | ||
| 0 points | 2 (13.3) | 13 (22.4) | ||
| 1points | 9 (60.0) | 40 (69.0) | ||
| 2points | 4 (26.7) | 5 (8.6) | ||
| Child-Pugh classification | 1.668 | 0.196 | ||
| A | 6 (40.0) | 34 (58.6) | ||
| B | 9 (60.0) | 24 (41.4) | ||
| Number of tumors | 1.237 | 0.744 | ||
| 1 | 3 (20.0) | 7 (12.1) | ||
| 2 | 1 (6.7) | 2 (3.4) | ||
| 3 | 0 (0.0) | 1 (1.7) | ||
| Multiple (> 3) | 11 (73.3) | 48 (82.8) | ||
| Barcelona staging | 0.434 | 0.510 | ||
| B | 9 (60) | 40 (69) | ||
| C | 6 (40) | 18 (31) |
The laboratory test indexes of patients before surgery and on the 3rd day after surgery are shown in Table 2. There were no statistical differences in the values of glutamic oxalyl transaminase, glutamic alanine transaminase, total bilirubin, serum albumin, leukocytes, red blood cells, and platelets before and after surgery in the drug-laden microsphere group and the conventional TACE group.
| Laboratory indicators | D-TACE (15 cases) | C-TACE (58 cases) | χ2 value | P value |
| AST changes before and after surgery (U/L) | 44.1 (10.4, 153.0) | 102.7 (52.4, 254.8) | -2.086 | 0.05 |
| ALT changes before and after surgery (U/L) | 133.1 (31.6, 181.8) | 110.3 (46.5, 309.7) | -1.382 | 0.176 |
| TBil changes before and after surgery (μmol/L) | 7.9 (5.4, 14.9) | 11.6 (5.8, 21.6) | -1.842 | 0.081 |
| ALB changes before and after surgery (g/L) | 4.9 (3.2, 5.4) | 2.6 (0.3, 5.3) | 1.992 | 0.055 |
| WBC changes before and after surgery (× 109/L) | 2.0 (0.9, 3.9) | 2.1 (1.0, 4.4) | -0.214 | 0.832 |
| RBC changes before and after surgery (× | 0.3 (0.1, 0.5) | 0.0 (-0.2, 0.3) | 1.995 | 0.058 |
| PLT changes before and after surgery (× | 30.0 (9.0, 43.5) | 36.0 (16.5, 59.2) | -1.616 | 0.120 |
| AFP changes 1 mo before and after surgery (ng/mL) | 0.8 (-0.3, 31.9) | 19.0 (0.0, 554.6) | -1.219 | 0.228 |
The common postoperative reactions of the patients are shown in Table 3. In terms of postoperative fever (maximum body temperature) in the drug-loaded microsphere group and the conventional TACE group, the drug-loaded microsphere group was 38.0 ± 0.9 °C, the conventional TACE group was 38.3 ± 0.7 °C, while the mean body temperature in the conventional TACE group was slightly higher but not statistically different between the two groups. In the postoperative 24 h PONV score and postoperative pain score, the traditional TACE group was higher than the drug-loaded microsphere group, and the data of the two groups were statistically different.
| Postoperative response | D-TACE (15 cases) | C-TACE (58 cases) | t/χ2 value | P value |
| Body temperature (℃) | 38.0 ± 0.9 | 38.3 ± 0.7 | -1.4141 | 0.162 |
| 24 h PONV score postoperative | 14.33 | 0.014 | ||
| 1 | 6 (40.0) | 3 (5.2) | ||
| 2 | 4 (26.7) | 20 (34.5) | ||
| 3 | 3 (20.0) | 20 (34.5) | ||
| 4 | 2 (13.3) | 9 (15.5) | ||
| 5 | 0 (0.0) | 4 (6.9) | ||
| 6 | 0 (0.0) | 2 (3.4) | ||
| Postoperative pain score | 32.967 | 0.000 | ||
| 0 | 5 (33.3) | 15 (25.9) | ||
| 2 | 7 (46.7) | 0 (0.0) | ||
| 4 | 3 (20.0) | 39 (67.2) | ||
| 6 | 0 (0.0) | 4 (6.9) |
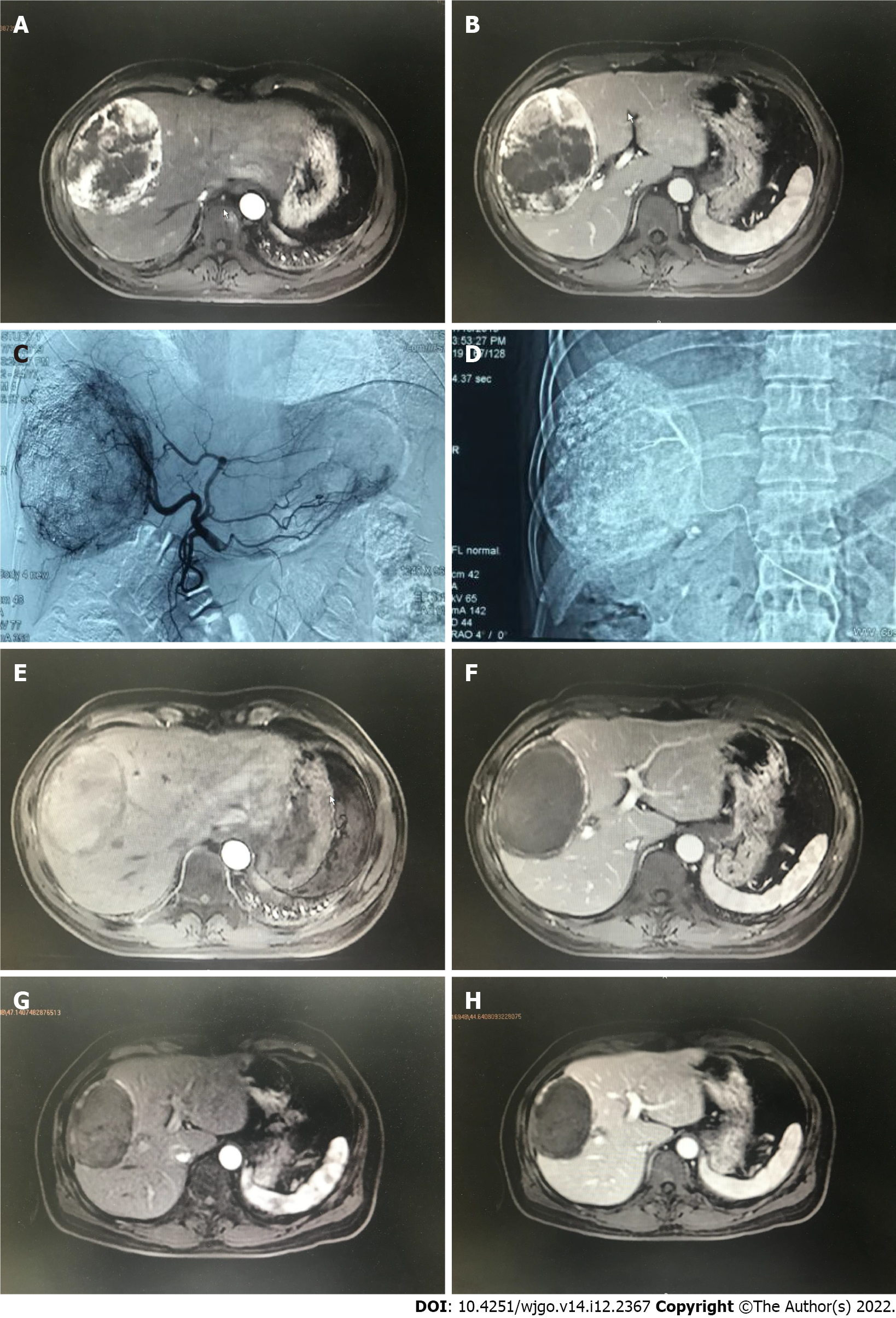
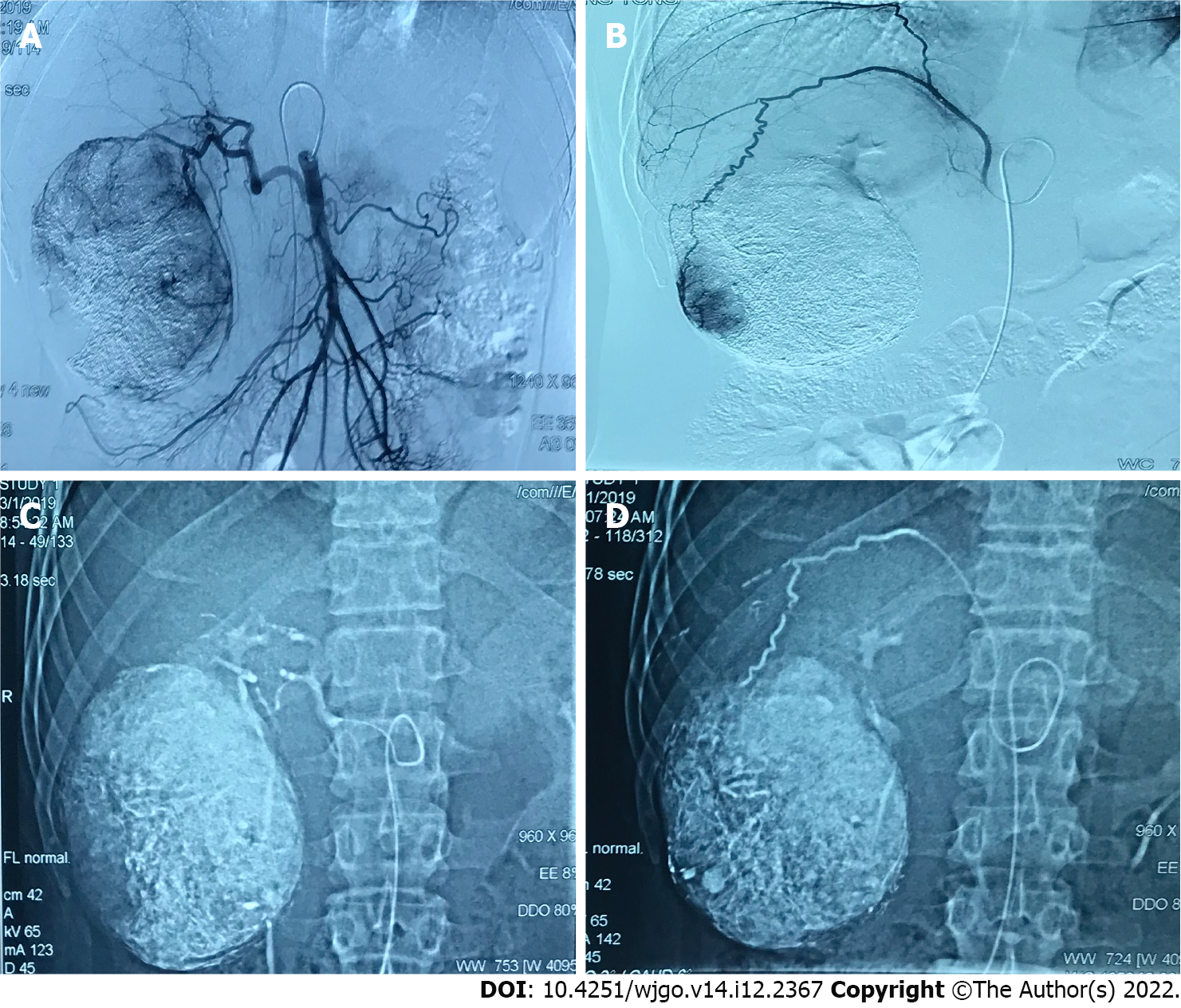
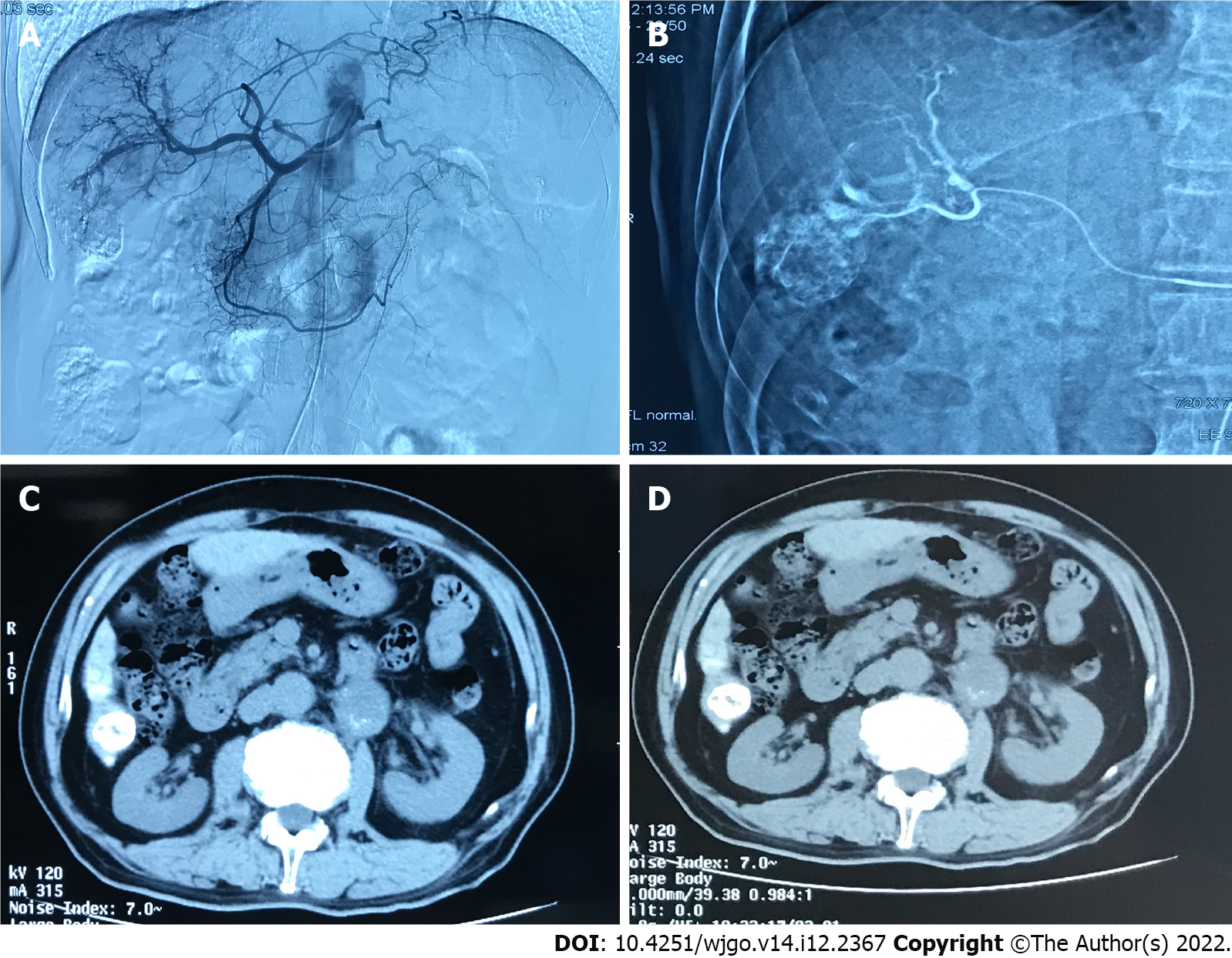
The tumor control status of the patients at 1 and 3 mo after surgery is shown in Table 4. The objective tumor response rate (CR + PR) was 66.6% and the disease control rate (CR + PR + SD) was 86.6% in the drug-loaded microsphere group, while the objective tumor response rate (CR + PR) was 70.7% and the disease control rate (CR + PR + SD) was 82.8% in the conventional TACE group at 1 mo after treatment. There was no statistical difference between the data of the two groups. The objective tumor response rate (CR + PR) and disease control rate (CR + PR + SD) were 60% at 3 mo after treatment in the drug-loaded microsphere group, while the objective tumor response rate (CR + PR) and disease control rate (CR + PR + SD) were 67.2% and 75.9% at 3 mo after treatment in the conventional TACE group, and there was no statistical difference between the two groups. The number of interventions received during the follow-up period was 1 in the drug-laden microsphere group; There were 32 patients who received 1 treatment, 24 cases received 2 treatments, and 2 patients received 3 treatments in the conventional TACE group, with a mean of 1.48 treatments. And the number of interventions in the two groups was statistically different. We briefly described three typical cases diagnosed as primary liver cancer for references (Figure 1, Figure 2, and Figure 3).
| Follow-up status | D-TACE (15 cases) | C-TACE (58 cases) | χ2 value | P value |
| Efficacy evaluation one month postoperative | 3.307 | 0.347 | ||
| CR | 2 (13.3) | 20 (34.5) | ||
| PR | 8 (53.3) | 21 (36.2) | ||
| SD | 3 (20.0) | 7 (12.1) | ||
| PD | 2 (13.3) | 10 (17.2) | ||
| Evaluation of curative effect in 3 mo postoperative | 4.091 | 0.252 | ||
| CR | 2 (13.3) | 18 (31.0) | ||
| PR | 7 (46.7) | 21 (36.2) | ||
| SD | 0 (0.0) | 5 (8.6) | ||
| PD | 6 (40.0) | 14 (24.1) | ||
| Number of interventional treatments | 10.444 | 0.005 | ||
| 1 | 15 (100.0) | 32 (55.2) | ||
| 2 | 0 (0.0) | 24 (41.4) | ||
| 3 | 0 (0.0) | 2 (3.4) |
HCC is the most common type of liver cancer and a main cause of tumor-related mortality[22]. As a palliative local treatment for advanced liver cancer, TACE is widely used and is a standard treatment for advanced liver cancer because it is not limited to the location and size of the tumor[23]. However, based on the adverse effects of systemic chemotherapy, HCC may be a tumor relatively resistant to chemotherapy, so the additional role of chemotherapeutic drugs in the embolization process has been controversial[24]. Also, delays or failures in successful treatment can also be attributed to poor health literacy and insurance-specific barriers[25]. We know that TACE acts mainly through embolization of the blood supply artery to the HCC, resulting in tumor tissue ischemia, hypoxia necrosis, and ischemia. Hypoxia may induce angiogenesis, resulting in tumor recurrence and metastasis. Previous studies have confirmed a correlation between polymorphisms of the angiopoietin-2 gene and prognosis in patients after TACE[26]. There is another view that incomplete hypoxia or incomplete devascularization of tumors is a powerful stimulating factor for angiogenesis[27].
Due to the complexity of the tumor blood supply (including the blood supply of the lateral hepatic artery and potential portal vein)[28] and the hemodynamics, it is difficult to achieve complete ischemia and hypoxia-induced tumor cell death after liver cancer embolization, which is the main reason for its recurrence and metastasis after tumor treatment. The basic principle of adding chemotherapeutic drugs to hepatic artery embolization is based on the assumption that chemotherapeutic drugs can enhance the antitumor effect of embolization agents in ischemia and hypoxia and counteract the stimulation of angiogenesis caused by ischemia and hypoxia. Therefore, during treatment, chemotherapeutic drugs are injected into the blood supply artery to improve the antitumor efficacy, and the enhanced cytotoxicity may contribute to the control and reduced recurrence of tumors. However, this hypothesis has not been confirmed in some clinical studies[29,30] because in these reports, TACE combined with local chemotherapy did not show significant survival benefits compared with TAE embolization alone.
There are two main reasons for this result. First, since cTACE has not been standardized in the implementation process, there are certain differences in the use of embolic materials, the selection of chemotherapeutic drugs, the selection of patients and operation technology among different regions and teams, especially in the selection of embolic agents and operation technology. This has caused certain difficulties in the formulation of treatment standards and efficacy evaluation, resulting in differences between different research results.
Therefore, in this study, to avoid the impact of these technical reasons on the results, the authors referred to the treatment recommendations jointly formulated by experts in the global TACE treatment field in 2016[31]. In terms of the selection of chemotherapeutic drugs, adriamycin or platinum-based chemotherapeutic drugs were mainly used in both groups. For the choice of embolization particles, the two groups both received 100-300 µm permanent embolization agents. To ensure the thoroughness of tumor blood supply artery embolization, a variety of imaging techniques (CT, MRI enhancement, CBCT) and digital subtracted angiography were used to identify the tumor blood supply arteries before and after embolization. The microcatheter superselective technique was used during the embolization. Second, during the process of cTACE, chemotherapeutic drugs are often mixed with iodinated oil and injected after emulsification. Due to the unstable physical and chemical properties of this mixture, the chemotherapeutic drugs quickly separate from the oil phase into the water phase. It has been reported that this emulsifier is released completely into the circulation within 4 h after reaching the tumor[32]. Li et al[33] studied the response of HepG2 cells to epirubicin and other chemotherapeutic drugs under different hypoxia and low nutrition conditions in vitro and confirmed that the inhibition rate of HepG2 cells at 24 h was significantly higher than that at 2 h. Similar results published in 2019[34] also confirmed that the cytotoxicity of adriamycin in HepG2 and Huh7 cell models increased over time. Therefore, the chemotherapy effect of cTACE has been controversial for a long time, which may be related to its inability to remain near the tumor cells after application.
In recent years, DEB has been gradually used in TACE. After drug loading, the half-life of DEBs with diameters of 100–300 μm reached 150 h, and the half-life of DEBs with diameters of 700–900 μm reached 1730 h. This feature greatly improved the local action time and concentration of the chemotherapeutic drugs after TACE and reduced the systemic toxicity. The drug loading microsphere preparation process and drug loading concentration are standardized operations, so it is easier to control the differences between different teams. Therefore, in this study, PVA embolization particles with similar embolization effects were used as controls to evaluate the effect of chemotherapy drugs under the new DEB conditions.
HBV infection is usually considered to be an important factor leading to liver cancer and it affects the long-term prognosis of patients with liver cancer. This study enrolled patients with inactive HBV after treatment, so it was not a priority factor affecting the short-term efficacy after treatment. Tumor load is an important factor affecting the short-term efficacy after treatment. From Table 1, we can see that the tumor load of the drug-loaded microsphere group was larger, the average tumor size was 102.7 ± 44.4 mm, and the average tumor size of the traditional TACE group was 75.0 ± 34.1 mm. In the case of a large tumor load, the drug-loaded microsphere group achieved the same short-term effect as the traditional TACE group. To some extent, this suggests that chemotherapy drugs can play a role in TACE. Since the blood supply of large HCC is often complex and there are more or even some portal veins involved in the potential blood supply compared with small HCC, it is difficult to completely block the blood supply by simple embolization. Drug-loaded microspheres can continuously release high-concentration chemotherapeutic drugs to the surrounding tumor for a long time and continue to kill tumor cells. Thus, it makes up for the deficiency of simple embolization treatment, improves the tumor control rate and reduces recurrence.
We also observed the safety of the two treatments. There was no significant difference in liver function biochemical or hematological indices between the two groups before and after the operation, suggesting that there was no significant difference in safety and toxicity between the two treatment methods. In terms of postoperative reactions, the traditional TACE group had more serious postoperative nausea, vomiting and pain symptoms. This is consistent with previous reports, indicating that drug-loaded microspheres can release chemotherapy drugs continuously and slowly, which can effectively reduce the incidence of systemic adverse drug reactions.
This study is a small sample retrospective study; inevitably, there is a certain bias. In addition, due to the many influencing factors on the long-term prognosis, its analysis was not performed. Therefore, the long-term prognosis of these two treatments for patients with advanced liver cancer needs to be further explored in large-sample randomized controlled trials.
In summary, the results of this study show that compared with traditional TACE, D-TACE may have more advantages in the short-term efficacy for treating large HCC in the middle and late stages, but the long-term clinical efficacy needs further follow-up studies. In terms of postoperative reactions, the patients in the drug-loaded microsphere group had less postoperative nausea, vomiting and pain and better subjective tolerance. In addition, the D-TACE can reduce the number of patients needing interventional treatment and improve their quality of life to a certain extent. Therefore, the D-TACE is worthy of clinical promotion.
Liver cancer is a malignant tumor with high morbidity and mortality. One of the main reasons is that conventional Transcatheter arterial chemoembolization (TACE) is not a standardized procedure, and there are some differences in the use of embolization materials, selection of chemotherapeutic drugs, and operation techniques among different regions and teams.
This study compared the effect of using the same size of embolization particles and drug-eluting bead (DEB) during TACE. We investigated the effect and systemic response of chemotherapy drugs in TACE under the new local drug delivery mode.
This study aimed to explore the short-term efficacy of drug-loaded microspheres TACE (D-TACE) and traditional TACE in the treatment of advanced liver cancer.
The clinical data of 73 patients with advanced liver cancer admitted to the First and Sixth Medical Centers of Chinese PLA General Hospital from January 2017 to October 2019 were retrospectively analyzed.
There were no statistical differences in the values of glutamic oxalyl transaminase, glutamic alanine transaminase, total bilirubin, serum albumin, leukocytes, red blood cells, and platelets before and after surgery in the drug-laden microsphere group and the conventional TACE group. In the postoperative 24 h postoperative nausea and vomiting score and postoperative pain score, the traditional TACE group was higher than the drug-loaded microsphere group, and the data of the two groups were statistically different.
The authors found that compared with traditional TACE, D-TACE may have more advantages in the short-term efficacy for treating large hepatocellular carcinoma in the middle and late stages.
D-TACE is worthy of clinical promotion. However, the long-term prognosis of D-TACE and traditional TACE for patients with advanced liver cancer needs to be further explored in large-sample randomized controlled trials.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Andersen JB, Denmark; Pandya S, United States; Shroff RT, United States S-Editor: Wang JL L-Editor: A P-Editor: Wang JL
| 1. | Yamashita T, Kaneko S. [Liver Cancer]. Rinsho Byori. 2016;64:787-796. [PubMed] |
| 2. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13211] [Article Influence: 1467.9] [Reference Citation Analysis (3)] |
| 3. | Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, Zhu AX, Murad MH, Marrero JA. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2107] [Cited by in RCA: 3025] [Article Influence: 432.1] [Reference Citation Analysis (3)] |
| 4. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6052] [Article Influence: 864.6] [Reference Citation Analysis (3)] |
| 5. | Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2800] [Cited by in RCA: 4102] [Article Influence: 586.0] [Reference Citation Analysis (6)] |
| 6. | Kudo M, Han KH, Ye SL, Zhou J, Huang YH, Lin SM, Wang CK, Ikeda M, Chan SL, Choo SP, Miyayama S, Cheng AL. A Changing Paradigm for the Treatment of Intermediate-Stage Hepatocellular Carcinoma: Asia-Pacific Primary Liver Cancer Expert Consensus Statements. Liver Cancer. 2020;9:245-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 7. | Tsochatzis EA, Fatourou E, O'Beirne J, Meyer T, Burroughs AK. Transarterial chemoembolization and bland embolization for hepatocellular carcinoma. World J Gastroenterol. 2014;20:3069-3077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 76] [Article Influence: 6.9] [Reference Citation Analysis (1)] |
| 8. | Facciorusso A. Drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma: Current state of the art. World J Gastroenterol. 2018;24:161-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 99] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (1)] |
| 9. | Kim KW, Van den Abbeele AD. Evolution of Transarterial Chemoembolization for the Treatment of Liver Cancer. Radiology. 2019;293:704-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Zhou GH, Han J, Sun JH, Zhang YL, Zhou TY, Nie CH, Zhu TY, Chen SQ, Wang BQ, Yu ZN, Wang HL, Chen LM, Wang WL, Zheng SS. Efficacy and safety profile of drug-eluting beads transarterial chemoembolization by CalliSpheres® beads in Chinese hepatocellular carcinoma patients. BMC Cancer. 2018;18:644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Yang B, Liang J, Qu Z, Yang F, Liao Z, Gou H. Transarterial strategies for the treatment of unresectable hepatocellular carcinoma: A systematic review. PLoS One. 2020;15:e0227475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 12. | Han T, Yang X, Zhang Y, Li G, Liu L, Chen T, Zheng Z. The clinical safety and efficacy of conventional transcatheter arterial chemoembolization and drug-eluting beads-transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: A meta-analysis. Biosci Trends. 2019;13:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 13. | Liu YS, Lin CY, Chuang MT, Tsai YS, Wang CK, Ou MC. Five-year outcome of conventional and drug-eluting transcatheter arterial chemoembolization in patients with hepatocellular carcinoma. BMC Gastroenterol. 2018;18:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 14. | Wu B, Zhou J, Ling G, Zhu D, Long Q. CalliSpheres drug-eluting beads versus lipiodol transarterial chemoembolization in the treatment of hepatocellular carcinoma: a short-term efficacy and safety study. World J Surg Oncol. 2018;16:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 15. | Zhang ZS, Li HZ, Ma C, Xiao YD. Conventional versus drug-eluting beads chemoembolization for infiltrative hepatocellular carcinoma: a comparison of efficacy and safety. BMC Cancer. 2019;19:1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Ma Y, Zhao C, Zhao H, Li H, Chen C, Xiang H, Zheng C, Ma C, Luo C, Qiu H, Yao Y, Hu H, Xiong B, Zhou J, Zhu H, Long Q. Comparison of treatment efficacy and safety between drug-eluting bead transarterial chemoembolization with CalliSpheres® microspheres and conventional transarterial chemoembolization as first-line treatment in hepatocellular carcinoma patients. Am J Transl Res. 2019;11:7456-7470. [PubMed] |
| 17. | Karalli A, Teiler J, Haji M, Seth E, Brismar TB, Wahlin S, Axelsson R, Stål P. Comparison of lipiodol infusion and drug-eluting beads transarterial chemoembolization of hepatocellular carcinoma in a real-life setting. Scand J Gastroenterol. 2019;54:905-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Kang YJ, Lee BC, Kim JK, Yim NY, Kim HO, Cho SB, Jeong YY. Conventional Versus Small Doxorubicin-eluting Bead Transcatheter Arterial Chemoembolization for Treating Barcelona Clinic Liver Cancer Stage 0/A Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2020;43:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Melchiorre F, Patella F, Pescatori L, Pesapane F, Fumarola E, Biondetti P, Brambillasca P, Monaco C, Ierardi AM, Franceschelli G, Carrafiello G. DEB-TACE: a standard review. Future Oncol. 2018;14:2969-2984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 20. | Cerrito L, Annicchiarico BE, Iezzi R, Gasbarrini A, Pompili M, Ponziani FR. Treatment of hepatocellular carcinoma in patients with portal vein tumor thrombosis: Beyond the known frontiers. World J Gastroenterol. 2019;25:4360-4382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 82] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (1)] |
| 21. | Lencioni R. New data supporting modified RECIST (mRECIST) for Hepatocellular Carcinoma. Clin Cancer Res. 2013;19:1312-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 22. | Mohamed AA, Omar AAA, El-Awady RR, Hassan SMA, Eitah WMS, Ahmed R, Khater A, Tantawi OMS, Mohamed AA. MiR-155 and MiR-665 Role as Potential Non-invasive Biomarkers for Hepatocellular Carcinoma in Egyptian Patients with Chronic Hepatitis C Virus Infection. J Transl Int Med. 2020;8:32-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Yang B, Li CL, Guo WH, Qin TQ, Jiao H, Fei ZJ, Zhou X, Duan LJ, Liao ZY. Intra-arterial ethanol embolization augments response to TACE for treatment of HCC with portal venous tumor thrombus. BMC Cancer. 2018;18:101. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Lanza E, Muglia R, Bolengo I, Poretti D, D'Antuono F, Ceriani R, Torzilli G, Pedicini V. Survival analysis of 230 patients with unresectable hepatocellular carcinoma treated with bland transarterial embolization. PLoS One. 2020;15:e0227711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 25. | Gomes C, Ginzberg D, Wong RJ. Delays and Gaps in Progressing Through the Hepatitis C Virus Cascade of Care: An Underserved Safety-net Hospital Experience. J Transl Int Med. 2020;8:261-267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Du Z, Tang CH, Li LJ, Kang L, Zhao J, Jin L, Wang CQ, Su CM. Angiopoietin-2 gene polymorphisms are biomarkers for the development and progression of colorectal cancer in Han Chinese. Int J Med Sci. 2020;17:97-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Malagari K, Pomoni M, Kelekis A, Pomoni A, Dourakis S, Spyridopoulos T, Moschouris H, Emmanouil E, Rizos S, Kelekis D. Prospective randomized comparison of chemoembolization with doxorubicin-eluting beads and bland embolization with BeadBlock for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2010;33:541-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 292] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 28. | Miyayama S, Matsui O, Zen Y, Yamashiro M, Hattori Y, Orito N, Matsui K, Tsuji K, Yoshida M, Sudo Y. Portal blood supply to locally progressed hepatocellular carcinoma after transcatheter arterial chemoembolization: Observation on CT during arterial portography. Hepatol Res. 2011;41:853-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Colombo GL, Cammà C, Attili AF, Ganga R, Gaeta GB, Brancaccio G, Franzini JM, Volpe M, Turchetti G. Patterns of treatment and costs of intermediate and advanced hepatocellular carcinoma management in four Italian centers. Ther Clin Risk Manag. 2015;11:1603-1612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Facciorusso A, Bellanti F, Villani R, Salvatore V, Muscatiello N, Piscaglia F, Vendemiale G, Serviddio G. Transarterial chemoembolization vs bland embolization in hepatocellular carcinoma: A meta-analysis of randomized trials. United European Gastroenterol J. 2017;5:511-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 76] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | de Baere T, Arai Y, Lencioni R, Geschwind JF, Rilling W, Salem R, Matsui O, Soulen MC. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. Cardiovasc Intervent Radiol. 2016;39:334-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 199] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 32. | Lewis AL, Gonzalez MV, Lloyd AW, Hall B, Tang Y, Willis SL, Leppard SW, Wolfenden LC, Palmer RR, Stratford PW. DC bead: in vitro characterization of a drug-delivery device for transarterial chemoembolization. J Vasc Interv Radiol. 2006;17:335-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 323] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 33. | Li Q, Zhu LZ, Yang RJ, Zhu X. Cytotoxic activity of anticancer drugs on hepatocellular carcinoma cells in hypoxic-hyponutritional culture. Int Surg. 2014;99:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Dubbelboer IR, Pavlovic N, Heindryckx F, Sjögren E, Lennernäs H. Liver Cancer Cell Lines Treated with Doxorubicin under Normoxia and Hypoxia: Cell Viability and Oncologic Protein Profile. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |