Published online Feb 15, 2021. doi: 10.4251/wjgo.v13.i2.109
Peer-review started: October 27, 2020
First decision: November 18, 2020
Revised: November 30, 2020
Accepted: December 11, 2020
Article in press: December 11, 2020
Published online: February 15, 2021
Processing time: 97 Days and 1.1 Hours
The results of the heat irrigating effect of radiofrequency ablation (RFA) are uncertain, and the accurate impact of the heat irrigating effect on regional liver tissue is unknown due to a lack of control experiments.
The aim of this study was to determine the influence of the heat irrigating effect of RFA on regional liver tissue in Bama miniature pigs.
Eight Bama miniature pigs were randomly divided into the observation group (group A) and the control group (group B), with 4 pigs/group. An RFA electrode needle was implanted near the hepatic segment vasculature (3-5 mm from the hepatic segment portal vein) under ultrasound guidance in group A. Similarly, an RFA electrode needle was implanted far from the hepatic segment vasculature (8-10 mm from the hepatic segment portal vein) in group B. The left internal lobe and right medial lobe were chosen as RFA sites in each pig. RFA was performed at the left internal lobe on day one in each pig, and at the right medial lobe 7 d later. Each RFA lasted 12 min. The general status of the pigs and serious complications were observed during the perioperative period. The pigs were sacrificed and the livers were removed immediately after RFA on the eighth day. The samples were roughly observed. Hematoxylin-eosin and Ki67 staining, as well as TUNEL detection, were performed on the tissue sections.
All 8 animals successfully underwent ultrasound-guided RFA. No serious complications, such as massive hemorrhage, biliary fistula, severe pleural effusion, pneumothorax, peripheral organ failure, or renal failure occurred in any of the animals during the perioperative period. The RFA coagulative necrosis lesion was spherical and the surrounding liver tissue showed an inflammatory response. The difference in the Suzuki score of the liver tissue surrounding the ablated portal vein, and its distal area between groups A and B, was statistically significant (P < 0.05). More apoptotic cells were seen in liver tissue surrounding the ablated portal vein and its distal area in group A, while fewer apoptotic cells in the same area were seen in group B. The difference in the apoptotic index of the above area between group A and group B was statistically significant (P < 0.05). Cells staining positive for Ki67 were observed in liver tissue at the left internal lobe around the ablated portal vein and its distal area in group A. No Ki67 staining positive cells were observed in other tissue sections. The difference in the Ki67 staining positive index in the above area was statistically significant (P < 0.05) between group A and group B.
Changes as a result of thermal damage occur in liver tissue around the ablated portal vein and its distal area due to the heat irrigating effect when the RFA electrode tip is close to (< 5 mm) the portal vein.
Core Tip: This is a basic experimental research paper on the heat irrigating effect of radiofrequency ablation (RFA). To determine the influence of the heat irrigating effect of RFA on the regional liver tissue in Bama miniature pigs, we designed a series of research experiments. We found changes due to thermal damage in the liver tissue around the ablated portal vein and its distal area as a result of the heat irrigating effect when the RFA electrode tip was close to (< 5 mm) the portal vein.
- Citation: Feng J, Wang S, Jiang K. Influence of the heat irrigating effect of radiofrequency ablation on regional liver tissue in Bama miniature pigs. World J Gastrointest Oncol 2021; 13(2): 109-118
- URL: https://www.wjgnet.com/1948-5204/full/v13/i2/109.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i2.109
Before the widespread application of radiofrequency ablation (RFA), it was found that in vivo testing had a smaller range of coagulative necrosis than that of in vitro testing under the same conditions. Therefore, it was speculated that the blood flowing along the ablation area takes some of heat the away, which was called the heat sink effect[1,2]. Jiang et al[3] observed that more postoperative complications occurred in patients if the tumor was within 5 mm from a large blood vessel (3 mm) during RFA of liver tumors. Therefore, we proposed the concept of the heat irrigating effect, which is, when a tumor close to the main blood vessel is ablated, some of the heat generated during RFA flows into the corresponding vascular perfusion area along the main blood vessel due to the heat sink effect. This causes thermal damage to normal liver tissue, especially the tissue close to the downstream arteries and portal veins. At the same time, the heat that is taken away by the hepatic vein causes the following effects during certain RFAs close to the hepatic vein, including a rise in body temperature, increase in metabolism, and an increase in body fluid consumption, among other effects, which is also called the heat irrigating effect or heat river-flow effect[2]. Jiang et al[4] used Bama miniature pigs to establish an animal model of RFA in order to study the heat irrigating effect, and initially observed the existence of this effect. The results were uncertain, and the accurate impact of the heat irrigating effect on regional liver tissue was unknown due to a lack of control experiments. Therefore, we used strictly designed and controlled animal experiments to observe the general status of 2 groups of animals after RFA. We also used hematoxylin-eosin (HE) staining, TUNEL detection, and Ki67 staining to assess the specific impact of the heat irrigating effect on regional liver tissue.
The experimental animals were selected with reference to the previously established RFA animal model using Bama miniature pigs[4]. Eight healthy male and female Bama miniature pigs weighing 20 to 25 kg, provided by the Experimental Animal Center of the General Hospital of the People's Liberation Army, were selected. These Bama miniature pigs were randomly divided into the observation group and the control group, with 4 pigs in each group. The RFA electrode was implanted near the hepatic blood vessels (the RFA electrode probe tip was 3 to 5 mm away from the hepatic portal vein) under ultrasound guidance for each RFA in the observation group, which was the near-blood-vessel group. The RFA electrode was implanted far away from the hepatic blood vessels (the RFA electrode probe tip was 8 to 10 mm away from the hepatic portal vein), in the same way, in the control group, which was the far-blood-vessel group. The operational procedures for the animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of PLA General Hospital.
Anesthesia procedure: Anesthesia was executed in accordance with the animal model of RFA established previously[4]. All animals were under adaptive breeding for 1 wk after entering the cage, and were inhibited from drinking water 24 h before surgery to ensure that the gastrointestinal tract was empty during surgery. Ten mg/kg ketamine and 1 mg atropine sulfate were intramuscularly injected for induction prior to anesthesia administration. Anesthesia was maintained with 0.5 mg/kg of 1% thiopental sodium. The ventilator was connected after tracheal intubation, and the heart rate and blood oxygen saturation were monitored.
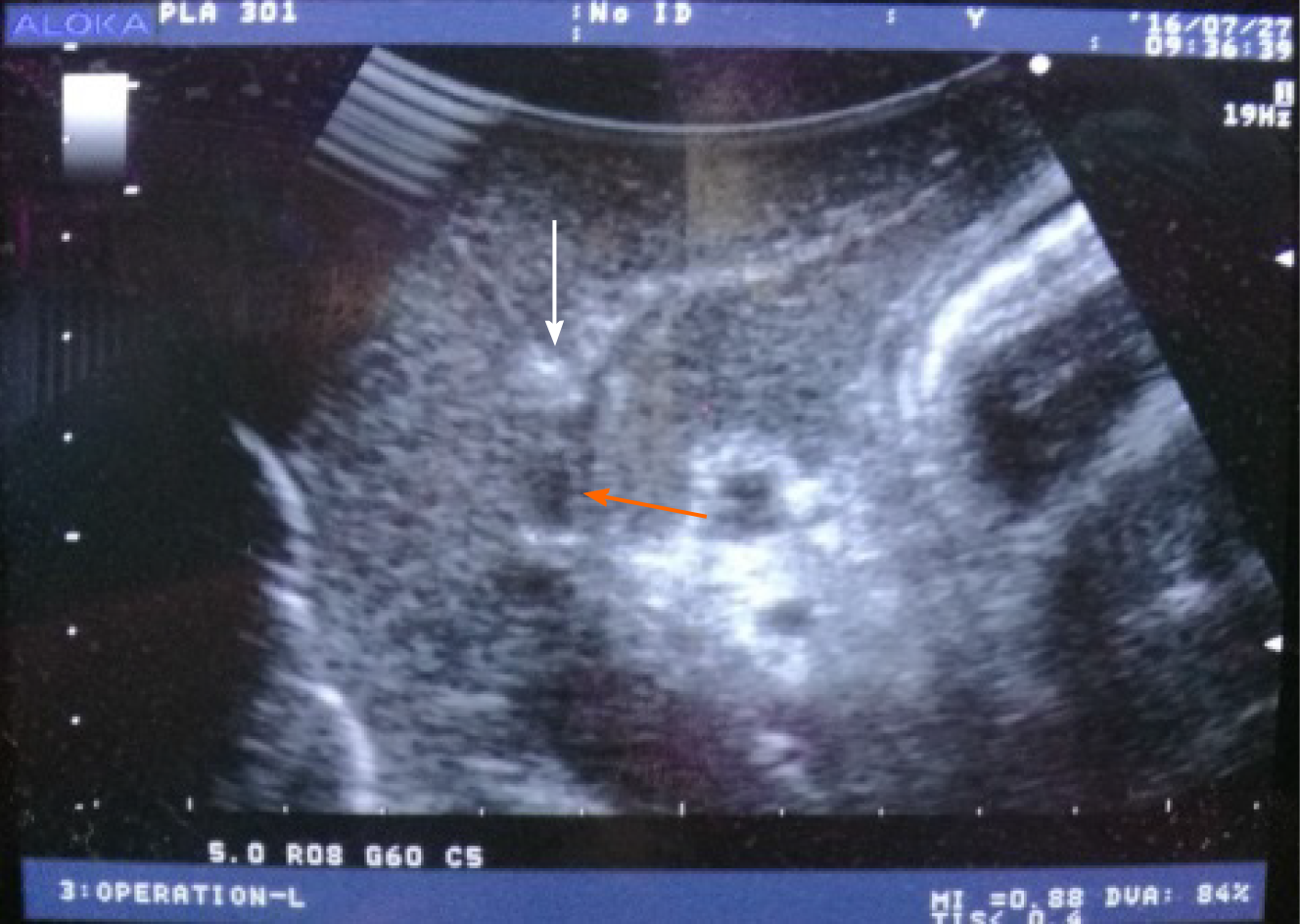
Surgery: Bama miniature pigs were placed in the supine position after anesthesia, and the abdomen was cut layer by layer with a median straight incision in the upper abdomen after draping and disinfection. After exploration, the incision was opened with a slit retractor to fully expose the liver. Intraoperative ultrasound was performed to detect the liver. Two target liver segments were selected in each animal, namely the left outer lobe and the right inner lobe. The left outer lobe was ablated by a single radiofrequency electrode probe under ultrasound guidance on the first day. The right inner lobe was ablated seven days later by the same method. The RFA electrode was implanted at the site 3-5 mm from the hepatic portal vein under ultrasound guidance for each RFA in group A, and 8-10 mm from the hepatic portal vein in group B. Figure 1 shows that under ultrasound guidance, the cold-circulation RFA electrodes were successfully and accurately placed at the target site. Potential damage to the bile duct or blood vessel was avoided during implantation of the RFA electrode probe. The RFA system was then connected. The cooling distilled water was circulated to maintain the electrode probe temperature at approximately 20 °C. Maximum power was used to ablate each site for 12 min, and attention was paid to isolating the surrounding organs during RFA. The abdomen was carefully closed layer by layer after RFA on the first day.
Postoperative observation and nursing: The animals were observed for survival, feeding, and activity after surgery. The animals were intramuscularly injected with penicillin G (0.96 g) sodium chloride solution before and the second day after surgery.
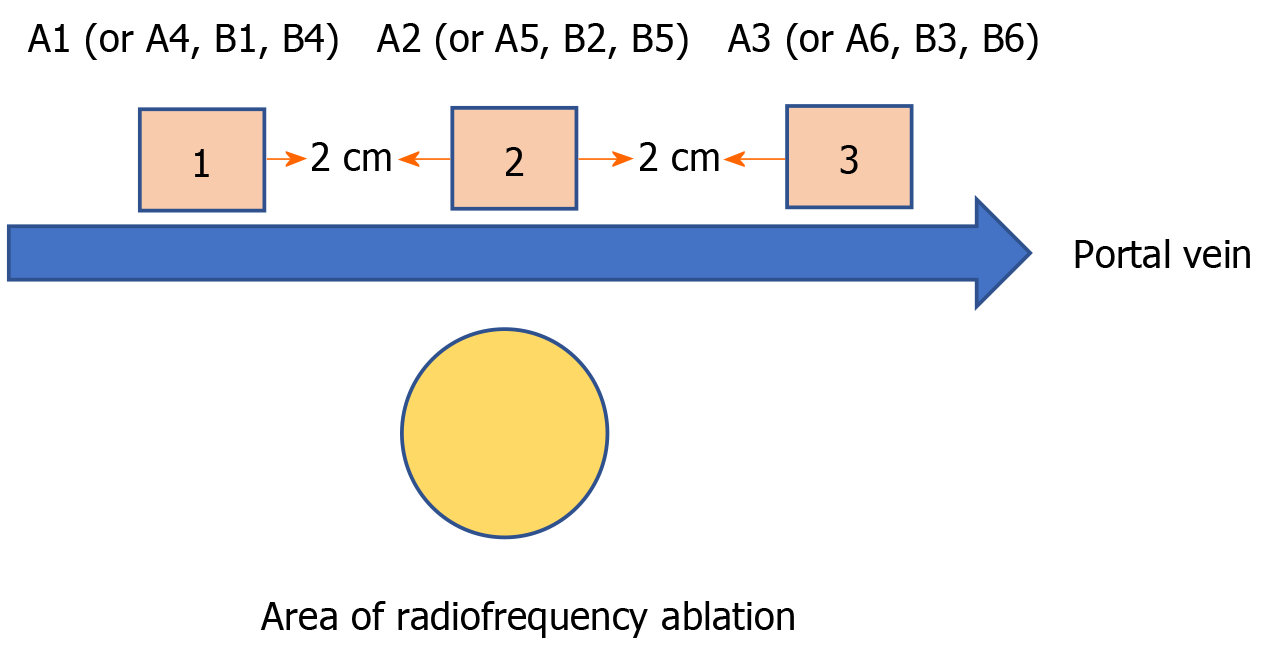
Animal sacrifice and specimen collection: The experimental animals were sacrificed by femoral artery bleeding on the eighth day after the end of ablation. The liver was completely removed and thoroughly washed with normal saline. Figure 2 shows how the liver specimen was cut along the long axis of the hepatic portal vein. On the first day, group A specimens 1, 2, and 3 were named A1, A2, and A3, respectively, while on the eighth day, group A specimens 1, 2, and 3 were named A4, A5, and A6, respectively. Similarly, the specimens obtained from group B were named B1, B2, B3, B4, B5, and B6. The specimens were fixed with 10% formalin solution after they were marked.
Intraoperative and postoperative animal survival, monitoring of heart rate and blood oxygen saturation during surgery, intraoperative and postoperative massive hemorrhage, biliary fistula, severe pleural effusion, pneumothorax, peripheral organ damage, and renal failure were assessed.
Gross observation of the liver: We observed the position, shape, and size of the damaged area around the RFA lesion.
HE staining: The liver tissue was embedded in paraffin, fixed, sectioned, and HE stained. Ten fields were randomly selected for each section and scored according to the Suzuki scoring standard under high power field (400 ×)[5].
TUNEL detection: TUNEL detection was performed on liver tissue paraffin sections according to the manufacturer’s instructions (In Situ Cell Death Detection Kit, POD Roche Version 14). Cells with the nucleus stained brownish yellow were positive cells. Ten fields were randomly selected for each section under high power field (400 ×). The percentage of positive cells in the total number of hepatocytes was calculated as the apoptosis index.
Ki67 staining: Ki67 staining of liver tissue paraffin sections was performed by peroxidase anti-peroxidase (PAP) immunohistochemistry. Cells with the nucleus stained brownish yellow were positive cells. Ten fields were randomly selected for each section under high power field (400 ×). The percentage of positive cells in the total number of hepatocytes was calculated as the positive rate of Ki67.
Data were statistically described using mean ± standard deviation (± SD). Suzuki scores of HE sections were analyzed using the rank sum test. The independent samples t-test was used for statistical analysis when the results of the apoptosis index and Ki67 positive index were compared between group A and B. All results were statistically analyzed using SPSS 17.0 (Statistical Package for the Social Sciences). P < 0.05 indicates that differences were statistically significant.
Intraoperative anesthesia was satisfactory in all animals, and vital signs were stable. No animals died during the perioperative period. Intraoperative or postoperative massive hemorrhage, biliary fistula, severe pleural effusion, pneumothorax, peripheral organ damage, or renal failure were not observed. One of the Bama miniature pigs developed an incision infection after the first surgery.
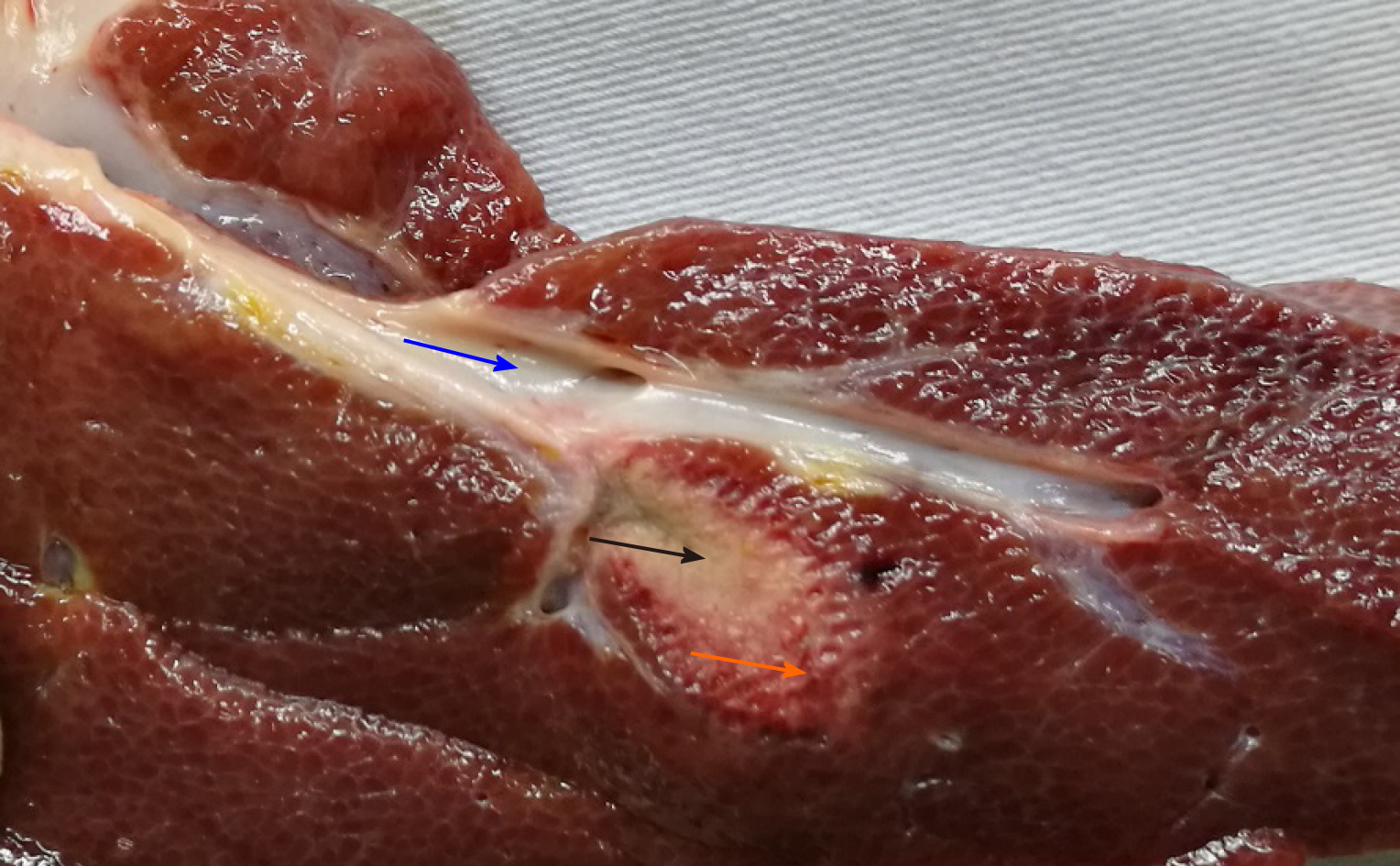
The damaged areas following RFA were similar in shape and were ellipsoidal. The central necrotic area was grayish and dry. The outer area was the RFA necrosis area-normal liver tissue transition area, the inflammatory response zone, which was brick red in color. The normal liver tissue around the RFA site was rosy and shiny. Both group A and B had good patency of the portal vein in the hepatic segment around the RFA site (Figure 3).
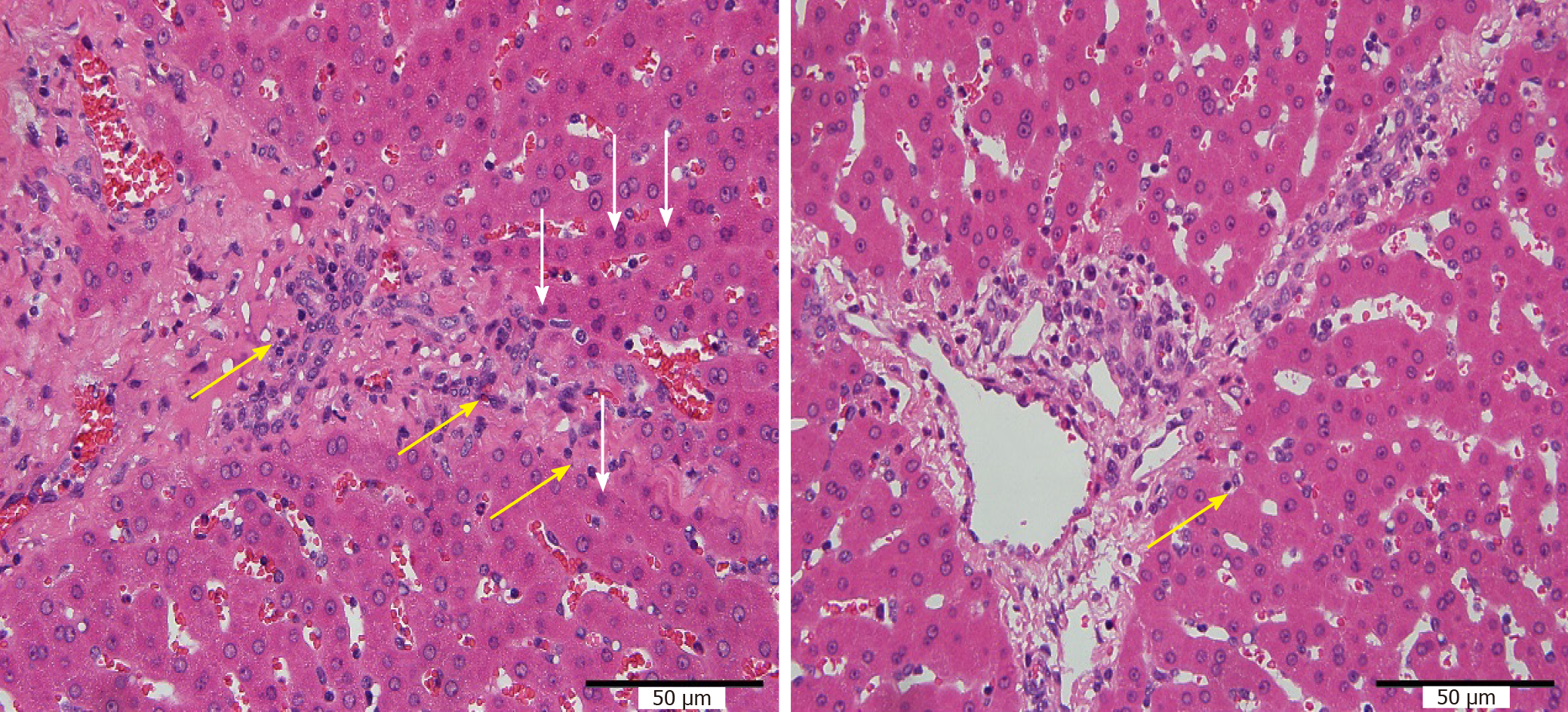
Coagulation necrosis occurred in liver tissue after RFA, the inflammatory response zone in the peripheral transition zone showed inflammatory cell infiltration and hyperemia changes, and gradually transitioned to normal liver tissue. The range of hepatic necrosis in group B was slightly wider than that in group A. More inflammatory cell infiltration was seen in the liver tissue around the portal area in group A than in group B as the degree of cell vacuolization was slightly more serious (Figure 4).
As shown in Table 1, there was a significant difference in HE staining Suzuki scores of the liver tissue around the distal portal vein beyond the RFA site, and no significant difference in HE staining Suzuki scores of the liver tissue around the proximal portal vein beyond the RFA site in the 2 groups.
| Group | Suzuki scores | Group | Suzuki scores | P values |
| A1 | 1.4 ± 0.70 | B1 | 1.5 ± 0.82 | 0.702 |
| A2 | 2.3 ± 0.483 | B2 | 1.7 ± 0.483 | 0.017 |
| A3 | 2.5 ± 0.53 | B3 | 1.4 ± 0.85 | 0.002 |
| A4 | 1.0 ± 0.67 | B4 | 1.4 ± 0.70 | 0.185 |
| A5 | 2.9 ± 0.57 | B5 | 2.2 ± 0.63 | 0.021 |
| A6 | 3.1 ± 0.74 | B6 | 2.1 ± 0.73 | 0.012 |
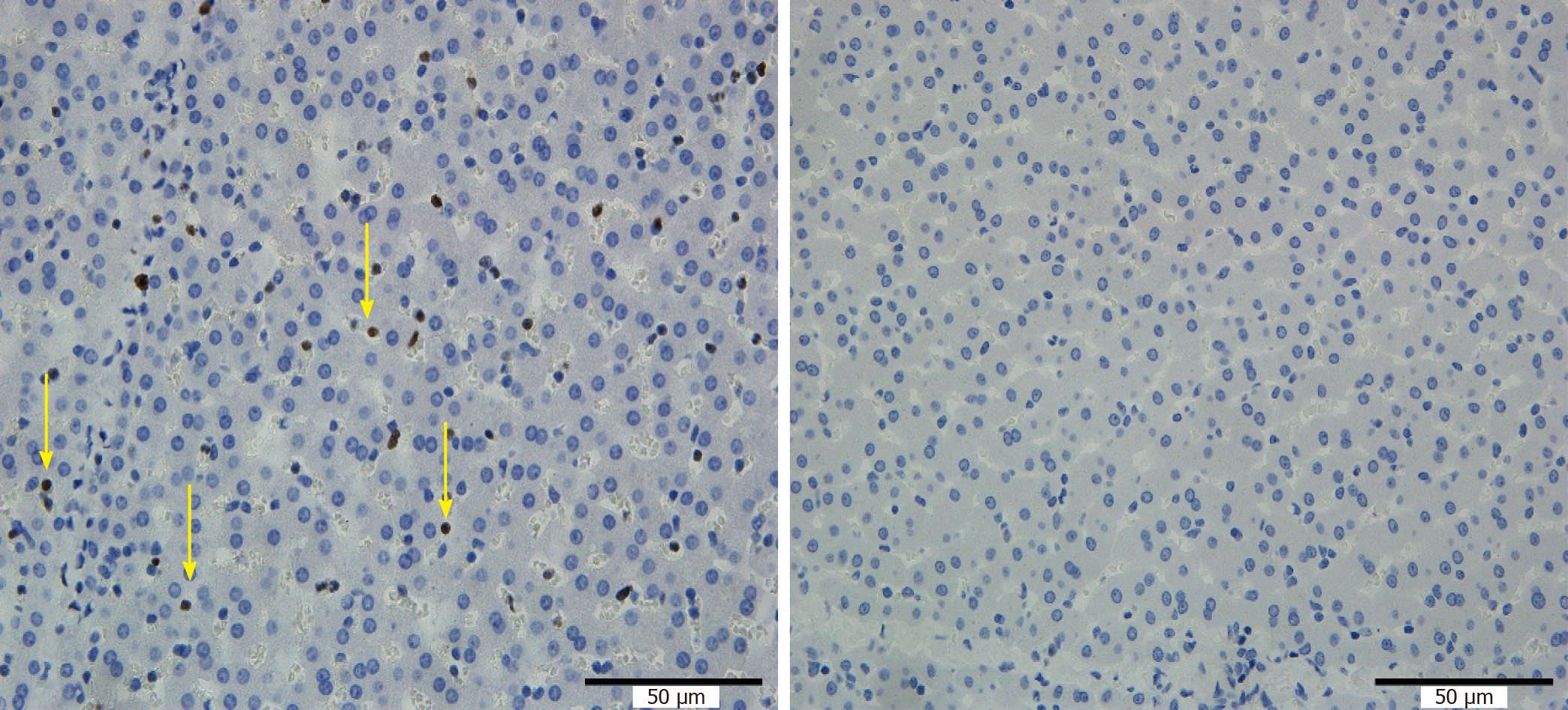
The hepatocyte apoptosis index around the RFA site and the distal portal vein in group A was higher than that in group B (Figure 5). Changes, such as more cytoplasmic condensation, vacuolization, and apoptosis bodies, were observed by HE staining of comparable sections, which was consistent with the results of the TUNEL detection microscopic observations.
As shown in Table 2, there was a statistically significant difference in the hepatocyte apoptosis index around the RFA site and the distal portal vein, and no significant difference in the hepatocyte apoptosis index around the proximal portal vein between the 2 groups.
| Group | Apoptosis indices (%) | Group | Apoptosis indices (%) | P values |
| A1 | 3.2 ± 1.03 | B1 | 3.1 ± 1.22 | 0.845 |
| A2 | 5.8 ± 0.83 | B2 | 2.9 ± 1.05 | < 0.01 |
| A3 | 14.0 ± 3.39 | B3 | 2.9 ± 0.65 | < 0.01 |
| A4 | 3.0 ± 0.97 | B4 | 3.0 ± 0.54 | 0.867 |
| A5 | 9.7 ± 1.12 | B5 | 3.1 ± 0.89 | < 0.01 |
| A6 | 8.0 ± 1.22 | B6 | 4.5 ± 0.83 | < 0.01 |
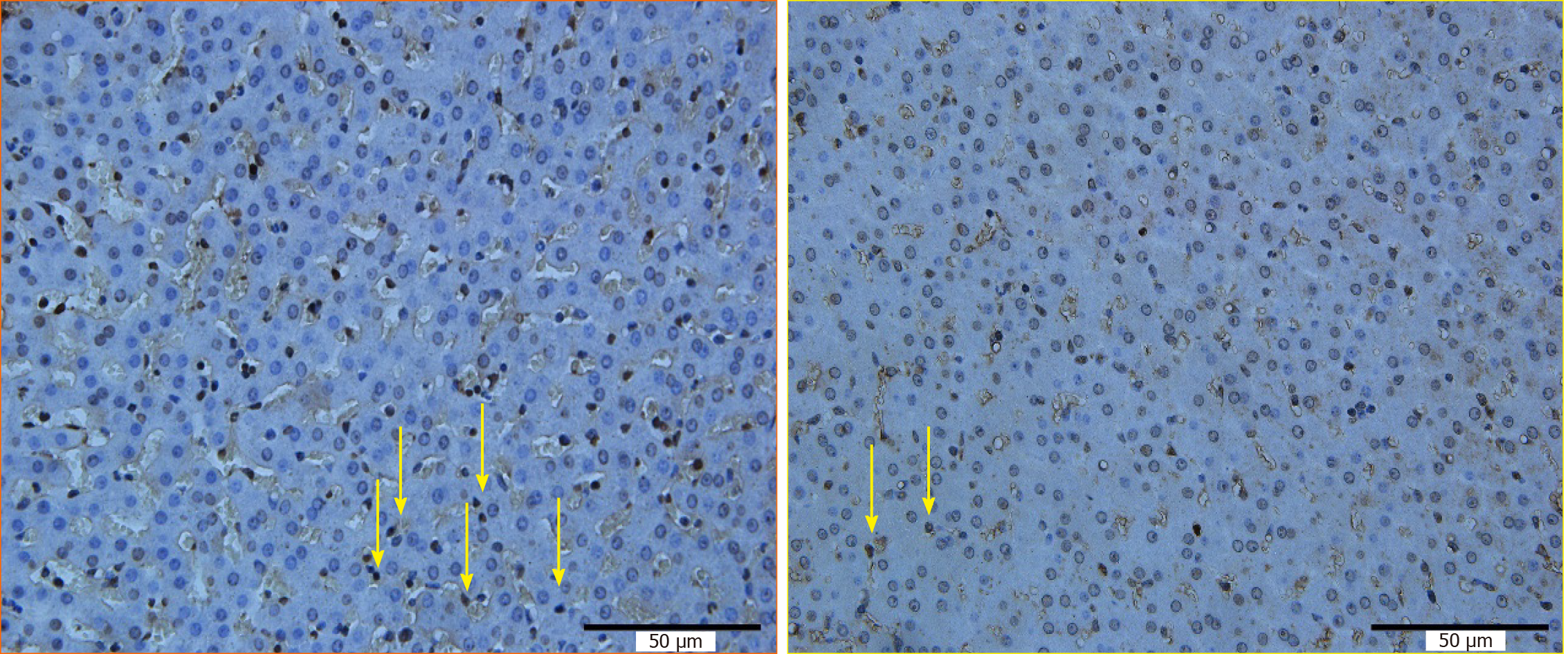
Ki67 staining was positive in specimens A2, A3, A5 and A6, but negative in the other specimens (Figure 6).
As shown in Table 3, the positive rate of Ki67 was not 0 in specimens A2, A3, A5, or A6, and was 0 in the other specimens, suggesting that there was an obvious injury-proliferation reaction in the liver tissue around the portal vein at the RFA site and the distal site in group A.
| Group positions | Ki67 positive rates (%) |
| A2 | 3.3 ± 0.64 |
| A3 | 5.34 ± 0.67 |
| A5 | 3.2 ± 1.46 |
| A6 | 4.96 ± 1.17 |
| A1, A6, B1-6 | 0 |
The heat irrigating effect of RFA is defined according to the heat sink effect, as discussed in the introduction. They are the description of the same phenomenon from different aspects and are inseparable. The heat sink effect means that a large blood vessel removes the heat of RFA when the procedure is performed close to the vessel, thus reducing the damaged area of ablation, leading to more incomplete ablation of liver cancer in clinical treatment. The heat irrigating effect implies that the heat removed by the large blood vessel affects the liver tissue and even the body, resulting in changes such as liver tissue damage and an increase in body temperature. The heat sink effect can be indirectly observed when there is a short distance (generally < 5-8 mm) between the RFA probe and the large blood vessel. Similarly, the heat irrigating effect may also be observed when the RFA probe is close enough to the large vessel. Therefore, we set up two groups with different distances between the RFA probe and the large vessel, including the observation group (3-5 mm) and the control group (8-10 mm). Specimen 1 (or 4) was located near the proximal portal vein, while specimen 3 (or 6) was located around the distal portal vein. Therefore, we were able to compare the regional liver tissue changes caused by the heat irrigating effect. This experiment was performed based on the Bama miniature pig ablation model[4] established previously as well as relevant anatomical studies. Thus, it had the advantages of good operability and reproducibility.
The effect of RFA on liver tissue is multifaceted. RFA, on the one hand, can cause damage to liver tissue in the form of cell necrosis and apoptosis[6-12]. On the other hand, RFA can cause changes in the immune status of the liver, resulting in a series of secondary changes, including an inflammatory reaction and proliferative effects, etc.[13-17]. In this experiment, there were significant differences in HE staining Suzuki scores in liver tissue around the distal portal vein between the two groups, but there were no significant differences in HE staining Suzuki scores in liver tissue around the proximal portal vein, indicating that when ablation was performed near the main hepatic portal vein, obvious damage to the distal portal vein occurred due to the heat irrigating effect. The range of liver tissue revealed by the TUNEL assay was larger than that of HE staining[9] after RFA, which increased the sensitivity for detecting injury following RFA. Ki67 is a nuclear antigen that responds to cell proliferation and has a function closely related to mitosis. Liver regeneration is a secondary reaction after liver injury. Therefore, Ki67 in this experiment also reflects the effect of RFA on liver tissue from another aspect. TUNEL detection and the Ki67 staining results were consistent with the results of the HE staining Suzuki scores, which further confirms the above viewpoint. The typical necrotic area-transition area-normal liver tissues seen on gross examination of specimens and HE staining after RFA are consistent with the description in the literature[10] for RFA carried out far from the hepatic portal vein. Following RFA near the hepatic portal vein, the liver tissue in the portal vein region, especially the liver tissue around the portal vein, showed apoptosis and the injury-proliferation reaction, which were in addition to the above general status. We studied the impact of the heat irrigating effect near the portal vein in this experiment. Similarly, the same conclusion was drawn when RFA was performed near the hepatic artery.
The scope of resection of liver cancer surgery needs to be larger than that of the tumor in order to remove as much tumor as possible due to tiny satellite foci around the tumor, thus leaving a "safe range" in the clinical treatment of liver cancer. Similarly, to ensure that RFA completely kills the tumor, the range of RFA needs to exceed the range of the tumor. It is easier to achieve a range of RFA larger than the tumor range, in liver cancer less than 3 cm. However, it often requires using a cluster radiofrequency probe, applying the probe multiple times, increasing the RFA power, and extending the RFA time to achieve a range of RFA larger than the tumor range, for liver cancer with a diameter greater than 3 cm. This inevitably increases the difficulty of treatment and the probability of related complications such as thermal injury to normal liver tissue and surrounding normal organs. Therefore, the safety and effectiveness of RFA are also intrinsically linked. The power required for RFA is low and the duration is short, when RFA is performed on tumors close to blood vessels and the tumor volume is small; thus, the injury caused by the heat irrigating effect on regional liver tissue is small. The power required for RFA is high and the duration is long to achieve complete inactivation of the tumor; thus, the injury caused by the heat irrigating effect on the regional liver tissue is greater, especially when the tumor is close to the main blood vessel or even the first hepatic portal vessel when the tumor is large. Special attention should be paid to complications such as liver dysfunction or even liver failure caused by the heat irrigating effect as there is a wide range of liver tissue in the corresponding vascular area.
In summary, the heat irrigating effect during RFA causes thermal damage to liver tissue and the corresponding portal vein, which can be manifested as hepatocyte necrosis, apoptosis, etc., when the RFA electrode probe is close to the portal vein (< 5 mm). This has important guiding significance for the RFA of liver lesions. The main limitations in this study are that the observation time in the animal experiments was too short, and the response of liver tissue in the late ablation stage was not studied.
Changes due to thermal damage occur in the liver tissue around the ablated portal vein and its distal area as a result of the heat irrigating effect when the RFA electrode tip is close to (< 5 mm) the portal vein.
The results of the heat irrigating effect of radiofrequency ablation (RFA) are uncertain, and the accurate impact of the heat irrigating effect on regional liver tissue is unknown due to a lack of control experiments.
We carried out strictly designed and controlled animal experiments to determine the general status of 2 groups of Bama miniature pigs after RFA.
We assessed the influence of the heat irrigating effect of RFA on the regional liver tissue of Bama miniature pigs.
Eight Bama miniature pigs were randomly divided into the observation group (group A) and the control group (group B), with 4 pigs/group. An RFA electrode needle was implanted near the hepatic segment vasculature (3-5 mm far from the hepatic segment portal vein) under ultrasound guidance in group A. Similarly, an RFA electrode needle was implanted far from the hepatic segment vasculature (8-10 mm from the hepatic segment portal vein) in group B. The left internal lobe and right medial lobe were chosen as RFA sites in each pig. RFA was performed at the left internal lobe on one day, and then at the right medial lobe 7 d later. Each RFA lasted 12 min. The general status and serious complications in these animals were observed during the perioperative period. The pigs were sacrificed and the livers were removed immediately after RFA on the eighth day. The samples were roughly observed. Hematoxylin-eosin and Ki67 staining, as well as TUNEL detection, were performed on the tissue sections.
All 8 animals successfully underwent ultrasound-guided RFA. No serious complications, such as massive hemorrhage, biliary fistula, severe pleural effusion, pneumothorax, peripheral organ failure, or renal failure occurred in any of the animals during the perioperative period. The RFA coagulative necrosis lesion was spherical and the surrounding liver tissue showed an inflammatory response. The difference in the Suzuki score of the liver tissue surrounding the ablated portal vein, and its distal area between groups A and B, was statistically significant (P < 0.05). More apoptotic cells were seen in liver tissue surrounding the ablated portal vein and its distal area in group A, while fewer apoptotic cells were observed in the same area in group B. The difference in the apoptotic index of the above area between group A and group B was statistically significant (P < 0.05). Cells staining positive for Ki67 were observed in liver tissue at the left internal lobe around the ablated portal vein and its distal area in group A, while no Ki67 staining positive cells were seen in other tissue sections. The difference in the Ki67 staining positive index of the above area between group A and group B was statistically significant (P < 0.05).
Changes due to thermal damage occurred in the liver tissue around the ablated portal vein and its distal area as a result of the heat irrigating effect when the RFA electrode tip was close to (< 5 mm) the portal vein.
When the tip of the RFA electrode is close to the portal vein (5 mm), the thermal irrigation effect during RFA causes thermal injury to liver tissue and the corresponding portal vein drainage area.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arredondo M, Ismail M, Martelotto S S-Editor: Gao CC L-Editor: Webster JR P-Editor: Li JH
| 1. | Goldberg SN, Gazelle GS, Halpern EF, Rittman WJ, Mueller PR, Rosenthal DI. Radiofrequency tissue ablation: importance of local temperature along the electrode tip exposure in determining lesion shape and size. Acad Radiol. 1996;3:212-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 237] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 2. | Chen R, Lu F, Wu F, Jiang T, Xie L, Kong D. An analytical solution for temperature distributions in hepatic radiofrequency ablation incorporating the heat-sink effect of large vessels. Phys Med Biol. 2018;63:235026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Jiang K, Dong J, Zhang W, Liu Y, Su M, Zhao X, Wang J, Yao M, Huang Z. Effect of one-off complete tumor radiofrequency ablation on liver function and postoperative complication in small hepatocellular carcinoma. Eur J Surg Oncol. 2014;40:576-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Jiang K, Chen J, Liu Y, Liu J, Liu A, Dong J, Huang Z. Heat-Irrigate Effect' of Radiofrequency Ablation on Relevant Regional Hepatocyte in Living Swine Liver-Initial Study on Pathology. Cell Biochem Biophys. 2015;72:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Suzuki S, Toledo-Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 699] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 6. | Aref MH, Aboughaleb IH, El-Sharkawy YH. Tissue characterization utilizing hyperspectral imaging for liver thermal ablation. Photodiagnosis Photodyn Ther. 2020;31:101899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Itoh T, Orba Y, Takei H, Ishida Y, Saitoh M, Nakamura H, Meguro T, Horita S, Fujita M, Nagashima K. Immunohistochemical detection of hepatocellular carcinoma in the setting of ongoing necrosis after radiofrequency ablation. Mod Pathol. 2002;15:110-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Song KD, Lee MW, Rhim H, Kang TW, Cha DI, Yang J. Chronological changes of radiofrequency ablation zone in rabbit liver: an in vivo correlation between gross pathology and histopathology. Br J Radiol. 2017;90:20160361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Netto GJ, Altrabulsi B, Katabi N, Martin P, Burt K, Levy M, Sanchez E, Watkins DL, Jennings L, Klintmalm G, Goldstein R. Radio-frequency ablation of hepatocellular carcinoma before liver transplantation: a histologic and 'TUNEL' study. Liver Int. 2006;26:746-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Bale R, Schullian P, Eberle G, Putzer D, Zoller H, Schneeberger S, Manzl C, Moser P, Oberhuber G. Stereotactic Radiofrequency Ablation of Hepatocellular Carcinoma: a Histopathological Study in Explanted Livers. Hepatology. 2019;70:840-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 59] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 11. | Cornelis FH, Durack JC, Kimm SY, Wimmer T, Coleman JA, Solomon SB, Srimathveeravalli G. A Comparative Study of Ablation Boundary Sharpness After Percutaneous Radiofrequency, Cryo-, Microwave, and Irreversible Electroporation Ablation in Normal Swine Liver and Kidneys. Cardiovasc Intervent Radiol. 2017;40:1600-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Stadlbauer V, Lang-Olip I, Leber B, Mayrhauser U, Koestenbauer S, Tawdrous M, Moche M, Sereinigg M, Seider D, Iberer F, Wiederstein-Grasser I, Portugaller RH, Stiegler P. Immunohistochemical and radiological characterization of wound healing in porcine liver after radiofrequency ablation. Histol Histopathol. 2016;31:115-129. [PubMed] |
| 13. | Kim D, Erinjeri JP. Postablation Immune Microenvironment: Synergy between Interventional Oncology and Immuno-oncology. Semin Intervent Radiol. 2019;36:334-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Rozenblum N, Zeira E, Bulvik B, Gourevitch S, Yotvat H, Galun E, Goldberg SN. Radiofrequency Ablation: Inflammatory Changes in the Periablative Zone Can Induce Global Organ Effects, including Liver Regeneration. Radiology. 2015;276:416-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Kumar G, Goldberg SN, Wang Y, Velez E, Gourevitch S, Galun E, Ahmed M. Hepatic radiofrequency ablation: markedly reduced systemic effects by modulating periablational inflammation via cyclooxygenase-2 inhibition. Eur Radiol. 2017;27:1238-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Ypsilantis P, Lambropoulou M, Evagellou A, Papadopoulos N, Simopoulos C. Immune and Inflammatory Responses of the Intestinal Mucosa following Extended Liver Radiofrequency Ablation. Gastroenterol Res Pract. 2017;2017:3450635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Dumolard L, Ghelfi J, Roth G, Decaens T, Macek Jilkova Z. Percutaneous Ablation-Induced Immunomodulation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |