Published online Jul 15, 2021. doi: 10.4251/wjgo.v13.i7.684
Peer-review started: March 4, 2021
First decision: March 29, 2021
Revised: April 5, 2021
Accepted: June 18, 2021
Article in press: June 18, 2021
Published online: July 15, 2021
Processing time: 127 Days and 20.8 Hours
Afferent loop obstruction (ALO) is defined as duodenal or jejunal mechanical obstruction at the proximal anastomosis site of a gastrojejunostomy. With advances in chemotherapy, the incidence of malignant ALO is increasing. Malignant ALO can be complicated by ischemia, gangrenous bowel, pancreatitis, and ascending cholangitis. Moreover, the general condition of patients with recurrent cancer is often poor. Therefore, accurate and rapid diagnosis and minimally invasive treatments are required. However, no review articles on the diagnosis and treatment of malignant ALO have been published. Through literature searching, we reviewed related articles published between 1959 and 2020 in the PubMed database. Herein, we present recent advances in the diagnosis and treatment of malignant ALO and describe future perspectives. Endoscopic transluminal self-expandable metal stent (SEMS) placement is considered the standard treatment for malignant ALO, as this procedure is well established and less invasive. However, with the development of interventional endoscopic ultrasound (EUS) in recent years, the usefulness of EUS-guided gastrojejunostomy has been reported. Moreover, through indirect comparison, this approach has been reported to be superior to transluminal SEMS placement. It is expected that a safer and less invasive treatment method will be established through the continued advancement and innovation of interventional endoscopy techniques.
Core Tip: Afferent loop obstruction (ALO) is defined as duodenal or jejunal mechanical obstruction at the proximal anastomosis site of a gastrojejunostomy. With advances in chemotherapy, the incidence of malignant ALO is increasing, and this condition can be complicated by ischemia, gangrenous bowel, pancreatitis, and ascending cholangitis. Moreover, the general condition of patients with recurrent cancer is often poor. Therefore, accurate and rapid diagnosis and minimally invasive treatments are required. Herein, we present recent advances in the diagnosis and treatment of malignant ALO and describe future perspectives.
- Citation: Sakai A, Shiomi H, Masuda A, Kobayashi T, Yamada Y, Kodama Y. Clinical management for malignant afferent loop obstruction. World J Gastrointest Oncol 2021; 13(7): 684-692
- URL: https://www.wjgnet.com/1948-5204/full/v13/i7/684.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i7.684
Afferent loop obstruction (ALO) is defined as duodenal or jejunal obstruction at the proximal anastomosis site of a gastrojejunostomy. The etiology of ALO can be classified as benign or malignant depending on the cause of the obstruction[1]. With advances in surgery, the prevalence of benign ALO seems to be decreasing, whereas with advances in chemotherapy, the prevalence of malignant ALO is increasing[2]. The diagnosis of malignant ALO has become easier owing to breakthroughs in cross-sectional imaging. In the past, malignant ALO was surgically managed. However, the general condition of patients with recurrent cancer is often not good indicated for surgery. Percutaneous intervention for malignant ALO was reported in the late 1980s, and endoscopic intervention was reported in the 2000s. Endoscopic self-expandable metal stent (SEMS) placement for malignant ALO is considered the current standard. Although most cases of malignant ALO can be successfully treated with SEMS placement, if the stricture is long or an angulated loop, endoscopic SEMS placement across the stricture is challenging. Recently, endoscopic ultrasound (EUS)-guided gastrojejunostomy (EUS-GJ) using a lumen-apposing metal stent (LAMS) has been reported.
The aim of this review is to present the development of diagnosis and treatment for malignant ALO. We identified 104 articles in our search of the PubMed database for English-language literature using keywords ‘malignant ALO’. After the screening of articles, we reviewed 60 English-language articles on the diagnosis and treatment of malignant ALO published between 1959 and 2020.
The afferent loop is the duodenum and jejunum located proximal to a gastrojejunostomy[3]. ALO is duodenal or jejunal mechanical obstruction at the proximal anastomosis site of a gastrojejunostomy[4]. It can result in an increased internal pressure caused by the accumulation of bile, pancreatic, and intestinal fluid[5]. ALO can be complicated by ischemia, gangrenous bowel, pancreatitis, and ascending cholangitis. Patients with hepaticojejunostomy develop biliary symptoms more frequently than those without, especially those with complete ALO, owing to loss of papillary function[3].
The etiology of ALO can be classified as benign or malignant depending on the nature of the obstructive site[1]. Malignant ALO is associated with locoregional tumor recurrence, which obstructs the afferent loop at the anastomotic site. Other causes of obstruction include regional lymphadenopathy and peritoneal carcinomatosis[3]. There are few reports on the incidence of malignant ALO. Aoki et al[6] reported the incidence of ALO after gastrectomy with Roux-en-Y reconstruction performed using an open approach[6]. They reported that of the 1908 patients who underwent distal gastrectomy followed by Ruox-en-Y reconstruction through an open approach (detailed patient background was not shown), 4 patients (0.2%) developed ALO, and only 1 patient had malignant ALO. Juan et al[7] reported that of 1100 patients who had undergone gastroenterostomy reconstruction (detailed patient background was not shown), 22 (2%) patients were diagnosed with ALO after surgery, including Roux-en-Y gastroenterostomy (n = 9), Billroth II gastrojejunostomy (n = 7), and Whipple’s operation (n = 6)[7]. In a series of 186 pancreatic cancer patients treated with PD, Pannala et al[8] reported 24 cases (13%) of ALO, of which 8 were due to cancer recurrence with median follow up duration of 1.2 years[8].
Malignant ALO was diagnosed by clinical presentation, laboratory data, and computed tomography (CT) findings. Malignant ALO often develops into cholangitis or pancreatitis. When a patient with a history of gastrointestinal (GI) reconstruction for malignant disease develops pancreatitis or cholangitis, malignant ALO should be suspected.
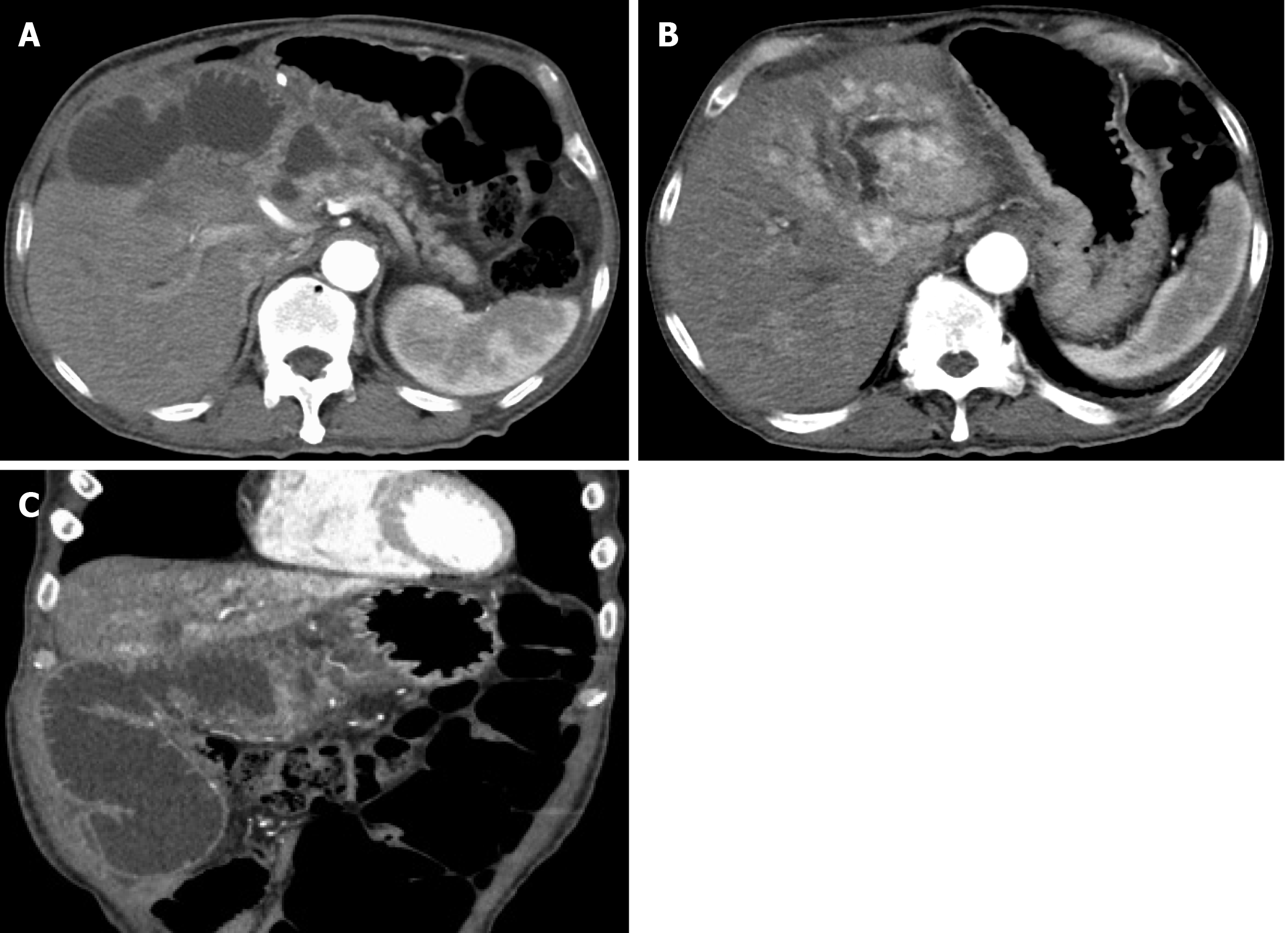
In the late 1970, cases of ALO diagnosed using abdominal CT were reported[9,10]. CT has been the mainstay of diagnostic imaging for ALO. CT scans revealed marked dilatation of the afferent loop due to bowel obstruction caused by a recurrent cancer (Figure 1). Juan YH reported the diagnosis of ALO following gastroenterostomy reconstruction using multidetector row CT (MDCT)[7]. They reported that a fluid-filled C-shaped afferent loop in combination with valvulae connivences projecting into the lumen was the most common MDCT feature of ALO and that the etiologies of ALO and its associated complications can be predicted to be 100% by MDCT. If malignant ALO can be listed in the differential diagnosis based on patient’s medical history, symptoms, and laboratory data, the diagnosis is not difficult due to advances in CT imaging[7,9-14].
Conventionally, malignant ALO is managed surgically[4,15-18]. For the management of ALO, surgical revisions, such as jejunojejunostomy or Roux-en-Y conversion, have been established[4]. However, the general condition of patients with recurrent cancer is not suitable for surgery. Reoperation is a difficult undertaking, and reoperation itself may cause further morbidity and mortality[1]. As nonsurgical treatment is clearly desirable, percutaneous or endoscopic treatment has been reported.
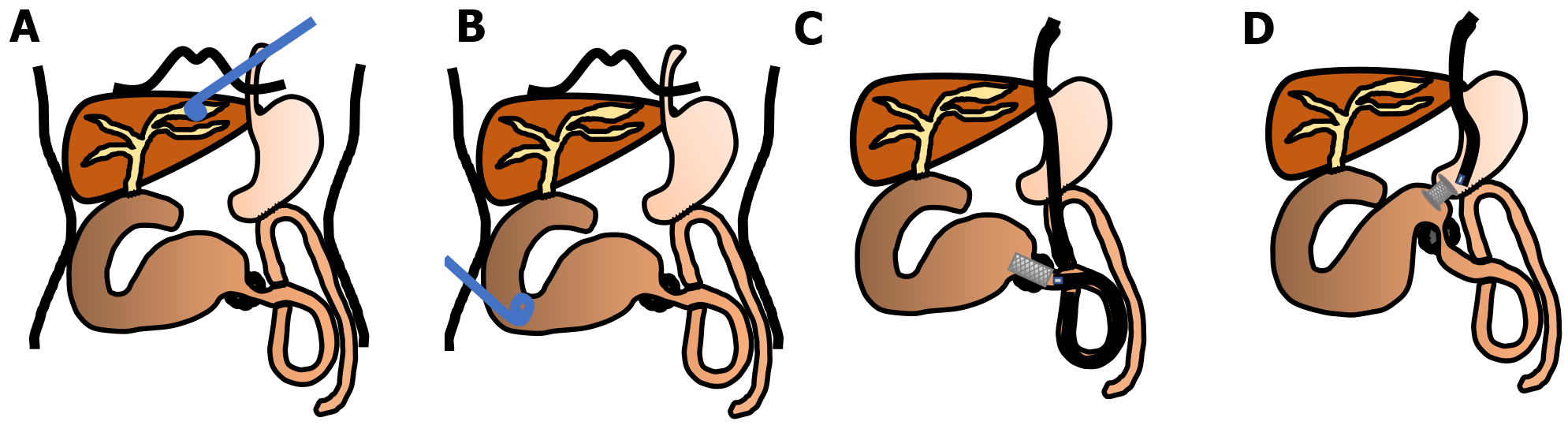
Regardless of the clinical presentation, dilated afferent loop is the most reasonable therapeutic target for malignant ALO. A majority of the reported cases of malignant ALO developed due to obstructive jaundice. For cases that present with obstructive jaundice, it is possible to approach the dilated bile duct. Malignant ALO with obstructive jaundice has been treated with percutaneous transhepatic biliary drainage[19-23] (Figure 2A). However, the procedure is known to cause bacteremia due to severe ascending cholangitis[20,21]. Since the development of enteral SEMS, SEMS placement via the transhepatic route for ALO was reported[24-30].
A direct percutaneous approach to the dilated afferent loop is available if the dilated afferent loop underlies the anterior abdominal wall[31,32] (Figure 2B). Sato et al[32] performed percutaneous drainage via the dilated afferent loop in eight patients. Of these, five patients had malignant ALO, and SEMS placement was subsequently performed via the afferent loop to address malignant ALO in two patients; however, in the remaining three cases, the external catheter could not be removed. No patients developed specific major complications, such as septic shock, peritonitis, or other procedure-related complications[32].
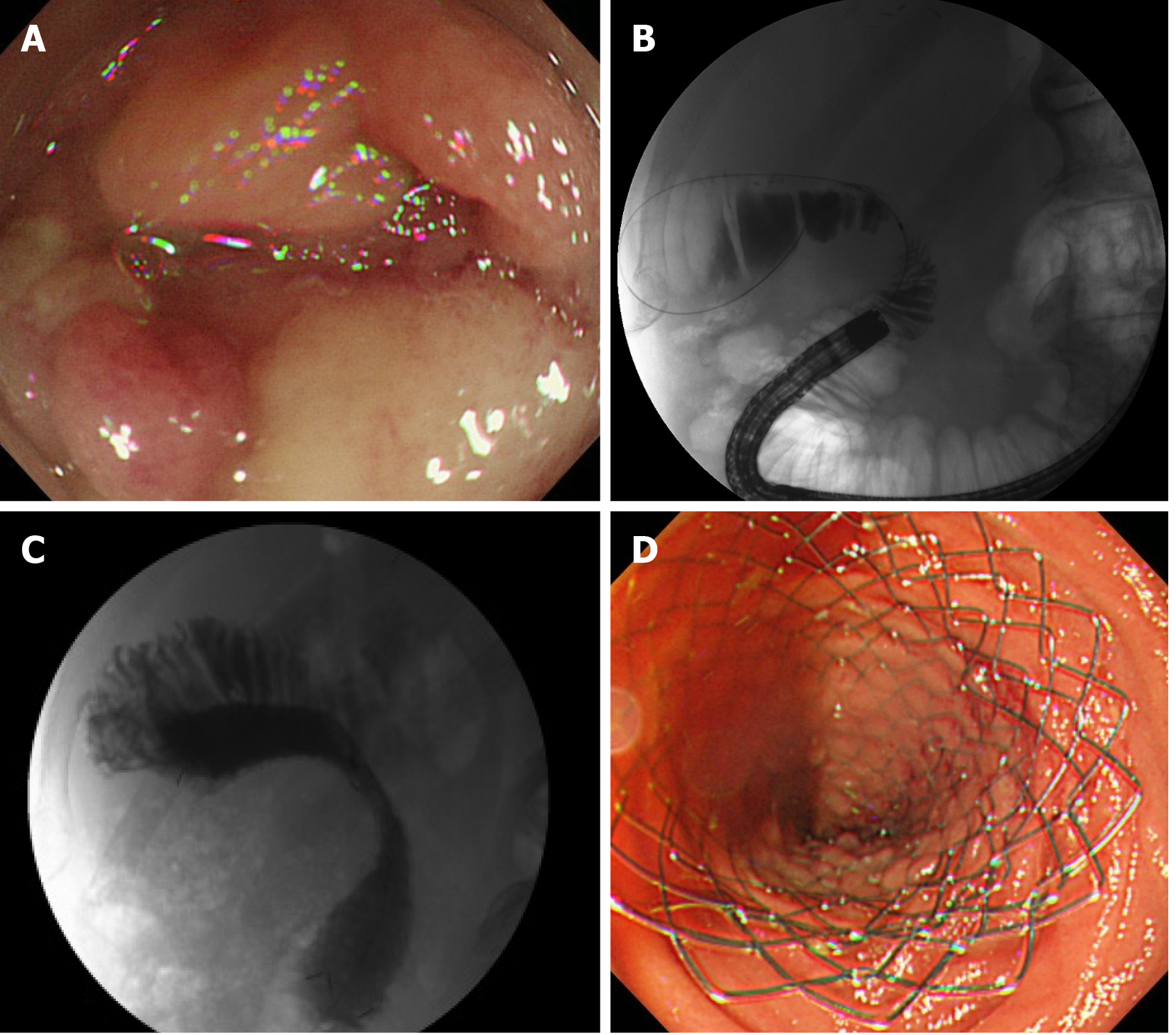
Since the 2000s, endoscopic transluminal SEMS placement for malignant ALO has been reported. An endoscopic treatment would be less invasive and technically easier than a percutaneous treatment[5,33-40] (Figures 2C and 3). Kida et al[41] conducted a retrospective analysis of 11 malignant ALO patients who underwent endoscopic transluminal SEMS placement[41]. The procedure was attempted in 13 sessions for 11 patients, and successful in 12 of 13 sessions; there were no adverse events, and the clinical efficacy was high in successful SEMS placement patients. The median survival time after the procedure was 118 d. Ten patients died of primary disease, and one patient died of severe cholangitis after the failure of the procedure. Malignant ALO recurred, and the procedure was repeated for 2 of the 10 patients who ultimately died due to the primary disease. In another study, we reported a detailed method of endoscopic transluminal SEMS placement for malignant ALO. Briefly, an N-tube was inserted into the dilated afferent loop by fluoroscopy guidance, and then SEMS was placed through the stricture after improvement of the physical condition[5]. In our case series, we retrospectively examined the records of seven patients who underwent endoscopic SEMS placement for malignant ALO following PD[40]. All cases were clinically successful. The median procedure time was 30 min (range, 15-50 min). There were no cases of stent dysfunction, and no procedure-related adverse events were observed. All patients died of their primary disease, and the median overall survival period was 155 d (range, 96-374 d). We pointed out that our two-step approach would be safe and useful method of endoscopic SEMS placement for malignant ALO. Recently, balloon-assisted endoscopy (BAE) has also been used to place a SEMS in cases where it cannot be reached to the ALO by a standard scope[41-51]. The recently designed BAE had a 3.2-mm working channel, enabled through-the-scope SEMS placement, which had been previously challenging because the large diameter of the SEMS delivery system did not allow stent placement by the conventional BAE with the 2.8-mm working channels[48].
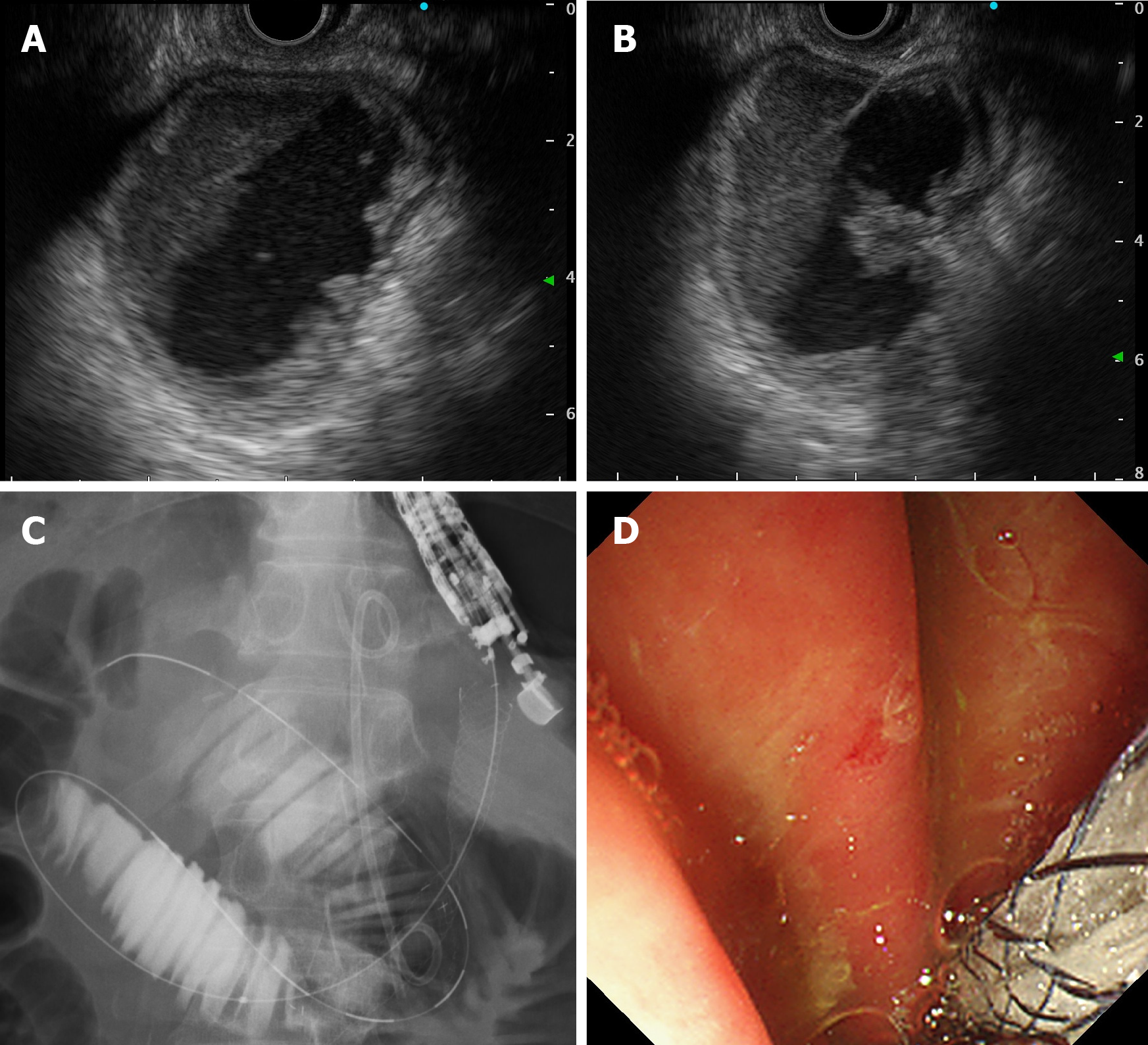
The creation of a GI anastomosis has been useful for relieving pancreatobiliary symptoms in patients with malignant ALO. However, surgical techniques are costly and time-consuming, and can be associated with high mortality and morbidity. Technological and clinical innovations in interventional endoscopy have allowed endoscopists to create a GI anastomosis with minimally invasive manner[52]. EUS-GJ has been shown to be a safe and effective method to bypass portions of bowel. Since it was first reported by Fritscher-Ravens et al[53] in 2003, technological advancements and technique alterations have made EUS-GJ a safe approach. The case of malignant ALO that was treated with EUS-GJ using a LAMS was first reported in 2015[54,55] (Figure 2D). EUS-GJ with LAMS has been reported to be useful for malignant ALO[56-58]. A multicenter retrospective study showed that complete resolution of symptoms was higher in patients treated with EUS-guided entero-enterostomy (EUS-EE), with less need for re-interventions, than in those undergoing enteroscopy-assisted luminal stent placement[59]. In this study, 18 patients underwent EUS-EE. Clinical success included resolution of symptoms (88.9 %) and improvement in hospital discharge (11.1 %). Technical success was achieved in 100% of the cases, with a mean procedure time of 29.7 min. The most common procedure was gastrojejunostomy (72.2%). Three adverse events (16.7 %) occurred (two mild, one moderate). When compared with data on endoscopic transluminal SEMS placement, patients treated with EUS-EE required fewer re-interventions (16.6 % vs 76.5 %; P < 0.001). However, although LAMS has the advantages of preventing both stent migration and fluid leakage, it is not available in many countries and is more expensive than that is conventional SEMS. We reported a method involving a conventional biliary SEMS with antimigration properties, comprising a large loop double-pigtail plastic stent within a fully covered biliary SEMS (Figure 4)[60].
This review outlines the clinical management of malignant ALO. The prevalence of malignant ALO is increasing because of advances in chemotherapy for advanced cancers. As the diagnosis of malignant ALO has become easier with the development of cross-sectional imaging, it is possible to diagnose it earlier. At this point, endoscopic transluminal SEMS placement is considered the standard treatment for malignant ALO because the procedure is well established and less invasive. However, with the development of interventional EUS, the usefulness of EUS-GJ has been reported in recent years. It is expected that a safer and less invasive treatment method will be established through the continued advancement and innovation of interventional endoscopy techniques.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujimori N S-Editor: Fan JR L-Editor: A P-Editor: Wang LL
| 1. | Blouhos K, Boulas KA, Tsalis K, Hatzigeorgiadis A. Management of afferent loop obstruction: Reoperation or endoscopic and percutaneous interventions? World J Gastrointest Surg. 2015;7:190-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 2. | Song KB, Yoo D, Hwang DW, Lee JH, Kwon J, Hong S, Lee JW, Youn WY, Hwang K, Kim SC. Comparative analysis of afferent loop obstruction between laparoscopic and open approach in pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2019;26:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Termsinsuk P, Chantarojanasiri T, Pausawasdi N. Diagnosis and treatment of the afferent loop syndrome. Clin J Gastroenterol. 2020;13:660-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Aimoto T, Uchida E, Nakamura Y, Katsuno A, Chou K, Tajiri T, Naito Z. Malignant afferent loop obstruction following pancreaticoduodenectomy: report of two cases. J Nippon Med Sch. 2006;73:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Sakai A, Shiomi H, Okabe Y, Yagi Y, Kobayashi T, Shiomi Y, Takenaka M, Hoshi N, Arisaka Y, Kutsumi H, Azuma T. Effectiveness of endoscopic self-expandable metal stent placement for afferent loop obstruction caused by pancreatic cancer recurrence after pancreaticoduodenectomy. Clin J Gastroenterol. 2015;8:103-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Aoki M, Saka M, Morita S, Fukagawa T, Katai H. Afferent loop obstruction after distal gastrectomy with Roux-en-Y reconstruction. World J Surg. 2010;34:2389-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Juan YH, Yu CY, Hsu HH, Huang GS, Chan DC, Liu CH, Tung HJ, Chang WC. Using multidetector-row CT for the diagnosis of afferent loop syndrome following gastroenterostomy reconstruction. Yonsei Med J. 2011;52:574-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Pannala R, Brandabur JJ, Gan SI, Gluck M, Irani S, Patterson DJ, Ross AS, Dorer R, Traverso LW, Picozzi VJ, Kozarek RA. Afferent limb syndrome and delayed GI problems after pancreaticoduodenectomy for pancreatic cancer: single-center, 14-year experience. Gastrointest Endosc. 2011;74:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 9. | Feiss JS, Raskin MM, Wolfe J, Plevy DJ, Luckman GS. A case of afferent loop obstruction secondary to recurrent carcinoma of the stomach with ultrasound and C.T. scan findings. Am J Gastroenterol. 1977;68:77-80. [PubMed] |
| 10. | Warrier RK, Steinheber FU. Afferent loop obstruction presenting as obstructive jaundice. Dig Dis Sci. 1979;24:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Gale ME, Gerzof SG, Kiser LC, Snider JM, Stavis DM, Larsen CR, Robbins AH. CT appearance of afferent loop obstruction. AJR Am J Roentgenol. 1982;138:1085-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Kim HC, Han JK, Kim KW, Kim YH, Yang HK, Kim SH, Won HJ, Lee KH, Choi BI. Afferent loop obstruction after gastric cancer surgery: helical CT findings. Abdom Imaging. 2003;28:624-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Zissin R. CT findings of afferent loop syndrome after a subtotal gastrectomy with Roux-en-Y reconstruction. Emerg Radiol. 2004;10:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Gayer G, Barsuk D, Hertz M, Apter S, Zissin R. CT diagnosis of afferent loop syndrome. Clin Radiol. 2002;57:835-839. [PubMed] |
| 15. | Ballon HC, Niloff PH. Elevation of serum amylase with post-gastrectomy jejunal obstruction and perforation. Can Med Assoc J. 1959;80:339-342. [PubMed] |
| 16. | Beranbaum SL, Lawrence L, Schwartz S. Roentgen exploration of the afferent loop. Radiology. 1968;91:932-941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Feiss JS, Plevy DJ, Luckman GS, Lenit OS Jr. A case of postgastrectomy afferent loop obstruction secondary to cancer of the stomach simulating pseudocyst of the pancreas. Am J Dig Dis. 1975;20:995-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Bakes D, Cain C, King M, Dong Xda E. Management of afferent loop obstruction from recurrent metastatic pancreatic cancer using a venting gastrojejunostomy. World J Gastrointest Oncol. 2013;5:235-239. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Lee LI, Teplick SK, Haskin PH, Sammon JK, Wolferth C, Amron G. Refractory afferent loop problems: percutaneous transhepatic management of two cases. Radiology. 1987;165:49-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Morita S, Takemura T, Matsumoto S, Odani R. Septic shock after percutaneous transhepatic drainage of obstructed afferent loop: case report. Cardiovasc Intervent Radiol. 1989;12:66-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Moriura S, Ikeda S, Kimura A, Iwatsuka Y, Ikezawa T, Naiki K. Jaundice due to afferent loop obstruction following hepatectomy for a hilar cholangiocarcinoma. Abdom Imaging. 1996;21:226-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Yao NS, Wu CW, Tiu CM, Liu JM, Whang-Peng J, Chen LT. Percutaneous transhepatic duodenal drainage as an alternative approach in afferent loop obstruction with secondary obstructive jaundice in recurrent gastric cancer. Cardiovasc Intervent Radiol. 1998;21:350-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Kim KH, Lee HB, Kim SH, Kim MC, Jung GJ. Role of percutaneous transhepatic biliary drainage in patients with complications after gastrectomy. Int Surg. 2015;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Caldicott DG, Ziprin P, Morgan R. Transhepatic insertion of a metallic stent for the relief of malignant afferent loop obstruction. Cardiovasc Intervent Radiol. 2000;23:138-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Johnsson E, Delle M, Lundell L, Liedman B. Transhepatic placement of an enteral stent to treat jaundice in a tumor recurrence obstructed afferent loop after a whipple procedure. Dig Surg. 2003;20:329-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Yoshida H, Mamada Y, Taniai N, Kawano Y, Mizuguchi Y, Shimizu T, Takahashi T, Okuda T, Miyashita M, Tajiri T. Percutaneous transhepatic insertion of metal stents with a double-pigtail catheter in afferent loop obstruction following distal gastrectomy. Hepatogastroenterology. 2005;52:680-682. [PubMed] |
| 27. | Gwon DI. Percutaneous transhepatic placement of covered, self-expandable nitinol stent for the relief of afferent loop syndrome: report of two cases. J Vasc Interv Radiol. 2007;18:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Hosokawa I, Kato A, Shimizu H, Furukawa K, Miyazaki M. Percutaneous transhepatic metallic stent insertion for malignant afferent loop obstruction following pancreaticoduodenectomy: a case report. J Med Case Rep. 2012;6:198. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Jinno N, Naitoh I, Nagura Y, Fujioka K, Mizuno Y, Momose J, Ooyama M, Hayashi K, Miyaki T, Nakamura M, Joh T. Percutaneous Transhepatic Self-expanding Metallic Stent Placement for the Treatment of Malignant Afferent Loop Obstruction. Intern Med. 2018;57:333-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Cha RR, Cho SB, Kim WS, Kim JJ, Lee JM, Lee SS, Kim HJ, Cho JK. Self-expanding metal stent procedure for afferent loop syndrome with ascending cholangitis caused by remnant gastric cancer: A case report. Medicine (Baltimore). 2018;97:e13072. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Chevallier P, Novellas S, Motamedi JP, Gugenheim J, Brunner P, Bruneton JN. Percutaneous jejunostomy and stent placement for treatment of malignant Roux-en-Y obstruction: a case report. Clin Imaging. 2006;30:283-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Sato Y, Inaba Y, Murata S, Yamaura H, Kato M, Kawada H, Shimizu Y, Ishiguchi T. Percutaneous drainage for afferent limb syndrome and pancreatic fistula via the blind end of the jejunal limb after pancreatoduodenectomy or bile duct resection. J Vasc Interv Radiol. 2015;26:566-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 33. | Burdick JS, Garza AA, Magee DJ, Dykes C, Jeyarajah R. Endoscopic management of afferent loop syndrome of malignant etiology. Gastrointest Endosc. 2002;55:602-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Song HY, Kim TH, Choi EK, Kim JH, Kim KR, Shin JH, Lee SK, Kim TW, Yook JH, Kim BS. Metallic stent placement in patients with recurrent cancer after gastrojejunostomy. J Vasc Interv Radiol. 2007;18:1538-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 35. | Kim JK, Park CH, Huh JH, Park JY, Park SW, Song SY, Chung J, Bang S. Endoscopic management of afferent loop syndrome after a pylorus preserving pancreatoduodenecotomy presenting with obstructive jaundice and ascending cholangitis. Clin Endosc. 2011;44:59-64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Kwong WT, Fehmi SM, Lowy AM, Savides TJ. Enteral stenting for gastric outlet obstruction and afferent limb syndrome following pancreaticoduodenectomy. Ann Gastroenterol. 2014;27:413-417. [PubMed] |
| 37. | Huang J, Hao S, Yang F, Di Y, Yao L, Li J, Jiang Y, Zhong L, Fu D, Jin C. Endoscopic metal enteral stent placement for malignant afferent loop syndrome after pancreaticoduodenectomy. Wideochir Inne Tech Maloinwazyjne. 2015;10:257-265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 38. | Kanno Y, Ohira T, Harada Y, Koike Y, Yamagata T, Tanaka M, Shimada T, Ito K. Metal Stent Placement in the Afferent Loop Obstructed by Peritoneal Metastases-Experience of Five Cases. Clin Endosc. 2018;51:299-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Takeuchi H, Abe N, Kondou E, Tsurumi M, Hashimoto Y, Ooki A, Nagao G, Masaki T, Mori T, Sugiyama M. Endoscopic self-expandable metal stent placement for malignant afferent loop obstruction caused by peritoneal recurrence after total gastrectomy. Int Cancer Conf J. 2018;7:98-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 40. | Sakai A, Shiomi H, Iemoto T, Nakano R, Ikegawa T, Kobayashi T, Masuda A, Kodama Y. Endoscopic Self-Expandable Metal Stent Placement for Malignant Afferent Loop Obstruction After Pancreaticoduodenectomy: A Case Series and Review. Clin Endosc. 2020;53:491-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Kida A, Matsuda K, Noda Y. Endoscopic metallic stenting by double-balloon enteroscopy and its overtube for malignant gastrointestinal obstruction as palliative treatment. Dig Endosc. 2013;25:552-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Sasaki T, Isayama H, Kogure H, Yamada A, Aoki T, Kokudo N, Koike K. Double-balloon enteroscope-assisted enteral stent placement for malignant afferent-loop obstruction after Roux-en-Y reconstruction. Endoscopy. 2014;46 Suppl 1 UCTN:E541-E542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 43. | Fujii M, Ishiyama S, Saito H, Ito M, Fujiwara A, Niguma T, Yoshioka M, Shiode J. Metallic stent insertion with double-balloon endoscopy for malignant afferent loop obstruction. World J Gastrointest Endosc. 2015;7:665-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Nakahara K, Okuse C, Matsumoto N, Suetani K, Morita R, Michikawa Y, Ozawa S, Hosoya K, Kobayashi S, Otsubo T, Itoh F. Enteral metallic stenting by balloon enteroscopy for obstruction of surgically reconstructed intestine. World J Gastroenterol. 2015;21:7589-7593. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 45. | Shugo H, Hodo Y, Yoneshima M. Endoscopic metallic stent insertion for malignant afferent loop obstruction using balloon-assisted enteroscopy: a case report. Am J Gastroenterol. 2015;110:355-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Minaga K, Kitano M, Takenaka M. Through-the-scope enteral metal stent placement using a short-type single-balloon enteroscope for malignant surgically reconstructed jejunal stenosis (with video). Dig Endosc. 2016;28:758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Nakahara K, Sato Y, Suetani K, Morita R, Michikawa Y, Kobayashi S, Itoh F. Endoscopic Double Metallic Stenting in the Afferent and Efferent Loops for Malignant Afferent Loop Obstruction with Billroth II Anatomy. Clin Endosc. 2016;49:97-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Shimatani M, Takaoka M, Tokuhara M, Kato K, Miyoshi H, Ikeura T, Okazaki K. Through-the-scope self-expanding metal stent placement using newly developed short double-balloon endoscope for the effective management of malignant afferent-loop obstruction. Endoscopy. 2016;48 Suppl 1 UCTN:E6-E7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 49. | Tsutsumi K, Kato H, Okada H. Impact of a Newly Developed Short Double-Balloon Enteroscope on Stent Placement in Patients with Surgically Altered Anatomies. Gut Liver. 2017;11:306-311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Sasaki T, Yamada I, Matsuyama M, Sasahira N. Enteral stent placement for malignant afferent loop obstruction by the through-the-scope technique using a short-type single-balloon enteroscope. Endosc Int Open. 2018;6:E806-E811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Yane K, Katanuma A, Hayashi T, Takahashi K, Kin T, Nagai K, Tanaka K, Komatsu N, Endo M, Kobayashi Y, Takigawa Y, Utsunomiya R. Enteral self-expandable metal stent placement for malignant afferent limb syndrome using single-balloon enteroscope: report of five cases. Endosc Int Open. 2018;6:E1330-E1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 52. | Marrache MK, Itani MI, Farha J, Fayad L, Sharara SL, Kalloo AN, Khashab MA, Kumbhari V. Endoscopic gastrointestinal anastomosis: a review of established techniques. Gastrointest Endosc. 2021;93:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 53. | Fritscher-Ravens A, Mosse CA, Mukherjee D, Mills T, Park PO, Swain CP. Transluminal endosurgery: single lumen access anastomotic device for flexible endoscopy. Gastrointest Endosc. 2003;58:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Ikeuchi N, Itoi T, Tsuchiya T, Nagakawa Y, Tsuchida A. One-step EUS-guided gastrojejunostomy with use of lumen-apposing metal stent for afferent loop syndrome treatment. Gastrointest Endosc. 2015;82:166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 55. | Shah A, Khanna L, Sethi A. Treatment of afferent limb syndrome: novel approach with endoscopic ultrasound-guided creation of a gastrojejunostomy fistula and placement of lumen-apposing stent. Endoscopy. 2015;47 Suppl 1 UCTN:E309-E310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 56. | Yamamoto K, Tsuchiya T, Tanaka R, Mitsuyoshi H, Mukai S, Nagakawa Y, Itoi T. Afferent loop syndrome treated by endoscopic ultrasound-guided gastrojejunostomy, using a lumen-apposing metal stent with an electrocautery-enhanced delivery system. Endoscopy. 2017;49:E270-E272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 57. | Ligresti D, Amata M, Messina M, Traina M, Tarantino I. Single-step EUS-guided jejunojejunostomy with a lumen-apposing metal stent as treatment for malignant afferent limb syndrome. VideoGIE. 2020;5:154-156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Monino L, Barthet M, Gonzalez JM. Endoscopic ultrasound-guided management of malignant afferent loop syndrome after gastric bypass: from diagnosis to therapy. Endoscopy. 2020;52:E84-E85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Brewer Gutierrez OI, Irani SS, Ngamruengphong S, Aridi HD, Kunda R, Siddiqui A, Dollhopf M, Nieto J, Chen YI, Sahar N, Bukhari MA, Sanaei O, Canto MI, Singh VK, Kozarek R, Khashab MA. Endoscopic ultrasound-guided entero-enterostomy for the treatment of afferent loop syndrome: a multicenter experience. Endoscopy. 2018;50:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 60. | Shiomi H, Kobayashi T, Sakai A, Shiomi Y, Masuda A, Bondoc EM, Kodama Y. Endoscopic ultrasound-guided gastrojejunostomy using fully covered metal stent combined with large-loop double-pigtail stent for malignant afferent loop syndrome. Endoscopy. 2019;51:E303-E304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |