Published online Jan 15, 2022. doi: 10.4251/wjgo.v14.i1.295
Peer-review started: May 16, 2021
First decision: June 16, 2021
Revised: August 7, 2021
Accepted: November 30, 2021
Article in press: November 30, 2021
Published online: January 15, 2022
Processing time: 239 Days and 11.2 Hours
Colorectal cancer (CRC) accounts for 9.4% of overall cancer deaths, ranking second after lung cancer. Despite the large number of factors tested to predict their outcome, most patients with similar variables show big differences in survival. Moreover, right-sided CRC (RCRC) and left-sided CRC (LCRC) patients exhibit large differences in outcome after surgical intervention as assessed by preoperative blood leukocyte status. We hypothesised that stronger indexes than circulating (blood) leukocyte ratios to predict RCRC and LCRC patient outcomes will result from combining both circulating and infiltrated (tumour/peritumour fixed tissues) concentrations of leukocytes.
To seek variables involving leukocyte balances in peripheral blood and tumour tissues and to predict the outcome of CRC patients.
Sixty-five patients diagnosed with colon adenocarcinoma by the Digestive Surgery Service of the La Paz University Hospital (Madrid, Spain) were enrolled in this study: 43 with RCRC and 22 with LCRC. Patients were followed-up from January 2017 to March 2021 to record overall survival (OS) and recurrence-free survival (RFS) after surgical interventions. Leukocyte concentrations in peripheral blood were determined by routine laboratory protocols. Paraffin-fixed samples of tumour and peritumoural tissues were assessed for leukocyte concentrations by immunohistochemical detection of CD4, CD8, and CD14 marker expression. Ratios of leukocyte concentration in blood and tissues were calculated and evaluated for their predictor values for OS and RFS with Spearman correlations and Cox univariate and multivariate proportional hazards regression, followed by the calculation of the receiver-operating characteristic and area under the curve (AUC) and the determination of Youden’s optimal cutoff values for those variables that significantly correlated with either RCRC or LCRC patient outcomes. RCRC patients from the cohort were randomly assigned to modelling and validation sets, and clinician-friendly nomograms were developed to predict OS and RFS from the respective significant indexes. The accuracy of the model was evaluated using calibration and validation plots.
The relationship of leukocyte ratios in blood and peritumour resulted in six robust predictors of worse OS in RCRC: CD8+ lymphocyte content in peritumour (CD8pt, AUC = 0.585, cutoff < 8.250, P = 0.0077); total lymphocyte content in peritumour (CD4CD8pt, AUC = 0.550, cutoff < 10.160, P = 0.0188); lymphocyte-to-monocyte ratio in peritumour (LMRpt, AUC = 0.807, cutoff < 3.185, P = 0.0028); CD8+ LMR in peritumour (CD8MRpt, AUC = 0.757, cutoff < 1.650, P = 0.0007); the ratio of blood LMR to LMR in peritumour (LMRb/LMRpt, AUC = 0.672, cutoff > 0.985, P = 0.0244); and the ratio of blood LMR to CD8+ LMR in peritumour (LMRb/CD8MRpt, AUC = 0.601, cutoff > 1.485, P = 0.0101). In addition, three robust predictors of worse RFS in RCRC were found: LMRpt (AUC = 0.737, cutoff < 3.185, P = 0.0046); LMRb/LMRpt (AUC = 0.678, cutoff > 0.985, P = 0.0155) and LMRb/CD8MRpt (AUC = 0.615, cutoff > 1.485, P = 0.0141). Furthermore, the ratio of blood LMR to CD4+ LMR in peritumour (LMRb/CD4MRpt, AUC = 0.786, cutoff > 10.570, P = 0.0416) was found to robustly predict poorer OS in LCRC patients. The nomograms showed moderate accuracy in predicting OS and RFS in RCRC patients, with concordance index of 0.600 and 0.605, respectively.
Easily obtainable variables at preoperative consultation, defining the status of leukocyte balances between peripheral blood and peritumoural tissues, are robust predictors for OS and RFS of both RCRC and LCRC patients.
Core Tip: This was a prospective study involving 65 patients with colorectal cancer, seeking to find robust predictors of survival after surgical intervention amongst the leukocyte balances in peripheral blood, tumour, and peritumoural tissues. A number of these variables are shown to predict overall survival and recurrence-free survival in both right-sided colorectal cancer and left-sided colorectal cancer patients, thus allowing the improvement of pre- and postoperative patient treatments.
- Citation: Cantero-Cid R, Montalbán-Hernández KM, Guevara J, Pascual-Iglesias A, Pulido E, Casalvilla JC, Marcano C, Serrano CB, Valentín J, Bonel-Pérez GC, Avendaño-Ortiz J, Terrón V, Lozano-Rodríguez R, Martín-Quirós A, Marín E, Pena E, Guerra-Pastrián L, López-Collazo E, Aguirre LA. Intertwined leukocyte balances in tumours and peripheral blood as robust predictors of right and left colorectal cancer survival. World J Gastrointest Oncol 2022; 14(1): 295-318
- URL: https://www.wjgnet.com/1948-5204/full/v14/i1/295.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v14.i1.295
Despite the great medical and scientific achievements attained over the last decades in the fields of cancer understanding, early detection, and care, cancer continues to be a majorly threatening disease worldwide. Amongst the many pathologies gathered under this term, colorectal cancer (CRC) accounts for 9.4% of overall cancer deaths, ranking second just after lung cancer[1]. CRC treatments vary depending on tumour location and stage of diagnosis; standard colectomy (along with lymphadenectomy) without adjuvant therapy is the usual treatment in early stages I and II, while most patients in advanced stages III and IV follow with chemo- and/or radiotherapy to reduce the risk of recurrence[2]. However, a large proportion of these patients present with (synchronous; 15%-25%) or will develop (metachronous; 40%-75%) metastases, mainly in the liver[3], which constitutes the major cause of deaths[4]. Therefore, a 5-year relative survival rate is reduced from 90% in early-stage detection to 12% in advanced cases[2]. Thus, finding robust markers before surgery to predict patient outcomes constitutes a safe strategy in order to stratify those groups with a high risk of recurrence and design personalised pre- and postoperative therapies.
A wide variety of factors, mainly based on clinical and pathological features, have been tested as prognostic markers for CRC development, such as: weight loss, haemoglobin levels, tumour-nodes-metastasis classification (TNM) staging and tumour differentiation, mismatch-repair proficiency, lymph node involvement, or response to (neo-) adjuvant therapies[5-7]. Moreover, since a clear distinction between the behaviour of right-sided CRC (RCRC) and left-sided CRC (LCRC) patients is well established, much effort has been put into categorising putative prognostic markers according to their respective characteristics, though still with controversial results[8].
Currently, an increasing number of research and clinical trials are supporting evidence of the influence of the systemic inflammatory response in cancer progression[5]. A measure of this response has been assessed by combining the number of peripheral circulating leukocytes: lymphocyte-to-monocyte ratio (LMR), neutrophil-to-lymphocytes ratio (NLR), and platelet-to-lymphocyte ratio (PLR). These analyses have shown interesting prognostic associations in several cancer types including urothelial, nasopharyngeal, osteosarcoma, lung carcinomas[9-12], and CRC[13-16]. Nevertheless, few studies have been directed towards the prognostic value of intertwined relationships across circulating and tumour-infiltrated populations of leucocytes on solid tumour progression[17-19].
Herein, we aimed to delve deep into the prognostic value of leukocyte distribution ratios, in both blood and tumour tissues, for CRC patient outcomes after surgery. We hypothesised that stronger indexes than circulating (blood) leukocyte ratios to predict patient outcome will result from combining both circulating and infiltrated (tumour/peritumoural tissues) concentrations of leukocytes. We show six robust predictors for RCRC overall survival (CD8pt, CD4CD8pt, LMRpt, CD8MRpt, LMRb/LMRpt, LMRb/CD8MRpt), three for RCRC recurrence-free survival (LMRpt, CD8MRpt, LMRb/LMRpt, LMRb/CD8MRpt), and another one for LCRC overall survival (LMRb/CD4MRpt), all these being based on the ratios between blood and peritumoural tissue concentration of lymphocytes and monocytes. Moreover, we highlight the importance of these variables in designing ad hoc surgical strategies, due to the ease with which surgeons can build a protocol by taking samples of peripheral blood and peritumoural tissue during a preoperative colonoscopy.
Sixty-five patients diagnosed with colon adenocarcinoma, with no records of previous neo-adjuvant therapy, were recruited at the Digestive Surgery Service of La Paz University Hospital (Madrid, Spain) from January 2017 to September 2019. They were surgically treated according to each patient’s condition for right (caecum, ascending, or transverse colon) or left (descending or sigmoid colon) hemicolectomies followed by anastomosis, with partial hepatectomy if synchronous metastasis was present. Patients’ clinical records were followed-up until March 2021. Overall survival (OS) was then defined as the length of time since surgery until exitus or the end of the study, whilst recurrence-free survival (RFS) was considered the interval from surgery until relapse, either from disease-free or (synchronous/metachronous) metastases-free statuses. All patients signed written consent, in accordance with the ethical guidelines of the 1975 Declaration of Helsinki and the Ethics Committee for Clinical Research of La Paz University Hospital (PI-1958), for further uses of blood samples and surgically resected organs for research purposes.
Only patients with adenomas or rectum adenocarcinoma were excluded from the study.
Venous blood samples were collected in 10 mL EDTA-tubes in the hospital room, 24 h prior to surgery and routinely tested for white blood cell, lymphocyte (L), monocyte (M), neutrophil (N) and platelet (P) counts at the Central Laboratory (CORE) of the La Paz University Hospital. Preoperative blood LMR (LMRb), NLR (NLRb), and PLR (PLRb) were then calculated for each patient by dividing the absolute counts of the respective populations in the peripheral blood (Table 1).
| Characteristics | Frequency | % | |
| All patients (n = 65) | |||
| Age (yr) ± SD | 73.54 ± 9.51 | ||
| (range) | (52-92) | ||
| Gender | |||
| Female | 29 | 44.62 | |
| Male | 36 | 55.38 | |
| Tumour localisation | |||
| Right colorectal cancer | 43 | 66.15 | |
| Caecum | 13 | 30.23 | |
| Ascending colon | 23 | 53.49 | |
| Transverse colon | 7 | 16.28 | |
| Left colorectal cancer | 22 | 33.85 | |
| Descending colon | 11 | 50.00 | |
| Sigma | 11 | 50.00 | |
| Emergency surgery | |||
| Yes | 1 | 1.54 | |
| No | 64 | 98.46 | |
| Surgical procedure | |||
| Laparoscopic hemicolectomy | 48 | 73.85 | |
| Open hemicolectomy | 17 | 26.15 | |
| Development at surgery | |||
| Non-metastasised | 37 | 56.92 | |
| Metastases | 28 | 43.08 | |
| Liver synchronous | 13 | 46.43 | |
| Liver metachronous | 8 | 28.57 | |
| Other organs | 13 | 46.43 | |
| MMR status | |||
| pMMR | 56 | 86.15 | |
| dMMR | 5 | 7.69 | |
| Unknown | 4 | 6.15 | |
| TNM stage | |||
| 0 | 3 | 4.62 | |
| I | 7 | 10.77 | |
| IIA | 21 | 32.31 | |
| IIB | 5 | 7.69 | |
| IIIA | 2 | 3.08 | |
| IIIB | 8 | 12.31 | |
| IIIC | 5 | 7.69 | |
| IV | 1 | 1.54 | |
| IVA | 10 | 15.38 | |
| IVB | 3 | 4.62 | |
| Adjuvant chemotherapy | |||
| Yes | 30 | 46.15 | |
| No | 35 | 53.85 | |
| Blood leukocytes counting (× 103/μL), (normal range) | RCRC | LCRC | P value |
| White blood cells count (3.6-10.5) | 7.41 (3.52-16.2) | 8.19 (4.83-15.8) | 0.271 |
| Lymphocytes (1.1-4.5) | 1.77 (0.46-4.46) | 2.04 (0.32-4.87) | 0.235 |
| Monocytes (0.1-0.9) | 0.54 (0.20-1.26) | 0.50 (0.22-1.11) | 0.493 |
| Neutrophils (1.5-7.7) | 4.86 (1.76-15.3) | 5.34 (2.96-13.0) | 0.480 |
| Platelets (150-370) | 275.65 (101.0-602.0) | 272.41 (142.0-725.0) | 0.910 |
| LMRb | 3.54 (0.42-7.96) | 4.64 (0.58-11.88) | 0.046 |
| NLRb | 3.65 (0.69-25.93) | 4.61 (0.93-30.66) | 0.481 |
| PLRb | 188.58 (52.24-551.22) | 213.93 (29.16-1187.50) | 0.585 |
Samples from the middle part (avoiding both the epicentre and the edge) of the tumours, 5 cm-adjacent peritumoural (non-neoplastic), and liver (in case of synchro
Organ samples were washed with PBS solution containing 56 μg/mL gentamicin (Braun, Melsungen, Germany; 636159), 2.5 μg/mL fungizome/anphotericin-B (Gibco, Amarillo, TX, United States; 15290-018), and 1% penicillin/streptomycin (Sigma-Aldrich, Saint Louis, MO, United States; P4333-100mL) and gently shaken for 30 min at room temperature. Then they were fixed in 4% paraformaldehyde for 16 h, washed with PBS for 24 h, and paraffin-embedded by standard procedures.
Tissue microarrays (TMA) recipient paraffin-blocks (24 mm × 2.0 mm) were prepared with a TMA builder kit (Histopathology Ltd., Baranya, 7632, Hungary; 20010.2) and filled with properly matched samples of previous patients’ blocks, following manufacturer’s protocol.
Thin sections (5 μm thick) of TMAs were cut with a Leica (RM2255) ultrathin-microtome and allowed to completely adhere to slides for 30 min at 60°C, before staining with commercially available antibodies against assessed surface markers was performed by standardised protocols (see Supplementary Table 1 for a complete list of primary and secondary antibodies used). Briefly, sections were deparaffinised with xylene, rehydrated through graded (100% to 70%) ethanol, and blocked for endo
An average of four photographs per sample (in order to cover the whole field for each sample on the TMA sections) were taken with an Olympus BX-41 microscope and blind-analysed by two independent observers with ImageJ (v1.52p), for the calculus of the relative areas to each antibody corresponding surface marker expression (CD4, CD8, and CD14). For a detailed description of the image processing see Supple
Total tumour and peritumour LMRs (respectively, LMRt and LMRpt) were calculated by dividing the sum of the areas for CD4 and CD8 by the area for CD14, e.g., LMRt=(A(CD4t)+A(CD8t))/A(CD14t). Individual subpopulation ratios were also analysed for both tumour and peritumour samples (CD4MRt, CD8MRt and CD4MRpt, CD8MRpt, respectively), e.g., CD4MRt=A(CD4t)/A(CD14t). Then, blood-to-tissue ratios for all previous tumour and peritumour subpopulation ratios (LMRb/LMRt, LMRb/CD4MRt, LMRb/CD8MRt and LMRb/LMRpt, LMRb/CD4MRpt, LMRb/CD8MRpt, respectively) were also reported for each patient.
All RCRC patients from the cohort were randomly divided into training (60%) and validation (40%) sets to establish and validate the clinician-friendly nomograms. For each nomogram to predict the probability of OS or RFS, the six or the three respectively significant predictive factors found early were used to formulate the nomograms with several R packages. The discriminatory ability of the nomogram was assessed by calculating the Harrell’s concordance index (C-index).
Data are represented as mean ± standard deviation. Student’s t test was used for pairwise comparisons. Mann-Whitney U analysis was applied for equal standard deviations, otherwise Welch’s correction was used. The distribution of the variables was assessed by a nonparametric test. Spearman r correlations were used to evaluate the association between the variables and ratios with the OS and RFS observed in our patients. Survival and population ratio relationships were analysed using Cox proportional hazard ratios; statistically significant variables in univariate analysis were further evaluated with the Cox multivariate step-by-step backward method to identify those with independent prognostic value. The Kaplan-Meier method was used to calculate the differences in OS and RFS rates for RCRC and LCRC over time (months), and significance was compared using the log-rank (Mantel-Cox) test; median time (months) survival proportions and P accuracy were reported. We calculated the receiver-operating characteristic (ROC) curve and the area under the curve (AUC) to determine whether the different variables and ratios could be used to predict OS and RFS in our cohort. We indicated the sensitivity, the specificity, the positive and negative predictive values, and 95% confidence interval for AUC and P accuracy. Optimal cutoff values, as determined with Youden’s index, Harrell’s C-index, and P accuracy, were calculated with R software. P values of 0.05 or less were considered indicative of statistical significance, and all these were two-sided. All statistics were performed in either Prism 6.0 (GraphPad, San Diego, CA, United States) or SPSS version 23 (IBM, NY, United States) software.
The cohort included in this study was exclusively recruited by one team of surgeons, from their assigned patients for surgically treated disorders of the digestive tract, thus only a fraction is constituted of the whole figure of CRC patients attended at La Paz University Hospital during the period of recruitment. Detailed clinicopathological characterisation of patients is shown in Table 1.
A total of 65 patients with a mean age of 73.5 years, of whom 43 (66.1%) presented with RCRC and 22 (33.8%) with LCRC, were finally enrolled. Of these, 29 (44.6%) were women and 36 (55.4%) were men. With the exception of one case, all had been programmed for surgery without an emergency condition. Forty-eight (73.8%) were hemicolectomised by minimally invasive laparoscopic procedure. They ranged from stages 0 to IV, based on TNM classification; 28 (43.1%) were presenting metastasis (either synchronous or metachronous at the time of surgery), and 30 (46.1%) received adjuvant therapy after surgery. Fifty-six (86.1%) of the tumours were found proficient for the mismatch-repair machinery at the histological level.
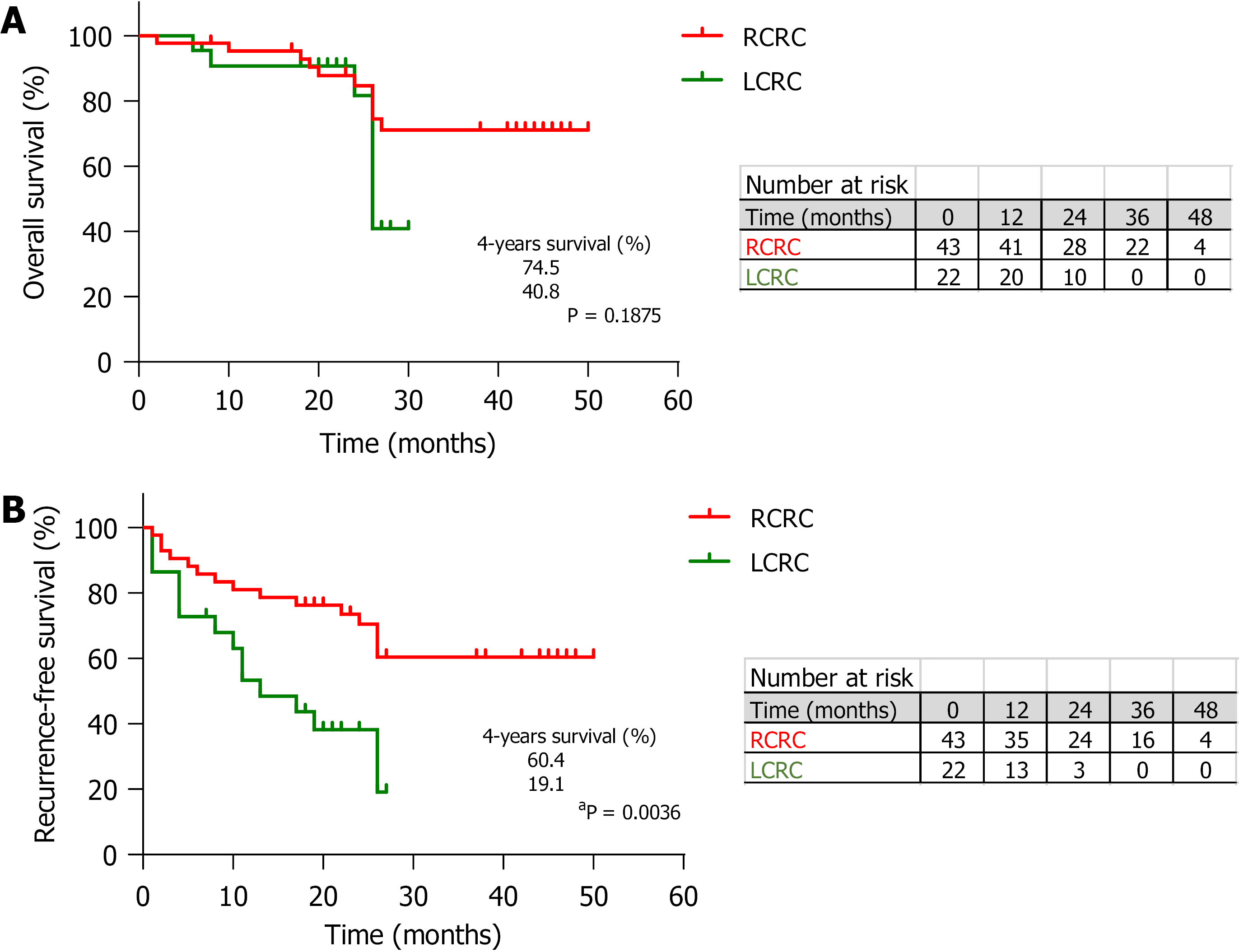
The survival analysis, with a median follow-up of 26 mo, showed no differences for OS between RCRC and LCRC patients (Figure 1A) but a trend towards poorer outcome for the latter (74.5% vs 40.8%, P = 0.1875). However, in the analysis of RFS (Figure 1B), we observed significantly better outcomes for RCRC compared to LCRC patients (60.4% vs 19.1%, P = 0.0036).
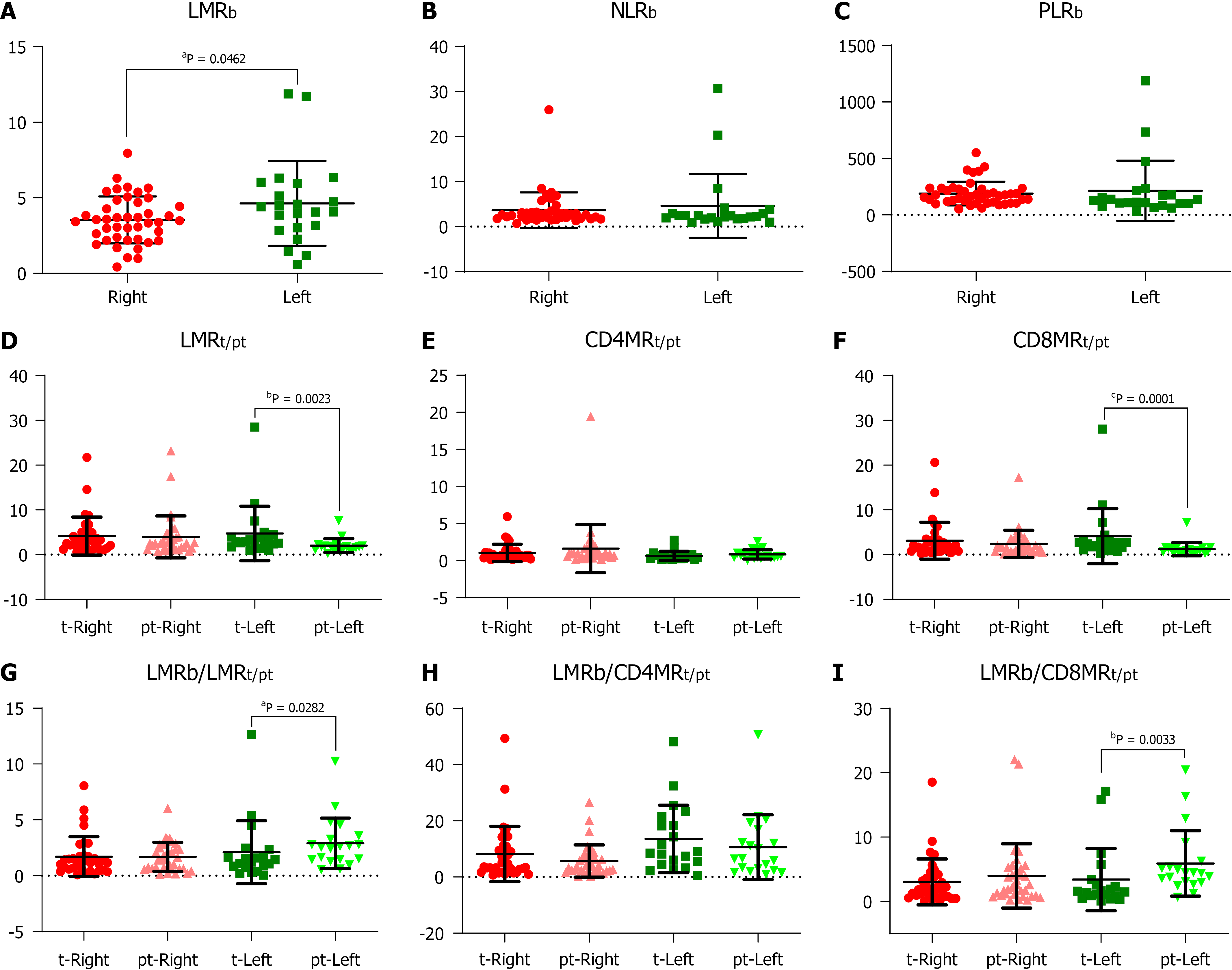
We found no differences (Table 1) in total leukocyte counts nor in individual populations of circulating lymphocytes, monocytes, neutrophils, or platelets between RCRC and LCRC patient peripheral blood. However, though all mean counts for both groups were within the normal physiological ranges, RCRC patients showed a trend towards low circulating lymphocytes. Thus, their LMRb was lower (P = 0.0462) than LCRC patients (Figure 2A). Neither NLRb nor PLRb showed differences between RCRC and LCRC patients (Figure 2B and C).
Tissues from 54 out of the total 65 patients included in the study, 34 from RCRC patients (63%) and 20 from LCRC patients (37%), could be assessed for leukocyte infiltration analyses. This fact was mainly due to the morphological characteristics of 11 tumours, which made it impossible to separate pieces for research purposes without affecting the global diagnostics by pathologists.
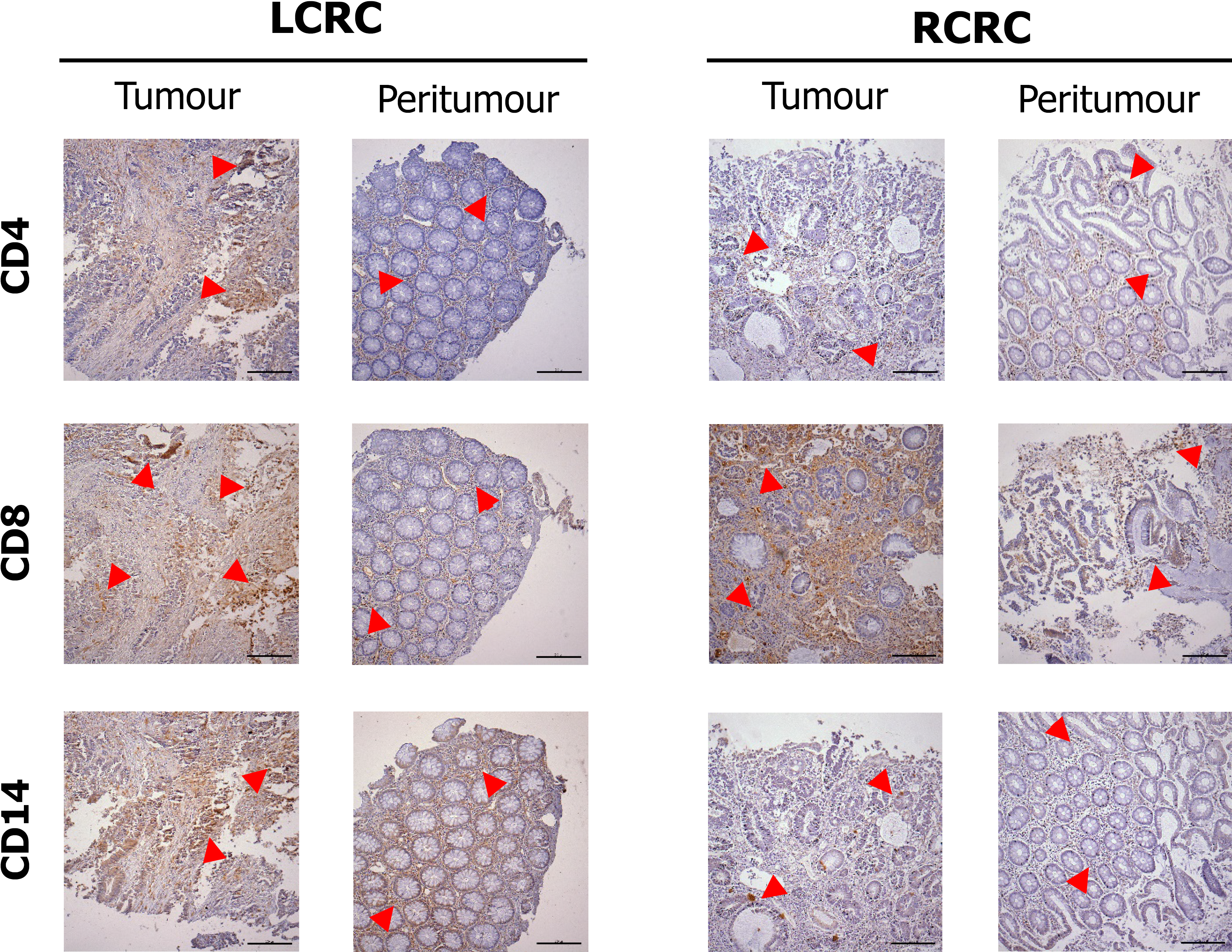
Figure 3 shows the staining pattern for CD4, CD8, and CD14 cells in tumour and peritumour samples from two representative patients of LCRC and RCRC. The distribution of total (CD4+ plus CD8+) lymphocytes, CD4+ lymphocytes, CD8+ lymphocytes, and CD14+ monocytes, in all analysed tissues, is shown in Supplementary Figure 2. Higher total lymphocyte content in tumours than peritumours from LCRC patients (13.06 ± 2.123 vs 7.57 ± 1.794, P = 0.0095) seemed due to the proportional increase of CD8+ lymphocytes (11.19 ± 2.158 vs 5.13 ± 1.757, P = 0.0020), as we detected no differences amongst infiltrated CD4+ lymphocytes in these tissues. No differences were found for lymphocyte infiltration in right tumours with respect to right peritumoural tissues. Moreover, infiltrated-leukocyte content in right tumours showed no differences to right peritumours.
The analysis of resulting ratios for lymphocyte and monocyte counts in tumour (t) and peritumoural (pt) tissues showed a higher LMRt with respect to LMRpt (4.128 ± 1.363 vs 2.022 ± 0.3432, P = 0.0023) beside higher CD8MRt than CD8MRpt (4.121 ± 1.374 vs 1.218 ± 0.3297, P = 0.0001) in LCRC patients (Figure 2D-F). No differences were detected for these ratios amongst RCRC respective tissues. Consequently, the analysis of blood-to-tissue ratios (Figure 2G-I) showed LCRC patients exhibited lower LMRb/LMRt than LMRb/LMRpt (2.104 ± 0.601 vs 2.900 ± 0.5061, P = 0.0282) as well as lower LMRb/CD8MRt than LMRb/CD8MRpt (3.381 ± 1.083 vs 5.898 ± 1.138, P = 0.0033). There were no differences in these ratios for RCRC respective tissues.
In order to assess the degree to which leukocyte balance (i.e. both the concentration and ratios of leukocytes for blood, tumour, and peritumours described above) was associated with RCRC and LCRC patient OS and RFS, we first conducted a Spearman correlation analysis (Table 2). We found that for RCRC LMRb (r = -0.3039, P = 0.0476), LMRpt (r = -0.4301, P = 0.0111), and CD8MRpt (r =-0.3596, P=0.0367) were negatively correlated with OS; LMRt (r = -0.4775, P = 0.0043), LMRpt (r = -0.3846, P = 0.0247), and CD8MRt (r = -0.4422, P = 0.0088) negatively correlated, but LMRb/LMRt (r = 0.3621, P = 0.0363) positively correlated with RFS. LMRb/CD8MRt (r = 0.3364, P = 0.0517) also showed a trend towards being positively correlated. For LCRC, CD14pt (r = 0.5677, P = 0.009) and LMRb/CD4MRpt (r = 0.4541, P=0.0443) positively correlated, but CD4MRpt (r = -0.473, P = 0.0352) negatively correlated with OS, whilst both CD14pt (r = 0.6018, P = 0.005) and CD8MRt (r = 0.4779, P = 0.331) positively correlated with RFS, and CD4CD8pt (r = 0.4425, P = 0.0507) also showed a trend towards being positively correlated.
| Index | Total patients | 4-year OS | 4-year RFS | ||||||
| n (%) | Spearman r | 95%CI | P | Spearman r | 95%CI | P | |||
| LMRb | |||||||||
| Right | 43 (66.2) | -0.3039 | -0.5601 to 0.005346 | 0.0476 | < 0.05 | -0.1946 | -0.4748 to 0.1214 | 0.211 | NS |
| Left | 22 (33.8) | 0.1615 | -0.2914 to 0.5553 | 0.4727 | NS | -0.07447 | -0.4912 to 0.3700 | 0.7419 | NS |
| NLRb | |||||||||
| Right | 43 (66.2) | 0.2262 | -0.08868 to 0.5000 | 0.1446 | NS | 0.1062 | -0.2094 to 0.4017 | 0.498 | NS |
| Left | 22 (33.8) | -0.06922 | -0.4872 to 0.3746 | 0.7595 | NS | 0.1192 | -0.3305 to 0.5247 | 0.5974 | NS |
| PLRb | |||||||||
| Right | 43 (66.2) | 0.2307 | -0.08405 to 0.5035 | 0.1367 | NS | 0.06684 | -0.2470 to 0.3680 | 0.6702 | NS |
| Left | 22 (33.8) | 0.1615 | -0.2914 to 0.5553 | 0.4727 | NS | 0.1192 | -0.3305 to 0.5247 | 0.5974 | NS |
| CD4t | |||||||||
| Right | 34 (63.0) | -0.05561 | -0.3954 to 0.2976 | 0.7548 | NS | 0.05447 | -0.2986 to 0.3944 | 0.7596 | NS |
| Left | 20 (37.0) | 0.01892 | -0.4387 to 0.4687 | 0.9369 | NS | -0.0354 | -0.4815 to 0.4253 | 0.8822 | NS |
| CD4pt | |||||||||
| Right | 34 (63.0) | 0.03708 | -0.3144 to 0.3796 | 0.8351 | NS | 0.07371 | -0.2809 to 0.4106 | 0.6787 | NS |
| Left | 20 (37.0) | -0.142 | -0.5597 to 0.3334 | 0.5505 | NS | 0.3276 | -0.1483 to 0.6803 | 0.1586 | NS |
| CD8t | |||||||||
| Right | 34 (63.0) | -0.08526 | -0.4202 to 0.2702 | 0.6316 | NS | -0.2083 | -0.1958 to 0.6531 | 0.2372 | NS |
| Left | 20 (37.0) | 0.03784 | -0.4233 to 0.4834 | 0.8741 | NS | 0.2832 | -0.3863 to 0.3074 | 0.2263 | NS |
| CD8pt | |||||||||
| Right | 34 (63.0) | -0.1186 | -0.4476 to 0.2386 | 0.504 | NS | -0.04486 | -0.3863 to 0.3074 | 0.8011 | NS |
| Left | 20 (37.0) | 0.3406 | -0.1340 to 0.6881 | 0.1417 | NS | 0.3186 | -0.1581 to 0.6749 | 0.171 | NS |
| CD4CD8t | |||||||||
| Right | 34 (63.0) | -0.1372 | -0.4626 to 0.2208 | 0.4392 | NS | -0.1955 | -0.5084 to 0.1630 | 0.268 | NS |
| Left | 20 (37.0) | 0.03784 | -0.4233 to 0.4834 | 0.8741 | NS | 0.2655 | -0.2142 to 0.6420 | 0.2579 | NS |
| CD4CD8pt | |||||||||
| Right | 34 (63.0) | -0.07044 | -0.4079 to 0.2839 | 0.6922 | NS | -0.009612 | -0.3559 to 0.3389 | 0.957 | NS |
| Left | 20 (37.0) | 0.3595 | -0.1127 to 0.6993 | 0.1195 | NS | 0.4425 | -0.01421 to 0.7464 | 0.0507 | NS |
| CD14t | |||||||||
| Right | 34 (63.0) | 0.1891 | -0.1695 to 0.5034 | 0.2842 | NS | 0.2467 | -0.1102 to 0.5472 | 0.1595 | NS |
| Left | 20 (37.0) | 0.05677 | -0.4076 to 0.4978 | 0.8121 | NS | -0.1239 | -0.5470 to 0.3496 | 0.6028 | NS |
| CD14pt | |||||||||
| Right | 34 (63.0) | 0.3003 | -0.05262 to 0.5865 | 0.0844 | NS | 0.2596 | -0.09658 to 0.5568 | 0.1382 | NS |
| Left | 20 (37.0) | 0.5677 | 0.1533 to 0.8122 | 0.009 | < 0.01 | 0.6018 | 0.2035 to 0.8292 | 0.005 | < 0.01 |
| LMRt | |||||||||
| Right | 34 (63.0) | -0.2929 | -0.5812 to 0.06070 | 0.0927 | NS | -0.4775 | -0.7075 to -0.1559 | 0.0043 | < 0.01 |
| Left | 20 (37.0) | 0.07569 | -0.3916 to 0.5119 | 0.7511 | NS | 0.4425 | -0.01421 to 0.7464 | 0.0507 | NS |
| LMRpt | |||||||||
| Right | 34 (63.0) | -0.4301 | -0.6764 to -0.09719 | 0.0111 | < 0.05 | -0.3846 | -0.6457 to -0.04285 | 0.0247 | < 0.05 |
| Left | 20 (37.0) | 0 | -0.4538 to 0.4538 | > 0.9999 | NS | -0.0354 | -0.4815 to 0.4253 | 0.8822 | NS |
| CD4MRt | |||||||||
| Right | 34 (63.0) | -0.2781 | -0.5704 to 0.07674 | 0.1113 | NS | -0.3173 | -0.5987 to 0.03388 | 0.0675 | NS |
| Left | 20 (37.0) | 0.04736 | -0.4154 to 0.4907 | 0.8428 | NS | 0.1949 | -0.2841 to 0.5960 | 0.4102 | NS |
| CD4MRpt | |||||||||
| Right | 34 (63.0) | -0.2781 | -0.5704 to 0.07670 | 0.1112 | NS | -0.2596 | -0.5568 to 0.09650 | 0.1381 | NS |
| Left | 20 (37.0) | -0.473 | -0.7631 to -0.02445 | 0.0352 | NS | -0.0885 | -0.5214 to 0.3806 | 0.7106 | NS |
| CD8MRt | |||||||||
| Right | 34 (63.0) | -0.2039 | -0.5149 to 0.1545 | 0.2474 | NS | -0.4422 | -0.6845 to -0.1120 | 0.0088 | < 0.01 |
| Left | 20 (37.0) | 0.1135 | -0.3588 to 0.5396 | 0.6337 | NS | 0.4779 | 0.03071 to 0.7657 | 0.0331 | < 0.05 |
| CD8MRpt | |||||||||
| Right | 34 (63.0) | -0.3596 | -0.6285 to -0.01393 | 0.0367 | < 0.05 | -0.2788 | -0.5709 to 0.07601 | 0.1104 | NS |
| Left | 20 (37.0) | 0.1893 | -0.2894 to 0.5923 | 0.4241 | NS | -0.03541 | -0.4815 to 0.4253 | 0.8822 | NS |
| LMRb/LMRt | |||||||||
| Right | 34 (63.0) | 0.1075 | -0.2492 to 0.4386 | 0.545 | NS | 0.3621 | 0.01678 to 0.6302 | 0.0353 | < 0.05 |
| Left | 20 (37.0) | -0.05677 | -0.4978 to 0.4076 | 0.8121 | NS | -0.4248 | -0.7366 to 0.03599 | 0.0619 | NS |
| LMRb/LMRpt | |||||||||
| Right | 34 (63.0) | 0.241 | -0.1162 to 0.5430 | 0.1698 | NS | 0.2884 | -0.06561 to 0.5779 | 0.0981 | NS |
| Left | 20 (37.0) | 0.1325 | -0.3420 to 0.5531 | 0.5778 | NS | -0.0531 | -0.4950 to 0.4106 | 0.8241 | NS |
| LMRb/CD4MRt | |||||||||
| Right | 34 (63.0) | 0.1372 | -0.2208 to 0.4626 | 0.4392 | NS | 0.2467 | -0.1101 to 0.5473 | 0.1595 | NS |
| Left | 20 (37.0) | -0.09461 | -0.5259 to 0.3754 | 0.6915 | NS | -0.177 | -0.5839 to 0.3010 | 0.4554 | NS |
| LMRb/CD4MRpt | |||||||||
| Right | 34 (63.0) | 0.1446 | -0.2136 to 0.4685 | 0.4146 | NS | 0.189 | -0.1695 to 0.5034 | 0.2843 | NS |
| Left | 20 (37.0) | 0.4541 | 0.0003499 to 0.7528 | 0.0443 | < 0.05 | 0.1062 | -0.3653 to 0.5343 | 0.6559 | NS |
| LMRb/CD8MRt | |||||||||
| Right | 34 (63.0) | 0.04078 | -0.3111 to 0.3828 | 0.8189 | NS | 0.3364 | -0.01245 to 0.6123 | 0.0517 | NS |
| Left | 20 (37.0) | -0.03784 | -0.4834 to 0.4233 | 0.8741 | NS | -0.4071 | -0.7267 to 0.05735 | 0.0748 | NS |
| LMRb/CD8MRpt | |||||||||
| Right | 34 (63.0) | 0.1409 | -0.2172 to 0.4655 | 0.4267 | NS | 0.1859 | -0.1727 to 0.5009 | 0.2926 | NS |
| Left | 20 (37.0) | -0.1703 | -0.5794 to 0.3073 | 0.4729 | NS | -0.1416 | -0.5595 to 0.3337 | 0.5515 | NS |
Next, the effect of these variables on survival was assessed by Cox proportional hazards regression. For OS (Table 3), the univariate analysis revealed that besides previously found LMRb (P = 0.043), LMRpt (P = 0.024), and CD8MRpt (P = 0.031) in RCRC patients, NLRb (P = 0.038) also significantly correlated with OS; LMRb/CD4MRpt (P = 0.026) was also confirmed to be significantly correlated with OS of LCRC patients. After adjusting for confounding variables through the multivariate analysis, NLRb (P = 0.038), CD8MRpt (P = 0.011), and LMRb/CD8MRpt (P = 0.016) resulted in a significant association with OS of RCRC patients; CD8pt (P = 0.058) also showed a trend towards being associated.
| Total patients | Univariate analysis | Multivariate analysis | |||||||||
| n (%) | HR | 95%CI | P | HR | 95%CI | P | |||||
| Variables | Low | High | Low | High | |||||||
| LMRb | |||||||||||
| Right | 34 (63.0) | 0.565 | 0.325 | 0.982 | 0.043 | < 0.05 | 0.133 | 0.000 | 71.041 | 0.529 | |
| Left | 20 (37.0) | 1.141 | 0.867 | 1.502 | 0.346 | 1.141 | 0.867 | 1.502 | 0.346 | ||
| NLRb | |||||||||||
| Right | 34 (63.0) | 1.416 | 1.019 | 1.967 | 0.038 | < 0.05 | 1.416 | 1.019 | 1.967 | 0.038 | < 0.05 |
| Left | 20 (37.0) | 1.043 | 0.944 | 1.152 | 0.410 | 1.126 | 0.987 | 1.284 | 0.078 | ||
| PLRb | |||||||||||
| Right | 34 (63.0) | 1.005 | 1.000 | 1.011 | 0.064 | 0.989 | 0.719 | 1.361 | 0.946 | ||
| Left | 20 (37.0) | 1.001 | 0.999 | 1.004 | 0.292 | 0.997 | 0.937 | 1.060 | 0.912 | ||
| CD4t | |||||||||||
| Right | 34 (63.0) | 0.923 | 0.638 | 1.334 | 0.670 | ||||||
| Left | 20 (37.0) | 0.843 | 0.493 | 1.439 | 0.531 | ||||||
| CD4pt | |||||||||||
| Right | 34 (63.0) | 0.959 | 0.817 | 1.124 | 0.604 | ||||||
| Left | 20 (37.0) | 0.813 | 0.454 | 1.456 | 0.487 | ||||||
| CD8t | |||||||||||
| Right | 34 (63.0) | 0.995 | 0.897 | 1.105 | 0.931 | 1.032 | 0.913 | 1.167 | 0.611 | ||
| Left | 20 (37.0) | 0.954 | 0.860 | 1.057 | 0.364 | ||||||
| CD8pt | |||||||||||
| Right | 34 (63.0) | 0.792 | 0.617 | 1.016 | 0.066 | 0.800 | 0.636 | 1.007 | 0.058 | ||
| Left | 20 (37.0) | 1.018 | 0.957 | 1.083 | 0.570 | 1.033 | 0.962 | 1.110 | 0.372 | ||
| CD4CD8t | |||||||||||
| Right | 34 (63.0) | 0.987 | 0.892 | 1.093 | 0.806 | ||||||
| Left | 20 (37.0) | 0.937 | 0.833 | 1.054 | 0.277 | ||||||
| CD4CD8pt | |||||||||||
| Right | 34 (63.0) | 0.891 | 0.761 | 1.043 | 0.150 | 1.073 | 0.821 | 1.401 | 0.606 | ||
| Left | 20 (37.0) | 1.015 | 0.953 | 1.082 | 0.641 | ||||||
| CD14t | |||||||||||
| Right | 34 (63.0) | 1.048 | 0.845 | 1.300 | 0.670 | 1.077 | 0.836 | 1.388 | 0.565 | ||
| Left | 20 (37.0) | 0.979 | 0.703 | 1.362 | 0.898 | ||||||
| CD14pt | |||||||||||
| Right | 34 (63.0) | 1.113 | 0.889 | 1.394 | 0.351 | 1.342 | 0.987 | 1.826 | 0.060 | ||
| Left | 20 (37.0) | 1.053 | 0.723 | 1.533 | 0.788 | 0.700 | 0.381 | 1.286 | 0.250 | ||
| LMRt | |||||||||||
| Right | 34 (63.0) | 0.635 | 0.365 | 1.103 | 0.107 | ||||||
| Left | 20 (37.0) | 1.000 | 0.908 | 1.101 | 0.997 | 0.976 | 0.555 | 1.716 | 0.933 | ||
| LMRpt | |||||||||||
| Right | 34 (63.0) | 0.416 | 0.194 | 0.889 | 0.024 | < 0.05 | |||||
| Left | 20 (37.0) | 1.030 | 0.712 | 1.490 | 0.876 | 0.031 | 0.000 | 4.228 | 0.166 | ||
| CD4MRt | |||||||||||
| Right | 34 (63.0) | 0.270 | 0.039 | 1.850 | 0.182 | ||||||
| Left | 20 (37.0) | 0.759 | 0.146 | 3.954 | 0.743 | ||||||
| CD4MRpt | |||||||||||
| Right | 34 (63.0) | 0.431 | 0.112 | 1.660 | 0.221 | ||||||
| Left | 20 (37.0) | 0.135 | 0.004 | 4.148 | 0.252 | ||||||
| CD8MRt | |||||||||||
| Right | 34 (63.0) | 0.712 | 0.364 | 1.394 | 0.321 | ||||||
| Left | 20 (37.0) | 1.001 | 0.910 | 1.100 | 0.986 | ||||||
| CD8MRpt | |||||||||||
| Right | 34 (63.0) | 0.223 | 0.057 | 0.872 | 0.031 | < 0.05 | 0.024 | 0.001 | 0.430 | 0.011 | < 0.05 |
| Left | 20 (37.0) | 1.078 | 0.775 | 1.500 | 0.654 | ||||||
| LMRb/LMRt | |||||||||||
| Right | 34 (63.0) | 0.957 | 0.603 | 1.519 | 0.853 | ||||||
| Left | 20 (37.0) | 1.163 | 0.935 | 1.446 | 0.175 | ||||||
| LMRb/LMRpt | |||||||||||
| Right | 34 (63.0) | 1.253 | 0.824 | 1.907 | 0.292 | ||||||
| Left | 20 (37.0) | 1.196 | 0.845 | 1.695 | 0.313 | ||||||
| LMRb/CD4MRt | |||||||||||
| Right | 34 (63.0) | 1.009 | 0.950 | 1.073 | 0.767 | 1.282 | 0.931 | 1.765 | 0.128 | ||
| Left | 20 (37.0) | 1.027 | 0.961 | 1.099 | 0.428 | ||||||
| LMRb/CD4MRpt | |||||||||||
| Right | 34 (63.0) | 1.028 | 0.926 | 1.142 | 0.600 | 1.291 | 0.447 | 3.728 | 0.636 | ||
| Left | 20 (37.0) | 1.097 | 1.011 | 1.190 | 0.026 | < 0.05 | 0.991 | 0.420 | 2.341 | 0.984 | |
| LMRb/CD8MRt | |||||||||||
| Right | 34 (63.0) | 0.973 | 0.747 | 1.269 | 0.842 | ||||||
| Left | 20 (37.0) | 1.103 | 0.940 | 1.294 | 0.229 | 1.971 | 0.258 | 15.069 | 0.513 | ||
| LMRb/CD8MRpt | |||||||||||
| Right | 34 (63.0) | 1.053 | 0.880 | 1.260 | 0.572 | 0.484 | 0.268 | 0.873 | 0.016 | < 0.05 | |
| Left | 20 (37.0) | 1.026 | 0.834 | 1.262 | 0.812 | 0.952 | 0.009 | 96.530 | 0.983 | ||
Regarding RFS (Table 4), the univariate analysis showed that in addition to previously found LMRt (P = 0.021) and LMRb/LMRt (P = 0.040) in RCRC, LMRb/CD8MRt (P = 0.025) also significantly correlated with RFS, and CD8MRt (P = 0.052) showed a trend towards being associated. In addition to previously found CD14pt (P = 0.010), NLRb (P = 0.020) and PLRb (P = 0.018) were also significantly correlated with RFS in LCRC patients. After the multivariate analysis, several variables emerged as independent prognostic factors for RFS in RCRC patients: NLRb (P = 0.039), PLRb (P = 0.037), CD14t (P = 0.026), LMRpt (P = 0.014), LMRb/LMRpt (P = 0.042), and LMRb/CD8MRt (P = 0.006). In LCRC patients, NLRb (P = 0.009), CD8pt (P = 0.020), CD4CD8t (P = 0.039), and CD8MRt (P = 0.019) were found, together with a trend observed for CD4CD8pt (P = 0.053).
| Total patients | Univariate analysis | Multivariate analysis | |||||||||
| n (%) | HR | 95%CI | P | HR | 95%CI | P | |||||
| Variables | Low | High | Low | High | |||||||
| LMRb | |||||||||||
| Right | 34 (63.0) | 0.865 | 0.593 | 1.262 | 0.453 | ||||||
| Left | 20 (37.0) | 0.977 | 0.770 | 1.239 | 0.848 | 0.156 | 0.001 | 24.118 | 0.470 | ||
| NLRb | |||||||||||
| Right | 34 (63.0) | 1.135 | 0.841 | 1.532 | 0.407 | 2.760 | 1.050 | 7.254 | 0.039 | < 0.05 | |
| Left | 20 (37.0) | 1.094 | 1.094 | 1.180 | 0.020 | < 0.05 | 1.156 | 1.038 | 1.288 | 0.009 | < 0.01 |
| PLRb | |||||||||||
| Right | 34 (63.0) | 1.001 | 0.996 | 1.007 | 0.596 | 0.978 | 0.958 | 0.999 | 0.037 | < 0.05 | |
| Left | 20 (37.0) | 1.002 | 1.000 | 1.004 | 0.018 | < 0.05 | 0.987 | 0.954 | 1.022 | 0.468 | |
| CD4t | |||||||||||
| Right | 34 (63.0) | 1.010 | 0.864 | 1.181 | 0.902 | 0.802 | 0.457 | 1.407 | 0.441 | ||
| Left | 20 (37.0) | 0.981 | 0.617 | 1.559 | 0.934 | ||||||
| CD4pt | |||||||||||
| Right | 34 (63.0) | 0.963 | 0.851 | 1.091 | 0.556 | 1.486 | 0.124 | 17.828 | 0.755 | ||
| Left | 20 (37.0) | 1.120 | 0.795 | 1.577 | 0.516 | ||||||
| CD8t | |||||||||||
| Right | 34 (63.0) | 0.962 | 0.872 | 1.062 | 0.446 | 1.821 | 0.451 | 7.347 | 0.400 | ||
| Left | 20 (37.0) | 1.032 | 0.971 | 1.097 | 0.310 | ||||||
| CD8pt | |||||||||||
| Right | 34 (63.0) | 0.906 | 0.780 | 1.053 | 0.199 | 1.117 | 0.848 | 1.472 | 0.431 | ||
| Left | 20 (37.0) | 1.050 | 0.988 | 1.115 | 0.116 | 1.098 | 1.015 | 1.189 | 0.020 | < 0.05 | |
| CD4CD8t | |||||||||||
| Right | 34 (63.0) | 0.972 | 0.893 | 1.058 | 0.515 | ||||||
| Left | 20 (37.0) | 1.033 | 0.970 | 1.100 | 0.307 | 0.436 | 0.198 | 0.960 | 0.039 | < 0.05 | |
| CD4CD8pt | |||||||||||
| Right | 34 (63.0) | 0.944 | 0.857 | 1.041 | 0.248 | ||||||
| Left | 20 (37.0) | 1.052 | 0.992 | 1.117 | 0.093 | 0.008 | 0.000 | 1.071 | 0.053 | ||
| CD14t | |||||||||||
| Right | 34 (63.0) | 1.123 | 0.949 | 1.329 | 0.178 | 1.467 | 1.048 | 2.054 | 0.026 | < 0.05 | |
| Left | 20 (37.0) | 0.918 | 0.713 | 1.180 | 0.502 | ||||||
| CD14pt | |||||||||||
| Right | 34 (63.0) | 1.075 | 0.891 | 1.297 | 0.449 | 1.790 | 0.592 | 5.406 | 0.302 | ||
| Left | 20 (37.0) | 1.472 | 1.095 | 1.978 | 0.010 | < 0.05 | |||||
| LMRt | |||||||||||
| Right | 34 (63.0) | 0.555 | 0.337 | 0.915 | 0.021 | < 0.05 | 0.641 | 0.213 | 1.926 | 0.428 | |
| Left | 20 (37.0) | 1.084 | 0.999 | 1.176 | 0.052 | 0.165 | 0.003 | 9.203 | 0.380 | ||
| LMRpt | |||||||||||
| Right | 34 (63.0) | 0.691 | 0.458 | 1.042 | 0.078 | 0.312 | 0.123 | 0.793 | 0.014 | < 0.05 | |
| Left | 20 (37.0) | 1.066 | 0.688 | 1.653 | 0.775 | ||||||
| CD4MRt | |||||||||||
| Right | 34 (63.0) | 0.588 | 0.220 | 1.572 | 0.290 | 0.876 | 0.382 | 2.010 | 0.756 | ||
| Left | 20 (37.0) | 0.950 | 0.381 | 2.370 | 0.912 | ||||||
| CD4MRpt | |||||||||||
| Right | 34 (63.0) | 0.734 | 0.327 | 1.648 | 0.454 | 7.229 | 0.515 | 101.544 | 0.142 | ||
| Left | 20 (37.0) | 0.583 | 0.199 | 1.709 | 0.325 | ||||||
| CD8MRt | |||||||||||
| Right | 34 (63.0) | 0.497 | 0.246 | 1.005 | 0.052 | ||||||
| Left | 20 (37.0) | 1.083 | 0.999 | 1.174 | 0.052 | 1.123 | 1.020 | 1.238 | 0.019 | < 0.05 | |
| CD8MRpt | |||||||||||
| Right | 34 (63.0) | 0.584 | 0.333 | 1.023 | 0.060 | ||||||
| Left | 20 (37.0) | 1.191 | 0.815 | 1.741 | 0.365 | 0.293 | 0.026 | 3.345 | 0.323 | ||
| LMRb/LMRt | |||||||||||
| Right | 34 (63.0) | 1.311 | 1.013 | 1.697 | 0.040 | < 0.05 | |||||
| Left | 20 (37.0) | 0.958 | 0.730 | 1.258 | 0.760 | ||||||
| LMRb/LMRpt | |||||||||||
| Right | 34 (63.0) | 1.248 | 0.887 | 1.756 | 0.203 | 0.404 | 0.169 | 0.969 | 0.042 | < 0.05 | |
| Left | 20 (37.0) | 1.030 | 0.787 | 1.347 | 0.830 | 1.132 | 0.859 | 1.493 | 0.378 | ||
| LMRb/CD4MRt | |||||||||||
| Right | 34 (63.0) | 1.012 | 0.965 | 1.060 | 0.632 | 1.056 | 0.973 | 1.146 | 0.192 | ||
| Left | 20 (37.0) | 0.991 | 0.937 | 1.048 | 0.742 | ||||||
| LMRb/CD4MRpt | |||||||||||
| Right | 34 (63.0) | 1.031 | 0.948 | 1.121 | 0.479 | 1.393 | 0.875 | 2.220 | 0.163 | ||
| Left | 20 (37.0) | 1.023 | 0.969 | 1.079 | 0.412 | 0.925 | 0.847 | 1.011 | 0.087 | ||
| LMRb/CD8MRt | |||||||||||
| Right | 34 (63.0) | 1.146 | 1.017 | 1.292 | 0.025 | < 0.05 | 1.301 | 1.078 | 1.571 | 0.006 | < 0.01 |
| Left | 20 (37.0) | 0.941 | 0.775 | 1.143 | 0.542 | 1.036 | 0.591 | 1.816 | 0.903 | ||
| LMRb/CD8MRpt | |||||||||||
| Right | 34 (63.0) | 1.022 | 0.894 | 1.169 | 0.746 | 1.390 | 0.304 | 6.350 | 0.671 | ||
| Left | 20 (37.0) | 0.968 | 0.844 | 1.109 | 0.638 | 0.847 | 0.576 | 1.244 | 0.397 | ||
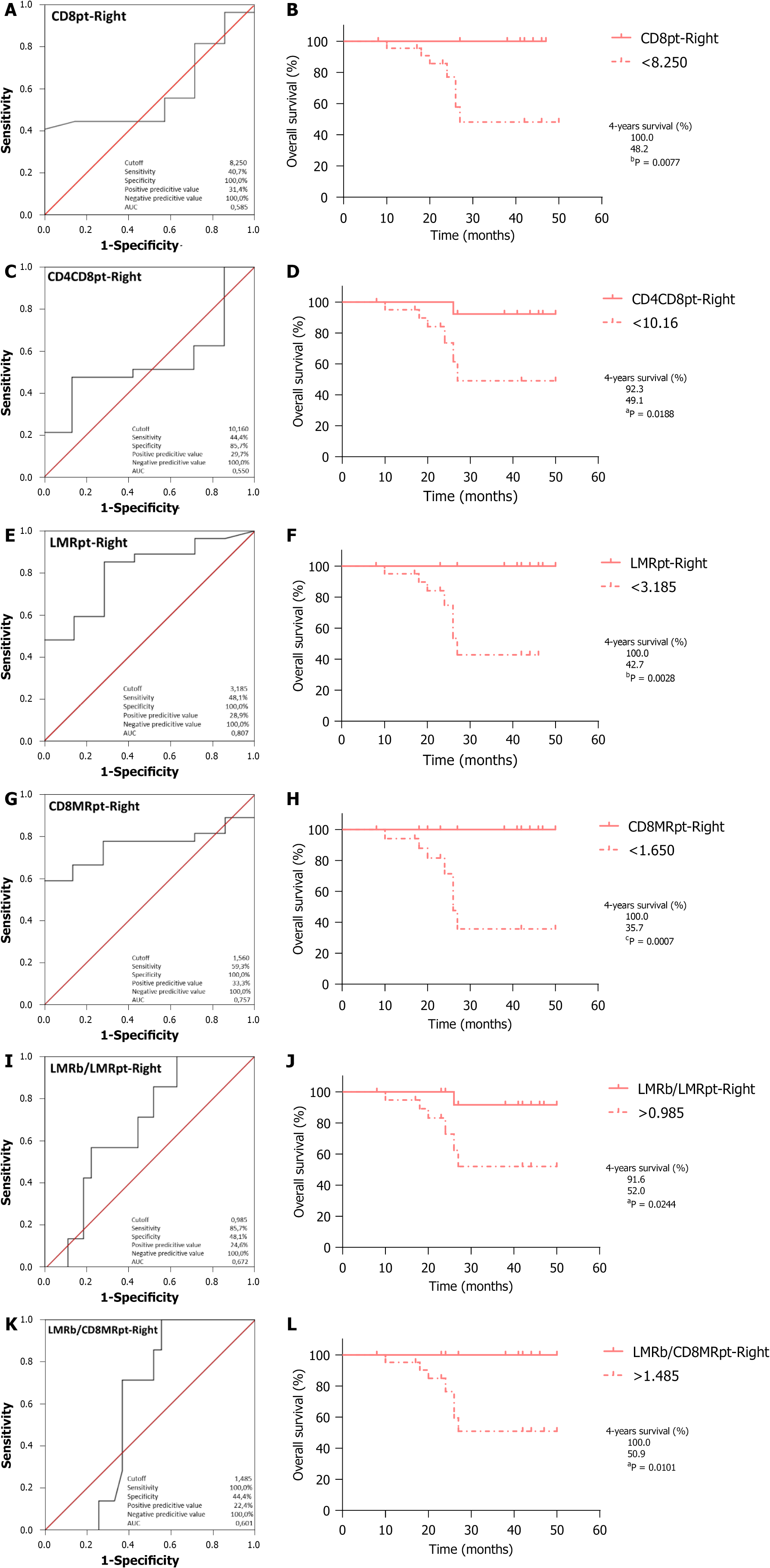
Taking into account all previous correlations, we then calculated the optimal cutoff values by ROC analyses for those variables significantly correlated with OS or RFS, using respectively cancer-specific death or relapse as the endpoints for both RCRC (Figures 4 and 6) and LCRC (Figure 5) patients after surgical intervention.
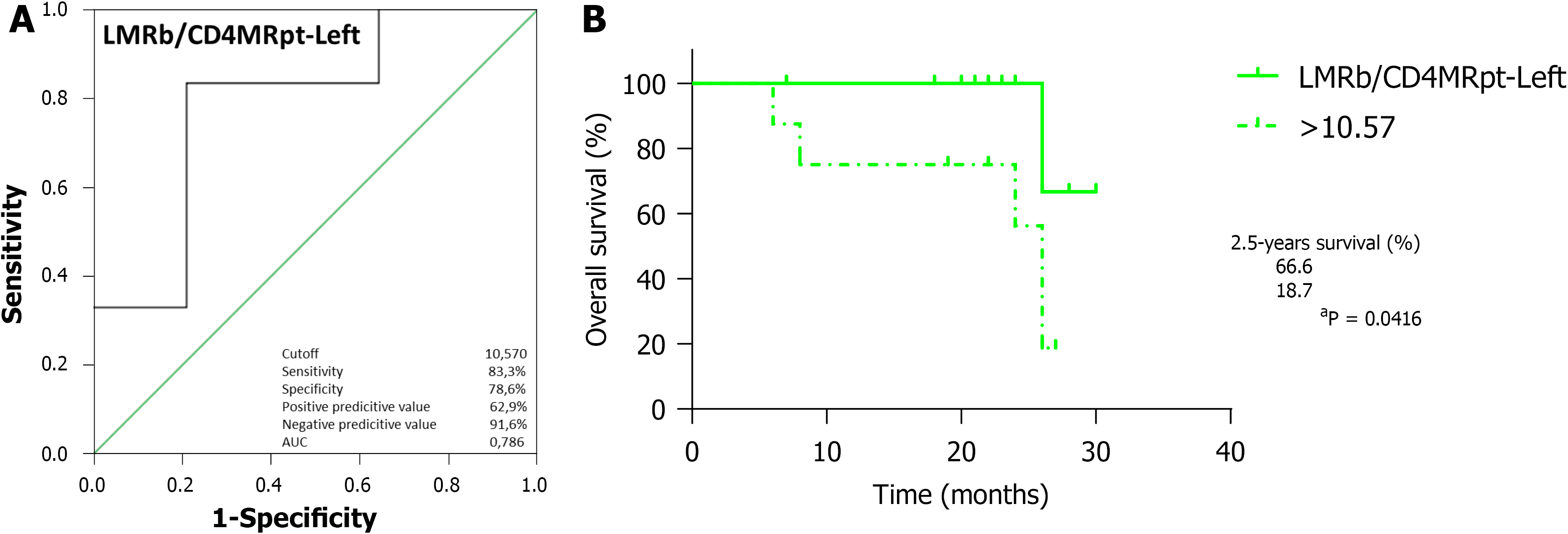
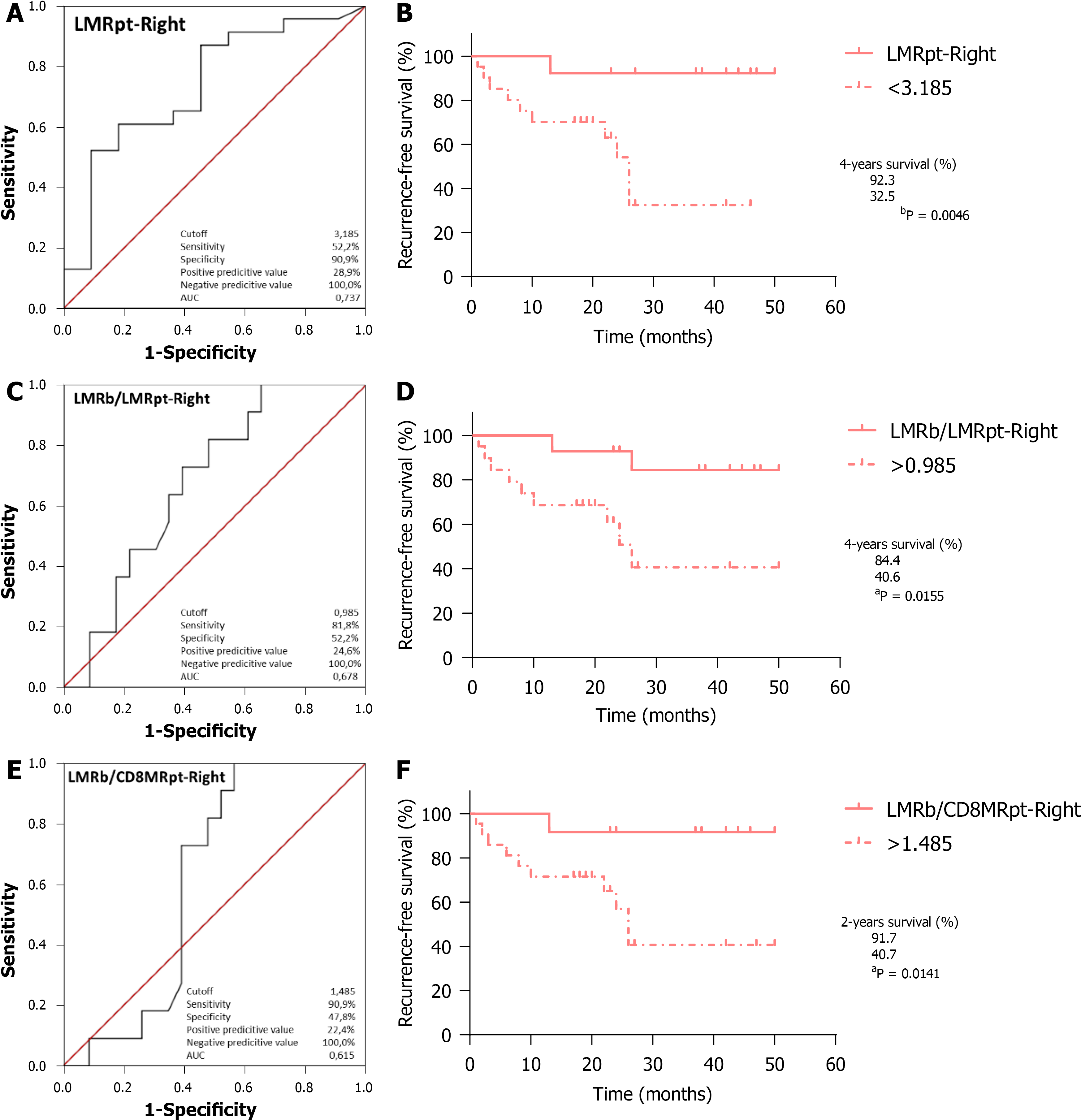
Regarding OS, ROC curve analysis of CD8pt (Figure 4A; AUC = 0.585, 95%CI: 0.376-0.793, P = 0.496) identified the optimal cutoff point at 8.250, which entails significantly worse outcomes for RCRC patients ranking below this (Figure 4B; 100% vs 48.2%, P = 0.0077). CD4CD8pt analysis (Figure 4C; AUC = 0.550, 95%CI: 0.334-0.766, P = 0.686) identified 10.16 as the optimal cutoff, with worse outcomes for RCRC patients ranking below this (Figure 4D; 92.3% vs 49.1%, P = 0.0188). LMRpt analysis (Figure 4E; AUC = 0.807, 95%CI: 0.641-0.973, P = 0.013) identified 3.185 as the optimal cutoff, with worse outcomes for RCRC patients ranking below this (Figure 4F; 100% vs 42.7%, P = 0.0028). CD8MRpt analysis (Figure 4G; AUC = 0.757, 95%CI: 0.600-0.914, P = 0.039) identified 1.650 as the optimal cutoff, with worse outcomes for RCRC patients ranking below this (Figure 4H; 100% vs 35.7%, P = 0.0007). LMRb/LMRpt analysis (Figure 4I; AUC = 0.672, 95%CI: 0.479-0.865, P = 0.166) identified 0.985 as the optimal cutoff, with worse outcomes for RCRC patients ranking above this (Figure 4J; 91.6% vs 52.0%, P = 0.0244). LMRb/CD8MRpt analysis (Figure 4K; AUC = 0.601, 95%CI: 0.419-0.782, P = 0.418) identified 1.485 as the optimal cutoff, with worse outcomes for RCRC patients ranking above this (Figure 4L; 100% vs 50.9%, P = 0.0101). Finally, LMRb/CD4MRpt analysis (Figure 5A; AUC = 0.786, 95%CI: 0.564-1.000, P = 0.048) identified 10.57 as the optimal cutoff, with worse outcomes for LCRC patients ranking above this (Figure 5B; 66.6% vs 18.7%, P = 0.0416). In addition, ROC curve analyses (Supplementary Figure 3) of CD4CD8t, PLRb, CD4CD8pt, and CD8MRt, though they showed significant AUC (0.524, 0.619, 0.726 and 0.571, respectively), rendered optimal cutoff values with no significant differences for LCRC survival.
With respect to RFS, ROC curve analysis of LMRpt (Figure 6A; AUC = 0.737, 95%CI: 0.554-0.920, P = 0.027) identified 3.185 as the optimal cutoff, with worse outcomes for RCRC patients ranking below this (Figure 6B; 92.3% vs 32.5%, P = 0.0046). LMRb/LMRpt analysis (Figure 6C; AUC = 0.678, 95%CI: 0.499-0.857, P = 0.098) identified 0.985 as the optimal cutoff, with worse outcomes for RCRC patients ranking above this (Figure 6D; 84.4% vs 40.6%, P = 0.0155). LMRb/CD8MRpt analysis (Figure 6E; AUC = 0.615, 95%CI: 0.427-0.802, P = 0.286) identified 1.485 as the optimal cutoff, with worse outcomes for RCRC patients ranking above this (Figure 6F; 91.7% vs 40.7%, P = 0.0141). The ROC analyses in RCRC patients of CD8MRpt, CD8pt, and CD4CD8pt (Supple
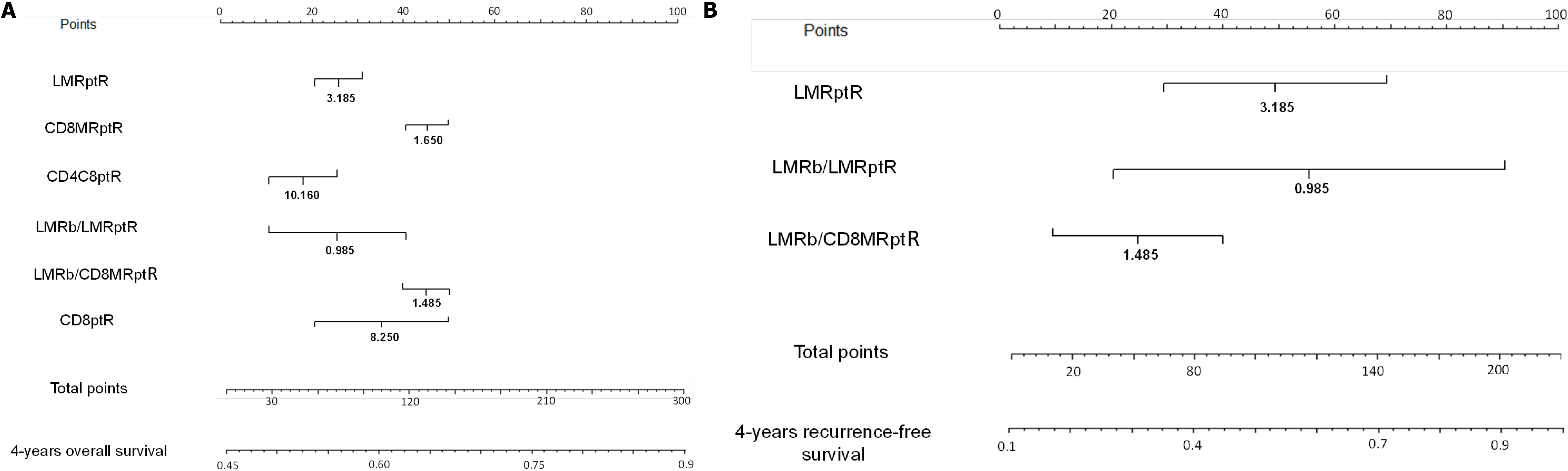
In order to avoid conflicts in handling the different values of the predictive indexes for RCRC patients, clinician-friendly nomograms were developed for both OS (Figure 7A) and RFS (Figure 7B) of these patients. The six significant predictive variables found for OS and the three found for RFS were used to construct the respective nomograms, with data from the training set of RCRC patients. The calibration of these nomograms revealed C-indexes of 0.600 (95%CI: 0.561-0.639) and 0.605 (95%CI: 0.579-0.631), respectively (Supplementary Figure 6A-B). Moreover, the reliability of the nomograms was evaluated with the validation set of RCRC patients, showing a moderate accuracy, with C-indexes of 0.500 (95%CI: 0.475-0.525) and 0.570 (95%CI: 0.541-0.599) for OS and RFS, respectively (Supplementary Figure 6C-D).
The segment of the large intestine proximal to the splenic flexure, i.e. the right colon (comprising caecum, ascending colon, and proximal two-thirds of the transverse colon), derives from the embryonic midgut; whereas the left colon (comprising the distal third part of the transverse colon and descending and sigmoid colon) derives from the embryonic hindgut[21]. Distinct embryologic origin of right and left sides of the colon markedly determines important physiological differences, mainly: cell motility, vasculature, lymphatic drainage, extrinsic innervation, development of the endocrine components, and the expression and patterns of epigenetic marks of crucial molecular factors for cell development[21,22].
Since seminal contributions by Bufill et al[23], an increasing number of studies have supported the hypothesis that these differences in origin may explain why RCRC and LCRC constitute two distinct clinical entities, which arise through different pathogenetic mechanisms[22,24,25]. Thus, differential aspects such as incidence, presentation, microbiome composition, genetic burden, or immunogenicity could be explained on these grounds[26-31]. In a large study with more than 17000 CRC patients, Benedix et al[32] showed that RCRC represents a more distinct tumour entity than LCRC, mainly because of its higher incidence in women and older people, poor differentiation, locally advanced carcinomas, a distinct pattern of metastatic spread, and worse outcome.
Likewise, survival after surgical intervention to remove the tumour should constitute a prominent feature to differentiate both pathologies. In this line, controversial results arise throughout the literature. Thereby, some studies support RCRC patients having poorer overall and disease-free survival rates[8], whilst others call attention to the stage of the disease, with better rates for RCRC being limited to stage II and better rates for LCRC being limited to stage III[33]. In our cohort, perhaps due to the stage’s heterogeneity of the patients, both OS and RFS were found side-dependent, with better outcomes in RCRC patients, reinforcing the idea that prognostic markers for the two pathologies should be studied separately.
A number of studies have stressed the importance of the systemic inflammatory response in CRC development and the search for variables involving its components as a valuable tool to drive prognosis[15,34]. Important prognostic records have been obtained in several research works[16,35], which avail the use of blood leukocyte ratios as predictors in CRC progression after surgery. However, some studies have highlighted inherent failures to these analyses. Thus, Zhang et al[36] warn against the impact of the use of distinct factors, within different studies, to adjust possible confounders for multivariate hazard ratio determination, which can make the latter at risk of bias and heterogeneity, in turn making LMR fail to reach significance in survival. Likewise, sample size, race heterogeneity, and most of all the pre/post
Notably, we report tissue leukocyte ratios, both alone and combined with preoperative blood LMRb, as six variables with a strong predictor value for RCRC overall survival (CD8pt, CD4CD8pt, LMRpt, CD8MRpt, LMRb/LMRpt, LMRb/CD8MRpt), three variables for recurrence-free survival (LMRpt, CD8MRpt, LMRb/LMRpt, LMRb/CD8MRpt), and another robust variable to predict LCRC overall survival (LMRb/CD4MRpt). In addition, to avoid conflicts when interpreting the different survival predictors of RCRC, physician-friendly nomograms are proposed for both OS and RFS. Albeit much effort has been made in describing and associating the leukocyte content of tumour tissues with CRC survival[38], most studies have been performed on disaggregated tumour and peritumour samples, and only a few of them have attempted to measure leukocyte expression in fixed samples of these tissues to associate them with circulating ratios[19] or to correlate them with patient survival[18,39]. Hence, this could be the first study in which leukocyte measures in both blood and fixed tissues are put together into predictor indexes for CRC survival.
It is worth noting that, in addition to the well-established predictor value of blood leukocyte ratios, the 10 indexes involve leukocyte concentrations in peritumoural zones of the bowel but not in the tumour mass. A peritumour constitutes an easily obtainable tissue during a preoperative exploration of the patient (this could be the colonoscopy), which might be safely biopsied without affecting the tumour environment in an adenoma-like surgical extraction protocol. Therefore, on a routine basis, surgeons might access both preoperative peripheral blood parameters as well as non-neoplastic peritumoural tissue (without disturbing the tumour itself) and make use of the described ratios and nomograms to predict the patient’s outcome after surgery. Thus, ad hoc surgical strategies can be designed to allow physicians to continue with surgery as programmed or delay the intervention until better scores are achieved after personalised treatments to correct the leukocyte levels in the patient.
Altogether, these indexes could be implemented in the first line of prognosis, making it easier to predict the outcome of patients after surgery depending on the tumour location and leukocyte distribution in both peripheral blood and biopsies of the peritumoural region.
Our study is mainly limited by the cohort size. It might be expected that the extension of these variables to a greater cohort would reinforce our conclusions or even make foregoing unobserved interactions surface.
Herein we present important remarks on the value of combining circulating leukocyte ratios and tissue infiltrated leukocyte ratios on the sustaining of valuable prognosis tools for physicians in order to stratify patients regarding their putative outcome. In the era of personalised medicine, such indexes will provide benefits to improving both resources and well-being of CRC patients after surgery.
Colorectal cancer (CRC) points to 9.4% of cancer deaths worldwide, ranking second after lung cancer. Despite the wide variety of factors tested to predict their outcome, most patients with similar variables show big differences in survival. Moreover, right-sided CRC (RCRC) and left-sided CRC (LCRC) patients exhibit large differences in outcome after surgical intervention as assessed by preoperative blood leukocyte ratios [today, the most extended parameters used to assess a patient’s overall survival (OS) and recurrence-free survival (RFS) after surgery]. However, few efforts have been made to link tumour infiltrated leukocyte ratios to patient outcomes.
To determine whether both RCRC and LCRC patient outcomes could be accurately predicted based on the counting of infiltrated leukocytes in tumour and peritumoural tissues.
The aim of this study was to find stronger indexes than circulating (blood) leukocyte ratios to predict RCRC and LCRC patient outcomes.
A prospective study was performed with CRC patients who had undergone surgical intervention to resect the tumours. Leukocyte concentrations in peripheral blood, tumour, and non-neoplastic peritumoural tissues were determined. Ratios of these parameters were evaluated as predictors for OS and RFS using Spearman correlations, Cox univariate and multivariate proportional hazards regression followed by the calculation of the receiver-operating characteristic and area under the curve (AUC) and the determination of Youden’s optimal cutoff values for those variables that significantly correlated with either RCRC or LCRC patient outcomes. Clinician-friendly nomograms were developed to predict OS and RFS from the prediction indexes. The accuracy of the model was evaluated using calibration and validation analyses.
We obtained six robust predictors of worse OS in RCRC: CD8+ lymphocyte content in peritumour (CD8pt, AUC = 0.585, cutoff < 8.250, P = 0.0077), total lymphocyte content in peritumour (CD4CD8pt, AUC = 0.550, cutoff < 10.160, P = 0.0188), lymphocyte-to-monocyte ratio in peritumour (LMRpt, AUC = 0.807, cutoff < 3.185, P = 0.0028), CD8+ LMR in peritumour (CD8MRpt, AUC = 0.757, cutoff < 1.650, P = 0.0007), the ratio of blood LMR to LMR in peritumour (LMRb/LMRpt, AUC = 0.672, cutoff > 0.985, P = 0.0244), and the ratio of blood LMR to CD8+ LMR in peritumour (LMRb/CD8MRpt, AUC = 0.601, cutoff > 1.485, P = 0.0101). In addition, three robust predictors of worse RFS in RCRC were found: LMRpt (AUC = 0.737, cutoff < 3.185, P = 0.0046), LMRb/LMRpt (AUC = 0.678, cutoff > 0.985, P = 0.0155), and LMRb/CD8MRpt (AUC = 0.615, cutoff > 1.485, P = 0.0141). Furthermore, the ratio of blood LMR to CD4+ LMR in peritumour (LMRb/CD4MRpt, AUC = 0.786, cutoff > 10.570, P = 0.0416) was found to robustly predict poorer OS in LCRC patients. The developed nomograms to predict OS and RFS of RCRC patients showed C-indexes of 0.600 (95% confidence interval: 0.561-0.639) and 0.605 (95% confidence interval: 0.579-0.631), respectively.
Easily obtainable variables at preoperative consultation, defining the status of leukocyte balances between peripheral blood and peritumoural tissue, have been shown to render indexes that accurately predict OS and RFS of CRC patients after surgical ablation of the tumours.
We hope these indexes could be implemented in the first line of prognosis, making it easier to predict the outcome of patients after surgery depending on the tumour location and leukocyte distribution in both peripheral blood and biopsies of the peritumoural region.
We thank Dr. María Teresa Vallejo-Cremades and Onys Camps-Ortega from the Service of Image and Immunohistochemistry of IdiPAZ for helpful assistance, and Dr. Jair A. Tenorio for software support.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Wu ZQ, Xie Q, Zeng YY S-Editor: Chang KL L-Editor: Filipodia P-Editor: Chang KL
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64442] [Article Influence: 16110.5] [Reference Citation Analysis (176)] |
| 2. | Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69:363-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2417] [Cited by in RCA: 3071] [Article Influence: 511.8] [Reference Citation Analysis (0)] |
| 3. | Augestad KM, Merok MA, Ignatovic D. Tailored Treatment of Colorectal Cancer: Surgical, Molecular, and Genetic Considerations. Clin Med Insights Oncol. 2017;11:1179554917690766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Forster S, Radpour R. Molecular Immunotherapy: Promising Approach to Treat Metastatic Colorectal Cancer by Targeting Resistant Cancer Cells or Cancer Stem Cells. Front Oncol. 2020;10:569017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Tan D, Fu Y, Tong W, Li F. Prognostic significance of lymphocyte to monocyte ratio in colorectal cancer: A meta-analysis. Int J Surg. 2018;55:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 6. | Mao Y, Chen D, Duan S, Zhao Y, Wu C, Zhu F, Chen C, Chen Y. Prognostic impact of pretreatment lymphocyte-to-monocyte ratio in advanced epithelial cancers: a meta-analysis. Cancer Cell Int. 2018;18:201. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Peng J, Li H, Ou Q, Lin J, Wu X, Lu Z, Yuan Y, Wan D, Fang Y, Pan Z. Preoperative lymphocyte-to-monocyte ratio represents a superior predictor compared with neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios for colorectal liver-only metastases survival. Onco Targets Ther. 2017;10:3789-3799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 8. | Guo D, Li X, Xie A, Cao Q, Zhang J, Zhang F, Li W, Chen J. Differences in oncological outcomes and inflammatory biomarkers between right-sided and left-sided stage I-III colorectal adenocarcinoma. J Clin Lab Anal. 2020;34:e23132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Altan M, Haberal HB, Akdoğan B, Özen H. A critical prognostic analysis of neutrophil-lymphocyte ratio for patients undergoing nephroureterectomy due to upper urinary tract urothelial carcinoma. Int J Clin Oncol. 2017;22:964-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Lu A, Li H, Zheng Y, Tang M, Li J, Wu H, Zhong W, Gao J, Ou N, Cai Y. Prognostic Significance of Neutrophil to Lymphocyte Ratio, Lymphocyte to Monocyte Ratio, and Platelet to Lymphocyte Ratio in Patients with Nasopharyngeal Carcinoma. Biomed Res Int. 2017;2017:3047802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Liu B, Huang Y, Sun Y, Zhang J, Yao Y, Shen Z, Xiang D, He A. Prognostic value of inflammation-based scores in patients with osteosarcoma. Sci Rep. 2016;6:39862. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 70] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Biswas T, Kang KH, Gawdi R, Bajor D, Machtay M, Jindal C, Efird JT. Using the Systemic Immune-Inflammation Index (SII) as a Mid-Treatment Marker for Survival among Patients with Stage-III Locally Advanced Non-Small Cell Lung Cancer (NSCLC). Int J Environ Res Public Health. 2020;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Ying HQ, Liao YC, Sun F, Peng HX, Cheng XX. The Role of Cancer-Elicited Inflammatory Biomarkers in Predicting Early Recurrence Within Stage II-III Colorectal Cancer Patients After Curable Resection. J Inflamm Res. 2021;14:115-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Dell'Aquila E, Cremolini C, Zeppola T, Lonardi S, Bergamo F, Masi G, Stellato M, Marmorino F, Schirripa M, Urbano F, Ronzoni M, Tomasello G, Zaniboni A, Racca P, Buonadonna A, Allegrini G, Fea E, Di Donato S, Chiara S, Tonini G, Tomcikova D, Boni L, Falcone A, Santini D. Prognostic and predictive role of neutrophil/lymphocytes ratio in metastatic colorectal cancer: a retrospective analysis of the TRIBE study by GONO. Ann Oncol. 2018;29:924-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 15. | Clarke SJ, Burge M, Feeney K, Gibbs P, Jones K, Marx G, Molloy MP, Price T, Reece WHH, Segelov E, Tebbutt NC. The prognostic role of inflammatory markers in patients with metastatic colorectal cancer treated with bevacizumab: A translational study [ASCENT]. PLoS One. 2020;15:e0229900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Ying HQ, Deng QW, He BS, Pan YQ, Wang F, Sun HL, Chen J, Liu X, Wang SK. The prognostic value of preoperative NLR, d-NLR, PLR and LMR for predicting clinical outcome in surgical colorectal cancer patients. Med Oncol. 2014;31:305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 226] [Article Influence: 20.5] [Reference Citation Analysis (1)] |
| 17. | Wu Y, Ye S, Goswami S, Pei X, Xiang L, Zhang X, Yang H. Clinical significance of peripheral blood and tumor tissue lymphocyte subsets in cervical cancer patients. BMC Cancer. 2020;20:173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Hamada T, Ishizaki H, Haruyama Y, Hamada R, Yano K, Kondo K, Kataoka H, Nanashima A. Neutrophil-to-Lymphocyte Ratio and Intratumoral CD45RO-Positive T Cells as Predictive Factors for Longer Survival of Patients with Colorectal Liver Metastasis after Hepatectomy. Tohoku J Exp Med. 2020;251:303-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Guo G, Wang Y, Zhou Y, Quan Q, Zhang Y, Wang H, Zhang B, Xia L. Immune cell concentrations among the primary tumor microenvironment in colorectal cancer patients predicted by clinicopathologic characteristics and blood indexes. J Immunother Cancer. 2019;7:179. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 64] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 20. | Cantero-Cid R, Casas-Martin J, Hernández-Jiménez E, Cubillos-Zapata C, Varela-Serrano A, Avendaño-Ortiz J, Casarrubios M, Montalbán-Hernández K, Villacañas-Gil I, Guerra-Pastrián L, Peinado B, Marcano C, Aguirre LA, López-Collazo E. PD-L1/PD-1 crosstalk in colorectal cancer: are we targeting the right cells? BMC Cancer. 2018;18:945. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Wilson DJ, Bordoni B. Embryology, Bowel. Treasure Island (FL): StatPearls, 2021. Available from: https://www.ncbi.nlm.nih.gov/pubmed/31424831/. |
| 22. | Kostouros A, Koliarakis I, Natsis K, Spandidos DA, Tsatsakis A, Tsiaoussis J. Large intestine embryogenesis: Molecular pathways and related disorders (Review). Int J Mol Med. 2020;46:27-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113:779-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 550] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 24. | Kwak HD, Ju JK. Immunological Differences Between Right-Sided and Left-Sided Colorectal Cancers: A Comparison of Embryologic Midgut and Hindgut. Ann Coloproctol. 2019;35:342-346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 25. | Lee GH, Malietzis G, Askari A, Bernardo D, Al-Hassi HO, Clark SK. Is right-sided colon cancer different to left-sided colorectal cancer? Eur J Surg Oncol. 2015;41:300-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 313] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 26. | Álvaro E, Cano JM, García JL, Brandáriz L, Olmedillas-López S, Arriba M, Rueda D, Rodríguez Y, Cañete Á, Arribas J, Inglada-Pérez L, Pérez J, Gómez C, García-Arranz M, García-Olmo D, Goel A, Urioste M, González-Sarmiento R, Perea J. Clinical and Molecular Comparative Study of Colorectal Cancer Based on Age-of-onset and Tumor Location: Two Main Criteria for Subclassifying Colorectal Cancer. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Baek SK. Laterality: Right-Sided and Left-Sided Colon Cancer. Ann Coloproctol. 2017;33:205-206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Baran B, Mert Ozupek N, Yerli Tetik N, Acar E, Bekcioglu O, Baskin Y. Difference Between Left-Sided and Right-Sided Colorectal Cancer: A Focused Review of Literature. Gastroenterology Res. 2018;11:264-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 337] [Article Influence: 48.1] [Reference Citation Analysis (1)] |
| 29. | Ghidini M, Petrelli F, Tomasello G. Right Versus Left Colon Cancer: Resectable and Metastatic Disease. Curr Treat Options Oncol. 2018;19:31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 30. | Jung MK, Shin US, Ki YJ, Kim YB, Moon SM, Sung SJ. Is the Location of the Tumor Another Prognostic Factor for Patients With Colon Cancer? Ann Coloproctol. 2017;33:210-218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Stintzing S, Tejpar S, Gibbs P, Thiebach L, Lenz HJ. Understanding the role of primary tumour localisation in colorectal cancer treatment and outcomes. Eur J Cancer. 2017;84:69-80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 32. | Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H; Colon/Rectum Carcinomas (Primary Tumor) Study Group. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 552] [Article Influence: 36.8] [Reference Citation Analysis (0)] |
| 33. | Huang ZS, Wu JW, Li Y, Lin YH, Li XY. Effect of sidedness on survival among patients with early-stage colon cancer: a SEER-based propensity score matching analysis. World J Surg Oncol. 2021;19:127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Patel M, McSorley ST, Park JH, Roxburgh CSD, Edwards J, Horgan PG, McMillan DC. The relationship between right-sided tumour location, tumour microenvironment, systemic inflammation, adjuvant therapy and survival in patients undergoing surgery for colon and rectal cancer. Br J Cancer. 2018;118:705-712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 35. | Guo G, Chen X, He W, Wang H, Wang Y, Hu P, Rong Y, Fan L, Xia L. Establishment of inflammation biomarkers-based nomograms to predict prognosis of advanced colorectal cancer patients based on real world data. PLoS One. 2018;13:e0208547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Zhang J, Chen L, Zhou R, Sun H, Liao Y, Liao W. Pretreatment Lymphocyte Monocyte Ratio Predicts Long-Term Outcomes in Patients with Digestive System Tumor: A Meta-Analysis. Gastroenterol Res Pract. 2016;2016:9801063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Li Z, Zhao R, Cui Y, Zhou Y, Wu X. The dynamic change of neutrophil to lymphocyte ratio can predict clinical outcome in stage I-III colon cancer. Sci Rep. 2018;8:9453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 60] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 38. | Idos GE, Kwok J, Bonthala N, Kysh L, Gruber SB, Qu C. The Prognostic Implications of Tumor Infiltrating Lymphocytes in Colorectal Cancer: A Systematic Review and Meta-Analysis. Sci Rep. 2020;10:3360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 39. | Forssell J, Oberg A, Henriksson ML, Stenling R, Jung A, Palmqvist R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin Cancer Res. 2007;13:1472-1479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |