Published online Apr 15, 2021. doi: 10.4251/wjgo.v13.i4.265
Peer-review started: December 23, 2020
First decision: February 14, 2021
Revised: February 16, 2021
Accepted: March 22, 2021
Article in press: March 22, 2021
Published online: April 15, 2021
Processing time: 106 Days and 23.9 Hours
Remnant gastric cancer (RGC) is a carcinoma arising in the stomach remnant after previous gastric resection. It is frequently reported as a tumor with a poor prognosis and distinct biological features from primary gastric cancer (PGC). However, as it is less frequent, its profile regarding the current molecular classifications of gastric cancer has not been evaluated.
To evaluate a cohort of RGC according to molecular subtypes of GC using a panel of immunohistochemistry and in situ hybridization to determine whether the expression profile is different between PGC and RGC.
Consecutive RGC patients who underwent gastrectomy between 2009 and 2019 were assessed using seven GC panels: Epstein-Barr virus in situ hybridization, immunohistochemistry for mismatch repair proteins (MutL homolog 1, MutS homolog 2, MutS homolog 6, and PMS1 homolog 2), p53 protein, and E-cadherin expression. Clinicopathological characteristics and survival of these patients were compared to 284 PGC patients.
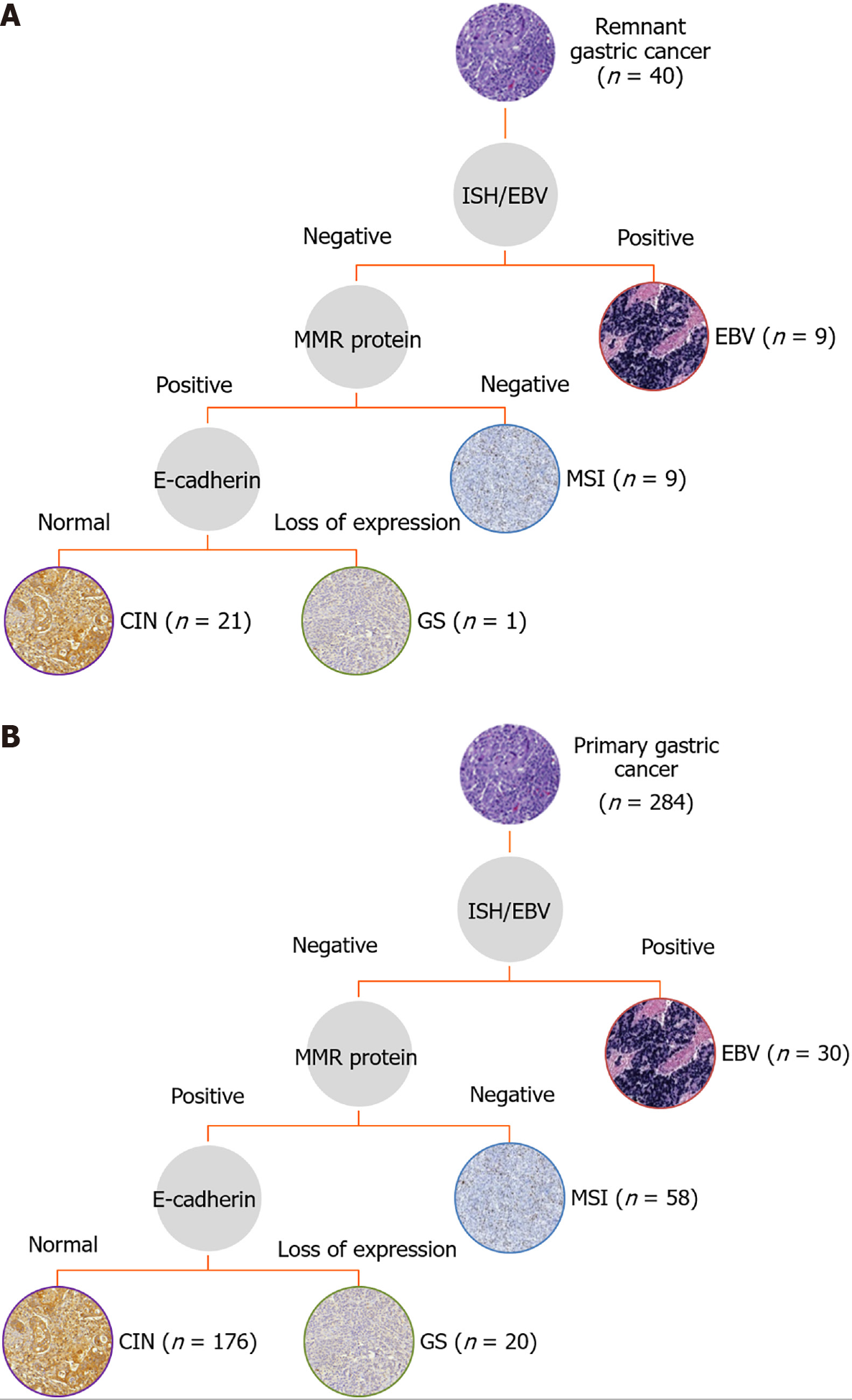
A total of 40 RGC patients were enrolled in this study. Compared to PGC, older age (P < 0.001), male (P < 0.001), lower body mass index (P = 0.010), and lower hemoglobin level (P < 0.001) were associated with RGC patients. No difference was observed regarding Lauren’s type and pathologic Tumor Node Metastasis stage between the groups. Regarding the profiles evaluated, EBV-positive tumors were higher in RGC compared to PGC (P = 0.039). The frequency of microsatellite instability, aberrant p53 immunostaining, and loss of E-cadherin expression were similar between RGC and PGC. Higher rates of simultaneous alterations in two or more profiles were observed in RGC compared to PGC (P < 0.001). According to the molecular classification, the subtypes were defined as EBV in nine (22.5%) cases, microsatellite instability in nine (22.5%) cases, genomically stable in one (2.5%) case, and chromosomal instability in 21 (52.5%) cases. There was no significant difference in survival between molecular subtypes in RGC patients.
RGC was associated with EBV positivity and higher rates of co-altered expression profiles compared to PGC. According to the molecular classification, there was no significant difference in survival between the subtypes of RGC.
Core Tip: This is a retrospective study to evaluate the molecular subtypes of gastric cancer (GC) using a panel by immunohistochemistry and in situ hybridization in remnant GC (RGC). We also compared the RGC patients with primary GC (PGC) to investigate whether the expression profiles were different between both types of GC. The findings indicated that RGC was associated with the Epstein-Barr virus. RGC also exhibited higher rates of simultaneous changes in two or more profiles compared to PGC. However, molecular subtypes of GC had no significant prognostic impact in terms of survival in RGC patients.
- Citation: Ramos MFKP, Pereira MA, Cardili L, de Mello ES, Ribeiro Jr U, Zilberstein B, Cecconello I. Expression profiles of gastric cancer molecular subtypes in remnant tumors. World J Gastrointest Oncol 2021; 13(4): 265-278
- URL: https://www.wjgnet.com/1948-5204/full/v13/i4/265.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i4.265
Gastric cancer (GC) remains one of the most frequent malignancies and the third-leading cause of cancer-related deaths[1]. Remnant GC (RGC) accounts for 1%-8% of all GC and is defined as a carcinoma arising in the stomach remnant after the previous gastrectomy for benign disease or after 5 years of previous GC resection[2-4].
In recent years, the number of patients who underwent gastrectomy for peptic ulcers has declined due to the improvement of drug therapy for duodenal and gastric ulcers. However, it is expected that the incidence of RGC after partial gastrectomy for malignant disease will increase, largely attributable to the increase in the number of patients diagnosed with early GC based on routine examinations and due to the improvement in the prognosis of patients with GC[5].
RGC has potential differences in carcinogenesis from primary GC (PGC). Duodenogastric reflux is considered one of the most important etiologic factors after Billroth II reconstruction, where both bile acids and pancreatic juice seem to be carcinogenic factors[6]. Denervation of the gastric mucosa also promotes carcinogenesis in the remnant stomach. In RGC after GC, some precancerous circumstances that already have existed at the time of initial surgery—such as atrophic gastritis and intestinal metaplasia—are considered factors associated with the tumor development in the residual stomach[2,7].
RGC is frequently diagnosed at an advanced stage and is associated with a low rate of curative resection and dismal prognosis, suggesting that RGC may have distinct biological features from PGC[5,8,9]. Thus, difficulties in predicting the prognosis and the best therapeutic approach in these patients are still challenges found in clinical practice.
Recently, GC was classified into four distinct molecular subtypes according to The Cancer Genome Atlas study: Epstein-Barr virus (EBV), microsatellite instability (MSI), genomically stable (GS), and chromosomal instability (CIN). This new classification elucidated the heterogeneity that GC can present with subtypes with distinct characteristics, prognosis, and therapeutic response[10,11]. However, as RGC occurs less often, its profiles, distribution of molecular subtypes, and prognostic impact have not been evaluated.
Therefore, in this study, we analyzed a cohort of RGC according to molecular subtypes of GC using immunohistochemistry (IHC) and in situ hybridization (ISH). Clinical and pathological results were also compared with PGC to determine whether the expression profile and prognosis are different between PGC and RGC.
All GC patients who underwent gastrectomy at our Institute between 2009 and 2019 were retrospectively evaluated. Inclusion criteria were: (1) Tumor located in the gastric remnant; (2) Curative intent resection; (3) Histological diagnosis of adenocarcinoma; and (4) Formalin-fixed paraffin-embedded tissue blocks available for analysis.
Patients with RGC were enrolled in this study, regardless of the previous resection due to benign or malignant disease. Patients with PGC who underwent D2-gastrectomy with curative intent were selected as the comparison group. Palliative resections, non-adenocarcinoma histology, Siewert I-II tumors, and patients with the previous resection due to GC less than 5 years apart were excluded.
The clinical variables collected included age, sex, preoperative body mass index (BMI), albumin level, hemoglobin level, neutrophil-lymphocyte ratio, American Society of Anesthesiologists classification, and comorbidities following Charlson-Deyo Comorbidity Index (without the inclusion of age and GC as comorbidity)[12]. Clinicopathological and follow-up data of these patients were collected from our database of GC patients.
The preoperative staging was performed through abdominal and pelvis computed tomography, endoscopy, and laboratory tests. Adjuvant or perioperative platinum-based chemotherapy (CMT) was administered according to clinical indication (T3/T4 and/or N+).
Gastrectomy and lymphadenectomy extension followed the recommendations of guidelines and were defined by the attending surgeon to achieve a complete R0 resection[3]. All patients were operated at a high-volume center by specialist surgeons. The final stage was classified according to the 8th edition of the Tumor Node Metastasis (TNM)-Union for International Cancer Control-American Joint Committee on Cancer classification[13].
Postoperative follow-up appointments were performed every 3 mo for the first year, and every 6 mo in the following years. Follow-up image tests for recurrence detection were performed based on the presence of symptoms. Lost to follow-up was defined as an absence for more than 12 mo in follow-up appointments. The study protocol was approved by the Institutional Review Board at our Hospital (CAAE: 37009120. 0.0000.0068).
Hematoxylin and eosin (HE) stained slides from all patients selected were reviewed for tissue microarray construction. Briefly, three representative tumor cores (1 mm in diameter) and two non-tumor gastric mucosa cores were obtained from the formalin-fixed paraffin-embedded tissue block in each case. Tissue cylinders were punched from representative tissue areas of each donor tissue block and brought into one recipient paraffin block using a precision mechanized system. Serial 4 μm sections were prepared and used for HE staining, IHC, and ISH.
The primary antibodies used for IHC were anti-MutL homolog 1 (MLH1) (clone M1), anti-MutS homolog 2 (MSH2) (clone G219-1129), anti-MutS homolog 6 (MSH6) (clone 44), anti-PMS1 homolog 2 (PMS2) (clone EPR 3947), anti-E-cadherin (clone 36B5), and anti-p53 (clone DO-7).
MLH1, MSH2, MSH6, and PMS2 IHC were performed with a BenchMark ULTRA fully automated slide processing system (Ventana, Oro Valley, AZ, United States) according to the manufacturer’s instructions.
For p53 and E-cadherin staining, the sections were deparaffinized in xylene, dehydrated with graded ethanol, and then immersed in methanol with 0.3% hydrogen peroxidase to block endogenous peroxidase activity. Antigen retrieval was carried out in a pressure cooker. The slides were then incubated overnight at 4 °C with the primary antibody. Avidin-biotin-free short polymer-based peroxidase amplification system and diaminobenzidine solution as chromogen were used for the development of reaction products. The sections were counterstained with hematoxylin.
GC was defined as MSI only if at least one of the markers showed a complete absence of nuclear staining in the tumor cells. Tumors that maintained expressions of mismatch repair (MMR) protein were considered to be microsatellite stable.
E-cadherin was evaluated using a score of 0 to 3 (0 = complete loss; 1 = cytoplasmic expression; 2 = cytoplasmic and membrane labeling; 3 = membrane labeling). Scores 0 and 1 were considered as loss of expression[14]. The p53 immunoexpression was assessed as previously described and classified in p53-normal or p53-aberrant (strong nuclear staining in > 70% of tumor cells or loss of p53 expression)[15].
The presence of EBV infection was determined by ISH using probes against Epstein-Barr encoded ribonucleic acid 1 (EBER1-Y5200). Cases with dark-blue staining in tumor cell nuclei were classified as EBV-positive.
All analyses were carried out by two pathologists. When divergence occurred, a third one was consulted, and slides were re-evaluated using a multiheaded microscope.
According to the results of the IHC and ISH, the subtypes of GC were classified according to the following hierarchy: EBV-positive (positive by ISH), MSI (loss of MMR protein expression), GS (loss of E-cadherin expression), and CIN (remaining tumors, including those with p53-aberrant expression)[10,16].
Statistical analysis was performed using SPSS software (v.20.0; Armonk, NY, United States). The independent-samples t-test, Mann-Whitney, or analysis of variance test was used for continuous data, and the chi-squared test and Fisher’s exact test were used for categorical data, as appropriate.
Survival curves were analyzed using the Kaplan-Meier method, and survival rates were compared between groups using the log-rank test. Disease-free survival (DFS) was defined as the interval between the date of surgery and the date of recurrence, death, or the last observation. Overall survival (OS) was calculated from the time of surgery until death or the last observation for surviving patients. All tests were two-sided, and differences were considered significant when P < 0.05.
A total of 40 RGC patients who met the inclusion criteria were enrolled in this study. The previous gastrectomy was due to benign disease in 36 (90%) cases and adenocarcinoma in the remaining 4 (10%) cases. The type of the previous reconstruction was Billroth II and Roux-en-Y in 38 and 2 patients, respectively.
The mean age of patients at the first surgery was 35.2 years [standard deviation (SD) = 13.7, range 19-73.7 years], and the average time from the previous operation to the diagnosis of RGC was 33.8 years (SD = 12.2, range 5.8-54.5 years). In 6 cases, the tumor in the remnant was located in the cardia (Siewert III) and in the body/fundus in the remaining 34 cases.
For the PGC group, a total of 284 GC patients were included for analyses, of which 60.6% underwent subtotal gastrectomy, and the remaining 39.4% underwent total gastrectomy. Clinicopathological and surgical characteristics of the RGC and PGC groups are shown in Table 1.
| Primary GC | Remnant GC | ||
| Variables | n 284 (%) | n 40 (%) | P value |
| Sex | < 0.001a | ||
| Female | 118 (41.5) | 5 (12.5) | |
| Male | 166 (58.5) | 35 (87.5) | |
| Age (yr) | < 0.001a | ||
| mean (SD) | 61.6 (12) | 69.0 (7.7) | |
| BMI (kg/cm²) | 0.010a | ||
| mean (SD) | 24.4 (5.4) | 22.0 (4.1) | |
| Albumin (g/dL) | 0.241 | ||
| mean (SD) | 4.2 (2.0) | 3.8 (0.6) | |
| Hemoglobin (g/dL) | < 0.001a | ||
| mean (SD) | 12.3 (2.2) | 10.6 (2.1) | |
| Neutrophil lymphocyte ratio | 0.600 | ||
| mean (SD) | 2.56 (2.25) | 2.75 (1.20) | |
| Charlson-Deyo Comorbidity Index | 0.956 | ||
| 0-1 | 200 (70.4) | 28 (70) | |
| > 1 | 84 (29.6) | 12 (30) | |
| ASA classification | 0.136 | ||
| I/II | 251 (88.4) | 32 (80) | |
| III/IV | 33 (11.6) | 8 (20) | |
| Tumor size (cm) | 0.206 | ||
| mean (SD) | 4.87 (3.14) | 5.6 (3.3) | |
| Lauren’s type | 0.764 | ||
| Intestinal | 149 (52.5) | 22 (55) | |
| Diffuse/mixed | 135 (47.5) | 18 (45) | |
| Grade of histological differentiation | 0.709 | ||
| Well/moderately differentiated | 126 (44.4) | 19 (47.5) | |
| Poorly differentiated | 158 (55.6) | 21 (52.5) | |
| Lymphatic invasion | 0.900 | ||
| No | 138 (48.9) | 20 (50) | |
| Yes | 144 (51.1) | 20 (50) | |
| Venous invasion | 0.292 | ||
| No | 188 (66.7) | 30 (75) | |
| Yes | 94 (33.3) | 10 (25) | |
| Perineural invasion | 0.642 | ||
| No | 144 (51.4) | 19 (47.5) | |
| Yes | 136 (48.6) | 21 (52.5) | |
| Number of lymph nodes | < 0.001a | ||
| mean (SD) | 41.9 (17.8) | 23.4 (13.5) | |
| pT | 0.590 | ||
| T1/T2 | 112 (39.4) | 14 (35) | |
| T3/T4 | 172 (60.6) | 26 (65) | |
| pN | 0.617 | ||
| N0 | 123 (43.3) | 19 (47.5) | |
| N+ | 161 (56.7) | 21 (52.5) | |
| pTNM | 0.518 | ||
| I/II | 155 (54.6) | 24 (60) | |
| III/IV | 129 (45.4) | 16 (40) |
Older age (P < 0.001), male (P < 0.001), lower BMI (P = 0.010), and lower hemoglobin level (P < 0.001) were associated with RGC patients. On average, 41.9 (± 17.8) and 23.4 (± 13.5) lymph nodes were dissected in PGC and RGC cases, respectively (P < 0.001). No difference was observed regarding Lauren’s type, depth of tumor invasion, lymph node metastasis, and pathologic TNM stage between the groups. Regarding CMT, 52.8% of patients in the PGC group and 30% of patients in the RGC group received some perioperative or adjuvant treatment (P = 0.007).
Concerning the IHC and ISH results (Table 2), a higher incidence of EBV-positive tumors was found in RGC compared to PGC (P = 0.039). Although not significant, the aberrant p53 immunostaining was seen most frequently in the RGC (50% vs 37.2%, P = 0.121). The frequency of MSI and loss of E-cadherin expression were also similar between groups. When evaluating the number of alterations in the expression of one or more profiles (EBV, MSI, E-cadherin, and p53), the RGC had a greater number of cases with simultaneous alteration compared to the PGC. Figure 1 shows the frequency of cases according to the profiles evaluated in the RGC and PGC groups and the co-altered expression profiles.
| Primary GC | Remnant GC | ||
| Variables | n 284 (%) | n 40 (%) | P value |
| EBV | 0.039a | ||
| Negative | 254 (89.4) | 31 (77.5) | |
| Positive | 30 (10.6) | 9 (22.5) | |
| MSI/MSS status | 0.362 | ||
| MSS | 224 (78.9) | 29 (72.5) | |
| MSI | 60 (21.1) | 11 (27.5) | |
| p53 | 0.121 | ||
| Normal | 177 (62.8) | 20 (50) | |
| Aberrant | 105 (37.2) | 20 (50) | |
| E-cadherin | 0.565 | ||
| Normal | 255 90.7) | 35 (87.5) | |
| Loss of expression | 26 (9.3) | 5 (12.5) | |
| Profiles with altered expression (EBV, MSI, E-cadherin, p53) | < 0.001a | ||
| One or none | 261 (91.9) | 26 (65) | |
| Two or more | 23 (8.1) | 14 (35) | |
| Number of altered profiles | - | ||
| 0 | 86 (30.3) | 13 (32.5) | |
| 1 | 175 (61.6) | 13 (32.5) | |
| 2 | 23 (8.1) | 11 (27.5) | |
| 3 | 0 (0) | 2 (5) | |
| 4 | 0 (0) | 1 (2.5) |
After the assessment of the expression profiles, the patients were divided according to the four molecular subtypes proposed by the Cancer Genome Atlas (Figure 2). For RGC, the subtypes were defined as EBV in 9 (22.5%) cases, MSI in 9 (22.5%) cases, GS in 1 (2.5%) case, and CIN in 21 (52.5%) cases. For PGC, the subtypes were determined as EBV in 30 (10.6%) cases, MSI in 58 (20.4%) cases, GS in 20 (7%) cases, and CIN in 176 (62%) cases. Clinical and pathological characteristics of RGC patients according to the molecular subtypes are presented in Supplementary Table 1. No statistical difference was observed between groups for the variables evaluated, including sex, age, histological type, and TNM stage. The presence of changes in p53 expression was greater in the MSI group (P = 0.044).
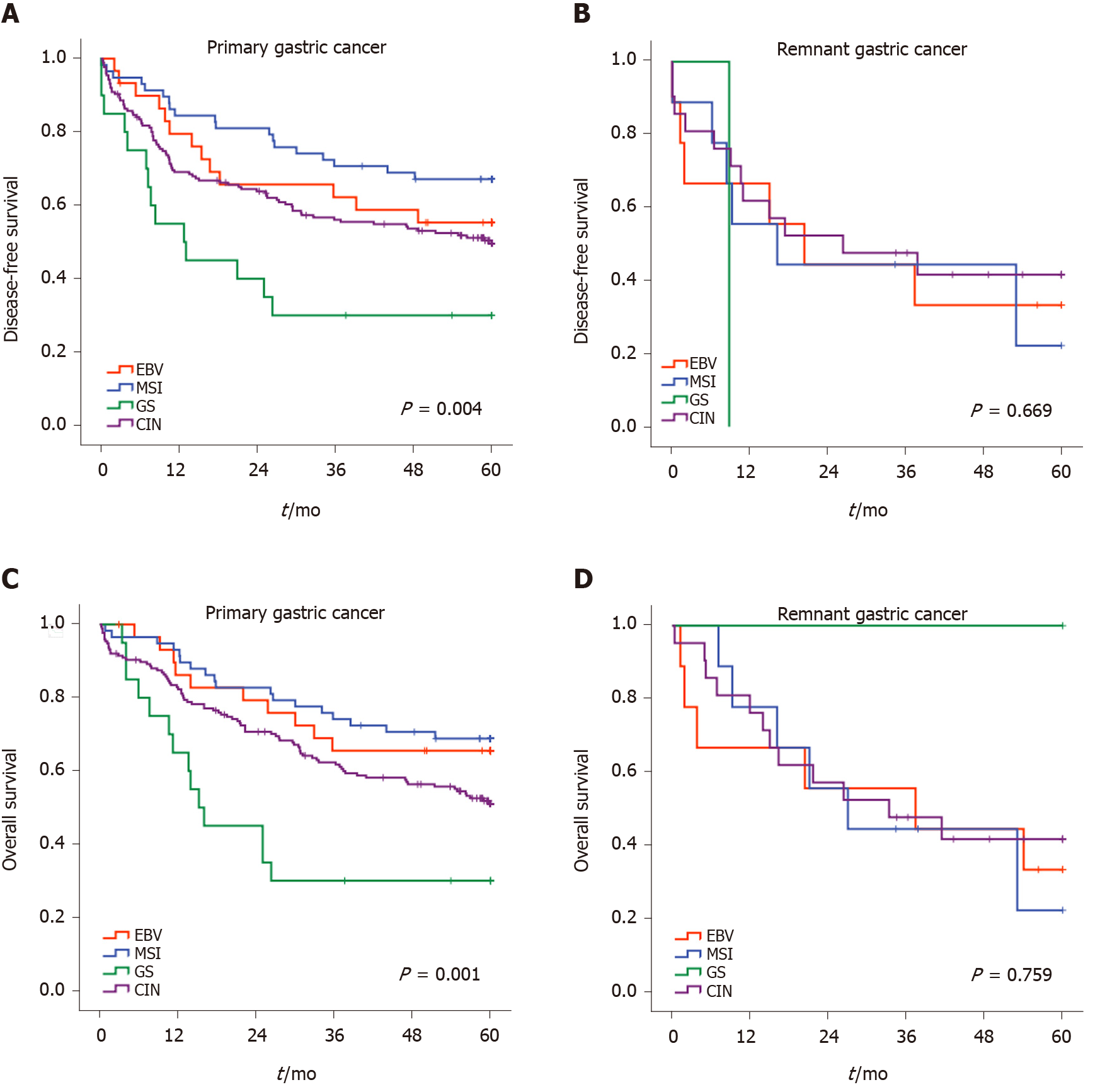
The median follow-up period for the entire cohort was 51.6 mo. The DFS rates for PGC and RGC were 53.3.5% and 34.9%, respectively (P = 0.022). OS survival rates were 55.6% and 36.6% for PGC and RGC, respectively (P = 0.028).
Regarding the recurrence site, among the 17 RGC patients with relapse of disease, they had a predominantly peritoneal recurrence (52.9%), followed by locoregional (41.2%) and distance recurrence (35.3%). Of the 79 PGC patients with relapse, a higher frequency of locoregional recurrence (44.3%), and an equal proportion of peritoneal and distant recurrence (40.5% for both sites) were observed. Also, 22.8% (18 out of 79 cases) and 23.5% (4 out of 17 cases) of patients in the PGC and RGC groups had a relapse of the disease in two or more sites.
According to the classification of GC subtypes, the survival curves for RGC and PGC are demonstrated in Figure 3. In the RGC group, the subtypes of molecular classification did not identify subsets of patients in the DFS analysis. The median DFS were 20.4, 16.2, 8.8, and 26.3 mo for EBV, MSI, GS, and CIN groups, respectively (P = 0.669). Conversely, molecular subtypes were significantly associated with DFS in PGC (P = 0.004). A better DFS rate was observed for MSI, while the GS subtype had worse survival.
For OS, the Kaplan-Meier plot for survival also showed overlapping curves for subtypes in RGC. The median OS for EBV, MSI, and CIN groups were 37.4, 27.0, and 33.3 mo, respectively and not reached for the GS group (P = 0.759). In PGC, we observed a relatively ordered distribution of OS according to the subtypes, with better survival attributed to the MSI, followed by EBV, CIN, and GS subtype (P = 0.001).
In the present study, the distinct GC molecular subtypes were investigated in 40 patients with RGC and compared to PGC using routinely applicable techniques. Our results demonstrated that RGCs, based on the panel evaluated, comprise a group of tumors with a more varied spectrum of changes in their expression profile compared to PGC, which may be due to differences related to the process of carcinogenesis in this type of GC.
Currently, new classifications based on molecular subtypes have provided a convenient screening tool and facilitate the development of targeted agents in clinical trials[11,17]. However, RGC is often excluded from trials due to surgical changes related to lymph node spread and due to their potential differences in molecular car
Consequently, even though determining molecular marker may be important to identify patients susceptible to developing RGC in clinical practice[19], these subtypes and characteristics of profile expression have not been fully exploited in RGC.
Some molecular alterations have been described in RGC, mainly related to changes in the remnant stomach following distal gastrectomy that contribute to tumor development[6]. Among these factors, EBV plays a role in gastric carcinogenesis, particularly in RGC[20]. EBV-associated GC represents about 10% of GC cases worldwide[10], and a high prevalence of EBV positivity in RGC has been reported recently—ranging from 22.2%-41.8%[20,21]. In our cohort, 22.5% of RGC patients were positive for EBV, which was significantly higher compared to PGC (10.6% in our PGC), confirming the relationship between EBV and RGC. It has been suggested that repetitive injuries to the gastric mucosa, such as bile reflux and changes in the microenvironment, may be involved in the development of EBV-associated GC in the remnant stomach[20,22].
Atrophic gastritis of remnant stomach, especially after Billroth II anastomosis, is considered the carcinogenic background for EBV-positive RGC. Tanigawa et al[21] reported in a series of RGC after distal gastrectomy for peptic ulcer or GC that Billroth II cases were frequently associated with EBV infection. In our study, the type of the previous reconstruction was Billroth II in 95% of cases, and we also found a high rate of EBV infection. The changes of anatomical circumstances in RGC may be the main factor, as they directly change the physiological environment and pH value of the gastric remnant. These could act as a cofactor mediating EBV infection of the epithelial cells or facilitate EBV entering the mucosa epithelia by inducing fusion of EBV carrying B cells and epithelial cells[23].
Since the time for tumor development is usually long, and the mean interval between the first and the second surgery may be over 30 years, RGC is often associated with older age of presentation[2,4]. Accordingly, epigenetic abnormalities may accumulate in apparently normal tissue over time, where deoxyribonucleic acid (DNA) methylation often correlates with the risk of carcinogenesis[4,24]. As a result, carcinogenesis in the remnant stomach is also found to be associated with the MSI pathway due to the inactivation of the DNA MMR system[25,26]. In our study, patients with RGC were older than PGC (69 years vs 61.6 years, P < 0.001), and the time between the first and second surgery for RGC was long (mean of 33.8 years). However, although our frequency of MSI in RGC is higher than that described for GC in the literature, no statistical difference in MSI was observed compared with our cohort of PGC (27% vs 21.1%, P = 0.362, respectively). This may be due to the greater proportion of distal tumors among PGC (> 60%), which are generally associated with the presence of MSI. When compared only to primary proximal tumors, a previous study showed that the MSI rate is higher in RGC (27.5% vs 9.4%, P = 0.022)[27].
In addition to MLH1, other genes may also be associated with hypermethylation in RGCs, such as the E-cadherin[19]. E-cadherin plays an important role in cell adhesion among epithelial cells, and its mutations are associated with hereditary diffuse GC and sporadic GC[14]. A previous study with RGC showed that E-cadherin hypermethylation was significantly higher in initially obtained specimens of non-cancerous mucosa from patients with the previous diagnosis of benign disease than in all other specimens of non-cancerous mucosa[19]. DNA methylation is well known to occur as part of carcinogenesis, and risk accumulation in the surrounding non-cancerous mucosa is thought to lead to cancer[19,28]. In our study, only one patient experienced a single loss of E-cadherin expression while the others had associated positive EBV or MSI, which suggests a predominance of alterations by DNA methylation in these patients. Thus, it is suggested that establishing a risk model for the investigation of hypermethylation of genes involved in gastric carcinogenesis could help in post-gastrectomy endoscopic surveillance in these patients.
Remarkably, half of our RGC patients had an aberrant p53 expression. A similar result was found in a previous analysis, where p53 overexpression was observed in 51.1% of RGC patients[21]. Indeed, p53 alterations are more commonly described in proximal tumors than in distal ones, suggesting that the molecular events leading to the development of GC may be different in proximal vs distal tumors[15]. Furthermore, it is suggested that p53 alterations occur early in the development of gastric carcinoma, being present even in the non-neoplastic mucosa and increasing in frequency during the pathway of development and progression of GC[3,21]. The presence of positive epithelial cells for p53 is known to be present in inflammatory conditions, and the increase of p53 positive cells in the anastomosis area—especially after BII reconstruction—was suggested as a reflection of increased DNA damage by active gastritis[21]. In invasive GC, the p53 immunoreactivity is seen in 17%-90.7% of cases[15]. In this sense, surveillance of the status of p53 in follow-up endoscopic biopsies could serve as a marker for early detection in patients with a previous gastrectomy.
Regarding the other clinicopathological variables, due to the often late diagnosis, RGC is commonly related to advanced stages and worse prognostic compared to those for primary GC[5,8,9]. In our cohort, older age, male patients, lower BMI, lower hemoglobin level, and an inferior number of resected lymph nodes were related to patients with RGC. No difference, however, was observed concerning the TNM stage between patients, which have also been described in other series[29-31]. Despite the similar stage, fewer patients in the RGC group received chemotherapeutic treatment. A lower rate of adjuvant treatment in the RGC was also reported by other authors[32]. This may be attributed to the advanced age and postoperative complications related to the completion of gastrectomy, which together with other factors contribute to the lower adherence to CMT in these patients[33].
The prognostic impact of RGC has long been a matter of debate[2,9]. Compared to PGC, patients with RGC had worse survival in our study. Regarding the profiles evaluated, previous studies reported that survival was relatively better in MSI and EBV GC[10,16], although this was not observed in our RGC patients. This finding may be partially attributed to the low number of cases available. Another factor that may likely contribute to this finding was the high number of markers that were simultaneously altered in RGC. In our study, 35% of RGC patients had changes in expression related to two or more profiles, while in primary tumors, only 8% exhibited simultaneous changes for EBV status, MSI, p53, and E-cadherin expression.
Some hypotheses may explain these differences. It was found that the binding of the proteins encoded by the EBV to p53 can inactivate this tumor suppressor pathway[34]. The EBV encoded Epstein-Barr nuclear antigen-5 protein (also designated EBNA-LP), which can form a molecular complex with the p53 tumor suppressor protein, may cause a prolonged lifespan of wild-type p53 in the EBV-infected remnant carcinomas, resulting in positive p53 IHC[34]. The increased DNA damage associated with the MSI in RGC development may also contribute to p53 positivity, which is known to be present in inflammatory conditions. In our study, 44.4% and 88.9% of patients in the EBV and MSI subtypes had aberrant p53 expression, respectively—both superior to that found in the CIN group (38.1%), which was expected to have a predominance of p53 abnormal expression. Intriguingly, although EBV infection and MSI are reported as mutually exclusive in PGC[10,11], we found that 5% of RGC were both EBV-positive and MSI, compared to 0.7% in PGC. Still, the loss of E-cadherin expression was also evidenced along with alterations in other markers in RGC, suggesting that alterations in expression may be predominantly related to methylation events.
The present study has some limitations. Firstly, there was selection bias in this study due to its retrospective nature. Only cases that underwent gastrectomy were included, so we do not know whether non-resected patients and those undergoing palliative treatment have different characteristics from patients undergoing curative treatment. Secondly, the sample size was relatively small. In this sense, some analyses were limited. We did not analyze according to the indication for the previous operation or the previous reconstruction method. The GC panel was evaluated through the tissue microarray construction, in which representative tumor samples were selected. However, we examined three tissue cores per case, as previously recommended[35], to avoid bias due to tumor heterogeneity.
As for strengths, we investigated in RGC patients a classification of tumors into four groups based on applicable and easily reproducible techniques in clinical practice. All patients were treated in a single western center, and we provide a cohort of PGC as a comparative group. This allows a precise estimate of the IHC/ISH diagnostic accuracy and lower prognostic influence of surgical complications and perioperative care.
Thus, clarifying the characteristics and expression profile of RGC is still required to improve oncological outcomes in affected patients. As the frequency of simultaneous changes in the expression determined by IHC and ISH that characterize more than one molecular subtype is high, further investigations with the addition of other molecular techniques should be conducted to assess which markers could best define the GC subtypes and stratify patients with RGC into the appropriate screening, surveillance, or treatment programs.
RGC was associated with EBV positivity and higher rates of co-altered expression profiles compared to PGC. There was no difference in survival in RGC according to the molecular subtypes of GC.
Remnant gastric cancer (RGC) is defined as a carcinoma arising in the stomach remnant after a previous gastrectomy. Despite the improvement in diagnosis and treatment, difficulties in predicting the prognosis and the best therapeutic approach in RGC patients are still challenges in clinical practice.
New classifications based on molecular subtypes have provided a promising prognostic tool and facilitate the development of targeted agents in clinical trials. However, gastric cancer (GC) profiles and the distribution of molecular subtypes have not been evaluated for RGC.
This study aimed to evaluate RGC according to molecular subtypes and determine whether the expression profile is different between RGC and primary GC (PGC).
RGC patients who underwent gastrectomy between 2009 and 2019 were assessed using a panel of immunohistochemistry (IHC) and in situ hybridization (ISH): Epstein-Barr virus (EBV) ISH, IHC for mismatch repair proteins (MutL homolog 1, MutS homolog 2, MutS homolog 6, and PMS1 homolog 2), p53 protein, and E-cadherin expression.
A total of 40 RGC patients were included, and 284 PGC served as a comparison group. EBV-positive tumors were higher in RGC compared to PGC (P = 0.039). The frequency of microsatellite instability, aberrant p53 immunostaining, and loss of E-cadherin expression were similar between RGC and PGC. Higher rates of simultaneous changes in two or more profiles were observed in RGC compared to PGC. According to the molecular classification, there was no significant difference in survival between the subtypes of RGC.
The presence of EBV-positive was significantly higher in patients with RGC compared to PGC. In addition, they also exhibited higher rates of co-altered expression profile profiles compared to PGC.
Our findings provide new data regarding the profiles of RGC according to the subtypes of molecular classification, reflecting potential differences from PGC that may assist in determining which markers could best define GC subtypes and stratify patients with RGC to the appropriate screening and treatment programs.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Merrett ND S-Editor: Gao CC L-Editor: Filipodia P-Editor: Li X
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55853] [Article Influence: 7979.0] [Reference Citation Analysis (132)] |
| 2. | Ohira M, Toyokawa T, Sakurai K, Kubo N, Tanaka H, Muguruma K, Yashiro M, Onoda N, Hirakawa K. Current status in remnant gastric cancer after distal gastrectomy. World J Gastroenterol. 2016;22:2424-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 55] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2014 (ver. 4). Gastric Cancer. 2017;20:1-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1575] [Cited by in RCA: 1915] [Article Influence: 239.4] [Reference Citation Analysis (1)] |
| 4. | Ramos MFKP, Pereira MCM, Oliveira YS, Pereira MA, Barchi LC, Dias AR, Zilberstein B, Ribeiro Junior U, Cecconello I. Surgical results of remnant gastric cancer treatment. Rev Col Bras Cir. 2020;47:e20202703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Song XH, Liu K, Sun LF, Chen XL, Zhao LY, Zhang WH, Chen XZ, Yang K, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. Clinicopathological characteristics and prognostic factors of remnant gastric cancer: A single-center retrospective analysis of 90 patients. Int J Surg. 2018;51:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Kondo K. Duodenogastric reflux and gastric stump carcinoma. Gastric Cancer. 2002;5:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 7. | Sinning C, Schaefer N, Standop J, Hirner A, Wolff M. Gastric stump carcinoma - epidemiology and current concepts in pathogenesis and treatment. Eur J Surg Oncol. 2007;33:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 100] [Article Influence: 5.3] [Reference Citation Analysis (1)] |
| 8. | Piso P, Meyer HJ, Edris C, Jähne J. Surgical therapy of gastric stump carcinoma--a retrospective analysis of 109 patients. Hepatogastroenterology. 1999;46:2643-2647. [PubMed] |
| 9. | Takeno S, Hashimoto T, Maki K, Shibata R, Shiwaku H, Yamana I, Yamashita R, Yamashita Y. Gastric cancer arising from the remnant stomach after distal gastrectomy: a review. World J Gastroenterol. 2014;20:13734-13740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 51] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202-209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5015] [Cited by in RCA: 4859] [Article Influence: 441.7] [Reference Citation Analysis (2)] |
| 11. | Sohn BH, Hwang JE, Jang HJ, Lee HS, Oh SC, Shim JJ, Lee KW, Kim EH, Yim SY, Lee SH, Cheong JH, Jeong W, Cho JY, Kim J, Chae J, Lee J, Kang WK, Kim S, Noh SH, Ajani JA, Lee JS. Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project. Clin Cancer Res. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 345] [Article Influence: 43.1] [Reference Citation Analysis (0)] |
| 12. | Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32099] [Cited by in RCA: 38328] [Article Influence: 1008.6] [Reference Citation Analysis (0)] |
| 13. | Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM. AJCC Cancer Staging Manual. 8th ed. Springer International Publishing: American Joint Commission on Cancer, 2017. |
| 14. | Carpenter PM, Al-Kuran RA, Theuer CP. Paranuaclear E-cadherin in gastric adenocarcinoma. Am J Clin Pathol. 2002;118:887-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 15. | Fenoglio-Preiser CM, Wang J, Stemmermann GN, Noffsinger A. TP53 and gastric carcinoma: a review. Hum Mutat. 2003;21:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 168] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 16. | Ahn S, Lee SJ, Kim Y, Kim A, Shin N, Choi KU, Lee CH, Huh GY, Kim KM, Setia N, Lauwers GY, Park DY. High-throughput Protein and mRNA Expression-based Classification of Gastric Cancers Can Identify Clinically Distinct Subtypes, Concordant With Recent Molecular Classifications. Am J Surg Pathol. 2017;41:106-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | Yu Y. Molecular classification and precision therapy of cancer: immune checkpoint inhibitors. Front Med. 2018;12:229-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 18. | Xu J, Zhu J, Wei Q. Adjuvant Radiochemotherapy vs Chemotherapy Alone for Gastric Cancer: Implications for Target Definition. J Cancer. 2019;10:458-466. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kojima K, Minatani N, Ushiku H, Ishii S, Tanaka T, Yokoi K, Nishizawa N, Ooizumi Y, Igarashi K, Hosoda K, Moriya H, Mieno H, Watanabe M, Yamashita K. Prediction of onset of remnant gastric cancer by promoter DNA methylation of CDO1/HOPX/Reprimo/E-cadherin. Oncotarget. 2019;10:2423-2434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Nishikawa J, Yanai H, Hirano A, Okamoto T, Nakamura H, Matsusaki K, Kawano T, Miura O, Okita K. High prevalence of Epstein-Barr virus in gastric remnant carcinoma after Billroth-II reconstruction. Scand J Gastroenterol. 2002;37:825-829. [PubMed] |
| 21. | Tanigawa H, Uesugi H, Mitomi H, Saigenji K, Okayasu I. Possible association of active gastritis, featuring accelerated cell turnover and p53 overexpression, with cancer development at anastomoses after gastrojejunostomy. Comparison with gastroduodenostomy. Am J Clin Pathol. 2000;114:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer (review). Int J Oncol. 2015;46:1421-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 23. | Lu C, Zhang H, Zhou W, Wan X, Li L, Yu C. Epstein-Barr virus infection and genome polymorphisms on gastric remnant carcinoma: a meta-analysis. Cancer Cell Int. 2020;20:401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Nakajima T, Akiyama Y, Shiraishi J, Arai T, Yanagisawa Y, Ara M, Fukuda Y, Sawabe M, Saitoh K, Kamiyama R, Hirokawa K, Yuasa Y. Age-related hypermethylation of the hMLH1 promoter in gastric cancers. Int J Cancer. 2001;94:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Aya M, Yashiro M, Nishioka N, Onoda N, Hirakawa K. Carcinogenesis in the remnant stomach following distal gastrectomy with billroth II reconstruction is associated with high-level microsatellite instability. Anticancer Res. 2006;26:1403-1411. [PubMed] |
| 26. | Nakachi A, Miyazato H, Shimoji H, Hiroyasu S, Isa T, Shiraishi M, Muto Y. Microsatellite instability in patients with gastric remnant cancer. Gastric Cancer. 1999;2:210-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Ramos MFKP, Pereira MA, de Castria TB, Ribeiro RRE, Cardili L, de Mello ES, Zilberstein B, Ribeiro-Júnior U, Cecconello I. Remnant gastric cancer: a neglected group with high potential for immunotherapy. J Cancer Res Clin Oncol. 2020;146:3373-3383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 28. | Loh M, Liem N, Vaithilingam A, Lim PL, Sapari NS, Elahi E, Mok ZY, Cheng CL, Yan B, Pang B, Salto-Tellez M, Yong WP, Iacopetta B, Soong R. DNA methylation subgroups and the CpG island methylator phenotype in gastric cancer: a comprehensive profiling approach. BMC Gastroenterol. 2014;14:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 29. | Lo SS, Wu CW, Hsieh MC, Lui WY. Is gastric remnant cancer clinically different from primary gastric cancer? Hepatogastroenterology. 1997;44:299-301. [PubMed] |
| 30. | Imada T, Rino Y, Takahashi M, Shiozawa M, Hatori S, Noguchi Y, Amano T, Kobayashi O, Sairenji M, Motohashi H. Clinicopathologic differences between gastric remnant cancer and primary cancer in the upper third of the stomach. Anticancer Res. 1998;18:231-235. [PubMed] |
| 31. | Thorban S, Böttcher K, Etter M, Roder JD, Busch R, Siewert JR. Prognostic factors in gastric stump carcinoma. Ann Surg. 2000;231:188-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Takahashi M, Takeuchi H, Tsuwano S, Nakamura R, Takahashi T, Wada N, Kawakubo H, Saikawa Y, Kitagawa Y. Surgical Resection of Remnant Gastric Cancer Following Distal Gastrectomy: A Retrospective Clinicopathological Study. Ann Surg Oncol. 2016;23:511-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Ramos MFKP, de Castria TB, Pereira MA, Dias AR, Antonacio FF, Zilberstein B, Hoff PMG, Ribeiro U Jr, Cecconello I. Return to Intended Oncologic Treatment (RIOT) in Resected Gastric Cancer Patients. J Gastrointest Surg. 2020;24:19-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 34. | Szekely L, Selivanova G, Magnusson KP, Klein G, Wiman KG. EBNA-5, an Epstein-Barr virus-encoded nuclear antigen, binds to the retinoblastoma and p53 proteins. Proc Natl Acad Sci USA. 1993;90:5455-5459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 190] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 35. | Ilyas M, Grabsch H, Ellis IO, Womack C, Brown R, Berney D, Fennell D, Salto-Tellez M, Jenkins M, Landberg G, Byers R, Treanor D, Harrison D, Green AR, Ball G, Hamilton P; National Cancer Research Institute (UK) Biomarker and Imaging Clinical Studies Group. Guidelines and considerations for conducting experiments using tissue microarrays. Histopathology. 2013;62:827-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |