Published online Feb 15, 2021. doi: 10.4251/wjgo.v13.i2.92
Peer-review started: October 30, 2020
First decision: December 12, 2020
Revised: December 25, 2020
Accepted: January 28, 2021
Article in press: January 28, 2021
Published online: February 15, 2021
Processing time: 93 Days and 23.4 Hours
Tumor-initiating cells (TICs) or cancer stem cells are believed to be responsible for gastrointestinal tumor initiation, progression, metastasis, and drug resistance. It is hypothesized that gastrointestinal TICs (giTICs) might originate from cell-cell fusion. Here, we systemically evaluate the evidence that supports or opposes the hypothesis of giTIC generation from cell-cell fusion both in vitro and in vivo. We review giTICs that are capable of initiating tumors in vivo with 5000 or fewer in vivo fused cells. Under this restriction, there is currently little evidence demonstrating that giTICs originate from cell-cell fusion in vivo. However, there are many reports showing that tumor generation in vitro occurs with more than 5000 fused cells. In addition, the mechanisms of giTIC generation via cell-cell fusion are poorly understood, and thus, we propose its potential mechanisms of action. We suggest that future research should focus on giTIC origination from cell-cell fusion in vivo, isolation or enrichment of giTICs that have tumor-initiating capabilities with 5000 or less in vivo fused cells, and further clarification of the underlying mechanisms. Our review of the current advances in our understanding of giTIC origination from cell-cell fusion may have significant implications for the understanding of carcinogenesis and future cancer therapeutic strategies targeting giTICs.
Core Tip: Currently, there are many controversial hypotheses concerning the generation of gastrointestinal tumor-initiating cells (giTICs). Here, we mainly review the current advances in the understanding of giTIC origination from the cell-cell fusion of cancer cells and bone marrow-derived cells.
- Citation: Zhou Y, Cheng JT, Feng ZX, Wang YY, Zhang Y, Cai WQ, Han ZW, Wang XW, Xiang Y, Yang HY, Liu BR, Peng XC, Cui SZ, Xin HW. Could gastrointestinal tumor-initiating cells originate from cell-cell fusion in vivo? World J Gastrointest Oncol 2021; 13(2): 92-108
- URL: https://www.wjgnet.com/1948-5204/full/v13/i2/92.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i2.92
Tumors are composed of cells with different levels of differentiation, and tumor-initiating cells (TICs) are the least differentiated cancer cells, which are then capable of giving rise to other cancer cells[1,2]. TICs are the source of gastrointestinal tumor initiation, progression, metastasis, and drug and radiation resistance. Moreover, they are capable of self-renewal, can differentiate into multiple cell lineages (such as cancer cells), and can undergo asymmetric cell division. TICs are the most carcinogenic subpopulation of cells in most cancer types[3-5], including gastrointestinal cancers[6]. The origin of TICs remains unknown; however, many hypotheses[7] have been proposed to explain it, including those involving gene mutations[8], endogenous reprogramm-ing[9,10], and cell-cell fusion[11-14].
Gastrointestinal TICs (giTICs) may originate from gene mutations[15]. Some hypothesized that gastrointestinal stem cells, similar to other types of stem cells, have protective mechanisms that reduce tumorigenesis. These mechanisms include asymmetric cell division via chromosomal segregation and relatively slow cell cycles[16], which can protect cells from DNA damage and cellular stress[17]. To form giTICs, these mechanisms must be circumvented. The development and progression of colorectal cancer (CRC) are associated with a number of identified gene mutations, in genes such as KRAS, adenomatous polyposis coli (APC), and p53, that promote the conversion of normal epithelial mucosal tissue to cancerous tissue[18,19]. The tumor suppressor gene p53 ensures the genomic stability of stem cells, and can therefore act as a barrier to the formation of TICs[20]. Wild-type p53 can be experimentally replaced with a mutant version of p53 via PCR, CRISPR/Cas9, and knock-in techniques. When a related gene mutation occurs, p53 loses its tumor-suppressing ability and acquires additional carcinogenic capabilities. This process is termed as mutant p53 gain of function (GOF). Experimental evidence suggests that mutant p53 GOF can mediate cancerous properties, such as cell death resistance, sustained proliferation, metastasis and invasion, and tumor-promoting inflammation[21-23]. Mutant p53 is highly expressed in colorectal TICs and CRC tissues[8]. Most evidence that supports this hypothesis arises from the observation that common mutations in CRC would affect normal stem cell behavior. For example, deletion or inactivation of the APC gene is often the initiating step in colorectal carcinogenesis[18] and as such, acts as a gatekeeper in CRC. The absence of APC is rare and APC is commonly found in gastrointestinal cells, including normal populations of gastrointestinal stem cells, as it plays a major role in regulating normal stem cell function[24]. There is little direct evidence demonstrating that giTICs originate from gene mutations in stem cells. Regardless, it is generally believed that giTICs originate from mutated stem cells because stem cells are long-lived gastrointestinal cell types. Thus, there is sufficient time for them to accumulate oncogenic mutations[19]. In addition, TICs and normal stem cells have many identical or similar properties, indicating that they have a common source or originate from the same ancestor.
Another hypothesis is that giTICs may originate from endogenous reprogramming. A specific combination of transcription factors can reprogram differentiated cells into pluripotent stem cells[25]. Following the same reasoning, gastrointestinal epithelial cells can be dedifferentiated into progenitor/stem cells via specific matched signal transduction pathways. Notably, bidirectional transformation between TICs and non-TICs was observed in intestinal tumors. Nuclear factor kappa-B (NF-κβ) induces the stabilization of β-catenin and activation of the β-catenin/T-cell factor transcription complex, which, together with the cancer-causing Kras, can induce dedifferentiation of non-stem colon cancer cells into stem-like cancer cells[9,26] or TICs[27,28]. However, the mechanisms underlying their regulation remain unclear[28]. Epithelial-mesenchymal transition (EMT) may also be involved in endogenous reprogramming[29] by inducing overexpression of the transcription factors Snail[30-33] and zinc finger E-box-binding protein 1 (Zeb1)[34-37]. It is worth noting that Zeb1 activation is associated with Slug (Snai2) in TICs[36]. Zeb1, a transcription factor known to be involved in EMT, is necessary for the conversion of non-TICs to TICs. EMT in TICs also induces the expression of CD44, which was shown to be highly expressed in giTICs[36].
Cell-cell fusion can be easily induced in vitro by physicochemical or biological molecules but also occurs in vivo, such as the fusion of sperm and egg cells. Cell fusion is an essential physiological process, which plays a role in fertilization, virus entry, muscle differentiation, and placenta development. It was also reported to be closely associated with the occurrence and development of cancer. Fused cells display the genotype and phenotype of the maternal cells, and hybrids produced by the fusion of different cell types have distinct properties. Cell-cell fusion can be identified by cell size and shape, karyotypes, DNA, gene expression, cell-specific markers, and other properties. Both fused cells and TICs display aneuploidy, such as being tetraploid, and chromosomal instability, as well as have the ability to induce metastasis and drug resistance[38], which suggests that cell-cell fusion may produce TICs. In other words, cell-cell fusion may be a better explanation of TIC generation than the aforementioned conventional gene mutation and endogenous reprogramming hypotheses. In addition, cell-cell fusion may play a role in giTIC formation by introducing endogenous reprogramming, as cell fusion hybrids retain transcripts from both parental cells and also express a unique subset of transcripts[39].
Cell-cell fusion in vivo and tumor-initiating capacity in vivo should be the criteria used to determine whether giTICs originate from cell-cell fusion. Non-tumor initiating cancer cells can also proliferate and generate tumors when enough of such cells are used. However, theoretically, only TICs can initiate tumor formation using a limited number of cells. Generally, unsorted cancer cells contain both TICs and non-TICs. Therefore, it is difficult to determine which cells are responsible for tumor initiation. Here, we review giTICs that can initiate tumors in vivo using 5000 or fewer in vivo fused cells, as well as tumor-initiating like cells (TILCs) that can generate tumors using more than 5000 fused cells. However, we do not exclude the possibility that more than 5000 hybrid cells may be needed to initiate tumors when cell-cell fusion occasionally induces rare genetic changes that lead to tumor development.
The fusion of human cells in vivo was confirmed by reports describing the fusion of melanoma cells and osteoclasts in 2007 and the fusion of BRAF mutated melanoma and stromal cells in 2016[40,41]. The fusion of macrophages and peripheral blood melanoma cells, which was discovered in 2015, also provided evidence for human cell fusion in vivo. Moreover, studies have shown that fusion cells exhibit high expression levels of cell fusion factors, including the cell fusion molecule chemokine receptor 4 (CXCR4), as well as that fusion cells may cause tumor metastasis. However, in the tumorigenic experiments described, 5 × 106 fusion cells were inoculated into mice to generate primary tumors at a number that was much higher than the 5000 cells criteria used for in vivo tumor generation. Therefore, the fusion cells were not concluded to be TICs when using the aforementioned restrictions[42].
Currently, there are no reports of giTICs originating from cell-cell fusion in vivo[43]. We summarize the reports regarding giTICs originating from cell-cell fusion, including the fusion of gastrointestinal cells with various cell types, the study methods used (in vitro or in vivo), evidence of cell-cell fusion, tumorigenic and tumor-initiating properties of the fused cells, and possible mechanisms of cell-cell fusion (Table 1).
| Cell I | Cell II | Cell fusion method | Cell fusion evidence | Tumor initiating method | Tumor initiating evidence | Mechanism | Ref. |
| Colorectal cancer cell | Human dendritic cell | In vitro. DCs and cancer cells fused using PEG | Purified cells | Enhanced induction of antigen-specific CTL | Streptococcal preparation OK-432 promotes fusion efficiency | [69] | |
| Human esophageal carcinomas cell | Human dendritic cell | In vitro. ECs and DCs fused using PEG | Co-expression of MHC class II, CD86, and MUC1 | Induced specific antitumor response | [55] | ||
| Human gastrointestinal cancer cell | Human dendritic cell | In vitro. Fusion via PEG and electroporation | Th1/Th2 and Tc1/Tc2 balance improved | [73] | |||
| Human gastric cancer cell | Human dendritic cell | FACS analysis | Induced CTLs, reduced metastases | [56] | |||
| Human gastric cancer cell (HGC-27 or SGC-7901) | hucMSC | In vitro. GC-DIO and hucMSCs-DID fusion using PEG | Double positive cells | BALB/C nude mice (2 × 106 cells) | In vivo. Tumors formed from fused cells | [44] | |
| Human breast cancer cell (MDA-MB-231) | Human MSC | In vivo. 2 × 106 MSC300415-GFP and 2 × 106 MDA-MB-231-cherry subcutaneously injected into 5 female NOD/SCID mice | Hybrid cells GFP/cherry fluorescence | 1 × 106 MDA-hyb3-GFP/cherry cells were injected subcutaneously into 3 female NOD/SCID mice | In vivo. Tumors formed from fused cells | [80] | |
| Human colon adenocarcinoma cell | Human HeLa cell (D98OR) | In vitro. Fused using PEG, isolation of hybrid cells by selecting isolated colonies | Flow cytometry analysis had more DNA than expected. A range of 71–140 chromosomes | Fusion cell characteristics were consistent with cancer cells | [75] | ||
| Human colon cancer cell (SW480) | Human dendritic cell | In vitro. Tumor cells- PKH26-red and DCs- PKH67-green fused using PEG | Dual red and green fluorescence and highly expressed CD80, CD86, and HLA-DR | CD8+ T lymphocytes co-cultured with dendritic cells at a ratio of 10:1 | CTLs were activated to proliferate and the number of T cells increased | [55] | |
| Human colon cancer cell (SW620) | Human dendritic cell | In vivo. DCs and tumor cells fused using PEG | Fusion efficacy was evaluated by FM and FC | In vivo. 1 × 107 fusion hybrids injected intraperitoneally | Cellular immune responses, significant inhibition of tumor growth | [55] | |
| Human colon carcinoma line (VACO-411) | Human breast cancer line (MCF-7) | In vitro. VACO-411 (1 × 106 cells) and MCF-7 (1 × 106 cells) fused using PEG | Morphology of VACO-411 × MCF-7 fused cells | In vitro. The fused cells were treated with TGF-β | Fusion cells were inhibited by TGF-β | [76] | |
| Human colon epithelial cancer cell | Human normal colon cell | In vitro. Cancer cells and normal cells (1:10) fused using PEG | Comparison of DNA synthesis (P < 0.01) | Male mice nu/nu injected subcutaneously with1 × 106 fused cells | The fused cells could not grow into tumors | [77] | |
| Human colorectal carcinoma cell | Human dendriticcell | In vitro. DCs-CMFDA-green,colorectal carcinoma-CMTMR-red cells fused using PEG/electrofusion | Double-positivecells | Efficiently activated autologous tumor-specific T cells | [68] | ||
| Human esophageal cancer cell (EC109) | Human dendritic cell | In vitro. DCs and ECs (5:1-10:1) fused using PEG | Co-expression of MHC-CiaSSII and CD86 and MUC1 antigens | Cytotoxic T lymphocytes | Antitumor capabilities | [60] | |
| Human esophageal cancer cell (EC9706) | hucMSC | In vitro. ECs-DiO hMSCs- DiD fused using PEG | Double positive hybrids are yellow and multinuclear | In vivo. Xenograft assays in immunodeficient mice | Both ECs and their self-fusion groups developed tumors | ||
| Human esophageal carcinoma cell (EC9706) | Human hemopoietic stem cell | In vitro. ECs and HSCs (10:1) fused using PEG | CD34+CD38-Scal+ cells isolated using immunomagnetic beads; HSCs cannot grow in DMEM supplemented with 10% FBS | In vivo. 5 × 105 fused cells injected into 12 NOD/SCID mice | All mice formed tumors; however, the tumor weight of the fused cell group was lower than that of the EC9706 group | [54] | |
| Human esophageal carcinoma cell | Human dendritic cell | In vitro. DCs and ECs (5:1) fused using PEG; incubated with FA-FITC and CD80-PE | Analysis using FATICan | In vivo. Fusion vaccine (0.2 mL; 1 × 106 cells) injected | Anti-tumor effects | [58] | |
| Human esophageal carcinoma cell (EC109) | Human dendritic cell | In vitro. DCs and ECs (5:1) fused using PEG. FA-FITC CD80-PE mAbs-CD80, CD83 and CD86 | FC | In vitro. Cytotoxicity assays | Antitumor activity | [59] | |
| Human female pancreatic adenoepithelial neoplasm cell | Human male BMDC | In vivo. 4 female pancreatic cancer patients transplanted with male BMDCs | Peripheral blood cell: EpCAM (yellow)/CD45 (green), Y chromosome, CK+/CD45+, MФ proteinsCD14, CD16, CD11c, CD163 MUC4 | [43] | |||
| Mouse colon cancer cell (MC38) | Mouse R26R- YFP Cre mice | In vivo. MC38 cells were injected ventrally into r26R-YFP Cre mice | RFP+ YFP+ | [51] | |||
| Mouse primary melanoma cell | Mouse MФ | Melanoma cells were injected into mice intradermally | RFP and GFP cells | 300 and 3000 cells, respectively injected into mice (n = 9, 3) | Tumor initiation | The characteristics of parental cells provided adhesive affinity | [51] |
| Human gastric cancer cell (MKN-1) | Dendritic cell | In vitro. DCs- PKH-26 and GC cells-PKH-67 fused via PEG/electrofusion | Double positive cell populations | In vitro. Co-cultured DCs (1 × 105 cells) with 1 × 106 T cells | Induced tumor antigen-specific CD8+ T cells | [70] | |
| Human gastric epithelial cell (GES-1) | CM-MSC | In vitro. GES-1- PHK-26 (2 × 106 cells) and CM-MSCs- CFSE (1 × 107) cells fused using PEG | Most cells express PKH26 and CFSE | In vivo. The fused cells (1 × | Tumors from the fused cells formed in vivo | [47] | |
| Human gastric cancer cell (SGC7901) | Human dendritic cell | In vitro. SGC7901 and DCs fused using PEG | Pure fused cells were obtained by selective culture with HAT/HT culture system | In vivo. Fusion cells (5 × 108) were injected into BALB/c mice | In vivo. The fused cells could not grow into tumors | [61] | |
| Human gastric cancer cell (SGC7901) | Human dendritic cell | In vitro. SGC7901 and DCs fused using PEG | Selective culture with the HAT/HT culture system | In vivo. Fused cells (5 × 106/mL) + T cells (5 × 106/mL) | In vivo. The fused cells could not grow into tumors | [62] | |
| Human hepatobiliary stem/progenitor cell | Human hematopoietic precursor-derived myeloid intermediate | In vitro. Cultures of CD34+ LTICs and xenograft cells (the xenografts were produced by CD34+ hybrid cells) | CD34+ LTICs co-expressed liver stem cell and myelomonocytic cell markers | HSPCs were fused with a | |||
| Human hepatocellular carcinoma cell (HepG2) | Human embryonic stem cell | In vitro. HepG2-red mitochondrion selective probe and hESCs-Oct-GFP cells fused via laser-induced single-cell fusion | Transfer of cytoplasmic GFP from hESCs to HepG2 cells | In vivo. HepG2 cells and the fused cells (5 × 104 and 1 × 105 cells, respectively) were injected into nude mice | Tumors were generated from fused cells | ||
| Human hepatocellular carcinoma cell (HepG2) | Mouse MSC | In vitro. MSCs- DiI (5 × 105 cells) and HepG2-eGFP (1 × 105 cells) fused using PEG | Dual fluorescence, two nuclei | In vivo. The fused cells were injected into 7 nude mice/group with 2.4 × 107 cells/group | Tumors were formed from fused cells | ||
| Human intestinal cancer cell (HT-29) | Human MSC | In vitro. PM7-eGFP and HT-29-DsRED cells were cocultured | eGFP and DsRED double positive cells | Acquired epithelial characteristics | [51] | ||
| Human intestinal epithelial cells | Human hematopoietic cell | In vitro. X- and Y-chromosome determined by FISH. Female recipients of hematopoietic cell transplant from male donors | Stained for X- (green) and Y- (red) chromosomes and Lamin B1 (white) | In mice, hematopoietic fusion with non-hematopoietic cell types occurs endogenously in the absence of disease | [86] | ||
| Mouse intestinal epithelial cell | Mouse bone marrow-derived cell | In vivo. CMV-CreGFP+ mice BM were transplanted into iDTR mice | Co-staining for GFP and EpCAM.GFP+ cells in the intestine | Cell fusion is dispensable for tissue homeostasis | [52] | ||
| Mouse intestinal stem cell | Human bone marrow-derived cell | In vivo. Donor female mice BMDCs-GFP, male recipient mice | EGFP expression in all principal intestinal epithelial lineages | [63] | |||
| Mouse colon adenocarcinoma cell (CT26) | Mouse dendritic cell | In vitro. Tumor cells- PKH67-Green and DCs fused using PEG | Assessedvia the trypan-blue exclusion test | In vivo. BALB/c mice injected with 5 × 105 cells | The fused cells could not generate tumors | [64] | |
| Mouse colon adenocarcinoma cell line (CT26) | Mouse dendritic cell | In vitro. DCs-anti-CD11cmAb and tumor cells- CFSE fused using PEG | Analyzed by FC | In vivo. Injection of 1 × 104, | The fused cells did not generate tumors. CTL anti-tumor effects | [72] | |
| Mouse colon carcinoma cells (CT26CL25) | Allogeneic and semi allogeneic dendritic cells | In vitro. DCs-PKH26-red andCT26CL25-PKH67-green fused using PEG | Analyzedby FC | In vivo. 1 × 106 fused cells and 5.0 × 105 CT26CL25 cells | Anti-tumor effects in vivo | [65] | |
| Mouse colon epithelial cell | Mouse BMDC | In vivo. Female mice BMDCs-GFP (1 × 107 cells) transplanted into irradiated male mice | Co-expression of GFP and the Y chromosome | In vivo. Parabiosis surgery (GFP and ROSA mice) | Bone-marrow/epithelial cell fusion causes genetic reprogramming | Inflammation and proliferation act together to mediate intestinal cell fusion | [87] |
| Mouse gastric epithelial cell | Mouse BMDC | In vivo. Male irradiated C57BL/6 mice received female C57BL/6 mice BMDC-GFP | Direct. Positive for the Y chromosome and expressed GFP as determined by FM | In vivo. GCs were induced with a carcinogen | Tumor formed from fused cells in vivo | Chronic inflammation (adenocarcinoma, glandular stomach, not squamous cell carcinoma) | [48] |
| Mouse hepatocellular carcinoma cell | Mouse dendritic cell | In vitro. HCCs PKH-26-red and DCs-PKH-2-green fused using PEG | The fusion cells were yellow under the confocal microscope | In vitro. CTL assay | In vitro. Activated cytotoxic T lymphocytes | [66] | |
| Mouse hepatoma cell line (Hepa1-6) | Mouse embryonic stem cell | In vitro. Cancer cells-GFP and ES cells-RFP fused using PEG | Double fluorescence-positive | In vivo. 1 × 106 ES-cancer fused cells injected into nude mice | Tumor formed from fused cells in vivo | ||
| Mouse intestinal epithelial cancer cell | Mouse WBM (macrophage) | In vivo. WBM-GFP (5 × 106 cells) injected in recipient mice (male WT, ApcMin/+, ROSA26, ROSA26/ApcMin/+). Parabiosis | Co-localization of GFP (green) and β-galactosidase (red) | Nuclear reprogramming | Fusion between circulating blood-derived cells and tumor epithelium origin at the natural course of tumorigenesis | [39] | |
| Mouse intestinal epithelial cells (IEC-6). Human cervical adenocarcinoma cells (HeLa) | Mouse intestinal epithelial cells (IEC-6) Human cervical adenocarcinoma cells (HeLa) | In vitro. IEC-6- CFSE andIEC-6- SNARF-1 (HeLa -Cy3-dUTP-red and HeLa- Cy5-dUTP-green) fused using PEG | The fused cell emits both CFSE and SNARF-1 fluorescence (IEC-6). Eight daughter cells contain both dyes (HeLa) | In vivo. The IEC-6 fused cells (Two million cells) were injected in 18 mice | Tumor formed from the fused cells in vivo (n = 11 generated tumors) | [78] | |
| Mouse intestine stromal cell | Mouse bone marrow-derived macrophage | In vivo. Female mice BMDCs-GFP injected in male recipient mice | Co-localization of GFP and Y-chromosome | Organ fibrosis | Depleting macrophages genetically reduced the number of cells | [53] | |
| Mouse prostate cancer cell (PCa) | Mouse BMDC | In vivo. 2 × 106 cells/mice BMDCs-GFP transplanted into 10 C57BL/6 mice | Co-expression of GFP and CK8 | C57BL/6 mice-GFP, induced prostate cancer by MNU | GFP-positive cells in the prostate cancer tissue | [79] | |
| Whole tumor cell | Human dendritic cell | In vitro. The purified DCs and tumor cells fused using PEG | [67] |
Xue et al[44] fused DIO-labeled (green) HGC-27 gastric cancer cells with DID-labeled (red) human umbilical cord mesenchymal stem cells (hucMSCs) using polyethylene glycol (PEG) 1500 in vitro. The fused cells with double nuclei were then stained with Hoechst 33342 (blue) and DIO-GC and DID-hucMSC double labels (yellow) were observed after 7 d by sorting via flow cytometry. Then, 20 male BALB/C nude mice were injected subcutaneously with 2 × 106 gastric cancer cells or fused cells. Mice in the fusion group exhibited tumor nodules at 4 d post-injection, while mice in the gastric cancer group showed no tumor nodules. Moreover, the fusion cells were shown to form more colonies than their parental cells and had higher Cyclin D1 and proliferating cell nuclear antigen (PCNA) expression levels. Cyclin D1 and PCNA expression in tumor tissues is usually positively correlated with cancer cell proliferation. The expression levels of the stem cell transcription factors Sox2, Oct4, Nanog, and Lin28, as well as those of the cancer cell markers CD133 and CD44, were also shown to be increased in the fused cells[44]. In addition, real-time RT-PCR analysis revealed that E-cadherin mRNA expression was decreased in fused cells, whereas that of mesenchymal markers, such as α-SMA, FAP, vimentin, snail, N-cadherin, slug, and twist, was significantly increased, indicating that the fused cells underwent EMT. EMT is associated with the metastatic ability and invasiveness of cancer cells. As such, the obtained fusion cells were shown to have EMT properties, which is similar to TICs[44-46]. Cell fusion in vitro between gastric epithelial cells and MSC also resulted in fusion cells with tumorigenic capabilities that underwent EMT[47]. However, these hybrid cells were formed in vitro and the number of cells used for the tumorigenic experiments was much higher than 5000 cells.
In a report by Yan et al[48], the bone marrow of green fluorescent protein (GFP) transgenic female C57BL/6 mice was transplanted into irradiated male homologous mice (68/68), all of which survived. Then, the transplanted bone marrow-derived cells (BMDCs) became the main bone marrow cells of the chimeric mice. Tumors were induced using the tumor-causing drug 3-methylcholanthrene. Three of the 12 treated mice successfully developed tumors. Hematoxylin & eosin staining showed two diffuse-type carcinomas in the glandular stomach and one squamous cell carcinoma (SCC). Analysis of CK-18 (mostly expressed in epithelial cancer cells and determined via immunohistochemistry staining) and GFP expression (fluorescence microscopy) showed that cells derived from both cancer types were positive for CK-18 and GFP expression, indicating that they are epithelial tumors originating from BMDCs. Moreover, co-expression of the Y chromosome and GFP in the cytoplasm was detected in a large number of adenocarcinoma cells via fluorescence in situ hybridization (FISH) and immunofluorescence microscopy. In SCC tissues, GFP expression was mainly detected in the interstitium and keratin pearl, but FISH did not detect the presence of the Y chromosome. Instead, the Y chromosome and GFP were co-expressed in the epithelial cells surrounding the SCC. Gastric cancer may originate from the BMDCs of transplant donors and develop initially via trans-differentiation and then cell-cell fusion[48-50]. These authors revealed that BMDC-gastric epithelial cell fusion may contribute to the renewal of the gastric mucosa and lead to increased carcinogenesis potential. Additionally, the aforementioned experiments demonstrated that fusion cells exhibit stem cell and cancer cell markers in chemical-induced tumor tissues in vivo but did not prove that fusion cells can initiate tumors. As such, it is not possible to distinguish between drug-induced tumors or fusion cell-induced tumors.
In a previous study[51], researchers directly co-cultured PM7 cells, which are eGFP-labeled bone marrow-derived MSCs, with the DsRED-labeled colon cancer cell line HT-29. After co-culture, some cells showed eGFP and DsRED double-positive labels and these fused cells were shown to be positive for epithelial-specific antigen (ESA) and cytokeratin expression. However, the authors did not investigate the tumorigenic capacity and other stemness properties of the fused cells. Other reports have found similar results[52,53].
Notably, a study revealed that the fusion of intestinal epithelial cancer cells and macrophages from BMDCs in vivo leads to nuclear reprogramming and the authors suggested that the fusion cells may play a role in tumor development and metastasis[39].
In a previous study, human embryonic stem cells (hESCs) were labeled with Oct-GFP and HepG2 hepatocytes and stained with a mitochondrial (red) probe, and the cells were then fused via laser-induced fusion. Later, it was shown that GFP was transferred from hESCs (green) to liver cancer cells (red), confirming the successful generation of fusion cells. Subsequently, different amounts of 5 × 104 - 1 × 106 fused cells were injected subcutaneously into nude mice, and mice injected with normal liver cancer cells were used as controls. The fused cell group exhibited a tumor incidence of 9/12, while the liver cancer cell group had a tumor incidence of only 1/8. Moreover, a lower number of fused cells were necessary for tumor generation when compared to the liver cancer cell group. These results demonstrated that in vitro cell fusion between liver cancer cells and stem cells could generate cells with giTILC properties. The tumorigenicity of the fusion-generated giTILCs was also shown to be significantly higher than that of the maternal cancer cells. However, the number of cells used for tumorigenicity experiments was higher than 5000 and cell-cell fusion was induced in vitro. Similar reports have also shown that in vitro cell-cell fusion produces tumorigenic hybrid cells or giTILCs.
CD34+ hybrid cells extracted from liver cancer cell lines were shown to express high levels of hepatic stem cell and bone marrow mononuclear cell markers. The cells were also shown to be drug-resistant and express some TIC markers. As such, these results suggested that liver TILCs may be formed by the fusion of hepatobiliary stem/progenitor cells and hematopoietic precursor-derived myeloid cells.
The fusion of gastrointestinal cells and dendritic cells does not produce giTICs or giTILCs, which is generally used to generate tumor vaccines[54-73]. In addition, it was shown that cell fusion in the pancreas and esophagus did not generate giTICs or giTILCs[54,74]. Moreover, in vitro fusion cells obtained from human colon adenocarci-noma cells and metastatic human cervical cancer HeLa cells were shown to possess cancer cell properties but were not considered to be giTICs or giTILCs[75]. Currently, cell-cell fusion between tumor cells and tumor cells has not been shown to lead to the generation of TICs[76]. Notably, cell-cell fusion between human colon epithelial cancer cells and normal colon cells not only fail to induce TICs or TILCs but also to generate tumorigenic hybrids in some cases[77]. Similarly, cell-cell fusion between intestinal epithelium cells did not generate giTICs or giTILCs[78].
Gast et al[43] intradermally injected mouse primary melanoma cells (RFP+, actin–green fluorescent protein, 5 × 104 cells) into mice with GFP+ macrophages (actin–green fluorescent protein, n = 12). This resulted in tumor formation and mouse macrophages (MФ, GFP) and melanocyte fusion cells (RFP+GFP+) were detected in the tumors. The researchers then implanted 300 RFP+GFP+ cells, which were isolated by fluorescence activated cell sorting (FACS), into 19 recipient mice (300 cells per mouse) and found that the fusion cells led to tumor growth. Then, 3000 fusion cells per mouse were implanted into three mice for time-dependent analysis of tumor growth characteristics. It was found that the fusion hybrids obtained in vivo had different tumor growth rates, which indicated that the obtained hybrid cells had heterogeneous growth abilities. Therefore, the authors demonstrated that melanoma TICs originate from cell-cell fusion in vivo[43]. Notably, MФ-tumor fusion cells were found in the peripheral blood and were shown to have a stronger ability to metastasize and proliferate. Moreover, the authors showed that the presence of hybrid cells in the peripheral blood of female pancreatic cancer patients with bone marrow transplants from male donors was correlated with disease stage and patient survival.
Xie et al[79] reported that glioma stem cells reorganized the inflammatory microenvironment at the implanting site in mice. Cell-cell fusion between glioma cells and immunoinflammatory cells was also demonstrated in vitro and the fusion cells were shown to be tumorigenic in nude mice and have TILC characteristics.
The formation of cancer cell/MSC hybrids was observed in breast and prostate cancers. Researchers transplanted stem cells into experimental mice and identified in situ CK8+ prostate tumors derived from GFP-labeled transplanted stem cells. This demonstrates that 1 × 106 fusion cells formed from stem cells and breast cancer cells can generate tumors. However, due to the excessive number of cells used for the tumorigenesis experiment, these fusion cells may not be TICs[79,80].
Cell-cell fusion is a process involving cell chemotactic trafficking, membrane fusion, intramembrane structure fusion (including nucleus), and formation of functional fusion cells[81,82]. Moreover, it requires two or more cells to undergo cell membrane merging. However, nuclear fusion is not necessary for the formation of functional fused cells. After the first mitotic division, the binuclear hybrid may undergo nuclear fusion to produce mononuclear cells[43,48,83]. Membrane fusion involves the physical merging of membranes from different cells into a single bilayer, allowing for the exchange of cellular contents[84,85]. Generally, cells undergo cell fusion as an adaptation to unfavorable environments or factors and for the acquisition of favorable phenotypes.
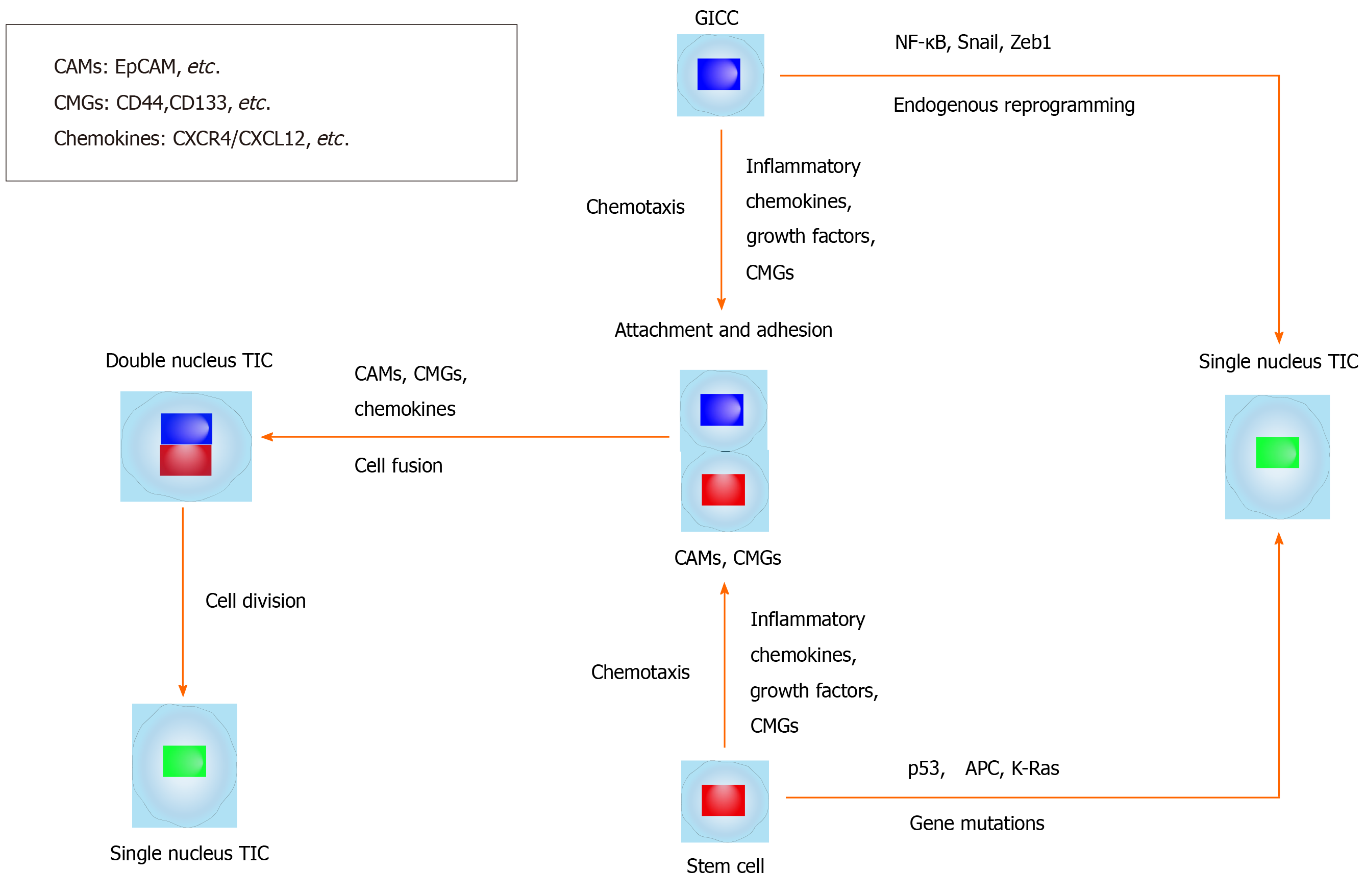
Here, we summarize and hypothesize the mechanisms of giTIC generation from cell-cell fusion (Table 2 and Figure 1). The generation of TICs via cell-cell fusion may involve several fusion partners: (1) BMDCs fusing with local differentiated cells; (2) BMDCs fusing with local stem cells; (3) Local differentiated cells fusing with local stem cells; and (4) Migratory cells from different tissues fusing with local stem cells. In all cases, fusion cells may inherit the self-renewal ability of stem cells[7,11].
| Tumor type | Cell surface molecules involved in cell fusion | Intracellular molecules involved in cell fusion | Signaling pathways involved in cell fusion | giTIC molecules |
| Gastric | CD44, CD133, EpCAM, CXCR4, Lgr5, CD54[3], ALDH1[3] | OCT4, SOX2[120], Twist, Slug[47], Nanog, Lin28[44] | CXCL12/CXCR4, Lgr5[3] | CD44[3], CD133[111] |
| Colorectal | CD44, CD133, EpCAM, CXCR4, CD166[3]. CD81, CD9, GTP-binding protein α13, radixin[85], Syncytin 1, CD47 | APC, p53, Kras, NF-κB, OCT4, SOX2[3]. ADAM10, myosin regulatory light chain, RhoA[85] | CXCL12/CXCR4, Wnt/β-catenin[3], c-Jun | CD133, CD44, ALDH1, EpCAM, CD44, CD166, CD24, LGR5, CD26[3] |
| Liver | CD44, CD133, CD13, EpCAM, CD24, CD90[3], E-cadherin, matrix metalloproteinase | p53[3], OCT4, SOX2[119]. Vimentin, Twist, Snail[113] | CXCL12/CXCR4[77], Wnt, TGF-β, Notch, Hedgehog[3] | CD133, CD49f, CD90, CD13[3] |
| Esophageal | CD44, CD133, EpCAM[115], CXCR4[3] | OCT4, SOX2 | Lgr5[3], CXCL12/CXCR4 | CD44, ALDH1[3] |
| Pancreatic | CD44, CD133, EpCAM, CXCR4, CD24[3], ALDH1[3] | KRAS, TP53, SMAD4, OCT4, SOX2[116] | CXCL12/CXCR4[3], Lgr5[118] | CD133, CD44, CD24, ESA, CXCR4[3] |
The mechanisms of giTIC generation via cell-cell fusion in vivo are very rarely elaborated. Cell-cell fusion between gastrointestinal cells and stem cells may be spontaneous[39,86], unexplained[44], or induced by carcinogens (carcinogenic chemicals) or carcinogenic factors[48,87], such as chronic inflammation and body damage. It is hypothesized that stem cells may initiate changes in the local microenvironment, which then recruits differentiated cells and leads to the fusion of local stem cells with differentiated cells, thereby generating TICs via cell-cell fusion. Similarly, Helicobacter pylori can cause chronic inflammation leading to gastric epithelial mucosal damage, which may recruit BMDCs. These BMDCs can differentiate through cell fusion with local gastric epithelial cells, leading to giTIC formation via cell-cell fusion and adenocarcinoma development[49].
Fusion proteins, also called fusogens, play an important role in mediating membrane fusion[84,85]. Cell adhesion molecules (CAMs) and cell membrane glycoproteins can mediate cell fusion. Most CAMs are involved in the process of membrane fusion and some in cell transfer[88,89]. CAMs, such as CD44, EpCAM[89,90], and cell membrane glycoprotein CD133, are highly expressed in gastrointestinal tumors, especially in giTICs or giTILCs[3,91].
CXCR4, which is a receptor of the chemokine CXCL12, is preferentially expressed in gastrointestinal tumors and promotes invasion and metastasis of gastrointestinal cancer cells[92-103]. The binding of CXCL12 to CXCR4 promotes the directed migration and homing of BMDCs[104,105]. Moreover, CXCL12 was shown to attract organ-specific metastases of CXCR4-expressing tumor cells[106,107] and CXCR4-positive MSCs were shown to migrate to the destination area, such as the stem-cell initiated tumor microenvironment[108]. Moreover, CXCL12 was shown to attract organ-specific metastases of CXCR4-expressing tumor cells and CXCR4-positive MSCs were shown to migrate to the destination area, such as the stem-cell initiated tumor microenvironment, thereby clarifying the mechanism of the induction/activation-cell migration-adhesion-cell fusion process[82,109]. Fujita et al[110] found that diffuse-type gastric cancer-derived CXCR4-positive stem-like cells penetrate into the gastric wall and migrate to the CXCL12-expressing peritoneum, resulting in the formation of peritoneal tumor lymph nodes and malignant ascites in an immunodeficient mouse model[110], which were also found to contain tumorigenic hybrid cells. Many factors, such as inflammatory factors, exosome secretion, cancer-related signal transduction pathways, and chemokines (such as CXCR4/CXCL12), can promote or cause cell chemotaxis; however, no report has shown that these factors are actually involved in the membrane fusion process[111].
In 2019, the cell-cell fusion of two colon cancer cell lines (HCT116 and LoVo) using cobalt chloride showed that syncytin 1, CD9, CD47, and c-Jun were overexpressed in the polyploid giant cancer cells (fusion cells), while PKARIα and JNK1 expression was decreased. Molecules that mediate cell fusion are usually highly expressed in fusion partner cells and hybrid cells. These highly expressed molecules or molecular pathways may be further studied as candidate cell fusion molecules that mediate cell-cell fusion.
The molecules or molecular pathways summarized in Tables 1 and 2 are likely involved in cell-cell fusion processes and the properties of TICs. As such, they may have potential as cell-cell fusion and TIC markers[112-118].
Understanding giTIC generation from cell-cell fusion may have significant implications for the understanding of carcinogenesis and the development of future cancer therapeutic strategies targeting giTICs. Under the aforementioned restrictions for giTICs and TILCs, to date, there is little evidence demonstrating that giTICs originate from cell-cell fusion in vivo, although there are reports showing that giTILCs and mouse TICs can form in vivo via the cell-cell fusion of melanoma cells and macrophages[4,5,43]. Human cell-cell fusion in vivo has also been reported, namely, the fusion of stem cells with microglia and mature neurons after the transplantation of bone marrow-derived stem cells[119]. In addition, the mechanisms of giTIC generation via cell-cell fusion are poorly understood. As such, we propose potential mechanisms involving a multi-step cell fusion process of different cell fusion partners, which is mediated by chemokine and fusogen molecules. Studies on in vitro cell-cell fusion may promote our understanding of the possible mechanisms of giTICs generation via cell-cell fusion in vivo. We suggest that future research should focus on giTIC generation via cell-cell fusion in vivo, isolation of giTICs that have tumor-initiating capabilities when using 5000 or less in vivo fused cells, and the understanding of their underlying mechanisms.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Tanabe S S-Editor: Fan JR L-Editor: Wang TQ P-Editor: Li JH
| 1. | Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5422] [Cited by in RCA: 5562] [Article Influence: 264.9] [Reference Citation Analysis (0)] |
| 2. | Wahl GM. BS1-1: Stem Cells, Cancer, and Cancer Stem Cells. Cancer Res. 2011;71:BS1-1. [DOI] [Full Text] |
| 3. | Taniguchi H, Moriya C, Igarashi H, Saitoh A, Yamamoto H, Adachi Y, Imai K. Cancer stem cells in human gastrointestinal cancer. Cancer Sci. 2016;107:1556-1562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 4. | Qureshi-Baig K, Ullmann P, Haan S, Letellier E. Tumor-Initiating Cells: a criTICal review of isolation approaches and new challenges in targeting strategies. Mol Cancer. 2017;16:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Bansal N, Banerjee D. Tumor initiating cells. Curr Pharm Biotechnol. 2009;10:192-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Kim WT, Ryu CJ. Cancer stem cell surface markers on normal stem cells. BMB Rep. 2017;50:285-298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 221] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 7. | Bastida-Ruiz D, Van Hoesen K, Cohen M. The Dark Side of Cell Fusion. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 8. | Solomon H, Dinowitz N, Pateras IS, Cooks T, Shetzer Y, Molchadsky A, Charni M, Rabani S, Koifman G, Tarcic O, Porat Z, Kogan-Sakin I, Goldfinger N, Oren M, Harris CC, Gorgoulis VG, Rotter V. Mutant p53 gain of function underlies high expression levels of colorectal cancer stem cells markers. Oncogene. 2018;37:1669-1684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 91] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 9. | Schwitalla S, Fingerle AA, Cammareri P, Nebelsiek T, Göktuna SI, Ziegler PK, Canli O, Heijmans J, Huels DJ, Moreaux G, Rupec RA, Gerhard M, Schmid R, Barker N, Clevers H, Lang R, Neumann J, Kirchner T, Taketo MM, van den Brink GR, Sansom OJ, Arkan MC, Greten FR. Intestinal tumorigenesis initiated by dedifferentiation and acquisition of stem-cell-like properties. Cell. 2013;152:25-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 821] [Cited by in RCA: 833] [Article Influence: 69.4] [Reference Citation Analysis (0)] |
| 10. | Zheng YW, Nie YZ, Taniguchi H. Cellular reprogramming and hepatocellular carcinoma development. World J Gastroenterol. 2013;19:8850-8860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 11. | Lu X, Kang Y. Cell fusion hypothesis of the cancer stem cell. Adv Exp Med Biol. 2011;714:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Houghton J. Bone-marrow-derived cells and cancer--an opportunity for improved therapy. Nat Clin Pract Oncol. 2007;4:2-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Lu X, Kang Y. Cell fusion as a hidden force in tumor progression. Cancer Res. 2009;69:8536-8539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 14. | Dittmar T, Nagler C, Niggemann B, Zänker KS. The dark side of stem cells: triggering cancer progression by cell fusion. Curr Mol Med. 2013;13:735-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Davies EJ, Marsh V, Clarke AR. Origin and maintenance of the intestinal cancer stem cell. Mol Carcinog. 2011;50:254-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Potten CS, Booth C, Pritchard DM. The intestinal epithelial stem cell: the mucosal governor. Int J Exp Pathol. 1997;78:219-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 352] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 17. | Potten CS, Owen G, Booth D. Intestinal stem cells protect their genome by selective segregation of template DNA strands. J Cell Sci. 2002;115:2381-2388. [PubMed] |
| 18. | Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8087] [Cited by in RCA: 8006] [Article Influence: 228.7] [Reference Citation Analysis (1)] |
| 19. | Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807-1816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 856] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 20. | Shetzer Y, Solomon H, Koifman G, Molchadsky A, Horesh S, Rotter V. The paradigm of mutant p53-expressing cancer stem cells and drug resistance. Carcinogenesis. 2014;35:1196-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell. 2014;25:304-317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1147] [Cited by in RCA: 1159] [Article Influence: 105.4] [Reference Citation Analysis (0)] |
| 22. | Solomon H, Madar S, Rotter V. Mutant p53 gain of function is interwoven into the hallmarks of cancer. J Pathol. 2011;225:475-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Bouaoun L, Sonkin D, Ardin M, Hollstein M, Byrnes G, Zavadil J, Olivier M. TP53 Variations in Human Cancers: New Lessons from the IARC TP53 Database and Genomics Data. Hum Mutat. 2016;37:865-876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 567] [Cited by in RCA: 577] [Article Influence: 64.1] [Reference Citation Analysis (0)] |
| 24. | Fevr T, Robine S, Louvard D, Huelsken J. Wnt/beta-catenin is essential for intestinal homeostasis and maintenance of intestinal stem cells. Mol Cell Biol. 2007;27:7551-7559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 492] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 25. | Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17989] [Cited by in RCA: 18204] [Article Influence: 958.1] [Reference Citation Analysis (0)] |
| 26. | Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5:749-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2227] [Cited by in RCA: 2420] [Article Influence: 121.0] [Reference Citation Analysis (0)] |
| 27. | Gupta PB, Chaffer CL, Weinberg RA. Cancer stem cells: mirage or reality? Nat Med. 2009;15:1010-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 718] [Cited by in RCA: 715] [Article Influence: 44.7] [Reference Citation Analysis (0)] |
| 28. | Friedmann-Morvinski D, Verma IM. Dedifferentiation and reprogramming: origins of cancer stem cells. EMBO Rep. 2014;15:244-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 376] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 29. | O'Brien-Ball C, Biddle A. Reprogramming to developmental plasticity in cancer stem cells. Dev Biol. 2017;430:266-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133:704-715. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6972] [Cited by in RCA: 6810] [Article Influence: 400.6] [Reference Citation Analysis (0)] |
| 31. | Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, Bapat SA. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059-2068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 461] [Cited by in RCA: 525] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 32. | Yang L, Ping YF, Yu X, Qian F, Guo ZJ, Qian C, Cui YH, Bian XW. Gastric cancer stem-like cells possess higher capability of invasion and metastasis in association with a mesenchymal transition phenotype. Cancer Lett. 2011;310:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | da Silva-Diz V, Lorenzo-Sanz L, Bernat-Peguera A, Lopez-Cerda M, Muñoz P. Cancer cell plasticity: Impact on tumor progression and therapy response. Semin Cancer Biol. 2018;53:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 142] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 34. | Liu Y, Siles L, Postigo A, Dean DC. Epigenetically distinct sister chromatids and asymmetric generation of tumor initiating cells. Cell Cycle. 2018;17:2221-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, D'Alessio AC, Young RA, Weinberg RA. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 599] [Cited by in RCA: 695] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 36. | Ye X, Tam WL, Shibue T, Kaygusuz Y, Reinhardt F, Ng Eaton E, Weinberg RA. Distinct EMT programs control normal mammary stem cells and tumour-initiating cells. Nature. 2015;525:256-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 489] [Cited by in RCA: 571] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 37. | Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schüler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487-1495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1262] [Cited by in RCA: 1352] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 38. | Melsheimer P, Vinokurova S, Wentzensen N, Bastert G, von Knebel Doeberitz M. DNA aneuploidy and integration of human papillomavirus type 16 e6/e7 oncogenes in intraepithelial neoplasia and invasive squamous cell carcinoma of the cervix uteri. Clin Cancer Res. 2004;10:3059-3063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Powell AE, Anderson EC, Davies PS, Silk AD, Pelz C, Impey S, Wong MH. Fusion between Intestinal epithelial cells and macrophages in a cancer context results in nuclear reprogramming. Cancer Res. 2011;71:1497-1505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 170] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 40. | Andersen TL, Boissy P, Sondergaard TE, Kupisiewicz K, Plesner T, Rasmussen T, Haaber J, Kølvraa S, Delaissé JM. Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: a new type of cancer-host partnership? J Pathol. 2007;211:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 41. | Kurgyis Z, Kemény LV, Buknicz T, Groma G, Oláh J, Jakab Á, Polyánka H, Zänker K, Dittmar T, Kemény L, Németh IB. Melanoma-Derived BRAF(V600E) Mutation in Peritumoral Stromal Cells: Implications for in Vivo Cell Fusion. Int J Mol Sci. 2016;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 42. | Clawson GA, Matters GL, Xin P, Imamura-Kawasawa Y, Du Z, Thiboutot DM, Helm KF, Neves RI, Abraham T. Macrophage-tumor cell fusions from peripheral blood of melanoma patients. PLoS One. 2015;10:e0134320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 43. | Gast CE, Silk AD, Zarour L, Riegler L, Burkhart JG, Gustafson KT, Parappilly MS, Roh-Johnson M, Goodman JR, Olson B, Schmidt M, Swain JR, Davies PS, Shasthri V, Iizuka S, Flynn P, Watson S, Korkola J, Courtneidge SA, Fischer JM, Jaboin J, Billingsley KG, Lopez CD, Burchard J, Gray J, Coussens LM, Sheppard BC, Wong MH. Cell fusion potentiates tumor heterogeneity and reveals circulating hybrid cells that correlate with stage and survival. Sci Adv. 2018;4:eaat7828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 218] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 44. | Xue J, Zhu Y, Sun Z, Ji R, Zhang X, Xu W, Yuan X, Zhang B, Yan Y, Yin L, Xu H, Zhang L, Zhu W, Qian H. Tumorigenic hybrids between mesenchymal stem cells and gastric cancer cells enhanced cancer proliferation, migration and stemness. BMC Cancer. 2015;15:793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 45. | Aiello NM, Kang Y. Context-dependent EMT programs in cancer metastasis. J Exp Med. 2019;216:1016-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 422] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 46. | Liao TT, Yang MH. Revisiting epithelial-mesenchymal transition in cancer metastasis: the connection between epithelial plasticity and stemness. Mol Oncol. 2017;11:792-804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 173] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 47. | He X, Li B, Shao Y, Zhao N, Hsu Y, Zhang Z, Zhu L. Cell fusion between gastric epithelial cells and mesenchymal stem cells results in epithelial-to-mesenchymal transition and malignant transformation. BMC Cancer. 2015;15:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 48. | Yan Y, Hsu Y, He X, Lu N, Wei W, Zhang Z, Zhu L. Evidence of cell fusion in carcinogen-induced mice gastric carcinoma. Tumour Biol. 2015;36:5089-5094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 49. | Bessède E, Dubus P, Mégraud F, Varon C. Helicobacter pylori infection and stem cells at the origin of gastric cancer. Oncogene. 2015;34:2547-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Houghton J, Stoicov C, Nomura S, Rogers AB, Carlson J, Li H, Cai X, Fox JG, Goldenring JR, Wang TC. Gastric cancer originating from bone marrow-derived cells. Science. 2004;306:1568-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 861] [Cited by in RCA: 840] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 51. | Ferrand J, Noël D, Lehours P, Prochazkova-Carlotti M, Chambonnier L, Ménard A, Mégraud F, Varon C. Human bone marrow-derived stem cells acquire epithelial characteristics through fusion with gastrointestinal epithelial cells. PLoS One. 2011;6:e19569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 52. | de Jong JH, Rodermond HM, Zimberlin CD, Lascano V, De Sousa E Melo F, Richel DJ, Medema JP, Vermeulen L. Fusion of intestinal epithelial cells with bone marrow derived cells is dispensable for tissue homeostasis. Sci Rep. 2012;2:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Yeh MH, Chang YH, Tsai YC, Chen SL, Huang TS, Chiu JF, Ch'ang HJ. Bone marrow derived macrophages fuse with intestine stromal cells and contribute to chronic fibrosis after radiation. Radiother Oncol. 2016;119:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Fan H, Lu S. Fusion of human bone hemopoietic stem cell with esophageal carcinoma cells didn't generate esophageal cancer stem cell. Neoplasma. 2014;61:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 55. | Deng YJ, Zhang LJ, Su XD, Zhang DK, Rong TH, Wang QJ, Xia JC. [Dendritic cell-tumor cell fusion vaccine prevents growth of subcutaneous transplanted esophageal carcinomas]. Ai Zheng. 2009;28:1067-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Imura K, Hayashi T, Yano Y, Naito K, Kouhara J, Ueda Y, Nakane K, Matsuura Y, Takeda A, Takeda T, Kawai K, Yamagishi H. [Immunogenic reactivity of CTLs induced by electrofusion cells of human dendritic cells and gastric cancer cells]. Gan To Kagaku Ryoho. 2004;31:1797-1799. [PubMed] |
| 57. | Xu F, Ye YJ, Wang S. In vitro antitumor immune response induced by fusion of dendritic cells and colon cancer cells. World J Gastroenterol. 2004;10:1162-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 58. | Guo GH, Chen SZ, Yu J, Zhang J, Luo LL, Xie LH, Su ZJ, Dong HM, Xu H, Wu LB. In vivo anti-tumor effect of hybrid vaccine of dendritic cells and esophageal carcinoma cells on esophageal carcinoma cell line 109 in mice with severe combined immune deficiency. World J Gastroenterol. 2008;14:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 59. | Guo G, Chen S, Zhang J, Luo L, Yu J, Dong H, Xu H, Su Z, Wu L. Antitumor activity of a fusion of esophageal carcinoma cells with dendritic cells derived from cord blood. Vaccine. 2005;23:5225-5230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Deng YJ, Xia JC, Zhou J, Wang QJ, Zhang PY, Zhang LJ, Rong TH. [Antitumor efficacy of fusion cells from esophageal carcinoma cells and dendritic cells as a vaccine in vitro]. Ai Zheng. 2007;26:137-141. [PubMed] |
| 61. | Zhang K, Gao PF, Yu PW, Rao Y, Zhou LX. Study on biological characters of SGC7901 gastric cancer cell-dendritic cell fusion vaccines. World J Gastroenterol. 2006;12:3438-3441. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 62. | Zhang K, Yu PW, Gao PF, Rao Y. [Inhibitive effects of gastric cancer cell-dendritic cell fusion vaccine on tumor cell proliferation cycle]. Zhonghua Wei Chang Wai Ke Zazhi. 2006;9:345-348. [PubMed] |
| 63. | Rizvi AZ, Swain JR, Davies PS, Bailey AS, Decker AD, Willenbring H, Grompe M, Fleming WH, Wong MH. Bone marrow-derived cells fuse with normal and transformed intestinal stem cells. Proc Natl Acad Sci USA. 2006;103:6321-6325. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 64. | Kao JY, Gong Y, Chen CM, Zheng QD, Chen JJ. Tumor-derived TGF-beta reduces the efficacy of dendritic cell/tumor fusion vaccine. J Immunol. 2003;170:3806-3811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 65. | Yasuda T, Kamigaki T, Kawasaki K, Nakamura T, Yamamoto M, Kanemitsu K, Takase S, Kuroda D, Kim Y, Ajiki T, Kuroda Y. Superior anti-tumor protection and therapeutic efficacy of vaccination with allogeneic and semiallogeneic dendritic cell/tumor cell fusion hybrids for murine colon adenocarcinoma. Cancer Immunol Immunother. 2007;56:1025-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 66. | Sheng XL, Zhang H. In-vitro activation of cytotoxic T lymphocytes by fusion of mouse hepatocellular carcinoma cells and lymphotactin gene-modified dendritic cells. World J Gastroenterol. 2007;13:5944-5950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 67. | Koido S, Gong J. Cell fusion between dendritic cells and whole tumor cells. Methods Mol Biol. 2015;1313:185-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 68. | Draube A, Beyer M, Schumer S, Thomas RK, von Tresckow B, Koslowsky TC, Krieglstein CF, Schultze JL, Wolf J. Efficient activation of autologous tumor-specific T cells: a simple coculture technique of autologous dendritic cells compared to established cell fusion strategies in primary human colorectal carcinoma. J Immunother. 2007;30:359-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Koido S, Hara E, Homma S, Torii A, Mitsunaga M, Yanagisawa S, Toyama Y, Kawahara H, Watanabe M, Yoshida S, Kobayashi S, Yanaga K, Fujise K, Tajiri H. Streptococcal preparation OK-432 promotes fusion efficiency and enhances induction of antigen-specific CTL by fusions of dendritic cells and colorectal cancer cells. J Immunol. 2007;178:613-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 70. | Matsumoto S, Saito H, Tsujitani S, Ikeguchi M. Allogeneic gastric cancer cell-dendritic cell hybrids induce tumor antigen (carcinoembryonic antigen) specific CD8(+) T cells. Cancer Immunol Immunother. 2006;55:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 71. | Xu F, Ye YJ, Liu W, Kong M, He Y, Wang S. Dendritic cell/tumor hybrids enhances therapeutic efficacy against colorectal cancer liver metastasis in SCID mice. Scand J Gastroenterol. 2010;45:707-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 72. | Yasuda T, Kamigaki T, Nakamura T, Imanishi T, Hayashi S, Kawasaki K, Takase S, Ajiki T, Kuroda Y. Dendritic cell-tumor cell hybrids enhance the induction of cytotoxic T lymphocytes against murine colon cancer: a comparative analysis of antigen loading methods for the vaccination of immunotherapeutic dendritic cells. Oncol Rep. 2006;16:1317-1324. [PubMed] |
| 73. | Iinuma H, Okinaga K, Fukushima R, Inaba T, Iwasaki K, Arai T, Tamura J, Kumagai H. [Reduction of immunosuppression and shift to Th1 response by tumor-DC (dendritic cells) fusion vaccine]. Gan To Kagaku Ryoho. 2004;31:1640-1642. [PubMed] |
| 74. | Sumi S, Yanai G. Fusion of mesenchymal stem cells and islet cells for cell therapy. Methods Mol Biol. 2015;1313:107-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 75. | O'Donnell RW, Leary JF, Penney DP, Budd HS, Marquis DM, Spennacchio JL, Henshaw EC, McCune CS. Somatic cell hybridization of human tumor samples. Somat Cell Mol Genet. 1984;10:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 76. | Traicoff JL, Periyasamy S, Brattain MG, Grady W, Casey G. Reconstitution of TGF-beta sensitivity in the VACO-411 human colon carcinoma line by somatic cell fusion with MCF-7. J Biomed Sci. 2003;10:253-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 77. | Johnson TL, Moyer MP. Normal human colon cells suppress malignancy when fused with colon cancer cells. In Vitro Cell Dev Biol. 1990;26:1095-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 78. | Zhou X, Merchak K, Lee W, Grande JP, Cascalho M, Platt JL. Cell Fusion Connects Oncogenesis with Tumor Evolution. Am J Pathol. 2015;185:2049-2060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 79. | Xie X, Wang CY, Cao YX, Wang W, Zhuang R, Chen LH, Dang NN, Fang L, Jin BQ. Expression pattern of epithelial cell adhesion molecule on normal and malignant colon tissues. World J Gastroenterol. 2005;11:344-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 80. | Luo F, Liu T, Wang J, Li J, Ma P, Ding H, Feng G, Lin D, Xu Y, Yang K. Bone marrow mesenchymal stem cells participate in prostate carcinogenesis and promote growth of prostate cancer by cell fusion in vivo. Oncotarget. 2016;7:30924-30934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Melzer C, von der Ohe J, Hass R. In Vivo Cell Fusion between Mesenchymal Stroma/Stem-Like Cells and Breast Cancer Cells. Cancers (Basel). 2019;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 82. | Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171:386-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 386] [Cited by in RCA: 438] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 83. | Aguilar PS, Baylies MK, Fleissner A, Helming L, Inoue N, Podbilewicz B, Wang H, Wong M. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013;29:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 196] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 84. | Pattnaik GP, Meher G, Chakraborty H. Exploring the Mechanism of Viral Peptide-Induced Membrane Fusion. Adv Exp Med Biol. 2018;1112:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 85. | Hernández JM, Podbilewicz B. The hallmarks of cell-cell fusion. Development. 2017;144:4481-4495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 139] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 86. | Carloni V, Mazzocca A, Mello T, Galli A, Capaccioli S. Cell fusion promotes chemoresistance in metastatic colon carcinoma. Oncogene. 2013;32:2649-2660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 87. | Silk AD, Gast CE, Davies PS, Fakhari FD, Vanderbeek GE, Mori M, Wong MH. Fusion between hematopoietic and epithelial cells in adult human intestine. PLoS One. 2013;8:e55572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 88. | Davies PS, Powell AE, Swain JR, Wong MH. Inflammation and proliferation act together to mediate intestinal cell fusion. PLoS One. 2009;4:e6530. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 89. | AlHajri SM, Cunha CW, Nicola AV, Aguilar HC, Li H, Taus NS. Ovine Herpesvirus 2 Glycoproteins B, H, and L Are Sufficient for, and Viral Glycoprotein Ov8 Can Enhance, Cell-Cell Membrane Fusion. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Chaudry MA, Sales K, Ruf P, Lindhofer H, Winslet MC. EpCAM an immunotherapeutic target for gastrointestinal malignancy: current experience and future challenges. Br J Cancer. 2007;96:1013-1019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 91. | Fang C, Fan C, Wang C, Huang Q, Meng W, Yu Y, Yang L, Hu J, Li Y, Mo X, Zhou Z. Prognostic value of CD133+ CD54+ CD44+ circulating tumor cells in colorectal cancer with liver metastasis. Cancer Med. 2017;6:2850-2857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 92. | Margolin DA, Myers T, Zhang X, Bertoni DM, Reuter BA, Obokhare I, Borgovan T, Grimes C, Green H, Driscoll T, Lee CG, Davis NK, Li L. The critical roles of tumor-initiating cells and the lymph node stromal microenvironment in human colorectal cancer extranodal metastasis using a unique humanized orthotopic mouse model. FASEB J. 2015;29:3571-3581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 93. | Feng H, Liu Y, Bian X, Zhou F, Liu Y. ALDH1A3 affects colon cancer in vitro proliferation and invasion depending on CXCR4 status. Br J Cancer. 2018;118:224-232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 94. | Shirafkan N, Shomali N, Kazemi T, Shanehbandi D, Ghasabi M, Baghbani E, Ganji M, Khaze V, Mansoori B, Baradaran B. microRNA-193a-5p inhibits migration of human HT-29 colon cancer cells via suppression of metastasis pathway. J Cell Biochem. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 95. | V.I. Gavrilov. Acta Virol. 1975;19:510. [PubMed] |
| 96. | Fang HY, Münch NS, Schottelius M, Ingermann J, Liu H, Schauer M, Stangl S, Multhoff G, Steiger K, Gerngroß C, Jesinghaus M, Weichert W, Kühl AA, Sepulveda AR, Wester HJ, Wang TC, Quante M. CXCR4 Is a Potential Target for Diagnostic PET/CT Imaging in Barrett's Dysplasia and Esophageal Adenocarcinoma. Clin Cancer Res. 2018;24:1048-1061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 97. | Gu X, Zhang Q, Zhang W, Zhu L. Curcumin inhibits liver metastasis of gastric cancer through reducing circulating tumor cells. Aging (Albany NY). 2019;11:1501-1509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 98. | Qi W, Sun L, Liu N, Zhao S, Lv J, Qiu W. Tetraspanin family identified as the central genes detected in gastric cancer using bioinformatics analysis. Mol Med Rep. 2018;18:3599-3610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 99. | Wang M, Yang X, Wei M, Wang Z. The Role of CXCL12 Axis in Lung Metastasis of Colorectal Cancer. J Cancer. 2018;9:3898-3903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 100. | Zhu X, Han S, Wu S, Bai Y, Zhang N, Wei L. Dual role of twist1 in cancer-associated fibroblasts and tumor cells promoted epithelial-mesenchymal transition of esophageal cancer. Exp Cell Res. 2019;375:41-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 101. | Qin LF, Qin JM, Zhang JQ, Lv XP, Huang LY, Wang JJ. CXCL12 and CXCR4 polymorphisms and expressions in peripheral blood from patients of hepatocellular carcinoma. Future Oncol. 2018;14:1261-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Xue LJ, Mao XB, Ren LL, Chu XY. Inhibition of CXCL12/CXCR4 axis as a potential targeted therapy of advanced gastric carcinoma. Cancer Med. 2017;6:1424-1436. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 103. | Kaemmerer D, Träger T, Hoffmeister M, Sipos B, Hommann M, Sänger J, Schulz S, Lupp A. Inverse expression of somatostatin and CXCR4 chemokine receptors in gastroenteropancreatic neuroendocrine neoplasms of different malignancy. Oncotarget. 2015;6:27566-27579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 104. | Melo RCC, Ferro KPV, Duarte ADSS, Olalla Saad ST. CXCR7 participates in CXCL12-mediated migration and homing of leukemic and normal hematopoietic cells. Stem Cell Res Ther. 2018;9:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 105. | Alekhina O, Marchese A. β-Arrestin1 and Signal-transducing Adaptor Molecule 1 (STAM1) Cooperate to Promote Focal Adhesion Kinase Autophosphorylation and Chemotaxis via the Chemokine Receptor CXCR4. J Biol Chem. 2016;291:26083-26097. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 106. | Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 866] [Cited by in RCA: 902] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 107. | Barbieri F, Bajetto A, Stumm R, Pattarozzi A, Porcile C, Zona G, Dorcaratto A, Ravetti JL, Minuto F, Spaziante R, Schettini G, Ferone D, Florio T. Overexpression of stromal cell-derived factor 1 and its receptor CXCR4 induces autocrine/paracrine cell proliferation in human pituitary adenomas. Clin Cancer Res. 2008;14:5022-5032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 81] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 108. | Shibata W, Ariyama H, Westphalen CB, Worthley DL, Muthupalani S, Asfaha S, Dubeykovskaya Z, Quante M, Fox JG, Wang TC. Stromal cell-derived factor-1 overexpression induces gastric dysplasia through expansion of stromal myofibroblasts and epithelial progenitors. Gut. 2013;62:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Oren-Suissa M, Podbilewicz B. Evolution of programmed cell fusion: common mechanisms and distinct functions. Dev Dyn. 2010;239:1515-1528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 110. | Fujita T, Chiwaki F, Takahashi RU, Aoyagi K, Yanagihara K, Nishimura T, Tamaoki M, Komatsu M, Komatsuzaki R, Matsusaki K, Ichikawa H, Sakamoto H, Yamada Y, Fukagawa T, Katai H, Konno H, Ochiya T, Yoshida T, Sasaki H. Identification and Characterization of CXCR4-Positive Gastric Cancer Stem Cells. PLoS One. 2015;10:e0130808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Sung BH, Weaver AM. Exosome secretion promotes chemotaxis of cancer cells. Cell Adh Migr. 2017;11:187-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 112. | Basati G, Mohammadpour H, Emami Razavi A. Association of High Expression Levels of SOX2, NANOG, and OCT4 in Gastric Cancer Tumor Tissues with Progression and Poor Prognosis. J Gastrointest Cancer. 2020;51:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 113. | Attia S, Atwan N, Arafa M, Shahin RA. Expression of CD133 as a cancer stem cell marker in invasive gastric carcinoma. Pathologica. 2019;111:18-23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 114. | Sun D, Qin L, Xu Y, Liu JX, Tian LP, Qian HX. Influence of adriamycin on changes in Nanog, Oct-4, Sox2, ARID1 and Wnt5b expression in liver cancer stem cells. World J Gastroenterol. 2014;20:6974-6980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 115. | Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer. 2010;103:1671-1679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 147] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 116. | Mokrowiecka A, Veits L, Falkeis C, Musial J, Kordek R, Lochowski M, Kozak J, Wierzchniewska-Lawska A, Vieth M, Malecka-Panas E. Expression profiles of cancer stem cell markers: CD133, CD44, Musashi-1 and EpCAM in the cardiac mucosa-Barrett's esophagus-early esophageal adenocarcinoma-advanced esophageal adenocarcinoma sequence. Pathol Res Pract. 2017;213:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 117. | Cheng Y, Cheung AK, Ko JM, Phoon YP, Chiu PM, Lo PH, Waterman ML, Lung ML. Physiological β-catenin signaling controls self-renewal networks and generation of stem-like cells from nasopharyngeal carcinoma. BMC Cell Biol. 2013;14:44. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 118. | Yu W, Ma Y, Shankar S, Srivastava RK. Chronic ethanol exposure of human pancreatic normal ductal epithelial cells induces cancer stem cell phenotype through SATB2. J Cell Mol Med. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 119. | Andrikou K, Santoni M, Piva F, Bittoni A, Lanese A, Pellei C, Conti A, Loretelli C, Mandolesi A, Giulietti M, Scarpelli M, Principato G, Falconi M, Cascinu S. Lgr5 expression, cancer stem cells and pancreatic cancer: results from biological and computational analyses. Future Oncol. 2015;11:1037-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 120. | Cusulin C, Monni E, Ahlenius H, Wood J, Brune JC, Lindvall O, Kokaia Z. Embryonic stem cell-derived neural stem cells fuse with microglia and mature neurons. Stem Cells. 2012;30:2657-2671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |