Published online Nov 15, 2022. doi: 10.4251/wjgo.v14.i11.2295
Peer-review started: September 2, 2022
First decision: September 19, 2022
Revised: September 25, 2022
Accepted: October 17, 2022
Article in press: October 17, 2022
Published online: November 15, 2022
Processing time: 74 Days and 2 Hours
Adenocarcinoma has the highest incidence among malignant tumors of the small intestine (SI). Squamous cell carcinoma (SCC) often occurs in organs covered with squamous epithelium. Primary or metastatic SCC originating from the SI is very rare, with very few cases reported in the literature.
This case report involves a 69-year-old man who developed abdominal pain after lunch. After admission, an abdominal computed tomography scan revealed perforation of the alimentary canal and multiple abnormal low-density lesions in the liver. During laparotomy, an approximately 4 cm × 3 cm-sized solid tumor was found in the jejunum, located 30 cm from the Treitz ligament, with a perforation. An intestinal segment of approximately 15 cm was removed, including the perforated portion. The pathological result was SCC. In combination with liver imaging, a diagnosis of SI SCC with multiple liver metastases was considered. The patient died from hepatic failure 1 mo after the operation.
SI tumors are very rare compared to those originating in other digestive organs. Due to its insidious onset, the diagnosis of this disease is usually delayed. Clinicians must pay close attention to digestive symptoms such as persistent abdominal pain and melena.
Core Tip: Squamous cell carcinoma (SCC) in the small bowel is a rare pathologic category. Clinical symptoms are not evident, and it is challenging to determine whether it is the small intestine’s primary or metastatic SCC. This paper describes a 69-year-old male patient diagnosed with SCC of the small intestine and hepatic metastases. Effective diagnosis and early treatment are vital in improving the prognosis of malignant small bowel tumors. Radical resection should be undertaken if no metastases are found.
- Citation: Xiao L, Sun L, Zhang JX, Pan YS. Rare squamous cell carcinoma of the jejunum causing perforated peritonitis: A case report. World J Gastrointest Oncol 2022; 14(11): 2295-2301
- URL: https://www.wjgnet.com/1948-5204/full/v14/i11/2295.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i11.2295
Small intestinal (SI) tumors are very rare compared to other digestive organs[1]. The incidence of small bowel tumors accounts for only 0.6% of all malignant tumors, including about 1%-3% of gastrointestinal malignancies[2]. Previous studies of malignant tumors have reported that approximately 30% to 50% are adenocarcinoma, 25% to 30% are carcinoid, and 15% to 20% are lymphoma[3]. Primary squamous cell carcinoma (SCC) of the SI is extremely rare, with only a few reports in the literature[4-8]. This paper describes a surgically treated patient with SCC arising from the jejunum with perforated peritonitis and multiple liver metastases.
The patient’s main complaints were epigastric pain after eating, nausea and vomiting, and then gradually full abdominal distension.
The patient developed epigastric pain and nausea half an hour after lunch. He then began vomiting; the vomitus was the stomach contents. Finally, he experienced full abdominal distension.
The patient was diagnosed with hypertension and diabetes, which were well-controlled with oral medications.
The patient had no history of SCC, and his family was negative for cancer.
After admission, the patient’s blood pressure was 125/71 mmHg, heart rate was 89 bpm, and body temperature was 36.7 °C. Abdominal tenderness, rebounding pain, muscle tension, and acute peritonitis were noted. Notably, no enlarged lymph nodes were found during the physical examination.
Blood analysis revealed a white blood cell count of 10.96 × 109/L, hemoglobin concentration of 124 g/L, neutrophil count of 8.96 × 109/L, and hypersensitive C-reactive protein level of 53 mg/L. Creatinine (141.7 Umol/L), albumin (40 g/L), alanine aminotransferase, and aspartate aminotransferase levels were normal. His glucose level was 15.6 mmol/L, prothrombin international normalized ratio was 1.14, and D-dimer was 0.77 mg/L.
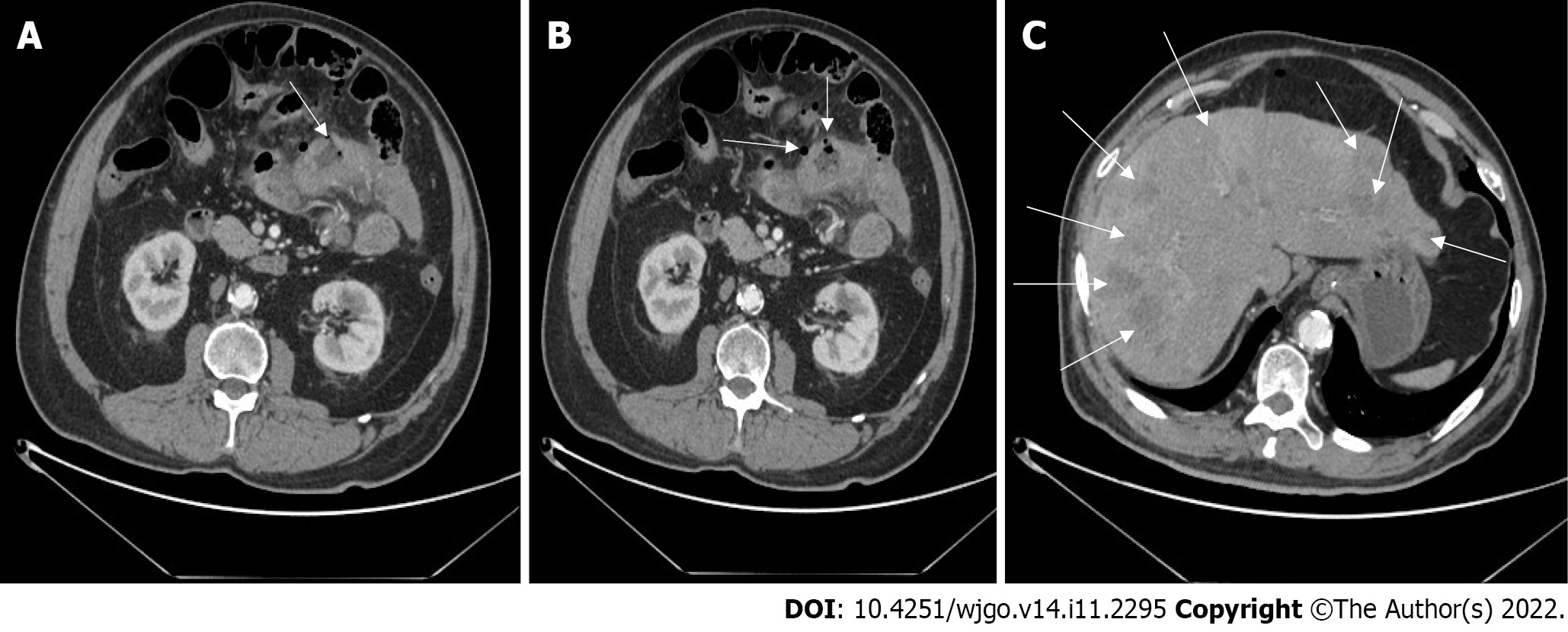
A computed tomography (CT) scan showed that the SI wall of the left upper abdomen was irregularly thickened, and free gas appeared in the abdominal cavity. Multiple round low-density nodules of varying sizes can be seen in the liver parenchyma (Figure 1).
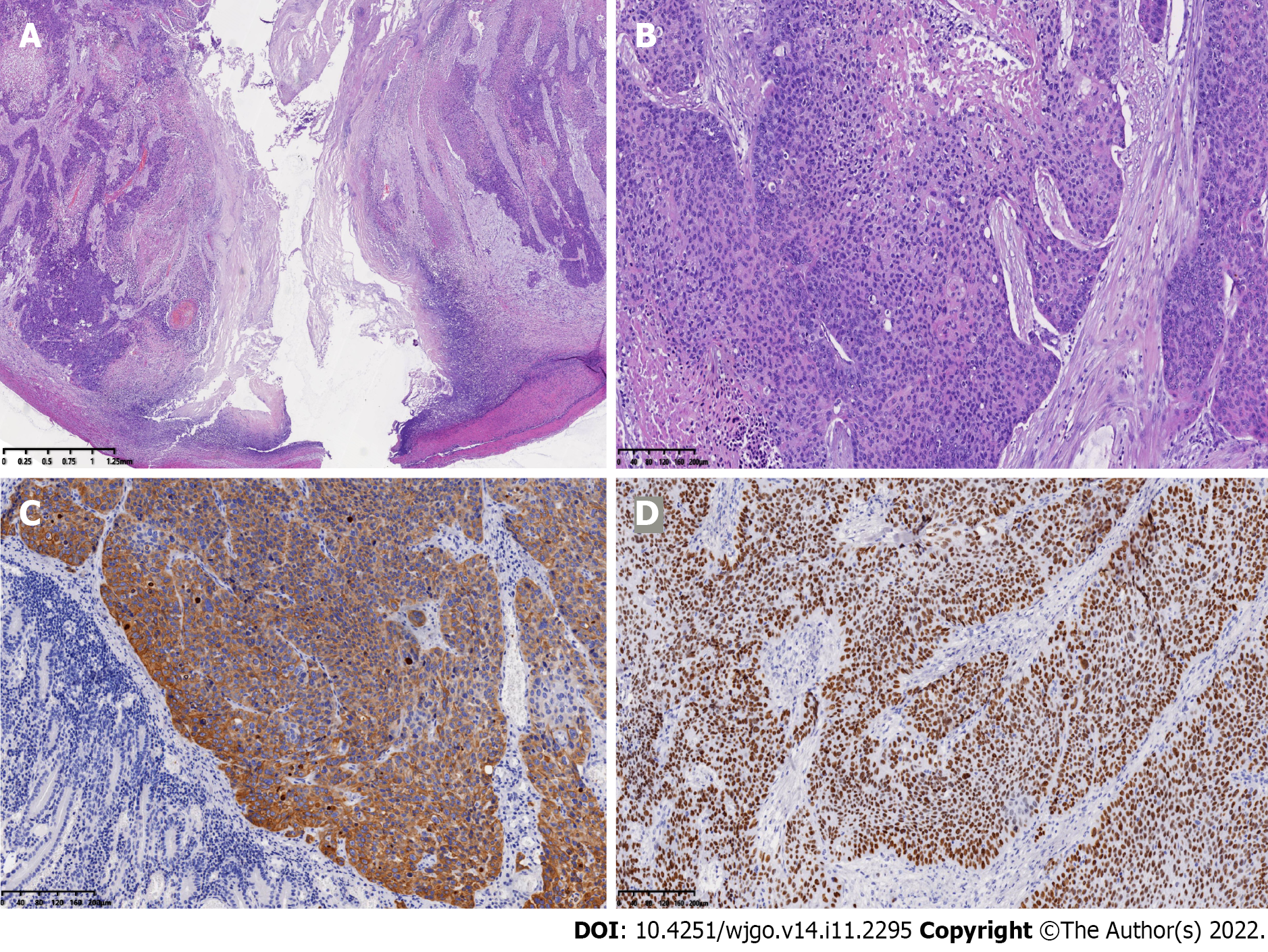
Postoperative pathology showed an approximately 4 cm × 3 cm × 1 cm-sized ulcerative tumor of the SI from the jejunum, which had infiltrated the entire thickness of the intestinal wall. Tumor cells presented as a poorly differentiated carcinoma, growing in nests, and intracellular dyskeratosis was visible (Figure 2A and B). No tumor cells were seen in the corresponding mesenteric adipose tissue and lymph nodes. Immunohistochemical findings demonstrated strong positivity for cytokeratin and antioncogene P40 (Figure 2C and D). These results were consistent with a diagnosis of SCC.
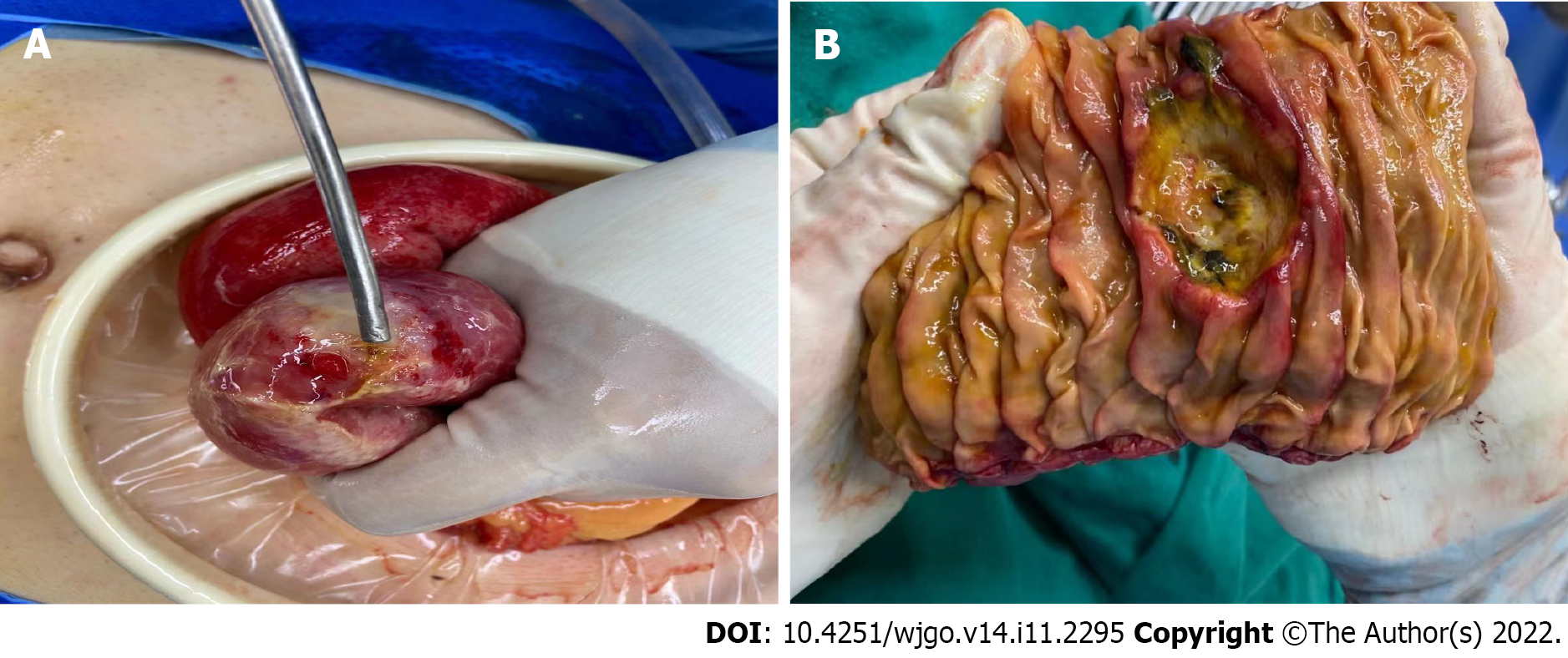
Laparotomy revealed that the SI was extensively edematous. A mass-like lesion about 4 cm in diameter with perforation (Figure 3) was identified. An intestinal segmental resection of about 15 cm, including the perforation site and the corresponding mesentery, was removed. An end-to-end intestinal anastomosis was performed, and the abdominal cavity was flushed with physiological saline solution.
The surgery was completed successfully. Palliative chemotherapy combined with immunotherapy was recommended, according to the opinion of chemotherapy specialists. Due to the patient’s poor physical condition, his family refused further treatment and only relieved his pain. The patient was discharged on postoperative day 6. However, he had advanced-stage malignancy and died from hepatic failure 1 mo after the operation.
The SI represents the longest part of the digestive tract, accounting for about 75% of the total length of the gastrointestinal canal and more than 90% of the mucosal surface. However, malignant tumors rarely develops in the SI[9]. The unique environment in the small bowel, including complex factors such as pH, immune function, and various enzymes, may be related to the low incidence of small bowel tumors[10]. Small bowel tumors are rare globally, and according to the “age standard of the world population”, the global incidence rate is less than 1.0 per 100000, ranging from 0.3 to 2.0[11]. SCC is even rarer among SI malignancies. Generally, SCC occurs in parts of the body covered by squamous epithelium, such as the skin, oral cavity, esophagus, and cervix. Some organs not covered by squamous epithelium can develop SCC through squamous epithelial metaplasia, such as the bronchus and gallbladder. SCC of the SI is extremely rare compared to other gastrointestinal tumors, accounting for approximately 2% among 1312 specimens of SI tumors[12]. More commonly, SCC detected in the intestine represents metastatic cancer from other organs. Lung cancer commonly metastasizes to the SI[13-15]. Other cancers known to metastasize to the SI include mandibular gingiva, esophagus, and cervix cancers[16-20]. Metastatic SCC of the SI is 2.5 times more common than primary SCC of the SI at autopsy[21].
The origin of primary SCC of the SI may be related to the malignant transformation of undifferentiated basal cells of the SI mucosal epithelium. There are three possible mechanisms of SCC developing in the SI: (1) Pluripotent stem cells differentiate into malignant squamous cells; (2) malignant transformation of ectopic squamous epithelium; and (3) malignant changes in squamous metaplasia caused by chronic mucosal damage[22]. These three pathways were supported by Platt et al[23]. The diagnosis of SCC must be rigorous, and key considerations are: (1) The characteristics of a malignant tumor, such as apparent atypia and nested distribution; (2) the characteristics of the epithelial cells, such as the formation of a keratinized pearl; (3) lack of glandular components and glandular epithelium; and (4) no evidence of involvement of primary SCC of other organs. Pathologically, it is challenging to identify tumor cells as a primary or metastatic feature of SCC in the SI, especially when metastatic tumors reach mucosal surfaces[24]. For rare SCC of the SI, when the histology is atypical and the cytokeratin and intercellular bridge structure are not obvious, it should be distinguished from carcinoids in the SI. Immunohistochemistry and neuroendocrine granules can be used to make such a differentiation.
In this case study, the patient was admitted to hospital with acute abdominal pain. Emergency surgery was performed because of peritonitis due to jejunal perforation, identified by relevant imaging and physical examinations. Postoperative pathology revealed disorderly growth of the squamous epithelial cells in large nests with pink keratin in the center. Immunohistochemical findings demonstrated that staining for cytokeratin-5/6 and antioncogene P40 was both strongly positive. Additionally, no other tissues or organs yield positive findings, including the respiratory, alimentary, and urogenital tracts.
In contrast, computed tomography imaging identified multiple low-density masses in the liver. The patient had no history of SCC, so a diagnosis of SCC of the SI, adenocarcinoma, and carcinoids is excluded. Despite showing multiple lesions on liver imaging, the patient refused to undergo contrast-enhanced MRI or liver puncture for pathology, due to poor physical condition. The multiple liver metastases of SI SCC via hematogenous spread were the considered diagnosis and may be related to the liver’s perfusion of the portal vein system. There is currently no postoperative adjuvant therapy for small bowel SCC other than surgical resection worldwide. Chemotherapy (taxanes and platinums) combined with immunotherapy was recommended, referring to the treatment for esophageal and lung SCC, but with no evidence support. The patient’s family refused further medical treatment due to his poor physical condition and only relieved his pain. He died from hepatic failure 1 mo after the operation.
Neoplasms of the SI are rare, and several different histological types of cancer can occur in the SI. The clinical symptoms are not specific. It is challenging to access the SI via conventional endoscopy, making the diagnosis of SI tumors difficult. Most patients are hospitalized for complications of the disease, with surgical R0 resections challenging because of the advanced stages of the disease at diagnosis. Capsule endoscopy is considered the best way to visualize the entire SI. It is also considered the first diagnostic method for gastrointestinal bleeding of unknown origin after a negative upper gastrointestinal endoscopy and colonoscopy. Many advances have been made in the clinical treatment of adenocarcinoma as well as stromal and neuroendocrine tumors arising from the SI[25]. As SI squamous tumors are rare, more extensive cases and studies are necessary to achieve a well-designed clinical trial. The comprehensive treatment of SI SCC is challenging and requires further medical research. Once a small bowel tumor is diagnosed, radical resection should be performed as soon as possible, representing resections of at least 10 cm of the involved region and the corresponding mesenteric lymph nodes to improve overall survival[26].
Malignant tumors of the SI are uncommon cancers and are easily misdiagnosed in the clinic. Therefore, most small bowel tumors are in the advanced stages when patients are admitted to the hospital. Early detection and diagnosis are of great significance for the optimal prognosis of patients. Clinicians should pay close attention to the symptoms of patients presenting with acute abdominal pain, such as acute peritonitis, bowel obstruction, and intussusception, during clinical diagnosis and treatment. Surgical resection is currently the most effective treatment for malignant SI tumors. It is also necessary to treat the patient’s underlying disease to assist them in restoring their health. Further clinical studies and reports of similar cases are required to improve our knowledge of SCC in the SI and ensure the best clinical outcomes.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S, Algeria; Şahin EA, Turkey S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9172] [Cited by in RCA: 9958] [Article Influence: 995.8] [Reference Citation Analysis (0)] |
| 2. | Sarosiek T, Stelmaszuk M. [Small intestine neoplasms]. Pol Merkur Lekarski. 2018;44:45-48. [PubMed] |
| 3. | Gill SS, Heuman DM, Mihas AA. Small intestinal neoplasms. J Clin Gastroenterol. 2001;33:267-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 4. | Sun DS, Shin OR, Ku YM, Kim YS, Seo KJ. Squamous cell carcinoma of the small bowel manifesting as a jejunal perforation: a case report. Int J Clin Exp Pathol. 2014;7:6345-6349. [PubMed] |
| 5. | Battal M, Bostancı O, Basak T, Kartal K, Ekiz F. Pure squamous cell carcinoma of the duodenum. Case Rep Surg. 2015;2015:714640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Bao Y, Zhong ZX, Yu YW. Squamous cell carcinoma of small intestine: a case report. Chin Med Sci J. 2014;29:239-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Nandedkar SS, Trivedi KK, Malukani K. Primary squamous cell carcinoma of the small intestine. J Cancer Res Ther. 2013;9:739-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 8. | Mumtaz S, Ahmad Z, Fatima S, Qureshi A. Squamous cell carcinoma in the small intestine. BMJ Case Rep. 2011;2011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Pan SY, Morrison H. Epidemiology of cancer of the small intestine. World J Gastrointest Oncol. 2011;3:33-42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 111] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (1)] |
| 10. | Condino G, Aratari A, Papi C, Catarci M. Gastrointestinal bleeding and severe anaemia: An uncommon presentation of small bowel carcinoma complicating ileal Crohn's disease. Dig Liver Dis. 2015;47:899-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Schottenfeld D, Beebe-Dimmer JL, Vigneau FD. The epidemiology and pathogenesis of neoplasia in the small intestine. Ann Epidemiol. 2009;19:58-69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 204] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 12. | Terada T. Malignant tumors of the small intestine: a histopathologic study of 41 cases among 1,312 consecutive specimens of small intestine. Int J Clin Exp Pathol. 2012;5:203-209. [PubMed] |
| 13. | Costa RS, Vieira AL, Costa JM, Fernandes B, Ferreira A. Metastatic small bowel occlusion as initial presentation of squamous cell carcinoma of the lung. Turk J Gastroenterol. 2019;30:492-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Yamada H, Akahane T, Horiuchi A, Shimada R, Shibuya H, Hayama T, Nozawa K, Ishihara S, Matsuda K, Watanabe T. A case of lung squamous cell carcinoma with metastases to the duodenum and small intestine. Int Surg. 2011;96:176-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Yuksel O, Uyar P, Sahin TT, Demirhan B. Small bowel perforation due to metastatic lung squamous cell carcinoma. Saudi Med J. 2007;28:631-633. [PubMed] |
| 16. | Okamura T, Beppu T, Tokumaru T, Yamada M, Sugiyama T, Koide N, Tani M, Kaneko M, Hamahata A, Nishimura Y, Fukuda T. Cancer of the mandibular gingiva metastasizing to the small intestine. Auris Nasus Larynx. 2019;46:479-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Li R, Chen Z, Wen Q. Metastatic squamous cell carcinoma from hand skin causing small bowel obstruction: an unusual case presentation. World J Surg Oncol. 2014;12:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Wang M, Patel J, Casey TT, Kieffer R, Dunn GD. Metastatic squamous cell carcinoma from the esophagus occurring as small bowel obstruction. South Med J. 1985;78:884-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Hulecki SJ, Klein FA, Davis JE. Squamous cell carcinoma of cervix metastatic to ileal loop. Urology. 1985;26:579-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 20. | Deshpande SH, Kini S, Nawalkar PR, Pandya JS. Metastasis of carcinoma of buccal mucosa to small intestine causing ileal perforation. BMJ Case Rep. 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132:931-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | Wang FD, Wang ZW, Xue HD, Wu HW, Zhang Y, Yu JC, Jin ZY. Primary Squamous Cell Carcinoma of the Small Intestine: Pathogenesis and Clinical Features. Chin Med J (Engl). 2016;129:2131-2133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Platt CC, Haboubi NY, Schofield PF. Primary squamous cell carcinoma of the terminal ileum. J Clin Pathol. 1991;44:253-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Estrella JS, Wu TT, Rashid A, Abraham SC. Mucosal colonization by metastatic carcinoma in the gastrointestinal tract: a potential mimic of primary neoplasia. Am J Surg Pathol. 2011;35:563-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Puccini A, Battaglin F, Lenz HJ. Management of Advanced Small Bowel Cancer. Curr Treat Options Oncol. 2018;19:69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 26. | Xie X, Zhou Z, Song Y, Dang C, Zhang H. Surgical Management and Prognostic Prediction of Adenocarcinoma of Jejunum and Ileum. Sci Rep. 2017;7:15163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |