Published online Jun 15, 2021. doi: 10.4251/wjgo.v13.i6.600
Peer-review started: January 27, 2021
First decision: March 8, 2021
Revised: April 13, 2021
Accepted: May 22, 2021
Article in press: May 22, 2021
Published online: June 15, 2021
Processing time: 131 Days and 10.3 Hours
Hepatocellular carcinoma (HCC) accounts for 8.2% of all cancer-related deaths worldwide. Being a vascular tumor, vascular endothelial growth factor (VEGF) plays a vital role in HCC pathogenesis, growth, and spread.
To determine the accuracy of serum VEGF and VEGF/platelet (PLT) as tumor markers in the early detection of HCC cases in patients with hepatitis C virus (HCV)-related liver cirrhosis.
We conducted a case-control study with HCV patients from the outpatient and inpatient hepatology clinics. Patients were classified into three groups: (1) HCC group; (2) Cirrhosis group; and (3) HCV without cirrhosis (control group). Patients were clinically evaluated, and blood samples were drawn for the analysis; serum VEGF levels were measured by a specific VEGF human recombinant enzyme-linked immunosorbent assay kit. Data from the three study groups were compared by the one-way analysis of variance or Kruskal-Wallis test. Receivers operating characteristic curves were constructed to determine the optimal cut-off values of alpha fetoprotein (AFP), VEGF, and VEGF/PLT that provided the best diagnostic accuracy. The sensitivity and specificity at the optimal cut-off value of each biomarker were then calculated.
This study included one hundred patients (HCC, cirrhosis, and control groups: n = 40, 30, 30, respectively). HCC patients had significantly higher serum VEGF and VEGF/PLT levels than the non-HCC groups (P = 0.001). Serum VEGF and VEGF/PLT showed significant positive correlations with and HCC tumor size, stage, vascular invasion, and Child-Pugh classification. Moreover, a VEGF cut-off the value of 250 pg/mL provided 80% sensitivity and 81.7% specificity for discriminating HCC patient from non-HCC patients. Similarly, the ratio of VEGF/PLT provided sensitivity and specificity of 77.5% and 80%, respectively which is higher than the accuracy provided by AFP. The combination of AFP, VEGF, and VEGF/PLT increases the accuracy of diagnosing HCC to > 95%.
In HCV patients, serum VEGF and VEGF/PLT separately or in combination with AFP are reliable biomarkers for early and accurate HCC diagnosis.
Core Tip: We conducted an observational study with 100 patients to assess the usefulness of serum vascular endothelial growth factor (VEGF) and VEGF/platelet (PLT) as tumor markers for hepatocellular carcinoma (HCC) diagnosis in hepatitis C virus-related cirrhotic patients, and comparing them to serum alpha fetoprotein (AFP); the conventional marker of HCC. It was found that serum VEGF and VEGF/PLT appear to be additional diagnostic markers for HCC detection and prognostic markers during HCC patients follow up. Also, combined measurement of serum VEGF, VEGF/PLT and AFP significantly increase the sensitivity, specificity and accuracy in detection of HCC among cirrhotic patients rather than using of AFP, VEGF, or VEGF/PLT separately.
- Citation: Alzamzamy A, Elsayed H, Abd Elraouf M, Eltoukhy H, Megahed T, Aboubakr A. Serum vascular endothelial growth factor as a tumor marker for hepatocellular carcinoma in hepatitis C virus-related cirrhotic patients. World J Gastrointest Oncol 2021; 13(6): 600-611
- URL: https://www.wjgnet.com/1948-5204/full/v13/i6/600.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i6.600
Liver cancer is the sixth most prevalent cancer worldwide with about 841000 new cases in 2018 according to the recent Global Cancer Observatory estimates[1]. Also, liver cancer is the fourth leading cause of cancer deaths worldwide accounting for 782000 deaths annually. Hepatocellular carcinoma (HCC) comprises about 75% to 85% of liver cancers[1].
Most of HCC patients are diagnosed at a late stage which makes HCC associated with low survival and poor prognosis[2]. The available screening methods for early detection of HCC in patients with liver cirrhosis are inadequate. The most established and well-studied HCC biomarker is serum alpha fetoprotein (AFP), and abdominal ultrasound performed every six months. However, this screening method has several limitations: (1) The limited sensitivity of AFP; AFP is not secreted in all cases of HCC and may be normal in about 40% of patients with early HCC; and (2) Abdominal ultrasound is highly dependent on the skills and experience of the examiner[3,4]. Recently, an unmet need to find novel tumor markers with high sensitivity and specificity for early diagnosis of HCC and to differentiate HCC from benign lesions has been demonstrated.
Most of the current HCC biomarkers have limited sensitivity or specificity which can be explained by the substantial heterogeneity of HCC. Therefore, it is suggested that the combination of two or three biomarkers with high specificity might provide early HCC optimal diagnosis.
Vascular endothelial growth factor (VEGF) is responsible for angiogenesis, and it appears to be the most important angiogenic factor in HCC. Hypoxia was suggested as the central stimulus of angiogenesis and liver carcinogenesis via upregulation of VEGF gene expression.
In HCC patients, a positive correlation between platelet count and serum VEGF was found[5]. Platelets store and transport VEGF to target cells; therefore, they have been reported to play a substantial role in tumor angiogenesis, progression, and prognosis. Serum VEGF/platelet (PLT) was used to overcome the variations in serum VEGF levels in HCC patients with different platelet counts. Therefore, VEGF/PLT has been proposed as an indirect theoretical estimate of VEGF in platelets.
Because HCC is characterized by high vascularity and the tumor relies on the formation of new blood vessels to grow and spread, angiogenic factors, such as VEGF play an important role in HCC pathogenesis[6]. VEGF acts on the VEGF receptor-2 (VEGFR2) and leads to induction of cancer stem cells and formation of microscopic blood vessels that facilitate tumor growth, invasion, and spread[7,8]. The role of VEGF in tumor pathogenesis, invasion, and spread has been described in many types of cancer, including HCC. Several studies report VEGF overexpression in HCC. Matsui et al[9] found higher levels of VEGF in patients with more advanced stages of the disease, larger tumor sizes, and more vascular invasion. As a prognostic factor, overexpression of VEGF was associated with an increase in vascular invasion and poor overall survival of HCC patients[10].
Cumulative evidence from the literature supports the idea that VEGF might be useful as a tumor marker for early HCC detection. Although several studies have been conducted to measure VEGF levels in HCC patients in different clinical settings, to the best of our knowledge few data are available about the HCC diagnostic performance of VEGF. Therefore, we conducted this observational study to assess the usefulness of serum VEGF and VEGF/PLT as tumor markers in patients with hepatitis C virus (HCV)-related liver cirrhosis and HCC and compare them to serum AFP, the conventional marker of HCC. Also, this study aimed to verify the possibility of using combined measurements of serum VEGF, VEGF/PLT, and AFP for HCC diagnosis.
We report this manuscript according to the Strengthening the Reporting of Observational Studies in Epidemiology statement guidelines[11]. The study was approved by the ethics committee of the Faculty of Medicine, Al Azhar University and Egypt center for research and regenerative medicine, Cairo, Egypt.
We performed a case-control study at the inpatient and outpatient clinics of the Gastroenterology and Hepatology Department, Maadi Armed Forces Medical Complex, Cairo, Egypt. The study was conducted on patients attending the study setting from January 2015 to June 2017.
Study subjects were selected according to several criteria: (1) Male and female patients aged between 18 and 60 years; and (2) Patients with chronic HCV infection confirmed by seropositive anti-HCV antibody detection using the third generation enzyme-linked immunosorbent assay (ELISA) and HCV-RNA seropositive histopathological criteria indicating chronic HCV liver disease.
We excluded patients with certain conditions: (1) Patients with any type of cancer other than HCC (such as breast, lung, brain, gastrointestinal, renal, bladder and ovarian); (2) Patients with collagen diseases (rheumatoid arthritis, psoriasis, and systemic sclerosis); (3) Patients with heart failure, chronic obstructive pulmonary disease, pulmonary hypertension and, acute respiratory distress syndrome; (4) Patients with diabetes mellitus, diabetic retinopathy, age-related macular degeneration; (5) Patients with sickle cell anaemia, pregnancy and preeclampsia; (6) Patients with co-infection with hepatitis B virus; and (7) Patients with alcoholic liver disease.
The study included three groups: (1) HCC group: This group included patients with HCC secondary to liver cirrhosis and chronic HCV, confirmed by HCV RNA polymerase chain reaction (PCR), abdominal ultrasound (US), triphasic spiral computed tomography (CT) of the abdomen, and/or dynamic abdominal magnetic resonance imaging (MRI); (2) Cirrhosis group: This group included patients with HCV-related liver cirrhosis without HCC. The diagnosis of cirrhosis was based on the clinical picture, US and laboratory findings suggestive liver cirrhosis; and (3) This group included patients with chronic HCV without cirrhosis or HCC.
The control group consisted of HCV patients who were free from cirrhosis and HCC. We used the medical records of the participants to confirm that they did not have cirrhosis or HCC.
Clinical assessment: All participants underwent a full clinical assessment, and medical histories were obtained. The clinical evaluation focused on the assessment of several factors: (1) Jaundice; (2) Ascites; (3) Palmar erythema; (4) Variceal bleeding; (5) Spider nevi; (6) Pallor; (7) Flapping tremors; and (8) Hepatic encephalopathy.
Laboratory assessment: For all participants, the following laboratory tests were done: (1) HCV viral markers: Anti-HCV antibody, HCV-RNA based on PCR, hepatitis B surface antigen, hepatitis B core antibody, and human immunodeficiency Ab complete blood picture; (2) Liver biochemical profile: Alanine aminotransferase, aspartate transaminase, alkaline phosphatase, gamma glutamyl transferase, serum albumin, serum bilirubin (total and direct), prothrombin time, and international normalized ratio; (3) Renal function tests: blood urea and serum creatinine; (4) Fasting blood glucose; (5) Postprandial blood glucose; (6) Glycosylated hemoglobin (normal < 6%); (7) Quantitative measurement of serum AFP; (8) Quantitative measurement of serum VEGF using ELISA kits; and (9) Quantitative measurement of serum VEGF/PLT by dividing serum VEGF concentration by the platelet count.
For imaging, all participants underwent abdominal US with emphasis on the signs suggestive of liver cirrhosis: (1) Any focal lesion (its number, site, and size); (2) Portal vein (patency and its diameter); (3) Splenic size; and (4) The presence of ascites (mild, moderate, severe).
Also, abdominal triphasic spiral CT with or without dynamic abdomen MRI was done to confirm the diagnosis of any suspected focal lesions suspected based on the US.
We measured the total serum VEGF with a human recombinant ELISA kit that is designed to measure human VEGF concentration in serum (Glory Science Co., Ltd, United States). We followed the established principals and the steps of VEGF kit assay according to the manufacturer`s instructions.
Categorical data were summarized as frequencies and percentages. While continuous data were initially tested for normality using the Kolmogorov-Smirnov test. Continuous data were presented as means and standard deviations. For comparison of categorical variables, we used chi-square and Fischer’s exact tests. For comparison of two means, the student's t- and Mann-Whitney tests were used for the normally and non-normally distributed data, respectively. To compare the quantitative variables between the three groups, we used either a one-way analysis of variance (ANOVA) followed by Tukey`s post-hoc or the Kruskal-Wallis test according to the type and distribution of the data. To study the relationship between two variables, Pearson’s correlation test was used, and the correlation coefficient (r) was calculated. To determine the optimal cut-off point for serum VEGF and VEGF/PLT ratio in which these measures achieved the highest sensitivity and specificity in diagnosing HCC, we constructed a receiver operating characteristic curve (ROC) which allowed us to plot the sensitivity against the 1-specificity at each point. An alpha level below 0.05 was considered statistically significant. All analyses were done using the Statistical Package for Social Science (SPSS software version 18 for Windows).
Our study included 100 participants (HCC group/group I: n = 40, cirrhosis group/group II: n = 30, and control group/group III: n = 30). Of them, 67 patients were males, and 33 were females. The demographic characteristics of the study groups are shown in Table 1. There was no statistically significant difference between the three studied groups as regard age and gender (Table 1). Moreover, there was no significant correlation between VEGF, AFP, and VEGF/PLT values and age and sex of the patients. Also, there was no significant correlation between tumor characteristics by Barcelona Clinic Liver Cancer (BCLC) and age and sex of the patients.
| HCC group | Cirrhosis group | Control group | P value | ||
| Gender | Male | 30 | 17 | 20 | > 0.05 |
| Female | 10 | 13 | 10 | ||
| Age (years) | mean (SD) | 60.07 ± 5.34 | 58.33 ± 8.07 | 55.2 ± 7.6 | > 0.05 |
| Range | 50-68 | 37-67 | 34-62 | ||
| Child-Pugh score, n (%) | A | 24 (60.0) | 19 (63.3) | Not applicable | |
| B | 11 (27.5) | 8 (26.7) | Not applicable | ||
| C | 5 (12.5) | 3 (10.0) | Not applicable |
The Child-Pugh classification for group I (HCC group) was A for 24 patients, B for 11 patients, and C for five patients while the Child-Pugh classification in group II (cirrhosis group) was A for 19 patients, B for eight patients, and C for three patients (Table 1).
In HCC group, 20 patients were classified as stage I, five patients as stage II, three patients as stage IIIA, 11 patients as stage IIIB, and one patient as stage IVB with respect to tumor/node/metastasis (TNM). While six patients were classified as stage 0 disease, 13 patients as stage A, eight patients as stage B, 11 patients as stage C, and two patients as stage D with respect to the BCLC tumor stage. Also, 12 patients with vascular invasion were noted, and only one patient had distal metastasis.
HCC group had significantly higher AFP levels than the two non-HCC groups (cirrhosis group and control group). Moreover, the VEGF levels were significantly higher in the HCC group when compared with the cirrhosis group (1409 pg/mL vs 233.3 pg/mL) and when compared with the control group (1409 pg/mL vs 204 pg/mL). The ratio of VEGF/PLT was 13.2 in the HCC group, which was much higher than the 1.68 reported in the cirrhosis group, and the 0.95 reported in the control group. The biomarker levels in the three study groups are shown in Table 2.
Statistically highly significant differences in the levels of the studied markers (AFP, VEGF, and VEGF/PLT) among the HCC, the cirrhotic, and the control groups were found.
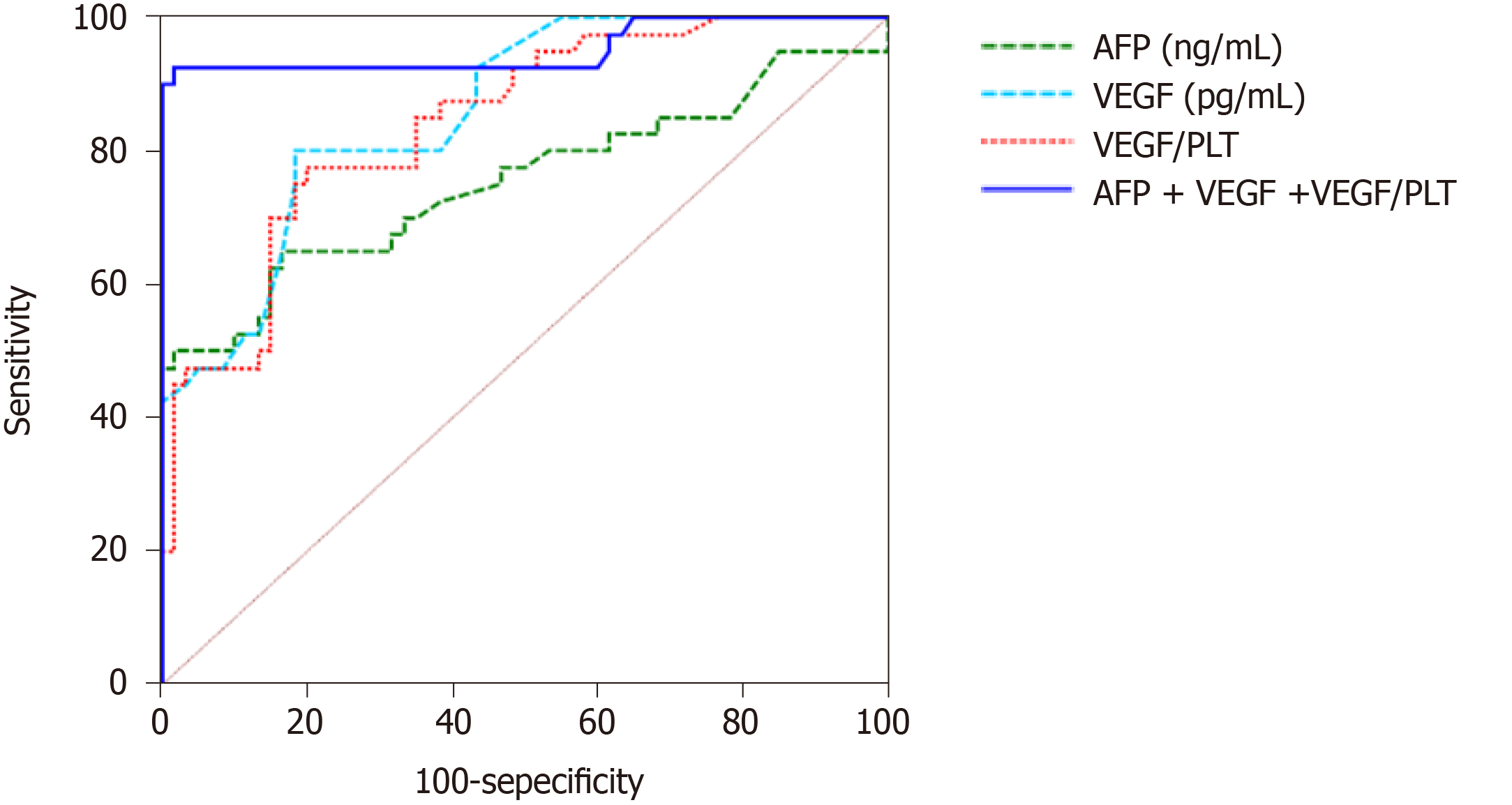
At a cut-off value of 40 ng/mL, serum AFP provided an accuracy of 74.1% when diagnosing HCC (a sensitivity of 65% and specificity of 83.3%). At a cut-off value of 250 pg/mL, serum VEGF provided 80% and 81.7% sensitivity and specificity, respectively, for diagnosing HCC. At a cut-off value of 2.31, serum VEGF/PLT provided a sensitivity and specificity of 77.5% and 80%, respectively. When the three parameters were combined, the highest accuracy was achieved with a sensitivity of 92.5% and a specificity of 98.3%. The ROC curve of the three HCC diagnostic markers and their combinations is shown in Figure 1. The diagnostic performance of the three parameters is shown in Table 3.
| Cut-off point | AUC | Sensitivity | Specificity | Accuracy | PPV | NPV | P value | |
| AFP | 40 | 0.754 | 65.00 | 83.33 | 74.17% | 72.2 | 78.1 | < 0.0001 |
| VEGF | 250 | 0.859 | 80.00 | 81.67 | 80.84 | 74.4 | 86.0 | < 0.0001 |
| VEGF/PLT | 2.31 | 0.842 | 77.50 | 80.00 | 78.75 | 72.1 | 84.2 | < 0.0001 |
| Combination | -- | 0.953 | 92.50 | 98.33 | 95.42 | 97.4 | 95.2 | < 0.0001 |
VEGF showed the highest sensitivity among the serum tumor markers. Also, ROC curves for the three studied tumor markers indicated that the specificity of VEGF was very high and other serum tumor markers were similar.
The area under the ROC curve (AUC) for serum VEGF was 0.859, while the AUC values for serum AFP and VEGF/PLT were 0.754 and 0.842, respectively. In addition, the accuracy of VEGF was 80.84%, while that of AFP and VEGF/PLT was 74.17% and 78.75%, respectively.
For diagnosis of HCC, serum VEGF showed higher sensitivity than the other tumor markers and it had the largest AUC on ROC analysis in addition to the highest accuracy. These results indicate that the serum VEGF level was more useful for the diagnosis of HCC than the other two tumor markers (serum AFP and VEGF/PLT) in patients with HCV-related liver cirrhosis
In this study, a significant increase in the AUC, sensitivity, specificity, accuracy, and positive and negative predictive values for detection of HCC in cirrhotic patients was detected, when we use combined measurements of serum VEGF, VEGF/PLT, and AFP rather than using AFP, VEGF, or VEGF/PLT separately (Table 3).
AFP was significantly correlated with the size of the tumor and the BCLC stage of the tumor but not with the TNM staging. Both VEGF and VEGF/PLT showed significantly positive correlations with the size of the tumor, TNM staging, and BLCL tumor staging (Table 4).
| AFP (ng/mL) | VEGF (pg/mL) | VEGF/PLT | ||
| Size of tumor | Correlation coefficient | 0.492 | 0.662 | 0.483 |
| P value | 0.001 | 0.0001 | 0.002 | |
| TNM stage of tumor | Correlation coefficient | 0.247 | 0.795 | 0.696 |
| P value | 0.124 | 0.0001 | 0.0001 | |
| BCLC stage of tumor | Correlation coefficient | 0.382 | 0.889 | 0.740 |
| P value | 0.015 | 0.0001 | 0.0001 | |
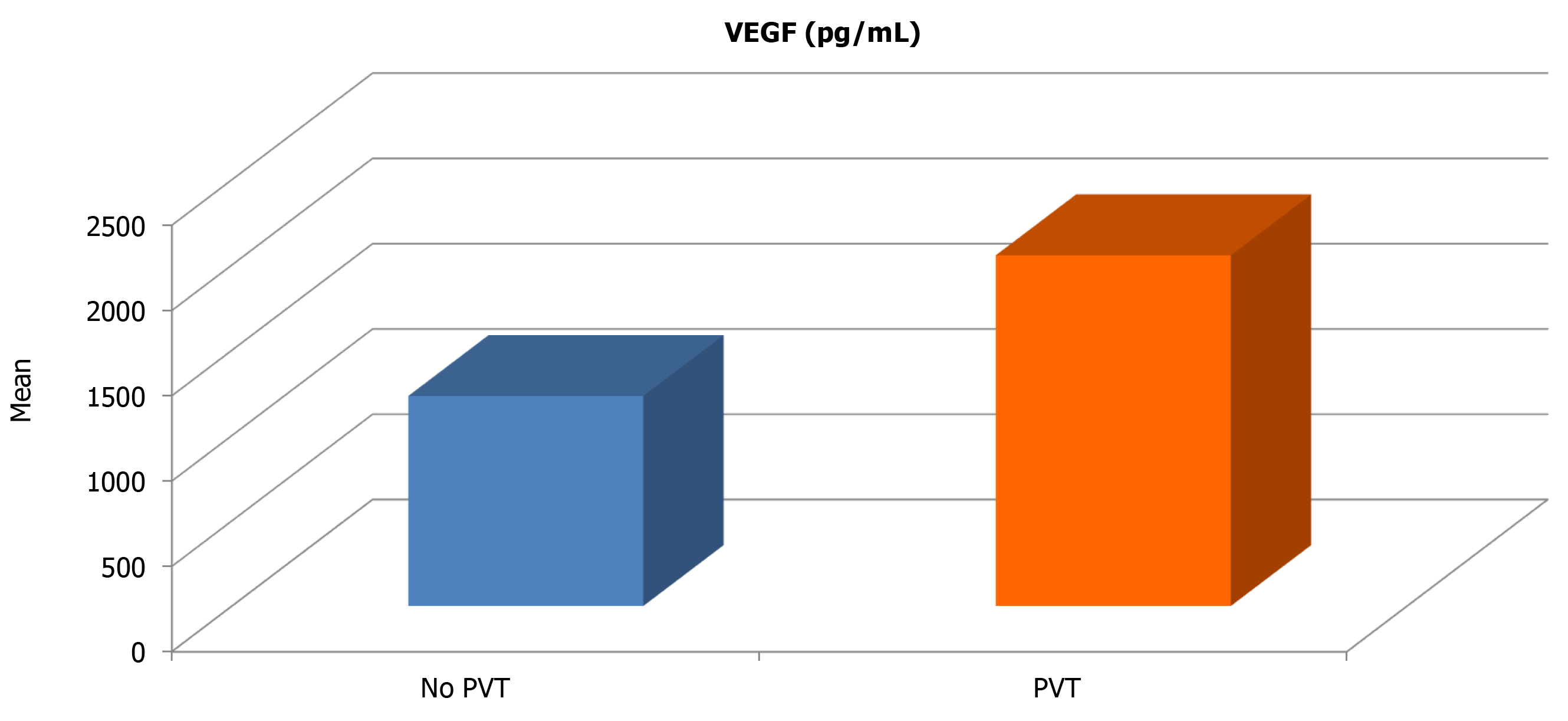
HCC cases with portal vein thrombosis had significantly higher VEGF (2056.3 vs 1231.6; P = 0.021) and VEGF/PLT levels (20.85 vs 11.35; P = 0.019) compared with those with patent portal vein (Table 5). Thus, statistically significant positive correlations between serum VEGF and serum VEGF/PLT levels; and the presence of portal vein thrombosis among HCC cases were found. On the other hand, no significant correlation between serum AFP and PVT was found.
| No PVT | PVT | Independent t-test | ||||
| Mean | SD | Mean | SD | t-value | P value | |
| AFP (ng/mL) | 580.64 | 854.69 | 1143.63 | 923.42 | −1.641 | 0.109 |
| VEGF (pg/mL) | 1231.56 | 907.97 | 2056.25 | 661.94 | −2.404 | 0.021 |
| VEGF/PLT | 11.35 | 9.98 | 20.85 | 9.12 | −2.446 | 0.019 |
In the patients with HCC, a statistically significant correlation between serum VEGF level and platelet count (r = 0.668; P = 0.02) was noted (Table 6 and Figure 2). However, no correlation between serum VEGF levels and platelet count in the patients with liver cirrhosis and the control group (P = 0.970; P = 0.781, respectively) was found (Table 6).
No correlation between serum AFP and VEGF levels among HCC cases was detected (Table 7). Also, no correlation between serum AFP and VEGF/PLT levels among HCC cases was found (Table 8). A statistically significant correlation between serum VEGF and VEGF/PLT levels among HCC cases was shown (Table 9).
| AFP (ng/mL) | ||
| r | P value | |
| VEGF (pg/mL) | 0.119 | 0.4631 |
The main aim of this work was to assess the usefulness of serum VEGF and VEGF/PLT as tumor markers in patients with HCV-related liver cirrhosis and HCC and to compare them with the control group (patients with chronic HCV without cirrhosis or HCC) in order to detect their sensitivity and specificity as diagnostic markers for HCC. These two markers were also compared to AFP, the conventional marker of HCC, and were then correlated with tumor size, stage, vascular invasion, and Child-Pugh classification. Also, this study aimed to verify the possibility of using combined measurement of serum VEGF, VEGF/PLT, and AFP for diagnosing HCC early and accurately.
Our findings showed that HCC patients had significantly higher serum VEGF and VEGF/PLT levels compared with non-HCC patients. Also, serum VEGF and VEGF/PLT levels were positively correlated with the tumor size, stage, vascular invasion and Child-Pugh classification. Serum VEGF and VEGF/PLT achieved higher accuracy (higher sensitivity and specificity) than AFP for diagnosing HCC. Also, the combined measurement of serum VEGF, VEGF/PLT and AFP significantly increased the sensitivity, specificity, accuracy, AUC, and positive and negative predictive values in detection of HCC among cirrhotic patients.
Results of this study can be explained by the essential role of VEGF in HCC pathogenesis and spread. Therefore, high serum VEGF levels in HCC patients were associated with larger tumor size and more advanced disease stages. Moreover, a VEGF cut-off the value of 250 pg/mL provided 80% sensitivity and 81.7% specificity for discriminating HCC patients from non-HCC patients. Similarly, the ratio of VEGF/PLT provided sensitivity and specificity of 77.5% and 80%, respectively, which is higher than the accuracy provided by AFP.
It is suggested that hypoxia stimulates angiogenesis and liver carcinogenesis via upregulation of VEGF gene expression. While the production of AFP occurs due to the de-differentiation of cancer cells, which does not usually occur in early stages since HCC often starts as a well-differentiated tumor and then undergoes de-differentiation as the tumor grows.
A positive correlation between serum VEGF levels and tumor VEGF expression as assessed by immunohistochemical study was reported and suggests that serum VEGF levels at least in part reflects the tumor VEGF expression.
In HCC cases, serum VEGF correlates with the platelet counts. VEGF is produced by tumor cells and is stored and transported by platelets[8,12]. The reservoir of VEGF in platelets might be indicative of HCC angiogenesis and invasion. Serum VEGF/PLT was shown to correlate with HCC, suggesting a role for VEGF/PLT as a standard measure of circulating VEGF[10].
A large body of evidence suggests that VEGF might be used as a tumor marker for HCC. First, HCC is a vascular tumor that depends on angiogenesis for tumor growth and survival. Second, high VEGF levels were associated with poor clinical characteristics of the tumor and poor prognosis; it was reported that HCC patients treated with sorafenib who have higher levels of VEGF had significantly poorer overall survival and less response to the treatment compared to those with low VEGF levels, suggesting that VEGF might be a useful tool for predicting patient response to sorafenib treatment[13]. Third, anti-VEGF agents, such as sorafenib, are effective HCC treatments that could improve patient survival, A recent meta-analysis of seven randomized controlled trials showed that tyrosine kinase inhibitors targeting VEGF were effective for the treatment of unresectable metastatic HCC and that anti-VEGF could extend the overall survival and time till disease progression in HCC patient[14]. Fourth, siRNA silencing of VEGF through hepatic artery perfusion could lead to suppression of HCC proliferation, induction of tumor cell apoptosis, and reduction in tumor angiogenesis[15].
It is evident that VEGF plays two roles in HCC pathogenesis: (1) Formation of blood vessels that lead to tumor growth and spread; and (2) Activation of VEGFR2 induces HCC cancer stem cells that lead to tumor recurrence[16], which makes it a reliable biomarker that is positively correlated with the clinical HCC stage, and is useful for predicting patient response to anti-VEGF treatment predicting tumor recurrence after HCC treatment.
Several previous studies have evaluated the accuracy of VEGF for diagnosing HCC.
According to our results, at a cut-off 250 pg/mL, the sensitivity was 80%, specificity was 81.67%, accuracy was 80.84%, the AUC was 0.859, positive predictive value was 74.4%, and negative predictive value was 86.0%. The present results were comparable to those of Mukozu et al[17] who reported a sensitivity of 86.4%, and specificity of 96.2% when the cut-off value was 108 pg/mL; in that study, accuracy was 89.4%, and the AUC was 0.988[17]. Also, our results were comparable to those of Sabry et al[18] who found that the sensitivity of VEGF was 88% and the specificity was 60% at a cut-off value of 228 pg/mL, accuracy was 82%, positive predictive value 90%, and negative predictive value 55%.
Another study of VEGF in HCC patients showed that VEGF had 90% sensitivity and specificity at a cut-off value of 271.85 pg/mL with an AUC of 0.972[19]. When combining serum VEGF with AFP, the accuracy increased to 100% and 98.7% for the sensitivity and specificity, respectively, which is in agreement with our findings[19]. In another study, the optimal AFP cut-off value of 15 ng/mL provided a sensitivity and specificity of 76% and 62%, respectively, while the serum VEGF cut-off value of 108 pg/mL achieved a sensitivity and specificity of 98% and 46%, respectively. They concluded that among the many studied serum biomarkers, VEGF had the highest sensitivity in diagnosing HCC[18]. Yvamoto et al[20] found that VEGF levels had a sensitivity and specificity of 65% and 85%, respectively, while AFP had sensitivity and specificity of 28% and 99% for diagnosing HCC, respectively.
Our study expands the literature by providing information about the accuracy of serum VEGF and VEGF/PLT in diagnosing HCC. Both serum VEGF and VEGF/PLT achieved better diagnostic performance than the traditional AFP. Moreover, when the three parameters were combined, the highest accuracy (> 95%) was achieved with a sensitivity and specificity of 92.5%, 98.3%, respectively.
The strength points of our study include three main point: (1) Validation of VEGF diagnostic accuracy in the HCV population, rather than in the general population, which makes these results applicable in the clinical setting; (2) The study was powered enough to demonstrate the accuracy of serum VEGF and VEGF/PLT in diagnosing HCC; and (3) Inclusion of a third group of cirrhotic patients to validate the specificity of VEGF and exclude cirrhotic HCV patients without HCC.
We recommend greater number of patients to gain greater insight into potential usefulness of serum VEGF and VEGF/PLT in patients with HCC. Also, we recommend more studies which are much more precise: (1) Serum or plasma VEGF; and (2) Serum VEGF or serum VEGF/PLT. Follow up of patients over several years is recommended to detect the association between serum VEGF levels and response to treatment in comparison to serum AFP levels. Future studies are recommended to explore the relationship between serum VEGF levels and the presence of HCC with or without portal vein invasion before and after treatment.
Serum VEGF and VEGF/PLT appear to be additional diagnostic markers for HCC detection and prognostic markers during the follow-up of HCC patients since these markers showed significant correlations with tumor size, and stage, and the presence of vascular invasion among HCC cases.
Combined measurements of serum VEGF, VEGF/PLT, and AFP significantly increase the sensitivity, specificity, accuracy, AUC, and positive and negative predictive values for detecting HCC among cirrhotic patients rather than using AFP, VEGF, or VEGF/PLT separately.
Hepatocellular carcinoma (HCC) is diagnosed at a late stage. Therefore, the prognosis of patients with HCC is generally poor. The recommended screening strategy for patients with liver cirrhosis includes the determination of serum alpha fetoprotein (AFP) levels and an abdominal ultrasound every 6 mo to detect HCC at an earlier stage. AFP, however, is a marker characterized by poor sensitivity and specificity, and abdominal ultrasound is highly dependent on the operator's experience. Vascular endothelial growth factor (VEGF) is a primary driving force for both physiological and pathological angiogenesis, and its overexpression is observed in HCC. VEGF is one of the most important angiogenic factors and it promotes angiogenesis in most human tumors. One of the notable features of most HCCs is hypervascularity and it has been reported that VEGF expression is correlated with tumor vascularity. The circulating VEGF level was reported to be correlated with the stage of HCC and the highest VEGF levels are found in patients with metastasis
Try to determine the accuracy of serum VEGF and VEGF/platelet (PLT) as tumor markers in the early detection of HCC cases in patients with hepatitis C virus (HCV)-related liver cirrhosis.
The present study provides important data for the early diagnosis of HCC, enabling an increase in the number of cases treated worldwide. The main aim of this work was to assess the usefulness of serum VEGF and VEGF/PLT as tumor markers in patients with HCV-related liver cirrhosis and HCC and to compare them with the control group (patients with chronic HCV without cirrhosis or HCC) in order to detect their sensitivity and specificity as diagnostic markers for HCC. These two markers were also compared to AFP, the conventional marker of HCC, and were then correlated with tumor size, stage, vascular invasion, and Child-Pugh classification. Also, this study aimed to verify the possibility of using combined measurement of serum VEGF, VEGF/PLT, and AFP for diagnosing HCC early and accurately.
We conducted a case-control study with HCV patients from the outpatient and inpatient hepatology clinics. Patients were classified into three groups: (1) HCC group; (2) Cirrhosis group; and (3) HCV without cirrhosis (control group). Patients were clinically evaluated, and blood samples were drawn for the analysis; serum VEGF levels were measured by a specific VEGF human recombinant enzyme-linked immunosorbent assay kit. Data from the three study groups were compared by the one-way analysis of variance or Kruskal-Wallis test. Receivers operating characteristic curves (ROC) were constructed to determine the optimal cut-off values of AFP, VEGF, and VEGF/PLT that provided the best diagnostic accuracy. The sensitivity and specificity at the optimal cut-off value of each biomarker were then calculated.
This study included one hundred patients (HCC, cirrhosis, and control groups: n = 40, 30, 30, respectively). HCC patients had significantly higher serum VEGF and VEGF/PLT levels than the non-HCC groups (P = 0.001). Serum VEGF and VEGF/PLT showed significant positive correlations with and HCC tumor size, stage, vascular invasion, and Child Pugh classification. Moreover, a VEGF cut-off the value of 250 pg/mL provided 80% sensitivity and 81.7% specificity for discriminating HCC patient from non-HCC patients. Similarly, the ratio of VEGF/PLT provided sensitivity and specificity of 77.5% and 80%, respectively which is higher than the accuracy provided by AFP. The combination of AFP, VEGF, and VEGF/PLT increases the accuracy of diagnosing HCC to > 95%.
Serum VEGF and VEGF/PLT appear to be additional diagnostic markers for HCC detection and prognostic markers during the follow-up of HCC patients since these markers showed significant correlations with tumor size, and stage, and the presence of vascular invasion among HCC cases. Combined measurements of serum VEGF, VEGF/PLT, and AFP significantly increase the sensitivity, specificity, accuracy, area under the ROC curve, and positive and negative predictive values for detecting HCC among cirrhotic patients rather than using AFP, VEGF, or VEGF/PLT separately.
Most of the current biomarkers for early and accurate diagnosis of HCC have limited sensitivity or specificity, so patients with HCC are diagnosed at a late stage and have low survival and poor prognosis. Therefore, a combination of two or three biomarkers with high specificity might provide the optimal diagnosis of early HCC is suggested. In this study, we conducted an observational study with 100 patients to assess the usefulness of serum VEGF and VEGF/PLT as tumor markers for early and accurate diagnosis of HCC in patients with HCV-related liver cirrhosis, and comparing them to serum AFP, the conventional marker of HCC. Also, this study aimed to verify the possibility of using combined measurement of serum VEGF, VEGF/PLT, and AFP for HCC diagnosis.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society for Gastrointestinal Endoscopy; and United European Gastroenterology.
Specialty type: Oncology
Country/Territory of origin: Egypt
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fernandes SA S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55833] [Article Influence: 7976.1] [Reference Citation Analysis (132)] |
| 2. | Golabi P, Fazel S, Otgonsuren M, Sayiner M, Locklear CT, Younossi ZM. Mortality assessment of patients with hepatocellular carcinoma according to underlying disease and treatment modalities. Medicine (Baltimore). 2017;96:e5904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 191] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 3. | El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Frenette C. Surveillance for Hepatocellular Carcinoma. Gastroenterol Hepatol (NY). 2016;12:394-396. |
| 5. | George ML, Eccles SA, Tutton MG, Abulafi AM, Swift RI. Correlation of plasma and serum vascular endothelial growth factor levels with platelet count in colorectal cancer: clinical evidence of platelet scavenging? Clin Cancer Res. 2000;6:3147-3152. [PubMed] |
| 6. | Moon WS, Rhyu KH, Kang MJ, Lee DG, Yu HC, Yeum JH, Koh GY, Tarnawski AS. Overexpression of VEGF and angiopoietin 2: a key to high vascularity of hepatocellular carcinoma? Mod Pathol. 2003;16:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A. A vascular niche and a VEGF-Nrp1 Loop regulate the initiation and stemness of skin tumors. Nature. 2011;478:399-403. [RCA] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 364] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 8. | Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer. 2013;13:871-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 963] [Article Influence: 80.3] [Reference Citation Analysis (0)] |
| 9. | Matsui D, Nagai H, Mukozu T, Ogino YU, Sumino Y. VEGF in patients with advanced hepatocellular carcinoma receiving intra-arterial chemotherapy. Anticancer Res. 2015;35:2205-2210. [PubMed] |
| 10. | Choi SB, Han HJ, Kim WB, Song TJ, Choi SY. VEGF Overexpression Predicts Poor Survival in Hepatocellular Carcinoma. Open Med (Wars). 2017;12:430-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5754] [Cited by in RCA: 9950] [Article Influence: 585.3] [Reference Citation Analysis (0)] |
| 12. | Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3536] [Cited by in RCA: 3555] [Article Influence: 98.8] [Reference Citation Analysis (0)] |
| 13. | Cao G, Li X, Qin C, Li J. Prognostic Value of VEGF in Hepatocellular Carcinoma Patients Treated with Sorafenib: A Meta-Analysis. Med Sci Monit. 2015;21:3144-3151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | Chintalacheruvu LM, Buddam A, Kanmanthareddy A, Ganti AK. Efficacy and safety of anti-VEGF therapy in metastatic unresectable hepatocellular carcinoma: A meta-analysis. J Clin Oncol. 2017;35:e15632-e15632. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 15. | Zou Y, Guo CG, Zhang MM. Inhibition of human hepatocellular carcinoma tumor angiogenesis by siRNA silencing of VEGF via hepatic artery perfusion. Eur Rev Med Pharmacol Sci. 2015;19:4751-4761. [PubMed] |
| 16. | Liu K, Hao M, Ouyang Y, Zheng J, Chen D. CD133+ cancer stem cells promoted by VEGF accelerate the recurrence of hepatocellular carcinoma. Sci Rep. 2017;7:41499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 17. | Mukozu T, Nagai H, Matsui D, Kanekawa T, Sumino Y. Serum VEGF as a tumor marker in patients with HCV-related liver cirrhosis and hepatocellular carcinoma. Anticancer Res. 2013;33:1013-1021. [PubMed] |
| 18. | Sabry HS, Nouh MA, Yoffe B, El-Sebaai HM, Mohamed HI, Mohamed SA. Study of the Diagostic Role of Vascular Endothelial Growth Factor in Hepatocellular Carcioma. J Am Sci. 2012;8:273-279. |
| 19. | Atta MM, Atta HM, Gad MA, Rashed LA, Said EM, Hassanien Sel-S, Kaseb AO. Clinical significance of vascular endothelial growth factor in hepatitis C related hepatocellular carcinoma in Egyptian patients. J Hepatocell Carcinoma. 2016;3:19-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Yvamoto EY, Ferreira RF, Nogueira V, Pinhe MA, Tenani GD, Andrade JG, Baitello ME, Gregório ML, Fucuta PS, Silva RF, Souza DR, Silva RC. Influence of vascular endothelial growth factor and alpha-fetoprotein on hepatocellular carcinoma. Genet Mol Res. 2015;14:17453-17462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |