Published online Apr 15, 2021. doi: 10.4251/wjgo.v13.i4.197
Peer-review started: December 21, 2020
First decision: January 11, 2021
Revised: January 14, 2021
Accepted: March 11, 2021
Article in press: March 11, 2021
Published online: April 15, 2021
Processing time: 108 Days and 22.6 Hours
Hepatocellular carcinoma (HCC) is the fourth leading cause of cancer-related deaths worldwide. The prognosis of patients with HCC remains poor largely due to the late diagnosis and lack of effective treatments. Despite being widely used, alpha-fetoprotein serology and ultrasonography have limited diagnostic performance for early-stage HCC. The emergence of omics strategies has contributed to significant advances in the development of non-invasive biomarkers for the early diagnosis of HCC including proteins, metabolites, circulating tumor deoxyribonucleic acid, and circulating non-coding ribonucleic acid. Early diagnosis is beneficial to patients as it increases the proportion who can be treated with curative treatment, thus prolonging survival outcomes. Currently, multiple clinical trials involving locoregional, systemic therapies, and combinations of these modalities are changing therapeutic strategies for different stage HCC. Success in several preclinical trials that involve immunotherapeutic innovations has created the potential to complement and enforce other treatment strategies in the future. This review summarizes the most recent advances in non-invasive early molecular detection, current therapy strategies, and potential immunotherapeutic innovations of HCC.
Core Tip: Long-term survival relies upon early diagnosis and timely treatment for patients with hepatocellular carcinoma (HCC). In this review, an update on the non-invasive early molecular detection and therapeutic strategies associated with HCC is discussed, focusing on omics-related biomarkers (e.g., proteins, metabolites, circulating tumor deoxyribonucleic acid, circulating non-coding ribonucleic acid), locoregional and systemic therapies for treating different stages of HCC, as well as potential immunotherapeutic innovations (e.g., adoptive cell transfer therapy).
- Citation: Guan MC, Wang MD, Liu SY, Ouyang W, Liang L, Pawlik TM, Xu QR, Huang DS, Shen F, Zhu H, Yang T. Early diagnosis and therapeutic strategies for hepatocellular carcinoma: From bench to bedside. World J Gastrointest Oncol 2021; 13(4): 197-215
- URL: https://www.wjgnet.com/1948-5204/full/v13/i4/197.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i4.197
Hepatocellular carcinoma (HCC) accounts for the predominant type of primary liver cancer, ranking as the sixth most common malignancy and representing the fourth leading cause of cancer-related deaths worldwide[1]. According to annual estimates, liver cancer will cause more than 1 million deaths in 2030, with a 5-year survival of 18%[2]. Although early diagnosis and prompt treatment may improve long-term outcomes, most HCC patients are diagnosed with advanced stages of disease and face a generally poor prognosis with limited treatment options.
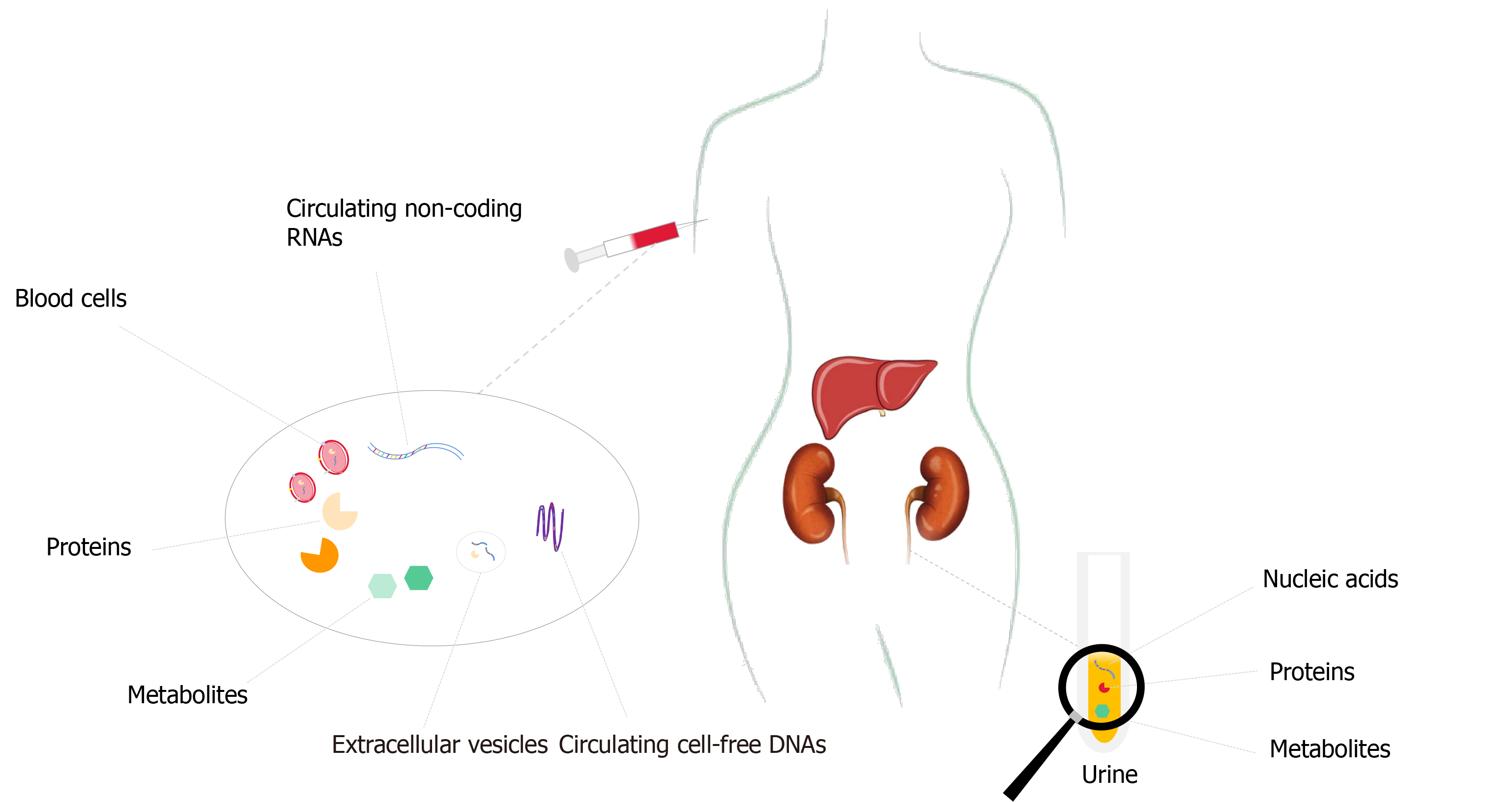
Therefore, the precise and early diagnosis are crucial for the management of HCC to facilitate the best chance of long-term patient survival. Despite being commonly used to screen high-risk populations, ultrasonography (US) remains a suboptimal method to detect HCC at an early stage, with a relatively low sensitivity of only 40%-50%[3]. Moreover, the accuracy of US is often influenced by the operator’s clinical experience and various patient-related factors such as obesity[1]. In turn, much attention has been focused on the development of non-invasive, reproducible, and quantifiable biomarkers that may allow for earlier diagnosis and better therapeutic interventions. Over the past decade, multiple studies have noted the potential and clinical value of omics-related biomarkers as a possible alternative option to facilitate the non-invasive diagnosis of tumors (Figure 1).
Therapeutic options to treat HCC have substantially improved over the last several decades, especially with the advancements made in molecular targeted drugs and immunotherapies. Clinical trials regarding locoregional and systemic therapies have paved the way for further improvement in patient outcomes. Nevertheless, identifying the best therapeutic strategies for different stage HCC remains a challenge due to the high tumor heterogeneity.
In this review, we summarize the most recent advances in non-invasive early detection and treatment options for HCC.
The emergence of omics strategies has recently led to significant progress in the non-invasive early detection of HCC. These omics-related biomarkers are mainly derived from body fluid (e.g., blood and urine) and include proteins, metabolites, circulating tumor DNA, and circulating non-coding RNA (Table 1).
| Type of biomarker | Examples |
| Proteins | AFP; DCP; GALAD score; HES algorithm; ASAP model |
| Metabolites | A panel based on phenylalanyl-tryptophan and glycocholate |
| Circulating cell-free DNAs | Somatic mutations; DNA methylation; 5-hydroxymethylcytosine |
| Circulating non-coding RNAs | Micro-RNAs (mirR-125b, miR-122, and miR-21); Circular RNAs; Long non-coding RNAs; Exosomal non-coding RNAs |
Alpha-fetoprotein (AFP) and des-gamma-carboxy prothrombin (DCP) are the most widely accepted serological diagnostic biomarkers for HCC in clinical practice. The sensitivity and specificity of a single biomarker for the early diagnosis of HCC vary considerably, suggesting that a single biomarker to detect early-stage HCC is insufficient and has unsatisfactory diagnostic performance. In contrast, the combination of several biomarkers increases early diagnostic rates. The combination of biomarkers with other parameters (e.g., sex and age) into prediction models to generate an aggregate score to help detect early HCC has been more promising, as demonstrated by such tools as the GALAD model, the ASAP model, and HES algorithm[4-6] (Table 2).
| Model | Parameters | Ref. | Notes |
| GALAD score | Gender, Age, AFP-L3, AFP, and DCP | [4,7,8] | Validated in different cohorts from Germany, Japan, Hong Kong, the United Kingdom; Surpassing the ability of ultrasound to predict HCC; Excelling in diagnosing early-stage HCC in the setting of cirrhosis or CHB or NASH |
| HES algorithm | Current level of AFP, rate of AFP change, level of alanine aminotransferase, platelet count, and age | Validated in the detection of HCC in patients with cirrhosis of any etiology; Superior to the AFP measuring alone in detecting early-stage HCC | |
| ASAP model | Age, gender, AFP, and DCP | [6] | Validated in the presence of HCC in patients with CHB; Exhibiting 73.8% sensitivity and 90.0% specificity for detecting BCLC stage 0-A HCC |
The GALAD model, which combines age and gender into an algorithm based on serum AFP, lens culinaris agglutinin-reactive fraction of AFP (AFP-L3), and DCP levels, was developed to determine the risk of HCC in high-risk individuals. Of note, the GALAD model has been validated in different cohorts from Germany, Japan, Hong Kong, and the United Kingdom[4]. Given its proper diagnostic value, Yang et al[7] compared the performance of the GALAD model vs the use of abdominal US to detect HCC. Interestingly, the GALAD score surpassed the ability of US to predict HCC with a sensitivity of 92%, specificity of 79%, and an area under curve (AUC) of 0.92 at a cut-off of-1.18, especially among HCC patients with Barcelona Clinic Liver Cancer (BCLC) stage 0-A. Given the increasing prevalence of nonalcoholic steatohepatitis (NASH)-based HCC and the lack of effective screening strategies for early-stage NASH-HCC, Best et al[8] estimated the performance of GALAD model among patients with early NASH-HCC without cirrhosis. To detect BCLC stage A NASH-HCC, the model had an AUC of 0.92 with 86.2% sensitivity and 90.9% specificity at a cut-off of-1.334. To identify stage A NASH-HCC without cirrhosis, the AUC value was 0.94 with 85.7% sensitivity and 96.2% specificity at a-0.63 cut-off. As such, the GALAD score may facilitate the surveillance of individuals with NASH, thereby helping to offset the limitation of US to detect HCC in this high-risk population.
The HES algorithm, which includes current AFP level, rate of AFP change, alanine aminotransferase level, platelet count, and age, was initially constructed to predict the development of HCC among patients with hepatitis C and cirrhosis[5]. The score was further validated in subsequent studies that evaluated the identification of HCC among patients with different etiologies of cirrhosis[9]. With a specificity of 90%, the HES algorithm was able to identify HCC with 52.56% sensitivity vs 48.13% for AFP alone (P < 0.0005) 6 mo prior to ultimate diagnosis. After incorporating the etiologic cause of cirrhosis, the updated HES remained superior to AFP alone to detect early-stage HCC with nearly no additional cost and the sensitivity improved by 5.18% (P = 0.0015)[10]. The ASAP, which incorporates age, gender, serum AFP and DCP levels, was another model developed to predict the occurrence of HCC in patients with chronic hepatitis B[6]. To detect BCLC stage 0-A HCC, the ASAP reportedly has 73.8% sensitivity and 90.0% specificity. Despite this, the best model with highest diagnostic accuracy remains controversial, due to the complexity and heterogeneity of HCC related to different etiologies, environmental, and genetic factors.
To improve the early detection rate of HCC, many studies have recently evaluated the diagnostic performance of other protein biomarkers such as plasma heat shock protein 90alpha[11], serum aldo-keto reductase family 1 member B10[12], and site-specific N-glycopeptides in serum haptoglobin[13]. In addition, urinary proteomic analysis has also gradually been increasingly investigated as a possible means to obtain biomarkers for HCC screening, diagnosis, and surveillance[14,15]. Unfortunately, to date, none of these protein biomarkers have been utilized in clinical practice or recommended by professional associations, highlighting the challenges and present status of biomarker development.
Metabolomics plays a vital role in providing insights into the etiology and mechanisms of HCC. In turn, metabolites in body fluids, which could directly and uniquely reflect the metabolic changes in the liver, may have the potential to become novel candidate markers for early diagnosis of HCC. To this point, analyzing plasma components, Di Poto et al[16] identified 11 metabolites and combined these metabolites with three clinical variables to propose a diagnostic strategy to detect early-stage HCC among patients with liver cirrhosis. Luo et al[17] discovered and validated another serum metabolite biomarker panel, which consisted of phenylalanyl-tryptophan and glycocholate; the panel demonstrated a good diagnostic performance for early detection of HCC among high-risk populations. In addition to identifying small or AFP false-negative HCCs, the panel had high sensitivity ranging from 71.4% to 80.0% to detect “preclinical” HCC from 1 to 3 years before a confirmed HCC diagnosis. Kim et al[18] constructed another prediction model, which included methionine, proline, ornithine, pimelylcarnitine, and octanoylcarnitine, that demonstrated excellent ability to detect early HCC. While several studies have investigated plasma metabolites, high-quality studies are lacking on the use of urinary metabolites. Future large prospective studies are needed to validate whether metabolite-based panels can improve current HCC surveillance and management strategies.
Circulating cell-free DNAs (cfDNAs) are composed of extracellular DNA fragments shed into the blood via any moribund normal or malignant cells[19]. Circulating tumor DNA (ctDNA), which accounts for a fraction of cfDNA, carries tumor-specific genetic or epigenetic variation, such as genetic mutations and DNA methylation. Several studies have suggested that ctDNA can assist in cancer screening and diagnostic strategies through the use of non-invasive liquid biopsies (i.e. molecular testing of solid malignancies via blood analysis). To date, a variety of tumor-specific genetic and/or epigenetic changes have been detected that may be promising ctDNA-based biomarkers for the diagnosis of early HCC. In particular, the combined detection of cfDNA somatic mutations and protein markers has the potential to identify early-stage HCC from asymptomatic HBsAg-seropositive individuals[20]. A recent systematic review, which included 10 studies reporting on different biomarker combinations, demonstrated that the combined analysis of cfDNA and serum AFP level yielded better detection rates of HCC than AFP alone[21].
To date, DNA methylation profiling of ctDNA has been the most promising approach for early identification of HCC among high-risk patients[22]. Given that the septin 9 (SEPT9) promoter in cfDNA is hypermethylated in HCC, methylated SEPT9 testing has been proposed as a useful blood epigenetic biomarker for HCC diagnosis among cirrhotic patients[23]. Xu et al[24] developed a diagnostic prediction model based on ten methylation markers in ctDNA to differentiate between HCC and benign liver diseases; this model had high diagnostic specificity and sensitivity (both P < 0.001). Kisiel et al[25] constructed a novel panel based on six plasma methylated DNA markers for HCC detection, which was significantly superior to AFP alone. In the phase II clinical validation trial that included 95 HCC patients, 51 cirrhotic controls, and 98 healthy volunteers, the panel had an AUC of 0.96 with a sensitivity of 75% for BCLC stage 0 HCC and a sensitivity of 93% for stage A HCC. A separate study demonstrated that combined assays of blood methylated DNA markers and serum AFP values had a superior capacity to distinguish HCC individuals from healthy subjects vs AFP alone[26]. Specifically, this panel, which included three methylated DNA markers (homeobox A1, empty spiracles homeobox 1, and testis-specific Y-encoded-like protein 5), beta-1,3-galactosyltransferase 6, and two protein markers (AFP and AFP-L3), had a sensitivity of 71% and a 90% specificity to diagnose early-stage HCC, which was superior to the GALAD score or AFP alone. Thus, the analysis of ctDNA can serve as a complementary strategy coupled with AFP testing for early HCC detection.
Besides alterations in methylation patterns, another epigenetic change in cfDNA, 5-hydroxymethylcytosine (5hmC), may facilitate early diagnosis of cancer[27,28]. 5hmC is not only associated with active DNA demethylation, but also has a relatively stable DNA epigenetic signature[28]. Cai et al[29] constructed a diagnostic model based on 5hmC markers identified in cfDNA and exhibited high capability for differentiating patients with early HCC from individuals with chronic hepatitis B virus (HBV) infection or cirrhosis, as well as from those with benign liver diseases and healthy controls. Taken together, the assays of cfDNAs, including ctDNA in body fluids, may provide a solid foundation for early detection and precise cancer diagnosis. More large-scale studies are required for further validation of these conclusions.
Data have suggested a regulatory role of non-coding RNAs (ncRNAs) in the development and progression of HCC[30]. A rapidly growing area of basic research given the advancements in sequencing techniques in recent years, circulating ncRNAs research has identified this area as ncRNAs as potential molecular biomarkers to diagnose cancer. In particular, microRNAs (miRNAs), circular RNAs (circRNAs), and long non-coding RNAs (lncRNAs) have increasingly attracted considerable attention.
miRNAs, a class of single-stranded ncRNAs with about 20 nucleotides length, mainly function as regulatory RNAs that control gene expression at the post-transcriptional levels. Several circulating miRNAs have already been identified to be relative to HCC, with different diagnostic values. One study demonstrated that miR-3197 had the potential to identify individuals at risk of HCC during the period of anti-viral treatment, since its expression levels in serum varied significantly with disease progression[31]. Besides, mirR-125b[32], miR-122[33,34], and miR-21[35] may serve as promising biomarkers for the early diagnosis of HCC. Subgroup analysis in a recent meta-analysis noted that the pooled sensitivity, specificity, and AUC of miR-125b for detecting HBV-related HCC patients were 0.95, 0.79, and 0.95, respectively[32]. Another meta-analysis of 13 studies including 920 HCC patients and 1217 controls revealed that the pooled sensitivities, specificities, and AUCs of serum miR-122 were 0.76, 0.75, and 0.82 for distinguishing HCC patients from overall controls; and 0.79, 0.82, and 0.87 for differentiating HCC from HBV or hepatitis C virus infection; respectively[33]. MiR-21 might be another useful biomarker for early detection of HCC, with the pooled sensitivity, specificity, and AUC of 85.2%, 79.2%, and 89%, respectively[35]. However, prospective population-based studies with larger sample sizes and different ethnic groups are needed to further validate these findings.
The diagnostic performance of a single miRNA biomarker may vary considerably, however, among different studies. Like cfDNAs, miR-panels are likely to improve the specificity and sensitivity of HCC diagnosis by integrating multiple miRNAs and some clinical parameters. For example, combining miR-27b-3p and miR-192-5p into a panel yielded considerable higher diagnostic value to detect HCC, especially among patients with AFP-negative tumors[36]. Of note, another miRNA panel that consisted of seven serum miRNAs (miR-29a, miR-29c, miR-133a, miR-143, miR-145, miR-192, and miR-505) was constructed by Lin et al[37] using multicenter data. This miRNA panel performed well to detect small HCC, early-stage HCC, and AFP-negative HCC among high-risk individuals, thus contributing to HCC screening and surveillance[37]. In a recent meta-analysis that compared the diagnostic effectiveness of single miRNAs and miRNA panels, the authors noted that combining circulating miRNAs and AFP performed better to diagnosis HCC; miRNAs did, however, have higher accuracy than AFP alone for early detection of HCC[38].
Extracellular vesicles (EVs), including exosomes, microvesicles, and apoptotic bodies, are small vesicular bodies with phospholipid bilayer membrane structure that are released into body fluid by various cells[39]. EVs usually capsulize miRNAs, lncRNAs, and other small molecules, to protect them from degradation. EVs and these molecules are involved in facilitating tumor development and progression. In turn, exosomal miRNAs have been investigated as potential diagnostic biomarkers and therapeutic targets for HCC. For the early detection of HCC, serum exo-miR-10b-5p has been reported to be a promising biomarker, with 90.7% sensitivity and 75.0% specificity at the cut-off value of 1.8-fold[40]. Notably, a novel panel comprising exo-miR-4661-5p and exomiR-4746-5p yielded a best fit AUC of 0.947 with a sensitivity of 75% and a specificity of 91.7% for early-stage HCC[41]. A separate study reported that the combination of exo-miR-122, exo-miR-148a, and AFP was able to distinguish early HCC from cirrhosis with an AUC of 0.931[42]. In addition, mounting evidence also has indicated that lncRNAs with a length of > 200 nucleotides and circRNAs with a covalently closed loop structure could serve as novel diagnostic biomarkers for early-stage HCC such as serum EV-derived lncRNA LINC00853[43] and LINC00853[43], and plasma circRNA panels[44].
Liver resection (LR) is a commonly used curative approach for eligible early-stage HCC individuals. Compared with other solid organs, the liver is characterized by an extremely strong regenerating capacity that can facilitate a return to a fully functional mass in a short time after losing major tissue[45]. The applied definitions of tumor resectability have varied among different institutions and are debated. Generally, LR is applicable to patients with no more than three lesions, and without vascular invasion or extrahepatic metastases[46]. Moreover, compared with other alternative treatments, LR can provide a survival benefit for patients with BCLC stage B/C HCCs[47], as well as individuals with portal vein tumor thrombus[48]. Although LR can achieve a 5-year overall survival (OS) of 60%-70%[49], the high incidence of HCC recurrence following LR remains a major challenge. Among patients with intrahepatic recurrence or extrahepatic recurrence after resection, re-LR may improve long-term survival[50].
More recently, minimally invasive approaches to LR have been increasingly adopted due to proven safety and effectiveness reported in many studies. Specifically, in two recent meta-analyses, laparoscopic LR achieved equivalent long-term oncological outcomes and exhibited excellent short-term clinical advantages compared with open LR, with less intraoperative blood loss and blood transfusions, fewer complications, and a shorter hospital stay[51,52]. Even among patients with post-hepatectomy recurrent HCC, laparoscopic LR may be a safe and effective treatment option[53]. Nevertheless, there is still a lack of large-scale randomized controlled trials to evaluate the superiority of either the open vs minimally invasive approach.
Liver transplantation (LT) represents the best therapeutic option for early-stage unresectable HCC within the Milan criteria (MC) for many patients. In particular, LT can provide a 10-year recurrence-free survival (RFS) of 50%-70%[54]. MC (single lesion < 5 cm, or multiple lesions with less than 3 nodules and a maximum diameter of less than 3 cm) has long been established as the standard criteria for LT patient selection. Over the last decade, there has been controversy regarding the expansion of current MC for liver transplantation. Some data have noted that patients meeting expanded criteria had equivalent post-transplant survival outcomes compared with individuals within MC[55]. Given that locoregional treatment has been shown to effectively downstage HCCs from beyond to within MC, Mazzaferro et al[56] conducted a randomized controlled phase IIb/III trial to determine the efficacy of LT among patients who achieved clinical downstaging of HCC beyond MC. As expected, LT improved tumor event-free survival and OS compared with non-transplantation therapies. Another multicenter study also demonstrated that effective clinical downstaging resulted in favorable post-LT outcomes[57]. Thus, the practice of downstag-ing therapies before LT may be beneficial for the expansion of patient selection. Early recurrence after LT (< 12 mo) is usually associated with a poor prognosis, but systemic or locoregional treatments may provide treatment options for these patients[58].
Ablation therapy is another commonly used treatment for patients with HCC ≤ 3 cm in size. Radiofrequency ablation (RFA) and microwave ablation (MWA), the two most common types of ablation therapy, are mainly performed using radiofrequency or microwave-generated thermal effects to cause local tumor necrosis[59]. A Japanese study that reported on actual 10-year survival after MWA for HCC noted that median OS, recurrence-free survival, and 10-year OS was 5.5 years, 2.4 years, and 23.8%, respectively[60]. Of note, among patients with lesions less than or equal to 4 cm in diameter, both MWA and RFA achieved similar therapeutic effects[61].
Given that the therapeutic efficacy of RFA and LR varied according to tumor size, Zheng et al[62] compared clinical outcomes after RFA and LR for HCC less than 5 cm. Interestingly, both RFA and LR achieved comparable OS and cancer-specific survival for HCC patients with lesions less than 3 cm, but LR was associated with increased long-term survival among patients with HCC ranging from 3.1 to 5 cm in diameter. A recent meta-analysis demonstrated, however, that LR provided more survival benefits, including better RFS and lower local recurrence compared with RFA[63]. Generally, ablation alone is not recommended for lesions > 5 cm, but MWA can achieve a desirable radical curative efficacy after downstaging by other therapies[64].
The introduction of advanced stereotactic navigation technology into ablation approaches has further expanded its application and scope. Stereotactic radiofrequency ablation has been reported to be an effective therapeutic option for HCC, even if the lesion size is > 3 cm[65]. Of note, stereotactic microwave ablation was safe and feasible among HCC patients, with acceptable short- and long-term clinical outcomes, particularly for tumors with complex anatomic locations[66,67].
Traditionally, radiation therapy (RT) had a limited role in HCC treatment. According to present guidelines, RFA, and not RT, is recommended as first-line therapy for unresectable early-stage HCC. Of note, stereotactic body radiation therapy (SBRT) is a highly conformal technique that enables delivering high doses of radiation to targeted lesions under high-precision image guidance. More studies have suggested that SBRT may be well-tolerated and effective treatment alternative for patients with unresectable HCC[68,69], especially for subphrenic tumors more than 3 cm in diameter, or lesions with progression after transarterial chemoembolization (TACE)[70]. For patients with intrahepatic recurrent HCC, repeat SBRT has yielded promising oncologic effects with a median OS of 71 mo for the 1st SBRT vs 44 mo for the 2nd SBRT[71]. A recent meta-analysis also confirmed that SBRT could achieve better local control for HCC vs RFA, but OS with SBRT was significantly shorter compared with RFA, warranting further investigation[72].
Conventional TACE is recommended as standard therapy for intermediate-stage HCC. TACE generally involves the mixture of chemotherapeutic agents (e.g., doxorubicin or cisplatin) and an embolic material (e.g., lipiodol) injected into the target artery via a catheter[73]. The efficacy and safety of lipiodol-based TACE were the topic of a systematic review with a reported objective response rate (ORR), 1-year OS, 3-year OS, 5-year OS, median OS, and overall mortality of 52.5%, 70.3%, 40.4%, 32.4%, 19.4 mo, and 0.6%, respectively[74]. An elevation in post-treatment transaminase levels and postembolization syndrome are typically the most frequently observed complications[74]. Prophylactic use of dexamethasone can reduce the occurrence of TACE-related fever, anorexia, and nausea[75]. cTACE has also been examined as an adjuvant therapy among patients at high-risk of recurrence after resection of HBV-related HCC[76]. Additionally, TACE with drug eluting beads has been reported to achieve comparable results as cTACE, but with lower systemic toxicity[73]. A prospective single-arm phase II study reported the efficacy of TACE with drug eluting beads using idarubicin for unresectable HCC; this study noted a 6-mo ORR, median progression-free survival (PFS), and median OS of 52%, 6.6 mo, and 18.6 mo, respectively[77]. The presence of macroscopic vascular invasion has been associated with poor prognosis among patients with advanced HCCs. Several studies noted that combination therapy of TACE and RT was able to achieve better survival outcomes in these patients compared with TACE plus systemic therapy[78,79].
After a decade of negative clinical trials, systemic therapy with sorafenib, an oral small molecule multikinase inhibitor, was approved as a standard first-line treatment option for advanced HCC in 2007. Prospective data demonstrated that sorafenib delayed the progression of the disease and prolonged patient survival compared with placebo[80]. Multiple clinical trials investigating the efficacy of novel molecular targeted drugs have subsequently failed to exhibit superiority or non-inferiority to sorafenib until the recent emergence of another multikinase inhibitor, lenvanitib. In 2018, lenvatinib was demonstrated to yield a similar survival time as sorafenib in treatment of patients with advanced HCC (median OS, 13.6 mo vs 12.3 mo) in a randomized phase III trial[81]. Since that study, additional therapeutic strategies for advanced HCC have evolved. Specifically, lenvatinib is now recommended as the first-line treatment by several clinical guidelines, and another two multikinase inhibitors (i.e. regorafenib and cabozantinib) are recommended as second-line options. A meta-analysis demonstrated that regorafenib was associated with a median OS, median PFS, as well as pooled objective response rate of 11.08 mo, 3.24 mo, and 10.1%, respectively[82].
Given that angiogenesis can lead to tumor growth and metastasis and HCC is a typical blood-rich tumor, antiangiogenic inhibitors (e.g., ramucirumab and bevacizu
The clinical benefits of immunotherapeutic drugs for HCC have also been a topic of investigation. The implementation of a single programmed cell death protein 1/ programmed death ligand 1 inhibitor (e.g., nivolumab, pembrolizumab, camrelizu
The combination of locoregional and systemic therapies has also gained attraction in the treatment of patients with intermediate- or advanced-stage HCC. To date, several studies have evaluated the efficacy of the combination of TACE and sorafenib compared with single therapy[89-91]. There is still no consensus, however, on whether this combination is superior for HCC patients. It is important to note that variations in treatment intervals between sorafenib and TACE may yield a different efficacy. Future efforts should be directed toward defining the optimal combination of therapeutic approaches for unresectable HCC.
At present, the basic mechanisms of HCC-induced immunosuppression remain less investigated, but the immunotherapeutic strategies are mainly based upon two fundamental rules: the capability to release current immunologic responses; and the requirement to trigger other immunologic responses[92]. Accumulating evidence suggests that anti-tumor immunotherapy can be achieved by regulating the function or number of immune cells, immune receptors, or corresponding ligands, and so on.
Recent advancements in adoptive cell transfer therapy, an approach to deliver autologous engineered immunocytes, have brought new hope for patients with cancer. Generally, these modified cells include chimeric antigen receptor T cells (CAR-T), T cell receptor (TCR)-engineered T cells (TCR-T), and cytokine-induced killer cells (CIK). With the approval and clinical implementation of CD19-targeted CAR-T cells for acute lymphoblastic leukemia and lymphoma, this therapy also achieves excellent results in solid tumors, e.g., HCC.
After modified by viral vectors, CAR-T cells are equipped with a positioning navigation device, i.e. an extracellular single-chain variable fragment of an antibody (scFv), specifically identifying tumor cells. Once CAR identifies tumor-associated antigens (TAA), T cell activation and proliferation is triggered by an intracellular signaling domain (CD3-ζ) and the additional costimulatory domains (e.g., cluster of differentiation 28 [CD28], 4-1BB, and CD134). Then the activated T cells rapidly perform immune functions to kill targeted cells[93-95]. Unrestricted by major histocompatibility complex (MHC), CAR-T cells can directly recognize tumor cells and avoid the immune-evasion mechanism of tumor cells via down-regulation of MHC[96]. Given that glypican-3 (GPC3), a heparan sulfate proteoglycan located in the cell membrane, is rarely expressed in healthy liver tissue but overexpressed in HCC, it may serve as a promising tumor antigen targeted by CAR-T[97]. To this point, GPC3-CAR-T therapy has been the focus of several recent clinical trials. In a phase I trial of GPC3-specific CAR-T for 13 advanced-stage HCC patients with lymphodepletion induced by chemotherapy, Shi et al[98] demonstrated that it was a safe, well-tolerated, and practical approach, with 1- and 3-year OS of 42.0% and 10.5% with reasonable toxic side effects. In contrast, in mouse experiments, a soluble programmed cell death 1-chimeric 3 [PD1-CH3] fusion protein derived from PD1 and IgG4 was introduced into GPC3-CAR-T and exhibited enhanced anti-tumor activities[99]. Transgenic expression of interleukin 15 (IL-15) and IL-21 also elevated the anti-tumor capability of GPC3-CAR-T against HCC in vivo[100]. GPC3- or NKG2D-based CAR natural killer cells may also be a promising immunotherapeutic target for HCC[101,102].
Given that most tumor antigens are not expressed on the cell membrane but in the cytoplasm, TCR-T cells, genetically modified with TCRs specific for TAAs, are able to recognize specifically tumor antigen peptides presented by MHC, which overcomes the deficiency of tumor surface antigens[94]. Several HCC-related antigens targeted by TCR have been identified, e.g., AFP-, GPC3-, virus-, NY-ESO-1-, and hTERT-specific TCRs[95]. Despite the success of NY-ESO-1-targeted TCR-T cells to treat multiple myeloma and synovial sarcoma, recent studies about TCR-T for HCC focus on targeting AFP[95]. The generation of affinity-optimized TCRs with specificity to AFP-positive tumors calls for the identification of novel AFP peptides; for example, the construction of HLA-A2/AFP158-specific TCRs and HLA-A24/AFP2-11-specific TCR forms the foundation to develop effective and safe TCR-T cells-based immunothera
CIK cells are generated by culturing T lymphocytes with cytokines in vitro. The primary effector cells are CD3+/CD56+ T cells, which integrate the dual function of both T cells and natural killer cells. As adjuvant immunotherapy, CIK therapy has achieved remarkable results for HCC treatment in several recent clinical trials. In a phase III trial, Lee et al[106] demonstrated that adjuvant CIK therapy improved RFS and OS among patients after curative resection for HCC, with median RFS of 44.0 mo and OS not reached. After an extended 5-year follow-up, the superiority of this adjuvant immunotherapy for HCC was further estimated, with marked improvement in RFS and OS and lower risk of recurrence or death[107]. However, not all patients responded to this therapy. Yu et al[108] noted that targeting myeloid-derived suppressor cells might be an effective therapeutic strategy to enhance the anti-tumor efficacy of CIKs for HCC treatment. With more in-depth research and more robust clinical trials ongoing, CIK therapy will be an appealing strategy to treat HCC.
Immune checkpoints are regulatory molecules involved with inhibitory receptors and signaling pathways, which can inhibit the function of T cells under normal physiological conditions, thereby allowing tumors to escape from host immune system and avoid being eliminated. In addition to cytotoxic T-lymphocyte-associated antigen 4 and PD-1 and its ligands, other inhibitory checkpoint molecules have also been identified (e.g., T-cell immunoglobulin and mucin-domain containing-3, lymphocyte-activation gene 3, B and T lymphocyte attenuator, and T cell immunoreceptor with Ig and ITIM domains [TIGIT]). Recently, Chiu et al[109] reported that tumor resistance to PD-1 inhibitors in nude mouse xenograft model was mediated by inhibitory Poliomyelitis receptor associated protein 1 antibody/pulmonary vascular resistance/ TIGIT axis, indicating that TIGIT was a promising target molecule to reverse the anti-PD1 resistance. In contrast, Ostroumov et al[110] demonstrated that overexpression of TIGIT was associated with the process of T cell exhaustion in liver cancer. The combination of its antibody with the PD1 inhibitor may effectively hinder the growth of liver cancer in immunocompetent mice. T-cell immunoglobulin and mucin-domain containing-3 was also involved in hepatocarcinogenesis, such as mediating effector T cell exhaustion[111].
Vaccines derived from tumor antigen peptides or dendritic cells allow for activation of the immune system to eliminate tumors. GPC3-derived vaccine was previously studied and the results of a phase II study involving the vaccine as adjuvant therapy for HCC were published in 2016. The study demonstrated that GPC3-peptide vaccine reduced the risk of 1-year recurrence among patients with GPC3-positive HCC[112]. On long-term follow-up, this adjuvant therapy provided better survival outcomes by activating cytotoxic T lymphocyte[113]. More preclinical studies to optimize GPC3 vaccine are currently being conducted. Recently, XCL1-GPC3 fusion molecules were constructed as a novel HCC vaccine by integrating the XCL1 chemokine with GPC3, which can stimulate the activation of immune system in mice and have a synergistic anti-tumor effect with anti-PD-1[114]. Dendritic cells (DCs), as potent antigen-presenting cells, are a weapon for killing cancer cells; in turn, DC vaccines may be a favorable potential immunotherapy for HCC. A phase II study of adjuvant therapy with autologous dendritic cells for HCC has been completed by Lee et al[115]. This study noted reduction in the risk of tumor recurrence among HCC patients who received standard therapies with autologous DC[115]. Concerning combination therapy of DC vaccine and programmed death-ligand 1 inhibitor, Teng et al[116] also noted an excellent anti-tumor effect in mice, with longer OS and smaller tumor size.
Oncolytic virus therapy is another emerging treatment approach, with viral vectors (e.g., adenovirus, reovirus, M1 virus, and herpes virus) loading anti-cancer oncogenes. Owing to the risk of systemic adverse reactions, the delivery of these viruses to targeted lesions remains a major challenge. Yoon et al[117] constructed mesenchymal stem cells with the characteristic of tumor-homing as a promising carrier to deliver targeted modified oncolytic adenovirus safely into tumors to enhance anti-HCC efficacy. Lin et al[118] reported that oncolytic herpes simplex virus with a single-chain variable fragment against PD1 could change the immunosuppressive tumor microenvironment in HCC and elevate the anti-tumor effect.
In this review, we summarized the latest research progress in non-invasive biomarkers for early diagnosis of HCC (e.g., proteins, metabolites, circulating cfDNAs, and ncRNAs), current therapeutic strategies, and potential immunotherapeutic innovations. For early diagnosis (Figure 1), prediction models combining protein biomarkers (e.g., AFP and DCP) with other parameters may soon be applied in clinical practice. These models hold the promise of high accuracy to identify early-stage HCC within high-risk populations such as individuals with cirrhosis or NASH. The utilization of currently available data in the clinic not only enables reducing additional healthcare costs, but also facilitates physicians making an objective and accurate diagnosis. In particular, cfDNA methylation scores to facilitate early detection of HCC in high-risk individuals seem to be the most promising strategy for early diagnostic workup rather than traditional HCC surveillance approaches (i.e. US with or without AFP). Meanwhile, miRNAs and metabolites have also exhibited great diagnostic potential to detect early-stage HCC. Candidate biomarkers or panels that enable identification of tumors within 6-12 mo before imaging examinations will provide more early curative treatment opportunities for patients. However, several limitations exist. Many studies to date have lacked appropriate study populations and enough sample size. Specifically, comparisons between early HCC patients and high-risk individuals with cirrhosis or with NAFLD, rather than any stage HCC and the healthy subjects, are needed. In addition, the establishment of standardization to collect, handle, storage, and analytic methods are required to ensure consistency in clinical application.
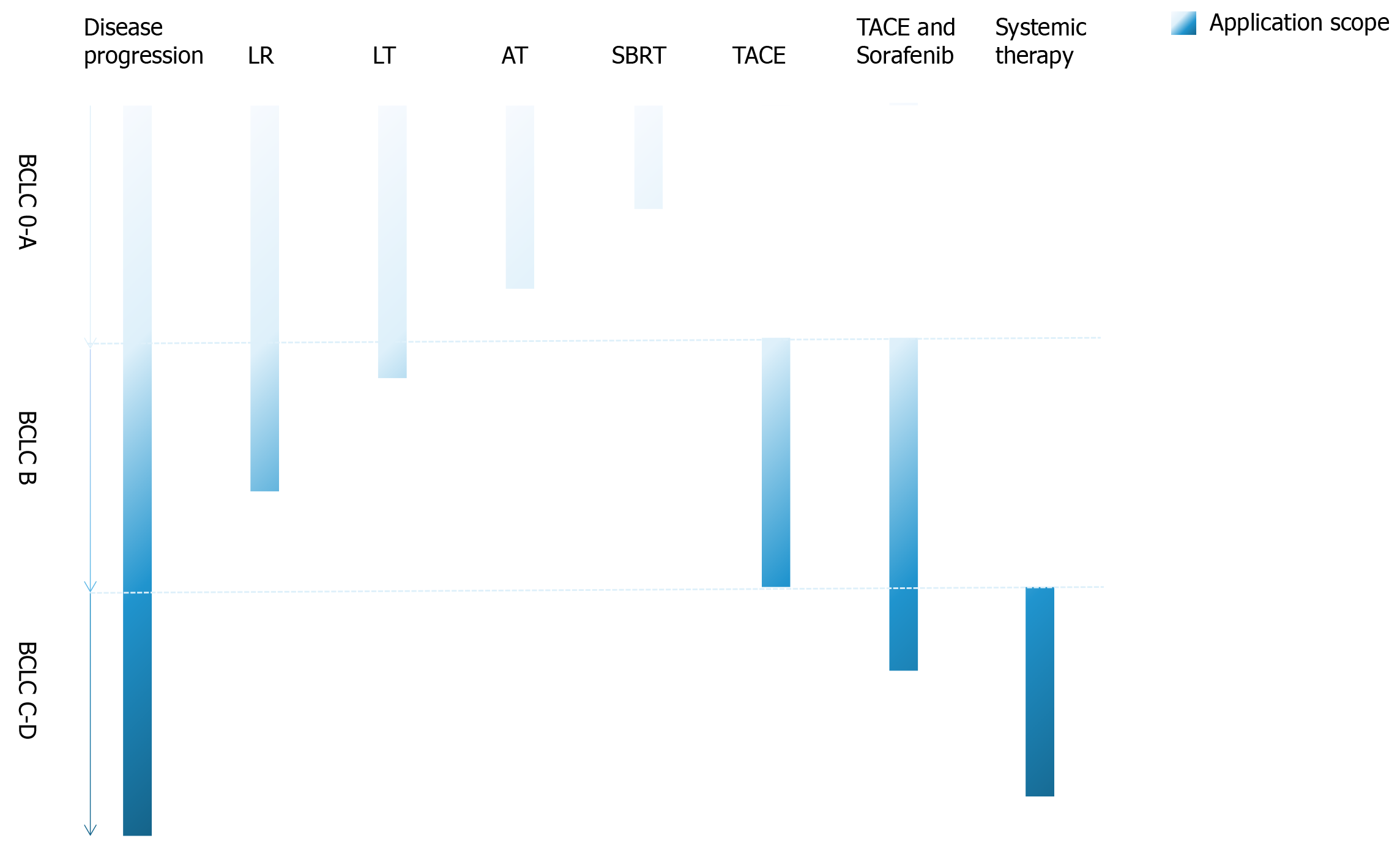
Regarding therapeutic strategies (Figure 2), patients with early-stage HCC should be elevated for curative treatments including hepatectomy, liver transplantation, or ablation depending on the patient performance status, underlying liver function, as well as disease-specific characteristics. Although there remains some controversy surrounding treatment options for intermediate-stage HCC, based on the latest evidence, TACE with or without sorafenib combination is likely the most appropriate strategy for unresectable HCCs. As for advanced-stage HCC, sorafenib, or a combination of atezolizumb and bevacizumab is the preferred treatment strategy. In addition, results from clinical trials involving systemic therapies or combinations of locoregional and systemic therapies have suggested these approaches may be effective for intermediate- and advanced-stage HCC. Also, adoptive cell transfer therapy, tumor vaccination, and oncolytic virus may complement or even change current treatment strategies for HCC in the future, especially CAR-T and CIK therapy. The success of CAR-T therapy for HCC is, however, hindered by the selection of TAA, an efficient migration and infiltration of CAR-T into the tumor lesions, as well as the survivability of CAR-T in the tumor microenvironment, which urgently needs to be solved[119].
In conclusion, the early diagnosis and multidisciplinary collaboration will promote the ongoing emergence of novel therapies for HCC. Future well-designed clinical trials are needed to improve early detection of HCC and thus contribute to a better prognosis.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Akbulut S, Tanabe S S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li JH
| 1. | Singal AG, Lampertico P, Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J Hepatol. 2020;72:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 526] [Cited by in RCA: 769] [Article Influence: 153.8] [Reference Citation Analysis (0)] |
| 2. | Villanueva A. Hepatocellular Carcinoma. N Engl J Med. 2019;380:1450-1462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2066] [Cited by in RCA: 3145] [Article Influence: 524.2] [Reference Citation Analysis (37)] |
| 3. | Tzartzeva K, Obi J, Rich NE, Parikh ND, Marrero JA, Yopp A, Waljee AK, Singal AG. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018; 154: 1706-1718. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 638] [Cited by in RCA: 801] [Article Influence: 114.4] [Reference Citation Analysis (0)] |
| 4. | Berhane S, Toyoda H, Tada T, Kumada T, Kagebayashi C, Satomura S, Schweitzer N, Vogel A, Manns MP, Benckert J, Berg T, Ebker M, Best J, Dechêne A, Gerken G, Schlaak JF, Weinmann A, Wörns MA, Galle P, Yeo W, Mo F, Chan SL, Reeves H, Cox T, Johnson P. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin Gastroenterol Hepatol 2016; 14: 875-886. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 223] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB, Kanwal F, Davila JA, Kramer J, Richardson P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology 2014; 146: 1249-55. e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Yang T, Xing H, Wang G, Wang N, Liu M, Yan C, Li H, Wei L, Li S, Fan Z, Shi M, Chen W, Cai S, Pawlik TM, Soh A, Beshiri A, Lau WY, Wu M, Zheng Y, Shen F. A Novel Online Calculator Based on Serum Biomarkers to Detect Hepatocellular Carcinoma among Patients with Hepatitis B. Clin Chem. 2019;65:1543-1553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 7. | Yang JD, Addissie BD, Mara KC, Harmsen WS, Dai J, Zhang N, Wongjarupong N, Ali HM, Ali HA, Hassan FA, Lavu S, Cvinar JL, Giama NH, Moser CD, Miyabe K, Allotey LK, Algeciras-Schimnich A, Theobald JP, Ward MM, Nguyen MH, Befeler AS, Reddy KR, Schwartz M, Harnois DM, Yamada H, Srivastava S, Rinaudo JA, Gores GJ, Feng Z, Marrero JA, Roberts LR. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol Biomarkers Prev. 2019;28:531-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 149] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 8. | Best J, Bechmann LP, Sowa JP, Sydor S, Dechêne A, Pflanz K, Bedreli S, Schotten C, Geier A, Berg T, Fischer J, Vogel A, Bantel H, Weinmann A, Schattenberg JM, Huber Y, Wege H, von Felden J, Schulze K, Bettinger D, Thimme R, Sinner F, Schütte K, Weiss KH, Toyoda H, Yasuda S, Kumada T, Berhane S, Wichert M, Heider D, Gerken G, Johnson P, Canbay A. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients With Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2020; 18: 728-735. e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 185] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 9. | Tayob N, Christie I, Richardson P, Feng Z, White DL, Davila J, Corley DA, Kanwal F, El-Serag HB. Validation of the Hepatocellular Carcinoma Early Detection Screening (HES) Algorithm in a Cohort of Veterans With Cirrhosis. Clin Gastroenterol Hepatol 2019; 17: 1886-1893. e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 10. | Tayob N, Corley DA, Christie I, Almers L, Rahal AK, Richardson P, White DL, Davila J, Kanwal F, El-Serag HB. Validation of the Updated Hepatocellular Carcinoma Early Detection Screening Algorithm in a Community-Based Cohort of Patients With Cirrhosis of Multiple Etiologies. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | Fu Y, Xu X, Huang D, Cui D, Liu L, Liu J, He Z, Zheng S, Luo Y. Plasma Heat Shock Protein 90alpha as a Biomarker for the Diagnosis of Liver Cancer: An Official, Large-scale, and Multicenter Clinical Trial. EBioMedicine. 2017;24:56-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 87] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 12. | Ye X, Li C, Zu X, Lin M, Liu Q, Liu J, Xu G, Chen Z, Xu Y, Liu L, Luo D, Cao Z, Shi G, Feng Z, Deng H, Liao Q, Cai C, Liao DF, Wang J, Jin J, Cao D. A Large-Scale Multicenter Study Validates Aldo-Keto Reductase Family 1 Member B10 as a Prevalent Serum Marker for Detection of Hepatocellular Carcinoma. Hepatology. 2019;69:2489-2501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 62] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 13. | Zhu J, Huang J, Zhang J, Chen Z, Lin Y, Grigorean G, Li L, Liu S, Singal AG, Parikh ND, Lubman DM. Glycopeptide Biomarkers in Serum Haptoglobin for Hepatocellular Carcinoma Detection in Patients with Nonalcoholic Steatohepatitis. J Proteome Res. 2020;19:3452-3466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 14. | Zhan Z, Guan Y, Mew K, Zeng W, Peng M, Hu P, Yang Y, Lu Y, Ren H. Urine α-fetoprotein and orosomucoid 1 as biomarkers of hepatitis B virus-associated hepatocellular carcinoma. Am J Physiol Gastrointest Liver Physiol. 2020;318:G305-G312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Zhao Y, Li Y, Liu W, Xing S, Wang D, Chen J, Sun L, Mu J, Xing B, Sun W, He F. Identification of noninvasive diagnostic biomarkers for hepatocellular carcinoma by urinary proteomics. J Proteomics. 2020;225:103780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Di Poto C, Ferrarini A, Zhao Y, Varghese RS, Tu C, Zuo Y, Wang M, Nezami Ranjbar MR, Luo Y, Zhang C, Desai CS, Shetty K, Tadesse MG, Ressom HW. Metabolomic Characterization of Hepatocellular Carcinoma in Patients with Liver Cirrhosis for Biomarker Discovery. Cancer Epidemiol Biomarkers Prev. 2017;26:675-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Luo P, Yin P, Hua R, Tan Y, Li Z, Qiu G, Yin Z, Xie X, Wang X, Chen W, Zhou L, Li Y, Chen H, Gao L, Lu X, Wu T, Wang H, Niu J, Xu G. A Large-scale, multicenter serum metabolite biomarker identification study for the early detection of hepatocellular carcinoma. Hepatology. 2018;67:662-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 193] [Cited by in RCA: 283] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 18. | Kim DJ, Cho EJ, Yu KS, Jang IJ, Yoon JH, Park T, Cho JY. Comprehensive Metabolomic Search for Biomarkers to Differentiate Early Stage Hepatocellular Carcinoma from Cirrhosis. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Ye Q, Ling S, Zheng S, Xu X. Liquid biopsy in hepatocellular carcinoma: circulating tumor cells and circulating tumor DNA. Mol Cancer. 2019;18:114. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 265] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 20. | Qu C, Wang Y, Wang P, Chen K, Wang M, Zeng H, Lu J, Song Q, Diplas BH, Tan D, Fan C, Guo Q, Zhu Z, Yin H, Jiang L, Chen X, Zhao H, He H, Li G, Bi X, Zhao X, Chen T, Tang H, Lv C, Wang D, Chen W, Zhou J, Cai J, Wang X, Wang S, Yan H, Zeng YX, Cavenee WK, Jiao Y. Detection of early-stage hepatocellular carcinoma in asymptomatic HBsAg-seropositive individuals by liquid biopsy. Proc Natl Acad Sci USA. 2019;116:6308-6312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 138] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 21. | Chen VL, Xu D, Wicha MS, Lok AS, Parikh ND. Utility of Liquid Biopsy Analysis in Detection of Hepatocellular Carcinoma, Determination of Prognosis, and Disease Monitoring: A Systematic Review. Clin Gastroenterol Hepatol 2020; 18: 2879-2902. e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 22. | von Felden J, Garcia-Lezana T, Schulze K, Losic B, Villanueva A. Liquid biopsy in the clinical management of hepatocellular carcinoma. Gut. 2020;69:2025-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (1)] |
| 23. | Oussalah A, Rischer S, Bensenane M, Conroy G, Filhine-Tresarrieu P, Debard R, Forest-Tramoy D, Josse T, Reinicke D, Garcia M, Luc A, Baumann C, Ayav A, Laurent V, Hollenbach M, Ripoll C, Guéant-Rodriguez RM, Namour F, Zipprich A, Fleischhacker M, Bronowicki JP, Guéant JL. Plasma mSEPT9: A Novel Circulating Cell-free DNA-Based Epigenetic Biomarker to Diagnose Hepatocellular Carcinoma. EBioMedicine. 2018;30:138-147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 120] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 24. | Xu RH, Wei W, Krawczyk M, Wang W, Luo H, Flagg K, Yi S, Shi W, Quan Q, Li K, Zheng L, Zhang H, Caughey BA, Zhao Q, Hou J, Zhang R, Xu Y, Cai H, Li G, Hou R, Zhong Z, Lin D, Fu X, Zhu J, Duan Y, Yu M, Ying B, Zhang W, Wang J, Zhang E, Zhang C, Li O, Guo R, Carter H, Zhu JK, Hao X, Zhang K. Circulating tumour DNA methylation markers for diagnosis and prognosis of hepatocellular carcinoma. Nat Mater. 2017;16:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 626] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 25. | Kisiel JB, Dukek BA, V S R Kanipakam R, Ghoz HM, Yab TC, Berger CK, Taylor WR, Foote PH, Giama NH, Onyirioha K, Abdallah MA, Burger KN, Slettedahl SW, Mahoney DW, Smyrk TC, Lewis JT, Giakoumopoulos M, Allawi HT, Lidgard GP, Roberts LR, Ahlquist DA. Hepatocellular Carcinoma Detection by Plasma Methylated DNA: Discovery, Phase I Pilot, and Phase II Clinical Validation. Hepatology. 2019;69:1180-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 125] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 26. | Chalasani NP, Ramasubramanian TS, Bhattacharya A, Olson MC, Edwards V DK, Roberts LR, Kisiel JB, Reddy KR, Lidgard GP, Johnson SC, Bruinsma JJ. A Novel Blood-Based Panel of Methylated DNA and Protein Markers for Detection of Early-Stage Hepatocellular Carcinoma. Clin Gastroenterol Hepatol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 84] [Article Influence: 21.0] [Reference Citation Analysis (1)] |
| 27. | Song CX, Yin S, Ma L, Wheeler A, Chen Y, Zhang Y, Liu B, Xiong J, Zhang W, Hu J, Zhou Z, Dong B, Tian Z, Jeffrey SS, Chua MS, So S, Li W, Wei Y, Diao J, Xie D, Quake SR. 5-Hydroxymethylcytosine signatures in cell-free DNA provide information about tumor types and stages. Cell Res. 2017;27:1231-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 211] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 28. | Li W, Zhang X, Lu X, You L, Song Y, Luo Z, Zhang J, Nie J, Zheng W, Xu D, Wang Y, Dong Y, Yu S, Hong J, Shi J, Hao H, Luo F, Hua L, Wang P, Qian X, Yuan F, Wei L, Cui M, Zhang T, Liao Q, Dai M, Liu Z, Chen G, Meckel K, Adhikari S, Jia G, Bissonnette MB, Zhao Y, Zhang W, He C, Liu J. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic biomarkers for human cancers. Cell Res. 2017;27:1243-1257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 259] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 29. | Cai J, Chen L, Zhang Z, Zhang X, Lu X, Liu W, Shi G, Ge Y, Gao P, Yang Y, Ke A, Xiao L, Dong R, Zhu Y, Yang X, Wang J, Zhu T, Yang D, Huang X, Sui C, Qiu S, Shen F, Sun H, Zhou W, Zhou J, Nie J, Zeng C, Stroup EK, Chiu BC, Lau WY, He C, Wang H, Zhang W, Fan J. Genome-wide mapping of 5-hydroxymethylcytosines in circulating cell-free DNA as a non-invasive approach for early detection of hepatocellular carcinoma. Gut. 2019;68:2195-2205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 30. | Wong CM, Tsang FH, Ng IO. Non-coding RNAs in hepatocellular carcinoma: molecular functions and pathological implications. Nat Rev Gastroenterol Hepatol. 2018;15:137-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 341] [Article Influence: 48.7] [Reference Citation Analysis (0)] |
| 31. | Pascut D, Cavalletto L, Pratama MY, Bresolin S, Trentin L, Basso G, Bedogni G, Tiribelli C, Chemello L. Serum miRNA Are Promising Biomarkers for the Detection of Early Hepatocellular Carcinoma after Treatment with Direct-Acting Antivirals. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 32. | Jin X, Cai C, Qiu Y. Diagnostic Value of Circulating microRNAs in Hepatitis B Virus-Related Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. J Cancer. 2019;10:4754-4764. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 33. | Zhao XF, Li N, Lin DD, Sun LB. Circulating MicroRNA-122 for the Diagnosis of Hepatocellular Carcinoma: A Meta-Analysis. Biomed Res Int. 2020;2020:5353695. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Wei XY, Ding J, Tian WG, Yu YC. MicroRNA-122 as a diagnostic biomarker for hepatocellular carcinoma related to hepatitis C virus: a meta-analysis and systematic review. J Int Med Res. 2020;48:300060520941634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Qu J, Yang J, Chen M, Cui L, Wang T, Gao W, Tian J, Wei R. MicroRNA-21 as a diagnostic marker for hepatocellular carcinoma: A systematic review and meta-analysis. Pak J Med Sci. 2019;35:1466-1471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Zhu HT, Liu RB, Liang YY, Hasan AME, Wang HY, Shao Q, Zhang ZC, Wang J, He CY, Wang F, Shao JY. Serum microRNA profiles as diagnostic biomarkers for HBV-positive hepatocellular carcinoma. Liver Int. 2017;37:888-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 37. | Lin XJ, Chong Y, Guo ZW, Xie C, Yang XJ, Zhang Q, Li SP, Xiong Y, Yuan Y, Min J, Jia WH, Jie Y, Chen MS, Chen MX, Fang JH, Zeng C, Zhang Y, Guo RP, Wu Y, Lin G, Zheng L, Zhuang SM. A serum microRNA classifier for early detection of hepatocellular carcinoma: a multicentre, retrospective, longitudinal biomarker identification study with a nested case-control study. Lancet Oncol. 2015;16:804-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 220] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 38. | Peng C, Ye Y, Wang Z, Guan L, Bao S, Li B, Li W. Circulating microRNAs for the diagnosis of hepatocellular carcinoma. Dig Liver Dis. 2019;51:621-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 39. | Mann J, Reeves HL, Feldstein AE. Liquid biopsy for liver diseases. Gut. 2018;67:2204-2212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 77] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 40. | Cho HJ, Eun JW, Baek GO, Seo CW, Ahn HR, Kim SS, Cho SW, Cheong JY. Serum Exosomal MicroRNA, miR-10b-5p, as a Potential Diagnostic Biomarker for Early-Stage Hepatocellular Carcinoma. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 41. | Cho HJ, Baek GO, Seo CW, Ahn HR, Sung S, Son JA, Kim SS, Cho SW, Jang JW, Nam SW, Cheong JY, Eun JW. Exosomal microRNA-4661-5p-based serum panel as a potential diagnostic biomarker for early-stage hepatocellular carcinoma. Cancer Med. 2020;9:5459-5472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 42. | Wang Y, Zhang C, Zhang P, Guo G, Jiang T, Zhao X, Jiang J, Huang X, Tong H, Tian Y. Serum exosomal microRNAs combined with alpha-fetoprotein as diagnostic markers of hepatocellular carcinoma. Cancer Med. 2018;7:1670-1679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 43. | Kim SS, Baek GO, Ahn HR, Sung S, Seo CW, Cho HJ, Nam SW, Cheong JY, Eun JW. Serum small extracellular vesicle-derived LINC00853 as a novel diagnostic marker for early hepatocellular carcinoma. Mol Oncol. 2020;14:2646-2659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 57] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 44. | Yu J, Ding WB, Wang MC, Guo XG, Xu J, Xu QG, Yang Y, Sun SH, Liu JF, Qin LX, Liu H, Yang F, Zhou WP. Plasma circular RNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma: A large-scale, multicenter study. Int J Cancer. 2020;146:1754-1763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 45. | Petrowsky H, Fritsch R, Guckenberger M, De Oliveira ML, Dutkowski P, Clavien PA. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat Rev Gastroenterol Hepatol. 2020;17:755-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 148] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 46. | Yang JD, Heimbach JK. New advances in the diagnosis and management of hepatocellular carcinoma. BMJ. 2020;371:m3544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 241] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 47. | Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK, Seo YS, Yim HJ, Yeon JE, Byun KS. Hepatic resection compared to chemoembolization in intermediate- to advanced-stage hepatocellular carcinoma: A meta-analysis of high-quality studies. Hepatology. 2018;68:977-993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 157] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 48. | Liang L, Chen TH, Li C, Xing H, Han J, Wang MD, Zhang H, Lau WY, Wu MC, Shen F, Yang T. A systematic review comparing outcomes of surgical resection and non-surgical treatments for patients with hepatocellular carcinoma and portal vein tumor thrombus. HPB (Oxford). 2018;20:1119-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Roberts LR, Heimbach JK. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2121] [Cited by in RCA: 3214] [Article Influence: 459.1] [Reference Citation Analysis (1)] |
| 50. | Yoh T, Seo S, Taura K, Iguchi K, Ogiso S, Fukumitsu K, Ishii T, Kaido T, Uemoto S. Surgery for Recurrent Hepatocellular Carcinoma: Achieving Long-term Survival. Ann Surg. 2019;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 73] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 51. | Xiangfei M, Yinzhe X, Yingwei P, Shichun L, Weidong D. Open vs laparoscopic hepatic resection for hepatocellular carcinoma: a systematic review and meta-analysis. Surg Endosc. 2019;33:2396-2418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 52. | Wang ZY, Chen QL, Sun LL, He SP, Luo XF, Huang LS, Huang JH, Xiong CM, Zhong C. Laparoscopic vs open major liver resection for hepatocellular carcinoma: systematic review and meta-analysis of comparative cohort studies. BMC Cancer. 2019;19:1047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 53. | Liang Y, Lin C, Zhang B, Cao J, Chen M, Shen J, Feng X, Xiao G, Pan L, Chen K, Maher H, Cai X. Perioperative outcomes comparing laparoscopic with open repeat liver resection for post-hepatectomy recurrent liver cancer: A systematic review and meta-analysis. Int J Surg. 2020;79:17-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 54. | Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol. 2020;72:262-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 55. | Xu X, Lu D, Ling Q, Wei X, Wu J, Zhou L, Yan S, Wu L, Geng L, Ke Q, Gao F, Tu Z, Wang W, Zhang M, Shen Y, Xie H, Jiang W, Wang H, Zheng S. Liver transplantation for hepatocellular carcinoma beyond the Milan criteria. Gut. 2016;65:1035-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 193] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 56. | Mazzaferro V, Citterio D, Bhoori S, Bongini M, Miceli R, De Carlis L, Colledan M, Salizzoni M, Romagnoli R, Antonelli B, Vivarelli M, Tisone G, Rossi M, Gruttadauria S, Di Sandro S, De Carlis R, Lucà MG, De Giorgio M, Mirabella S, Belli L, Fagiuoli S, Martini S, Iavarone M, Svegliati Baroni G, Angelico M, Ginanni Corradini S, Volpes R, Mariani L, Regalia E, Flores M, Droz Dit Busset M, Sposito C. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020;21:947-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 57. | Kardashian A, Florman SS, Haydel B, Ruiz RM, Klintmalm GB, Lee DD, Taner CB, Aucejo F, Tevar AD, Humar A, Verna EC, Halazun KJ, Chapman WC, Vachharajani N, Hoteit M, Levine MH, Nguyen MH, Melcher ML, Langnas AN, Carney CA, Mobley C, Ghobrial M, Amundsen B, Markmann JF, Sudan DL, Jones CM, Berumen J, Hemming AW, Hong JC, Kim J, Zimmerman MA, Nydam TL, Rana A, Kueht ML, Fishbein TM, Markovic D, Busuttil RW, Agopian VG. Liver Transplantation Outcomes in a U.S. Multicenter Cohort of 789 Patients with Hepatocellular Carcinoma Presenting Beyond Milan Criteria. Hepatology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 79] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 58. | Maccali C, Chagas AL, Boin I, Quiñonez E, Marciano S, Vilatobá M, Varón A, Anders M, Hoyos Duque S, Lima AS, Menendez J, Padilla-Machaca M, Poniachik J, Zapata R, Maraschio M, Chong Menéndez R, Muñoz L, Arufe D, Figueroa R, Soza A, Fauda M, Perales SR, Vergara Sandoval R, Bermudez C, Beltran O, Arenas Hoyos I, McCormack L, Mattera FJ, Gadano A, Parente García JH, Tani CM, Augusto Carneiro D'Albuquerque L, Carrilho FJ, Silva M, Piñero F. Recurrence of hepatocellular carcinoma after liver transplantation: Prognostic and predictive factors of survival in a Latin American cohort. Liver Int. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 59. | Izzo F, Granata V, Grassi R, Fusco R, Palaia R, Delrio P, Carrafiello G, Azoulay D, Petrillo A, Curley SA. Radiofrequency Ablation and Microwave Ablation in Liver Tumors: An Update. Oncologist. 2019;24:e990-e1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 353] [Article Influence: 58.8] [Reference Citation Analysis (1)] |
| 60. | Ryu T, Takami Y, Wada Y, Hara T, Sasaki S, Saitsu H. Actual 10-Year Survival After Surgical Microwave Ablation for Hepatocellular Carcinoma: A Single-Center Experience in Japan. Ann Surg Oncol. 2019;26:4126-4133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 61. | Vietti Violi N, Duran R, Guiu B, Cercueil JP, Aubé C, Digklia A, Pache I, Deltenre P, Knebel JF, Denys A. Efficacy of microwave ablation vs radiofrequency ablation for the treatment of hepatocellular carcinoma in patients with chronic liver disease: a randomised controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2018;3:317-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 212] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 62. | Zheng L, Zhang CH, Lin JY, Song CL, Qi XL, Luo M. Comparative Effectiveness of Radiofrequency Ablation vs. Surgical Resection for Patients With Solitary Hepatocellular Carcinoma Smaller Than 5 cm. Front Oncol. 2020;10:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 63. | Shin SW, Ahn KS, Kim SW, Kim TS, Kim YH, Kang KJ. Liver Resection Versus Local Ablation Therapies for Hepatocellular Carcinoma Within the Milan Criteria: A Systemic Review and Meta-analysis. Ann Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 64. | Shi F, Lian S, Mai Q, Mo Z, Zhuang W, Cui W, Shen L, Chen M, Wu P, Chen X. Microwave ablation after downstaging of hepatocellular carcinoma: outcome was similar to tumor within Milan criteria. Eur Radiol. 2020;30:2454-2462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Bale R, Schullian P, Eberle G, Putzer D, Zoller H, Schneeberger S, Manzl C, Moser P, Oberhuber G. Stereotactic Radiofrequency Ablation of Hepatocellular Carcinoma: a Histopathological Study in Explanted Livers. Hepatology. 2019;70:840-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 66. | Lachenmayer A, Tinguely P, Maurer MH, Frehner L, Knöpfli M, Peterhans M, Weber S, Dufour JF, Candinas D, Banz V. Stereotactic image-guided microwave ablation of hepatocellular carcinoma using a computer-assisted navigation system. Liver Int. 2019;39:1975-1985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 67. | Tinguely P, Frehner L, Lachenmayer A, Banz V, Weber S, Candinas D, Maurer MH. Stereotactic Image-Guided Microwave Ablation for Malignant Liver Tumors-A Multivariable Accuracy and Efficacy Analysis. Front Oncol. 2020;10:842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 68. | Durand-Labrunie J, Baumann AS, Ayav A, Laurent V, Boleslawski E, Cattan S, Bogart E, Le Deley MC, Steen V, Lacornerie T, Peiffert D, Mirabel X. Curative Irradiation Treatment of Hepatocellular Carcinoma: A Multicenter Phase 2 Trial. Int J Radiat Oncol Biol Phys. 2020;107:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 69. | Jang WI, Bae SH, Kim MS, Han CJ, Park SC, Kim SB, Cho EH, Choi CW, Kim KS, Hwang S, Kim JH, Chang AR, Park Y, Kim ES, Kim WC, Jo S, Park HJ. A phase 2 multicenter study of stereotactic body radiotherapy for hepatocellular carcinoma: Safety and efficacy. Cancer. 2020;126:363-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 70. | Kim N, Cheng J, Jung I, Liang J, Shih YL, Huang WY, Kimura T, Lee VHF, Zeng ZC, Zhenggan R, Kay CS, Heo SJ, Won JY, Seong J. Stereotactic body radiation therapy vs. radiofrequency ablation in Asian patients with hepatocellular carcinoma. J Hepatol. 2020;73:121-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 131] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 71. | Kimura T, Takeda A, Tsurugai Y, Kawano R, Doi Y, Oku Y, Hioki K, Miura H, Nagata Y. A Multi-Institutional Retrospective Study of Repeated Stereotactic Body Radiation Therapy for Intrahepatic Recurrent Hepatocellular Carcinoma. Int J Radiat Oncol Biol Phys. 2020;108:1265-1275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 72. | Pan YX, Fu YZ, Hu DD, Long Q, Wang JC, Xi M, Liu SL, Xu L, Liu MZ, Chen MS, Zhang YJ. Stereotactic Body Radiotherapy vs. Radiofrequency Ablation in the Treatment of Hepatocellular Carcinoma: A Meta-Analysis. Front Oncol. 2020;10:1639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Sieghart W, Hucke F, Peck-Radosavljevic M. Transarterial chemoembolization: modalities, indication, and patient selection. J Hepatol. 2015;62:1187-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 247] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 74. | Lencioni R, de Baere T, Soulen MC, Rilling WS, Geschwind JF. Lipiodol transarterial chemoembolization for hepatocellular carcinoma: A systematic review of efficacy and safety data. Hepatology. 2016;64:106-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 552] [Cited by in RCA: 516] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 75. | Ogasawara S, Chiba T, Ooka Y, Kanogawa N, Motoyama T, Suzuki E, Tawada A, Nagai K, Nakagawa T, Sugawara T, Hanaoka H, Kanai F, Yokosuka O. A randomized placebo-controlled trial of prophylactic dexamethasone for transcatheter arterial chemoembolization. Hepatology. 2018;67:575-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 58] [Article Influence: 8.3] [Reference Citation Analysis (1)] |
| 76. | Wang Z, Ren Z, Chen Y, Hu J, Yang G, Yu L, Yang X, Huang A, Zhang X, Zhou S, Sun H, Wang Y, Ge N, Xu X, Tang Z, Lau W, Fan J, Wang J, Zhou J. Adjuvant Transarterial Chemoembolization for HBV-Related Hepatocellular Carcinoma After Resection: A Randomized Controlled Study. Clin Cancer Res. 2018;24:2074-2081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 202] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 77. | Guiu B, Chevallier P, Assenat E, Barbier E, Merle P, Bouvier A, Dumortier J, Nguyen-Khac E, Gugenheim J, Rode A, Oberti F, Valette PJ, Yzet T, Chevallier O, Barbare JC, Latournerie M, Boulin M. Idarubicin-loaded Beads for Chemoembolization of Hepatocellular Carcinoma: The IDASPHERE II Single-Arm Phase II Trial. Radiology. 2019;291:801-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Yoon SM, Ryoo BY, Lee SJ, Kim JH, Shin JH, An JH, Lee HC, Lim YS. Efficacy and Safety of Transarterial Chemoembolization Plus External Beam Radiotherapy vs Sorafenib in Hepatocellular Carcinoma With Macroscopic Vascular Invasion: A Randomized Clinical Trial. JAMA Oncol. 2018;4:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 320] [Article Influence: 53.3] [Reference Citation Analysis (0)] |
| 79. | Shen L, Xi M, Zhao L, Zhang X, Wang X, Huang Z, Chen Q, Zhang T, Shen J, Liu M, Huang J. Combination Therapy after TACE for Hepatocellular Carcinoma with Macroscopic Vascular Invasion: Stereotactic Body Radiotherapy vs Sorafenib. Cancers (Basel). 2018;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Liu Z, Lin Y, Zhang J, Zhang Y, Li Y, Liu Z, Li Q, Luo M, Liang R, Ye J. Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma. J Exp Clin Cancer Res. 2019;38:447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 81. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3128] [Cited by in RCA: 3790] [Article Influence: 541.4] [Reference Citation Analysis (1)] |
| 82. | Facciorusso A, Abd El Aziz MA, Sacco R. Efficacy of Regorafenib in Hepatocellular Carcinoma Patients: A Systematic Review and Meta-Analysis. Cancers (Basel). 2019;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 83. | Sonbol MB, Riaz IB, Naqvi SAA, Almquist DR, Mina S, Almasri J, Shah S, Almader-Douglas D, Uson Junior PLS, Mahipal A, Ma WW, Jin Z, Mody K, Starr J, Borad MJ, Ahn DH, Murad MH, Bekaii-Saab T. Systemic Therapy and Sequencing Options in Advanced Hepatocellular Carcinoma: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2020;6:e204930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 152] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 84. | Finn RS, Ryoo BY, Merle P, Kudo M, Bouattour M, Lim HY, Breder V, Edeline J, Chao Y, Ogasawara S, Yau T, Garrido M, Chan SL, Knox J, Daniele B, Ebbinghaus SW, Chen E, Siegel AB, Zhu AX, Cheng AL; KEYNOTE-240 investigators. Pembrolizumab As Second-Line Therapy in Patients With Advanced Hepatocellular Carcinoma in KEYNOTE-240: A Randomized, Double-Blind, Phase III Trial. J Clin Oncol. 2020;38:193-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1365] [Cited by in RCA: 1327] [Article Influence: 265.4] [Reference Citation Analysis (0)] |
| 85. | Yau T, Hsu C, Kim TY, Choo SP, Kang YK, Hou MM, Numata K, Yeo W, Chopra A, Ikeda M, Kuromatsu R, Moriguchi M, Chao Y, Zhao H, Anderson J, Cruz CD, Kudo M. Nivolumab in advanced hepatocellular carcinoma: Sorafenib-experienced Asian cohort analysis. J Hepatol. 2019;71:543-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 86. | Qin S, Ren Z, Meng Z, Chen Z, Chai X, Xiong J, Bai Y, Yang L, Zhu H, Fang W, Lin X, Chen X, Li E, Wang L, Chen C, Zou J. Camrelizumab in patients with previously treated advanced hepatocellular carcinoma: a multicentre, open-label, parallel-group, randomised, phase 2 trial. Lancet Oncol. 2020;21:571-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 424] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 87. | Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M, Okusaka T, Kobayashi M, Kumada H, Kaneko S, Pracht M, Mamontov K, Meyer T, Kubota T, Dutcus CE, Saito K, Siegel AB, Dubrovsky L, Mody K, Llovet JM. Phase Ib Study of Lenvatinib Plus Pembrolizumab in Patients With Unresectable Hepatocellular Carcinoma. J Clin Oncol. 2020;38:2960-2970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 868] [Article Influence: 173.6] [Reference Citation Analysis (0)] |
| 88. | Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, Breder V, Merle P, Kaseb AO, Li D, Verret W, Xu DZ, Hernandez S, Liu J, Huang C, Mulla S, Wang Y, Lim HY, Zhu AX, Cheng AL; IMbrave150 Investigators. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med. 2020;382:1894-1905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2542] [Cited by in RCA: 4615] [Article Influence: 923.0] [Reference Citation Analysis (2)] |
| 89. | Kudo M, Ueshima K, Ikeda M, Torimura T, Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, Tsumura H, Kuzuya T, Isoda N, Yasui K, Aino H, Ido A, Kawabe N, Nakao K, Wada Y, Yokosuka O, Yoshimura K, Okusaka T, Furuse J, Kokudo N, Okita K, Johnson PJ, Arai Y; TACTICS study group. Randomised, multicentre prospective trial of transarterial chemoembolisation (TACE) plus sorafenib as compared with TACE alone in patients with hepatocellular carcinoma: TACTICS trial. Gut. 2020;69:1492-1501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 496] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 90. | Park JW, Kim YJ, Kim DY, Bae SH, Paik SW, Lee YJ, Kim HY, Lee HC, Han SY, Cheong JY, Kwon OS, Yeon JE, Kim BH, Hwang J. Sorafenib with or without concurrent transarterial chemoembolization in patients with advanced hepatocellular carcinoma: The phase III STAH trial. J Hepatol. 2019;70:684-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 150] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 91. | Kok VC, Chen YC, Chen YY, Su YC, Ku MC, Kuo JT, Yoshida GJ. Sorafenib with Transarterial Chemoembolization Achieves Improved Survival vs. Sorafenib Alone in Advanced Hepatocellular Carcinoma: A Nationwide Population-Based Cohort Study. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 92. | Johnston MP, Khakoo SI. Immunotherapy for hepatocellular carcinoma: Current and future. World J Gastroenterol. 2019;25:2977-2989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 121] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 93. | Zongyi Y, Xiaowu L. Immunotherapy for hepatocellular carcinoma. Cancer Lett. 2020;470:8-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 177] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 94. | Mizukoshi E, Kaneko S. Immune cell therapy for hepatocellular carcinoma. J Hematol Oncol. 2019;12:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 95. | Caraballo Galva LD, Cai L, Shao Y, He Y. Engineering T cells for immunotherapy of primary human hepatocellular carcinoma. J Genet Genomics. 2020;47:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | June CH, O'Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science. 2018;359:1361-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1289] [Cited by in RCA: 2036] [Article Influence: 290.9] [Reference Citation Analysis (0)] |
| 97. | Nishida T, Kataoka H. Glypican 3-Targeted Therapy in Hepatocellular Carcinoma. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 98. | Shi D, Shi Y, Kaseb AO, Qi X, Zhang Y, Chi J, Lu Q, Gao H, Jiang H, Wang H, Yuan D, Ma H, Li Z, Zhai B. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin Cancer Res. 2020;26:3979-3989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 234] [Article Influence: 46.8] [Reference Citation Analysis (0)] |
| 99. | Pan Z, Di S, Shi B, Jiang H, Shi Z, Liu Y, Wang Y, Luo H, Yu M, Wu X, Li Z. Increased antitumor activities of glypican-3-specific chimeric antigen receptor-modified T cells by coexpression of a soluble PD1-CH3 fusion protein. Cancer Immunol Immunother. 2018;67:1621-1634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 100. | Batra SA, Rathi P, Guo L, Courtney AN, Fleurence J, Balzeau J, Shaik RS, Nguyen TP, Wu MF, Bulsara S, Mamonkin M, Metelitsa LS, Heczey A. Glypican-3-Specific CAR T Cells Coexpressing IL15 and IL21 Have Superior Expansion and Antitumor Activity against Hepatocellular Carcinoma. Cancer Immunol Res. 2020;8:309-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 175] [Article Influence: 35.0] [Reference Citation Analysis (0)] |
| 101. | Yu M, Luo H, Fan M, Wu X, Shi B, Di S, Liu Y, Pan Z, Jiang H, Li Z. Development of GPC3-Specific Chimeric Antigen Receptor-Engineered Natural Killer Cells for the Treatment of Hepatocellular Carcinoma. Mol Ther. 2018;26:366-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 102. | Mantovani S, Oliviero B, Varchetta S, Mele D, Mondelli MU. Natural Killer Cell Responses in Hepatocellular Carcinoma: Implications for Novel Immunotherapeutic Approaches. Cancers (Basel). 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 52] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 103. | Zhu W, Peng Y, Wang L, Hong Y, Jiang X, Li Q, Liu H, Huang L, Wu J, Celis E, Merchen T, Kruse E, He Y. Identification of α-fetoprotein-specific T-cell receptors for hepatocellular carcinoma immunotherapy. Hepatology. 2018;68:574-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 104. | Docta RY, Ferronha T, Sanderson JP, Weissensteiner T, Pope GR, Bennett AD, Pumphrey NJ, Ferjentsik Z, Quinn LL, Wiedermann GE, Anderson VE, Saini M, Maroto M, Norry E, Gerry AB. Tuning T-Cell Receptor Affinity to Optimize Clinical Risk-Benefit When Targeting Alpha-Fetoprotein-Positive Liver Cancer. Hepatology. 2019;69:2061-2075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (1)] |
| 105. | Li Z, Gong H, Liu Q, Wu W, Cheng J, Mei Y, Chen Y, Zheng H, Yu X, Zhong S, Li Y. Identification of an HLA-A*24:02-restricted α-fetoprotein signal peptide-derived antigen and its specific T-cell receptor for T-cell immunotherapy. Immunology. 2020;159:384-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 106. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015; 148: 1383-91. e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 387] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 107. | Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, Gwak GY, Kim KM, Kim YJ, Lee JW, Yoon JH. Sustained efficacy of adjuvant immunotherapy with cytokine-induced killer cells for hepatocellular carcinoma: an extended 5-year follow-up. Cancer Immunol Immunother. 2019;68:23-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 68] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 108. | Yu SJ, Ma C, Heinrich B, Brown ZJ, Sandhu M, Zhang Q, Fu Q, Agdashian D, Rosato U, Korangy F, Greten TF. Targeting the crosstalk between cytokine-induced killer cells and myeloid-derived suppressor cells in hepatocellular carcinoma. J Hepatol. 2019;70:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 116] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 109. | Chiu DK, Yuen VW, Cheu JW, Wei LL, Ting V, Fehlings M, Sumatoh H, Nardin A, Newell EW, Ng IO, Yau TC, Wong CM, Wong CC. Hepatocellular Carcinoma Cells Up-regulate PVRL1, Stabilizing PVR and Inhibiting the Cytotoxic T-Cell Response via TIGIT to Mediate Tumor Resistance to PD1 Inhibitors in Mice. Gastroenterology. 2020;159:609-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 125] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 110. | Ostroumov D, Duong S, Wingerath J, Woller N, Manns MP, Timrott K, Kleine M, Ramackers W, Roessler S, Nahnsen S, Czemmel S, Dittrich-Breiholz O, Eggert T, Kühnel F, Wirth TC. Transcriptome profiling identifies TIGIT as a marker of T cell exhaustion in liver cancer. Hepatology. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 111. | Liu F, Liu Y, Chen Z. Tim-3 expression and its role in hepatocellular carcinoma. J Hematol Oncol. 2018;11:126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 112. | Sawada Y, Yoshikawa T, Ofuji K, Yoshimura M, Tsuchiya N, Takahashi M, Nobuoka D, Gotohda N, Takahashi S, Kato Y, Konishi M, Kinoshita T, Ikeda M, Nakachi K, Yamazaki N, Mizuno S, Takayama T, Yamao K, Uesaka K, Furuse J, Endo I, Nakatsura T. Phase II study of the GPC3-derived peptide vaccine as an adjuvant therapy for hepatocellular carcinoma patients. Oncoimmunology. 2016;5:e1129483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 136] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 113. | Taniguchi M, Mizuno S, Yoshikawa T, Fujinami N, Sugimoto M, Kobayashi S, Takahashi S, Konishi M, Gotohda N, Nakatsura T. Peptide vaccine as an adjuvant therapy for glypican-3-positive hepatocellular carcinoma induces peptide-specific CTLs and improves long prognosis. Cancer Sci. 2020;111:2747-2759. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 114. | Chen K, Wu Z, Zhao H, Wang Y, Ge Y, Wang D, Li Z, An C, Liu Y, Wang F, Bi X, Wang H, Cai J, Ma C, Qu C. XCL1/Glypican-3 Fusion Gene Immunization Generates Potent Antitumor Cellular Immunity and Enhances Anti-PD-1 Efficacy. Cancer Immunol Res. 2020;8:81-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 115. | Lee JH, Tak WY, Lee Y, Heo MK, Song JS, Kim HY, Park SY, Bae SH, Lee JH, Heo J, Kim KH, Bae YS, Kim YJ. Adjuvant immunotherapy with autologous dendritic cells for hepatocellular carcinoma, randomized phase II study. Oncoimmunology. 2017;6:e1328335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 116. | Teng CF, Wang T, Wu TH, Lin JH, Shih FY, Shyu WC, Jeng LB. Combination therapy with dendritic cell vaccine and programmed death ligand 1 immune checkpoint inhibitor for hepatocellular carcinoma in an orthotopic mouse model. Ther Adv Med Oncol. 2020;12:1758835920922034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 117. | Yoon AR, Hong J, Li Y, Shin HC, Lee H, Kim HS, Yun CO. Mesenchymal Stem Cell-Mediated Delivery of an Oncolytic Adenovirus Enhances Antitumor Efficacy in Hepatocellular Carcinoma. Cancer Res. 2019;79:4503-4514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 78] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 118. | Lin C, Ren W, Luo Y, Li S, Chang Y, Li L, Xiong D, Huang X, Xu Z, Yu Z, Wang Y, Zhang J, Huang C, Xia N. Intratumoral Delivery of a PD-1-Blocking scFv Encoded in Oncolytic HSV-1 Promotes Antitumor Immunity and Synergizes with TIGIT Blockade. Cancer Immunol Res. 2020;8:632-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 119. | Dal Bo M, De Mattia E, Baboci L, Mezzalira S, Cecchin E, Assaraf YG, Toffoli G. New insights into the pharmacological, immunological, and CAR-T-cell approaches in the treatment of hepatocellular carcinoma. Drug Resist Updat. 2020;51:100702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |