Published online Jun 15, 2021. doi: 10.4251/wjgo.v13.i6.589
Peer-review started: November 3, 2020
First decision: January 29, 2021
Revised: March 24, 2021
Accepted: May 7, 2021
Article in press: May 7, 2021
Published online: June 15, 2021
Processing time: 216 Days and 3.2 Hours
Solid pseudopapillary neoplasms (SPN) of the pancreas represents approximately 2% of non-endocrine tumors of the pancreas. It is described in the literature as a rare and predominant tumor in young women.
To report a case series with SPN and analyzing clinical, surgical, anatomopathological characteristics, as well as the prognosis and review of literature.
Retrospective analysis of patients undergoing surgery, with histological diagnosis of SPN between 1998 and 2018, using standardized and prospectively completed forms, performed at the Surgery Service of the Upper Digestive System at Hospital São Rafael/Rede D’Or in Salvador - BA. Review of literature through a database search in MEDLINE/PubMed of retrospective articles.
Fourteen female patients with the average age of 31.6 years (range min-max) were selected. Twelve patients (85.7%) were asymptomatic, being an incidental diagnosis or due to screening for other reasons. One patient had abdominal pain due to gastric compression and another patient had jaundice. The 14 patients were staged with computerized tomography or magnetic resonance imaging. None had evidence of metastasis. In 8 patients (57.1%), the tumor was in the tail and body. The average size was 6.7 cm (range min-18). The type of surgery was according to the anatomical location of the tumor. There was no lymph node involvement. In two cases, vascular resection with the use of a prosthesis was required for reconstruction. The surgical margins were free. In all cases, postoperative immunohistochemistry confirmed that it was a solid pseudo-papillary neoplasia of the pancreas. There has been no disease recurrence in any case so far.
The tumors had a benign, indolent and histopathological behavior compatible with the literature. Curative surgery is recommended in all cases.
Core Tip: Surgery is the only curative treatment for solid papillary neoplasm of the pancreas. Even in cases of large tumors, wherein extensive resections of both the main tumor and the metastases are an absolute requirement, surgery can be curative and allow a long, disease-free survival. Some of the patients in the service studied underwent tumor resection more than 13 years ago, without relapse of the disease and maintaining a good quality of life. This type of neoplasm is considered rare, but in the present study most cases were discovered incidentally through imaging examinations, with the number of cases increasing since 2012.
- Citation: Silano F, de Melo Amaral RB, Santana RC, Neves VC, Ardengh JC, do Amaral PCG. Yield of surgery in solid pseudopapillary neoplasms of the pancreas: A case series and literature review. World J Gastrointest Oncol 2021; 13(6): 589-599
- URL: https://www.wjgnet.com/1948-5204/full/v13/i6/589.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i6.589
Solid pseudopapillary neoplasm (SPN) is a rare tumor of the pancreas, which was first described in 1959[1,2]. It represents approximately 1 to 2% of pancreatic tumors, but a higher incidence has been reported in recent years[3,4]. It occurs in young women, its natural history is unknown, and it exhibits an indolent behavior. However, SPN is potentially aggressive, particularly when large masses are present, and it may cause symptoms due to the invasion of nearby organs and vascular structures; it may even send distant metastases[3,5,6]. Surgery is the only curative treatment for SPN. Even for large tumors, for which extensive resection is imperative, including metastases, surgical treatment may be curative and allow long-term disease-free survival[5,7-9].
The authors present herein a case series from a single center with extensive experience in pancreatic surgery and assess the postoperative results in a long-term follow-up of SPN patients with curative intent.
This study was approved by the Human Research Ethics Committee. The database from Surgery Service of the Upper Digestive System at the Hospital São Rafael/Rede D’Or, in Salvador- State of Bahia, Brazil, was retrospectively studied but with prospective data collection in a subsequent treatment by surgery. The authors identified all patients undergoing pancreatic resection for SPN between 1998 and 2018.
The clinical characteristics, perioperative, anatomopathological data, and long-term follow-up were analyzed. The occurrence of adverse events (AE) and the treatment was analyzed. The review of the literature, composed of retrospective articles, was conducted through a database search in MEDLINE/PubMed.
The Karnofsky Performance Status Scale was used in the initial evaluation of the patients, correlating the disease symptoms with physical impairment and self-care, on a scale of 10%-100%. A score ≥ 80% indicates an ability to perform daily activities without special care, despite the presence of symptoms of the disease. All surgeries were performed by surgeons experienced in pancreatic surgery. Pylorus-preserving pancreaticoduodenectomy (PPD) was the surgical technique of choice for tumors of the head of the pancreas, whereas distal pancreatectomy (DP) was performed for tumors of the body and tail of the pancreas. All patients who underwent DP were vaccinated against encapsulated bacteria 15 d before the procedure, including those whose spleen was preserved.
The Enhanced Recovery After Surgery protocol was used, except in DPs that were systematically drained, because reliable predictors for pancreatic fistula are lacking in the literature. The drain was removed on the third day after surgery if amylase was ≤ 5000 U/L in the drained fluid[10]. The amount of pancreatic tissue removed was determined in the DPs and related to the appearance of diabetes mellitus (DM). The mean size of the surgical specimens collected in all the DPs performed in center (8 cm) was used. The occurrence of fistulas was classified according to the criteria of the International Study Group on Pancreatic Fistula[11]. A fistula was defined as an amylase level in the drainage fluid that is more than 3 times the upper normal limit of serum amylase persisting beyond 3 wk.
The Clavien–Dindo classification was used to classify AEs, whether related to the surgery site or not, with the aim of categorizing them into minor events, which required the use of analgesics and prokinetics (Clavien–Dindo I) or blood transfusion associated with parenteral nutrition (Clavien–Dindo II), and major events, which required intervention ranging from endoscopy to surgery (Clavien–Dindo III)[12]. Surgery-related mortality was considered up to 90 d after the procedure[13].
The microscopic criterion for malignancy, as defined by the World Health Organization (WHO), was the invasion of perineural, angiolymphatic, capsular, or peripancreatic adipose tissues[14].
The patients were followed up on an outpatient basis for at least 5 years, except one case, which was followed for only one year. The following parameters were analyzed: tumor relapse, appearance of DM, and pre- and postoperative quality of life as perceived by the patient (Karnofsky score).
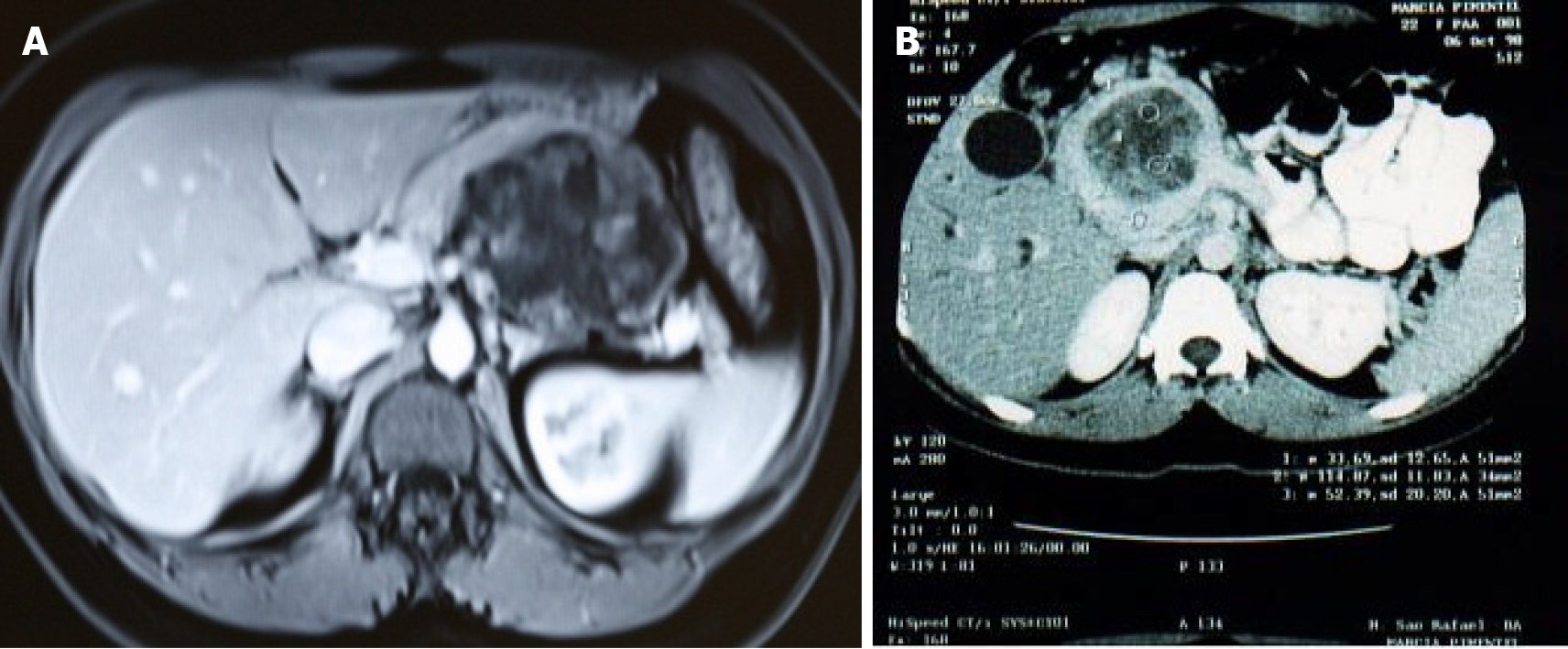
Fourteen patients with SPN submitted to curative surgery over a 20-year period were identified, all of them female. The mean age was 31.5 years (range: 20-55); 11 patients (78.6%) were aged 35 years or younger. Twelve patients (85.7%) were asymptomatic. The diagnosis was made through abdominal ultrasound (US), which had been requested due to other causes. One patient (7.1%) had abdominal pain along with nausea and vomiting due to an 11 cm tumor that affected the body and head of the pancreas and caused gastric compression (Figure 1A). Another patient presented with jaundice and weight loss due to a 9 cm mass in the head of the pancreas (Figure 1B). All cases had a Karnofsky score ≥ 80%.
All patients were staged using either computed tomography (CT) or abdominal magnetic resonance imaging (MRI). None had any evidence of liver, peritoneum, or lymph node metastases. Endosonography was performed in one of the patients (7.1%) and SPN was confirmed. The mean size of the tumors identified by the preoperative imaging examinations was 6.72 cm (range: 1.5-18 cm). The most common locations were the body and tail of the pancreas (Table 1).
| Demographic data (n = 14) | n (%) |
| Gender | |
| Female/male | 14 (100)/0 (0) |
| Mean age (yr); (range) | 31.6 (20-55) |
| 35 yr | 11 (78.6) |
| Symptoms | |
| Asymptomatic | 12 (85.7) |
| Abdominal pain and vomiting | 1 (7.1) |
| Jaundice/weight loss | 1 (7.1) |
| Radiological characteristics | |
| Largest injury (cm) | 18 |
| Minor injury (cm) | 1.5 |
| Average lesion size (cm); (range) | 6.7 (1.5-18) |
| Tumor location | |
| Head | 6 (42.8) |
| Body/tail | 8 (57.1) |
| Metastasis | |
| Yes | 0 |
| No | 14 (100) |
| Lymph node enlargement | 0 |
| Vascular invasion | 2 (14.3) |
Nine patients (64.3%) underwent open surgery and 5 (35.7%) had laparoscopic surgery. Splenectomy was performed in 7 patients (50%), and one patient (7.1%) required a transverse colon resection and a partial gastrectomy due to SPN invasion of these organs. Because of the tumor location, DP was the most common procedure (8 patients, 57%), while PPD was performed in 6 patients (43%). The mean intraoperative time was 346.6 minutes for PPD and 228.12 minutes for DP (Table 2).
| Intraoperative characteristics (n = 14) | n (%) |
| Access way | |
| Laproscopic | 5 (38.4) |
| Open Laparotomy | 9 (64.2) |
| Type of surgery | |
| Duodenopancreatectomy | 6 (42.8) |
| Distal pancreatectomy | 8 (57.1) |
| Vascular resection | |
| Yes | 2 (14.2) |
| No | 12 (85.7) |
| Resection of other organs | |
| Yes | 8 (57.1) |
| Spleen | (7) |
| Transverse colon | (1) |
| Stomach | (1) |
| No | 6 (42.8) |
| Blood transfusion | |
| Yes | 2 (14.2) |
| No | 12 (85.7) |
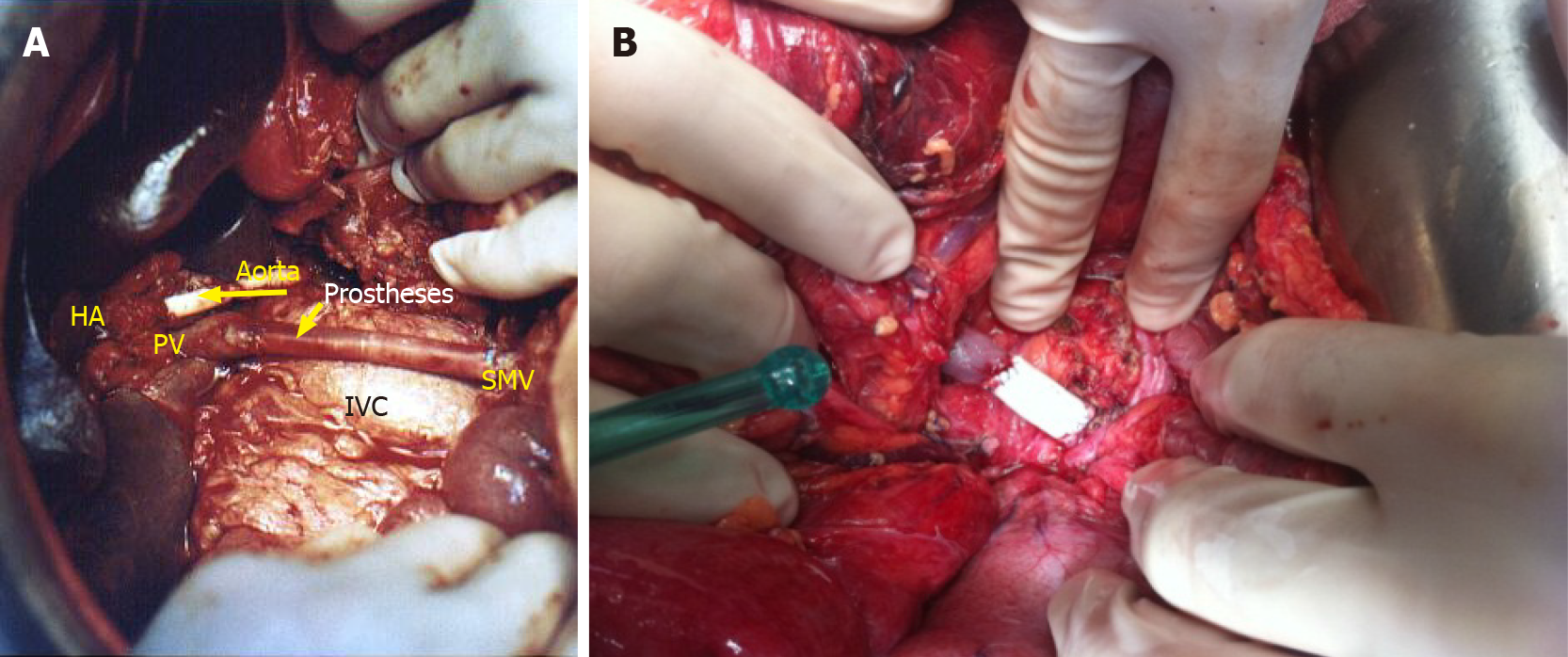
One PPD procedure (7.1%) required intraoperative vascular resection of the hepatic artery and superior mesenteric vein (Figure 2A), with the surgery lasting 17 h and the need for blood transfusion. The patient developed ascites and liver failure, caused by massive hypoperfusion of the liver due to the long period of ischemia. This patient’s treatment was conservative (Clavien–Dindo I). Another segmental resection of the superior mesenteric vein (Figure 2B) was necessary during a DP, with the surgery lasting 9 h and the need for blood transfusion. The patient had a satisfactory evolution.
Two patients (14.3%) submitted to PPD had AEs classified as Clavien–Dindo grade II (urinary and surgical wound infections, both treated with antibiotic therapy). Two patients (14.3%) who underwent DP developed fistulas, one had a gradeB fistula (Clavien–Dindo II) and the other had a gradeC fistula (Clavien–Dindo III) (Table 3).
| Postoperative characteristics (n = 14) | n (%) | Clavien-Dindo |
| Abdominal drain | 14 (100) | - |
| Surgical site (adverse events) | 4 (28.5) | - |
| Surgical wound infection (PD) | 1 (7.1) | II (Antibiotic) |
| Transient liver failure/ascites (PD) | 1 (7.1) | I |
| Grade C pancreatic fistula (CCP); Grade B pancreatic fistula (CCP) | 1 (7.1); 1 (7.1) | III (EL); II (abdominal drain) |
| Surgical site (adverse events outside) | 1 (7.1) | |
| Urinary infection (PD) | 1 (7.1) | II (Antibiotic) |
| Readmission | 1 (7.1) | Grade C fistula (EL) |
| Death up to 90 d | 0 |
In 2 patients (14.3%) among the 8 patients submitted to DP, the surgical specimen measured less than 8 cm, and in 85.7% of the patients, the specimen measured more than 8 cm, with a 9-18 cm range. Two patients (14.3%) submitted to DP developed DM at 18 mo and 4 years after surgery. These two patients had 9 cm and 15 cm of the pancreas resected, respectively. They did not have comorbid conditions, including obesity.
In the present study, the evaluation of the resected surgical specimens according to the WHO criterion did not show any features predictive of recurrence, except in one patient who had invasion of the peripancreatic adipose tissue and who was operated in 2012 with no subsequent relapse. In all surgeries, resection with free margins (R0) was achieved. No lymph node metastases were found in any case. The immunohistochemical analysis confirmed the SPN diagnosis in all surgical specimens (Table 4).
| Pathological characteristics | n (%) |
| Free surgical margins | 14 (100) |
| Average of resected lymph nodes | 6.5 |
| Lymph node metastasis | 0 |
| Perineural invasion | 0 |
| Angiolymphatic invasion | 0 |
| Microscopic extension of the tumor | 1 |
| Peripancreatic adipose tissue | 1 |
| Invasion of the capsule | 0 |
The mean follow-up period was 56.6 mo (12-156 mo). Ten patients were followed up for 5 years; 3 patients with less than 5 years since the surgery are still under follow-up. There were no cases of relapse. The 5year survival and disease-free survival rates were both 100% (Table 5). The mortality rate from causes related to the surgery was 0% in the group. There was one death from bronchopneumonia 12 mo postoperatively. However, one patient reported worsened quality of life, with a Karnofsky score < 80%.
Table 6 shows several series of SPN cases described in the literature.
| Ref. | Year | n | Study | Incidental (%) | Ap (%) | Other findings (%) |
| Papavramidis et al[15] | 2006 | 718 | R | 15.5 | 46.5 | Mass 34.8 |
| Lubezky et al[16] | 2017 | 32 | R | 28 | 48 | Unspecific 24 |
| Song et al[17] | 2017 | 53 | R | 39.6 | 37.7 | Mass 30.2 |
| Wright et al[6] | 2019 | 78 | R | 30.7 | 42.3 | Nauseas 14.1 |
| Lin et al[18] | 2019 | 60 | R | 65 | 31.7 | Strain 2.2 |
| Torres et al[19] | 2019 | 16 | R | - | 87.5 | Mass 12.5 |
| Liu et al[20] | 2019 | 243 | R | 63.4 | 19 | Unspecific |
| Farhat et al[21] | 2020 | 10 | R | 20 | 40 | Mass 40 |
SPN occurs in young women, with a prevalence ratio of 9.7:1 relative to men[15-25]. The mean age varies from 21.9 to 41.2 years[6,15-21], which is similar to that observed in our case series (31.5 years). Two patients in our study were aged 47 years or older, a finding that corroborates the statement that SPN does not occur exclusively in young individuals of reproductive age. β-estrogen and progesterone receptors found in SPN may be the cause of the predominance of the disease among young women, including tumor growth in a favorable hormonal environment such as pregnancy[26,27]. In the present study, a 20-year-old woman in the 8th week of pregnancy with an incidental diagnosis of SPN (6 cm) in the head of the pancreas during an evaluation of urinary infection underwent PPD without harm to the pregnancy. However, the occurrence of SPN in men suggests that the disease is not exclusively hormone-dependent[27,28].
The presence of symptoms in patients with SPN is related to the size of the abdominal mass, which compresses adjacent organs, and with the location of the mass, a tumor in the head of the pancreas may cause abdominal pain and jaundice[15,17,20,21]. Two patients (14.3%) in the present study had epigastric pain and nausea (one patient) and jaundice associated with weight loss (one patient). The former patient’s symptoms resulted from the compression of the stomach by an 11 cm SPN in the body of the pancreas, while the latter patient had a 9.5 cm SPN resting on the head of the pancreas. Most patients with SPN are asymptomatic and have an incidental diagnosis, similar to the cases of 85.7% of patients in the present study (Table 1)[20]. Torres et al[19], in a retrospective multicenter study of 16 patients with SPN located in the head of the pancreas, did not observe any case of jaundice, even for lesions ≥ 10 cm in diameter.
SPN is malignant but indolent when compared to other pancreatic tumors. Tumor aggressiveness occurs in 10%-15% of the cases and is detected either at diagnosis or during disease progression[16,25]. Characteristics of aggressiveness include invasion of nearby organs or of vascular structures, hepatic metastasis, and relapse after treatment[5,15,16,20,21]. There are reports in the literature that an incomplete capsule and solid components larger than cystic components on imaging exams are related to greater aggressiveness[25,29]. Tumor size appears to have a stronger association with aggressive SPNs. Butte et al[5] suggest that SPNs ≥ 7.8 cm have a greater correlation with malignancy (P < 0.005). Kang et al[23] studied 351 patients and multivariate analysis showed a correlation between tumors > 8 cm and relapse (P < 0.0018). In the present study, 42.8% of patients had SPN ≥ 9 cm but this characteristic was not found to be correlated with the presence of distant or lymph node metastases or with disease recurrence, including the two cases with vascular invasion.
Surgery is the treatment of choice for SPN and over 95% of tumors can be resected (R0). Surgery can be curative even with distant metastases[20]. Despite the indolent behavior seen in the present study, this neoplasm can invade nearby structures and organs. All patients in our study were treated with primary surgery (R0 resection), even the cases that required extensive resection of blood vessels (common hepatic artery and superior mesenteric vein) and of organs such as the transverse colon, stomach, and spleen, and the surgical treatment was effective. In a meta-analysis of highly heterogeneous retrospective studies, Yepuri et al[30] observed an SPN recur
The development of DM after DP is debatable. Studies have shown a low incidence of DM in these patients, between 4.8 and 8%[30]. The risk for DM appears to increase 40%-50% seven years after surgery, which may be correlated with the amount of resected pancreas tissue[31]. However, obesity appears to also influence the risk. It is not known how much remaining pancreas tissue is required for normal glucose metabolism to be maintained. In our study, 25% of the patients who underwent DP had DM; they had 9 to 15 cm of their pancreas resected and were not obese.
For years SPN has been described as rare, representing approximately 3% of all pancreatic tumors[20]. An increase in the number of diagnosed cases has been reported in specialized centers. In the literature, the incidence varies from 2.5% to 5.1%[16,21,32]. In the present study, the incidence was 6.3%, i.e., a significant increase in notified cases. In a series of cases from 1988 to 2008 at the Johns Hopkins Hospital, 41 out of the 78 patients were diagnosed in the last 10 years of the series[6]. Other studies indicate a recent increase in the number of diagnoses, with over 60% of cases reported in the last decade[15,22,23]. In the present study, 2 patients were diagnosed and treated before 1998 and the others were diagnosed after 2012 (Table 5). The authors indicate some factors that may contribute to the appearance of new cases, including greater access to specialist physicians, radiologists specialized in diseases of the pancreas, and advances in imaging methods such as CT, MRI, and endosonography. Social programs such as women’s health programs — in which screening exams, e.g., US — are performed, explain the finding of pancreatic tumors in asymptomatic patients.
In this study, SPN occurred in young women, most of which were asymptomatic. Surgery was curative for all patients and the tumors exhibited an indolent behavior in all cases.
Study with 14 patients, operated in a single center, regardless of the size of the tumor, obtained Margins R0, disease-free survival in 100% of cases, with a follow-up longer than 5 years.
Surgical treatment is the main pillar of cure for solid papillary neoplasm of the pancreas. The challenge is to improve access to imaging exams, screening programs and surgeons specialized in pancreatic surgery. The importance is in early diagnosis, greater chance of R0 surgery with less resection of other structures and consequently greater survival.
Show that solid pseudopapillary neoplasms, even when in large volumes and with the need to dry other noble structures, such as large vessels, has the possibility of cure in surgery. We were able to demonstrate in this study, with R0 surgeries and evidencing deonca-free survival and quality of life.
Retrospective study with analysis of a prospectively filled database.
This study was made up of female patients in its entirety, corroborating the literature on being a more frequent neoplasm in women. Approximately 85% asymptomatic, incidental discovery. In our series, we have seen an increase in cases since 2012. Perhaps due to greater accessibility to imaging methods. The fact that women have specific programs for women’s health with periodic examinations and screening, may contribute to the discovery of tumors such as Solid papillary neoplasm of the pancreas in asymptomatic patients, and that we are facing an underreported neoplasm, but it is evident that we need more studies to affirm this.
One theory would be that of an underdiagnosed neoplasm.
Follow-up of operated patients to assess the natural history of the disease.
Manuscript source: Unsolicited manuscript
Specialty type: Surgery
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Anastasiou I, Eysselein VE S-Editor: Yan JP L-Editor: A P-Editor: Yuan YY
| 1. | Frantz VK. Atlas of tumor patology. Tumor of the pancreas. Washington, DC: US force Armed Force institute of pathology, 1959: 32-33. |
| 2. | Kosmahl M, Seada LS, Jänig U, Harms D, Klöppel G. Solid-pseudopapillary tumor of the pancreas: its origin revisited. Virchows Arch. 2000;436:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 227] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | de Castro SM, Singhal D, Aronson DC, Busch OR, van Gulik TM, Obertop H, Gouma DJ. Management of solid-pseudopapillary neoplasms of the pancreas: a comparison with standard pancreatic neoplasms. World J Surg. 2007;31:1130-1135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Scholten L, van Huijgevoort NCM, van Hooft JE, Besselink MG, Del Chiaro M. Pancreatic Cystic Neoplasms: Different Types, Different Management, New Guidelines. Visc Med. 2018;34:173-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Butte JM, Brennan MF, Gönen M, Tang LH, D'Angelica MI, Fong Y, Dematteo RP, Jarnagin WR, Allen PJ. Solid pseudopapillary tumors of the pancreas. Clinical features, surgical outcomes, and long-term survival in 45 consecutive patients from a single center. J Gastrointest Surg. 2011;15:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Wright MJ, Javed AA, Saunders T, Zhu Y, Burkhart RA, Yu J, He J, Cameron JL, Makary MA, Wolfgang CL, Weiss MJ. Surgical Resection of 78 Pancreatic Solid Pseudopapillary Tumors: a 30-Year Single Institutional Experience. J Gastrointest Surg. 2020;24:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Guo N, Zhou QB, Chen RF, Zou SQ, Li ZH, Lin Q, Wang J, Chen JS. Diagnosis and surgical treatment of solid pseudopapillary neoplasm of the pancreas: analysis of 24 cases. Can J Surg. 2011;54:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Mao C, Guvendi M, Domenico DR, Kim K, Thomford NR, Howard JM. Papillary cystic and solid tumors of the pancreas: a pancreatic embryonic tumor? Surgery. 1995;118:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 189] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 9. | Reddy S, Cameron JL, Scudiere J, Hruban RH, Fishman EK, Ahuja N, Pawlik TM, Edil BH, Schulick RD, Wolfgang CL. Surgical management of solid-pseudopapillary neoplasms of the pancreas (Franz or Hamoudi tumors): a large single-institutional series. J Am Coll Surg. 2009;208:950-7; discussion 957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | McMillan MT, Malleo G, Bassi C, Allegrini V, Casetti L, Drebin JA, Esposito A, Landoni L, Lee MK, Pulvirenti A, Roses RE, Salvia R, Vollmer CM Jr. Multicenter, Prospective Trial of Selective Drain Management for Pancreatoduodenectomy Using Risk Stratification. Ann Surg. 2017;265:1209-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 11. | Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, Andersson R, Asbun HJ, Besselink MG, Conlon K, Del Chiaro M, Falconi M, Fernandez-Cruz L, Fernandez-Del Castillo C, Fingerhut A, Friess H, Gouma DJ, Hackert T, Izbicki J, Lillemoe KD, Neoptolemos JP, Olah A, Schulick R, Shrikhande SV, Takada T, Takaori K, Traverso W, Vollmer CR, Wolfgang CL, Yeo CJ, Salvia R, Buchler M; International Study Group on Pancreatic Surgery (ISGPS). The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161:584-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3041] [Cited by in RCA: 2964] [Article Influence: 370.5] [Reference Citation Analysis (35)] |
| 12. | Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18532] [Cited by in RCA: 24851] [Article Influence: 1183.4] [Reference Citation Analysis (0)] |
| 13. | Vin Y, Sima CS, Getrajdman GI, Brown KT, Covey A, Brennan MF, Allen PJ. Management and outcomes of postpancreatectomy fistula, leak, and abscess: results of 908 patients resected at a single institution between 2000 and 2005. J Am Coll Surg. 2008;207:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 146] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 14. | Tjaden C, Hassenpflug M, Hinz U, Klaiber U, Klauss M, Büchler MW, Hackert T. Outcome and prognosis after pancreatectomy in patients with solid pseudopapillary neoplasms. Pancreatology. 2019;19:699-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Papavramidis T, Papavramidis S. Solid pseudopapillary tumors of the pancreas: review of 718 patients reported in English literature. J Am Coll Surg. 2005;200:965-972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 512] [Cited by in RCA: 536] [Article Influence: 26.8] [Reference Citation Analysis (1)] |
| 16. | Lubezky N, Papoulas M, Lessing Y, Gitstein G, Brazowski E, Nachmany I, Lahat G, Goykhman Y, Ben-Yehuda A, Nakache R, Klausner JM. Solid pseudopapillary neoplasm of the pancreas: Management and long-term outcome. Eur J Surg Oncol. 2017;43:1056-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 17. | Song H, Dong M, Zhou J, Sheng W, Zhong B, Gao W. Solid Pseudopapillary Neoplasm of the Pancreas: Clinicopathologic Feature, Risk Factors of Malignancy, and Survival Analysis of 53 Cases from a Single Center. Biomed Res Int. 2017;2017:5465261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 18. | Lin X, Lin R, Lu F, Chen Y, Huang H. Surgical Management of Solid Pseudopapillary Neoplasms of Pancreas: A Single-Center Experience of 60 Patients. Dig Surg. 2020;37:348-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Torres OJM, Rezende MB, Waechter FL, Neiva RF, Moraes-Junior JMA, Torres CCS, Fernandes ESM. Pancreatoduodenectomy for solid pseudopapillary tumor of the pancreas: a multi-institution study. Arq Bras Cir Dig. 2019;32:e1442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Liu M, Liu J, Hu Q, Xu W, Liu W, Zhang Z, Sun Q, Qin Y, Yu X, Ji S, Xu X. Management of solid pseudopapillary neoplasms of pancreas: A single center experience of 243 consecutive patients. Pancreatology. 2019;19:681-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 21. | Farhat W, Ammar H, Amine Said M, Mizouni A, Bouazzi A, Abdessaied N, Ben Mabrouk M, Ben Ali A. Solid pseudopapillary neoplasm of the pancreas: a report of 10 cases and literature review. ANZ J Surg. 2020;90:1683-1688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Gordon-Dseagu VL, Devesa SS, Goggins M, Stolzenberg-Solomon R. Pancreatic cancer incidence trends: evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. Int J Epidemiol. 2018;47:427-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 145] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 23. | Kang CM, Choi SH, Kim SC, Lee WJ, Choi DW, Kim SW; Korean Pancreatic Surgery Club. Predicting recurrence of pancreatic solid pseudopapillary tumors after surgical resection: a multicenter analysis in Korea. Ann Surg. 2014;260:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 24. | Law JK, Ahmed A, Singh VK, Akshintala VS, Olson MT, Raman SP, Ali SZ, Fishman EK, Kamel I, Canto MI, Dal Molin M, Moran RA, Khashab MA, Ahuja N, Goggins M, Hruban RH, Wolfgang CL, Lennon AM. A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas. 2014;43:331-337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 241] [Article Influence: 21.9] [Reference Citation Analysis (0)] |
| 25. | You L, Yang F, Fu DL. Prediction of malignancy and adverse outcome of solid pseudopapillary tumor of the pancreas. World J Gastrointest Oncol. 2018;10:184-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 33] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (1)] |
| 26. | Farrell JJ. Prevalence, Diagnosis and Management of Pancreatic Cystic Neoplasms: Current Status and Future Directions. Gut Liver. 2015;9:571-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 27. | Morales A, Duarte-Rojo A, Angeles-Angeles A, Mery CM, Ruíz-Molina JM, Díaz-Sánchez V, Robles-Díaz G. The beta form of the estrogen receptor is predominantly expressed in the papillary cystic neoplasm of the pancreas. Pancreas. 2003;26:258-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Machado MC, Machado MA, Bacchella T, Jukemura J, Almeida JL, Cunha JE. Solid pseudopapillary neoplasm of the pancreas: distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery. 2008;143:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 160] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 29. | Reddy S, Wolfgang CL. Solid pseudopapillary neoplasms of the pancreas. Adv Surg. 2009;43:269-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Yepuri N, Naous R, Meier AH, Cooney RN, Kittur D, Are C, Jain A, Dhir M. A systematic review and meta-analysis of predictors of recurrence in patients with Solid Pseudopapillary Tumors of the Pancreas. HPB (Oxford). 2020;22:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 31. | King J, Kazanjian K, Matsumoto J, Reber HA, Yeh MW, Hines OJ, Eibl G. Distal pancreatectomy: incidence of postoperative diabetes. J Gastrointest Surg. 2008;12:1548-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Lam KY, Lo CY, Fan ST. Pancreatic solid-cystic-papillary tumor: clinicopathologic features in eight patients from Hong Kong and review of the literature. World J Surg. 1999;23:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 170] [Article Influence: 6.5] [Reference Citation Analysis (0)] |