Published online Jun 15, 2021. doi: 10.4251/wjgo.v13.i6.560
Peer-review started: February 5, 2021
First decision: March 29, 2021
Revised: March 31, 2021
Accepted: May 22, 2021
Article in press: May 22, 2021
Published online: June 15, 2021
Processing time: 122 Days and 2.1 Hours
The development of endoscopic treatment technology has further promoted the minimally invasive treatment of early gastric cancer (EGC). Endoscopic treatment has achieved better therapeutic effects in terms of safety and prognosis and is the preferred treatment method for patients who meet the indications for endoscopic treatment. However, the consequent problem is that some patients receiving endoscopic treatment may undergo non-curative resection, and the principle of follow-up management for non-curative resection patients deserves further attention. In addition, there are still debates on how to improve the accuracy of clinical staging, select a reasonable treatment method for patients who meet the expanded indications for endoscopic treatment, manage patients with positive endoscopic surgical margins, conduct research on function-preserving surgery, and manage the treatment of EGC under the current situation in China. Consequently, we aim to review current indications for endoscopic submucosal dissection of EGC in order to better inform treatment options.
Core Tip: Gastric cancer is a worldwide public health problem with a lower cure rate and worse prognosis. With the improvement of people’s health awareness and the popularization of physical examination, the detection rate of early gastric cancer is increasing each year. Helicobacter pylori and Epstein-Barr virus are important pathogenic factors for gastric cancer. For patients who meet with the absolute and expanded indications for endoscopic treatment, endoscopic submucosal dissection can have the same therapeutic effect as surgery while reducing surgical trauma. For non-curative resection, laparoscopic subtotal gastrectomy or function-preserving gastrectomy can be performed based on the patient’s condition.
- Citation: Zheng Z, Yin J, Liu XY, Yan XS, Xu R, Li MY, Cai J, Chen GY, Zhang J, Zhang ZT. Current indications for endoscopic submucosal dissection of early gastric cancer. World J Gastrointest Oncol 2021; 13(6): 560-573
- URL: https://www.wjgnet.com/1948-5204/full/v13/i6/560.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i6.560
Gastric cancer is a malignant tumor originating from the gastric mucosal epithelium, and its prognosis and outcomes are closely related to tumor stage. Most patients with early gastric cancer (EGC) have no obvious clinical symptoms. If gastric cancer screening is performed properly, gastric cancer can be detected at an early stage. However, unfortunately, patients are often diagnosed at the stage of advanced gastric cancer because they have not been screened for gastric cancer. This leads to a lower radical tumor resection rate and poor prognosis, and the 5-year survival rate is less than 30%[1]. However, patients with EGC have a better prognosis, with a 5-year survival rate of more than 90%. With the gradual popularization of diagnostic techniques and endoscopic screening in China, more patients with gastric cancer can be diagnosed in the early stage and receive therapy.
At present, radical surgery is still the acknowledged treatment for EGC, and whether it is accompanied by lymph node metastasis (LNM) is an important basis for the choice of surgery. In recent years, endoscopic submucosal dissection (ESD), a minimally invasive and effective technique, has become the preferred approach for the treatment of EGC. It is advantageous because under the premise of strict control of indications, the surgical trauma is significantly less than laparoscopic or open surgery, and the long-term prognosis is not worse than surgical treatment. Furthermore, it can maximize the preservation of gastric functions and improve the life quality of patients after surgery[2]. However, difficulty in accurately assessing the histopathological conditions, such as the depth of tumor invasion, the extent of lateral invasion, and vascular invasion before treatment, as well as the deficiency of endoscopic surgical technique leads to the occurrence of non-curative resection (NCR), which is also a disadvantage of endoscopic therapy. A study found that among 194 patients with EGC who received additional surgical treatment after NCR of ESD, 10 (5.2%) had tumor recurrence and 11 (5.7%) had LNM[3]. Although patients with NCR of EGC have a higher risk of LNM and should be treated by additional surgery, follow-up results showed that most patients do not have LNM after surgery, and some patients are unable or unwilling to receive surgical treatment due to advanced age and underlying diseases[4]. Therefore, how to minimize surgical trauma and choose the optimal treatment to ensure the tumor radical resection is the focus of ongoing research. Herein, we aim to review the current indications for ESD of EGC in order to better evaluate treatment options.
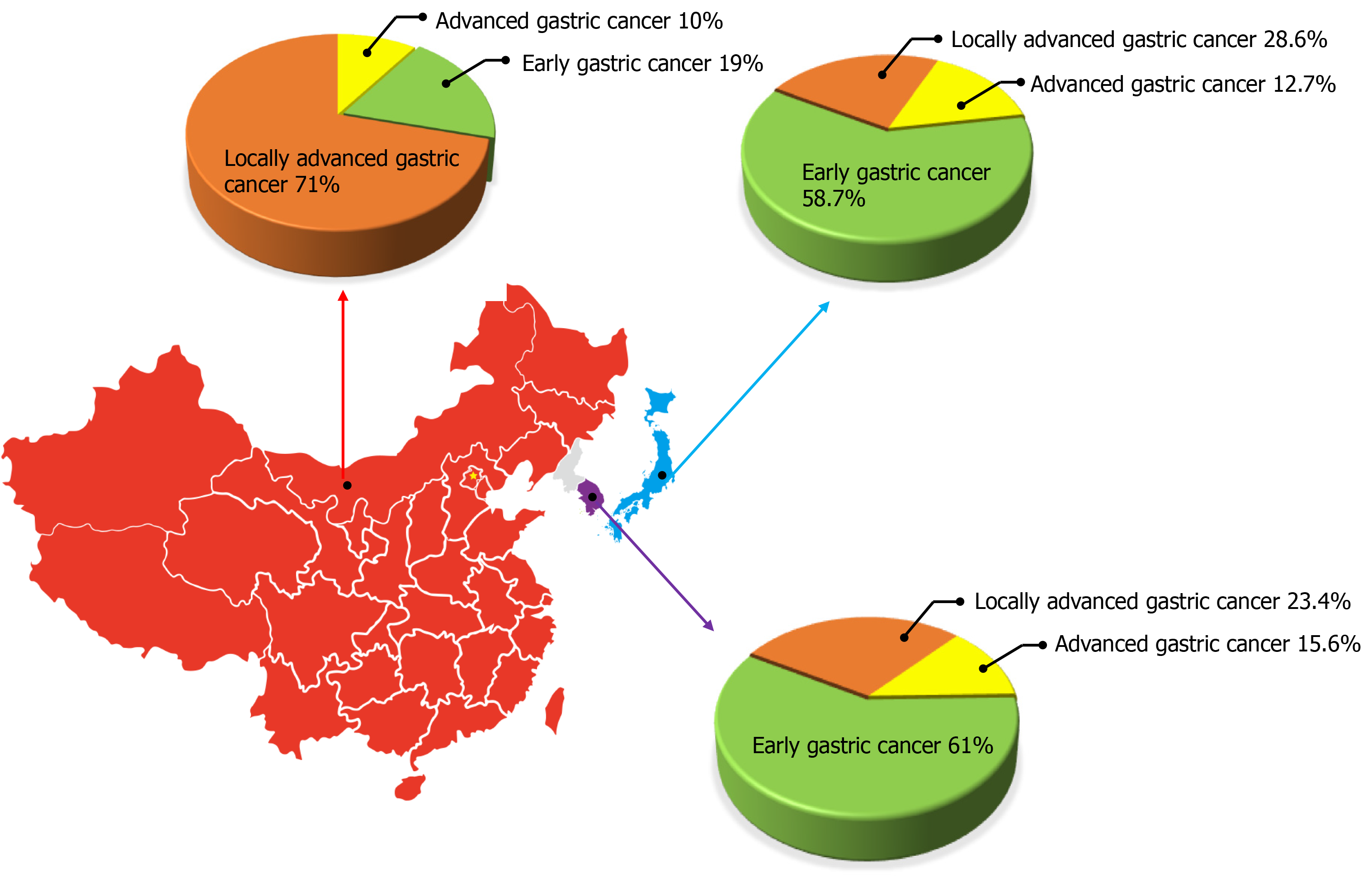
Gastric cancer is a worldwide public health problem with a lower cure rate and worse prognosis. According to the Global Cancer statistic report of the World Health Organization and the International Agency for Research on Cancer, there were 1 million new cases of gastric cancer worldwide in 2018, ranking fifth among new patients with malignant tumors. There were 783000 deaths accounting for the third highest number of cancer-related deaths[5]. There are obvious differences in the epidemiological characteristics of gastric cancer between East Asia and Western countries, among which Japan, South Korea, and China are the regions with a high incidence of gastric cancer, and the incidence in males is about twice that in females[5,6]. Based on the survey results of the National Central Cancer Registry of China, there were about 410000 new cases of gastric cancer and about 290000 deaths in China in 2014, making it the second common cause of morbidity and mortality among cancer patients[7]. Although the overall incidence and mortality of gastric cancer have shown a downward trend with the gradual improvement of diagnostic methods and strategies and the deepening understanding of its molecular mechanisms, it still faces huge challenges. In South Korea and Japan, due to the mature gastroscopy screening system, the detection rate of EGC accounts for 50%-60% of the overall proportion of gastric cancer[8-10]. In China, with the improvement of people’s health awareness and the popularization of physical examination, the detection rate of EGC in the overall incidence of gastric cancer is increasing each year. According to the statistics of the Chinese Association of Gastrointestinal Cancer Surgery from 2014 to 2016, EGC accounted for 19% of the total gastric cancer cases, which is still a considerable gap compared with Japan and South Korea[11] (Figure 1). Therefore, attention should be paid to the screening, early diagnosis, and treatment in order to further increase the detection rate of EGC and better improve the long-term prognosis of patients.
Helicobacter pylori (H. pylori) infection is an important pathogenic factor for gastric cancer. In addition, H. pylori is involved in tumor proliferation, apoptosis, and epigenetic modification of oncogenes, which ultimately leads to tumorigenesis associated with inflammatory lesions[12]. However, for patients with EGC undergoing surgery, whether H. pylori is routinely eradicated is still inconclusive, and whether radical H. pylori eradication can stop the progression from precancerous lesions to cancer is still debated. Studies have found that compared with the placebo group, the H. pylori eradication group showed a significantly reduced incidence of metachronous gastric cancer [hazard ratio (HR) = 0.50, 95% confidence interval (CI): 0.26-0.94, P = 0.03] after ESD in patients with EGC[13]. No serious adverse events occurred in either group, which fully affirmed the significance of eradicating H. pylori infection in precancerous lesions and EGC.
Epstein-Barr virus (EBV) mainly exists in gastric cancer cells and lymphoid stroma, while most normal epithelial cells do not have EBV. Currently, with the establishment of molecular classification of gastric cancer and the rise of immunotherapy, EBV-related gastric cancer has gradually attracted attention, but the mechanism of EBV in the pathogenesis of gastric cancer remains unclear[14]. In the Cancer Genome Atlas molecular classification, EBV type has higher CpG island methylation, phosphoinositide 3-kinase mutation, programmed death ligand 1/2 overexpression, silencing of cyclin-dependent kinase inhibitor 2A, and activation of immune-related signaling pathways, suggesting that EBV-associated gastric cancer may have its own independent biological and clinical characteristics[14,15]. A study found that the objective response rate of patients with EBV-positive metastatic gastric cancer was 100% after treatment with pembrolizumab, which preliminarily confirmed that EBV-positive status can be used as a potential molecular marker to predict the possibility of immunotherapy[16]. However, the limitation of this study was its retrospective design and the findings need to be further verified by prospective studies. It is believed that further studies on the molecular mechanism of EBV-related gastric cancer will provide a theoretical basis for the refinement of gastric cancer molecular classification and developing new drugs.
LNM is one of the important factors affecting the prognosis of patients with EGC and the choice of treatment. Therefore, endoscopic treatment is suitable for tumors with relatively limited primary lesions and an extremely low possibility of LNM[6]. At present, endoscopic resection of EGC mainly includes endoscopic mucosal resection (EMR) and ESD. ESD makes up for the problems that EMR cannot remove, such as large areas of lesions. Moreover, ESD can make more accurate judgments on the depth of tumor invasion and presence of vascular invasion. Thus, ESD has gradually replaced EMR as the preferred treatment for EGC[17]. However, there are still many controversies regarding the selection of ESD indications for EGC. Consequently, how to refine the indications for ESD treatment more effectively, reduce surgical trauma, and improve safety and rationality as far as possible under the premise of curative resection has gradually become a prominent problem in the treatment of EGC.
In 2016, the Japan Gastroenterological Endoscopy Society and the Japanese Gastric Cancer Association (JGCA) jointly issued the guidelines for ESD and EMR of EGC, which divided indications for endoscopic resection into absolute and expanded indications[18]. The former has good long-term prognostic evidence, while the latter lacks reliable long-term prognostic results.
Tumor lesions with a risk of LNM less than 1% for which endoscopic resection is considered to have the same effect as radical surgery are classified as absolute indications for ESD therapy[6]. Based on the research results of 5265 patients with EGC by Gotoda et al[19], Japanese gastric cancer treatment guidelines 2014 (version 4) clearly stated non-ulcerated and differentiated intramucosal carcinoma with a tumor diameter ≤ 2 cm as the absolute indication for endoscopic treatment of EGC[20] (Table 1). This absolute indication range is the same as that mentioned in guidelines for ESD and EMR for EGC[18]. Based on the results of the JCOG0607 trial[21], Japanese gastric cancer treatment guidelines 2018 (5th edition) adjusted tumor diameter > 2 cm, differentiated intramucosal carcinoma without ulcer lesions and tumor diameter ≤ 3 cm, and differentiated intramucosal carcinoma with ulcer lesions as the absolute indications for ESD. On the other hand, undifferentiated intramucosal carcinoma without ulcer and with a diameter ≤ 2 cm was considered an expanded indication for ESD[22] (Table 1). This version of guidelines is the same as the Korean Practice Guideline for Gastric Cancer 2018 for the absolute and expanded indications for ESD in EGC[23]. In addition, the ongoing study of JCOG1009/1010, which explores the efficacy and safety of ESD in the treatment of undifferentiated cT1a gastric cancer, has completed a 5-year follow-up. The results of the study confirmed that the patients with undifferentiated intramucosal carcinoma with tumor diameter < 2 cm, without ulcers had a satisfactory prognosis after endoscopic treatment, and no LNM occurred[24,25]. It is believed that this indication is expected to be included in the absolute indication range of endoscopic resection, which will lay a theoretical foundation for further elaboration of ESD indications.
| T1a | T1b | |||||||
| UL (-) | UL (+) | SM1 (< 500 µm) | SM2 (> 500 µm) | |||||
| ≤ 2 cm | > 2 cm | ≤ 3 cm | > 3 cm | ≤ 3 cm | > 3 cm | ≤ 3 cm | > 3 cm | |
| JGCA guideline (version 4, 2014) | ||||||||
| Differentiated | ESD | EXPANDED(JCOG0607) | EXPANDED(JCOG0607) | SURGERY | EXPANDED | SURGERY | SURGERY | SURGERY |
| Undifferentiated | EXPANDED | SURGERY | SURGERY | SURGERY | SURGERY | SURGERY | SURGERY | SURGERY |
| JGCA guideline (version 5, 2018) | ||||||||
| Differentiated | ESD | ESD | ESD | SURGERY | EXPANDED | SURGERY | SURGERY | SURGERY |
| Undifferentiated | EXPANDED(JCOG1009/1010) | SURGERY | SURGERY | SURGERY | SURGERY | SURGERY | SURGERY | SURGERY |
Due to the low incidence of EGC in European and American countries, endoscopic treatment still lacks relevant evidence-based medicine. Currently, research results of endoscopic therapy for EGC are mainly based on the data from related studies in Japan and South Korea. The European Society of Gastrointestinal Endoscopy guidelines and the National Comprehensive Cancer Network (NCCN) guidelines fully adopt the absolute indications for endoscopic treatment recommended by the JGCA for endoscopic resection[26-28]. However, whether the above ESD treatment indications are suitable for the Chinese population is yet to be validated in high-quality clinical trials. Therefore, some research centers in China are carrying out exploratory research on ESD indications for EGC, hoping to establish reasonable ESD indications that meet the characteristics of the Chinese population[29]. Meanwhile, a staging diagnosis scheme for EGC suitable for China’s national conditions was proposed to further achieve the purpose of precision treatment and improve the life quality and prognosis of patients.
The absolute indications for ESD for the treatment of EGC have been unanimously approved, but the application of expanded indications is still controversial. Among them, assessment of the risk of LNM is the key to determining the optimal therapy for patients with expanded indications. A study from Korea found that 17.6% of the patients who were assessed to meet the expanded indications for ESD before surgery were proved to be non-compliant with the ESD indications after surgery, while only 6.7% of patients who were assessed as absolute indications before surgery did not meet the indications[30]. It indicates that improving the accuracy of preoperative diagnosis of expanded indications is a prerequisite for the rational application of ESD in the treatment of EGC. Therefore, although expanded indications can benefit some patients with EGC, its exact efficacy is still being explored. A meta-analysis showed that en bloc resection rates (93.6% vs 97%, P < 0.0001) and radical resection rates (82.4% vs 94%, P < 0.0001) were significantly lower in patients eligible for the expanded indications than in those with absolute indications, but there was no statistically significant difference in long-term survival (P = 0.37)[31]. Another retrospective study from South Korea used propensity score matching to analyze 522 patients who were eligible for expanded indications and underwent surgery or endoscopic treatment[32]. The study found that the overall and tumor-specific survival rates were not statistically different between the two groups, but the 5-year relapse-free survival rate in the surgery group was better than that in the endoscopic group (96.7% vs 92.7%, P < 0.001)[32]. Further comparison of patient recurrence patterns showed that there was no significant statistical difference in LNM rate and distant metastasis rate between the two groups, but the metachronous metastasis rate in the endoscopic group was higher than that in the surgical group[32]. This result indicates that the recurrence pattern of patients with expanded indications for endoscopic treatment is mainly local recurrence. Therefore, endoscopic treatment may be a good option for patients with expanded indications under the condition of ensuring sufficient surgical margins and regular postoperative re-examination.
The JCOG0607 trial, which is conducted in Japan, suggested that ESD treatment is safe and effective for EGC patients with expanded indications. A total of 470 patients eligible for expanded indications for ESD were included in the study. The results showed that the en bloc rate of ESD was 99.1%, the curative resection rate was 67%, and the delayed bleeding and perforation rates were 8.5% and 2.6%, respectively. Of these, 86.8% of patients with NCR received surgical treatment, and the 5-year survival rate of all patients was 97%[21]. Based on the results of this study, it was confirmed that ESD was reasonable and safe in the treatment of patients with EGC who partially met the expanded indications. On this basis, the endoscopic treatment indications of the Japanese gastric cancer treatment guidelines 2018 (5th edition) have been revised to make the guidelines more in line with the needs of clinical treatment. Therefore, the current expanded indications for endoscopic treatment mainly include undifferentiated intramucosal carcinoma with tumor diameter ≤ 2 cm and without ulcer lesions and differentiated submucosal carcinoma with tumor diameter < 3 cm and invasion depth <500 µm[22]. Recently, with the publication of JCOG1009/1010 results, the application scope of existing ESD indications will be further expanded, benefiting more patients with EGC[24] (Table 1).
Although the above research results support the application of ESD expanded indications, some skeptical studies pointed out that compared with patients with absolute indications, patients with expanded indications had a higher rate of LNM, especially for undifferentiated intramucosal carcinoma with a diameter ≤ 2 cm [25/972 (2.6%), reference range = 6.79, P = 0.004] and differentiated submucosal carcinoma with a diameter < 3 cm [8/315 (2.5%), reference range = 6.30, P = 0.004][33]. Therefore, the current debate on expanded indications mainly focuses on undifferentiated carcinoma and submucosal infiltrating carcinoma. These two types of lesions seem to have a higher risk of LNM, which may not only increase the rate of NCR but also pose a challenge to preoperative evaluation of lesions. Therefore, we suggest that endoscopic therapy for patients with expanded indications should be selectively carried out by experienced centers in the context of clinical trials.
Curative resection refers to the complete resection of the lesions with negative margins and no vascular and lymphatic infiltration which meet the absolute and expanded indications. Complete resection is an important condition for curative resection and complete reconstruction after segmental resection of the lesions can also be considered as meeting the criteria for curative resection. According to the Japanese gastric cancer treatment guidelines 2014 (version 4)[20], complete resection, tumor diameter ≤ 2 cm, differentiated intramucosal carcinoma without ulceration, negative horizontal and vertical margins, and no lymph node or vascular infiltration are required for absolute indications. For expanded indications, one of the following four requirements is required: (1) Tumor diameter > 2 cm, differentiated intramucosal carcinoma without ulcer; (2) Tumor diameter ≤ 3 cm, differentiated intramucosal carcinoma with ulcer; (3) Tumor diameter ≤ 2 cm, undifferentiated intramucosal carcinoma without ulcer; and (4) Tumor diameter ≤ 3 cm, differentiated submucosal carcinoma with invasion depth < 500 µm. In addition, the horizontal and vertical resection margins should be negative without lymphatic and vascular infiltration (Table 2). Curative resection of EGC is the ultimate goal of endoscopic therapy and the key is that clinicians need to have a full grasp of the indications for endoscopic therapy. However, postoperative pathology confirmed that part of EGC did not reach the standard of curative resection after endoscopic treatment. Cho et al[34] analyzed the literature on the efficacy of ESD in the treatment of EGC in Eastern and Western countries in recent years and found that the en bloc rate was 92%-97% and curative resection rate was 73.6%, which indicated that NCR still had a certain proportion in postoperative pathological evaluation. Therefore, the Japanese gastric cancer treatment guidelines 2018 (5th edition) updated the expression of ‘curative/non-curative resection’ in the evaluation of ESD radical resection to ‘endoscopic curability (eCura)’[22]. In these guidelines, curative resection, expanded curative resection, and NCR were changed to eCura A, eCura B, and eCura C, respectively (Table 2). Studies have found that patients with curative resection still have a potential recurrence risk after surgery with a local recurrence rate of 0.13%-1.3%[35], an incidence of simultaneous carcinoma and metachronous carcinoma of 4.0%-12.9% and 2.5%-5.1%, respectively[35-37], and 5-year and 10-year cumulative risk rates as high as 9.5% and 22.7%, respectively[35]. Therefore, for patients with eCura A and eCura B, the Japanese guidelines recommend close follow-up observation to monitor the occurrence of metachronous gastric cancer and LNM[38], and regular high-quality endoscopy follow-up can detect more than 95% of metachronous carcinomas and regular abdominal computed tomography can monitor the presence or absence of LNM and distant organ metastasis.
| T1a | T1b | |||||||
| UL (-) | UL (+) | SM1 (< 500 µm) | SM2 (> 500 µm) | |||||
| ≤ 2 cm | > 2 cm | ≤ 3 cm | > 3 cm | ≤ 3 cm | > 3 cm | ≤ 3 cm | > 3 cm | |
| JGCA guideline (version 4, 2014) | ||||||||
| Differentiated | CR | CR | CR | NCR | CR | NCR | NCR | NCR |
| Undifferentiated | CR | NCR | NCR | NCR | NCR | NCR | NCR | NCR |
| JGCA guideline (version 5, 2018) | ||||||||
| Differentiated | eCura A | eCura A | eCura A | eCura C | eCura B | eCura C | eCura C | eCura C |
| Undifferentiated | eCura B | eCura C | eCura C | eCura C | eCura C | eCura C | eCura C | eCura C |
NCR refers to the situation that does not meet the criteria for curative resection or expanded curative resection after endoscopic resection, and its incidence is approximately 14.3%-21.4%[39-42]. NCR includes eCura C1 and eCura C2, among which eCura C1 refers to non-en bloc resection and positive horizontal margins, while other situations belong to eCura C2[22].
For patients with eCura C1, the guidelines recommend additional ESD remedial resection, surgical treatment, and close follow-up. Follow-up is a feasible strategy for patients having only positive horizontal margins with a low rate of LNM. A study found that in 77 patients with positive horizontal margins after ESD, only 11.9% had local recurrence without distant metastasis after 60 mo of follow-up, and the 5-year overall survival rate was 94.2%[43]. Other studies have found a higher risk of recurrence in patients whose tumors were partitioned, but no tumor-related deaths during the 10-year follow-up period were observed[44]. However, due to the lack of evidence from randomized controlled studies, there is still no accepted standard treatment for NCR of eCura C1.
For eCura C2 patients with high-risk factors for LNM, the guidelines recommend additional surgical treatment. Suzuki et al[45] divided 1969 EGC patients with NCR into the additional surgery group and the observation group and found that the 5-year overall survival rates of the two groups were 91% and 75.5% (P < 0.001), and the disease-specific survival rates was 99.0% and 96.8% (P = 0.013), respectively. Therefore, although the current treatment of eCura C2 is still controversial, most evidence shows that additional surgery can benefit patients’ survival[39,46-48]. However, salvage surgery also increases the risk of surgical complications and reduces the patient’s postoperative life quality, and it is possible for these patients to obtain postoperative pathological specimens without residual cancer.
Therefore, for patients diagnosed with NCR after ESD, two factors need to be considered in the formulation of remedial strategies: (1) Positive margin or local recurrence; and (2) LNM. In the absence of LNM, complete excision can be achieved by ESD again, regardless of positive margin or local recurrence. However, how to predict the risk of LNM after NCR of ESD is the key to guiding treatment after NCR[49].
To assess the risk factors for LNM in patients with NCR, Hatta et al[40] proposed the eCura scoring system to make treatment decisions. Five factors including tumor size (1 point), invasion depth (1 point), lymphatic invasion (3 points), venous invasion (1 point), and vertical margin positive (1 point) were included in the eCura scoring system. Patients with a total score of 0-1, 2-4, and 5-7 were classified as low-risk, medium-risk, and high-risk groups, with LNM rates of 2.5%, 6.7%, and 22.7%, respectively(Table 3). The eCura scoring system was used to conduct internal verification on 905 patients with EGC without additional surgical treatment. The results showed that the 5-year tumor-specific survival rates of the low-, medium-, and high-risk groups were 99.6%, 96%, and 90.2%, respectively (P < 0.01)[40]. In a follow-up study, compared to the patients with additional surgery, Hatta et al[41] demonstrated that a higher risk of tumor recurrence (HR = 3.13, P = 0.024) and no significant difference in specific tumor-related mortality (reference range = 2.66, P = 0.063) in high-risk patients as per the eCura scoring system. This indicated that the additional radical surgery after ESD is of great significance to improve the prognosis of the high-risk group, while close follow-up is also a feasible option for low-risk group. In addition, Niwa et al[50] retrospectively analyzed 47 patients with EGC and found that the eCura scoring system was also applicable to the selection of additional surgery after the NCR of ESD. However, in clinical practice, patients with undifferentiated EGC often choose radical gastrectomy, and there is a selection bias. Therefore, the study did not recommend the use of eCura scoring system to evaluate risk level and formulate therapy in patients with undifferentiated EGC. Consequently, how to put forward a more accurate model to predict LNM and tumor recurrence to reduce unnecessary surgical trauma is still a research hotspot in the future.
| Risk factor | Score | Risk grade | Total score1 | Lymph node metastasis rate (%) |
| Tumor diameter > 3 cm | 1 | Low | 0-1 | 2.5 |
| Submucosal invasion depth > 500 µm | 1 | Medium | 2-4 | 6.7 |
| Lymphatic invasion positive | 3 | |||
| Vascular invasion positive | 1 | High | 5-7 | 22.5 |
| Vertical incisal margin positive | 1 |
Thus, there are both correlations and differences between eCura and the eCura scoring system[22,40]. eCura is mainly used for the curative evaluation of EGC patients undergoing endoscopic resection. As per the guidelines, undifferentiated carcinoma or carcinoma with a tumor diameter > 2 cm that invades the submucosa are classified as eCura C2. However, the eCura scoring system is mainly for eCura C2 patients with EGC to predict the risk of LNM, so the histological type is not included as an evaluation index (Table 4).
| eCura system | eCura in JGCA guidelines version 5, 2018 | |
| Evaluation index | Predicting LNM | Curative resection criteria |
| Scope of application | Patients with EGC who do not meet the criteria of curative resection (eCura C2) | Patients with EGC who receive endoscopic resection |
| Category | Low risk; medium risk; high risk | eCura A; eCura B; eCura C1; eCura C2 (using eCura system predicted LNM rate) |
Whether to add surgery after NCR of EGC should be dependent on the risk of LNM. For patients with NCR, an accurate histopathological examination should be performed on the excised specimens, risk factors for LNM should be evaluated comprehensively, and the treatment strategies should be developed based on individual conditions. At present, radical surgery is still the main treatment for EGC patients with NCR. However, conventional surgery provides survival benefits for a small number of patients while it may impose additional surgical risks on some patients who do not have LNM. Therefore, there are still controversies about the choice of additional surgery after ESD. In recent years, although laparoscopic surgery has developed rapidly, it still lacks sufficient evidence-based medicine. With the application of the first case of laparoscopic radical gastrectomy in patients with EGC in 1991, it has shown great potential in terms of safety and curative effect[51], but whether it can achieve the same curative effect as traditional open surgery is still controversial. Therefore, the JCOG0912 and KLASS01 trials compared the short-term and long-term curative effects of laparoscopic radical gastrectomy and traditional open radical gastrectomy in the treatment of EGC. The results showed that compared with traditional open surgery, laparoscopic surgery had the same safety and radical curative effects for tumors, and recurrence rate and long-term survival rate were not significantly different. However, it had the advantages of less trauma, less bleeding, lower postoperative complication rate, and faster recovery[52,53]. Meanwhile, the CLASS02 trial from China also showed that the rate of overall morbidity and mortality (rate difference = -1.1%, 95%CI: -11.8% to 9.6%) and postoperative complication occurrence were not significantly different between the laparoscopic group and open group[54]. Therefore, these studies suggested that laparoscopic radical gastrectomy is a safe and feasible way to treat EGC.
For additional surgery after NCR, the range of gastric resection is not clearly specified in the guidelines, but the resection range of EGC can be referred to. According to the European Society for Medical Oncology guidelines, a distal gastrectomy should be performed if the proximal margin of resection is more than 5 cm from the tumor, otherwise a total gastrectomy should be considered[55]. According to the NCCN guidelines, adequate gastrectomy for T1b-T3 stage tumors is recommended to achieve a negative pathologic margin. Distal gastrectomy is preferred for distal gastric tumors, while both proximal and total gastrectomy are available for proximal gastric tumors[27]. Yamasaki et al[56] conducted a prospective multicenter controlled study on early upper stomach cancer, which confirmed that compared with total gastrectomy, patients with proximal gastrectomy had good safety and short-term and long-term efficacy. Therefore, the Japanese guidelines recommend that a safe margin of 2 cm should be ensured for T1 patients and preoperative endoscopic positioning should be performed for tumors with unclear boundaries. Distal gastrectomy should be performed for lower stomach cancer and pylorus-preserving gastrectomy (PPG) and proximal gastrectomy should be considered for tumors in the middle of the stomach (more than 4 cm from the pylorus) and upper stomach, respectively[22].
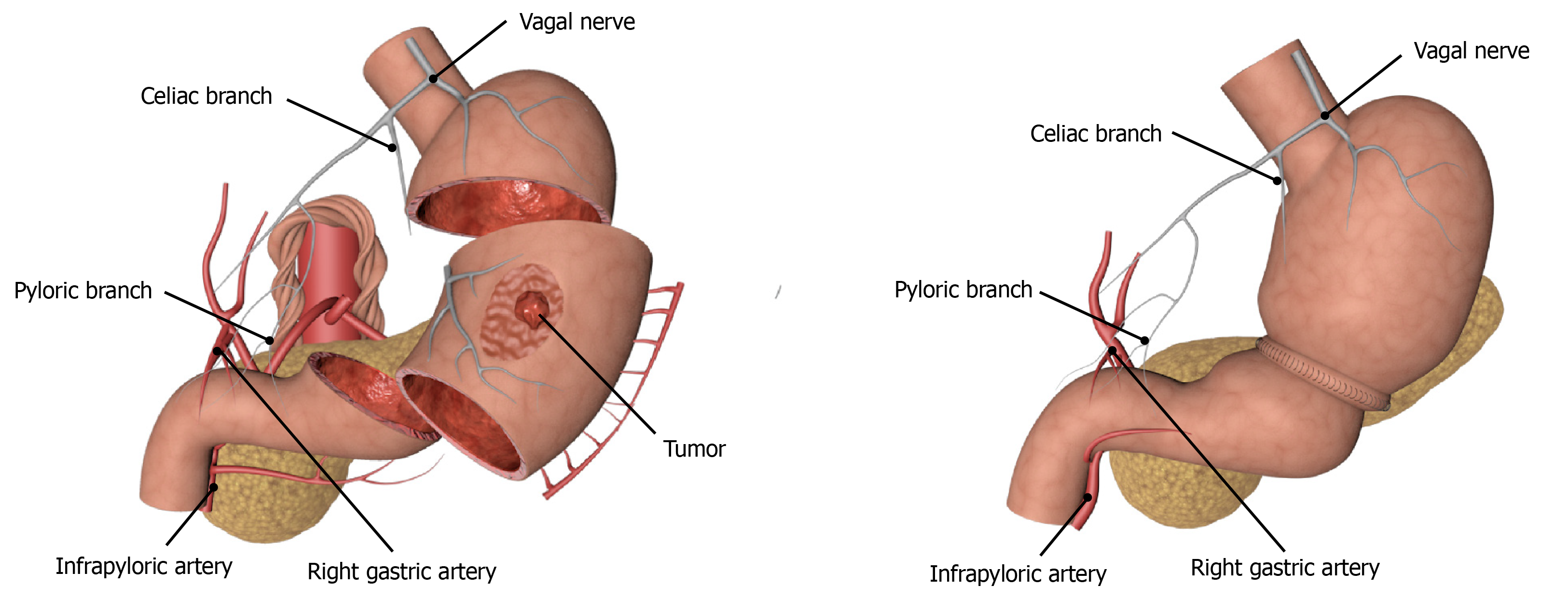
Function-preserving gastrectomy is performed to maximize the postoperative life quality of patients by ensuring the radical resection of the tumor. With the gradual improvement in people’s requirements for quality of life, the treatment of EGC has gradually shifted from radical gastrectomy to function-preserving gastrectomy which includes PPG, laparoscopic-endoscopic combined partial gastrectomy, proximal gastrectomy, segmental gastrectomy, and local gastrectomy. Although segmental gastrectomy and local gastrectomy can theoretically achieve the effects of radical oncology, there is still a lack of high-quality research evidence, so it is not often used in clinical practice. The indications for PPG, which have been studied extensively in recent years, are mainly for EGC patients with cT1N0, tumor lesions located at the greater curvature of the gastric body, and the distance from the pylorus of more than 4 cm. The advantages of surgery are mainly reflected in the reduced incidence of dumping syndrome and bile reflux due to pyloric resection, as well as better food storage[57,58] (Figure 2). Studies have confirmed that the probability of suprapyloric LNM in T1 stage EGC in the middle of the stomach is only 0.2%. Therefore, lymph node dissection in the suprapyloric region can be omitted or only partially dissected to preserve the hepatic branch, the celiac branch, and the pyloric branch of the vagus nerve and the right gastric vessel, so as to seek a balance between the radical resection of the tumor and the function preservation as far as possible, and at the same time reduce the incidence of postoperative gastric emptying and improve the postoperative life quality of patients[59,60]. We also think that anastomotic methods might be associated with gastric emptying disorder occurrence after PPG, that is, manual suture might be better than Stapler. However, we need to perform large-sample clinical trials to verify this in the future. In addition, another study conducted short- and long-term follow-ups of 2898 Japanese patients with EGC in the middle of the stomach who underwent either PPG or distal gastrectomy[61]. It was found that there were no statistically significant differences in mortality, incidence of postoperative complications, and 3-year and 5-year survival rates between the two groups[61]. Meanwhile, Tsujiura et al[62] evaluated the nutritional status of 465 patients undergoing PPG surgery and found that the serum total protein, albumin, and hemoglobin could be maintained at a good level, and the bodyweight ratio could be restored to 93.24% ± 7.29% one year after the surgery. Therefore, PPG can achieve the same therapeutic effects as distal gastrectomy for patients with T1N0 EGC in the middle of the stomach, but the grasp of indications, especially the accuracy of preoperative diagnosis, is an important factor affecting the therapeutic effect.
Diagnosis and treatment of EGC are the key to improving the prognosis of patients. Without affecting the radical effect of EGC, minimally invasive surgery can significantly improve the postoperative life quality of patients. For some patients with EGC, endoscopic resection is a safe and effective treatment. With the publication of JCOG1009/1010 results, the scope of indications for endoscopic therapy will be further expanded, and endoscopy will occupy an indispensable position in the treatment of EGC in the future. For patients with EGC who are not suitable for endoscopic resection or NCR, laparoscopic surgery is an appropriate treatment and may help achieve the same efficacy as traditional open surgery. Of course, though PPG preserves gastric functions and shows great potential in terms of patients’ life quality and curative effects, clinicians still need to be cautious about whether it is suitable for a wide range of clinical applications. It requires strict technical standardization and large-scale, multicenter clinical trials to evaluate its safety and efficacy, hoping to provide a theoretical basis for function-preserving surgery.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Masaki S, Shang Y S-Editor: Yan JP L-Editor: Wang TQ P-Editor: Li JH
| 1. | Tanabe S, Hirabayashi S, Oda I, Ono H, Nashimoto A, Isobe Y, Miyashiro I, Tsujitani S, Seto Y, Fukagawa T, Nunobe S, Furukawa H, Kodera Y, Kaminishi M, Katai H. Gastric cancer treated by endoscopic submucosal dissection or endoscopic mucosal resection in Japan from 2004 through 2006: JGCA nationwide registry conducted in 2013. Gastric Cancer. 2017;20:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 2. | Liu Q, Ding L, Qiu X, Meng F. Updated evaluation of endoscopic submucosal dissection versus surgery for early gastric cancer: A systematic review and meta-analysis. Int J Surg. 2020;73:28-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 110] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 3. | Kim ER, Lee H, Min BH, Lee JH, Rhee PL, Kim JJ, Kim KM, Kim S. Effect of rescue surgery after non-curative endoscopic resection of early gastric cancer. Br J Surg. 2015;102:1394-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Chang JW, Jung DH, Park JC, Shin SK, Lee SK, Lee YC. Long-Term Outcomes and Prognostic Factors of Endoscopic Submucosal Dissection for Early Gastric Cancer in Patients Aged ≥75 Years. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55650] [Article Influence: 7950.0] [Reference Citation Analysis (132)] |
| 6. | Hatta W, Gotoda T, Koike T, Masamune A. History and future perspectives in Japanese guidelines for endoscopic resection of early gastric cancer. Dig Endosc. 2020;32:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 7. | Yang L, Zheng R, Wang N, Yuan Y, Liu S, Li H, Zhang S, Zeng H, Chen W. Incidence and mortality of stomach cancer in China, 2014. Chin J Cancer Res. 2018;30:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 147] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 8. | Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Nunobe S, Kakeji Y, Nashimoto A; Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 354] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 9. | Hong S, Lee YY, Lee J, Kim Y, Choi KS, Jun JK, Suh M. Trends in Cancer Screening Rates among Korean Men and Women: Results of the Korean National Cancer Screening Survey, 2004-2018. Cancer Res Treat. 2021;53:330-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 10. | Information Committee of Korean Gastric Cancer Association. Korean Gastric Cancer Association Nationwide Survey on Gastric Cancer in 2014. J Gastric Cancer. 2016;16:131-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 150] [Article Influence: 16.7] [Reference Citation Analysis (1)] |
| 11. | Wang Y, Li Z, Shan F, Miao R, Xue K, Gao C, Chen N, Gao X, Li S, Ji J. [Current status of diagnosis and treatment of early gastric cancer in China--Data from China Gastrointestinal Cancer Surgery Union]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:168-174. [PubMed] |
| 12. | Wang F, Meng W, Wang B, Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345:196-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 607] [Cited by in RCA: 568] [Article Influence: 51.6] [Reference Citation Analysis (1)] |
| 13. | Choi IJ, Kook MC, Kim YI, Cho SJ, Lee JY, Kim CG, Park B, Nam BH. Helicobacter pylori Therapy for the Prevention of Metachronous Gastric Cancer. N Engl J Med. 2018;378:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 492] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 14. | Fang WL, Chen MH, Huang KH, Lin CH, Chao Y, Lo SS, Li AF, Wu CW, Shyr YM. The Clinicopathological Features and Genetic Alterations in Epstein-Barr Virus-Associated Gastric Cancer Patients after Curative Surgery. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4820] [Article Influence: 438.2] [Reference Citation Analysis (2)] |
| 16. | Kim ST, Cristescu R, Bass AJ, Kim KM, Odegaard JI, Kim K, Liu XQ, Sher X, Jung H, Lee M, Lee S, Park SH, Park JO, Park YS, Lim HY, Lee H, Choi M, Talasaz A, Kang PS, Cheng J, Loboda A, Lee J, Kang WK. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med. 2018;24:1449-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 1155] [Article Influence: 165.0] [Reference Citation Analysis (0)] |
| 17. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2802] [Article Influence: 560.4] [Reference Citation Analysis (5)] |
| 18. | Ono H, Yao K, Fujishiro M, Oda I, Nimura S, Yahagi N, Iishi H, Oka M, Ajioka Y, Ichinose M, Matsui T. Guidelines for endoscopic submucosal dissection and endoscopic mucosal resection for early gastric cancer. Dig Endosc. 2016;28:3-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 405] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 19. | Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1308] [Cited by in RCA: 1324] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 20. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1910] [Article Influence: 238.8] [Reference Citation Analysis (1)] |
| 21. | Hasuike N, Ono H, Boku N, Mizusawa J, Takizawa K, Fukuda H, Oda I, Doyama H, Kaneko K, Hori S, Iishi H, Kurokawa Y, Muto M; Gastrointestinal Endoscopy Group of Japan Clinical Oncology Group (JCOG-GIESG). A non-randomized confirmatory trial of an expanded indication for endoscopic submucosal dissection for intestinal-type gastric cancer (cT1a): the Japan Clinical Oncology Group study (JCOG0607). Gastric Cancer. 2018;21:114-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 22. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer. 2021;24:1-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 735] [Cited by in RCA: 1320] [Article Influence: 330.0] [Reference Citation Analysis (2)] |
| 23. | Guideline Committee of the Korean Gastric Cancer Association (KGCA); Development Working Group & Review Panel. . Korean Practice Guideline for Gastric Cancer 2018: an Evidence-based, Multi-disciplinary Approach. J Gastric Cancer. 2019;19:1-48. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 311] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 24. | Takizawa K, Ono H, Hasuike N, Takashima A, Minashi K, Boku N, Kushima R, Katayama H, Ogawa G, Fukuda H, Fujisaki J, Oda I, Yano T, Hori S, Doyama H, Hirasawa K, Yamamoto Y, Ishihara R, Tanabe S, Niwa Y, Nakagawa M, Terashima M, Muto M; Gastrointestinal Endoscopy Group (GIESG) and the Stomach Cancer Study Group (SCSG) of Japan Clinical Oncology Group. A nonrandomized, single-arm confirmatory trial of expanded endoscopic submucosal dissection indication for undifferentiated early gastric cancer: Japan Clinical Oncology Group study (JCOG1009/1010). Gastric Cancer. 2021;24:479-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 25. | Horiuchi Y, Ida S, Yamamoto N, Nunobe S, Ishizuka N, Yoshimizu S, Ishiyama A, Yoshio T, Hirasawa T, Tsuchida T, Kumagai K, Ohashi M, Sano T, Fujisaki J. Feasibility of further expansion of the indications for endoscopic submucosal dissection in undifferentiated-type early gastric cancer. Gastric Cancer. 2020;23:285-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 26. | Pimentel-Nunes P, Dinis-Ribeiro M, Ponchon T, Repici A, Vieth M, De Ceglie A, Amato A, Berr F, Bhandari P, Bialek A, Conio M, Haringsma J, Langner C, Meisner S, Messmann H, Morino M, Neuhaus H, Piessevaux H, Rugge M, Saunders BP, Robaszkiewicz M, Seewald S, Kashin S, Dumonceau JM, Hassan C, Deprez PH. Endoscopic submucosal dissection: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2015;47:829-854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 925] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 27. | Ajani JA, D'Amico TA, Almhanna K, Bentrem DJ, Chao J, Das P, Denlinger CS, Fanta P, Farjah F, Fuchs CS, Gerdes H, Gibson M, Glasgow RE, Hayman JA, Hochwald S, Hofstetter WL, Ilson DH, Jaroszewski D, Johung KL, Keswani RN, Kleinberg LR, Korn WM, Leong S, Linn C, Lockhart AC, Ly QP, Mulcahy MF, Orringer MB, Perry KA, Poultsides GA, Scott WJ, Strong VE, Washington MK, Weksler B, Willett CG, Wright CD, Zelman D, McMillian N, Sundar H. Gastric Cancer, Version 3.2016, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2016;14:1286-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 678] [Article Influence: 75.3] [Reference Citation Analysis (0)] |
| 28. | Qiu H, Zhou Z. [Updates and interpretation on NCCN clinical practice guidelines for gastric cancer 2017 version 5]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21:160-164. [PubMed] |
| 29. | Zheng Z, Yin J, Li Z, Ye Y, Wei B, Wang X, Tian Y, Li M, Zhang Q, Zeng N, Xu R, Chen G, Zhang J, Li P, Cai J, Yao H, Zhang Z, Zhang S. Protocol for expanded indications of endoscopic submucosal dissection for early gastric cancer in China: a multicenter, ambispective, observational, open-cohort study. BMC Cancer. 2020;20:801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 30. | Sohn SH, Lee SH, Kim KO, Jang BI, Kim TN. Therapeutic outcomes of endoscopic submucosal dissection for early gastric cancer: single-center study. Eur J Gastroenterol Hepatol. 2017;29:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Peng LJ, Tian SN, Lu L, Chen H, Ouyang YY, Wu YJ. Outcome of endoscopic submucosal dissection for early gastric cancer of conventional and expanded indications: systematic review and meta-analysis. J Dig Dis. 2015;16:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 32. | Lee S, Choi KD, Han M, Na HK, Ahn JY, Jung KW, Lee JH, Kim DH, Song HJ, Lee GH, Yook JH, Kim BS, Jung HY. Long-term outcomes of endoscopic submucosal dissection versus surgery in early gastric cancer meeting expanded indication including undifferentiated-type tumors: a criteria-based analysis. Gastric Cancer. 2018;21:490-499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Abdelfatah MM, Barakat M, Lee H, Kim JJ, Uedo N, Grimm I, Othman MO. The incidence of lymph node metastasis in early gastric cancer according to the expanded criteria in comparison with the absolute criteria of the Japanese Gastric Cancer Association: a systematic review of the literature and meta-analysis. Gastrointest Endosc. 2018;87:338-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 102] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 34. | Cho KB, Jeon WJ, Kim JJ. Worldwide experiences of endoscopic submucosal dissection: not just Eastern acrobatics. World J Gastroenterol. 2011;17:2611-2617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Min BH, Kim ER, Kim KM, Park CK, Lee JH, Rhee PL, Kim JJ. Surveillance strategy based on the incidence and patterns of recurrence after curative endoscopic submucosal dissection for early gastric cancer. Endoscopy. 2015;47:784-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 36. | Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Nakajima T, Sekiguchi M, Mori G, Taniguchi H, Sekine S, Katai H, Saito Y. Long-term surveillance and treatment outcomes of metachronous gastric cancer occurring after curative endoscopic submucosal dissection. Endoscopy. 2015;47:1113-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 98] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 37. | Jang MY, Cho JW, Oh WG, Ko SJ, Han SH, Baek HK, Lee YJ, Kim JW, Jung GM, Cho YK. Clinicopathological characteristics of synchronous and metachronous gastric neoplasms after endoscopic submucosal dissection. Korean J Intern Med. 2013;28:687-693. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Sun K, Chen S, Ye J, Wu H, Peng J, He Y, Xu J. Endoscopic resection versus surgery for early gastric cancer: a systematic review and meta-analysis. Dig Endosc. 2016;28:513-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 39. | Jeon MY, Park JC, Hahn KY, Shin SK, Lee SK, Lee YC. Long-term outcomes after noncurative endoscopic resection of early gastric cancer: the optimal time for additional endoscopic treatment. Gastrointest Endosc 2018; 87: 1003-1013. e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakaya N, Nakamura T, Shimosegawa T. A Scoring System to Stratify Curability after Endoscopic Submucosal Dissection for Early Gastric Cancer: "eCura system". Am J Gastroenterol. 2017;112:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 218] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 41. | Hatta W, Gotoda T, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakamura K, Hirano M, Esaki M, Matsuda M, Ohnita K, Shimoda R, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Nakamura T, Shimosegawa T. Is radical surgery necessary in all patients who do not meet the curative criteria for endoscopic submucosal dissection in early gastric cancer? J Gastroenterol. 2017;52:175-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 113] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 42. | Park JW, Ahn S, Lee H, Min BH, Lee JH, Rhee PL, Kim KM, Kim JJ. Predictive factors for lymph node metastasis in early gastric cancer with lymphatic invasion after endoscopic resection. Surg Endosc. 2017;31:4419-4424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 43. | Sekiguchi M, Suzuki H, Oda I, Abe S, Nonaka S, Yoshinaga S, Taniguchi H, Sekine S, Kushima R, Saito Y. Risk of recurrent gastric cancer after endoscopic resection with a positive lateral margin. Endoscopy. 2014;46:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 44. | Horiki N, Omata F, Uemura M, Suzuki S, Ishii N, Fukuda K, Fujita Y, Ninomiya K, Tano S, Katurahara M, Tanaka K, Gabazza EC, Takei Y. Risk for local recurrence of early gastric cancer treated with piecemeal endoscopic mucosal resection during a 10-year follow-up period. Surg Endosc. 2012;26:72-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 45. | Suzuki S, Gotoda T, Hatta W, Oyama T, Kawata N, Takahashi A, Yoshifuku Y, Hoteya S, Nakagawa M, Hirano M, Esaki M, Matsuda M, Ohnita K, Yamanouchi K, Yoshida M, Dohi O, Takada J, Tanaka K, Yamada S, Tsuji T, Ito H, Hayashi Y, Shimosegawa T. Survival Benefit of Additional Surgery After Non-curative Endoscopic Submucosal Dissection for Early Gastric Cancer: A Propensity Score Matching Analysis. Ann Surg Oncol. 2017;24:3353-3360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Suzuki H, Oda I, Abe S, Sekiguchi M, Nonaka S, Yoshinaga S, Saito Y, Fukagawa T, Katai H. Clinical outcomes of early gastric cancer patients after noncurative endoscopic submucosal dissection in a large consecutive patient series. Gastric Cancer. 2017;20:679-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 47. | Kawata N, Kakushima N, Takizawa K, Tanaka M, Makuuchi R, Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Sugino T, Kusafuka K, Shimoda T, Nakajima T, Terashima M, Ono H. Risk factors for lymph node metastasis and long-term outcomes of patients with early gastric cancer after non-curative endoscopic submucosal dissection. Surg Endosc. 2017;31:1607-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 48. | Kikuchi S, Kuroda S, Nishizaki M, Kagawa T, Kanzaki H, Kawahara Y, Kagawa S, Tanaka T, Okada H, Fujiwara T. Management of early gastric cancer that meet the indication for radical lymph node dissection following endoscopic resection: a retrospective cohort analysis. BMC Surg. 2017;17:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Kang HJ, Chung H, Kim SG, Kim J, Kim JL, Lee E, Jung HC. Synergistic Effect of Lymphatic Invasion and Venous Invasion on the Risk of Lymph Node Metastasis in Patients with Non-Curative Endoscopic Resection of Early Gastric Cancer. J Gastrointest Surg. 2020;24:1499-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Niwa H, Ozawa R, Kurahashi Y, Kumamoto T, Nakanishi Y, Okumura K, Matsuda I, Ishida Y, Hirota S, Shinohara H. The eCura system as a novel indicator for the necessity of salvage surgery after non-curative ESD for gastric cancer: A case-control study. PLoS One. 2018;13:e0204039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 51. | Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Katai H, Mizusawa J, Katayama H, Takagi M, Yoshikawa T, Fukagawa T, Terashima M, Misawa K, Teshima S, Koeda K, Nunobe S, Fukushima N, Yasuda T, Asao Y, Fujiwara Y, Sasako M. Short-term surgical outcomes from a phase III study of laparoscopy-assisted versus open distal gastrectomy with nodal dissection for clinical stage IA/IB gastric cancer: Japan Clinical Oncology Group Study JCOG0912. Gastric Cancer. 2017;20:699-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 285] [Article Influence: 35.6] [Reference Citation Analysis (1)] |
| 53. | Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Kim CY, Yang HK, Park DJ, Song KY, Lee SI, Ryu SY, Lee JH; Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Prospective randomized controlled trial (phase III) to comparing laparoscopic distal gastrectomy with open distal gastrectomy for gastric adenocarcinoma (KLASS 01). J Korean Surg Soc. 2013;84:123-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, Yu P, Li Y, Suo J, Zhao N, Zhang W, Li H, He H, Sun Y; Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group. Morbidity and Mortality of Laparoscopic vs Open Total Gastrectomy for Clinical Stage I Gastric Cancer: The CLASS02 Multicenter Randomized Clinical Trial. JAMA Oncol. 2020;6:1590-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 55. | Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D; ESMO Guidelines Committee. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38-v49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1114] [Article Influence: 123.8] [Reference Citation Analysis (0)] |
| 56. | Yamasaki M, Takiguchi S, Omori T, Hirao M, Imamura H, Fujitani K, Tamura S, Akamaru Y, Kishi K, Fujita J, Hirao T, Demura K, Matsuyama J, Takeno A, Ebisui C, Takachi K, Takayama O, Fukunaga H, Okada K, Adachi S, Fukuda S, Matsuura N, Saito T, Takahashi T, Kurokawa Y, Yano M, Eguchi H, Doki Y. Multicenter prospective trial of total gastrectomy versus proximal gastrectomy for upper third cT1 gastric cancer. Gastric Cancer. 2021;24:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 57. | Oh SY, Lee HJ, Yang HK. Pylorus-Preserving Gastrectomy for Gastric Cancer. J Gastric Cancer. 2016;16:63-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 58. | Eom BW, Park B, Yoon HM, Ryu KW, Kim YW. Laparoscopy-assisted pylorus-preserving gastrectomy for early gastric cancer: A retrospective study of long-term functional outcomes and quality of life. World J Gastroenterol. 2019;25:5494-5504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (2)] |
| 59. | Huang C, Yu F, Zhao G, Xia X. Postoperative quality of life after laparoscopy-assisted pylorus-preserving gastrectomy compared with laparoscopy-assisted distal gastrectomy for early gastric cancer. J Gastroenterol Hepatol. 2020;35:1712-1719. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 60. | Isozaki H, Okajima K, Momura E, Ichinona T, Fujii K, Izumi N, Takeda Y. Postoperative evaluation of pylorus-preserving gastrectomy for early gastric cancer. Br J Surg. 1996;83:266-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 61. | Aizawa M, Honda M, Hiki N, Kinoshita T, Yabusaki H, Nunobe S, Shibasaki H, Matsuki A, Watanabe M, Abe T. Oncological outcomes of function-preserving gastrectomy for early gastric cancer: a multicenter propensity score matched cohort analysis comparing pylorus-preserving gastrectomy versus conventional distal gastrectomy. Gastric Cancer. 2017;20:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 62. | Tsujiura M, Hiki N, Ohashi M, Nunobe S, Kumagai K, Ida S, Hayami M, Sano T, Yamaguchi T. Excellent Long-Term Prognosis and Favorable Postoperative Nutritional Status After Laparoscopic Pylorus-Preserving Gastrectomy. Ann Surg Oncol. 2017;24:2233-2240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (2)] |