Published online Sep 15, 2021. doi: 10.4251/wjgo.v13.i9.1210
Peer-review started: May 8, 2021
First decision: June 16, 2021
Revised: June 16, 2021
Accepted: August 9, 2021
Article in press: August 9, 2021
Published online: September 15, 2021
Processing time: 124 Days and 20.6 Hours
The use of liquid biopsies is a relatively new tool in diagnosis and management of gastrointestinal cancers and is actively being investigated. Liquid biopsies have become extremely popular in cholangiocarcinoma and pancreatic cancer research. With more prospective trials using this tool for early diagnosis, liquid biopsies may become an important part of cancer management.
Core Tip: Liquid biopsies are a novel method to help physicians in the early diagnosis and management of various cancer subtypes. Due to the east of performance, cost-effectiveness, and quick results associated with liquid biopsies, this technique will be integrated more into the treatment of aggressive cancer types such as cholangiocarcinoma and pancreatic cancers. More prospective clinical trials are needed to validate results on this tool.
- Citation: Khachfe HH. Use of liquid biopsies in gastrointestinal cancers. World J Gastrointest Oncol 2021; 13(9): 1210-1212
- URL: https://www.wjgnet.com/1948-5204/full/v13/i9/1210.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i9.1210
We read with interest a review by Rompianesi et al[1], who analyzed the current evidence present on liquid biopsy use in the diagnosis and management of patients affected with cholangiocarcinoma[1].
We agree with the authors’ insight that cholangiocarcinoma remains a dismal prognosis for patients, primarily due to the difficulty in diagnosis, minimal surgical resection options available, and high recurrence rates. The use of liquid biopsies in its various forms as a new biomarker is a novel technique in the diagnosis and management of cholangiocarcinoma and other gastrointestinal tumors due to its low risk, ease of performance, cost-effectiveness, and quick results. This is especially true when compared to other mainstay options of screening, diagnosis and follow up such as surgical or computed tomography-guided biopsy and various forms of imaging (Computed tomography, magnetic resonance imaging, etc.).
Because of the increased interest in liquid biopsy use in cancer management as a biomarker, there are currently over 1000 clinical trials (over 700 on circulating tumor cells and over 300 on circulating tumor derived DNA) listed in ClinicalTrials.gov concerning the topic[2]. We expect that even more will follow on detecting microRNA, proteins, cytokines, metabolites, and extracellular vesicles in most gastrointestinal cancers, including cholangiocarcinoma.
Liquid biopsies have reported sensitivities ranging from 38% to 100% in different cancers such as breast, prostate, colorectal and pancreatic[3]. Due to the need for fast and accurate diagnosis in aggressive cancer types (cholangiocarcinoma and pancreatic cancer for example), liquid biopsies offer clinicians a novel way to treat such diseases. Pancreatic cancer and cholangiocarcinoma rates have increased drastically over the past few decades, but 5-year survival rates remain at 5%-20% and 8%-25% respectively even with curative intent surgical resection and chemo/radiotherapy[4,5]. As such, innovative methods such as liquid biopsies are needed to increase survival odds by allowing earlier diagnosis and tracking new biomarkers preoperatively and postoperatively for surgical prognostication.
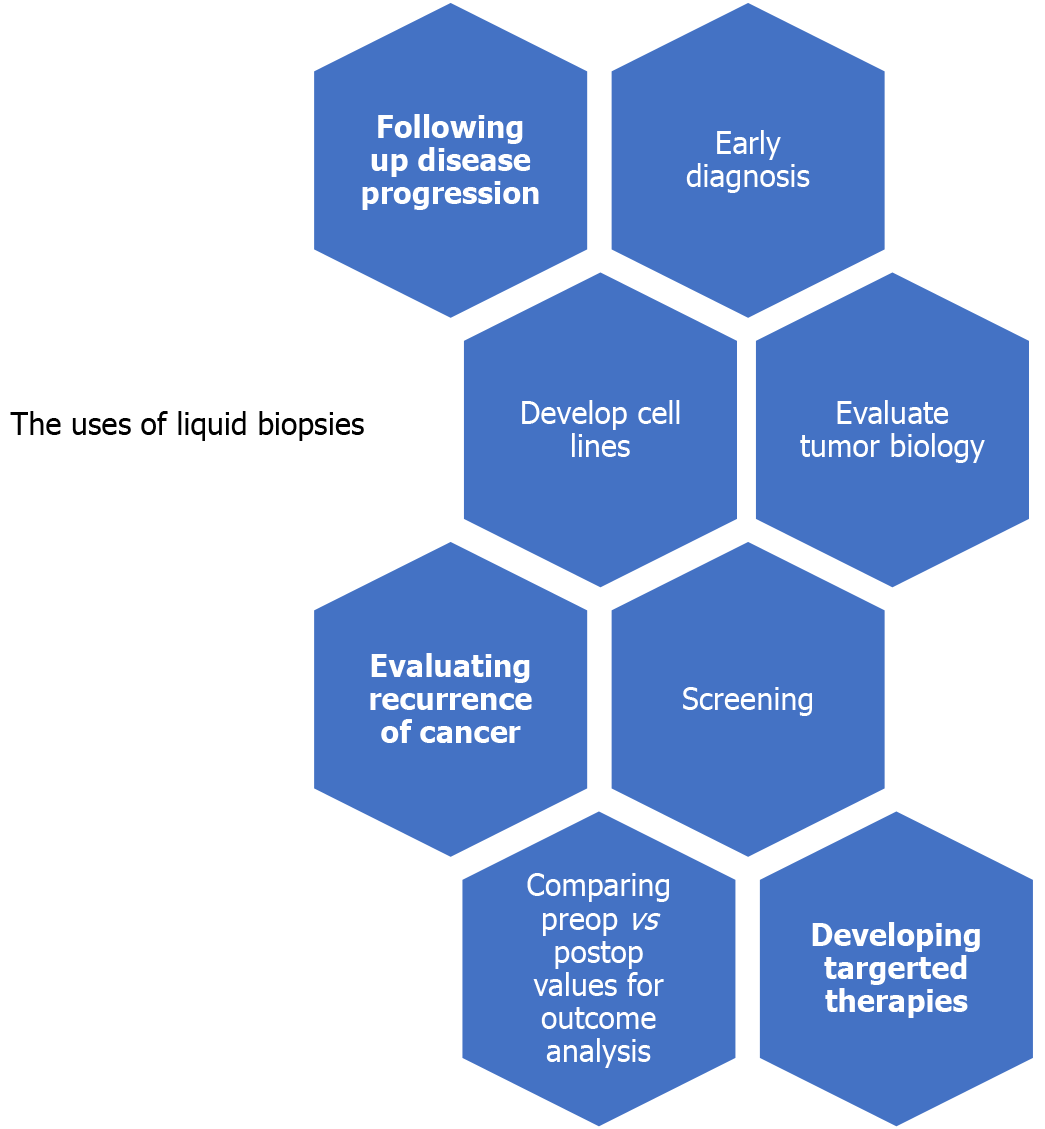
Liquid biopsies offer clinicians with insights on tumor biology, which might help in developing targeted therapies according to specific cancer pathologies. The uses of liquid biopsies are summarized in Figure 1. We believe this will be a key factor in future cholangiocarcinoma and other gastrointestinal treatment plans, especially in patients where surgical resection is not a viable option. These biopsies will be able to evaluate treatment response and provide “real-time” evidence of resistance to some chemo/targeted therapies.
We believe that liquid biopsies will become a crucial tool in the early diagnosis and management of gastrointestinal cancers, as is the case with cholangiocarcinoma. More large prospective clinical trials are needed to validate the results already present on the technique. More funding is needed to develop further this technique in order to increase the sensitivity and accuracy of liquid biopsy results.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Garg PK, Park WS S-Editor: Fan JR L-Editor: A P-Editor: Ma YJ
| 1. | Rompianesi G, Di Martino M, Gordon-Weeks A, Montalti R, Troisi R. Liquid biopsy in cholangiocarcinoma: Current status and future perspectives. World J Gastrointest Oncol. 2021;13:332-350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 41] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 2. | ClinicalTrials.gov. ClinicalTrials.gov is a database of privately and publicly funded clinical studies conducted around the world. [cited 10 May 2021]. Available from: https://clinicaltrials.gov/. |
| 3. | Lewis AR, Valle JW, McNamara MG. Pancreatic cancer: Are "liquid biopsies" ready for prime-time? World J Gastroenterol. 2016;22:7175-7185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71:7-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8287] [Cited by in RCA: 11933] [Article Influence: 2983.3] [Reference Citation Analysis (4)] |
| 5. | Puckett Y. Pancreatic Cancer. StatPearls [Internet]. StatPearls (ed): StatPearls Publishing, Treasure Island (FL), 2020. |