Published online Feb 15, 2021. doi: 10.4251/wjgo.v13.i2.119
Peer-review started: September 27, 2020
First decision: December 12, 2020
Revised: December 22, 2020
Accepted: January 7, 2021
Article in press: January 7, 2021
Published online: February 15, 2021
Processing time: 126 Days and 22.7 Hours
For locally advanced rectal cancer (LARC), standard therapy [consisting of neoadjuvant chemoradiotherapy (CRT), surgery, and adjuvant chemotherapy (ChT)] achieves excellent local control. Unfortunately, survival is still poor due to distant metastases, which remains the leading cause of death among these patients. In recent years, the concept of total neoadjuvant treatment (TNT) has been developed, whereby all systemic ChT-mainly affecting micrometastases-is applied prior to surgery.
To compare standard therapy and total neoadjuvant therapy for LARC patients with high-risk factors for failure.
In a retrospective study, we compared LARC patients with high-risk factors for failure who were treated with standard therapy or with TNT. High-risk for failure was defined according to the presence of at least one of the following factors: T4 stage; N2 stage; positive mesorectal fascia; extramural vascular invasion; positive lateral lymph node. TNT consisted of 12 wk of induction ChT with capecitabine and oxaliplatin or folinic acid, fluorouracil and oxaliplatin, CRT with capecitabine, and 6-8 wk of consolidation ChT with capecitabine and oxaliplatin or folinic acid, fluorouracil and oxaliplatin prior to surgery. The primary endpoint was pathological complete response (pCR). In total, 72 patients treated with standard therapy and 89 patients treated with TNT were included in the analysis.
Compared to standard therapy, TNT showed a higher proportion of pCR (23% vs 7%; P = 0.01), a lower neoadjuvant rectal score (median: 8.43 vs 14.98; P < 0.05), higher T-and N-downstaging (70% and 94% vs 51% and 86%), equivalent R0 resection (95% vs 93%), shorter time to stoma closure (mean: 20 vs 33 wk; P < 0.05), higher compliance during systemic ChT (completed all cycles 87% vs 76%; P < 0.05), lower proportion of acute toxicity grade ≥ 3 during ChT (3% vs 14%, P < 0.05), and equivalent acute toxicity and compliance during CRT and in the postoperative period. The pCR rate in patients treated with TNT was significantly higher in patients irradiated with intensity-modulated radiotherapy/volumetric-modulated arc radiotherapy than with 3D conformal radiotherapy (32% vs 9%; P < 0.05).
Compared to standard therapy, TNT provides better outcome for LARC patients with high-risk factors for failure, in terms of pCR and neoadjuvant rectal score.
Core Tip: Our data suggest that treatment of locally advanced rectal cancer (LARC) with high-risk factors for failure using total neoadjuvant therapy (TNT) is more effective than standard therapy, achieving a higher rate of pathological complete response, more favourable survival prognosis, higher proportion of T-and N-downstaging, shorter time to temporary stoma closure, better compliance, and lower toxicity grade 3-5 during systemic chemotherapy. The outcomes of TNT in patients with the most aggressive form of LARC are completely comparable to TNT in all patients with LARC.
- Citation: Tuta M, Boc N, Brecelj E, Peternel M, Velenik V. Total neoadjuvant therapy vs standard therapy of locally advanced rectal cancer with high-risk factors for failure. World J Gastrointest Oncol 2021; 13(2): 119-130
- URL: https://www.wjgnet.com/1948-5204/full/v13/i2/119.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i2.119
Neoadjuvant chemoradiotherapy (CRT), followed by surgery and adjuvant chemotherapy (ChT) is recommended as the standard of care for patients with locally advanced rectal cancer (LARC). While this approach has improved local control, survival remains poor due to distant metastases, which remain the leading cause of death among these patients. The role of adjuvant ChT in the treatment of LARC remains unclear. Adjuvant ChT is often associated with poor tolerance and compliance, the need for dose reduction, and delays in beginning adjuvant treatment due to postoperative complication[1,2]. In recent years, the concept of total neoadjuvant treatment (TNT) has been developed, whereby systemic ChT, which mainly affects micrometastasis, is applied with CRT prior to surgery.
Rectal cancer patients who achieve a pathological complete response (pCR) have better disease-free survival, fewer local recurrences, better distant metastasis-free survival, and better overall survival[3]. This fact has become an important guide in testing different strategies to improve the outcome of patients with LARC. Compared to standard treatment, preoperative systemic ChT shows better compliance with ChT, increased downstaging, more margin-negative resections, and a higher rate of pCR[4-7]. Therefore, in the future, this may represent a non-operative approach to selected patients. The highest risk of systemic and/or local failure is found in patients with the presence of at least one of the following factors: T4 status; N2 status; positive mesorectal fascia; extramural vascular invasion; and positive lateral lymph node[8-13].
The aim of this study was to compare the outcomes following TNT or standard therapy in LARC patients with high-risk factors for failure in the same time period.
This retrospective study included all adult patients with newly-diagnosed LARC with high-risk factors for failure who were treated with TNT or standard therapy at the Institute of Oncology, Ljubljana (Slovenia), from 2016 to 2019. The inclusion criteria were: Histologically-proven rectal adenocarcinoma with distal margin of 15 cm or less from the anal verge on magnetic resonance imaging; Clinical stage II or III; and The presence of at least one of the high-risk factors for failure (T4, N2, mesorectal fascia+, extramural vascular invasion+, and/or lateral lymph node+). Patients were excluded from the study if they had distant metastases, concomitant malignancy, inflammatory bowel disease, or malabsorption syndrome. The study was approved by the local institutional review boards and the National Medical Ethics Committee of Slovenia (No. 0120-298/2019/5).
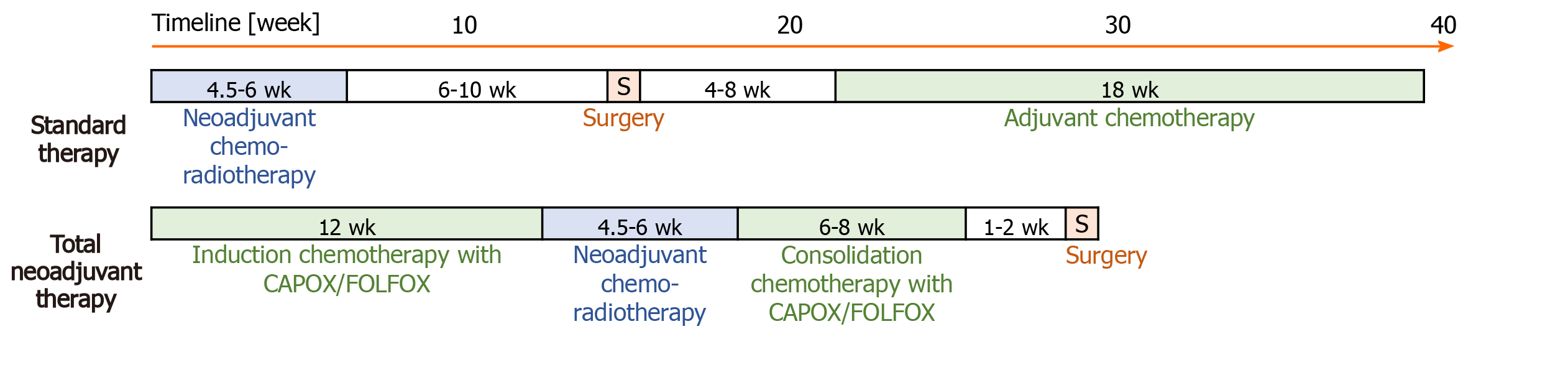
Standard therapy consists of capecitabine-based CRT, followed by surgery and in patients without pCR adjuvant ChT (Figure 1). As part of the preoperative standard therapy, all patients received external-beam radiotherapy using a three-dimensional conformal radiation therapy technique (3D CRT) to the pelvis (45.0 Gy in 25 fractions and a boost to the tumour at a dose of 50.4 Gy for T3 tumours and 54 Gy for T4 tumours, in three to five fractions) and intensity-modulated radiotherapy (IMRT) or volumetric modulated arc therapy (VMAT) to the pelvis (41.8 Gy and simultaneously integrated boost to the tumour at a dose of 46.2 Gy for T3 tumours or 48.4 Gy to T4 tumours in 22 fractions). Concomitant ChT was performed via administration of capecitabine at a daily dose of 825 mg/m2/12 h per os on irradiation day in 3D CRT or from the first to the last irradiation day in IMRT/VMAT. Surgery was scheduled to take place 6-10 wk after completion of CRT. In cases that did not achieve pCR, adjuvant ChT was started at 4-8 wk after surgery. In cases of microscopic residual disease (R1), adjuvant ChT consisted of eight cycles of capecitabine and oxaliplatin (CAPOX). In cases of poor compliance or heart failure, adjuvant ChT consisted of four cycles of 5-fluorouracil and leucovorin.
TNT consisted of induction ChT with CAPOX or with folinic acid, fluorouracil and oxaliplatin (FOLFOX), capecitabine-based CRT and consolidation ChT with CAPOX/FOLFOX prior to surgery. TNT with the CAPOX regimen was defined as four induction cycles (12 wk) of CAPOX, capecitabine-based CRT and two consolidation cycles (6 wk) of CAPOX before surgery. One cycle of the CAPOX regimen involved capecitabine (1000 mg/m2/12 h per os on days 1-14) and oxaliplatin (oxaliplatin 130 mg/m2 intravenous over 2 h on day 1) every 3 wk. In cases with expectation of poorer compliance, patients received the FOLFOX regimen instead of ChT according to the CAPOX regimen. TNT with the FOLFOX regimen was defined as 12 wk of induction ChT and 8 wk of consolidation ChT. The FOLFOX regimen involved 5-fluorouracil (400 mg/m2 intravenous bolus on day 1, then 1200 mg/m2/d for 2 d), oxaliplatin (85 mg/m2 intravenous over 2 h on day 1), and leucovorin (400 mg/m2 intravenous over 2 h on day 1) every 2 wk, twice. CRT in TNT was the same as in standard therapy. Surgery was scheduled to take place 8-10 wk after completion of CRT or 1-2 wk after completion of consolidation ChT.
The study was based upon a cohort of 161 LARC patients who had high-risk factors for failure and who underwent treatment between the years of 2016 and 2019. A total of 72 patients received standard therapy (standard group) and 89 patients received TNT (TNT group). The baseline characteristics for all evaluable patients are listed in Table 1. All patients treated with TNT were pre-treatment staged with computed tomography (CT) of the chest, abdomen and pelvis. Patients treated with standard therapy were pre-treatment staged with CT of the chest, abdomen and pelvis or positron emission tomography-CT (86%); only a minority of patients (14%) had a chest x-ray or abdominal ultrasound in combination with/without CT.
| Characteristic | Standard therapy, n = 72, (%) | TNT, n = 89, (%) | P value | |
| Age in year | < 65 | 29 (40) | 66 (74) | < 0.001 |
| ≥ 65 | 43 (60) | 23 (26) | ||
| Range | 40-84 | 33-79 | ||
| mean [SD] | 65.4 [10.5] | 57.5 [10.1] | < 0.001 | |
| Sex | M | 45 (63) | 54 (61) | 0.813 |
| F | 27 (38) | 35 (39) | ||
| PS WHO | 0 | 48 (67) | 67 (75) | 0.229 |
| 1 | 24 (33) | 22 (25) | ||
| High-risk factors for failure | cT4 | 15 (21) | 33 (37) | 0.025 |
| cN2 | 49 (68) | 62 (70) | 0.827 | |
| MRF+ | 39 (54) | 66 (74) | 0.008 | |
| EMVI+ | 27 (38) | 65 (73) | < 0.001 | |
| Lateral node | 15 (21) | 8 (9) | 0.033 | |
| cTN | T2N2 | 1 (1) | 1 (1) | 0.1381 |
| T3N0 | 1 (1) | 0 (0) | ||
| T3N1 | 21 (29) | 20 (22) | ||
| T3N2 | 34 (47) | 35 (39) | ||
| T4N1 | 1 (1) | 7 (8) | ||
| T4N2 | 14 (19) | 26 (29) | ||
| Distance from anal verge in cm | ≤ 5 | 27 (38) | 29 (33) | 0.370 |
| 5.1-10 | 37 (51) | 43 (48) | ||
| ≥ 10.1 | 8 (11) | 17 (19) | ||
The primary endpoint was pCR rate, which was defined as ypT0N0. Secondary endpoints were neoadjuvant rectal (NAR) score, proportion of T-and N-downstaging, rates of R0 resection, time to stoma closure, acute toxicity, and compliance during treatment. The NAR score was calculated using the equation [5 pN – 3 × (cT-pT) + 12]2 / 9.61 and further classified as low (< 8), intermediate (8-16), or high (< 16)[14,15]. T- or N-downstaging was defined as a reduction in the clinical stage relative to the pathohistological stage. The time to stoma closure was defined as the time from surgery to temporary stoma closure. Treatment-related toxicities were scored according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.0[16].
All statistical analyses were performed using the SPSS statistical software, version 26.0 (IBM Corp., Armonk, NY, United States). Patient and treatment parameters were compared with the χ2 test or Fisher’s exact test for categorical variables and with Student’s t-test for continuous variables. The normality of data distribution was estimated by graphical analysis. In the case of an expected count less than 5 in cells with categorical variables, a likelihood-ratio test was performed, where indicated. A P value of < 0.05 was considered statistically significant. Frequencies and percentages are given in the tables presented herein, unless otherwise indicated. The statistical methods of this study were reviewed by a biomedical statistician holding a PhD in statistics (Rho sigma, https://www.rosigma.si/en/rhosigma).
Patients treated with TNT had a statistically significantly higher proportion of pCR compared to those who received the standard treatment (23% vs 7%; P = 0.007). The odds of achieving pCR were determined to be 3.9-fold higher in the TNT group than in the standard group (odds ratio of 3.92 with a 95% confidence interval of 1.38 to 11.14). The two treatment groups differed significantly in age and in proportion of high-risk factors for failure, but these characteristics alone did not have a significant effect on the rate of pCR. Furthermore, the influence of different characteristics (cT, full dose of systemic ChT, full-dose radiotherapy, all planned preoperative treatment, time from completion of radiotherapy to surgery, presence of acute toxicity, irradiation technique) on the rate of pCR was tested. Among the previously mentioned characteristics, only the irradiation technique showed a statistically significant effect on the rate of pCR in the TNT group, in contrast to the standard therapy group. In detail, we showed a statistically significantly higher proportion of pCR with IMRT/VMAT than with 3D CRT (32% vs 9%; P = 0.03). Similar to the proportion of pCR achievement, the NAR score showed a more favourable distribution in TNT compared to standard treatment (median: 8.43 vs 14.98; P < 0.001) (Table 2).
| Characteristic | Standard therapy, n = 70, (%) | TNT, n = 82, (%) | P value | |
| pCR | 5 (7) | 19 (23) | 0.007 | |
| CR | 7 (10) | 23 (26) | 0.009 | |
| NAR score | mean [SD] | 16.8 [12.9] | 10.7 [10.8] | 0.002 |
| NAR classes | < 8 | 9 (13) | 35 (43) | < 0.001 |
| 8-16 | 38 (56) | 33 (40) | ||
| > 16 | 21 (31) | 14 (17) | ||
| Surgery | R0 | 65 (93) | 78 (95) | 0.4451 |
| R1 | 4 (6) | 4 (5) | ||
| R2 | 1 (1) | 0 (0) | ||
| Weeks to stoma closure | mean [SD] | 32.8 [18.6] | 20.1 [10.9] | < 0.001 |
When comparing compliance and toxicity, we found statistically significant differences between the two treatment groups only during the period of systemic ChT administration (Tables 3 and 4). There were no adverse events experienced by patients with grade 4-5 during systemic ChT nor by those with grade 5 during CRT. The proportion of patients completing all planned cycles of systemic ChT was statistically higher in the TNT group than in the standard group. A statistically significant association was observed between the toxicity and the type of treatment administered during systemic ChT. Specifically, in the TNT group there was a slightly higher proportion of patients who experienced toxicity (82% vs 76%) and a higher proportion of patients who experienced adverse events of grades < 3 (79% vs 62%). In spite of that, there was a lower proportion of patients who experienced adverse events of grades 3-5 (3% vs 14%). The most frequent adverse events during systemic ChT were hand-foot syndrome (40%) in the standard group and paraesthesia (61%) in the TNT group (Table 5). The most frequent adverse events of grade 3 during systemic ChT were hand-foot syndrome (10%) in the standard group and hand-foot syndrome (1%), infection (1%) and thromboembolic event (1%) in the TNT group.
| Standard therapy during CRT | TNT | P value | |
| Patients who received CRT | 72 (100) | 88 (99) | 0.2751 |
| Patients who received RT | 0 (0) | 1 (1) | |
| Full-dose ChT (CAP or 5-FU + LV) | 62 (86) | 67 (75) | 0.087 |
| Modification of concomitant ChT | 10 (14) | 22 (25) | |
| Modification of RT | 1 (1) | 0 (0) | |
| During adjuvant ChT | During systemic ChT | ||
| Without ChT due to pCR | 5 (7) | 0 (0) | |
| Patients who received ChT | 50 (72) | 89 (100) | |
| All planned cycle | |||
| 6c of systemic ChT (CAP or CAPOX) | 34 (68) | 76 (85) | 0.0481 |
| Other alternative schemes | 4 (8) | 2 (2) | |
| No | 12 (24) | 11 (12) | |
| Full-dose | |||
| 6c of systemic ChT (CAP or CAPOX) | 25 (50) | 56 (63) | 0.1581 |
| Other alternative schemes | 4 (8) | 2 (2) | |
| No | 21 (42) | 31 (35) | |
| Toxicity | Standard therapy, n (%) | TNT, n (%) | P value |
| During systemic ChT | 0.0371 | ||
| Without | 12 (24) | 16 (18) | |
| Grade 1-2 | 31 (62) | 70 (79) | |
| Grade 3 | 7 (14) | 3 (3) | |
| During CRT | 0.5531 | ||
| Without | 13 (18) | 21 (24) | |
| Grade 1-2 | 57 (79) | 64 (72) | |
| Grade 3-4 | 2 (3) | 4 (4) | |
| Postoperative complications | 0.140 | ||
| Without | 43 (61) | 62 (76) | |
| Grade 1-2 | 18 (26) | 15 (18) | |
| Grade 3-5 | 9 (13) | 5 (6) | |
| Toxicity during systemic ChT | Adjuvant ChT (standard therapy), 50 patients | During induction and consolidation ChT (TNT), 89 patients | |||||||||
| Grade 1-2 | Grade 3 | Grade 4 | Grade 1-2 | Grade 3 | Grade 4 | ||||||
| Thrombocytopenia | 7 | 14% | 0 | 0 | 11 | 12% | 0 | 0 | |||
| Anaemia | 15 | 30% | 0 | 0 | 12 | 13% | 0 | 0 | |||
| Neutropenia | 3 | 6% | 0 | 0 | 8 | 9% | 0 | 0 | |||
| Diarrhoea | 6 | 12% | 1 | 2% | 0 | 10 | 11% | 0 | 0 | ||
| Nausea | 2 | 4% | 1 | 2% | 0 | 29 | 33% | 0 | 0 | ||
| Vomiting | 2 | 4% | 1 | 2% | 0 | 8 | 9% | 0 | 0 | ||
| Hand foot syndrome | 15 | 30% | 5 | 10% | 0 | 10 | 11% | 1 | 1% | 0 | |
| Paraesthesia | 1 | 2% | 0 | 0 | 54 | 61% | 0 | 0 | |||
| Acute renal failure | 1 | 2% | 0 | 0 | 0 | 0 | 0 | ||||
| Rectal fistula | 0 | 1 | 2% | 0 | 0 | 0 | 0 | ||||
| Stoma site infection | 1 | 2% | 1 | 2% | 0 | 0 | 0 | 0 | |||
| Hepatotoxicity | 0 | 0 | 0 | 3 | 3 | 0 | 0 | ||||
| Infection | 5 | 10% | 0 | 0 | 2 | 2% | 1 | 1% | 0 | ||
| Chest pain | 0 | 0 | 0 | 1 | 1% | 0 | 0 | ||||
| Thromboembolism | 5 | 10% | 0 | 0 | 1 | 1% | 1 | 1% | 0 | ||
| Ileus | 1 | 2% | 0 | 0 | 1 | 1% | 0 | 0 | |||
| Enterocolitis | 0 | 0 | 0 | 1 | 1% | 0 | 0 | ||||
| Without toxicity | 12 (24%) | 16 (18%) | |||||||||
There were no differences between the groups in compliance and acute toxicities during CRT and surgery. In the standard group, 70 (97%) patients underwent surgery, of which 1 patient died a few days after surgery due to septic shock. Two patients who had a clinical complete response refused surgery. In the TNT group, 82 (92%) patients underwent surgery and 6 (7%) patients refused surgery, including 4 patients with clinical complete response among the latter. However, at the end of the first cycle of consolidation ChT, 1 patient developed perineal infection and underwent two non-radical operations at another hospital, dying after the second operation.
Through our study, we have confirmed that the treatment of LARC patients with high-risk factors for failure with TNT was statistically significantly better than with standard treatment, in terms of the pCR and NAR score. Despite the fact that the treatment groups differed in age distribution and in the proportions of some high-risk factors for failure, we showed by statistical analysis that these characteristics do not affect achievement of pCR. Moreover, the TNT group had overall greater extent of disease but achieved a higher proportion of pCR. Treatment of LARC patients with high-risk factors for failure by means of TNT is more effective than standard therapy because it achieves a higher rate of pCR, more favourable survival prognosis, higher proportion of T-and N-downstaging, shorter time to temporary stoma closure, better compliance, and lower toxicity grade 3-5 during systemic ChT. To our knowledge, our distribution of the NAR prognostic score in the TNT group is the most favourable of all published studies on the treatment of LARC with near TNT, CRT or neoadjuvant radiotherapy[17-21]. Our patients with the most aggressive form of LARC treated with TNT had a similar rate of pCR as all patients with LARC, which speaks in favour of TNT[22,23].
We showed a statistically significant influence of the irradiation technique on the proportion of pCR in the TNT group. This statistically significant influence was not found in the standard group but a similar trend was observed. One of the reasons why we failed to demonstrate the influence of the irradiation technique on the proportion of pCR in standard treatment could be the small sample size. In the standard group, only 17% of patients were irradiated with IMRT/VMAT, whereas in the TNT group, this was 61%. The higher rate of pCR in the TNT group irradiated with IMRT/VMAT is attributed to the fact that shortening of the overall treatment time, accuracy of irradiation technique and hypofractionation (higher dose per fraction, simultaneous integrated boost) were enabled with IMRT/VMAT application[24]. We believe that in the trials of different TNT approaches, greater emphasis should be placed on the choice of optimal radiotherapy.
To the best of our knowledge, this study is one of the first comparing standard therapy and TNT in LARC patients with high-risk factors for failure, in addition to the RAPIDO trial[25]. We achieved a slightly lower rate of pCR with both treatments than that reported for the RAPIDO trial (7% and 23% vs 14% and 28% respectively)[26]. Conversely, if we focus only on patients who were irradiated with more advanced irradiation techniques, i.e. IMRT/VMAT, we found higher proportions of pCR achievement (17% and 32% vs 14% and 28% respectively) compared to the RAPIDO trial. When comparing our study with the RAPIDO trial, it should be noted that patients in the latter were irradiated with a short-course radiotherapy, whereby the rate of tumour regression is lower and the occurrence of pCR is less likely[27]. On the other hand, it should be noted that in the RAPIDO trial, there was a longer interval between the end of radiotherapy and surgery, which may have affected the higher rate of pCR[3,28,29].
After analysing the results of treatment of this high-risk group of patients in two previous Slovenian studies, we reported 10.5% of pCR in 2011-2013 and 20% in 2014-2015[24,30,31]. Preoperative treatment was more intensive than standard treatment in both studies. In our present study, the standard group had 7% of pCR, which is less than in the two previous Slovenian studies, as expected. This fact suggests that the population of patients with LARC with high-risk factors for failure needs a more intensive preoperative treatment regimen as the only standard therapy, in order to achieve a higher rate of pCR. In addition to different treatment regimens, patients in the two previous Slovenian studies were treated with different irradiation techniques. Generally speaking, our TNT with 3D CRT differed from treatment in patients in 2011-2013 in the more aggressive preoperative systemic ChT, which, however, was not clearly reflected in a higher rate of pCR (9% vs 10.5%). On the other hand, our TNT with IMRT/VMAT differed from treatment in patients in 2014-2015 in additional preoperative systemic ChT, which was clearly reflected in a higher rate of pCR (32% vs 20%). These facts confirm the position of systemic ChT in the preoperative period to achieve more effective downstaging or a high proportion of pCR. In summary, we believe that systemic ChT has a place in the preoperative period but consideration should be given to choosing the more optimal scheme of preoperative systemic ChT. Therefore, the influence of a radiotherapy regimen and of the aggressiveness of systemic ChT on the outcome of treatment must be taken into account when comparing and researching different TNT schemes.
Compliance with all planned cycles of preoperative systemic ChT in the TNT group was statistically significantly better compared to that in the standard group. This is in line with the findings of other randomised studies in LARC patients and LARC patients with high-risk factors for failure[5,25]. Compliance with the systemic ChT of our TNT group is also comparable to compliance in TNT of LARC patients in other studies (86%-100%)[32].
As with compliance, the toxicity between both groups differs most during the period of systemic ChT. Standard treatment showed an 11% higher rate of toxicity grade ≥ 3 compared to TNT (14% vs 3%). Studies of the TNT and near TNT regimen in the treatment of LARC patients with high-risk factors have demonstrated a varied spectrum of the most common preoperative adverse reactions of grades ≥ 3. All rates of the most common grade ≥ 3 adverse reactions were higher (range: 9%–42%) than in our TNT group, where radiodermatitis and hand-foot syndrome were the most common (in 2%)[25,33-39]. It should be noted that our scheme of TNT is, according to the aforementioned studies, the least aggressive, but at the same time having a very comparable rate of pCR. We found a markedly lower rate of toxicity of grades 3-5 during TNT when comparing toxicities reported from the RAPIDO trial
The proportion of postoperative complications in both groups (39% for the standard group and 24% for the TNT group) was comparable to that of other studies researching TNT in LARC. These other studies showed 13%-51% of postoperative complications[32]. Comparing the proportions of postoperative complications with the largest randomised study in the field of treatment of LARC patients with high-risk factors, we found a fairly comparable or even a slightly lower proportion of postoperative complications with standard therapy (39% vs 47%) and, on the other hand, almost a one-half lower proportion of postoperative complications with TNT (24% vs 50%). One reason for this could be a more effective downstaging with our TNT regimen and consequently a lower rate of abdominoperineal excision compared to the RAPIDO trial (17% vs 58%), despite the fact that we had more patients with low-lying tumours (33% vs 22%)[25]. It is known that the rate of postoperative complications is higher after abdominoperineal excision than after sphincter-preserving surgery[40].
Our study has some limitations that need to be considered when favouring TNT over other treatment options for LARC patients with high-risk factors for failure. First, this study used a retrospective design and, therefore, has a lower level of data reliability than does a prospective or randomised study. Second, patients were followed for up to 3 mo after the end of treatment, which is a short period. A longer follow-up is required to determine the impact on local control, disease-free survival and overall survival. TNT is a relatively new approach in the treatment of LARC, and data on 5-year survival parameters are not yet available.
The outcome of TNT is better than that of standard treatment in LARC patients with high-risk factors for failure in terms of the pCR rate and the NAR prognostic score. Our study is one of the first to compare standard treatment and TNT in LARC patients with high-risk factors for failure. With TNT administration, we achieved a statistically significantly higher rate of pCR with IMRT/VMAT compared to 3D CRT. The reasons for the higher pCR are the accuracy of the irradiation technique and the possibility of hypofractionation (higher dose per fraction, simultaneous boost to the tumour) and thus shorter irradiation time.
Distant metastases remain the leading cause of death for patients with locally advanced rectal cancer. Systemic chemotherapy that mainly affects micrometastasis is administered with chemoradiotherapy prior to surgery in total neoadjuvant treatment.
Currently, it is unknown which treatment is better for patients with locally advanced rectal cancer and high-risk factors for treatment failure.
To compare the results of total neoadjuvant therapy and standard therapy in patients with locally advanced rectal cancer and high-risk factors for failure in the same time period.
We selected patients with locally advanced rectal cancer and high-risk factors for failure who were treated with standard therapy or with total neoadjuvant therapy. High-risk for failure were defined by the presence of at least one of the following factors: T4 status; N2 status; positive mesorectal fascia; extramural vascular invasion; and/or positive lateral lymph node.
This retrospective study showed that total neoadjuvant therapy yielded a higher proportion of pathological complete response (pCR), lower neoadjuvant rectal score, higher T-and N-downstaging, equivalent R0 resection, shorter time to stoma closure, higher compliance during systemic chemotherapy, lower proportion of acute toxicity grades ≥ 3 during chemotherapy, and equivalent acute toxicity and compliance during chemoradiotherapy and in the postoperative period. With total neoadjuvant therapy, we achieved a statistically significantly higher rate of pCR with intensity-modulated radiotherapy/volumetric modulated arc therapy compared to the three-dimensional conformal radiation therapy technique.
The outcome of total neoadjuvant therapy is better than that of standard treatment of locally advanced rectal cancer with high-risk factors for failure, in terms of the pCR rate and the neoadjuvant rectal prognostic score.
Randomized studies are needed to more reliably assess the benefits of total neoadjuvant therapy for locally advanced rectal cancer with high-risk factors for failure.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: Slovenia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed M, Cheng J S-Editor: Zhang L L-Editor: A P-Editor: Li JH
| 1. | Glynne-Jones R, Grainger J, Harrison M, Ostler P, Makris A. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: should we be more cautious? Br J Cancer. 2006;94:363-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 2. | Breugom AJ, van Gijn W, Muller EW, Berglund Å, van den Broek CBM, Fokstuen T, Gelderblom H, Kapiteijn E, Leer JWH, Marijnen CAM, Martijn H, Meershoek-Klein Kranenbarg E, Nagtegaal ID, Påhlman L, Punt CJA, Putter H, Roodvoets AGH, Rutten HJT, Steup WH, Glimelius B, van de Velde CJH. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo) radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol. 2015;26:696-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 294] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 3. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr, Suárez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1460] [Article Influence: 97.3] [Reference Citation Analysis (0)] |
| 4. | Chau I, Brown G, Cunningham D, Tait D, Wotherspoon A, Norman AR, Tebbutt N, Hill M, Ross PJ, Massey A, Oates J. Neoadjuvant capecitabine and oxaliplatin followed by synchronous chemoradiation and total mesorectal excision in magnetic resonance imaging-defined poor-risk rectal cancer. J Clin Oncol. 2006;24:668-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 5. | Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E, Mengual JL, Saigi E, Estevan R, Mira M, Polo S, Hernandez A, Gallen M, Arias F, Serra J, Alonso V. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 6. | Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM, Guillem JG, Paty PB, Yaeger R, Stadler ZK, Seier K, Gonen M, Segal NH, Reidy DL, Varghese A, Shia J, Vakiani E, Wu AJ, Crane CH, Gollub MJ, Garcia-Aguilar J, Saltz LB, Weiser MR. Adoption of Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer. JAMA Oncol. 2018;4:e180071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 447] [Article Influence: 63.9] [Reference Citation Analysis (1)] |
| 7. | Cercek A, Goodman KA, Hajj C, Weisberger E, Segal NH, Reidy-Lagunes DL, Stadler ZK, Wu AJ, Weiser MR, Paty PB, Guillem JG, Nash GM, Temple LK, Garcia-Aguilar J, Saltz LB. Neoadjuvant chemotherapy first, followed by chemoradiation and then surgery, in the management of locally advanced rectal cancer. J Natl Compr Canc Netw. 2014;12:513-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 165] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 8. | Gunderson LL, Sargent DJ, Tepper JE, Wolmark N, O'Connell MJ, Begovic M, Allmer C, Colangelo L, Smalley SR, Haller DG, Martenson JA, Mayer RJ, Rich TA, Ajani JA, MacDonald JS, Willett CG, Goldberg RM. Impact of T and N stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J Clin Oncol. 2004;22:1785-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 308] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 9. | Dresen RC, Peters EE, Rutten HJ, Nieuwenhuijzen GA, Demeyere TB, van den Brule AJ, Kessels AG, Beets-Tan RG, van Krieken JH, Nagtegaal ID. Local recurrence in rectal cancer can be predicted by histopathological factors. Eur J Surg Oncol. 2009;35:1071-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Chand M, Siddiqui MR, Swift I, Brown G. Systematic review of prognostic importance of extramural venous invasion in rectal cancer. World J Gastroenterol. 2016;22:1721-1726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 78] [Cited by in RCA: 74] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 11. | Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rödel C, Cervantes A, Arnold D; ESMO Guidelines Committee. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 292] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 12. | Blomqvist L, Glimelius B. The 'good', the 'bad', and the 'ugly' rectal cancers. Acta Oncol. 2008;47:5-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 13. | Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen YJ, Ciombor KK, Cohen S, Cooper HS. NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. 3th edition. 2019. Available from: http://guide.medlive.cn/guideline/19028. |
| 14. | George TJ Jr, Allegra CJ, Yothers G. Neoadjuvant Rectal (NAR) Score: a New Surrogate Endpoint in Rectal Cancer Clinical Trials. Curr Colorectal Cancer Rep. 2015;11:275-280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 122] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 15. | Yothers G, George TJ, Allegra CJ, Bosset J-F, Bujko K, Collette L, O’Connell MJ, Doyen J, Fernandez-Martos C, Seitz JF, Wolmark N. Predictive validity of NeoAdjuvant Rectal (NAR) Score and pathologic complete response (ypCR) for overall survival (OS) as surrogate endpoints in rectal cancer clinical trial. J Clin Oncol. 2016;34:3533-3533. [RCA] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | National Institutes of Health National Cancer Institute. Common Terminology Criteria for Adverse Events Version 4.0. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf. |
| 17. | Sun Y, Zhang Y, Wu X, Lin H, Lu X, Huang Y, Xu Z, Huang S, Wang X, Chi P. Prognostic significance of neoadjuvant rectal score in locally advanced rectal cancer after neoadjuvant chemoradiotherapy and construction of a prediction model. J Surg Oncol. 2018;117:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Roy A, Olsen JR, Myerson RJ, Markovina S, DeWees TA, Parikh PJ. Short-Term Endpoints for Neoadjuvant Rectal Cancer Therapy: Pathologic Complete Response or Neoadjuvant Rectal Cancer Score? Int J Radiat Oncol. 2016;96:E201. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Fokas E, Fietkau R, Hartmann A, Hohenberger W, Grützmann R, Ghadimi M, Liersch T, Ströbel P, Grabenbauer GG, Graeven U, Hofheinz RD, Köhne CH, Wittekind C, Sauer R, Kaufmann M, Hothorn T, Rödel C; German Rectal Cancer Study Group. Neoadjuvant rectal score as individual-level surrogate for disease-free survival in rectal cancer in the CAO/ARO/AIO-04 randomized phase III trial. Ann Oncol. 2018;29:1521-1527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 20. | Fokas E, Allgäuer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R, Kuhnt T, Staib L, Brunner T, Grosu AL, Schmiegel W, Jacobasch L, Weitz J, Folprecht G, Schlenska-Lange A, Flentje M, Germer CT, Grützmann R, Schwarzbach M, Paolucci V, Bechstein WO, Friede T, Ghadimi M, Hofheinz RD, Rödel C; German Rectal Cancer Study Group. Randomized Phase II Trial of Chemoradiotherapy Plus Induction or Consolidation Chemotherapy as Total Neoadjuvant Therapy for Locally Advanced Rectal Cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019;37:3212-3222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 356] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 21. | Kim SY, Joo J, Kim TW, Hong YS, Kim JE, Hwang IG, Kim BG, Lee KW, Kim JW, Oh HS, Ahn JB, Zang DY, Kim DY, Oh JH, Baek JY. A Randomized Phase 2 Trial of Consolidation Chemotherapy After Preoperative Chemoradiation Therapy Versus Chemoradiation Therapy Alone for Locally Advanced Rectal Cancer: KCSG CO 14-03. Int J Radiat Oncol Biol Phys. 2018;101:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 22. | Manthravadi S, Sun W, Saeed A, Baranda JC, Kasi A. Total neoadjuvant therapy compared with standard therapy in locally advanced rectal cancer: A systematic review and meta-analysis. J Clin Oncol. 2019;37:709-709. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Petrelli F, Trevisan F, Cabiddu M, Sgroi G, Bruschieri L, Rausa E, Ghidini M, Turati L. Total Neoadjuvant Therapy in Rectal Cancer: A Systematic Review and Meta-analysis of Treatment Outcomes. Ann Surg. 2020;271:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 245] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 24. | But-Hadzic J, Anderluh F, Brecelj E, Edhemovic I, Secerov-Ermenc A, Hudej R, Jeromen A, Kozelj M, Krebs B, Oblak I, Omejc M, Vogrin A, Velenik V. Acute Toxicity and Tumor Response in Locally Advanced Rectal Cancer After Preoperative Chemoradiation Therapy With Shortening of the Overall Treatment Time Using Intensity-Modulated Radiation Therapy With Simultaneous Integrated Boost: A Phase 2 Trial. Int J Radiat Oncol Biol Phys. 2016;96:1003-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | van der Valk MJM, Marijnen CAM, van Etten B, Dijkstra EA, Hilling DE, Kranenbarg EM, Putter H, Roodvoets AGH, Bahadoer RR, Fokstuen T, Ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes AMR, de Groot DJA, Nilsson PJ, Glimelius B, van de Velde CJH, Hospers GAP; Collaborative investigators. Compliance and tolerability of short-course radiotherapy followed by preoperative chemotherapy and surgery for high-risk rectal cancer - Results of the international randomized RAPIDO-trial. Radiother Oncol. 2020;147:75-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 144] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 26. | Hospers G, Bahadoer RR, Dijkstra EA, van Etten B, Marijnen C, Putter H, Meershoek – Klein Kranenbarg E, Roodvoets AG, Nagtegaal ID, Beets-Tan RG, Blomqvist LK, Fokstuen T, ten Tije AJ, Capdevila J, Hendriks MP, Edhemovic I, Cervantes A, Nilsson PJ, Glimelius B, et al Short-course radiotherapy followed by chemotherapy before TME in locally advanced rectal cancer: The randomized RAPIDO trial. J Clin Oncol. 2020;38:4006-4006. [RCA] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 27. | Bujko K, Bujko M. Point: short-course radiation therapy is preferable in the neoadjuvant treatment of rectal cancer. Semin Radiat Oncol. 2011;21:220-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Kerr SF, Norton S, Glynne-Jones R. Delaying surgery after neoadjuvant chemoradiotherapy for rectal cancer may reduce postoperative morbidity without compromising prognosis. Br J Surg. 2008;95:1534-1540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Tulchinsky H, Shmueli E, Figer A, Klausner JM, Rabau M. An interval >7 wk between neoadjuvant therapy and surgery improves pathologic complete response and disease-free survival in patients with locally advanced rectal cancer. Ann Surg Oncol. 2008;15:2661-2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 30. | Tuta M, Boc N, Brecelj E, Omejc M, Anderluh F, Ermenc AS, Peressutti AJ, Oblak I, Krebs B, Velenik V. Total neoadjuvant treatment of locally advanced rectal cancer with high risk factors in Slovenia. Radiol Oncol. 2019;53:465-472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Golo D, But-Hadzic J, Anderluh F, Brecelj E, Edhemovic I, Jeromen A, Omejc M, Oblak I, Secerov-Ermenc A, Velenik V. Induction chemotherapy, chemoradiotherapy and consolidation chemotherapy in preoperative treatment of rectal cancer - long-term results of phase II OIGIT-01 Trial. Radiol Oncol. 2018;52:267-274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 32. | Zaborowski A, Stakelum A, Winter DC. Systematic review of outcomes after total neoadjuvant therapy for locally advanced rectal cancer. Br J Surg. 2019;106:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 33. | Chua YJ, Barbachano Y, Cunningham D, Oates JR, Brown G, Wotherspoon A, Tait D, Massey A, Tebbutt NC, Chau I. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI-defined poor-risk rectal cancer: a phase 2 trial. Lancet Oncol. 2010;11:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 34. | Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A, Tait D, Brown G, Wotherspoon A, Gonzalez de Castro D, Chua YJ, Wong R, Barbachano Y, Oates J, Chau I. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol. 2012;30:1620-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 301] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 35. | Uehara K, Hiramatsu K, Maeda A, Sakamoto E, Inoue M, Kobayashi S, Tojima Y, Yoshioka Y, Nakayama G, Yatsuya H, Ohmiya N, Goto H, Nagino M. Neoadjuvant oxaliplatin and capecitabine and bevacizumab without radiotherapy for poor-risk rectal cancer: N-SOG 03 Phase II trial. Jpn J Clin Oncol. 2013;43:964-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 36. | Eisterer W, Piringer G, DE Vries A, Öfner D, Greil R, Tschmelitsch J, Samonigg H, Sölkner L, Gnant M, Thaler J; Austrian Breast and Colorectal Cancer Study Group. Neoadjuvant Chemotherapy with Capecitabine, Oxaliplatin and Bevacizumab Followed by Concomitant Chemoradiation and Surgical Resection in Locally Advanced Rectal Cancer with High Risk of Recurrence - A Phase II Study. Anticancer Res. 2017;37:2683-2691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Wang X, Yu Y, Meng W, Jiang D, Deng X, Wu B, Zhuang H, Wang C, Shen Y, Yang L, Zhu H, Cheng K, Zhao Y, Li Z, Qiu M, Gou H, Bi F, Xu F, Zhong R, Bai S, Wang Z, Zhou Z. Total neoadjuvant treatment (CAPOX plus radiotherapy) for patients with locally advanced rectal cancer with high risk factors: A phase 2 trial. Radiother Oncol. 2018;129:300-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 38. | Glynne-Jones R, Hall MR, Lopes A, Pearce S, Goh V, Bosompem S, Bridgewater J, Chau I, Wasan H, Moran B, Melcher L, West NP, Quirke P, Wong WL, Beare S, Hava N, Duggan M, Harrison M. BACCHUS: A randomised non-comparative phase II study of neoadjuvant chemotherapy (NACT) in patients with locally advanced rectal cancer (LARC). Heliyon. 2018;4:e00804. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 39. | Konishi T, Shinozaki E, Murofushi K, Taguchi S, Fukunaga Y, Nagayama S, Fujimoto Y, Akiyoshi T, Nagasaki T, Suenaga M, Chino A, Kawachi H, Yamamoto N, Ishikawa Y, Oguchi M, Ishizuka N, Ueno M, Yamaguchi K. Phase II Trial of Neoadjuvant Chemotherapy, Chemoradiotherapy, and Laparoscopic Surgery with Selective Lateral Node Dissection for Poor-Risk Low Rectal Cancer. Ann Surg Oncol. 2019;26:2507-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 40. | Kim JS, Hur H, Kim NK, Kim YW, Cho SY, Kim JY, Min BS, Ahn JB, Keum KC, Kim H, Sohn SK, Cho CH. Oncologic outcomes after radical surgery following preoperative chemoradiotherapy for locally advanced lower rectal cancer: abdominoperineal resection vs sphincter-preserving procedure. Ann Surg Oncol. 2009;16:1266-1273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |