Published online Oct 15, 2022. doi: 10.4251/wjgo.v14.i10.2061
Peer-review started: June 8, 2022
First decision: August 20, 2022
Revised: September 4, 2022
Accepted: September 13, 2022
Article in press: September 13, 2022
Published online: October 15, 2022
Processing time: 127 Days and 20.6 Hours
Targeted therapy (TT) has resulted in controversial efficacy as first-line treatment for biliary tract cancer (BTC). More efficacy comparisons are required to clarify the overall effects of chemotherapy (CT) combined with TT and CT alone on advanced BTC.
To conduct a meta-analysis of the available evidence on the efficacy of CT combined with TT for advanced BTC.
The PubMed, EMBASE, ClinicalTrials, Scopus and Cochrane Library databases were systematically searched for relevant studies published from inception to August 2022. Only randomized clinical trials (RCTs) including comparisons between the combination of gemcitabine-based CT with TT and CT alone as first-line treatment for advanced BTC were eligible (PROSPERO-CRD42022313001). The odds ratios (ORs) for the objective response rate (ORR) and hazard ratios (HRs) for both progression-free survival (PFS) and overall survival (OS) were calculated and analyzed. Subgroup analyses based on different targeted agents, CT regimens and tumor locations were prespecified.
Nine RCTs with a total of 1361 individuals were included and analyzed. The overall analysis showed a significant improvement in ORR in patients treated with CT + TT compared to those treated with CT alone (OR = 1.43, 95%CI: 1.11-1.86, P = 0.007) but no difference in PFS or OS. Similar trends were observed in the subgroup treated with agents targeting epidermal growth factor receptor (OR = 1.67, 95%CI: 1.17-2.37, P = 0.004) but not in the subgroups treated with agents targeting vascular endothelial growth factor receptor or mesenchymal-epithelial transition factor. Notably, patients who received a CT regimen of gemcitabine + oxaliplatin in the CT + TT arm had both a higher ORR (OR = 1.75, 95%CI: 1.20-2.56, P = 0.004) and longer PFS (HR = 0.83, 95%CI: 0.70-0.99, P = 0.03) than those in the CT-only arm. Moreover, patients with cholangiocarcinoma treated with CT + TT had significantly increased ORR and PFS (ORR, OR = 2.06, 95%CI: 1.27-3.35, PFS, HR = 0.79, 95%CI: 0.66-0.94).
CT + TT is a potential first-line treatment for advanced BTC that leads to improved tumor control and survival outcomes, and highlighting the importance of CT regimens and tumor types in the application of TT.
Core Tip: The clinical efficacy of adding targeted agents to first-line treatment of biliary tract cancer (BTC) remains unclear. Our study is the first meta-analysis of randomized clinical trials to evaluate the efficacy of the combination of targeted therapy (TT) with standard chemotherapy (CT) as first-line treatment in patients with advanced BTC. We assessed the efficacy of combined TT and CT in terms of objective response rate, progression-free survival and overall survival. Subgroup analyses were conducted based on different targeted agents, CT regimens and tumor locations.
- Citation: Bai XS, Zhou SN, Jin YQ, He XD. Combining of chemotherapy with targeted therapy for advanced biliary tract cancer: A systematic review and meta-analysis. World J Gastrointest Oncol 2022; 14(10): 2061-2076
- URL: https://www.wjgnet.com/1948-5204/full/v14/i10/2061.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i10.2061
Biliary tract cancer (BTC), including cholangiocarcinoma (CCA) (intrahepatic cholangiocarcinoma (iCCA), perihilar cholangiocarcinoma, or cholangiocarcinoma in the distal biliary tree) and gallbladder cancer (GBC), is a relatively rare invasive adenocarcinoma with a dismal prognosis. In recent decades, the incidence of BTC has shown a consistent increasing trend worldwide, particularly in Asian countries[1]. Surgery offers the only potentially curative treatment option for patients who have resectable disease. The high incidence of lymph node involvement and liver invasion are associated with worse clinical outcomes after surgery. However, given the frequent absence of symptoms and late diagnosis in patients with BTC, only a minority of patients (35% for CCA and 20% for GBC) are potential candidates for radical resection; even after resection with a negative surgical margin, the postoperative relapse rate is over 60%[2-4].
For patients with advanced BTC, including radically unresectable or metastatic adenocarcinoma[5], the available systemic therapeutics have limited effect, with a five-year survival of 4%[6,7]. Currently, the first-line treatment for advanced BTC remains gemcitabine-based chemotherapy regimens[1]. According to the ABC-02 trial in 2010, gemcitabine and cisplatin combination chemotherapy (CisGem) was verified to improve overall survival (OS), progression-free survival (PFS) and tumor control rate (TCR) compared with gemcitabine monotherapy in patients with CCA and GBC[6,8]. Gemcitabine and oxaliplatin (GemOx) combination therapy was also identified as an alternative to CisGem. The results of a phase III randomized controlled trial (RCT) in 2019 showed that modified GemOx might lead to a longer median OS (P = 0.57) and different toxicities than CisGem[9]. In addition, randomized phase 3 study trials evaluating efficacy have shown that the efficacy of gemcitabine and S1 combination regimens are noninferior to that of CisGem[10]. Nevertheless, no studies have verified if the superiority of such regimens over CisGem has statistical significance.
Due to the limited efficacy of current chemotherapy (CT) regimens for advanced BTC, new therapies need to be developed. In the past decade, through new parallel sequencing of malignancies, several genetic alterations and molecular characteristics for BTC have been further revealed, including isocitrate dehydrogenase (IDH)-1 and -2 mutations, fibroblast growth factor receptor (FGFR) fusions, neurotropic tyrosine kinase receptor fusions, V-raf murine sarcoma viral oncogene homolog B (BRAF) mutations and aberrations of human epidermal growth factor receptor (HER) family members[11-14]. Targeted therapies (TTs) based on monoclonal antibodies or tyrosine kinase inhibitors associated with actionable genetic alterations in BTC are being extensively explored. Recently, combinations of CT and TT have been attempted to improve the prognosis for advanced BTC, but the clinical efficacy remains to be further evaluated[15].
Considering that the high heterogeneity and low incidence of BTC impede the recruitment of large cohorts of patients to identify effective targets and regimens in clinical trials, meta-analysis is needed to further assess the value of TT and investigate the survival benefits of this treatment. This study is the first meta-analysis of RCTs to evaluate the efficacy of the combination of TT with standard CT as first-line treatment for patients with advanced BTC.
This systematic review and meta-analysis was conducted in accordance with the guidelines of the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) statement guidelines and was prospectively registered in The International Prospective Register of Systematic Reviews (PROSPERO, https://www.crd.york.ac.uk/prospero/) platform (registration number CRD 42022313001).
A systematic literature search of the PubMed/MEDLINE, Clinical Trials, EMBASE, SOCPUS and Cochrane Library databases was conducted from inception to August 2022. Various combinations of the following search terms were used in the database searches: "biliary tract cancer", "gallbladder neoplasms", "cholangiocarcinoma", "molecular targeted therapy" and "antineoplastic agents". Reference Citation Analysis (https://www.referencecitationanalysis.com) was used to avoid missing relevant studies. In addition, we searched the reference lists of the included literature and potentially relevant studies to retrieve studies from other sources. The detailed search strategy and results are described in the supplement (Supplementary Table 1).
Trials were eligible for inclusion if they met the following criteria: (1) Randomized controlled trials involving patients with BTC who were treated with targeted therapy and chemotherapy as first-line treatment; and (2) Advanced, unresectable, recurrent or metastatic BTC with PFS, OS, and/or objective response rate (ORR) reported. Studies involving the following were excluded: (1) No standard chemotherapy arm as a control; (2) Case reports, reviews, commentaries, notes and letters; and (3) Non-English language articles. Two independent reviewers conducted the assessment of all the searched studies. To avoid duplicate clinical data, the registration information in ClinicalTrials.gov was checked, and the most recent and most complete publication was incorporated.
Two authors independently extracted the data and information. In the event of a disagreement, the data source was checked, and a third reviewer was consulted to confirm the correct data. The following information was extracted: the first author’s name, journal name, publication year, study period, national clinical trial number, institution and country. The detailed demographic characteristics included the number of patients, age, sex and disease site. Regarding therapeutic interventions, information on the treatment regimen was collected. The efficacy outcomes extracted included the ORR, PFS and OS. If the hazard ratios (HRs) of OS or PFS were not reported in the literature, Engauge Digitizer 4.1 was used to plot points on the survival curves and extract the HR values.
The risk of bias and quality of the RCTs were assessed by two independent authors in accordance with the criterion of the Cochrane risk of bias tool (ROB) including the following seven dimensions: blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias. A third author was consulted to resolve any disagreements.
Pooled HRs with 95%CIs were calculated for time-to-event data, including OS and PFS. Estimated odds ratios (ORs) were calculated for discrete variables, including the ORR and adverse events. The selection of a fixed- or random-effects model was based on the level of heterogeneity of the data, which was assessed by Cochran’s Q-test and the Higgins I2 statistic. A fixed effects model was adopted for I2 < 50%, and a random effect model was adopted for I2 > 50%. The potential bias of the publications was presented as funnel plots and measured using Egger's tests. Subgroup analysis was performed based on the different targets of the agents, CT regimens and location of the BTC. P < 0.05 was considered statistically significant. All statistical analyses of the extracted data were conducted with Review Manager 5.4.1 (Cochrane Collaboration, Oxford, United Kingdom).
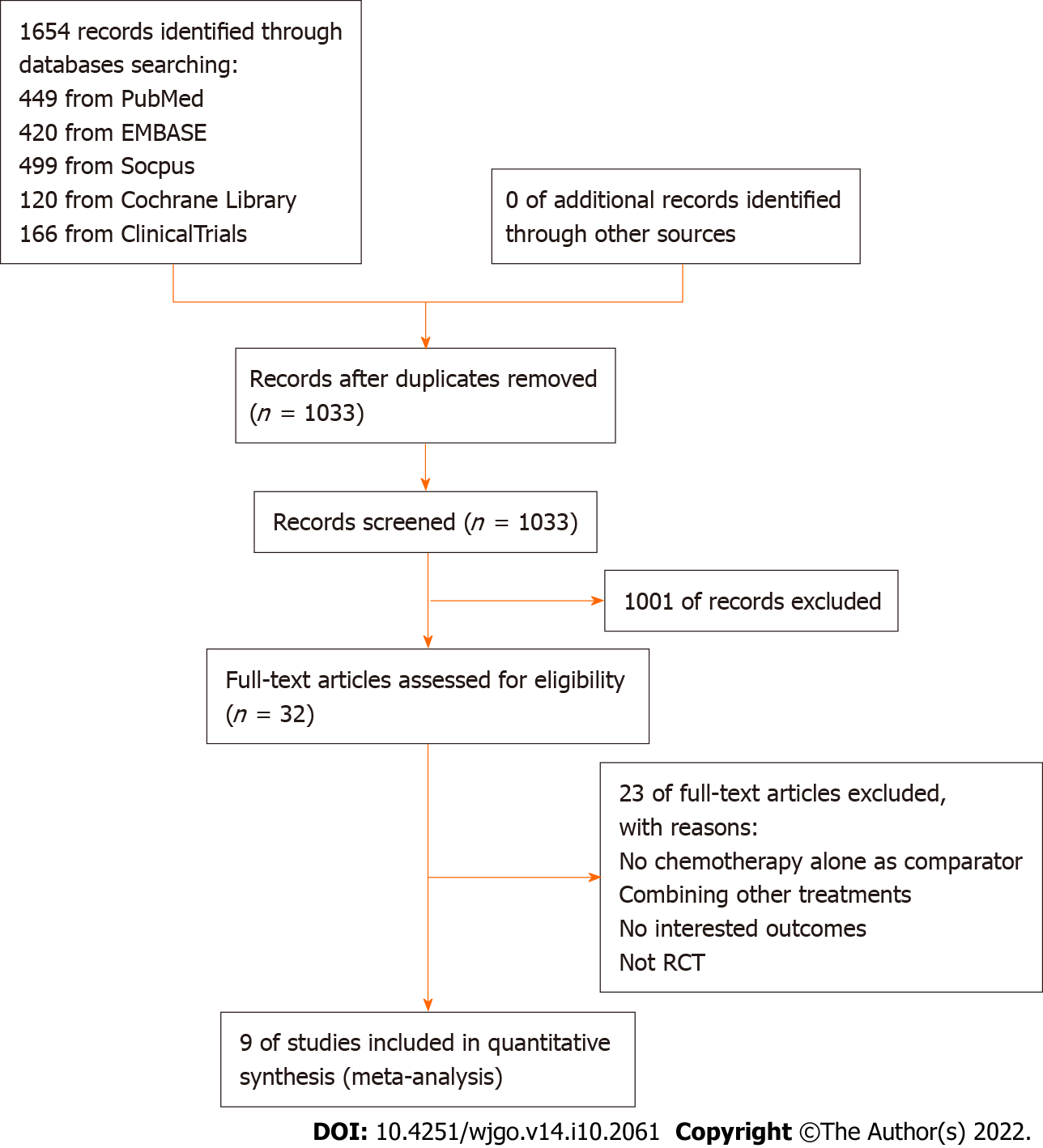
The PRISMA flowchart of the study search and selection process is presented in Figure 1. A total of 1654 records were retrieved in the initial search of PubMed, Embase, Scopus, Cochrane Library and ClinicalTrials.gov. A total of 621 records were duplicates and removed, and 1033 records were screened for eligibility using titles and abstracts. Of the 32 studies that underwent full-text assessment, nine RCTs met the prespecified inclusion criteria for the meta-analysis[16-24].
The design characteristics of the nine clinical trials are summarized in Table 1. All 9 studies included were RCTs. Data on a total of 1361 patients were provided in the nine included trials. Eight targeted treatment regimens (ramucirumab, merestinib, panitumumab, cediranib, vandetanib, cetuximab, sorafenib and erlotinib) and three gemcitabine-based first-line CT regimens (CisGem, GemOx and gemcitabine) were used. Four of the studies were designed with blinding using CT plus placebo as comparators. All studies reported final data for ORR, PFS and OS as endpoints, with an acceptable sample size of patients and adequate length of follow-up. The quality assessment of the included articles was evaluated with the ROB (Supplementary Figure 1).
| Ref. | NCT number | Country/regions | Study period | Number of patients | Chemotherapy regimen | |
| CT | CT + TT | |||||
| Valle et al[1], 2021 | NCT02711553 | 18 countries and regionsa | May, 2016 to Aug, 2017 | 101 | 106 | CisGem |
| 102 | ||||||
| Vogel et al[17], 2018 | NCT01320254 | Germany | Jul, 2011 to Dec, 2015 | 28 | 62 | CisGem |
| Leone et al[18], 2016 | NCT01389414 | Italy | Jun, 2010 to Sept, 2013 | 44 | 45 | GemOx |
| Valle et al[19], 2015 | NCT00939848 | UK | Apr, 2011 to Sept, 2012 | 60 | 62 | CisGem |
| Santoro et al[21], 2015 | NCT00753675 | Italy | Oct, 2008 to Sept, 2012 | 56 | 58 | Gemcitabine |
| Chen et al[20], 2015 | NCT01267344 | China | Dec, 2010 to May, 2012 | 60 | 62 | GemOx |
| Moehler et al[22], 2014 | NCT00661830 | Germany | May, 2008 to Jul, 2011 | 48 | 49 | Gemcitabine |
| Malka et al[23], 2014 | NCT00552149 | France and Germany | Oct, 2007 to Dec, 2009 | 74 | 76 | GemOx |
| Lee et al[24], 2012 | NCT01149122 | South Korea | Feb, 2009 to Aug, 2010 | 133 | 135 | GemOx |
The descriptions of all the trial patients are presented in Table 2. Overall, the median age of the patients ranged from 59 to 68 years old. Most patients in these cohorts had unresectable disease with metastases. There was no difference between the CT group and CT combined with TT group in the distribution of age, sex, Eastern Cooperative Oncology Group performance status, disease status or primary tumor site.
| Ref. | Design | Age, median | Males/females | ECOG 0/1-2 | Locally advanced/metastatic | Primary tumour site |
| Valle et al[1], 2021 | CisGem | 59 | 53/48 | 61/39 | 2/98 | iCCA, 55; GBC, 26; eCCA, 14; AoV, 5 |
| Ramucirumab | 64 | 46/60 | 45/58 | 3/103 | iCCA, 56; GBC, 24; eCCA, 18; AoV, 8 | |
| Merestinib | 62 | 48/54 | 52/50 | 4/98 | iCCA, 60; GBC, 22; eCCA, 14; AoV, 6 | |
| Vogel et al[17], 2018 | CisGem | 59.5 | 14/14 | 11/17 | 5/17 | GBC, 3; dCCA, 1; pCCA, 2; iCCA, 20; Others, 6 |
| Panitumumab | 62 | 36/26 | 21/39 | 13/42 | GBC, 11; dCCA, 7; pCCA, 2; iCCA, 41; Others, 6 | |
| Leone et al[18], 2016 | GemOx | 64.2 | 15/29 | 1/43 | 6/38 | iCCA, 21; eCCA, 7; GBC, 16 |
| Panitumumab | 63.9 | 17/28 | 0/45 | 8/37 | iCCA, 21; eCCA, 12; GBC, 12 | |
| Valle et al[19], 2015 | CisGem | 64.5 | 28/34 | 28/34 | 8/54 | iCCA, 15; eCCA, 24; GBC, 19; AoV, 4 |
| Cediranib | 68 | 34/28 | 27/35 | 12/50 | iCCA, 14; eCCA, 24; GBC, 20; AoV, 4 | |
| Santoro et al[21], 2015 | Gemcitabine | 64 | 25/31 | 34/21 | NR | iCCA, 29; eCCA, 13; GBC, 7; AoV, 6 |
| Vandetanib | 64.4 | 31/27 | 36/23 | NR | iCCA, 31; eCCA, 10; GBC, 13; AoV, 4 | |
| Chen et al[20], 2015 | GemOx | 59 | 30/30 | 17/43 | 17/43 | iCCA, 45; eCCA, 10; GBC, 5 |
| Cetuximab | 61 | 28/34 | 18/44 | 23/39 | iCCA, 44; eCCA, 9; GBC, 9; | |
| Moehler et al[22], 2014 | Gemcitabine | 64.5 | 25/23 | 9/35 | NR | GBC, 7; iCCA, 29 |
| Sorafenib | 64 | 29/20 | 17/30 | NR | GBC, 6; iCCA, 33 | |
| Malka et al[23], 2014 | GemOx | 62 | 42/32 | 27/43 | 15/59 | iCCA, 46; eCCA, 14; GBC, 11; AoV, 0 |
| Cetuximab | 61 | 43/33 | 35/36 | 17/59 | iCCA, 49; eCCA, 8; GBC, 11; AoV, 1 | |
| Lee et al[24], 2012 | GemOx | 61 | 79/54 | 20/113 | 0/133 | CCA, 84; GBC, 47; AoV, 2; |
| Erlotinib | 59 | 91/44 | 26/109 | 0/135 | CCA, 96; GBC, 35; AoV, 4 |
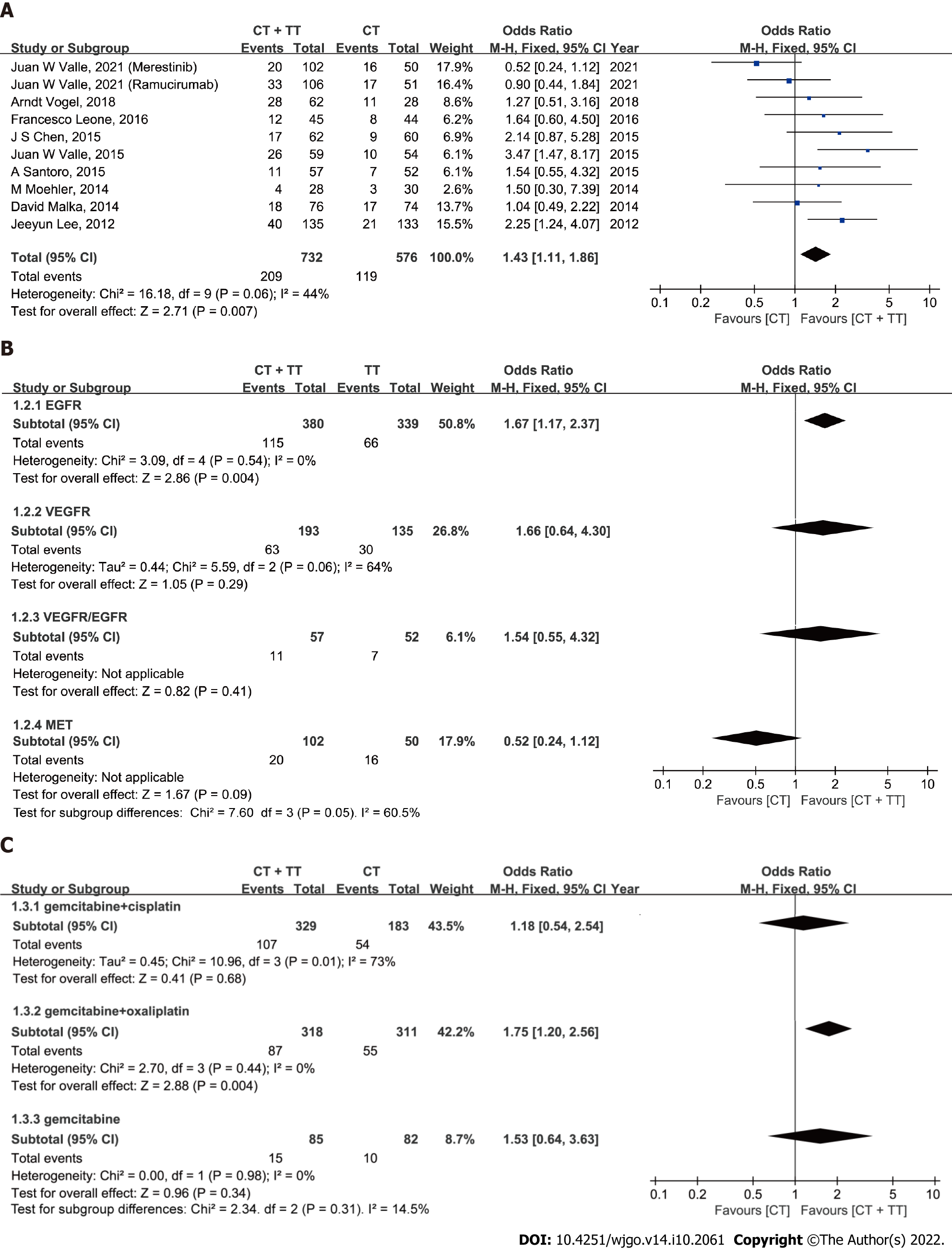
ORR: The ORR reported in the studies ranged from 19.3% to 44% in the CT + TT group and 10% to 39% in the CT group (Figure 2A). No significant heterogeneity was detected among studies, with I2= 44% and P = 0.06. Therefore, the fixed-effect model was adopted for the meta-analysis. The pooled data showed that CT + TT could significantly increase the ORR in BTC compared to CT (OR = 1.43, 95%CI: 1.11-1.86, P = 0.007). Subgroup analyses showed heterogeneity between different targeting molecules (I2 = 60.5%, P = 0.05), implying that different therapeutic targets might an interaction effect on the ORR of BTC patients. CT + TT targeting for epidermal growth factor receptor (EGFR) might more effectively enhance the ORR in BTC than CT alone (Figure 2B, OR = 1.67, 95%CI: 1.17-2.37, P = 0.004), but no difference was found for agents targeting vascular endothelial growth factor receptor (VEGFR) (P = 0.29), mesenchymal-epithelial transition factor (MET) (P = 0.09) or VEGFR/EGFR (P = 0.41). No significant heterogeneity was detected in the ORR among subgroups of different CT regimens (I2 = 14.5%, P = 0.31). In the GemOx subgroup, the ORR was higher in the CT + TT arm than in the CT-only arm (Figure 2C, OR = 1.75, 95%CI: 1.20-2.56, P = 0.004).
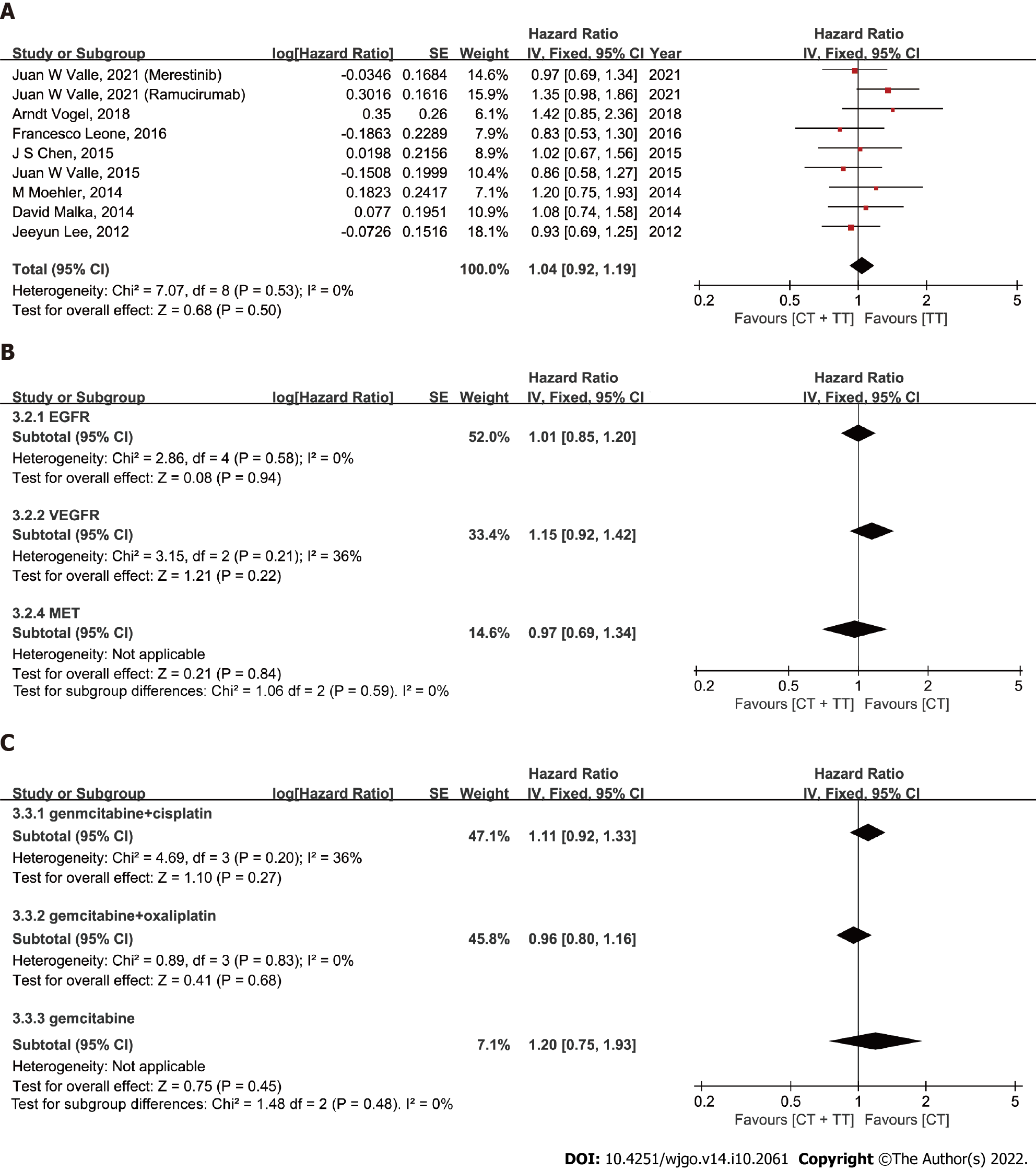
PFS: The median PFS ranged from 4.1 to 8.25 mo in the CT-only group and 3.0 to 8.0 mo in the CT combined with TT group. The overall pooled HR for OS was calculated based on a fixed-effect model (I2 = 32%, P = 0.15). The results showed that the BTC patients in the group treated with CT + TT had a longer PFS than those treated with CT alone, but this difference was not statistically significant (Figure 3A, HR = 0.96, 95%CI: 0.85-1.08, P = 0.47). In the subgroup analysis, the selection of different CT regimens was found to have an interaction effect on PFS in BTC patients (I2= 70.0%, P = 0.04). When the CT regimen was GemOx, a remarkable survival benefit was observed in the CT + TT group compared to the CT-only group (HR = 0.83, 95%CI: 0.70-0.99, P = 0.03). Similar to the ORR results, when targeting EGFR, the combination of CT with TT still tended to lead to better PFS for BTC patients, although no statistical significance was observed (OR = 0.88, 95%CI: 0.75-1.03, P = 0.11) (P = 0.59 when targeting VEGFR, P = 0.21 when targeting VEGFR/EGFR, P = 0.49 when targeting MET).
OS: The median OS ranged from 9.5 to 20.07 mo in the CT-only group and 8.4 to 14.1 mo in the CT combined with TT group. Except for the Santoro et al[21]. study, all of the studies reported OS with HR data and events as outcomes. The fixed-effect model was applied, with I2= 0% and P = 0.53. The pooled data showed no significant improvement in the OS of BTC patients treated with CT + TT compared to those treated with CT alone (Figure 4A, HR = 1.04, 95%CI: 0.92-1.19, P = 0.50). Subgroup analysis among different molecular targets and CT regimens failed to show differences between CT and CT + TT (both I2 = 0%).
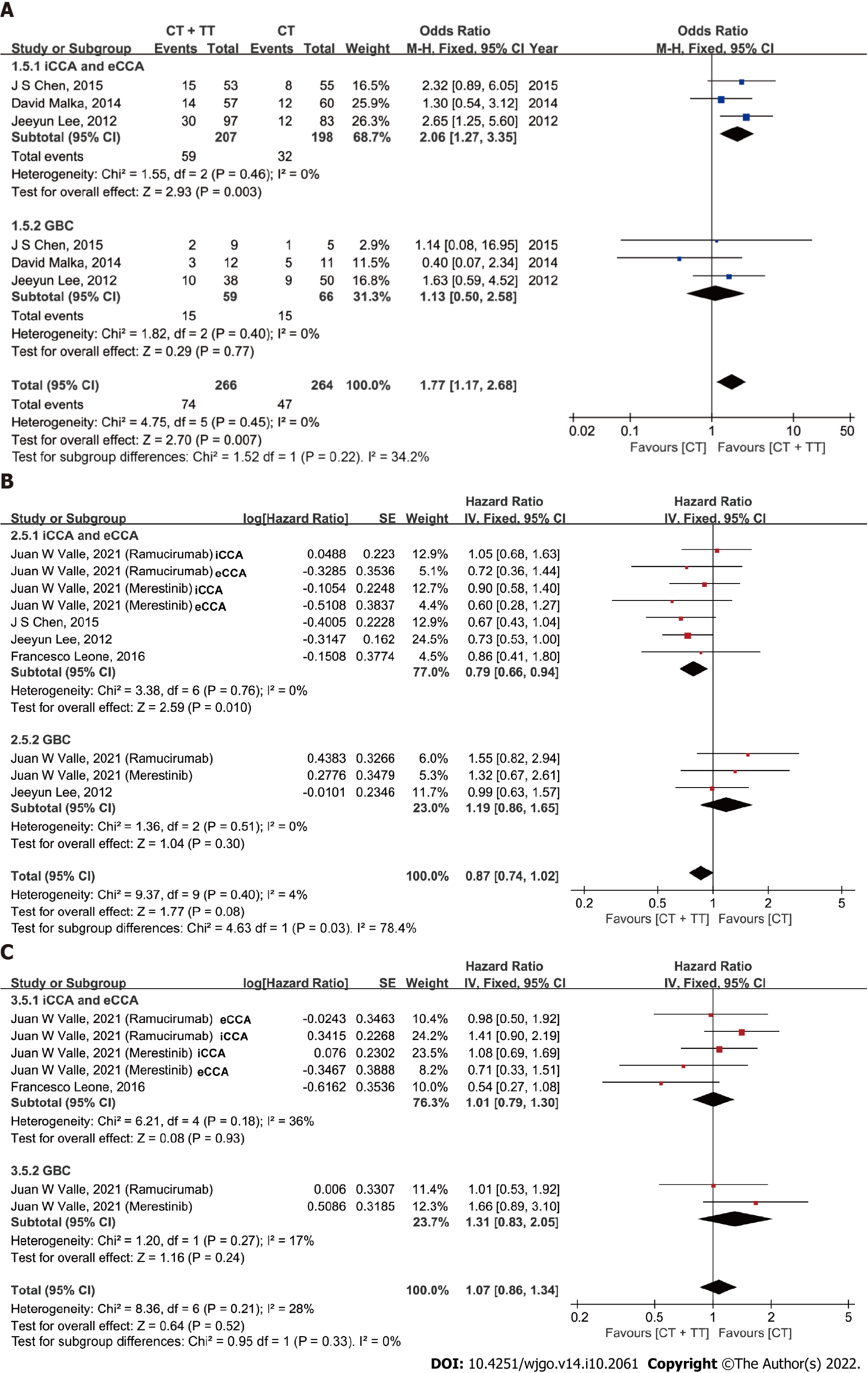
Exploratory analyses were performed to compare the effect of combining CT with TT according to the site of tumor origin (Figure 5). Among the 9 studies, two studies differentiated the data of PFS for GBC from BTC. Four studies reported PFS data for CCA [iCCA or extrahepatic cholangiocarcinoma (eCCA)]. After data pooling, heterogeneity was detected among subgroups in PFS according to tumor location (I2 = 78.4%, P = 0.03), with no significant heterogeneity within subgroups (both I2 = 0%). In the subgroup of patients with CCA, CT + TT conferred an improved ORR and PFS benefit compared to CT alone (ORR: OR = 2.06, 95%CI: 1.27, 3.35, P = 0.003; PFS: HR = 0.79, 95%CI: 0.66-0.94, P = 0.010). In contrast, the ORR and PFS did not differ among patients with GBC (ORR: P = 0.77, PFS: P = 0.30). The OS was similar between the CT and CT + TT groups for both CCA and GBC patients.
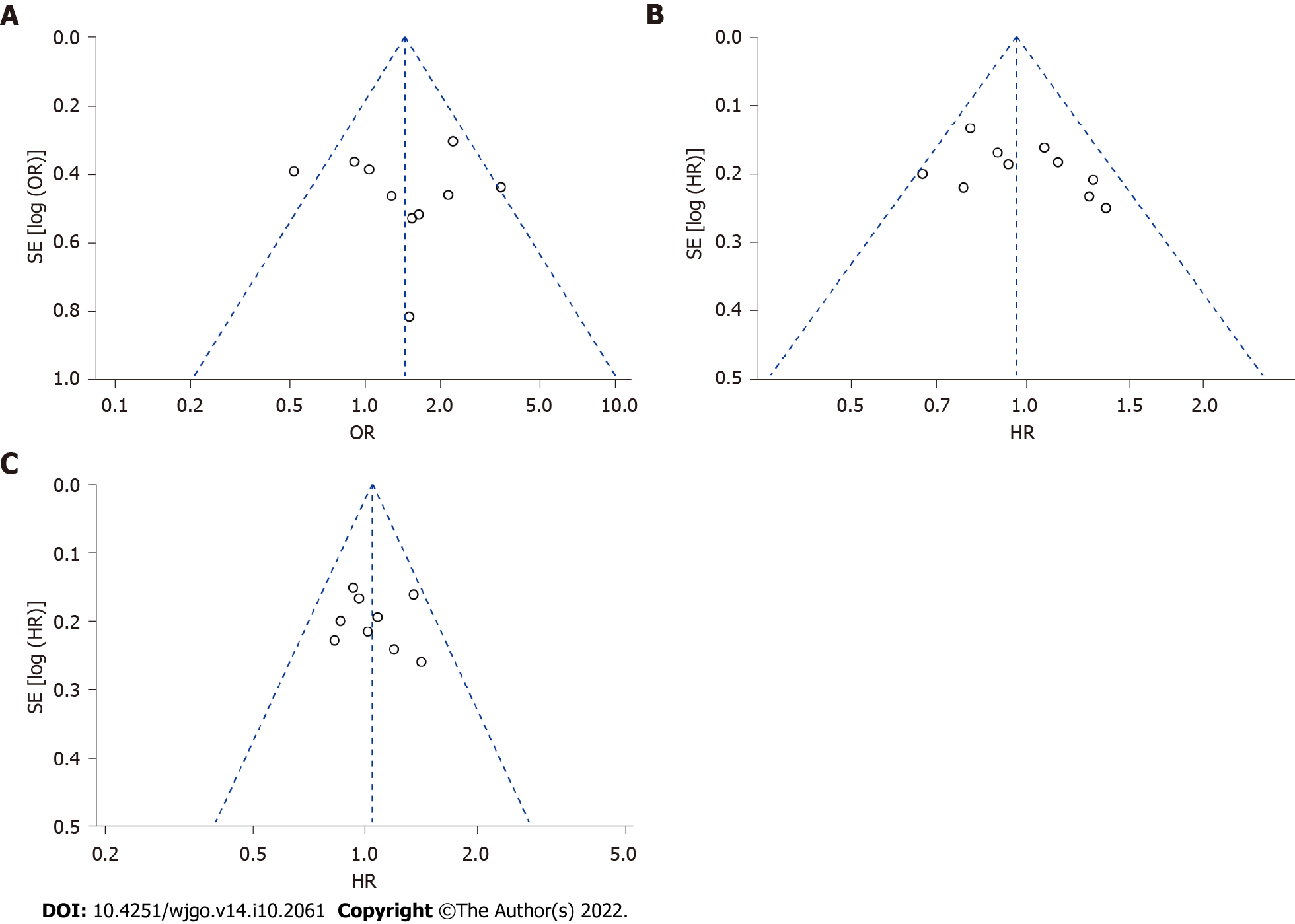
Publication bias was assessed by Egger’s test and is presented as a funnel plot. Both the P value from Egger’s test and the symmetry seen from the funnel plot indicate that there was no evidence of significant publication bias for ORR, PFS or OS in our meta-analysis (Figure 6, Egger’s test, P = 0.756, 0.171, 0.706, respectively).
BTC tends to be diagnosed late and is associated with a poor prognosis. Currently, gemcitabine-based CT is still the standard first-line treatment for unresectable advanced BTC. However, the median survival in gemcitabine-treated BTC patients is only approximately 12 mo[13]. To further improve patient prognosis, more effective first-line strategies need to be explored[25]. Therefore, triple CT combinations such as with the addition of S-1 or nab-paclitaxel to the standard of care CisGem, 5-fluorouracil, irinotecan and oxaliplatin as well as some new agents such as NUC-1031 have also been evaluated in phase 2 clinical trials and demonstrated favorable safety profile[26-28]. Thereinto, gemcitabine, cisplatin plus S-1 showed survival benefits and higher risk ratio than gemcitabine, cisplatin. However, further exploration is required with phase III clinical trials for improving the clinical outcomes of advanced BTC patients. Recently, with the further understanding and exploration of the molecular characteristics of BTC, several actionable mutations have been identified and have changed the treatment paradigm for BTC. Currently, inhibitors targeting FGFR fusions and IDH-1 and -2 mutations have been tested in clinical trials with encouraging outcomes for pretreated CCA. As one of the most promising targets, the survival benefit of IDH-1 inhibitors as a second-line treatment option in IDH-1-mutated CCA has been demonstrated in a phase III clinical trial[29]. In addition, some pre-clinical and early clinical studies on other potential targets including HER-2, BRAF and ring finger protein 43 mutations are currently undertaken[30]. However, their efficacy as first-line treatment for BTC is still being evaluated in ongoing clinical trials (NCT02386397). Due to the lack of adequate patient selection, whether the addition of TT to first-line treatment improves prognosis compared with CT alone has been controversial to date.
This analysis pooled and analyzed data from a total of 1361 individuals from 9 RCTs to compare the effects of CT with TT or CT alone, in terms of the ORR, PFS and OS, as BTC treatment. Our results suggest that combination TT with CT as first-line systemic treatment for advanced biliary tract malignancies might be associated with beneficial outcomes in some situations.
Overall, our meta-analysis showed a significant improvement in the ORR in unselected patients treated with CT + TT compared to that in patients treated with CT (28.6% vs 20.7%). All the trials included adopted gemcitabine-based CT schedules for first-line systemic therapy, which is consistent with the current standards of care. Therefore, the ORR of CisGem was superior to that of gemcitabine alone, similar to in the phase III ABC-02 study[6]. Oxaliplatin is sometimes substituted for cisplatin. The adoption of GemOx as first-line CT is based on the fact that oxaliplatin is easier to administer than cisplatin, as it does not require excessive hydration and reduces the risk of renal toxicity, but maintains a similar efficacy to CisGem[6,31,32]. However, there has been no direct comparison or validated superiority among different combinations (CisGem and GemOx) in advanced BTC. Our results demonstrate that combination TT with CT might significantly improve the ORR in BTC patients treated with GemOx (27.4% vs 17.7%, P = 0.004). Moreover, a similar significant advantage was observed in PFS for combination TT with CT, which we regard as a more unbiased outcome than OS, because OS is susceptible to the influence of subsequent therapies and other factors (HR = 0.83, P = 0.03)[33].
In the subgroup analysis of GemOx, the main driver of the OS benefit favoring the combination of TT and CT was the data from the study from Lee et al[24], in which erlotinib plus GemOx yielded a clear improvement in ORR (30% vs 15.8, P = 0.005) and a marginal superiority in PFS (5.8 mo vs 4.2 mo, HR = 0.80, 95%CI: 0.61-1.03, P = 0.087) compared with GemOx alone. Similar to our results, the PFS improved significantly with cetuximab plus gemcitabine treatment (HR = 0.66, 95%CI: 0.45-0.98, P = 0.04) in the study by Chen et al[20] that included Chinese patients. Unlike the other studies in this subgroup, Chen et al[20] adopted the modified GemOx regimen, which might have a better compliance rate than traditional GemOx, and observed a significantly longer treatment duration for GemOx plus cetuximab than for GemOx alone (P = 0.01). These results suggest that relatively mild CT regimens plus TT might be advantageous and beneficial for advanced BTC patients, especially Asian patients.
The heterogeneity of different anatomical locations and molecular profiles has been demonstrated to be associated with differences in clinicopathologic features and prognoses of advanced BTC. However, complete information about treatment and survival is usually absent in classifications that are based on molecular subtypes and anatomical location[34]. The subgroup analysis showed that the combination of EGFR agents with CT significantly improved the ORR compared with CT alone (30.3% vs 19.5%, P = 0.004), but no significant difference in PFS or OS was observed between the two groups. These results corroborate the finding of a pooled analysis by Eckel et al[35] that analyzed pooled data from 161 trials containing 6337 BTC patients treated with gemcitabine-based CT with or without TT. The study also demonstrates that the combination of EGFR-targeted agents with gemcitabine-based CT was more effective for tumor control and survival, with superior outcomes in the TCR, tumor progression and OS.
Nevertheless, most of the RCTs (except for the study from Chen et al[20]) could not independently validate a significant improvement with the combination of TT with CT. Given that differences in survival outcomes and molecular profiling have been reported between GBC and non-GBC BTCs, an exploratory analysis based on different tumor locations was conducted[6,36,37]. Previous studies have shown that patients with CCA tend to exhibit better chemosensitivity and prognoses than those with GBC[38]. Interestingly, in our meta-analysis, both the ORR and PFS in the iCCA and eCCA subgroups improved with the combination treatment of TT with CT (vs CT alone) (P = 0.003, P = 0.010, respectively), but no difference in ORR or PFS was observed for the GBC subgroup (combination of TT with CT vs CT alone). Our results suggest that CCA might be associated with better treatment response and survival outcomes than GBC. Due to the different characteristics and patterns of CCA and GBC, more clinical data evaluating tumor location-specific outcomes should be reported in the future.
There are some potential limitations in our study. First, due to our strict inclusion criteria, only nine studies were included in the meta-analysis. Even so, the nearly symmetrical funnel plot and Egger’s test indicated no evidence of publication bias. Second, the proportion of BTCs was imbalanced in terms of tumor location, which might have implications for overall tumor control and survival outcomes. Furthermore, the detailed data for subgroups of tumor location were incomplete, which might also influence the quality of the evaluation for overall outcomes. Moreover, the gender ratio, age and countries of patients were assumed to be similar, although they varied among the included studies. In addition, only English studies were included, which might result in a risk of language bias.
In conclusion, this meta-analysis provides supporting clinical evidence for the promise of TT as first-line systemic therapy for advanced BTC. Gemcitabine-based CT combined with TT, especially agents targeting EGFR, could evidently increase the ORR for advanced BTC compared to CT alone. However, the higher ORR did not appear to translate into a significant benefit in PFS or OS in most of the prospective trials. Despite this, we identified that the CT regimen and tumor location had significant interactions in assessing the effect of TT in advanced BTC. CT combined with TT significantly improved the survival outcome of advanced BTC in patients who received GemOx as first-line treatment or those with CCA but not GBC. A deeper understanding of TT is required and the results are promising for the development of novel treatment strategies for advanced BTC. Our results help facilitate the design of future clinical trials for advanced BTC.
The prognosis of patients with advanced biliary tract cancer (BTC) is poor. The clinical efficacy of combining chemotherapy (CT) with targeted therapy (TT) as first-line treatment remains controversial.
Currently, TT based on actionable genetic alterations in BTC are being extensively explored. However, the clinical efficacy of combination CT with TT as first-line treatment for advanced BTC is unclear. A meta-analysis is necessary to systematically and comprehensively evaluate the clinical value of TT for advanced BTC.
The purpose of this meta-analysis was to explore the value of CT combined with TT as first-line treatment for advanced BTC.
We systematically searched PubMed, EMBASE, ClinicalTrials, Scopus, and the Cochrane Library databases to screen and include randomized clinical trials (RCTs) on gemcitabine-based CT alone vs the combination of TT and CT as first-line treatment for advanced BTC. Review Manager 5.4.1 software was used to conduct the statistical analysis. Objective response rate (ORR), progression-free survival (PFS) and overall survival (OS) were analyzed as main outcomes. Subgroup analyses based on different targeted agents, CT regimens and tumor locations were performed.
Our meta-analysis showed a significant improvement in ORR in patients treated with CT + TT compared to those treated with CT alone (P = 0.007), but no difference in PFS or OS. Similar trends were observed in the subgroup treated with agents targeting EGFR (P = 0.004). Notably, patients who received a CT regimen of gemcitabine + oxaliplatin in the CT + TT arm had both a higher ORR (P = 0.004) and longer PFS (P = 0.03) than those in the CT-only arm. Moreover, patients with cholangiocarcinoma treated with CT + TT had significantly increased ORR and PFS.
Our study is the first meta-analysis of RCTs to evaluate the efficacy of the combining TT with standard CT as first-line treatment for advanced BTC. The meta-analysis has demonstrated that CT + TT is a promising first-line treatment for advanced BTC that leads to improved clinical outcomes.
In the future, more clinical studies are needed to explore the role of TT for advanced BTC. In addition, attention should be paid on the interactions of CT regimen and tumor location for assessing the clinical efficacy of TT in advanced BTC.
The authors thank Hai-Yu Pang for her support in statistical review of methodology and meta-analysis process. We thank Jie-Min Chen for his help in the process of editing and revising this work.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kapritsou M, Greece; Kitamura K, Japan; Tzeng IS, Taiwan S-Editor: Zhang H L-Editor: A P-Editor: Zhang H
| 1. | Valle JW, Kelley RK, Nervi B, Oh DY, Zhu AX. Biliary tract cancer. Lancet. 2021;397:428-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 624] [Article Influence: 156.0] [Reference Citation Analysis (2)] |
| 2. | Rizvi S, Khan SA, Hallemeier CL, Kelley RK, Gores GJ. Cholangiocarcinoma - evolving concepts and therapeutic strategies. Nat Rev Clin Oncol. 2018;15:95-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1052] [Cited by in RCA: 1141] [Article Influence: 163.0] [Reference Citation Analysis (0)] |
| 3. | Cao L, Bridle KR, Shrestha R, Prithviraj P, Crawford DHG, Jayachandran A. CD73 and PD-L1 as Potential Therapeutic Targets in Gallbladder Cancer. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Ishihara S, Horiguchi A, Miyakawa S, Endo I, Miyazaki M, Takada T. Biliary tract cancer registry in Japan from 2008 to 2013. J Hepatobiliary Pancreat Sci. 2016;23:149-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 101] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol. 2018;25:845-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 547] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 6. | Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J; ABC-02 Trial Investigators. Cisplatin plus gemcitabine vs gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273-1281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2617] [Cited by in RCA: 3167] [Article Influence: 211.1] [Reference Citation Analysis (1)] |
| 7. | Uson Junior PLS, Majeed U, Yin J, Botrus G, Sonbol MB, Ahn DH, Starr JS, Jones JC, Babiker H, Inabinett SR, Wylie N, Boyle AWR, Bekaii-Saab TS, Gores GJ, Smoot R, Barrett M, Nagalo B, Meurice N, Elliott N, Petit J, Zhou Y, Arora M, Dumbauld C, Barro O, Baker A, Bogenberger J, Buetow K, Mansfield A, Mody K, Borad MJ. Cell-Free Tumor DNA Dominant Clone Allele Frequency Is Associated With Poor Outcomes in Advanced Biliary Cancers Treated With Platinum-Based Chemotherapy. JCO Precis Oncol. 2022;6:e2100274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 8. | Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, Bridgewater J, Okusaka T. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 293] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 9. | Sharma A, Kalyan Mohanti B, Pal Chaudhary S, Sreenivas V, Kumar Sahoo R, Kumar Shukla N, Thulkar S, Pal S, Deo SV, Pathy S, Ranjan Dash N, Kumar S, Bhatnagar S, Kumar R, Mishra S, Sahni P, Iyer VK, Raina V. Modified gemcitabine and oxaliplatin or gemcitabine + cisplatin in unresectable gallbladder cancer: Results of a phase III randomised controlled trial. Eur J Cancer. 2019;123:162-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 10. | Morizane C, Okusaka T, Mizusawa J, Katayama H, Ueno M, Ikeda M, Ozaka M, Okano N, Sugimori K, Fukutomi A, Hara H, Mizuno N, Yanagimoto H, Wada K, Tobimatsu K, Yane K, Nakamori S, Yamaguchi H, Asagi A, Yukisawa S, Kojima Y, Kawabe K, Kawamoto Y, Sugimoto R, Iwai T, Nakamura K, Miyakawa H, Yamashita T, Hosokawa A, Ioka T, Kato N, Shioji K, Shimizu K, Nakagohri T, Kamata K, Ishii H, Furuse J; members of the Hepatobiliary and Pancreatic Oncology Group of the Japan Clinical Oncology Group (JCOG-HBPOG). Combination gemcitabine plus S-1 vs gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol. 2019;30:1950-1958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 215] [Article Influence: 35.8] [Reference Citation Analysis (1)] |
| 11. | Valle JW, Lamarca A, Goyal L, Barriuso J, Zhu AX. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017;7:943-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 455] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 12. | Jiao Y, Pawlik TM, Anders RA, Selaru FM, Streppel MM, Lucas DJ, Niknafs N, Guthrie VB, Maitra A, Argani P, Offerhaus GJA, Roa JC, Roberts LR, Gores GJ, Popescu I, Alexandrescu ST, Dima S, Fassan M, Simbolo M, Mafficini A, Capelli P, Lawlor RT, Ruzzenente A, Guglielmi A, Tortora G, de Braud F, Scarpa A, Jarnagin W, Klimstra D, Karchin R, Velculescu VE, Hruban RH, Vogelstein B, Kinzler KW, Papadopoulos N, Wood LD. Exome sequencing identifies frequent inactivating mutations in BAP1, ARID1A and PBRM1 in intrahepatic cholangiocarcinomas. Nat Genet. 2013;45:1470-1473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 538] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 13. | De Lorenzo S, Garajova I, Stefanini B, Tovoli F. Targeted therapies for gallbladder cancer: an overview of agents in preclinical and clinical development. Expert Opin Investig Drugs. 2021;30:759-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Bekaii-Saab TS, Bridgewater J, Normanno N. Practical considerations in screening for genetic alterations in cholangiocarcinoma. Ann Oncol. 2021;32:1111-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 81] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 15. | Casadio M, Biancaniello F, Overi D, Venere R, Carpino G, Gaudio E, Alvaro D, Cardinale V. Molecular Landscape and Therapeutic Strategies in Cholangiocarcinoma: An Integrated Translational Approach towards Precision Medicine. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Valle JW, Vogel A, Denlinger CS, He AR, Bai LY, Orlova R, Van Cutsem E, Adeva J, Chen LT, Obermannova R, Ettrich TJ, Chen JS, Wasan H, Girvan AC, Zhang W, Liu J, Tang C, Ebert PJ, Aggarwal A, McNeely SC, Moser BA, Oliveira JM, Carlesi R, Walgren RA, Oh DY. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: a randomised, double-blind, multicentre, phase 2 study. Lancet Oncol. 2021;22:1468-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 17. | Vogel A, Kasper S, Bitzer M, Block A, Sinn M, Schulze-Bergkamen H, Moehler M, Pfarr N, Endris V, Goeppert B, Merx K, Schnoy E, Siveke JT, Michl P, Waldschmidt D, Kuhlmann J, Geissler M, Kahl C, Evenkamp R, Schmidt T, Kuhlmann A, Weichert W, Kubicka S. PICCA study: panitumumab in combination with cisplatin/gemcitabine chemotherapy in KRAS wild-type patients with biliary cancer-a randomised biomarker-driven clinical phase II AIO study. Eur J Cancer. 2018;92:11-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 18. | Leone F, Marino D, Cereda S, Filippi R, Belli C, Spadi R, Nasti G, Montano M, Amatu A, Aprile G, Cagnazzo C, Fasola G, Siena S, Ciuffreda L, Reni M, Aglietta M. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer. 2016;122:574-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 19. | Valle JW, Wasan H, Lopes A, Backen AC, Palmer DH, Morris K, Duggan M, Cunningham D, Anthoney DA, Corrie P, Madhusudan S, Maraveyas A, Ross PJ, Waters JS, Steward WP, Rees C, Beare S, Dive C, Bridgewater JA. Cediranib or placebo in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer (ABC-03): a randomised phase 2 trial. Lancet Oncol. 2015;16:967-978. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 194] [Cited by in RCA: 214] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 20. | Chen JS, Hsu C, Chiang NJ, Tsai CS, Tsou HH, Huang SF, Bai LY, Chang IC, Shiah HS, Ho CL, Yen CJ, Lee KD, Chiu CF, Rau KM, Yu MS, Yang Y, Hsieh RK, Chang JY, Shan YS, Chao Y, Chen LT; Taiwan Cooperative Oncology Group. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann Oncol. 2015;26:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 21. | Santoro A, Gebbia V, Pressiani T, Testa A, Personeni N, Arrivas Bajardi E, Foa P, Buonadonna A, Bencardino K, Barone C, Ferrari D, Zaniboni A, Tronconi MC, Cartenì G, Milella M, Comandone A, Ferrari S, Rimassa L. A randomized, multicenter, phase II study of vandetanib monotherapy vs vandetanib in combination with gemcitabine vs gemcitabine plus placebo in subjects with advanced biliary tract cancer: the VanGogh study. Ann Oncol. 2015;26:542-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 22. | Moehler M, Maderer A, Schimanski C, Kanzler S, Denzer U, Kolligs FT, Ebert MP, Distelrath A, Geissler M, Trojan J, Schütz M, Berie L, Sauvigny C, Lammert F, Lohse A, Dollinger MM, Lindig U, Duerr EM, Lubomierski N, Zimmermann S, Wachtlin D, Kaiser AK, Schadmand-Fischer S, Galle PR, Woerns M; Working Group of Internal Oncology. Gemcitabine plus sorafenib vs gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer. 2014;50:3125-3135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Malka D, Cervera P, Foulon S, Trarbach T, de la Fouchardière C, Boucher E, Fartoux L, Faivre S, Blanc JF, Viret F, Assenat E, Seufferlein T, Herrmann T, Grenier J, Hammel P, Dollinger M, André T, Hahn P, Heinemann V, Rousseau V, Ducreux M, Pignon JP, Wendum D, Rosmorduc O, Greten TF; BINGO investigators. Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15:819-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 301] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 24. | Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, Jang JS, Jeung HC, Kang JH, Lee HW, Shin DB, Kang HJ, Sun JM, Park JO, Park YS, Kang WK, Lim HY. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 347] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 25. | Adeva J, Sangro B, Salati M, Edeline J, La Casta A, Bittoni A, Berardi R, Bruix J, Valle JW. Medical treatment for cholangiocarcinoma. Liver Int. 2019;39 Suppl 1:123-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 26. | Ioka T, Kanai M, Kobayashi S, Sakai D, Eguchi H, Baba H, Seo S, Taketomi A, Takayama T, Yamaue H, Takahashi M, Sho M, Kamei K, Fujimoto J, Toyoda M, Shimizu J, Goto T, Shindo Y, Yoshimura K, Hatano E, Nagano H; Kansai Hepatobiliary Oncology Group (KHBO). Randomized phase III study of Gemcitabine, Cisplatin plus S-1 (GCS) vs Gemcitabine, Cisplatin (GC) for Advanced Biliary Tract Cancer (KHBO1401-MITSUBA). J Hepatobiliary Pancreat Sci. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 128] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 27. | Phelip JM, Desrame J, Edeline J, Barbier E, Terrebonne E, Michel P, Perrier H, Dahan L, Bourgeois V, Akouz FK, Soularue E, Ly VL, Molin Y, Lecomte T, Ghiringhelli F, Coriat R, Louafi S, Neuzillet C, Manfredi S, Malka D; PRODIGE 38 AMEBICA Investigators/Collaborators. Modified FOLFIRINOX Versus CISGEM Chemotherapy for Patients With Advanced Biliary Tract Cancer (PRODIGE 38 AMEBICA): A Randomized Phase II Study. J Clin Oncol. 2022;40:262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 28. | McNamara MG, Bridgewater J, Palmer DH, Faluyi O, Wasan H, Patel A, Ryder WD, Barber S, Gnanaranjan C, Ghazaly E, Evans TRJ, Valle JW. A Phase Ib Study of NUC-1031 in Combination with Cisplatin for the First-Line Treatment of Patients with Advanced Biliary Tract Cancer (ABC-08). Oncologist. 2021;26:e669-e678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 29. | Abou-Alfa GK, Macarulla T, Javle MM, Kelley RK, Lubner SJ, Adeva J, Cleary JM, Catenacci DV, Borad MJ, Bridgewater J, Harris WP, Murphy AG, Oh DY, Whisenant J, Lowery MA, Goyal L, Shroff RT, El-Khoueiry AB, Fan B, Wu B, Chamberlain CX, Jiang L, Gliser C, Pandya SS, Valle JW, Zhu AX. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21:796-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 557] [Cited by in RCA: 714] [Article Influence: 142.8] [Reference Citation Analysis (0)] |
| 30. | Lamarca A, Barriuso J, McNamara MG, Valle JW. Molecular targeted therapies: Ready for "prime time" in biliary tract cancer. J Hepatol. 2020;73:170-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 260] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 31. | Fiteni F, Nguyen T, Vernerey D, Paillard MJ, Kim S, Demarchi M, Fein F, Borg C, Bonnetain F, Pivot X. Cisplatin/gemcitabine or oxaliplatin/gemcitabine in the treatment of advanced biliary tract cancer: a systematic review. Cancer Med. 2014;3:1502-1511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 32. | Azizi AA, Lamarca A, McNamara MG, Valle JW. Chemotherapy for advanced gallbladder cancer (GBC): A systematic review and meta-analysis. Crit Rev Oncol Hematol. 2021;163:103328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Jácome AA, Castro ACG, Vasconcelos JPS, Silva MHCR, Lessa MAO, Moraes ED, Andrade AC, Lima FMT, Farias JPF, Gil RA, Prolla G, Garicochea B. Efficacy and Safety Associated With Immune Checkpoint Inhibitors in Unresectable Hepatocellular Carcinoma: A Meta-analysis. JAMA Netw Open. 2021;4:e2136128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 34. | Jusakul A, Cutcutache I, Yong CH, Lim JQ, Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AWT, Ng LM, Choo SP, Myint SS, Thanan R, Nagarajan S, Lim WK, Ng CCY, Boot A, Liu M, Ong CK, Rajasegaran V, Lie S, Lim AST, Lim TH, Tan J, Loh JL, McPherson JR, Khuntikeo N, Bhudhisawasdi V, Yongvanit P, Wongkham S, Totoki Y, Nakamura H, Arai Y, Yamasaki S, Chow PK, Chung AYF, Ooi LLPJ, Lim KH, Dima S, Duda DG, Popescu I, Broet P, Hsieh SY, Yu MC, Scarpa A, Lai J, Luo DX, Carvalho AL, Vettore AL, Rhee H, Park YN, Alexandrov LB, Gordân R, Rozen SG, Shibata T, Pairojkul C, Teh BT, Tan P. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017;7:1116-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 678] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 35. | Eckel F, Schmid RM. Chemotherapy and targeted therapy in advanced biliary tract carcinoma: a pooled analysis of clinical trials. Chemotherapy. 2014;60:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Lamarca A, Frizziero M, McNamara MG, Valle JW. Clinical and Translational Research Challenges in Biliary Tract Cancers. Curr Med Chem. 2020;27:4756-4777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 37. | Ji JH, Song HN, Kim RB, Oh SY, Lim HY, Park JO, Park SH, Kim MJ, Lee SI, Ryou SH, Hwang IG, Jang JS, Kim HJ, Choi JY, Kang JH. Natural history of metastatic biliary tract cancer (BTC) patients with good performance status (PS) who were treated with only best supportive care (BSC). Jpn J Clin Oncol. 2015;45:256-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, Thomas CR Jr, Alberts SR, Dawson LA, Micetich KC, Thomas MB, Siegel AB, Blanke CD. SWOG S0809: A Phase II Intergroup Trial of Adjuvant Capecitabine and Gemcitabine Followed by Radiotherapy and Concurrent Capecitabine in Extrahepatic Cholangiocarcinoma and Gallbladder Carcinoma. J Clin Oncol. 2015;33:2617-2622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 274] [Article Influence: 27.4] [Reference Citation Analysis (0)] |