Published online Feb 15, 2021. doi: 10.4251/wjgo.v13.i2.131
Peer-review started: September 22, 2020
First decision: November 16, 2020
Revised: November 29, 2020
Accepted: December 11, 2020
Article in press: December 11, 2020
Published online: February 15, 2021
Processing time: 132 Days and 2.1 Hours
An increasing number of older patients is undergoing curative, surgical treatment of esophageal cancer. Previous meta-analyses have shown that older patients suffered from more postoperative morbidity and mortality compared to younger patients, which may lead to patient selection based on age. However, only studies including patients that underwent open esophagectomy were included. Therefore, it remains unknown whether there is an association between age and outcome in patients undergoing minimally invasive esophagectomy.
To perform a systematic review on age and postoperative outcome in esophageal cancer patients undergoing esophagectomy.
Studies comparing older with younger patients with primary esophageal cancer undergoing curative esophagectomy were included. Meta-analysis of studies using a 75-year age threshold are presented in the manuscript, studies using other age thresholds in the Supplementary material. MEDLINE, Embase and the Cochrane Library were searched for articles published between 1995 and 2020. Risk of bias was assessed with the Newcastle-Ottawa Scale. Primary outcomes were anastomotic leak, pulmonary and cardiac complications, delirium, 30- and 90-d, and in-hospital mortality. Secondary outcomes included pneumonia and 5-year overall survival.
Seven studies (4847 patients) using an age threshold of 75 years were included for meta-analysis with 755 older and 4092 younger patients. Older patients (9.05%) had higher rates of 90-d mortality compared with younger patients (3.92%), (confidence interval = 1.10-5.56). In addition, older patients (9.45%) had higher rates of in-hospital mortality compared with younger patients (3.68%), (confidence interval = 1.01-5.91). In the subgroup of 2 studies with minimally invasive esophagectomy, older and younger patients had comparable 30-d, 90-d and in-hospital mortality rates.
Older patients undergoing curative esophagectomy for esophageal cancer have a higher postoperative mortality risk. Minimally invasive esophagectomy may be important for minimizing mortality in older patients.
Core Tip: In this systematic review including articles from the last 25 years, it was found that older esophageal cancer patients suffer from a two-fold higher risk of in-hospital and 90-d mortality as compared to their younger counterparts. In addition, subgroup analysis showed that these differences did not occur when only studies on minimally invasive esophagectomy were analyzed. This implies that minimally invasive esophagectomy may be important for minimizing morbidity and mortality in older patients undergoing curative esophagectomy.
- Citation: Baranov NS, Slootmans C, van Workum F, Klarenbeek BR, Schoon Y, Rosman C. Outcomes of curative esophageal cancer surgery in elderly: A meta-analysis. World J Gastrointest Oncol 2021; 13(2): 131-146
- URL: https://www.wjgnet.com/1948-5204/full/v13/i2/131.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i2.131
Since the incidence of esophageal cancer is rising as well as the average age of the global population, more older patients are expected to be diagnosed with esophageal cancer[1,2]. In the West, already around 30 percent of patients undergoing curative esophagectomy for esophageal cancer is aged 70 years or older[3]. Increased use worldwide of minimally invasive esophagectomy (MIE) may benefit these older patients because it is associated with lower postoperative morbidity[4-7]. However, patients aged 75 or 80 years or older were excluded from all major trials that compared MIE with open esophagectomy (OE)[4-6].
One meta-analysis by Markar et al[8] from 2012 comparing OE outcome between older and younger patients showed that older patients had a higher rate of in-hospital mortality, pulmonary and cardiac complications and a lower 5-year overall survival. Two recent meta-analyses from 2019 and 2020, which included only studies using an age threshold of 80 years to distinguish old from young, reported similar results[9,10]. However, these three meta-analyses did not include studies on MIE, and excluded studies that used other age thresholds. In contrast, recent individual studies that compared older with younger patients who underwent MIE showed comparable rates of anastomotic leak as well as 30-d mortality[11-13]. Regarding pulmonary and cardiac complications, however, results are conflicting. No systematic review of recent literature including MIE studies that compared older with younger patients, has been undertaken to this date. Therefore, it remains unknown whether MIE could be beneficial to older patients regarding postoperative morbidity and mortality.
We aimed to investigate the association between patient age and outcome after curative (open and minimally invasive) esophagectomy for esophageal cancer.
The protocol for this review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under number CRD 42019121754. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline was followed[14].
The inclusion criteria were studies comparing older with younger patients undergoing esophagectomy with curative intent for primary esophageal cancer (regardless of age threshold). The exclusion criteria were: Studies with more than 20% stage IV patients, salvage or palliative esophagectomy, conference abstracts, cross-sectional studies, case series, case reports and letters to editors. Studies having more than two age groups were included only when it was possible to combine the age groups in an older and younger age group.
The electronic databases of MEDLINE, Embase (both through the Ovid interface) and The Cochrane Central Register of Controlled Trials were systematically searched. Reference lists from the included studies were also searched. The search strategy was composed in collaboration with a librarian from the Radboud University Medical Library.
The MEDLINE search strategy was: [exp ESOPHAGECTOMY/ or (Esophagectom* or Oesophagectom* or Esophagus or Oesophagus or Oesophageal or Esophageal) and (Resection* or Surger* or Laparoscop* or Thoracoscop*).ti,ab,kf.] AND (exp Esophageal Neoplasms/ or (Esophag* or oesophag*) and (cancer* or neoplasm* or carcinoma* or adenocarcinoma* or malignanc*).ti,ab,kf.) AND (Aged or Old* or Frail*).ti,kf. or (Older* or Elder* or Senior* or Geriatric).ti,ab,kf or Age Factors/ or exp *AGED/ or exp AGE DISTRIBUTION/. Comparable search strategies were used in Embase and the Cochrane Library. Complete search strategies were listed in the protocol for this review. No language restrictions were applied and all results up to January 1st, 2020 were included.
First, two reviewers (NB and CS) independently screened titles and abstracts for potentially relevant studies. Second, two reviewers (NB and CS) independently examined the full text of potentially relevant studies for eligibility. When disagreement occurred during this phase, a third reviewer (FvW) was consulted until consensus was reached. Two reviewers (NB and CS) screened reference lists from the included studies for potentially relevant articles repeating aforementioned processes when articles were deemed eligible.
When possible, data of the studies included in the systematic review were pooled for quantitative meta-analysis. Otherwise, the data was described. For the purpose of this review the following data was extracted: Patient and tumor characteristics, surgical technique and approach, operation characteristics, complications, hospital stay, mortality, survival and quality of life.
The Newcastle-Ottawa quality assessment scale was used to assess bias in studies included in this review[15]. This scale rates studies on three sources of bias based on eight criteria. Each criterion is worth one star except confounding, which is worth two stars. For this systematic review, studies scoring 7-9 stars were considered to be of high methodological quality, studies scoring 4-6 stars were considered to be of moderate methodological quality, and studies scoring 1-3 stars were considered to be of low methodological quality.
The primary outcomes were: The rate of anastomotic leak, pulmonary and cardiac complications, delirium, 30-d, 90-d and in-hospital mortality. After multiple thorough discussions between the authors, medical doctors from both the department of surgery and geriatrics, these outcomes were deemed most clinically relevant to answer our research question.
The secondary outcomes were: the rate of chylothorax, pneumonia, hospital length of stay, quality of life, 5-year survival and 5-year disease-specific survival.
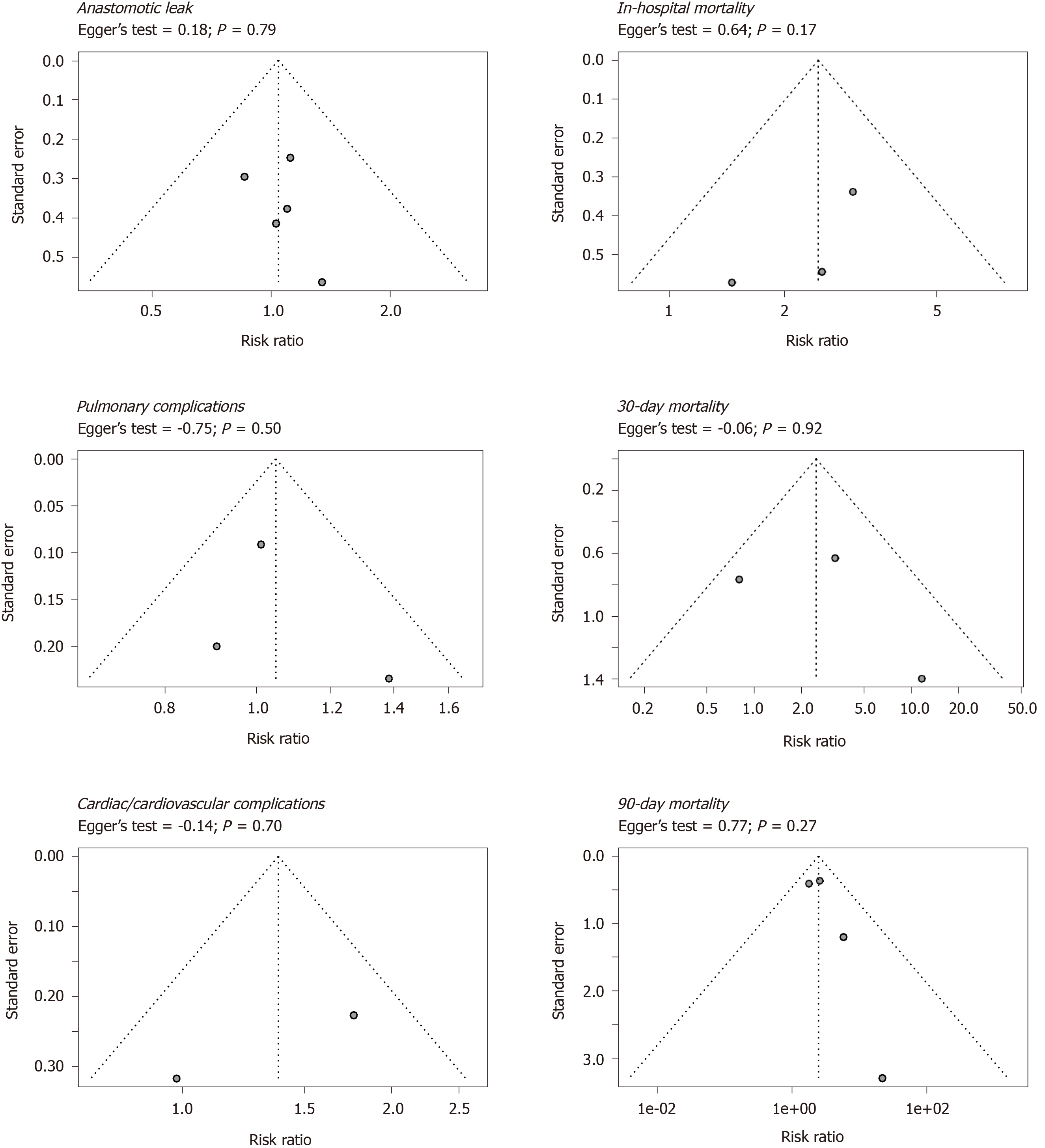
To assess publication bias, funnel plots with the effect measures on the x-axis and standard error on the y-axis were used for visualization and the Egger’s test was used for quantification. Funnel plots and Egger’s tests were performed for the primary outcome measures.
It was decided, after careful deliberation among the authors, to present in the current article the meta-analysis results of studies using an age threshold of 75 years, because it was deemed most relevant for current surgical practice, and prominent randomized controlled trials (TIME, MIRO) used this age threshold as an exclusion criterion[4,5]. Meta-analysis results of studies using other age thresholds as well as all age thresholds combined were presented in the supplementary only (Supplementary Table 1).
A quantitative synthesis of aggregate patient data (for OE, MIE and both groups together) was performed. Regardless of study homogeneity in terms of design and comparators and regardless of statistical homogeneity as expressed by the I², we conducted a meta-analysis using a random-effects model. Dichotomous data were analyzed by using relative risks with a 95% confidence interval (CI). Continuous outcomes were analyzed using weighted mean differences (with a 95%CI) or standardized mean differences (95%CI) if different measurement scales were used. If data was reported as median with ranges (maximum, minimum), the formula described by Wan et al[16] was used to estimate the mean, variance and standard deviation. If inter-quartile ranges were reported without minima and maxima, the data were presented descriptively when deemed necessary. If desired data was missing, authors were contacted when deemed necessary. The data was synthesized using the appropriate imputation methods, otherwise the data was presented descriptively. Statistical heterogeneity was tested using the Chi² test (significance level: 0.1) and I² statistic (0% to 40%: Might not be important; 30% to 60%: May represent moderate heterogeneity; 50% to 90%: May represent substantial heterogeneity; 75% to 100%: Considerable heterogeneity). Outcomes were combined and calculated using the statistical software program R with the package “meta” in accordance with Doing Meta-Analysis in R by Harrer et al[17-19].
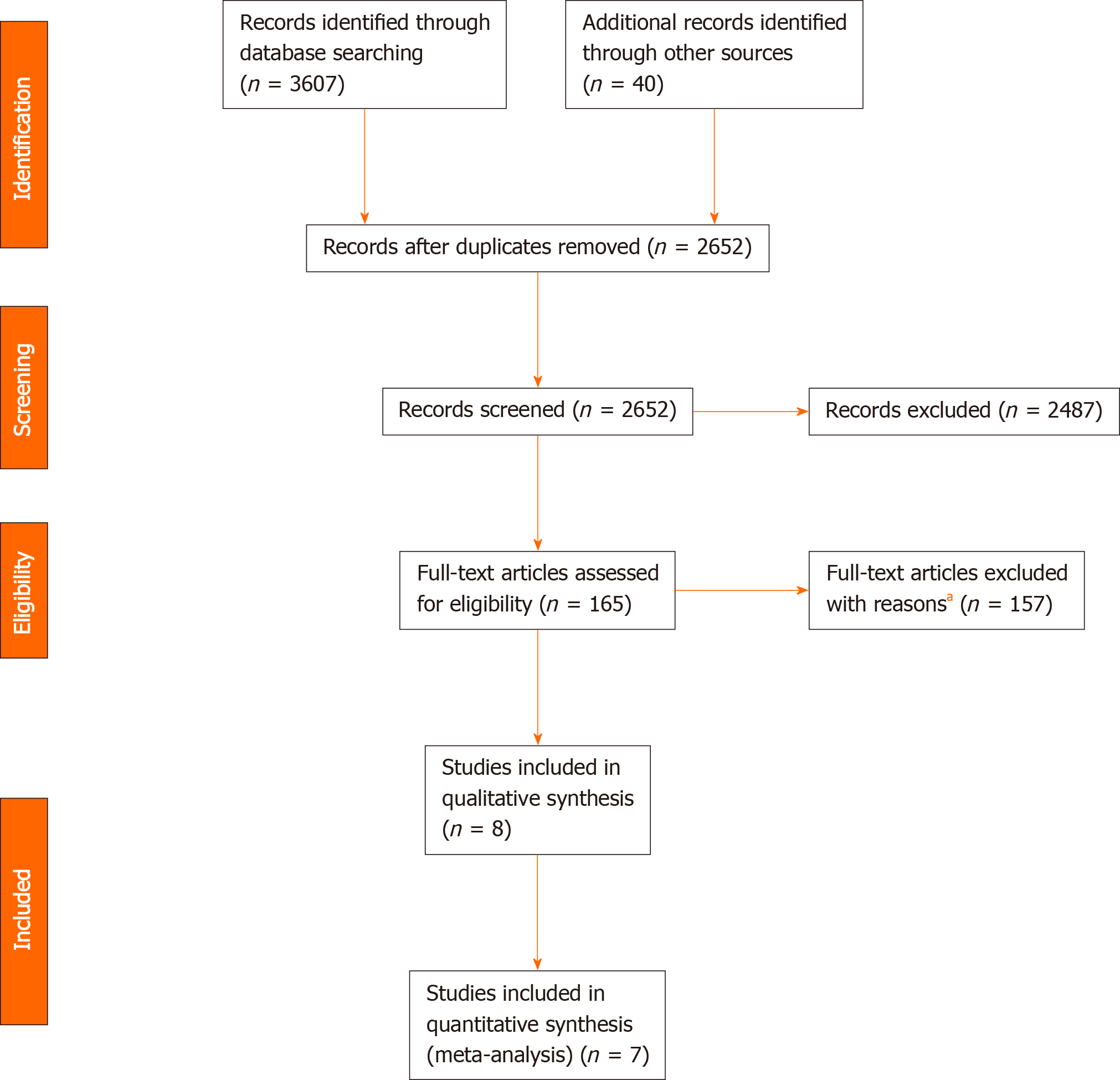
Figure 1 shows the study selection process. A total of 3647 records were identified through database searching and cross-referencing. Overall, 8 studies were included for qualitative synthesis of which 7 studies were included for quantitative analysis (meta-analysis) with 4991 and 4847 patients respectively[13,20-26].
One study was published in 2009 and the rest was published after 2010. Regarding operative approach, 2 studies were deemed as “MIE” (> 50% MIE), 3 as “OE”, and 3 as “Unknown” (studies of whom the surgical approach could not be determined).
From the 7 included studies for meta-analysis, 2 were of “High Methodological Quality”, 5 were of “Medium Methodological Quality” and none were of “Low Methodological Quality”. Individual studies’ star counts are shown in Table 1.
Older patients, had comparable preoperative comorbidity compared with younger patients, except for ASA score and clinical tumor stage (older patients had more clinical stage II cancer, while younger patients had more clinical stage III cancer) and neoadjuvant therapy (less often in older patients). The extend of resection (transthoracic or transhiatal) was comparable between both groups, but more older patients underwent minimally invasive esophagectomy (Table 2).
| Variable | Young | Old | Studies | P value | |||
| No. (% of total) | 4195 | 84.1% | 796 | 15.9% | 8 | ||
| n | % | n | % | n | % | ||
| Gender | < 0.001 | ||||||
| Male | 3572 | 85.1 | 623 | 78.3 | |||
| Female | 520 | 12.4 | 132 | 16.6 | |||
| Unknown | 103 | 2.5 | 41 | 5.2 | 1 | 12.5 | |
| Charlson comorbidity index | 0.058 | ||||||
| 0 | 202 | 4.8 | 38 | 4.8 | |||
| 1 | 90 | 2.1 | 28 | 3.5 | |||
| 2 | 65 | 1.5 | 23 | 2.9 | |||
| 3 | 0 | 0.0 | 0 | 0.0 | |||
| 4 | 0 | 0.0 | 0 | 0.0 | |||
| 5 | 0 | 0.0 | 0 | 0.0 | |||
| Unknown | 3838 | 91.5 | 707 | 88.8 | 7 | 87.5 | |
| Respiratory comorbidity | 0.272 | ||||||
| Yes | 43 | 1.0 | 8 | 1.0 | |||
| No | 361 | 8.6 | 104 | 13.1 | |||
| Unknown | 3791 | 90.4 | 684 | 85.9 | 6 | 75.0 | |
| Cardiac/Cardiovascular comorbidity | 0.244 | ||||||
| Yes | 88 | 2.1 | 28 | 3.5 | |||
| No | 316 | 7.5 | 84 | 10.6 | |||
| Unknown | 3791 | 90.4 | 684 | 85.9 | 6 | 75.0 | |
| Diabetes mellitus | 0.005 | ||||||
| Yes | 17 | 0.4 | 10 | 1.3 | |||
| No | 316 | 7.5 | 60 | 7.5 | |||
| Unknown | 3862 | 92.1 | 726 | 91.2 | 6 | 75.0 | |
| Renal insufficiency | 0.258 | ||||||
| Yes | 1 | 0.0 | 2 | 0.3 | |||
| No | 99 | 2.4 | 48 | 6.0 | |||
| Unknown | 4095 | 97.6 | 746 | 93.7 | 7 | 87.5 | |
| Liver cirrhosis | - | ||||||
| Yes | 0 | 0.0 | 0 | 0.0 | |||
| No | 0 | 0.0 | 0 | 0.0 | |||
| Unknown | 4195 | 100.0 | 796 | 100.0 | 8 | 100.0 | |
| ASA | < 0.001 | ||||||
| I | 164 | 3.9 | 13 | 1.6 | |||
| II | 598 | 14.3 | 117 | 14.7 | |||
| III | 194 | 4.6 | 66 | 8.3 | |||
| IV | 6 | 0.1 | 0 | 0.0 | |||
| V | 0 | 0.0 | 0 | 0.0 | |||
| Unknown | 3233 | 77.1 | 600 | 75.4 | 5 | 62.5 | |
| Tumor location in the esophagus | 0.239 | ||||||
| Cervical esophagus | 0 | 0.0 | 0 | 0.0 | |||
| Upper third | 25 | 0.7 | 4 | 0.5 | |||
| Middle third | 253 | 6.6 | 71 | 8.9 | |||
| Lower third | 79 | 2.1 | 14 | 1.8 | |||
| Junction | 0 | 0.0 | 0 | 0.0 | |||
| Cardia | 0 | 0.0 | 0 | 0.0 | |||
| Unknown | 0 | 0.0 | 0 | 0.0 | |||
| Unknown1 | 3838 | 100.0 | 707 | 88.8 | 7 | 87.5 | |
| Histological type | 0.022 | ||||||
| Adenocarcinoma | 2956 | 70.5 | 597 | 75.0 | |||
| Squamous cell carcinoma | 442 | 10.5 | 89 | 11.2 | |||
| Other | 21 | 0.5 | 0 | 0.0 | |||
| Unknown1 | 1 | 0.0 | 2 | 0.3 | |||
| Unknown | 775 | 18.5 | 108 | 13.6 | 2 | 25.0 | |
| cTNM | 0.039 | ||||||
| 0 | 56 | 1.3 | 4 | 0.5 | |||
| I | 234 | 5.6 | 40 | 5.0 | |||
| II | 525 | 12.5 | 94 | 11.8 | |||
| III | 547 | 13.0 | 83 | 10.4 | |||
| IV | 131 | 3.1 | 14 | 1.8 | |||
| Unknown1 | 70 | 1.7 | 3 | 0.4 | |||
| Unknown | 2632 | 62.7 | 558 | 70.1 | 4 | 50.0 | |
| pTNM | 0.648 | ||||||
| 0 | 1 | 0.0 | 0 | 0.0 | |||
| I | 36 | 0.9 | 21 | 2.6 | |||
| II | 20 | 0.5 | 9 | 1.1 | |||
| III | 36 | 0.9 | 19 | 2.4 | |||
| IV | 7 | 0.2 | 1 | 0.1 | |||
| Unknown1 | 0 | 0.0 | 0 | 0.0 | |||
| Unknown | 4095 | 97.6 | 746 | 93.7 | 7 | 87.5 | |
| Complete pathological response | 0.793 | ||||||
| Yes | 60 | 1.4 | 16 | 2.0 | |||
| No | 297 | 7.1 | 73 | 9.2 | |||
| Unknown | 3838 | 91.5 | 707 | 88.8 | 7 | 87.5 | |
| Neoadjuvant therapy | < 0.001 | ||||||
| Yes | 714 | 17.0 | 120 | 15.1 | |||
| No | 527 | 12.6 | 146 | 18.3 | |||
| Unknown | 2954 | 70.4 | 530 | 66.6 | 3 | 37.5 | |
| Resection type | - | ||||||
| Transthoracic | 590 | 14.1 | 109 | 13.7 | |||
| Transhiatal | 0 | 0.0 | 0 | 0.0 | |||
| Unknown | 3605 | 85.9 | 687 | 86.3 | 6 | 75.0 | |
| Surgical technique | 0.046 | ||||||
| Ivor lewis | 472 | 11.3 | 98 | 12.3 | |||
| McKeown | 17 | 0.4 | 1 | 0.1 | |||
| Orringer | 0 | 0.0 | 0 | 0.0 | |||
| Other | 101 | 2.4 | 10 | 1.3 | |||
| Unknown | 3605 | 85.9 | 687 | 86.3 | 6 | 75.0 | |
| Surgical approach | < 0.001 | ||||||
| Open | 705 | 16.8 | 116 | 14.6 | |||
| Minimally invasive | 525 | 12.5 | 129 | 16.2 | |||
| Hybrid | 11 | 0.3 | 21 | 2.6 | |||
| Unknown | 2954 | 70.4 | 530 | 66.6 | 3 | 37.5 | |
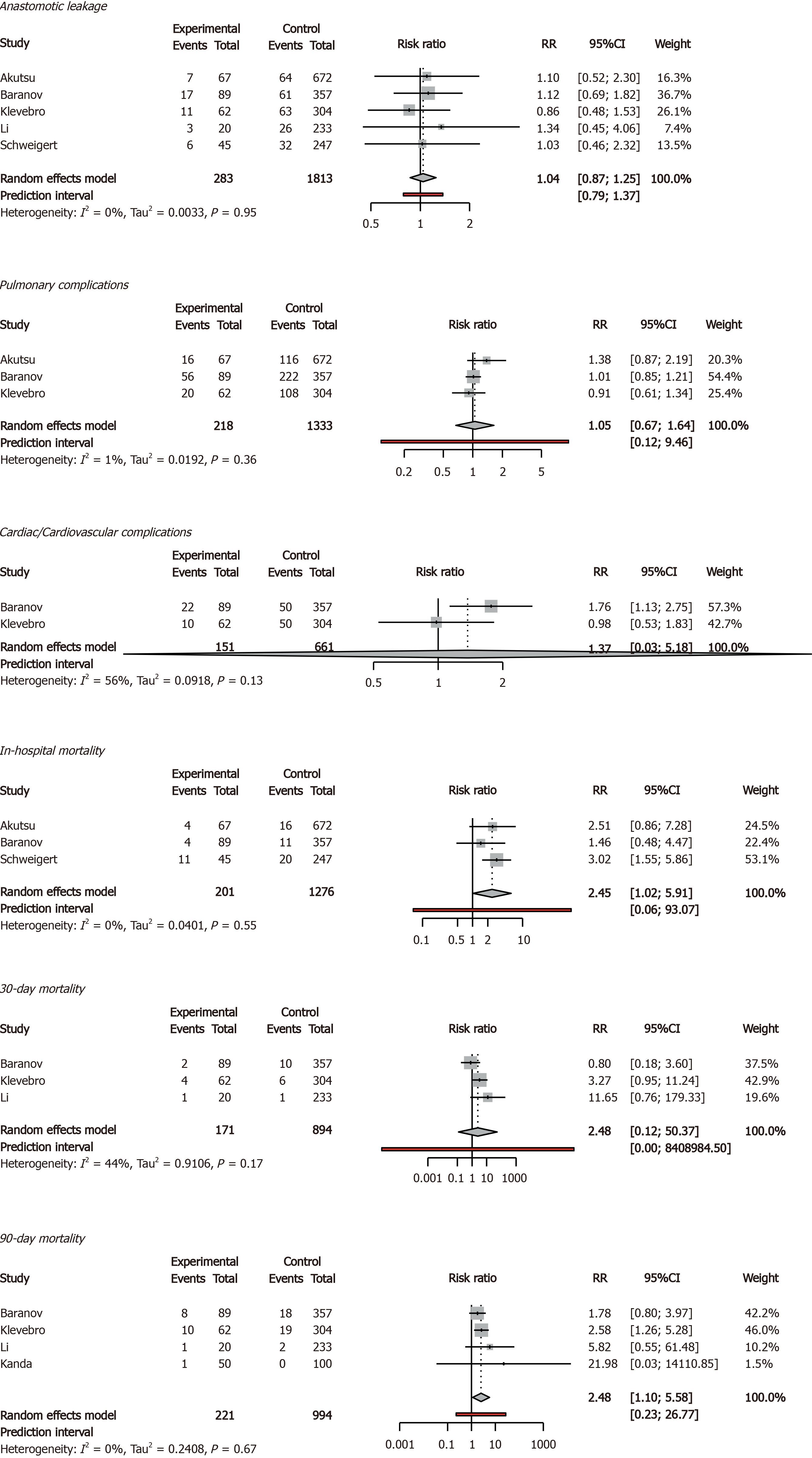
The 90-d mortality rate was 9.05% in older patients and 3.92% in younger patients (95%CI = 1.10-5.56). In addition, the in-hospital mortality rate was 9.45% in older patients and 3.68% in younger patients (95%CI = 1.01-5.91). Anastomotic leak rates, rates of pulmonary and cardiovascular complications, and 30-d mortality rates were comparable between older and younger patients. Delirium was reported only once, therefore no meta-analysis was possible (Figures 2 and 3).
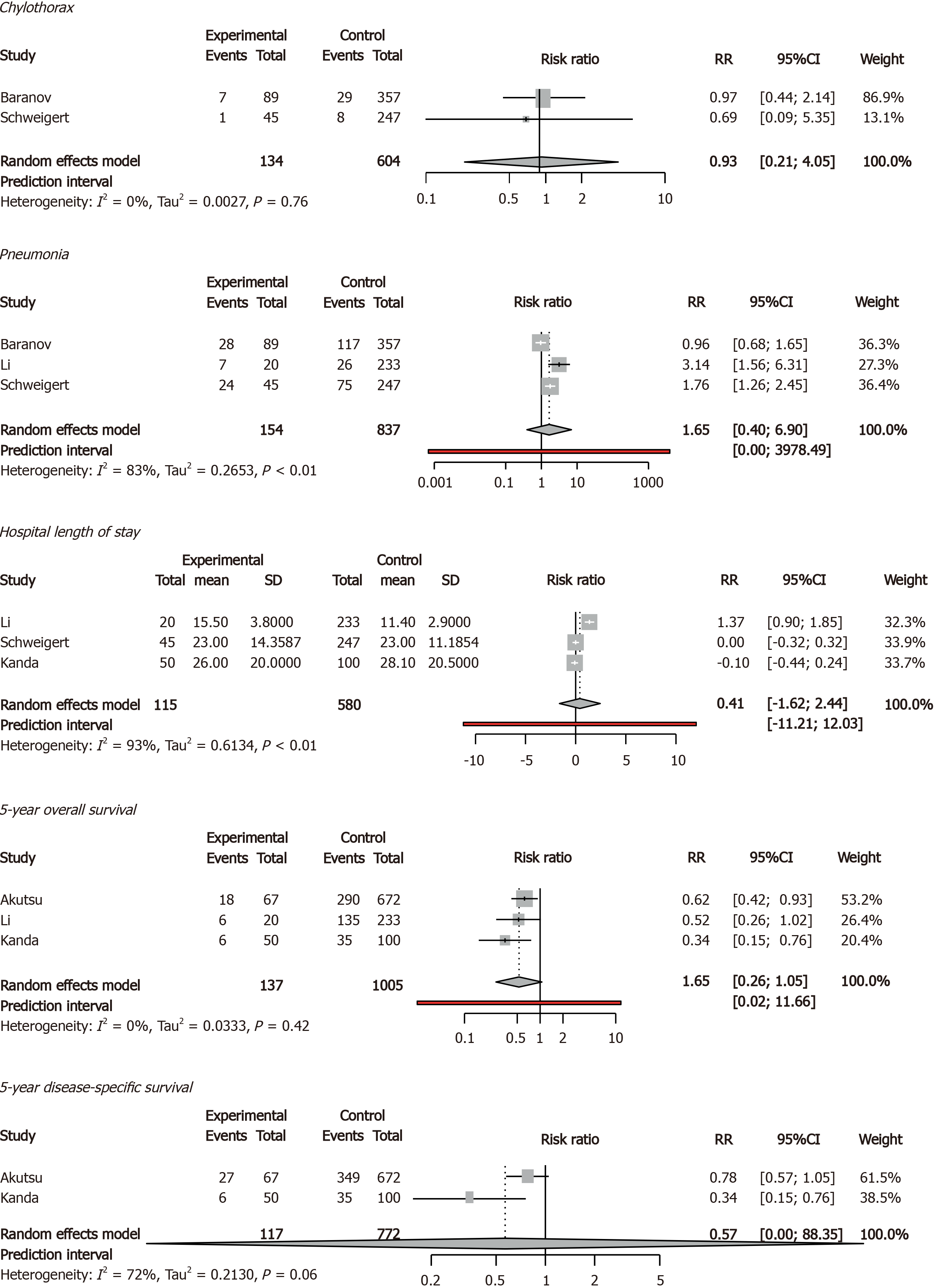
Older and younger patients had comparable rates of chylothorax, pneumonia, hospital length of stay, 5-year overall survival, and 5-year disease-specific survival. Quality of life was not reported (Figure 4).
Two studies with 812 patients (151 older and 661 younger). From the primary outcomes, older patients showed comparable anastomotic leak, 30-d, 90-d, and in-hospital mortality rates. From the secondary outcomes, older patients showed comparable rates of chylothorax, and pneumonia (Table 3).
| Studies | Participants | Younger | Older | RR | 95%CI | P value | ||
| n | n | % | % | |||||
| Primary outcomes | ||||||||
| Anastomotic leak | ||||||||
| OE | 2 | 545 | 12.08 | 13.85 | 1.1307 | 0.2235 | 5.7199 | 0.5120 |
| MIE | 2 | 812 | 18.76 | 18.54 | 0.9996 | 0.1877 | 5.3243 | 0.9979 |
| 30-d mortality rate | ||||||||
| OE | 1 | 253 | 0.43 | 5.00 | 11.6500 | 0.7568 | 179.3310 | 0.0784 |
| MIE | 2 | 812 | 2.42 | 3.97 | 1.7320 | 0.0002 | 12493.0189 | 0.5761 |
| 90-d mortality rate | ||||||||
| OE | 2 | 403 | 0.60 | 2.86 | 6.8396 | 0.0279 | 1675.2496 | 0.1410 |
| MIE | 2 | 812 | 5.60 | 25.71 | 2.1859 | 0.2111 | 22.6353 | 0.1471 |
| In-hospital morality rate | ||||||||
| OE | 1 | 292 | 8.10 | 24.44 | 3.0189 | 1.5547 | 5.8620 | 0.0011 |
| MIE | 1 | 446 | 3.08 | 4.49 | 1.4586 | 0.4757 | 4.4730 | 0.5091 |
| Secondary outcomes | ||||||||
| Chylothorax | ||||||||
| OE | 1 | 292 | 3.24 | 2.22 | 0.6861 | 0.0879 | 5.3534 | 0.7193 |
| MIE | 1 | 446 | 8.12 | 7.87 | 0.9682 | 0.4385 | 2.1377 | 0.9363 |
| Pneumonia | ||||||||
| OE | 2 | 545 | 21.04 | 47.69 | 2.1531 | 0.0639 | 72.5068 | 0.2205 |
| MIE | 1 | 446 | 32.77 | 31.46 | 0.9600 | 0.6828 | 1.3497 | 0.8141 |
Two studies with 545 patients (65 older and 480 younger). From the primary outcomes, older patients had higher rates of in-hospital mortality, while anastomotic leak, 30-d and 90-d mortality rates were comparable. From the secondary outcomes, chylothorax and pneumonia rates were comparable between older and younger patients (Table 3).
We found that patients older than 75 years who underwent esophagectomy have a higher risk of 90-d and in-hospital mortality. Postoperative complications, 30-d mortality rate and survival were comparable between older and younger patients. In the subgroup of studies with MIE, older patients had comparable 30-d, 90-d and in-hospital mortality rates compared to younger patients.
Strengths of this review are the subgroup analyses of MIE and OE, the large number of patients that were included, and the fact that the studies included in this study were not used in previous meta-analyses. Another strength is the inclusion and separate analysis of different age thresholds to ensure that no studies were left out. These analyses did not show substantially different results with regard to the main analysis. The most important limitations of this review are the fact that only 1 prospective study was included, and only 2 studies which included patients that underwent MIE.
The higher 90-d mortality rates in patients aged 75 years and older found in this review correspond to results of earlier systematic reviews that used other age thresholds (70 and 80 years)[8-10]. More recently published studies confirm these results[27-29]. Literature shows, that older patients have a worse comorbidity status preoperatively compared with their younger counterparts, and as such have a higher risk of (severe) complications and mortality[30-33]. This is especially true, when considering that older patients with a similar comorbidity status as younger patients, have comparable short-term outcomes[13,34]. Our study, however, showed that the comorbidity status between patients aged < 75 and ≥ 75 was comparable, and this may be the result of lack of data, since it was not possible to obtain comorbidity rates for meta-analysis from most studies.
Subgroup analyses of OE studies showed increased in-hospital mortality rates in older patients, while studies including patients that underwent MIE showed comparable in-hospital, 30-d and 90-d mortality rates between older and younger patients. MIE might give surgeons opportunities to expand indications for curative surgery to older patients, because of the lower physical burden and risk of operation trauma of minimally invasive surgery on patients. Alternatively, it can be argued that studies with MIE used different selection criteria, as the two MIE studies in this review suggest[13,22].
The results from this review underline the importance of age as a predictor of mortality in patients undergoing esophagectomy for esophageal cancer, and this should be taken into account by clinicians. The results of this review also suggest that MIE might give older patients a chance at curative resection without a higher risk of mortality as compared to younger patients. In our view, however, it remains important to take comorbidity, fitness, frailty and patients’ views into consideration in addition to patient age and surgical approach.
In conclusion, patients aged 75 years or older undergoing curative esophagectomy for esophageal cancer have a higher risk of mortality. Minimally invasive esophagectomy may be important for minimizing mortality in older patients.
Fit patients diagnosed with cT1-3N0-3M0 (resectable) esophageal cancer generally undergo curative esophagectomy. An increasing number of older patients is undergoing curative esophagectomy for resectable esophageal cancer. Previous meta-analyses have shown that older patients suffered from more postoperative morbidity and mortality compared to younger patients.
Increased morbidity and mortality in older patients after esophagectomy may lead to patient selection based on age. However, only studies including patients that underwent open esophagectomy were meta-analyzed. Therefore, it remains unknown whether there is an association between age and outcome in patients undergoing minimally invasive esophagectomy.
To perform a systematic review and meta-analysis on age and postoperative outcome in esophageal cancer patients undergoing esophagectomy, including minimally invasive esophagectomy.
Studies comparing older with younger patients with primary esophageal cancer undergoing curative esophagectomy were included. Meta-analysis of studies using a 75-year age threshold are presented in the manuscript, studies using other age thresholds in the Supplementary material. MEDLINE, Embase and the Cochrane Library were searched for articles published between 1995 and 2020. Risk of bias was assessed with the Newcastle-Ottawa Scale. Primary outcomes were anastomotic leak, pulmonary and cardiac complications, delirium, 30-and 90-d, and in-hospital mortality. Secondary outcomes included pneumonia and 5-year overall survival.
Seven studies (4847 patients) using an age threshold of 75 years were included for meta-analysis with 755 older and 4092 younger patients. Older patients (9.05%) had higher rates of 90-d mortality compared with younger patients (3.92%), (confidence interval = 1.10-5.56). In addition, older patients (9.45%) had higher rates of in-hospital mortality compared with younger patients (3.68%), (confidence interval = 1.01-5.91). In the subgroup of 2 studies with minimally invasive esophagectomy, older and younger patients had comparable 30-d, 90-d and in-hospital mortality rates.
Older patients undergoing curative esophagectomy for esophageal cancer have a higher postoperative mortality risk. Minimally invasive esophagectomy may be important for minimizing mortality in older patients.
Future studies with more patients are needed to investigate the effects of curative minimally invasive esophagectomy on morbidity, mortality and especially quality of life in older patients with resectable esophageal cancer. Currently, we are investigating this with population-based surgical oncology data from the Netherlands.
Manuscript source: Invited manuscript
Specialty type: Surgery
Country/Territory of origin: Netherlands
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujiwara Y S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Christensen K, Doblhammer G, Rau R, Vaupel JW. Ageing populations: the challenges ahead. Lancet. 2009;374:1196-1208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2275] [Cited by in RCA: 2237] [Article Influence: 139.8] [Reference Citation Analysis (0)] |
| 2. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55840] [Article Influence: 7977.1] [Reference Citation Analysis (132)] |
| 3. | Schlottmann F, Strassle PD, Nayyar A, Herbella FAM, Cairns BA, Patti MG. Postoperative outcomes of esophagectomy for cancer in elderly patients. J Surg Res. 2018;229:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JH, Hollmann MW, de Lange ES, Bonjer HJ, van der Peet DL, Cuesta MA. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1207] [Article Influence: 92.8] [Reference Citation Analysis (0)] |
| 5. | Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, D'Journo XB, Brigand C, Perniceni T, Carrère N, Mabrut JY, Msika S, Peschaud F, Prudhomme M, Bonnetain F, Piessen G; Fédération de Recherche en Chirurgie (FRENCH) and French Eso-Gastric Tumors (FREGAT) Working Group. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N Engl J Med. 2019;380:152-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 475] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 6. | van der Sluis PC, van der Horst S, May AM, Schippers C, Brosens LAA, Joore HCA, Kroese CC, Haj Mohammad N, Mook S, Vleggaar FP, Borel Rinkes IHM, Ruurda JP, van Hillegersberg R. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg. 2019;269:621-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 422] [Article Influence: 84.4] [Reference Citation Analysis (0)] |
| 7. | Haverkamp L, Seesing MF, Ruurda JP, Boone J, V Hillegersberg R. Worldwide trends in surgical techniques in the treatment of esophageal and gastroesophageal junction cancer. Dis Esophagus. 2017;30:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 8. | Markar SR, Karthikesalingam A, Thrumurthy S, Ho A, Muallem G, Low DE. Systematic review and pooled analysis assessing the association between elderly age and outcome following surgical resection of esophageal malignancy. Dis Esophagus. 2013;26:250-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Han Y, Liu S, Guo W, Zhang Y, Li H. Clinical outcomes of oesophagectomy in elderly versus relatively younger patients: a meta-analysis. Interact Cardiovasc Thorac Surg. 2019;29:897-905. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Lopez-Lopez V, Gómez-Ruiz AJ, Eshmuminov D, Cascales-Campos PA, Alconchel F, Arevalo-Perez J, Robles Campos R, Parrilla Paricio P. Surgical oncology in patients aged 80 years and older is associated with increased postoperative morbidity and mortality: A systematic review and meta-analysis of literature over 25 years. Surg Oncol. 2020;33:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 11. | Abbott A, Shridhar R, Hoffe S, Almhanna K, Doepker M, Saeed N, Meredith K. Robotic assisted Ivor Lewis esophagectomy in the elderly patient. J Gastrointest Oncol. 2015;6:31-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 12. | Zhao H, Liu G, Wei S, Liu H. Short- and long-term outcomes of minimally invasive esophagectomy in elderly patients with esophageal squamous cell carcinoma. J BUON. 2017;22:1540-1546. [PubMed] |
| 13. | Baranov NS, van Workum F, van der Maas J, Kouwenhoven E, van Det M, van den Wildenberg FJH, Polat F, Nieuwenhuijzen GAP, Luyer MDP, Rosman C. The Influence of Age on Complications and Overall Survival After Ivor Lewis Totally Minimally Invasive Esophagectomy. J Gastrointest Surg. 2019;23:1293-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 47195] [Article Influence: 2949.7] [Reference Citation Analysis (0)] |
| 15. | Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos DM, Tugwell P. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [cited 25 January 2021]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 16. | Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:, 135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3433] [Cited by in RCA: 7043] [Article Influence: 640.3] [Reference Citation Analysis (0)] |
| 17. | Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. 2019;22:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1954] [Cited by in RCA: 3154] [Article Influence: 525.7] [Reference Citation Analysis (0)] |
| 18. | Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing Meta-Analysis in R: A Hands-on Guide. 2019. [DOI] [Full Text] |
| 19. | Team R. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Vienna, Austra, 2010. Available from: https://www.bibsonomy.org/bibtex/2b73bfa376907e90089cf7434f67bfd53/yevb0. |
| 20. | Akutsu Y, Shuto K, Uesato M, Hoshino I, Matsubara H. [The problems of surgery in aged esophageal cancer]. Nihon Ronen Igakkai Zasshi. 2009;46:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Kanda M, Koike M, Tanaka C, Kobayashi D, Hayashi M, Yamada S, Nakayama G, Omae K, Kodera Y. Feasibility of subtotal esophagectomy with systematic lymphadenectomy in selected elderly patients with esophageal cancer; a propensity score matching analysis. BMC Surg. 2019;19:143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 22. | Klevebro F, Garritano S, Scandavini CM, Shetye A, Coppola A, Kamiya S, Nilsson M, Lundell L, Rouvelas I. Surgical outcomes of oesophagectomy or gastrectomy due to cancer for patients ≥75 years of age: a single-centre cohort study. ANZ J Surg. 2019;89:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Li CW, Wu HR, Xu GW, Xiong R, Xu MQ, Xie MR. Analysis of short- and long-term outcomes after esophagectomy in elderly cancer patients. hongguo Zhongliu Linchuang. 2018;45:508-512. [DOI] [Full Text] |
| 24. | Matsumoto Y, Kimura K, Zhou Q, Sasaki K, Saiki T, Moriyama M, Saijo Y. Treatments and outcomes of older patients with esophageal cancer: Comparison with younger patients. Mol Clin Oncol. 2019;11:383-389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Schweigert M, Solymosi N, Dubecz A, Stadlhuber RJ, Ofner D, Stein HJ. Current outcome of esophagectomy in the very elderly: experience of a German high-volume center. Am Surg. 2013;79:754-763. [PubMed] |
| 26. | Yang S, Li H, Jia C, Ma X, Guo W, Li H. Clinicopathological features and prognosis of patients <45 years old with esophageal adenocarcinoma comparing to other age groups. J Thorac Dis. 2016;8:2724-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 27. | Horne ZD, Wegner RE, Colonias A, Weksler B, Glaser SM, Kalash R, Beriwal S. Drivers of 30- and 90-day Postoperative Death After Neoadjuvant Chemoradiation for Esophageal Cancer. Ann Thorac Surg. 2020;109:921-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Madhavan A, Kamarajah SK, Navidi M, Wahed S, Immanuel A, Hayes N, Griffin SM, Phillips AW. The impact of age on patients undergoing transthoracic esophagectomy for cancer. Dis Esophagus. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 29. | Lagergren J, Bottai M, Santoni G. Patient Age and Survival After Surgery for Esophageal Cancer. Ann Surg Oncol. 2021;28:159-166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 30. | van Gestel YR, Lemmens VE, de Hingh IH, Steevens J, Rutten HJ, Nieuwenhuijzen GA, van Dam RM, Siersema PD. Influence of comorbidity and age on 1-, 2-, and 3-month postoperative mortality rates in gastrointestinal cancer patients. Ann Surg Oncol. 2013;20:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 31. | Divo MJ, Martinez CH, Mannino DM. Ageing and the epidemiology of multimorbidity. Eur Respir J. 2014;44:1055-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 430] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 32. | Backemar L, Lagergren P, Johar A, Lagergren J. Impact of co-morbidity on mortality after oesophageal cancer surgery. Br J Surg. 2015;102:1097-1105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Yamashita K, Watanabe M, Mine S, Fukudome I, Okamura A, Yuda M, Hayami M, Imamura Y. The impact of the Charlson comorbidity index on the prognosis of esophageal cancer patients who underwent esophagectomy with curative intent. Surg Today. 2018;48:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 34. | Pultrum BB, Bosch DJ, Nijsten MW, Rodgers MG, Groen H, Slaets JP, Plukker JT. Extended esophagectomy in elderly patients with esophageal cancer: minor effect of age alone in determining the postoperative course and survival. Ann Surg Oncol. 2010;17:1572-1580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |