Published online Nov 15, 2022. doi: 10.4251/wjgo.v14.i11.2238
Peer-review started: August 5, 2022
First decision: September 29, 2022
Revised: October 5, 2022
Accepted: October 27, 2022
Article in press: October 27, 2022
Published online: November 15, 2022
Processing time: 101 Days and 22 Hours
The features of gastric cancer based on the anatomic site remain unknown in northern China patients.
To analyze gastric cancer features and associated trends based on the anatomical site in northern China patients.
This cross-sectional study used incident gastric cancer case data from 10 Peking University-affiliated hospitals (2014 to 2018). The clinical and prevailing local features were analyzed.
A total of 10709 patients were enrolled, including antral (42.97%), cardia (34.30%), and stomach body (18.41%) gastric cancer cases. Cancer in the cardia had the highest male:female ratio, proportion of elderly patients, and patients with complications, including hypertension, diabetes, cerebrovascular, and coronary diseases (P < 0.001). gastric cancer involving the antrum showed the lowest proportion of patients from rural areas and accounted for the highest hospitalization rate and cost (each P < 0.001). The proportion of patients with cancer involving the cardia increased with an increase in the number of gastroesophageal reflux disease cases during the same period (P < 0.001). Multivariate analysis revealed that tumor location in the cardia increased the risk of in-hospital mortality (P = 0.046). Anatomical subsite was not linked to postoperative complications.
The features of gastric cancer based on the anatomical site differ between northern China and other regions, both globally and within the country. Social factors may account for these differences and should affect policy-making and clinical practice.
Core Tip: Cancer in the cardia has the highest male:female ratio, proportion of elderly patients, and patients with complications including hypertension, diabetes, cerebrovascular, and coronary diseases. Gastric cancer in the antrum has the lowest proportion of patients from rural areas and accounts for the highest hospitalization rate and cost. The proportion of patients with cancer in the cardia increases with an increase in the number of gastroesophageal reflux disease cases during the same period. Tumor location in the cardia increases the risk of in-hospital mortality. Anatomical subsite is not linked to postoperative complications.
- Citation: Qu RZ, Ma YP, Bao XY, Tao LY, Zhou X, Lu SY, Zhang Y, Wang BY, Li F, Tuo L, Zhang ZP, Fu W. Features of gastric cancer by anatomic subsite in northern China: A multi-center Health Science Report database study. World J Gastrointest Oncol 2022; 14(11): 2238-2252
- URL: https://www.wjgnet.com/1948-5204/full/v14/i11/2238.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i11.2238
Gastric cancer is among the most common digestive malignant tumors worldwide and is predicted to have 27600 incident cases and cause 11010 deaths in the United States of America[1]. It ranks fifth among cancer diagnoses (1089103 cases) and fourth in gross mortality (768793 cases) worldwide[2]. China is among the regions with the highest gastric cancer incidence, reporting over 450000 new cases and 300000 deaths[3]. China may account for approximately half of the annual incidence of gastric cancer in Eastern Asia[4]. High mortality rates are a major concern for gastric cancer. Gastric cancer-related disability-adjusted life-years are the third-highest worldwide[4], accounting for 24.2% of cases with a 5-year overall survival (OS) rate[5]. In this cohort, the 5-year OS of patients with stage IV disease is lower than 4%[6].
A few studies have demonstrated differences in clinical and epidemiological features of this tumor type based on its presence in the anatomical subsites of the stomach. These subsites are usually divided into cardia (including adjacent gastroesophageal junction) and non-cardia locations, including the gastric body and antrum[7]. The constituent ratio of patients with gastric cancer in the cardia tends to be relatively high in Western countries, including the United States of America and the United Kingdom[7-10]. However, this same constituent ratio decreases in some Asian countries, including Japan, with a significant increase in the number of patients with gastric cancer in the stomach corpus[11]. The divergent trends could result from different etiologies for cardia and non-cardia subsites of gastric cancer; e.g., non-cardia gastric cancer (specifically, antral gastric cancer) is directly associated with Helicoibacter pylori (H. pylori)-induced atrophic gastritis and accompanying hypochlorhydria[12,13]. In contrast, cancer involving the cardia (including cancers of the gastroesophageal junction) is closely related to gastroesophageal reflux disease (GERD)[14]. Gastric tumors at different anatomic locations may be distinct clinical entities[15].
Environmental and lifestyle factors affect the burden of gastric cancer. Smoking is an important risk factor in male patients[16], and a high-sodium diet is associated with gastric cancer in Eastern Asian patients, particularly in Chinese patients[4]. The incidence rates and distribution patterns of gastric cancer differ across various geographic regions[17], including within China. Despite the rise in the ratio of patients with cancer at the cardia of the stomach and the concomitant reduction among those with gastric cancer involving the antrum in the Chinese population, the rates of antral gastric cancer vary from 20% to 50%[18,19]. Both trends may result from a divergence in risk factors, including variations in environmental influences and eating habits that differ among regions; these factors may also determine differences among tumors at different locations[18]. However, few population-based studies have been conducted in China on this topic[18,19], and analyses of data from northern China are lacking. Herein, we aimed to examine the clinical features of gastric cancer at different anatomical sites in patients from northern China. We also aimed to examine the associated variability and trends.
Patient information was obtained from the Health Science Report (HSR) database of Peking University-affiliated hospitals[20,21]. As a patient-level database consisting of hospitalized populations from 10 comprehensive tertiary hospitals affiliated with Peking University, the HSR database is managed by the Department of Hospital Management, Peking University Health Science Center, including patients covering all of China (mainly from northern China), and handles 2097347 gastric cancer patients. HSRs are submitted annually by the hospitals, as determined by the guidelines of the National Health Commission of the People’s Republic of China. A system developed by the Medical Information Center of Peking University Health Science Center was applied for integration, storage, management, analysis, and display of the data, and controls for safety and quality were embedded in each layer. Demographic and clinical characteristics of selected patients were extracted, including the corresponding International Classification of Diseases 10 edition (ICD-10) codes, demographic characteristics (age, sex, and others), hospitalization information (route of admission, hospital stay, costs, among others), diagnosis, operation type, and pathological information. Ethical approval was obtained from the Ethics Committee of Peking University Third Hospital (IRB00006761-M2019387). The written informed consent requirement was waived because of the retrospective nature of the study.
According to the accessibility and quality of data, patients registered from January 1, 2014, to December 31, 2018, were chosen for analysis[20]. Individuals who (1) had pathology records with a diagnosis of gastric cancer, (2) were hospitalized in at least one of the included hospitals, and (3) had one or more sets of complete hospitalization records were included in the analysis. Patients who had (1) no pathological diagnosis or (2) tumors in the stomach that had metastasized from other organs were excluded from the analysis. Anonymization of personal information was conducted for data privacy protection.
ICD-10 codes were implemented for facilitated identification, and gastric cancer was designated as C16.0-4 as per published research[19]. Descriptive medical phrases were also applied to query for gastric/cardia/esophagogastric junction/non-cardia/body/antrum/pylorus cancer in the possible linguistic expressions in the Chinese language. Due to differences in anatomical, biological, and clinical characteristics by different subsites in gastric cancer, the selected patients were further divided according to tumor anatomical locations into gastric cancer involving the cardia (ICD-10 code: C16.0), gastric cancer involving the body (ICD-10 codes: C16.1-2), gastric cancer involving the antrum (ICD-10 codes: C16.3-4), and gastric cancers of multiple foci (ICD-10 code: C16.8). For cases without exact diagnosis on anatomical site (ICD-10 code: C16.5-6 and C16.9), diagnosis description and pathological results were screened by two senior gastroenterologists. Patients with unidentifiable subsites were categorized as “other type”. A fuzzy string-matching algorithm was also applied with the listed medical phrases to search for more potential patients to avoid omission. Validation was applied to the classification strategy. Data from a total of 1000 gastric cancer patients were extracted stochastically each time after primary selection, with a respective manual review of the diagnosis by two senior gastroenterologists for detection with the help of R (version 3.5.1), and the final consistency rate was over 99%.
The screened gastric cancer patients were classified by searching for different keywords on clinical, diagnostic, and surgical data with R (version 3.5.1). Only incident cases, which were identified as patients who were pathologically diagnosed through surgery and/or endoscopy for pathology (the gold standard for gastric cancer diagnosis), were defined as the study population. Those with multiple hospitalization records were identified by health care card numbers, and only their first visits were included to avoid duplication. The composition ratio of each anatomical subsite was calculated separately, and clinical characteristics, including age, sex, hospitalization costs, hospitalization stay, admission mode, and disease-related complications, were calculated based on tumor location. Alternation trends for some factors within the study period were calculated. According to worldwide guidelines that recommend endoscopy screening for gastric cancer from the age of 50 years, patients were categorized into age groups of ≤ 49 years, 50-74 years, and ≥ 75 years, and the age group of 50-74 years was further analyzed by dividing into four groups with 5-year increments. Patients with records of surgery (including laparoscopic or open tumor resection and excluding endoscopy and endoscopic resection) were selected, and the short-term postoperative complications, including anastomotic leakage, anastomotic hemorrhage, abdominal hemorrhage, abdominal infection, gastroparesis, incision infection, incision hemorrhage, incision dehiscence, and pancreatic fistula, were indexed.
Continuous variables are expressed as means ± SD, and categorical variables are expressed as frequencies and proportions. Student’s t-test was used to compare continuous variables, and the chi-squared test was used to compare categorical variables. SPSS (SPSS Inc., Armonk, Chicago, IL, United States, version 26.0) was used for all statistical analyses, and a two-sided test was considered statistically significant at P value of < 0.05.
The association between gastric cancer and risk of in-hospital death or short-term postoperative complications was examined in the involved gastric cancer patients (postoperative complications were examined in patients who had undergone surgery) by performing a multivariate analysis adjusted for sex, age, anatomical subsite, complications (including hypertension, diabetes, cerebrovascular disease, coronary disease, reflux syndrome, anemia, and hypoproteinemia), and operation (for analysis of in-hospital mortality). Logistic regression was used in indicators with occurrence higher than 5%, and Poisson regression was used in indicators with occurrence lower than 5%. Knots were used according to the principle of minimized Akaike information criterion. Adjusted odds and transformed odds ratios (aORs) were used to estimate the absolute risks (probabilities) with 95% confidence intervals (CIs).
Patients selected from the database originated from 10 affiliated hospitals of Peking University across nearly all provinces of China, while most came from northern China. In the aggregate, 2097347 hospitalizations between January 1, 2014, and December 31, 2018, were eligible (including 289561, 309776, 462175, 490020, and 545815 annual hospitalizations, respectively). After further selection, 10709 incident gastric cancer cases were chosen, including 2608, 2429, 2614, 2744, and 2811 cases from 2014 to 2018, respectively. A total of 72.71% of the patients were men. Patients originated nationwide but were mainly from northern China and Beijing, Inner Mongolia, and Hebei (Supplementary Figure 1). The average age of the patients with incident gastric cancer was 61.18 years ± 11.91 years (95%CI: 60.96-61.41). The mean hospitalization cost was 55.70 (95%CI: 54.79-56.60) thousand RMB (approximately 8.77 thousand USD).
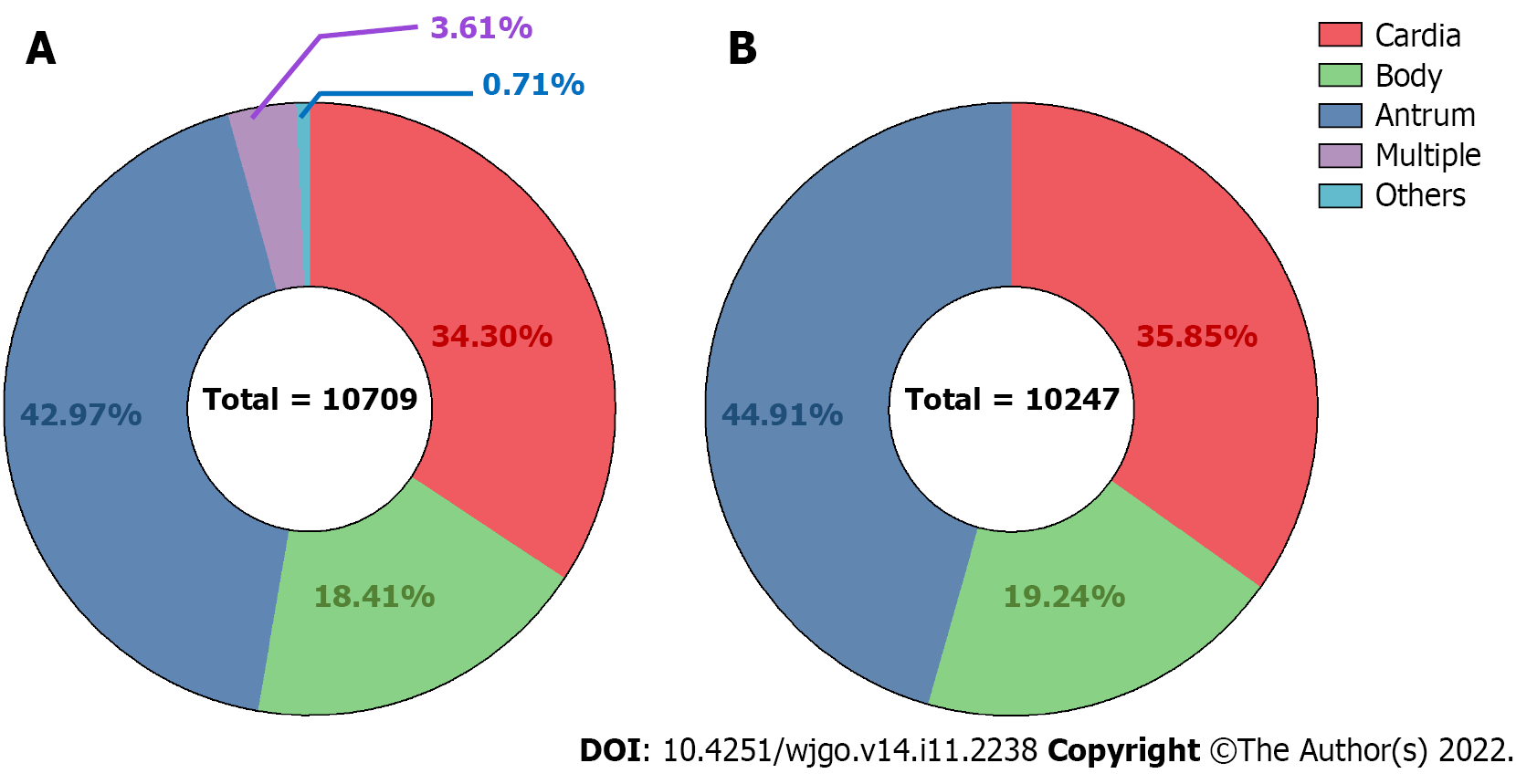
A total of 4602 (42.97%), 3673 (34.30%), 1972 (18.41%), 386 (3.61%), and 76 (0.71%) cases were antrum, cardia, gastric body, multiple site, and unclear site gastric cancer, respectively (Figure 1A). Among 10,247 cases extracted for proportion calculation, the ratios of the gastric antrum, cardia, and body cancers were 44.91%, 35.85%, and 19.24%, respectively (Figure 1B). Data from cases from different regions worldwide were collected from previous studies, and the proportions of cardia and non-cardia (including body and antrum) cases were recalculated (Table 1).
| Region | Ref. | Period | Anatomical site ratio (%)1 | |||||
| Cardia | Non-Cardia | |||||||
| Body | Antrum | |||||||
| East Asia | Northern China | This article | 2014-2018 | 35.85% | 19.24% | 44.91% | ||
| Southwest China | Liu et al[19], 2016 | 2008-2012 | 37.15% | 10.30% | 52.55% | |||
| Northwest China | Zhou et al[18], 2008 | 1993-2004 | 35.78% | 28.00% | 36.22% | |||
| Japan | Koizumi et al[11], 2018 | 2013-2015 | 9.82% | 53.58% | 36.60% | |||
| West Asia | Northwest Iran | Derakhshan et al[29], 2004 | 2000-2003 | 44.78% | 26.19% | 29.03% | ||
| North America | The USA | Camargo et al[7], 2011 | 1999-2007 | 41.41% | 11.13% | 47.46% | ||
| Europe | Central Switzerland | Schmassmann et al[26], 2009 | 1982-2007 | 26.02% | 73.98% | |||
| Spain | Aragonés et al[27], 2010 | 1980-2004 | 26.67% | 73.33% | ||||
| Netherland | Holster et al[28], 2014 | 1973-2011 | 31.82% | 30.30% | 37.88% | |||
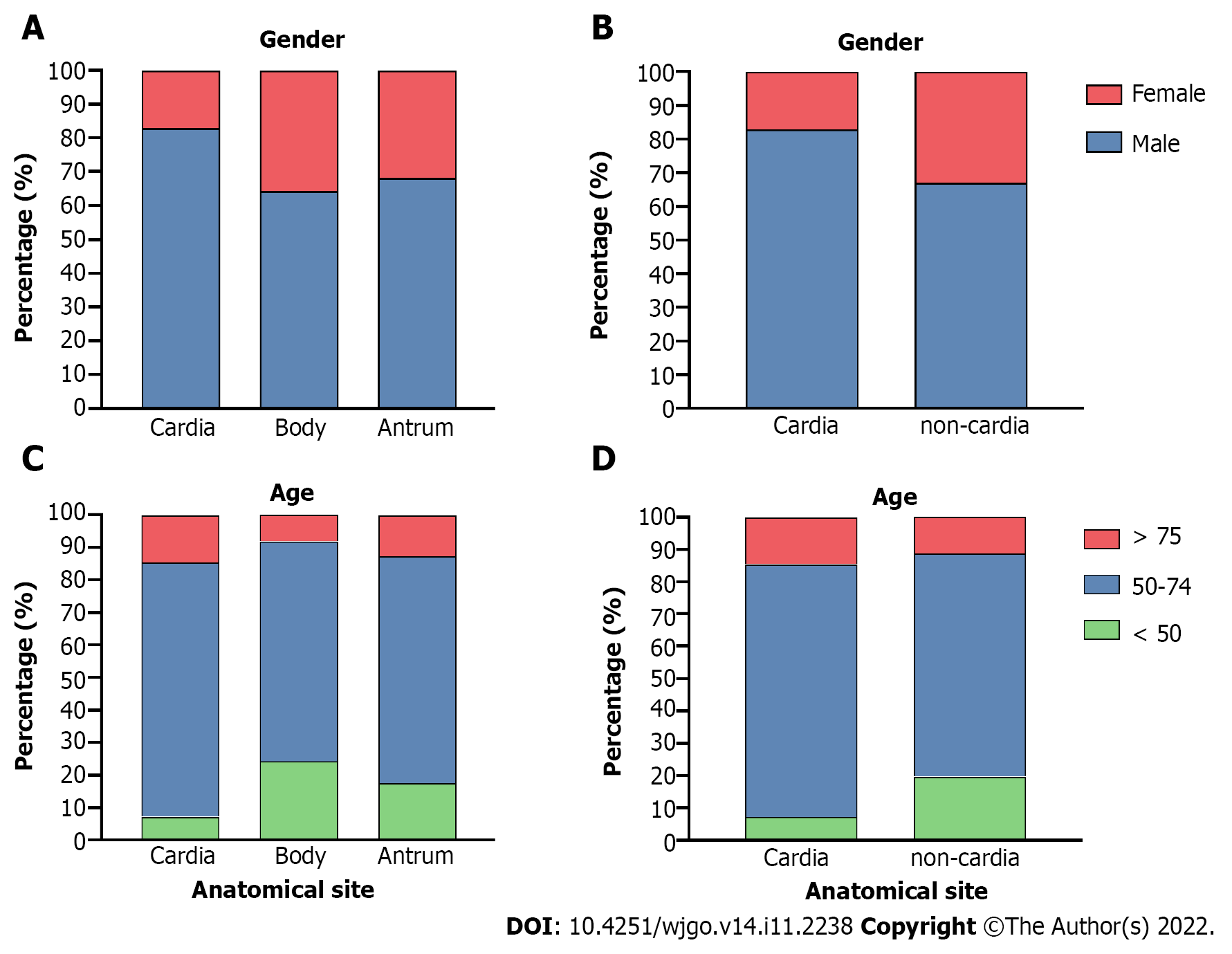
Gastric cardia, body, and antrum cancer cases were extracted for clinical feature analysis, and the body and antrum cases were further classified as “non-cardia cancer” for additional analyses. Both cardia and non-cardia cancers showed a higher proportion of male patients, while the male:female ratio in cardia cancer cases was approximately 5:1; it was approximately 2:1 in non-cardia cancer cases (Table 1, Figure 2A and B, P < 0.001 both in the comparison of the three subsites and of cardia and non-cardia cancer cases). The average age of patients with gastric cardia, body, and antrum cancers were 63.98, 57.57, and 60.32 years, respectively (Table 2, P < 0.001). After dividing the cases into three age subgroups (≤ 49 years, 50-74 years, and ≥ 75 years), both cardia and non-cardia cancers at the three anatomical subsites showed most patients in the age group of 50-74 years. However, non-cardia cancer was more prevalent among patients younger than 50 years, and cardia cancer was more prevalent among patients older than 75 years (Figure 2C and D, P < 0.001 both in three subsites and in cardia and non-cardia cancer cases). Due to the location of the involved hospitals, most patients came from urban areas, while gastric antrum cancer accounted for the lowest proportion of patients from the rural area compared to those from the upper subsites. This finding was further verified through insurance information: patients with cardia cancer had a higher proportion of rural medical insurance settlement (Table 2, P < 0.001, respectively). In addition, gastric cardia cancer had the largest proportion, and gastric body cancer had the lowest proportion of patients with hypertension, diabetes, cerebrovascular disease, and coronary disease (Table 2, P < 0.001, P = 0.03, P = 0.01, and P < 0.001 for hypertension and diabetes, respectively). Moreover, 11.46% of gastric cardia cancer patients had a combined diagnosis of GERD, which was higher than the proportion of non-cardia cancer patients, which included gastric body and antrum cancers (Table 2, P < 0.001). Non-cardia cancer cases accounted for a greater proportion of anemia and hypoproteinemia than cardia cancer cases (for anemia: 8.16% for gastric body cancer patients and 7.13% for antrum cancer patients; for hypoproteinemia: 4.56% for gastric body cancer patients and 3.78% for antrum cancer patients) (Table 2, P = 0.009 and 0.005, respectively). A higher proportion of obstruction syndrome was found in antrum cancer patients than in the other groups (Table 2, P < 0.001). Detailed P values of the pairwise comparisons among the three subsites are shown in Supplementary Table 1.
| Variables | Cardia (n = 3673) | Body (n = 1972) | Antrum (n = 4602) | P value | ||
| Clinical features | Basic information | Gender | < 0.001 | |||
| Male | 3046 (82.93%) | 1264 (64.10%) | 3140 (68.23%) | |||
| Female | 627 (17.07%) | 708 (35.90%) | 1462 (31.77%) | |||
| Age (yr) | < 0.001 | |||||
| ≤ 49 | 266 (7.24%) | 479 (24.29%) | 809 (17.58%) | |||
| 50-74 | 536 (78.16%) | 158 (67.70%) | 585 (69.71%) | |||
| ≥ 75 | 2871 (14.60%) | 1335 (8.01%) | 3208 (12.71%) | |||
| Mean (mean ± SD) | 63.98 ± 10.21 (63.65, 64.31) | 57.57 ± 12.68 (57.04, 58.10) | 60.32 ± 12.06 (59.97, 60.67) | < 0.001 | ||
| Patient source | < 0.001 | |||||
| Urban | 3000 (81.68%) | 1633 (82.81%) | 3969 (86.25%) | |||
| Rural | 673 (18.32%) | 339 (17.19%) | 633 (13.75%) | |||
| Insurance Source | < 0.001 | |||||
| MIUE | 1499 (40.81%) | 906 (45.94%) | 2004 (43.55%) | |||
| MIUR | 179 (4.88%) | 98 (4.97%) | 205 (4.45%) | |||
| NRCMI | 673 (18.32%) | 302 (15.31%) | 627 (13.62%) | |||
| Own expense | 885 (24.09%) | 476 (24.14%) | 1121 (24.36%) | |||
| Others | 437 (11.90%) | 190 (9.64%) | 645 (14.02%) | |||
| Complications | Hypertension | < 0.001 | ||||
| No | 2630 (71.60%) | 1550 (78.60%) | 3470 (75.40%) | |||
| Yes | 1043 (28.40%) | 422 (21.40%) | 1132 (24.60%) | |||
| Diabetes | 0.03 | |||||
| No | 3213 (87.48%) | 1767 (89.60%) | 4093 (88.94%) | |||
| Yes | 460 (12.52%) | 205 (10.40%) | 509 (11.06%) | |||
| GERD | < 0.001 | |||||
| No | 3252 (88.54%) | 1886 (95.64%) | 4387 (95.33%) | |||
| Yes | 421 (11.46%) | 86 (4.36%) | 215 (4.67%) | |||
| Cerebrovascular disease | 0.01 | |||||
| No | 3488 (94.96%) | 1895 (96.10%) | 4430 (96.26%) | |||
| Yes | 185 (5.04%) | 77 (3.90%) | 172 (3.74%) | |||
| Coronary disease | < 0.001 | |||||
| No | 3353 (91.29%) | 1843 (93.46%) | 4309 (93.63%) | |||
| Yes | 320 (8.71%) | 129 (6.54%) | 293 (6.37%) | |||
| Obstruction | < 0.001 | |||||
| No | 3616 (98.45%) | 1952 (98.99%) | 4136 (89.87%) | |||
| Yes | 57 (1.55%) | 20 (1.01%) | 466 (10.13%) | |||
| Anemia | 0.009 | |||||
| No | 3451 (93.96%) | 1811 (91.84%) | 4274 (92.87%) | |||
| Yes | 222 (6.04%) | 161 (8.16%) | 328 (7.13%) | |||
| Hypoproteinemia | 0.005 | |||||
| No | 3566 (97.09%) | 1882 (95.44%) | 4428 (96.22%) | |||
| Yes | 107 (2.91%) | 90 (4.56%) | 174 (3.78%) | |||
| Hospitalization features | Admission route | 0.007 | ||||
| Emergency | 100 (2.72%) | 28 (1.42%) | 101 (2.20%) | |||
| Non-emergency | 3573 (97.28%) | 1944 (98.58%) | 4501 (97.80%) | |||
| Stay (d) (mean ± SD) | 13.41 ± 12.17 (13.02, 13.80) | 14.08 ± 12.24 (13.57, 14.59) | 16.89 ± 12.24 (16.53, 17.24) | < 0.001 | ||
Gastric body cancer cases had the lowest emergency admission rate. No significant difference was found between the emergency proportion of the cardia and antrum cancers (Table 2, P = 0.007 in total, and P = 0.121 between the antrum and cardia cancers). Gastric antrum cancer led to the longest hospitalization duration among the three anatomical subsites; in contrast, gastric cardia cancer had the shortest hospitalization (Table 2, P < 0.001). Gastric antrum cancer had the highest total hospitalization (78.41 ± 54.69 thousand CNY, 95%CI: 76.83-79.98) and surgery costs [30.84 ± 20.43 thousand CNY, (95%CI: 30.24-31.43)], and cardia cancer had the lowest [total hospitalization: 50.33 ± 50.68 thousand CNY, (95%CI: 48.69-51.97); surgery: 17.86 ± 22.69 thousand CNY, (95%CI: 17.12-18.62)] (Supplementary Table 2, P < 0.001 for both total hospitalization and surgery costs). The detailed P values among the three subsites are shown in Supplementary Table 3.
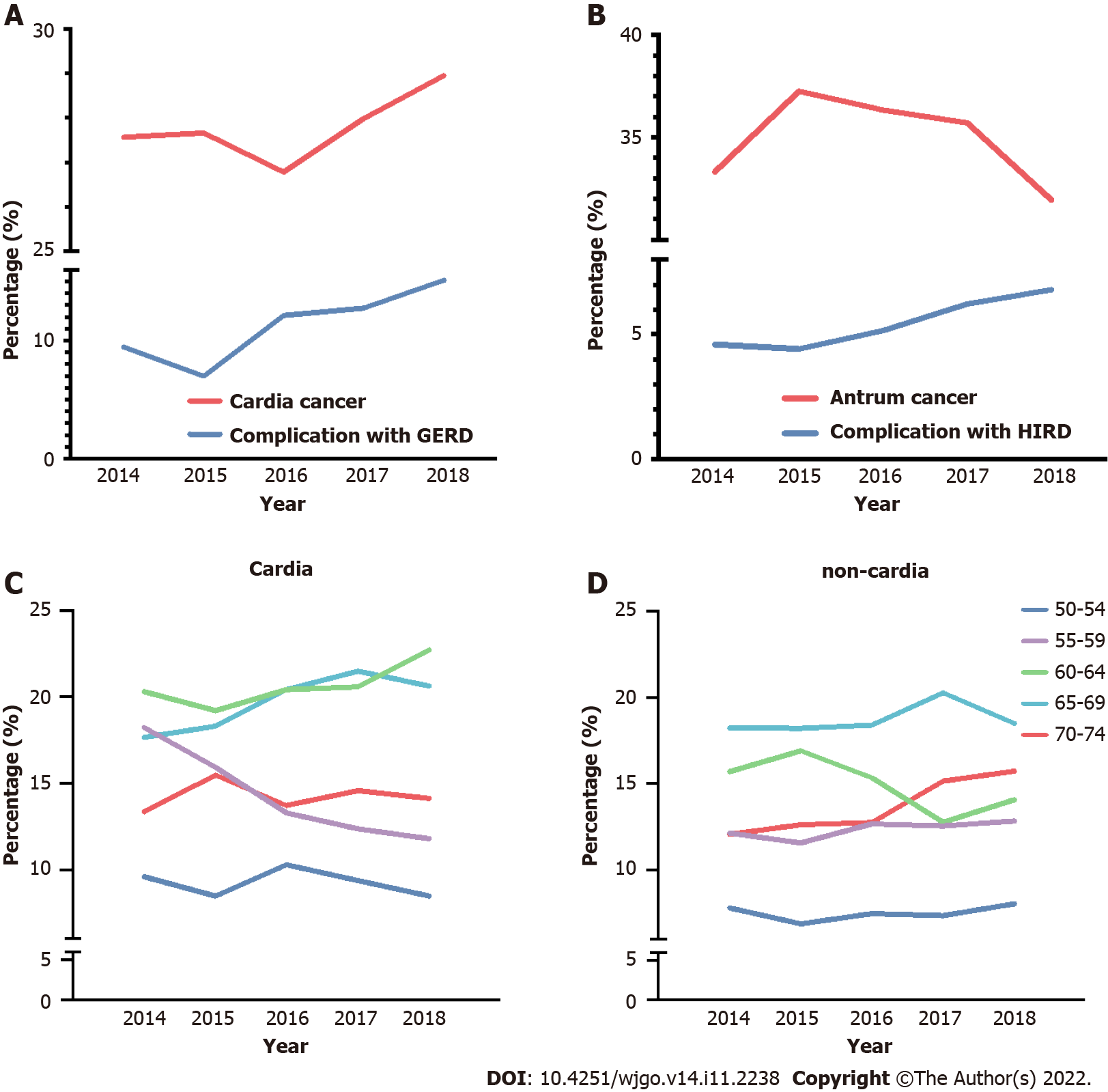
Gastric cardia and antrum cancers were chosen to examine the annual alternation in proportion between 2014 and 2018, combined with the proportion trend analysis of related complications, GERD, and H. pylori infection-related diseases (HIRD). A general trend of the increasing proportion of gastric cardia cancer was observed (ranging from 26.77% to 28.59%), along with an increase in the combined diagnosis of GERD (Figure 3A, P < 0.001). The complication ratio of GERD ranged from 6.99% to 15.11%, which is slightly higher than that in a previous report on Chinese patients[19]. A general decrease in trend was found for gastric cancers in the 5-year period, accompanied by an increase in the combined diagnostic proportion of HIRD (Figure 3B, P = 0.014), and the ratio of HIRD was lower than that in previous reports[22,23].
According to the World Health Organization, the age boundary between middle and old age is 60 years, and the cutoff between old and advanced age is 75 years in the Asia-Pacific region. Therefore, a detailed study based on anatomical subsites was conducted for the 50-74 years age group at increments of every 5 years to observe age-related trends. A significant decrease was found in the proportion of patients with gastric cardia cancer in the 55-59 years age group, with increasing trends between the 60-64 and 65-69 years age groups (Figure 3C, P = 0.02). For non-cardia cancer, although a slight increase was found in the 70-74 years age group, no significant difference was found in the five age groups (Figure 3D, P = 0.086).
In-hospital mortality and short-term postoperative complication rates reflect the prognosis of patients and the quality and safety of medical treatment. In this study, a total of 92 (0.86%) gastric cancer-related deaths were detected, including 37 (1.01%) patients with gastric cardia cancer, 13 (0.66%) with gastric body cancer, and 33 (0.72%) with antrum cancer (P = 0.243 among the three subsites, Sup
A total of 6,956 patients (6749 cancers at the three anatomical subsites) had a history of surgery during hospitalization. Among them, 605 (8.70%) had short-term postoperative complications, including 158 (8.55%) cardia cancer cases, 109 (9.08%) body cancer cases, and 324 (accounted for 8.76%) antrum cancer cases (P = 0.876 among the three subsites, Supplementary Table 3).
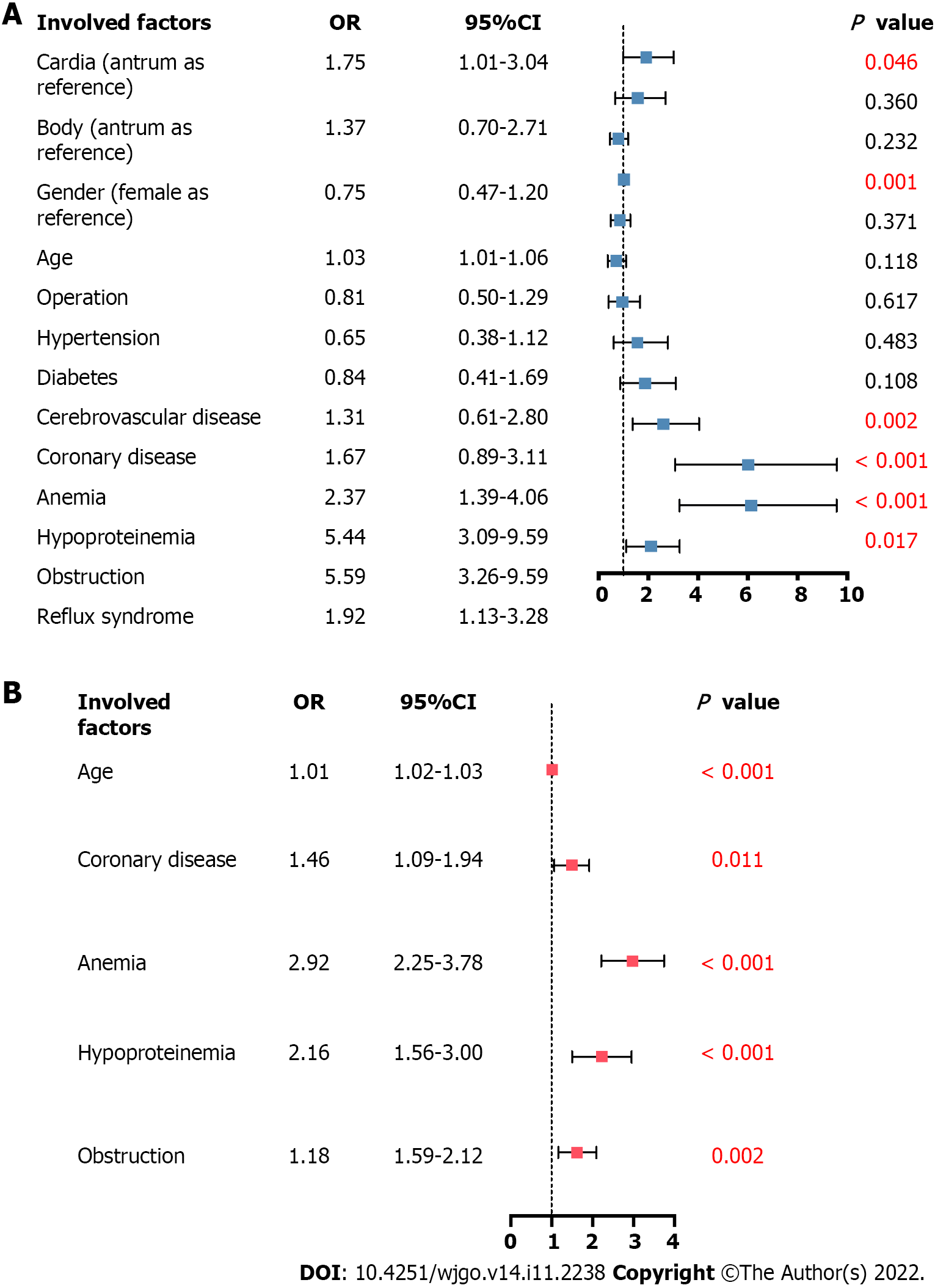
Multivariable regression analysis was performed to further explore the association between anatomical subsite and patient in-hospital clinical outcomes, including in-hospital mortality in all patients and postoperative complications in patients who had undergone surgery. Other vital factors, such as sex, age, operation (in the analysis of in-hospital mortality), and complications, were also included in the analysis. In-hospital mortality was associated with the anatomical sites, among which cardia cancer had a higher risk (aOR 1.75, 95%CI: 1.01-3.04, P = 0.046). It was also associated with age increase (aOR 1.03, 95%CI: 1.01-1.06, P = 0.001) and increased risks of complications, including anemia (aOR 2.37, 95%CI: 1.39-4.06, P = 0.002), hypoproteinemia (aOR 5.44, 95%CI: 3.09-9.59, P < 0.001), obstruction syndrome (aOR 5.59, 95%CI: 3.26-9.59, P < 0.001), and reflux syndrome (aOR 1.92, 95%CI: 1.13-3.28, P = 0.017) (Figure 4A). The higher risk of postoperative complications was not associated with anatomical sites (aOR 0.979, 95%CI: 0.79-1.21, P = 0.846); however, it was associated with age increase (aOR 1.01, 95%CI: 1.02-1.03, P < 0.001) and increased risks of complications, including coronary disease (aOR 1.46, 95%CI: 1.09-1.94, P = 0.011), anemia (aOR 2.92, 95%CI: 2.25-3.78, P < 0.001), hypoproteinemia (aOR 2.16, 95%CI: 1.56-3.00, P < 0.001), and obstruction syndrome (aOR 1.18, 95%CI: 1.59-2.12, P = 0.002) (Figure 4B).
In this study, we first examined the epidemiologic features of gastric cancer in northern China based on anatomical subsites, showing a higher male ratio, older age distribution, older age-related trend, increasing proportion, close relationship with GERD, and increased risk of in-hospital mortality in gastric cardia cancer than in other types. This cancer site was also associated with younger age distribution, increased likelihood of residence in the city, and decreasing trends in the proportion of antral cancer. Overall, the constituent ratio of gastric cardia cancer in northern China was higher than the average level in China (18-27%)[24,25] and Europe (26-31%)[26-28] and lower than that in North America and West Asia (both > 40%)[7,29]. Compared to previous reports in China, the constituent ratio of gastric cardia cancer in this study was slightly higher than that in the Gansu Province (northwest China)[18] and lower than that in southwest China[19] but showed high inner similarity compared with other global regions (Supplementary Table 5). Similarly, the proportion of antral gastric cancer is in the mid-level worldwide[24]. Regionality is a typical phenomenon in both cardia and non-cardia (including gastric body and antrum) cancers and is generally considered to be linked to race, unique eating habits, and the environment[17,30].
Gastric cardia cancer may account for a large proportion of all gastric cancers in countries/regions with a low incidence of gastric cancer[31,32], contributing to better control of some risk factors of non-cardia cancer, including H. pylori infection. However, with a higher incidence of gastric cancer in China, the proportion of cardia cancer in this study was also higher, compared with that in European countries[26-28]. One major reason might be the high prevalence of smoking in China, which is considered a major risk factor for cardia cancer-related risk factors, including GERD and cardia cancer[33,34]. In this study, five times more men than women had gastric cardia cancer; this ratio was higher than the global (by approximately 3:1)[25] and China-based (by approximately 4:1) values[24], further suggesting the effect of smoking, showing sex-based differences. Furthermore, a higher proportion of upper gastric cancer, including cardia cancer, has been found in rural residents (including those with rural medical insurance) that are usually considered to have a higher smoking prevalence[35]. Therefore, more attention should be paid to the implementation of smoking cessation policies, especially those targeting men and rural areas, to help prevent cardia cancer. The sex-based difference between cardia and non-cardia cancers could also be traced to the epidemiology of Epstein-Barr virus-associated gastric cancer (EBVaGC)[36]. The relationships among EBVaGC, cardia cancer, and male sex were tested in a meta-analysis, with a male to female ratio of approximately 2-3:1[37].
In this study, cardia cancer cases increased annually and proportionally to the cases at other subsites and concurrent GERD ratio, which was also found in southwest China[19]. Smoking, which is a risk factor for GERD and subsequent gastric cardia cancer, may contribute to this trend. The implementation of tobacco-controlling policies in China, specifically in northern and southwestern regions, remains insufficient, with a reported ratio of 60.2%-61.8% of adults in northern China who are passive smokers[34]. Moreover, the lifestyle among Chinese people, including youth, has been westernized[38]. Increased ingestion of animal-source foods has made obesity one of the main public health issues in China, especially in developed cities, and was shown to be a risk factor for both GERD and cardia cancer by increasing abdominal pressure and prolonging nocturnal acid exposure[39-41]. Reducing smoking and obesity may help prevent further increases in gastric cardia cancer rates as the Chinese population ages.
Association between higher in-hospitalization mortality and cardia anatomical subsite was found in this study, rather than non-cardia sites, in multivariable regression analysis after adjusting for age, sex, and basic complications. The rates of postoperative complications were comparable among the anatomical sites. The older age distribution and more severe previous complication status among cardia cancer patients in this study may account for the correlation with the increased risk of in-hospital mortality, as they affect surgical safety[42]. Moreover, the non-significant short-term postoperative complication risk difference among the anatomical sites might reflect the establishing of techniques in gastric cancer surgery, ensuring the safety of cardia cancer surgeries by helping achieve adequate anastomotic tension and blood supply[43]. The preoperative management of basic diseases may help improve recovery rates and safety profile.
H. pylori is a major risk factor for non-cardia cancer[22], especially for that located in the antrum[25]. The incidence of H. pylori infection is relatively low in northern China, which may account for the reported proportions of non-cardia cancers[44]. Eating habits are key factors in H. pylori infection. The constituent ratio of antrum cancer was higher in southwest China compared to our results[19], which could be correlated with the habit of spicy food consumption in this region, which might increase the incidence of H. pylori infection[45,46]. A generally decreasing trend of antral cancer proportion in the present study might be attributed to the popularization of screening, including that for gastric cancer and HIRD[47]. However, the proportion of malignancies located in the antrum was higher than that in some Western countries, including northern America, which could be attributed to the high virulence of H. pylori bacterial strains in East Asian populations[48]. Moreover, the hospitalization duration and financial burden remain significant in gastric antrum cancer, as observed in the present study; this finding may be related to the complex surgical methods involved and high complication rates[49]. The approximately 10-times higher incidence of obstruction in antrum cancer than in other cancer sites may affect treatment efficiency[50]. This finding suggests that further screening and radical cure of both H. pylori and antrum cancer are required in the future[51].
This study had several limitations. First, due to the data source used, which covers medical centers but not an entire region or province, this study involved patients from northern China. Second, detailed anatomic data were not available in some cases in the HSRs, resulting in the creation of the category named “other types”, and the anatomical information based on the “upper, middle, and lower” classification could not be re-traced. Third, due to the absence of some information in HSRs, including laboratory test results, imaging findings, or long-term prognostic information, the detailed figures, including tumor stage and patient prognosis, could not be extracted; the detailed operative procedures, details on the economic status of the patients (e.g., salary), and lifestyles were also not available, making it difficult to construct specific characteristics and risk factor features of gastric cancer patients in northern China, and should be further testified in the future. Fourth, the combined diagnosis proportion of HSRs was much lower than that in previous studies[22,23]. This reflects the defects of some HSRs that missed such diagnoses and the diagnostic failure of some clinicians that missed H. pylori infections, resulting in omissions. Finally, the results of the multivariate analysis should be further validated in more cohorts to increase their credibility.
In summary, this is the first study to report the composition ratio characteristics and changes in gastric cancer trends based on anatomical sites in patients in northern China. This study examined plausible explanations for these findings. Large-scale screening programs for gastric cancer and infection, increasing awareness and prevention of risk factors, reducing smoking and obesity, as well as patient stratification for treatment based on anatomical sites are required to reduce the burden of gastric cancer.
Gastric cancer is among the most common digestive malignant tumors worldwide. China is among the regions with the highest gastric cancer incidence. Differences in clinical and epidemiological features of this tumor type based on its presence in the anatomical subsites of the stomach have been reported.
Few population-based studies have been conducted in China to determine differences among tumors at different locations, and analyses of data from northern China are lacking.
To examine the clinical features of gastric cancer at different anatomical sites in patients from northern China. We also aimed to examine the associated variability and trends.
We conducted a cross-sectional study used incident gastric cancer case data from 10 Peking University-affiliated hospitals, and the clinical and prevailing local features were analyzed.
Ten thousand seven hundred and nine patients were enrolled, including antral, cardia, and stomach body gastric cancer cases. Cancer in the cardia had the highest male:female ratio, proportion of elderly patients, and patients with complications, including hypertension, diabetes, cerebrovascular, and coronary diseases (P < 0.001). gastric cancer involving the antrum showed the lowest proportion of patients from rural areas and accounted for the highest hospitalization rate and cost (each P < 0.001). The proportion of patients with cancer involving the cardia increased with an increase in the number of gastroesophageal reflux disease (GERD) cases during the same period (P < 0.001). Multivariate analysis revealed that tumor location in the cardia increased the risk of in-hospital mortality (P = 0.046). Anatomical subsite was not linked to postoperative complications.
In this study, we first examined the epidemiologic features of gastric cancer in northern China based on anatomical subsites, showing a higher male ratio, older age distribution, older age-related trend, increasing proportion, close relationship with GERD, and increased risk of in-hospital mortality in gastric cardia cancer than in other types. This cancer site was also associated with younger age distribution, increased likelihood of residence in the city, and decreasing trends in the proportion of antral cancer. Overall, the constituent ratio of gastric cardia cancer in northern China was higher than the average level in China and Europe, and lower than that in North America and West Asia. Compared to previous reports in China, the constituent ratio of gastric cardia cancer in this study was slightly higher than that in the northwest China and lower than that in southwest China but showed high inner similarity compared with other global regions. Similarly, the proportion of antral gastric cancer is in the mid-level worldwide. Regionality is a typical phenomenon in both cardia and non-cardia (including gastric body and antrum) cancers and is generally considered to be linked to race, unique eating habits, and the environment.
This is the first study to report the composition ratio characteristics and changes in gastric cancer trends based on anatomical sites in patients in northern China. This study examined plausible explanations for these findings. Large-scale screening programs for gastric cancer and infection, increasing awareness and prevention of risk factors, reducing smoking and obesity, as well as patient stratification for treatment based on anatomical sites are required to reduce the burden of gastric cancer.
We acknowledge the Department of Hospital Management from Peking University Health Science Center for the usage permission of database, and the Medical Information Center from Peking University Health Science Center for assistance with data analysis.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dilek ON, Turkey; Kinami S, Japan; Li L, New Zealand S-Editor: Chen YL L-Editor: A P-Editor: Chen YL
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020;70:7-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12667] [Cited by in RCA: 15320] [Article Influence: 3064.0] [Reference Citation Analysis (4)] |
| 2. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64704] [Article Influence: 16176.0] [Reference Citation Analysis (177)] |
| 3. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13215] [Article Influence: 1468.3] [Reference Citation Analysis (3)] |
| 4. | Ocvirk S, O'Keefe SJD. Dietary fat, bile acid metabolism and colorectal cancer. Semin Cancer Biol. 2021;73:347-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 158] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 5. | Berrino F, De Angelis R, Sant M, Rosso S, Bielska-Lasota M, Coebergh JW, Santaquilani M; EUROCARE Working group. Survival for eight major cancers and all cancers combined for European adults diagnosed in 1995-99: results of the EUROCARE-4 study. Lancet Oncol. 2007;8:773-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 591] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 6. | Thrumurthy SG, Chaudry MA, Chau I, Allum W. Does surgery have a role in managing incurable gastric cancer? Nat Rev Clin Oncol. 2015;12:676-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 87] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Camargo MC, Anderson WF, King JB, Correa P, Thomas CC, Rosenberg PS, Eheman CR, Rabkin CS. Divergent trends for gastric cancer incidence by anatomical subsite in US adults. Gut. 2011;60:1644-1649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 8. | Crane SJ, Locke GR 3rd, Harmsen WS, Diehl NN, Zinsmeister AR, Melton LJ 3rd, Romero Y, Talley NJ. Subsite-specific risk factors for esophageal and gastric adenocarcinoma. Am J Gastroenterol. 2007;102:1596-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Dolan K, Sutton R, Walker SJ, Morris AI, Campbell F, Williams EM. New classification of oesophageal and gastric carcinomas derived from changing patterns in epidemiology. Br J Cancer. 1999;80:834-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Kocher HM, Linklater K, Patel S, Ellul JP. Epidemiological study of oesophageal and gastric cancer in south-east England. Br J Surg. 2001;88:1249-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Koizumi S, Motoyama S, Watanabe N, Matsuhashi T, Iijima K. Chronological Changes in the Gastric Cancer Subsite in Akita, Japan: The Trends from the Data of a Hospital-Based Registration System. Tohoku J Exp Med. 2018;246:131-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3126] [Cited by in RCA: 3187] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 13. | Hansen S, Vollset SE, Derakhshan MH, Fyfe V, Melby KK, Aase S, Jellum E, McColl KE. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy and Helicobacter pylori status. Gut. 2007;56:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 128] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 14. | Derakhshan MH, Malekzadeh R, Watabe H, Yazdanbod A, Fyfe V, Kazemi A, Rakhshani N, Didevar R, Sotoudeh M, Zolfeghari AA, McColl KE. Combination of gastric atrophy, reflux symptoms and histological subtype indicates two distinct aetiologies of gastric cardia cancer. Gut. 2008;57:298-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 138] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Brown LM, Devesa SS. Epidemiologic trends in esophageal and gastric cancer in the United States. Surg Oncol Clin N Am. 2002;11:235-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 315] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 16. | Kumar S, Metz DC, Ellenberg S, Kaplan DE, Goldberg DS. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology. 2020;158:527-536.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 17. | Russo AE, Strong VE. Gastric Cancer Etiology and Management in Asia and the West. Annu Rev Med. 2019;70:353-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 117] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 18. | Zhou Y, Zhang Z, Wu J, Ren D, Yan X, Wang Q, Wang Y, Wang H, Zhang J, Zhu X, Yang Y, Luo C, Guo X, Tang C, Qiao L. A rising trend of gastric cardia cancer in Gansu Province of China. Cancer Lett. 2008;269:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (1)] |
| 19. | Liu K, Yang K, Zhang W, Chen X, Zhang B, Chen Z, Chen J, Zhao Y, Zhou Z, Chen L, Hu J. Changes of Esophagogastric Junctional Adenocarcinoma and Gastroesophageal Reflux Disease Among Surgical Patients During 1988-2012: A Single-institution, High-volume Experience in China. Ann Surg. 2016;263:88-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 20. | Qu R, Ma Y, Tao L, Bao X, Zhou X, Wang B, Li F, Lu S, Tuo L, Zhan S, Zhang Z, Fu W. Features of colorectal cancer in China stratified by anatomic sites: A hospital-based study conducted in university-affiliated hospitals from 2014 to 2018. Chin J Cancer Res. 2021;33:500-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 21. | Qu R, Ma Y, Zhang Z, Fu W. Increasing burden of colorectal cancer in China. Lancet Gastroenterol Hepatol. 2022;7:700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 52] [Reference Citation Analysis (0)] |
| 22. | Yang L, Kartsonaki C, Yao P, de Martel C, Plummer M, Chapman D, Guo Y, Clark S, Walters RG, Chen Y, Pei P, Lv J, Yu C, Jeske R, Waterboer T, Clifford GM, Franceschi S, Peto R, Hill M, Li L, Millwood IY, Chen Z; China Kadoorie Biobank Collaborative Group. The relative and attributable risks of cardia and non-cardia gastric cancer associated with Helicobacter pylori infection in China: a case-cohort study. Lancet Public Health. 2021;6:e888-e896. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 128] [Article Influence: 32.0] [Reference Citation Analysis (0)] |
| 23. | Wang RT, Wang T, Chen K, Wang JY, Zhang JP, Lin SR, Zhu YM, Zhang WM, Cao YX, Zhu CW, Yu H, Cong YJ, Zheng S, Wu BQ. Helicobacter pylori infection and gastric cancer: evidence from a retrospective cohort study and nested case-control study in China. World J Gastroenterol. 2002;8:1103-1107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 43] [Cited by in RCA: 43] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 24. | Colquhoun A, Arnold M, Ferlay J, Goodman KJ, Forman D, Soerjomataram I. Global patterns of cardia and non-cardia gastric cancer incidence in 2012. Gut. 2015;64:1881-1888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 264] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 25. | Arnold M, Ferlay J, van Berge Henegouwen MI, Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 367] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 26. | Schmassmann A, Oldendorf MG, Gebbers JO. Changing incidence of gastric and oesophageal cancer subtypes in central Switzerland between 1982 and 2007. Eur J Epidemiol. 2009;24:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Aragonés N, Izarzugaza MI, Ramos M, Chirlaque MD, Almar E, Martínez C; Oesophago-gastric Cancer Working Group. Trends in oesophago-gastric cancer incidence in Spain: analysis by subsite and histology. Ann Oncol. 2010;21 Suppl 3:iii69-iii75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Holster IL, Aarts MJ, Tjwa ET, Lemmens VE, Kuipers EJ. Trend breaks in incidence of non-cardia gastric cancer in the Netherlands. Cancer Epidemiol. 2014;38:9-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Derakhshan MH, Yazdanbod A, Sadjadi AR, Shokoohi B, McColl KE, Malekzadeh R. High incidence of adenocarcinoma arising from the right side of the gastric cardia in NW Iran. Gut. 2004;53:1262-1266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 45] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 30. | Saumoy M, Schneider Y, Shen N, Kahaleh M, Sharaiha RZ, Shah SC. Cost Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology. 2018;155:648-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 31. | Ferro A, Peleteiro B, Malvezzi M, Bosetti C, Bertuccio P, Levi F, Negri E, La Vecchia C, Lunet N. Worldwide trends in gastric cancer mortality (1980-2011), with predictions to 2015, and incidence by subtype. Eur J Cancer. 2014;50:1330-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 502] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 32. | Rauf A, Khalil AA, Rahman UU, Khalid A, Naz S, Shariati MA, Rebezov M, Urtecho EZ, de Albuquerque RDDG, Anwar S, Alamri A, Saini RK, Rengasamy KRR. Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit Rev Food Sci Nutr. 2022;62:6034-6054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 94] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 33. | Engel LS, Chow WH, Vaughan TL, Gammon MD, Risch HA, Stanford JL, Schoenberg JB, Mayne ST, Dubrow R, Rotterdam H, West AB, Blaser M, Blot WJ, Gail MH, Fraumeni JF Jr. Population attributable risks of esophageal and gastric cancers. J Natl Cancer Inst. 2003;95:1404-1413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 518] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 34. | Wang M, Luo X, Xu S, Liu W, Ding F, Zhang X, Wang L, Liu J, Hu J, Wang W. Trends in smoking prevalence and implication for chronic diseases in China: serial national cross-sectional surveys from 2003 to 2013. Lancet Respir Med. 2019;7:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 240] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 35. | Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, Taylor CE, Becker K, Xu J. Smoking in China: findings of the 1996 National Prevalence Survey. JAMA. 1999;282:1247-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 453] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 36. | Naseem M, Barzi A, Brezden-Masley C, Puccini A, Berger MD, Tokunaga R, Battaglin F, Soni S, McSkane M, Zhang W, Lenz HJ. Outlooks on Epstein-Barr virus associated gastric cancer. Cancer Treat Rev. 2018;66:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 37. | Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824-833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 378] [Cited by in RCA: 381] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 38. |
|
| 39. | Patti MG. An Evidence-Based Approach to the Treatment of Gastroesophageal Reflux Disease. JAMA Surg. 2016;151:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 40. | Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. 2021;9:373-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 893] [Article Influence: 223.3] [Reference Citation Analysis (0)] |
| 41. | Camilleri M, Malhi H, Acosta A. Gastrointestinal Complications of Obesity. Gastroenterology. 2017;152:1656-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 156] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 42. | D'Journo XB, Boulate D, Fourdrain A, Loundou A, van Berge Henegouwen MI, Gisbertz SS, O'Neill JR, Hoelscher A, Piessen G, van Lanschot J, Wijnhoven B, Jobe B, Davies A, Schneider PM, Pera M, Nilsson M, Nafteux P, Kitagawa Y, Morse CR, Hofstetter W, Molena D, So JB, Immanuel A, Parsons SL, Larsen MH, Dolan JP, Wood SG, Maynard N, Smithers M, Puig S, Law S, Wong I, Kennedy A, KangNing W, Reynolds JV, Pramesh CS, Ferguson M, Darling G, Schröder W, Bludau M, Underwood T, van Hillegersberg R, Chang A, Cecconello I, Ribeiro U Jr, de Manzoni G, Rosati R, Kuppusamy M, Thomas PA, Low DE; International Esodata Study Group. Risk Prediction Model of 90-Day Mortality After Esophagectomy for Cancer. JAMA Surg. 2021;156:836-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 43. | Ojima T, Nakamura M, Hayata K, Kitadani J, Katsuda M, Takeuchi A, Tominaga S, Nakai T, Nakamori M, Ohi M, Kusunoki M, Yamaue H. Short-term Outcomes of Robotic Gastrectomy vs Laparoscopic Gastrectomy for Patients With Gastric Cancer: A Randomized Clinical Trial. JAMA Surg. 2021;156:954-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 144] [Article Influence: 36.0] [Reference Citation Analysis (1)] |
| 44. | Liu WZ, Xie Y, Lu H, Cheng H, Zeng ZR, Zhou LY, Chen Y, Wang JB, Du YQ, Lu NH; Chinese Society of Gastroenterology, Chinese Study Group on Helicobacter pylori and Peptic Ulcer. Fifth Chinese National Consensus Report on the management of Helicobacter pylori infection. Helicobacter. 2018;23:e12475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 45. | Hwang MK, Bode AM, Byun S, Song NR, Lee HJ, Lee KW, Dong Z. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1. Cancer Res. 2010;70:6859-6869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | López-Carrillo L, López-Cervantes M, Robles-Díaz G, Ramírez-Espitia A, Mohar-Betancourt A, Meneses-García A, López-Vidal Y, Blair A. Capsaicin consumption, Helicobacter pylori positivity and gastric cancer in Mexico. Int J Cancer. 2003;106:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Lee YC, Chiang TH, Chou CK, Tu YK, Liao WC, Wu MS, Graham DY. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology. 2016;150:1113-1124.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 674] [Article Influence: 74.9] [Reference Citation Analysis (0)] |
| 48. | Yamaoka Y. Helicobacter pylori typing as a tool for tracking human migration. Clin Microbiol Infect. 2009;15:829-834. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 49. | So JB, Rao J, Wong AS, Chan YH, Pang NQ, Tay AYL, Yung MY, Su Z, Phua JNS, Shabbir A, Ng EKW. Roux-en-Y or Billroth II Reconstruction After Radical Distal Gastrectomy for Gastric Cancer: A Multicenter Randomized Controlled Trial. Ann Surg. 2018;267:236-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 50. | Grunwald D, Cohen J, Bartley A, Sheridan J, Chuttani R, Sawhney MS, Pleskow DK, Berzin TM, Mizrahi M. The location of obstruction predicts stent occlusion in malignant gastric outlet obstruction. Therap Adv Gastroenterol. 2016;9:815-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Glob Health. 2020;8:e180-e190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1319] [Article Influence: 263.8] [Reference Citation Analysis (0)] |