Published online Nov 15, 2022. doi: 10.4251/wjgo.v14.i11.2224
Peer-review started: July 23, 2022
First decision: August 18, 2022
Revised: August 25, 2022
Accepted: October 2, 2022
Article in press: October 2, 2022
Published online: November 15, 2022
Processing time: 115 Days and 3 Hours
Many biomarkers have predictive value for overall survival (OS) and disease-free survival (DFS) in tumor patients. However, the role of indirect bilirubin (IBIL) in local advanced rectal cancer (LARC) patients treated with neoadjuvant chemoradiotherapy (nCRT) has not been studied.
To explore the predictive value of IBIL before nCRT (pre-IBIL) for the OS and DFS of LARC patients treated with nCRT.
A total of 324 LARC patients undergoing nCRT with total mesorectal excision (TME) were enrolled. Preoperative clinical features and postoperative patho
Among 324 patients, the median pre-IBIL was 6.2 μmol/L (interquartile range: 4.6 μmol/L-8.4 μmol/L). In the Cox multivariate regression analysis, we found that pre-IBIL, smoking history, tumor regression grade (TRG), vascular invasion, and carbohydrate antigen 19-9 before nCRT (pre-CA19-9) were predictors of OS. Additionally, pre-IBIL, body mass index (BMI), nCRT with surgery interval, TRG, and vascular invasion were predictors of DFS. Predictive nomograms were developed to predict 5-year OS and 5-year DFS with area under the ROC curve values of 0.7518 and 0.7355, respectively. Good statistical performance on internal validation was shown by calibration plots and ROC curves.
This study demonstrated that pre-IBIL was an independent prognostic factor for OS and DFS in LARC patients treated with nCRT followed by TME. Nomograms incorporating pre-IBIL, BMI, smoking history, nCRT with surgery interval, TRG, vascular invasion, and pre-CA19-9 could be helpful to predict OS and DFS.
Core Tip: Our study demonstrated that indirect bilirubin measurement before neoadjuvant chemoradiotherapy (nCRT) (pre-IBIL) is an independent and significant risk factor for survival in local advanced rectal cancer patients treated with nCRT followed by total mesorectal excision. In addition, nomograms based on pre-IBIL can predict 5-year overall survival and 5-year disease-free survival with good agreement.
- Citation: Li SF, Wei R, Yu GH, Jiang Z. Predictive value of indirect bilirubin before neoadjuvant chemoradiotherapy in evaluating prognosis of local advanced rectal cancer patients. World J Gastrointest Oncol 2022; 14(11): 2224-2237
- URL: https://www.wjgnet.com/1948-5204/full/v14/i11/2224.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i11.2224
According to the National Comprehensive Cancer Network guidelines, local advanced rectal cancer (LARC) patients should be treated with neoadjuvant chemoradiotherapy (nCRT) combined with surgery[1]. The nCRT can reduce the tumor burden, and local recurrence rate, as well as improve the R0 resection rate and anus preservation rate of LARC[2]. However, approximately 20%-46% of LARC patients with available postoperative pathological data had tumor cells that did not regress significantly or did not regress at all. These patients did not respond significantly to treatment and had poor prognoses[3-5]. Moreover, there are few studies on the changes in biological characteristics of tumors after nCRT treatment, so the current prognosis of this group of patients is still evaluated by TNM staging, which may not be accurate[6-8]. Combined with the results of existing studies, most of the studies evaluated the prognosis of this group of patients based on the pathological or anatomical characteristics of the tumor while ignoring some biological characteristics of the patients themselves, such as blood biochemistry status and underlying diseases[9-11].
Indirect bilirubin (IBIL) is bilirubin that is not bound to glucuronic acid. Elevated serum IBIL is mainly associated with various hemolytic diseases. After the destruction of a large number of red blood cells, a large amount of hemoglobin is converted into IBIL, which exceeds the processing capacity of the liver to convert all of it into direct bilirubin, resulting in elevated IBIL in the blood. Its concentration reflects the conversion function of hepatocytes and the catabolic state of red blood cells. In recent years, an increasing number of studies have found a relationship between IBIL and tumor prognosis. Some studies have shown that bilirubin is associated with prognosis in cancer patients, such as ovarian cancer, lung cancer, nasopharyngeal cancer, and oral squamous cell carcinoma[12-15]. However, the predictive values of the IBIL before nCRT (pre-IBIL) for prognostic outcomes in LARC patients treated with nCRT are unknown. In this retrospective study, we aimed to perform statistical analysis of the clinicopathological data of a large number of LARC patients who underwent nCRT was performed to investigate the predictive value of pre-IBIL in the prognosis and to create a nomogram that could predict the prognosis of patients.
We retrospectively identified 324 rectal cancer patients who received nCRT between November 1, 2012 and October 30, 2018 in the National Cancer Center/Cancer Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, P.R. China). The inclusion criteria were as follows: (1) Completion of long course nCRT followed by total mesorectal excision (TME); (2) Pathologically confirmed rectal adenocarcinoma by colonoscopic biopsy; and (3) Imaging suggested cTNM stage II-III without distant metastasis at initial diagnosis. The exclusion criteria were as follows: (1) Absence of some clinical features before nCRT treatment; (2) After nCRT, distant metastasis was found by imaging examinations; and (3) Synchronous tumors.
All the available detailed clinical characteristics and pathological parameters were enrolled, including pre-IBIL, age, sex, body mass index (BMI), smoking history, drinking history, chronic disease history, cTNM stage, nCRT with surgery interval, inferior margin, tumor regression grade (TRG), vascular invasion, neural invasion, radiotherapy type before surgery, chemotherapy type before surgery, adjuvant chemotherapy, carcinoembryonic antigen before nCRT (pre-CEA), and carbohydrate antigen 19-9 before nCRT (pre-CA19-9), in which TRG uses Dworak stage.
All patients were treated with a 45-60 Gy dose of long-course nCRT. Three schemes of concurrent radiotherapy are volumetric modulated arc therapy (VMAT), intensity modulated radiation therapy (IMRT), and 3-dimensional conformal radiation therapy (3D-CRT). The concurrent chemotherapy schemes were capecitabine, capecitabine + platinum, capecitabine + bevacizumab, capecitabine + oxaliplatin and raltitrexed. All patients were treated with nCRT for at least 4 wk before undergoing TME.
All patients received postoperative reviews in the hospital every 3 mo for 2 years after surgery and every 6 mo for 3-5 years after surgery. The postoperative examinations included a physical examination, peripheral blood tumor markers, chest computed tomography (CT), abdominal CT, pelvic CT or magnetic resonance imaging, and a whole-body positron emission tomography-CT if necessary. Additionally, we followed up with patients at regular intervals until August 31, 2021. Overall survival (OS) was defined as the time from the date of diagnosis to the date of death due to any cause or the last follow-up. Disease-free survival (DFS) was defined as the time since radical surgery to disease recurrence, metastasis, the last follow-up, or patient death.
Fisher’s exact test and χ2 tests were employed to compare categorical data. Cox regression analysis was performed to evaluate the hazard ratio (HR) and 95% confidence interval (95%CI) of both OS and DFS. Parameters that were statistically significant in the univariate analysis were subsequently included in the multivariate analysis. Nomograms were constructed based on statistically significant factors identified by the multivariate analyses from the Cox regression model to predict 5-year OS and DFS. We assessed the predictive performance of the nomogram with calibration plots and receiver operating characteristic (ROC) curves. The Kaplan-Meier survival method was used to analyze OS and DFS, and survival differences were calculated by the log-rank test. All statistical analyses were performed using R version 4.1.0. Two-tailed P values less than 0.05 were considered statistically significant.
A total of 324 patients, 98 (30.25%) females and 226 (69.75%) males met the inclusion criteria for this study, and the median pre-IBIL (interquartile range) was 6.2 (4.6-8.4) μmol/L. Then, 6.2 μmol/L was used as cut-off value in grouping patients. Moreover, 48.46% (157/324) of patients had a smoking history, 41.67% (135/324) of patients had a drinking history and 37.04% (120/324) of patients had a chronic disease history. For radiotherapy type before surgery, 286 (88.27%) patients received the VMAT regimen, 30 (9.26%) patients received the IMRT regimen, and 8 (2.47%) patients received the 3D-CRT regimen. For chemotherapy type before surgery, 289 (89.20%) patients received the capecitabine regimen, and 18 (5.56%) patients received the capecitabine plus platinum regimen. The demographics and clinicopathological characteristics are presented in Table 1.
| Characteristic | Pre-IBIL ≤ 6.2 μmol/L (n = 163) | Pre-IBIL > 6.2 μmol/L (n = 161) | P value | All patients (n = 324) |
| Age, yr | 0.305 | |||
| < 60 | 88 (53.99) | 97 (60.25) | 185 (57.10) | |
| ≥ 60 | 75 (46.01) | 64 (39.75) | 139 (42.90) | |
| Sex | 0.007 | |||
| Female | 61 (37.42) | 37 (22.98) | 98 (30.25) | |
| Male | 102 (62.58) | 124 (77.02) | 226 (69.75) | |
| BMI, kg/m2 | 0.317 | |||
| < 18.5 | 7 (4.29) | 4 (2.48) | 11 (3.40) | |
| 18.5-23.9 | 86 (52.76) | 73 (45.34) | 159 (49.07) | |
| 24.0-27.9 | 52 (31.90) | 66 (40.99) | 118 (36.42) | |
| ≥ 28.0 | 18 (11.04) | 18 (11.18) | 36 (11.11) | |
| Smoking history | 0.581 | |||
| No | 87 (53.37) | 80 (49.69) | 167 (51.54) | |
| Yes | 76 (46.63) | 81 (50.31) | 157 (48.46) | |
| Drinking history | 0.925 | |||
| No | 96 (58.90) | 93 (57.76) | 189 (58.33) | |
| Yes | 67 (41.10) | 68 (42.24) | 135 (41.67) | |
| Chronic disease history | 0.624 | |||
| No | 100 (61.35) | 104 (64.60) | 204 (62.96) | |
| Yes | 63 (38.65) | 57 (35.40) | 120 (37.04) | |
| cTNM stage | 0.839 | |||
| II | 23 (14.11) | 25 (15.53) | 48 (14.81) | |
| III | 140 (85.89) | 136 (84.47) | 276 (85.19) | |
| nCRT with surgery interval, d | 0.573 | |||
| 31-60 | 88 (53.99) | 79 (49.07) | 167 (51.54) | |
| 61-90 | 63 (38.65) | 66 (40.99) | 129 (39.81) | |
| > 90 | 12 (7.36) | 16 (9.94) | 28 (8.64) | |
| Inferior margin | 0.373 | |||
| ≤ 5 cm | 109 (66.87) | 116 (72.05) | 225 (69.44) | |
| > 5 cm | 54 (33.13) | 45 (27.95) | 99 (30.56) | |
| TRG | 0.160 | |||
| 1 | 14 (8.59) | 16 (9.94) | 30 (9.26) | |
| 2 | 90 (55.21) | 72 (44.72) | 162 (50.00) | |
| 3 | 41 (25.15) | 43 (26.71) | 84 (25.93) | |
| 4 | 18 (11.04) | 30 (18.63) | 48 (14.81) | |
| Vascular invasion | 0.471 | |||
| Negative | 155 (95.09) | 149 (92.55) | 304 (93.83) | |
| Positive | 8 (4.91) | 12 (7.45) | 20 (6.17) | |
| Neural invasion | 0.846 | |||
| Negative | 130 (79.75) | 126 (78.26) | 256 (79.01) | |
| Positive | 33 (20.25) | 35 (21.74) | 68 (20.99) | |
| Radiotherapy type before surgery | 0.688 | |||
| VMAT | 146 (89.57) | 140 (86.96) | 286 (88.27) | |
| IMRT | 14 (8.59) | 16 (9.94) | 30 (9.26) | |
| 3D-CRT | 3 (1.84) | 5 (3.11) | 8 (2.47) | |
| Chemotherapy type before surgery | 0.975 | |||
| Capecitabine | 145 (88.96) | 144 (89.44) | 289 (89.20) | |
| Capecitabine + platinum | 9 (5.52) | 9 (5.59) | 18 (5.56) | |
| Other | 9 (5.52) | 8 (4.97) | 17 (5.25) | |
| Adjuvant chemotherapy | 0.383 | |||
| No | 57 (34.97) | 48 (29.81) | 105 (32.41) | |
| Yes | 106 (65.03) | 113 (70.19) | 219 (67.59) | |
| Pre-CEA | 0.832 | |||
| ≤ 5 ng/mL | 91 (55.83) | 87 (54.04) | 178 (54.94) | |
| > 5 ng/mL | 72 (44.17) | 74 (45.96) | 146 (45.06) | |
| Pre-CA19-9 | 0.931 | |||
| ≤ 27 U/mL | 131 (80.37) | 131 (81.37) | 262 (80.86) | |
| > 27 U/mL | 32 (19.63) | 30 (18.63) | 62 (19.14) |
All variables identified in Table 1 were selected for univariable Cox regression analysis to estimate OS in LARC patients treated with nCRT and TME. In the univariable analysis, there were many variables statistically significant for OS in these patients, including pre-IBIL, smoking history, TRG, vascular invasion, neural invasion, and pre-CA19-9. These statistically significant parameters in the univariate analysis were included in the multivariate model. In the multivariate analysis, we found that pre-IBIL (pre-IBIL > 6.2 μmol/L, adjusted HR = 1.77, 95%CI: 1.04-3.01, P = 0.035), smoking history (yes, adjusted HR = 0.58, 95%CI: 0.34-1.00. P = 0.048), TRG (TRG = 2, adjusted HR = 0.37, 95%CI: 0.18-0.75, P = 0.006; TRG = 3, adjusted HR = 0.27, 95%CI: 0.12-0.64, P = 0.003; TRG = 4, adjusted HR = 0.18, 95%CI: 0.06-0.53 P = 0.002), vascular invasion (positive, adjusted HR = 2.93, 95%CI: 1.39-6.21, P = 0.005), and pre-CA19-9 (pre-CA19-9 > 27 U/mL, adjusted HR = 2.05, 95%CI: 1.16-3.62, P = 0.014) were predictors of OS (Table 2).
| Characteristic | Univariable analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Pre-IBIL | ||||
| ≤ 6.2 μmol/L | Reference | Reference | ||
| > 6.2 μmol/L | 1.71 (1.01-2.87) | 0.045 | 1.77 (1.04-3.01) | 0.035 |
| Age, yr | ||||
| < 60 | Reference | |||
| ≥ 60 | 1.39 (0.84-2.31) | 0.199 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 1.16 (0.66-2.04) | 0.596 | ||
| BMI, kg/m2 | ||||
| < 18.5 | Reference | |||
| 18.5-23.9 | 0.74 (0.23-2.43) | 0.624 | ||
| 24.0-27.9 | 0.57 (0.17-1.93) | 0.370 | ||
| ≥ 28.0 | 0.27 (0.05-1.32) | 0.105 | ||
| Smoking history | ||||
| No | Reference | Reference | ||
| Yes | 0.57 (0.34-0.97) | 0.039 | 0.58 (0.34-1.00) | 0.048 |
| Drinking history | ||||
| No | Reference | |||
| Yes | 0.72 (0.42-1.23) | 0.227 | ||
| Chronic disease history | ||||
| No | Reference | |||
| Yes | 1.09 (0.65-1.82) | 0.749 | ||
| cTNM stage | ||||
| II | Reference | |||
| III | 2.54 (0.92-6.99) | 0.072 | ||
| nCRT with surgery interval, day | ||||
| 31-60 | Reference | |||
| 61-90 | 0.61 (0.35-1.07) | 0.083 | ||
| > 90 | 0.68 (0.24-1.92) | 0.469 | ||
| Inferior margin | ||||
| ≤ 5 cm | Reference | |||
| > 5 cm | 0.91 (0.53-1.59) | 0.752 | ||
| TRG | ||||
| 1 | Reference | Reference | ||
| 2 | 0.35 (0.18-0.67) | 0.002 | 0.37 (0.18-0.75) | 0.006 |
| 3 | 0.23 (0.10-0.52) | < 0.001 | 0.27 (0.12-0.64) | 0.003 |
| 4 | 0.17 (0.06-0.48) | < 0.001 | 0.18 (0.06-0.53) | 0.002 |
| Vascular invasion | ||||
| Negative | Reference | Reference | ||
| Positive | 3.79 (1.86-7.74) | < 0.001 | 2.93 (1.39-6.21) | 0.005 |
| Neural invasion | ||||
| Negative | Reference | Reference | ||
| Positive | 2.15 (1.26-3.69) | 0.005 | 1.27 (0.69-2.31) | 0.442 |
| Radiotherapy type before surgery | ||||
| VMAT | Reference | |||
| IMRT | 1.68 (0.85-3.35) | 0.137 | ||
| 3D-CRT | 0.69 (0.09-4.98) | 0.709 | ||
| Chemotherapy type before surgery | ||||
| Capecitabine | Reference | |||
| Capecitabine + platinum | 1.45 (0.57-3.66) | 0.431 | ||
| Other | 1.90 (0.76-4.78) | 0.172 | ||
| Adjuvant chemotherapy | ||||
| No | Reference | |||
| Yes | 0.60 (0.36-1.00) | 0.050 | ||
| Pre-CEA | ||||
| ≤ 5 ng/mL | Reference | |||
| > 5 ng/mL | 1.44 (0.87-2.39) | 0.158 | ||
| Pre-CA19-9 | ||||
| ≤ 27 U/mL | Reference | Reference | ||
| > 27 U/mL | 2.05 (1.19-3.54) | 0.010 | 2.05 (1.16-3.62) | 0.014 |
All variables identified in Table 1 were selected for univariable Cox regression analysis to estimate DFS in LARC patients treated with nCRT and TME. Univariable analysis revealed that factors including pre-IBIL, BMI, nCRT with surgery interval, TRG, vascular invasion, and neural invasion were independently associated with DFS in these patients. All statistically significant predictors in the univariate analysis were included in the multivariate model. The multivariate analysis revealed that pre-IBIL (pre-IBIL > 6.2 μmol/L, adjusted HR = 1.86, 95%CI: 1.22-2.84, P = 0.004), BMI (BMI ≥ 28.0, adjusted HR = 0.19, 95%CI: 0.05-0.66, P = 0.009), nCRT with surgery interval (nCRT with surgery interval = 61-90 d, adjusted HR = 0.63, 95%CI: 0.40-0.99, P = 0.044), TRG (TRG = 2, adjusted HR = 0.43, 95%CI: 0.24-0.78, P = 0.005; TRG = 3, adjusted HR = 0.30, 95%CI: 0.15-0.62, P = 0.001; TRG = 4, adjusted HR = 0.31, 95%CI: 0.14-0.70, P = 0.005), and vascular invasion (positive, adjusted HR = 2.24, 95%CI: 1.13-4.44, P = 0.021) were predictors of DFS (Table 3).
| Characteristic | Univariable analysis | Multivariable analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Pre-IBIL | ||||
| ≤ 6.2 μmol/L | Reference | Reference | ||
| > 6.2 μmol/L | 1.68 (1.12-2.53) | 0.013 | 1.86 (1.22-2.84) | 0.004 |
| Age, yr | ||||
| < 60 | Reference | |||
| ≥ 60 | 1.16 (0.78-1.74) | 0.456 | ||
| Sex | ||||
| Female | Reference | |||
| Male | 0.84 (0.55-1.28) | 0.414 | ||
| BMI, kg/m2 | ||||
| < 18.5 | Reference | Reference | ||
| 18.5-23.9 | 0.64 (0.26-1.61) | 0.346 | 0.46 (0.18-1.17) | 0.102 |
| 24.0-27.9 | 0.62 (0.24-1.57) | 0.312 | 0.50 (0.19-1.30) | 0.157 |
| ≥ 28.0 | 0.25 (0.07-0.87) | 0.029 | 0.19 (0.05-0.66) | 0.009 |
| Smoking history | ||||
| No | Reference | |||
| Yes | 0.72 (0.48-1.08) | 0.108 | ||
| Drinking history | ||||
| No | Reference | |||
| Yes | 0.71 (0.47-1.07) | 0.103 | ||
| Chronic disease history | ||||
| No | Reference | |||
| Yes | 1.07 (0.72-1.61) | 0.732 | ||
| cTNM stage | ||||
| II | Reference | |||
| III | 1.49 (0.80-2.80) | 0.210 | ||
| nCRT with surgery interval, d | ||||
| 31-60 | Reference | Reference | ||
| 61-90 | 0.64 (0.41-0.99) | 0.047 | 0.63 (0.40-0.99) | 0.044 |
| > 90 | 0.84 (0.40-1.77) | 0.655 | 0.63 (0.29-1.35) | 0.235 |
| Inferior margin | ||||
| ≤ 5 cm | Reference | |||
| > 5 cm | 0.80 (0.51-1.25) | 0.318 | ||
| TRG | ||||
| 1 | Reference | Reference | ||
| 2 | 0.45 (0.26-0.79) | 0.006 | 0.43 (0.24-0.78) | 0.005 |
| 3 | 0.29 (0.15-0.57) | < 0.001 | 0.30 (0.15-0.62) | 0.001 |
| 4 | 0.31 (0.15-0.66) | 0.002 | 0.31 (0.14-0.70) | 0.005 |
| Vascular invasion | ||||
| Negative | Reference | Reference | ||
| Positive | 2.54 (1.32-4.90) | 0.005 | 2.24 (1.13-4.44) | 0.021 |
| Neural invasion | ||||
| Negative | Reference | Reference | ||
| Positive | 1.82 (1.17-2.82) | 0.008 | 1.34 (0.82-2.18) | 0.238 |
| Radiotherapy type before surgery | ||||
| VMAT | Reference | |||
| IMRT | 1.07 (0.57-2.01) | 0.836 | ||
| 3D-CRT | 0.83 (0.20-3.37) | 0.791 | ||
| Chemotherapy type before surgery | ||||
| Capecitabine | Reference | |||
| Capecitabine + platinum | 0.98 (0.43-2.26) | 0.969 | ||
| Other | 1.56 (0.72-3.37) | 0.262 | ||
| Adjuvant chemotherapy | ||||
| No | Reference | |||
| Yes | 0.89 (0.59-1.35) | 0.584 | ||
| Pre-CEA | ||||
| ≤ 5 ng/mL | Reference | |||
| > 5 ng/mL | 1.33 (0.89-1.98) | 0.158 | ||
| Pre-CA19-9 | ||||
| ≤ 27 U/mL | Reference | |||
| > 27 U/mL | 1.47 (0.92-2.33) | 0.104 | ||
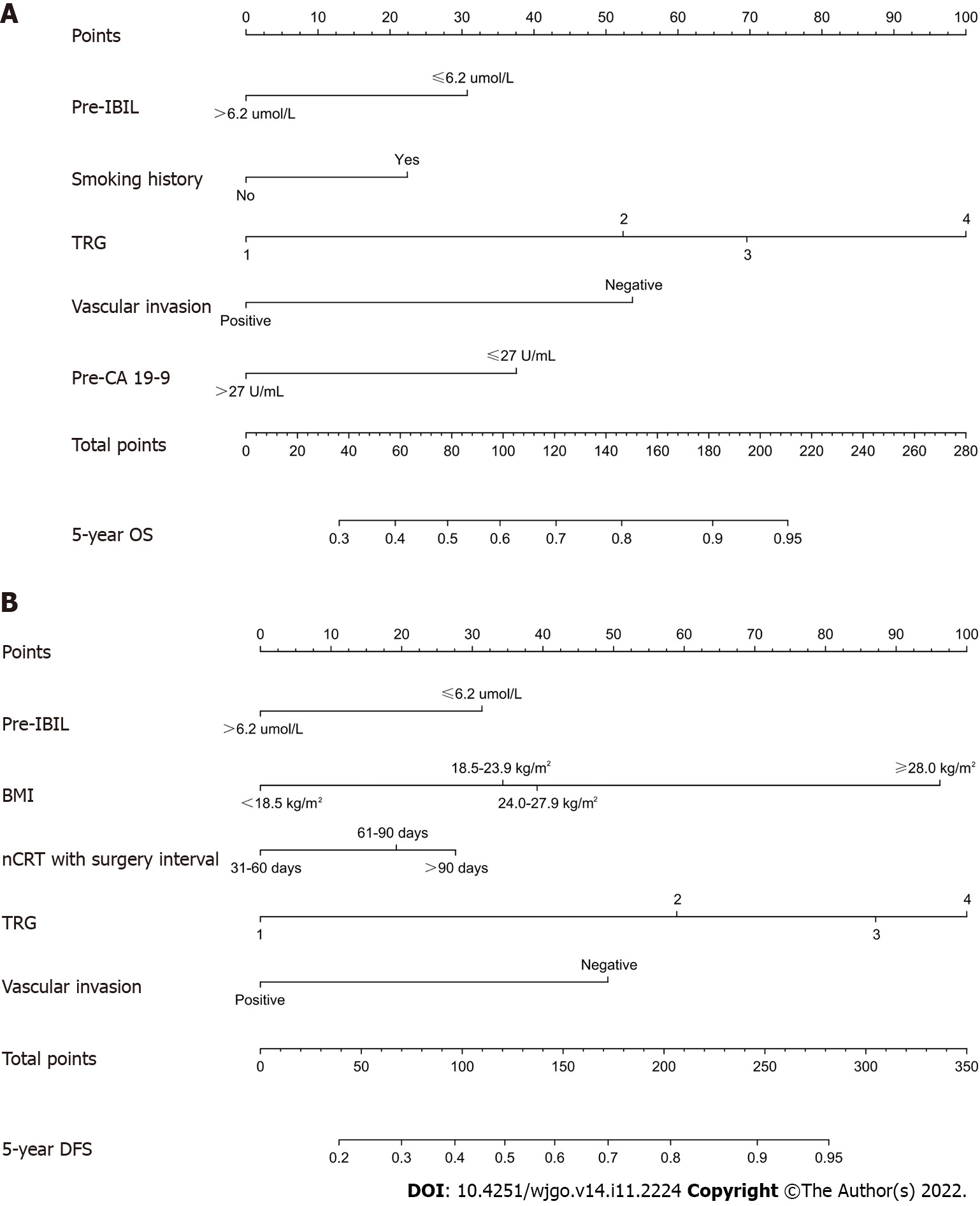
We established nomograms for the prediction of the 5-year OS and DFS using the independent variables (Figure 1). Pre-IBIL, smoking history, TRG, vascular invasion, and pre-CA19-9 were statistically significant predictors of OS on Cox multivariate analysis. Pre-IBIL, BMI, nCRT with surgery interval, TRG, and vascular invasion were statistically significant predictors of DFS on Cox multivariate analysis. Therefore, these variables were subsequently included in the nomogram. A weighted total score is calculated from these factors, which is applied to predict the OS and DFS for the LARC patients who received nCRT. We found that patients with a lower pre-IBIL had better OS and DFS.
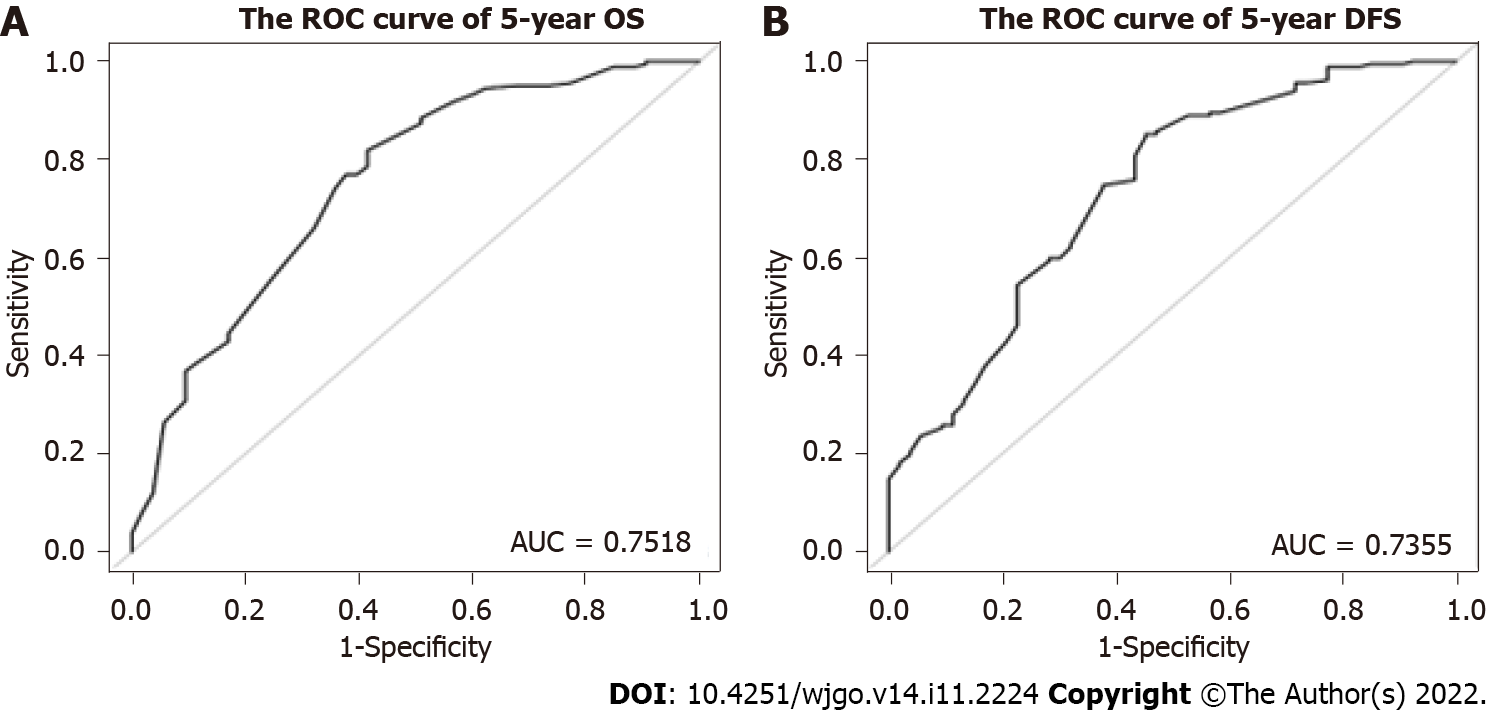
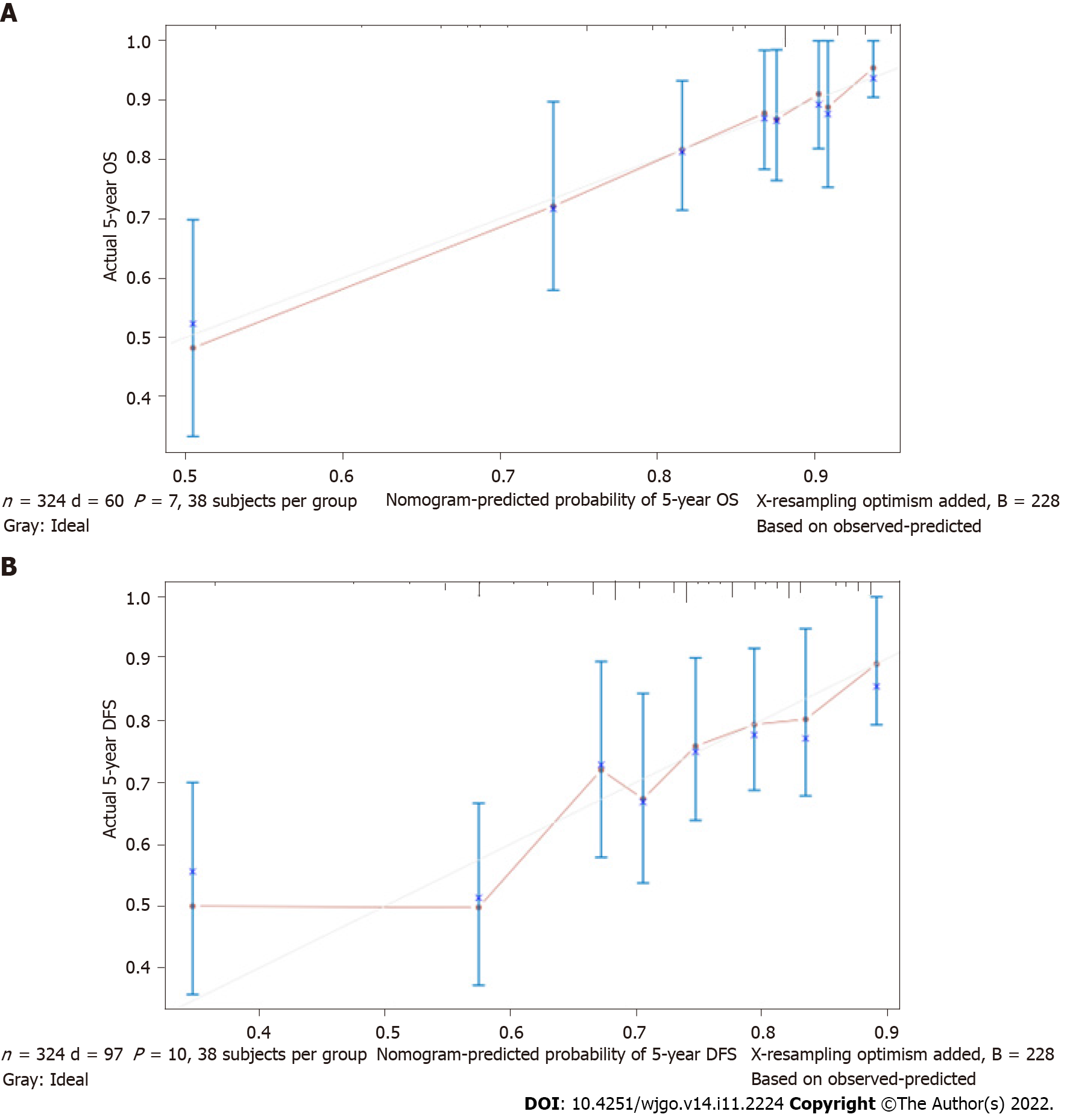
The nomogram for predicting OS and DFS in LARC patients treated with nCRT was developed based on the multivariate model in the cohort. The area under the ROC curve (AUC) was analyzed for the cohort, and the AUC values for the 5-year OS and DFS nomograms were 0.7518 and 0.7355, respectively (Figure 2). The model demonstrated good statistical performance for predicting OS and DFS, and calibration curves for the probability of 5-year OS and 5-year DFS indicated satisfactory consistency between actual observation and nomogram prediction (Figure 3).
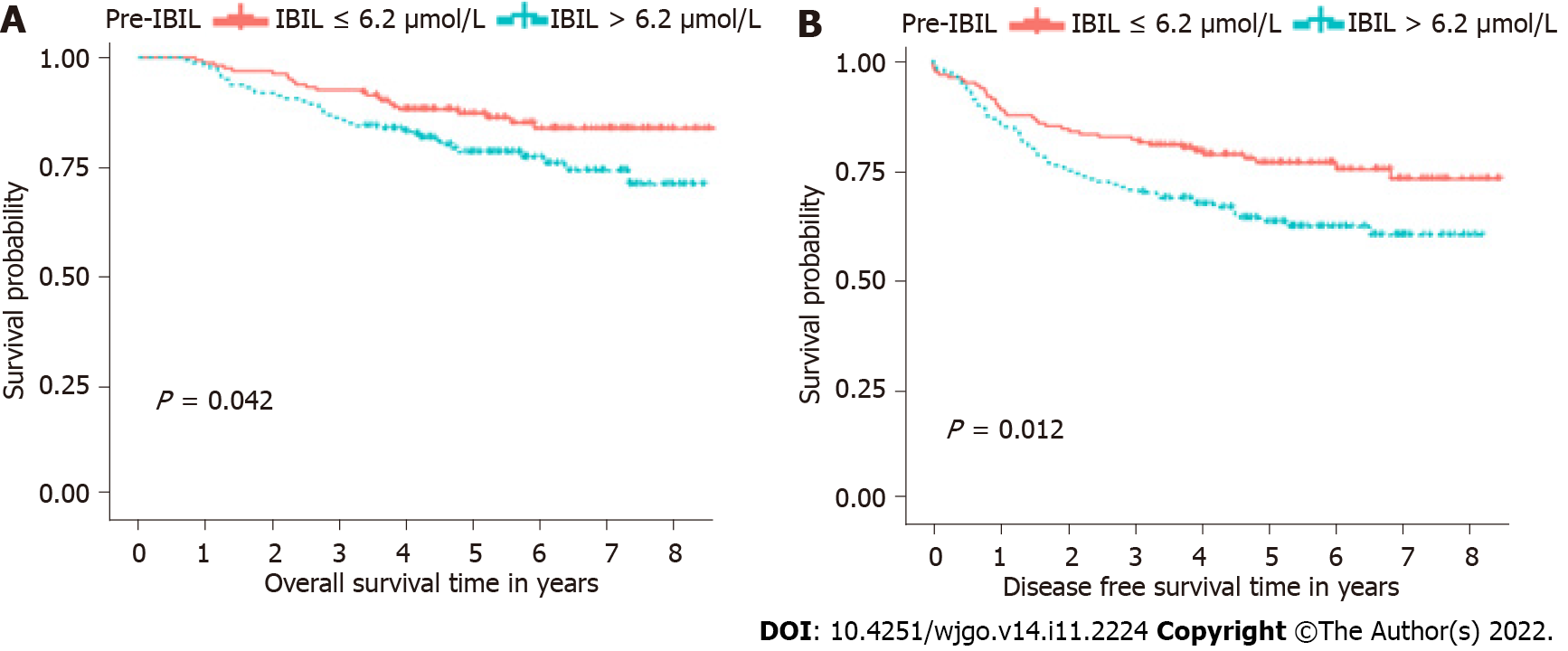
In Kaplan-Meier survival analysis, we found that pre-IBIL was related to OS (Figure 4A) and DFS (Figure 4B) in LARC patients treated with nCRT. For OS, the median was 5.28 years. The patients in the pre-IBIL ≤ 6.2 μmol/L group had a significantly longer OS than patients in the pre-IBIL > 6.2 μmol/L group (P = 0.042). The 5-year survival rate of the patients in the pre-IBIL ≤ 6.2 μmol/L group was 87.27%, higher than that of the patients in the pre-IBIL > 6.2 μmol/L group, 78.48%. For DFS, the median DFS was 4.71 years. Additionally, the patients in the pre-IBIL ≤ 6.2 μmol/L group also had a significantly longer DFS than patients in the pre-IBIL > 6.2 μmol/L group (P = 0.012). The 5-year recurrence rates of patients in the pre-IBIL ≤ 6.2 μmol/L and pre-IBIL > 6.2 μmol/L groups were 76.90% and 64.39%, respectively.
The nCRT combined with TME is the standard treatment for patients with LARC[16]. Although this treatment regimen has been shown to improve the local control rate in some LARC patients, there are still considerable differences in prognoses among patients[17]. Tumor markers are substances that are synthesized or released by tumor cells themselves or produced by the body in response to tumor cells during the process of tumor development. When these substances reach a certain level, they can indicate the existence of a specific tumor, and the changes in the levels of these substances can be used to monitor the recurrence and progression of the tumor[18]. At present, common tumor markers are not routinely tested in health checkups, which often leads to the missed diagnosis of some patients with early malignant tumors, thus indicating that it is important to identify new tumor markers during routine checkups.
Previous studies have focused attention on identifying pretreatment blood-based biomarkers and traditional tumor biomarkers, including CEA, CA19-9 and the neutrophil-to-lymphocyte ratio. These biomarkers have been proven to have prognostic effects in predicting the response and survival in LARC[19]. In recent years, liquid biopsy has received increasing attention in cancer research, and biomarkers based on liquid biopsy have also shown favorable significance in treatment and prognosis prediction[20,21]. For example, a prospective study including 119 Chinese LARC patients identified circulating tumor DNA as a good predictor for risk stratification and for guiding the treatment strategy[22]. However, these new biomarkers are still under investigation, and their clinical application may need to be further studied to obtain a consensus.
Biochemical tests for blood samples are routinely performed for each hospitalized patient, and bilirubin is one of the most important markers of the biochemical test. Bilirubin is a major toxic metabolite of iron porphyrin compounds in the body, which can cause irreversible damage to the nervous system; however, it has antioxidant properties and can inhibit the oxidation of linoleic acid and phospholipids. Recent studies have shown that bilirubin, as an endogenous antioxidant, can moderately increase the ability of cancer patients to scavenge oxidative free radicals in the body and affect the prognoses of cancer patients. Therefore, bilirubin levels are of great clinical significance as a novel tumor biomarker[23-27]. One study demonstrated that the direct bilirubin-to-IBIL ratio was an independent predictor of survival in resectable colorectal cancer patients[28]. Nevertheless, few studies have been conducted to explore the prognostic effect of bilirubin in LARC patients.
In this study, we collected clinical and pathological data of 324 LARC patients treated with nCRT combined with TME and followed up with each of these patients. We performed all factor Cox regression analyses in Table 1, and the characteristics with statistically significant results from the univariate analysis were included in the multivariate analysis. The multivariate analysis results showed that pre-IBIL, smoking history, TRG, vascular invasion and pre-CA19-9 were risk factors affecting OS. The multivariate analysis results showed that pre-IBIL, BMI, nCRT with surgery interval, TRG and vascular invasion were risk factors affecting DFS. In addition, we constructed nomograms to evaluate prognoses based on the above indicators.
Our research had the following advantages. First, we found a correlation of pre-IBIL with the prognosis of LARC patients after receiving nCRT, and the prognoses of those patients with pre-IBIL > 6.2 μmol/L were poorer, which indicated that pre-IBIL is a risk factor affecting the prognoses of patients. Second, we performed a Cox regression analysis of pre-IBIL with common characteristics and found that pre-IBIL was associated with the OS and DFS of LARC patients receiving nCRT. We also constructed nomograms that could predict OS and DFS. Third, we collected clinical characteristics, pathological characteristics, treatment information, hematological characteristics and sociological characteristics of patients and considered more comprehensive factors. However, our study had some limitations. First, this was a single-center study, the sample size was relatively small and the results need to be further validated by expanding the sample size. Second, in our study, with the limitations of the retrospective nature, we found an association between a blood sample and oncological outcomes in patients with LARC, and future prospective studies should be conducted to further explore the associations between pre-IBIL and the prognoses of LARC patients.
In conclusion, this study demonstrated that pre-IBIL was an independent prognostic factor for OS and DFS in LARC patients treated with nCRT followed by TME. Nomograms incorporating pre-IBIL, BMI, smoking history, nCRT with surgery interval, TRG, vascular invasion, and pre-CA19-9 could be helpful to predict OS and DFS. These findings provide a new direction for future clinical precision treatment and scientific research.
Neoadjuvant chemoradiotherapy (nCRT) has been regarded as the standard treatment for local advanced rectal cancer (LARC). Bilirubin has shown significance in the prognosis of various cancer types, including ovarian cancer and lung cancer. However, the predictive values of indirect bilirubin (IBIL) in the prognoses of LARC patients treated with nCRT remain unknown.
The present study attempted to identify the prognostic value of IBIL before nCRT (pre-IBIL) in LARC patients and to construct a nomogram based on pre-IBIL to predict the survival of the patients.
This study aimed to identify the prognostic value of pre-IBIL in LARC patients and to construct a nomogram to predict their 5-year overall survival (OS) and 5-year disease-free survival (DFS).
A total of 324 LARC patients undergoing nCRT with total mesorectal excision (TME) were enrolled. Preoperative clinical features and postoperative pathological characteristics were collected. A Cox regression analysis was performed, and a Cox-based nomogram was developed to predict OS and DFS. We also assessed the predictive performance of the nomogram with receiver operating characteristic (ROC) and curves calibration plots.
In the Cox multivariate regression analysis, we found that pre-IBIL, smoking history, tumor regression grade (TRG), vascular invasion and carbohydrate antigen 19-9 before nCRT were predictors of OS. Furthermore, pre-IBIL, body mass index, nCRT with surgery interval, TRG and vascular invasion were predictors of DFS. Predictive nomograms were developed to predict 5-year OS and 5-year DFS with areas under the ROC curve of 0.7518 and 0.7355, respectively. Good statistical performance on internal validation was shown via the calibration plots and ROC curves.
Pre-IBIL was an independent prognostic factor for OS and DFS in LARC patients treated with nCRT followed by TME. Nomograms based on pre-IBIL could be helpful for predicting survival in LARC patients.
Although our single-center study identified the prognostic value of pre-IBIL in LARC patients, a future prospective study with larger samples should be conducted to further explore the association between pre-IBIL and the prognosis of LARC patients.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Aurello P, Italy; Bustamante-Lopez LA, Brazil; Preziosi F, Italy S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ
| 1. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Garrido-Laguna I, Grem JL, Gunn A, Hoffe S, Hubbard J, Hunt S, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Nurkin S, Overman MJ, Parikh A, Patel H, Pedersen K, Saltz L, Schneider C, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Johnson-Chilla A, Gurski LA. NCCN Guidelines Insights: Rectal Cancer, Version 6.2020. J Natl Compr Canc Netw. 2020;18:806-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 2. | Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, Hui B, Liu R, Ma H, Ren J. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int J Biol Sci. 2016;12:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 164] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 3. | Keller DS, Berho M, Perez RO, Wexner SD, Chand M. The multidisciplinary management of rectal cancer. Nat Rev Gastroenterol Hepatol. 2020;17:414-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 202] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 4. | Sun Y, Huang Z, Chi P. An inflammation index-based prediction of treatment response to neoadjuvant chemoradiotherapy for rectal mucinous adenocarcinoma. Int J Clin Oncol. 2020;25:1299-1307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Sun Y, Zhang Y, Huang Z, Chi P. Prognostic Implication of Negative Lymph Node Count in ypN+ Rectal Cancer after Neoadjuvant Chemoradiotherapy and Construction of a Prediction Nomogram. J Gastrointest Surg. 2019;23:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Li J, Yi CH, Hu YT, Li JS, Yuan Y, Zhang SZ, Zheng S, Ding KF. TNM Staging of Colorectal Cancer Should be Reconsidered According to Weighting of the T Stage: Verification Based on a 25-Year Follow-Up. Medicine (Baltimore). 2016;95:e2711. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Gunderson LL, Jessup JM, Sargent DJ, Greene FL, Stewart AK. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 439] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 8. | Hashiguchi Y, Hase K, Kotake K, Ueno H, Shinto E, Mochizuki H, Yamamoto J, Sugihara K. Evaluation of the seventh edition of the tumour, node, metastasis (TNM) classification for colon cancer in two nationwide registries of the United States and Japan. Colorectal Dis. 2012;14:1065-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Ji D, Yi H, Zhang D, Zhan T, Li Z, Li M, Jia J, Qiao M, Xia J, Zhai Z, Song C, Gu J. Somatic Mutations and Immune Alternation in Rectal Cancer Following Neoadjuvant Chemoradiotherapy. Cancer Immunol Res. 2018;6:1401-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 10. | Lee IH, Kang K, Kang BW, Lee SJ, Bae WK, Hwang JE, Kim HJ, Park SY, Park JS, Choi GS, Kim JG. Genetic variations using whole-exome sequencing might predict response for neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Med Oncol. 2018;35:145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Tweedle EM, Khattak I, Ang CW, Nedjadi T, Jenkins R, Park BK, Kalirai H, Dodson A, Azadeh B, Terlizzo M, Grabsch H, Mueller W, Myint S, Clark P, Wong H, Greenhalf W, Neoptolemos JP, Rooney PS, Costello E. Low molecular weight heat shock protein HSP27 is a prognostic indicator in rectal cancer but not colon cancer. Gut. 2010;59:1501-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Geng Y, Mei Y, Xi Y, Yu J, Meng K, Zhang T, Ma W. Bilirubin Can Be Used as a Prognostic Factor for Lung Adenocarcinoma Patients with EGFR Mutations. Onco Targets Ther. 2020;13:11089-11095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Xi XX, Wang HL, Chen T, Dai JR, Hou SY, Chen YG. Prognostic value of preoperative serum bilirubin levels in ovarian cancer. Am J Transl Res. 2020;12:2267-2280. [PubMed] |
| 14. | Yao JJ, Kou J, Peng QH, Dong J, Zhang WJ, Lawrence WR, Zhang F, Zhou GQ, Wang SY, Sun Y. Prognostic value of serum bilirubin in southern Chinese patients with advanced nasopharyngeal carcinoma. Clin Chim Acta. 2018;484:314-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Zhang H, Li G, Zhu Z, Zheng Y, Wu Y, Zhang W, Gu N, Wang X, Song X. Serum bilirubin level predicts postoperative overall survival in oral squamous cell carcinoma. J Oral Pathol Med. 2018;47:382-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Liu H, Wei R, Li C, Zhao Z, Guan X, Yang M, Liu Z, Wang X, Jiang Z. BMI May Be a Prognostic Factor for Local Advanced Rectal Cancer Patients Treated with Long-Term Neoadjuvant Chemoradiotherapy. Cancer Manag Res. 2020;12:10321-10332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Liu H, Li C, Zhao Z, Guan X, Yang M, Liu Z, Tang Y, Jiang Z, Wang X. Safety and Long-Term Effect Assessment of Neoadjuvant Chemoradiotherapy for Elderly Patients With Locally Advanced Rectal Cancer: A CHN Single-Center Retrospective Study. Technol Cancer Res Treat. 2020;19:1533033820970339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Faria SC, Sagebiel T, Patnana M, Cox V, Viswanathan C, Lall C, Qayyum A, Bhosale PR. Tumor markers: myths and facts unfolded. Abdom Radiol (NY). 2019;44:1575-1600. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 19. | Mendis S, To YH, Tie J. Biomarkers in Locally Advanced Rectal Cancer: A Review. Clin Colorectal Cancer. 2022;21:36-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 20. | Alix-Panabières C, Pantel K. Liquid Biopsy: From Discovery to Clinical Application. Cancer Discov. 2021;11:858-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 585] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 21. | Dizdarevic E, Hansen TF, Jakobsen A. The Prognostic Importance of ctDNA in Rectal Cancer: A Critical Reappraisal. Cancers (Basel). 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 22. | Wang Y, Yang L, Bao H, Fan X, Xia F, Wan J, Shen L, Guan Y, Wu X, Xu Y, Shao Y, Sun Y, Tong T, Li X, Cai S, Zhu J, Zhang Z. Utility of ctDNA in predicting response to neoadjuvant chemoradiotherapy and prognosis assessment in locally advanced rectal cancer: A prospective cohort study. PLoS Med. 2021;18:e1003741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 23. | Gao C, Fang L, Li JT, Zhao HC. Significance and prognostic value of increased serum direct bilirubin level for lymph node metastasis in Chinese rectal cancer patients. World J Gastroenterol. 2016;22:2576-2584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Jiang D, Shi J, Yuan M, Duan X, Li L, Li Q. Levels of serum bilirubin in small cell lung cancer and non-small cell lung cancer patients. Cell Mol Biol (Noisy-le-grand). 2018;64:71-76. [PubMed] |
| 25. | Liu X, Meng QH, Ye Y, Hildebrandt MA, Gu J, Wu X. Prognostic significance of pretreatment serum levels of albumin, LDH and total bilirubin in patients with non-metastatic breast cancer. Carcinogenesis. 2015;36:243-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 26. | Sun H, He B, Nie Z, Pan Y, Lin K, Peng H, Xu T, Chen X, Hu X, Wu Z, Wu D, Wang S. A nomogram based on serum bilirubin and albumin levels predicts survival in gastric cancer patients. Oncotarget. 2017;8:41305-41318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 27. | Vítek L. [Role of bilirubin in the prevention of cardiovascular diseases and cancer]. Cas Lek Cesk. 2016;155:10-14. [PubMed] |
| 28. | Ma Y, Shi L, Lu P, Yao S, Xu H, Hu J, Liang X, Wei S. Creation of a Novel Nomogram Based on the Direct Bilirubin-To-Indirect Bilirubin Ratio and Lactate Dehydrogenase Levels in Resectable Colorectal Cancer. Front Mol Biosci. 2021;8:751506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |