Published online Oct 15, 2021. doi: 10.4251/wjgo.v13.i10.1506
Peer-review started: February 23, 2021
First decision: April 19, 2021
Revised: April 22, 2021
Accepted: July 21, 2021
Article in press: July 21, 2021
Published online: October 15, 2021
Processing time: 216 Days and 0.8 Hours
Tubular adenocarcinoma of the colon, which originates from the epithelium of the glands, is a major health concern worldwide. However, it is difficult to detect at an early stage. The lack of biomarkers is a main barrier to the diagnosis and treatment of tubular adenocarcinoma. Neutrophil gelatinase-associated lipocalin (NGAL) is a secreted protein that induces the expression of matrix metalloproteinase-9 (MMP-9) and is involved in various tumors. NGAL and MMP-9 have been reported to be associated with tumorigenesis and development. They may have potential as biomarkers for diagnosis of tubular adenocarcinoma of the colon.
To determine whether NGAL and MMP-9 can be used as potential biomarkers to indicate the progression of tubular adenocarcinoma of the colon.
Samples were collected from surgically excised tissue from various patients. The content of pro-gastrin-releasing peptide (pro-GRP) in the serum was measured by an electrochemiluminescence immunoassay. The expression patterns of NGAL and MMP-9 and the relationship between NGAL and MMP-9 were examined by quantitative real-time PCR, Western blotting and immunohistochemical analysis.
In this study, we found that NGAL and MMP-9 can be used as biomarkers for the detection of tubular adenocarcinoma of the colon and that their combination improved diagnostic accuracy. By analyzing the expression of NGAL in tubular adenocarcinoma at different levels, we found that NGAL expression was significantly upregulated in primary tubular adenocarcinoma tissues compared with normal tissues. The upregulation of NGAL expression was strongly correlated with both the degree of differentiation and the disease stage (I–III), indicating that NGAL could serve as a diagnostic biomarker for tubular adenocarcinoma. When using NGAL as a biomarker for diagnosis, the accuracy was similar to that achieved with the widely used biomarker pro-GRP, suggesting that NGAL is reliable. Moreover, the expression of MMP-9 was also strongly correlated with the differentiation stage, demonstrating that MMP-9 could be used as a biomarker to indicate the progression of tubular adenocarcinoma of the colon. More importantly, the combination of NGAL and MMP-9 produced a more accurate diagnosis of tubular adenocarcinoma, and these results were further confirmed by immunohistochemical analysis of tissue sections.
Our study demonstrated that both NGAL and MMP-9 can be used as biomarkers for the diagnosis of colon tubular adenocarcinoma and that the results could be further improved by combining them.
Core Tip: Neutrophil gelatinase-associated lipocalin (NGAL) is a secreted protein, which modulates the expression of matrix metalloproteinase-9 (MMP-9) and is present in various cancers. However, it has not been determined whether NGAL or MMP-9 can be used as biomarkers in the diagnosis of tubular adenocarcinoma of the colon. In the present study, we demonstrated that both NGAL and MMP-9 could be used as biomarkers for diagnosis of tubular adenocarcinoma of the colon. By using them together, diagnositic accuracy can be further improved.
- Citation: Yuan JH, Xie LS, Zhu YH, Wang XH, Zhang YJ, Wang XJ. Combination of neutrophil gelatinase-associated lipocalin and matrix metalloproteinase-9 are biomarkers for the detection of colon tubular adenocarcinoma . World J Gastrointest Oncol 2021; 13(10): 1506-1517
- URL: https://www.wjgnet.com/1948-5204/full/v13/i10/1506.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v13.i10.1506
Colorectal cancer (CRC) is the third most common cancer worldwide with 1.36 million people diagnosed in 2012, which contributed to 9.7% of cancer cases[1]. In China, CRC is the fourth most common malignant cancer, and has increased rapidly, especially in developing regions[2,3]. CRC is caused by numerous factors, which are associated with heritability, lifestyle, chronic inflammation and so on[4-6]. Although early stage I-II CRC can be cured via surgical excision, advanced stage III-IV CRC is usually lethal and incurable[7,8]. Most CRC cases are tubular adenocarcinomas, which comprise approximately 95%, and are characterized by peritoneal dissemination and infiltrative growth[9]. Early diagnosis of colorectal adenocarcinoma is obviously important in the treatment of primary or recurrent colorectal adenocarcinoma.
Advances in the understanding of colorectal carcinogenesis offer opportunities to identify biomarkers for earlier diagnosis. Recent studies demonstrated that neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9) could be used as diagnostic and prognostic biomarkers in various cancers, including breast cancer[10], bladder cancer[11,12], gastric cancer[13], endometrial cancer[14] and kidney tumors[15]. NGAL, also called lipocalin-2 (LCN2), is a small glycoprotein, which is encoded by the LCN2 gene. As a member of the lipocalin family, NGAL participates in the transportation of lipophilic substances. Additionally, it has also been reported that NGAL is associated with the delivery of iron from the extracellular space into the inner cell, which may promote tumor development and support the proliferation of neoplastic cells[16,17]. Previous studies revealed that increased expression of NGAL contributed to the progression of cancers[18,19], and in a number of malignancies, NGAL was over-expressed[20,21]. MMP-9 is one of the matrixmetalloproteinases, whose activity is modulated by NGAL[22]. MMP-9 can degrade the extracellular matrix (ECM), which is the barrier to cell invasion. Degradation of the ECM can provide a favorable environment for promoting the growth and dissemination of cancer cells. The increased expression level of MMP-9 has been observed in several cancers, which was related to the aggressiveness of cancer cells and the overall survival of patients[23]. In combination with NGAL, MMP-9 can avoid proteolytic degradation, which increases its enzymatic activity and the movability of malignancies[10].
NGAL, MMP-9, and their complex have shown value as diagnostic and prognostic biomarkers in several cancers. However, their value in tubular adenocarcinoma of the colon remains unknown. Therefore, in the present study, we evaluated the potential of NGAL and MMP-9 as biomarkers for the early detection of tubular adenocarcinoma of the colon and explored the possible application of combining these two biomarkers.
This study included 15 female and 32 male patients aged 45 to 80 years treated in the Provincial Hospital Affiliated to Shandong University. In total, 30 samples of tubular adenocarcinoma of the colon were collected from the patients who underwent surgical excision between 2015 and 2019. Ten patients had polyps (I), 10 patients had mild tubular adenocarcinoma (II) and 10 patients had severe tubular adenocarcinoma (III), respectively, confirmed by a pathologist. In addition, 10 normal samples were included as controls. All experiments were in compliance with the ethical standards of the World Medical Association Declaration of Helsinki, the samples were only used for research and consent was obtained from the patients before the start of the experiment.
Serum samples were tested using a commercial electrochemiluminescence immunoassay for pro-gastrin-releasing peptide (pro-GRP) with the Roche C6000 automated immunoassay analyzer (Roche Diagnostics GmbH, Penzberg, Germany)[24].
Total RNA was extracted from serum samples with TRIzol Reagent (DP405-02, TIANGEN Biotech, Beijing, China), after which cDNA was synthesized with reverse transcriptase (RR047B, Takara, Beijing, China) in accordance with the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) was performed using SYBR® Premix Ex Taq™ II (Tli RNaseH Plus, TaKaRa, Japan) to detect the mRNA levels of NGAL and MMP9. Reaction parameters were as follows: 95oC for 30 s, followed by 45 cycles at 95oC for 5 s and 60oC for 40 s. Data were collected and analyzed with GraphPad and SPSS 25.0. The expression of genes within a sample was normalized to GAPDH expression by employing the 2ΔΔCt method. The primers used in the present study were as follows: NGAL (Forward: ACAAAGACCCGCAAAAGATG; Reverse: TTGGGACAGGGAAGACGAT), and MMP9 (Forward: GAGCACGGAGACG
Western blotting for NGAL and MMP-9 was carried out. Serum sample lysates were thawed and mixed with an equal volume of 2X buffer. 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was used to separate proteins and the proteins were then transferred onto a polyvinylidene difluoride (PVDF) membrane. Anti-NGAL antibody (ab41105) and anti-MMP-9 antibody (ab38898) obtained from Abcam (Cambridge, United Kingdom) and B-actin monoclonal antibody (YM3028) obtained from Immunoway (Plano, United States) were employed as primary antibodies. The membrane was incubated with primary antibodies at 4oC overnight and secondary antibodies at 37oC for 40 min, respectively. Enhanced chemiluminescence (ECL, WBKLS0500, Millipore, MA, United States) solution was used to visualize the bands. The blots were subsequently scanned, and band intensity was quantified based on densitometry software (ImageJ, National Institutes of Health, Bethesda, MD, United States).
For immunohistochemical (IHC) analysis of tumor tissues, the samples were rinsed with PBS, fixed with 4% paraformaldehyde for 1 h and embedded in paraffin. Sections of 5 μm thickness were prepared for IHC staining. Briefly, the slides were rehydrated using a gradient. Sodium citrate buffer solution (pH 6.0) was used for antigen retrieval. To avoid the influence of endogenous peroxidase activity, 0.3% hydrogen peroxide was added for 10 min. In addition, 3% calf serum was applied to block the sections. The sections were subsequently stained with the same primary antibodies as above. Then the samples were incubated with the universal secondary antibody and VECTASTAIN Elite ABC reagent (PK6200, Vector, Germany), reacted with 3,3′-Diaminobenzidine tetra hydrochloride hydrate (DAB, Thermo Fisher Scientific, Waltham, United States), and counterstained with hematoxylin (H3404, Vector, Germany). The images were captured using an optical microscope.
Unless otherwise indicated, all experiments were carried out in triplicate. Error bars represent standard deviations. Data are presented as mean value ± SE from three independent measurements, and values of P < 0.05 were considered statistically significant. Graphs were plotted and analyzed using GraphPad and SPSS 25.0.
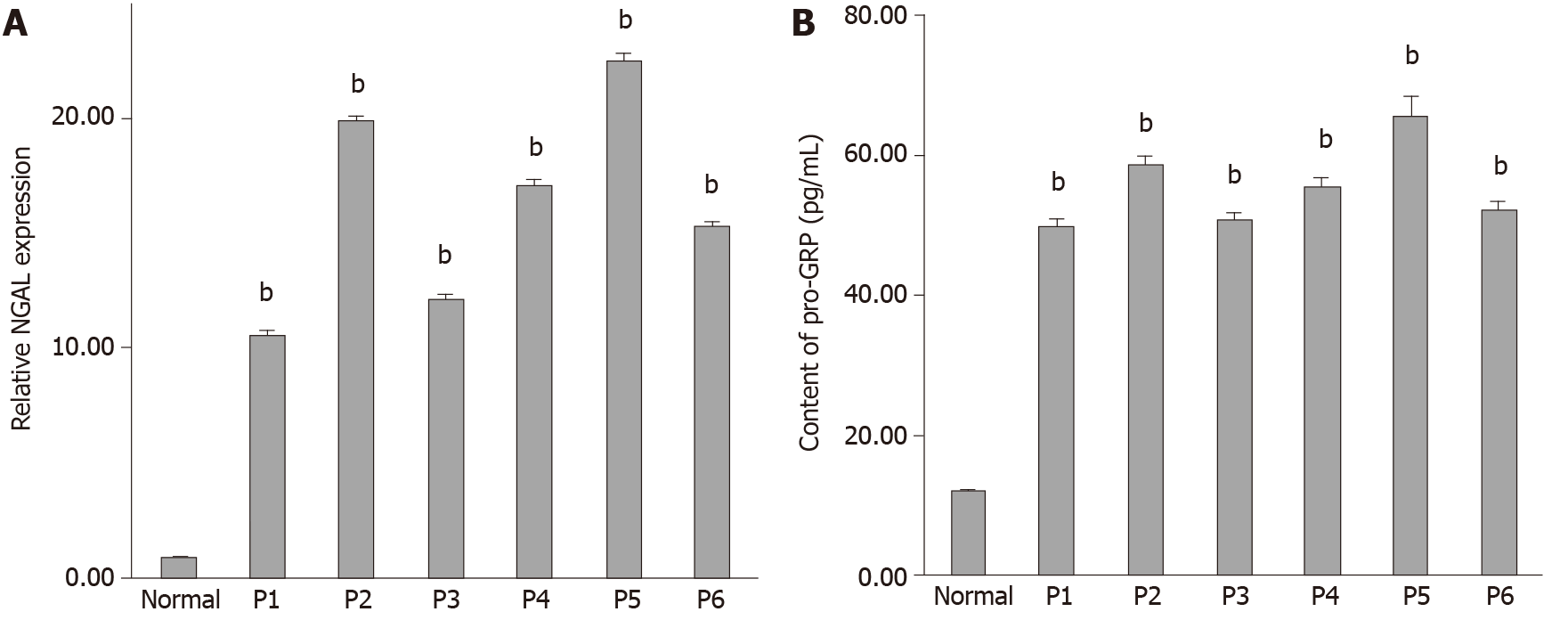
It has been reported that NGAL can be used as a biomarker to detect some cancers. However, the potential value of NGAL in tubular adenocarcinoma of the colon remains unknown. Therefore, we first analyzed 6 samples from patients (Table 1), who were diagnosed with tubular adenocarcinoma of the colon. The mRNA expression level of NGAL was evaluated by qRT-PCR. Figure 1A shows that the expression of NGAL was significantly higher in tubular adenocarcinoma of the colon in comparison with the control sample. The biomarker pro-GRP, is commonly used in the diagnosis of tubular adenocarcinoma[24,25]. As shown in Figure 1B and Table 1, pro-GRP was detected in the serum of these 6 patients. According to the results obtained, we found that the expression of NGAL was consistent with the expression of pro-GRP in various patients, suggesting that the expression of NGAL in serum was positively correlated with the occurrence of tubular adenocarcinomas of the colon.
| Case | Gender | Age | Pathologic grade | pro-GRP (pg/mL) | Relative NGAL expression |
| Normal | M | 64 | Polyps | 12.32 ± 0.30 | 1 ± 0.02 |
| P1 | M | 76 | Mild tubular adenocarcinoma | 50.02 ± 0.80 | 10.62 ± 0.10 |
| P2 | F | 78 | Mild tubular adenocarcinoma | 58.86 ± 1.31 | 19.90 ± 0.22 |
| P3 | F | 45 | Mild tubular adenocarcinoma | 50.99 ± 0.85 | 12.17 ± 0.15 |
| P4 | M | 74 | Mild tubular adenocarcinoma | 55.71 ± 1.15 | 17.14 ± 0.20 |
| P5 | M | 65 | Mild tubular adenocarcinoma | 65.87 ± 2.61 | 22.56 ± 0.27 |
| P6 | F | 80 | Mild tubular adenocarcinoma | 52.52 ± 1.01 | 15.34 ± 0.19 |
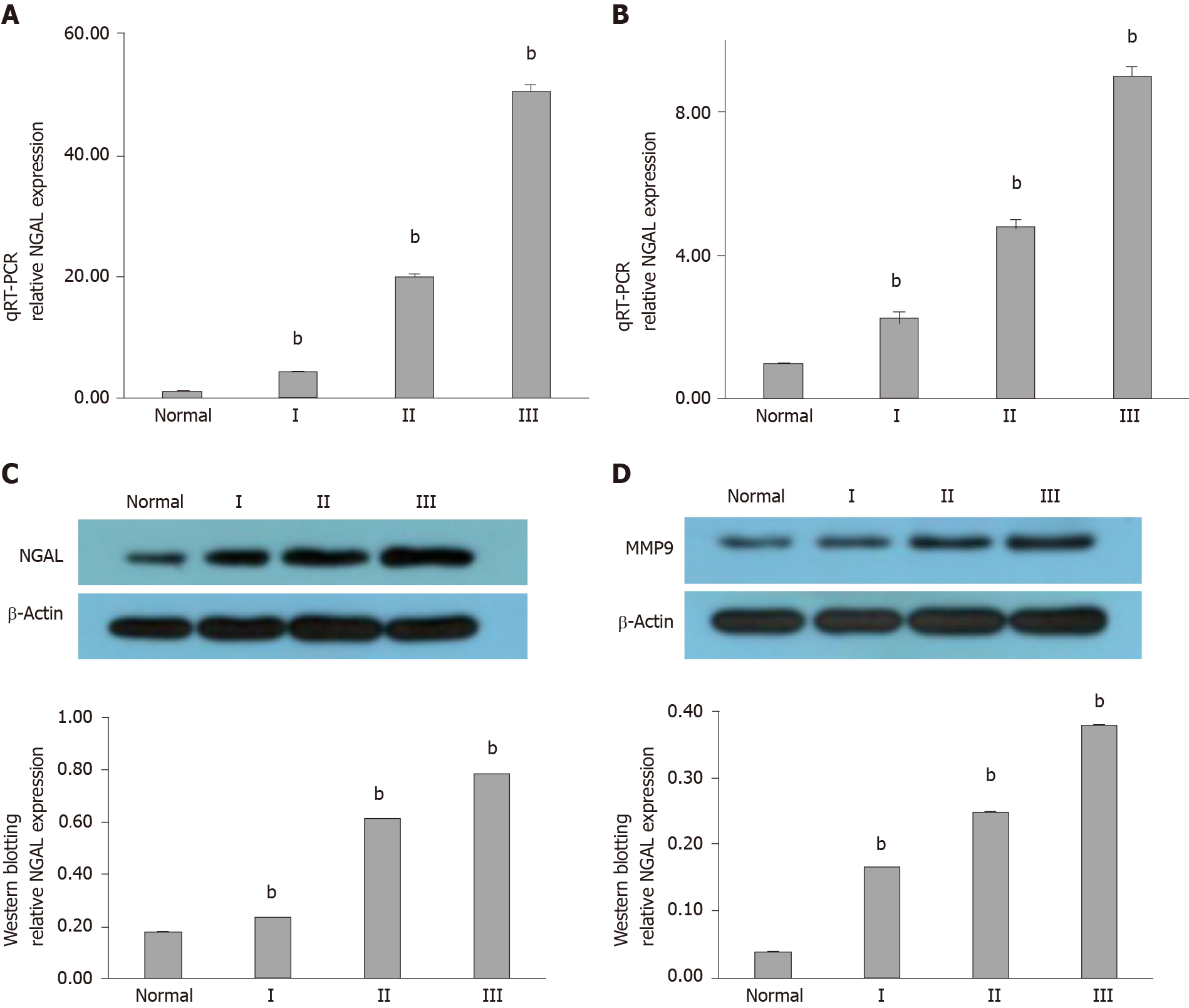
In order to confirm the relationship between NGAL and the occurrence of tubular adenocarcinoma, more samples were analyzed. We collected more samples of tubular adenocarcinoma of the colon, including polyps (I), mild tubular adenocarcinoma (II) and severe tubular adenocarcinoma (III), as shown in Table 2. The qRT-PCR results in Figure 2A reveal that increased gene expression of NGAL was found in all cases, while the increment varied. In addition, we also found that with the development of tubular adenocarcinoma, the mean expression levels of NGAL were increased, and the expression of NGAL was higher in stage II and III than that in stage I. We then assessed the expression of MMP-9, which can be induced by NGAL. As expected, both NGAL and MMP-9 gradually increased with progression of the disease, although the gene expression of MMP-9 was extremely low in stage I, it was still slightly higher than that in the control group (Figure 2B).
| Group | Case | Gender | Age | Pathologic grade | pro-GRP (pg/mL) | Relative NGAL expression | Relative MMP-9 expression |
| Normal | N-1 | M | 64 | -- | 11.51 ± 0.31 | 1.00 ± 0.02 | 1.00 ± 0.01 |
| Normal | N-2 | M | 62 | -- | 6.33 ± 0.13 | 0.81 ± 0.01 | 0.98 ± 0.01 |
| Normal | N-3 | M | 59 | -- | 8.54 ± 0.12 | 0.93 ± 0.03 | 0.94 ± 0.02 |
| Normal | N-4 | M | 67 | -- | 15.67 ± 0.37 | 1.03 ± 0.02 | 0.97 ± 0.01 |
| Normal | N-5 | M | 48 | -- | 8.34 ± 0.21 | 1.12 ± 0.03 | 1.02 ± 0.02 |
| Normal | N-6 | M | 52 | -- | 13.51 ± 0.33 | 0.96 ± 0.01 | 1.04 ± 0.02 |
| Normal | N-7 | M | 69 | -- | 16.62 ± 0.41 | 0.89 ± 0.01 | 1.00 ± 0.01 |
| Normal | N-8 | M | 56 | -- | 6.88 ± 0.22 | 1.08 ± 0.04 | 2.03 ± 0.03 |
| Normal | N-9 | M | 70 | -- | 17.36 ± 0.31 | 0.97 ± 0.03 | 0.96 ± 0.01 |
| Normal | N-10 | M | 65 | -- | 18.24 ± 0.35 | 1.21 ± 0.03 | 1.06 ± 0.02 |
| I | I-1 | M | 54 | Polyps | 15.68 ± 0.27 | 1.31 ± 0.02 | 1.02 ± 0.01 |
| I | I-2 | F | 65 | Polyps | 17.83 ± 0.31 | 1.42 ± 0.04 | 1.08 ± 0.02 |
| I | I-3 | F | 66 | Polyps | 20.12 ± 0.35 | 1.90 ± 0.04 | 1.28 ± 0.03 |
| I | I-4 | M | 74 | Polyps | 24.38 ± 0.46 | 2.84 ± 0.06 | 4.32 ± 0.12 |
| I | I-5 | M | 65 | Polyps | 29.67 ± 0.36 | 3.85 ± 0.07 | 2.35 ± 0.06 |
| I | I-6 | F | 71 | Polyps | 62.56 ± 0.83 | 4.24 ± 0.10 | 2.76 ± 0.07 |
| I | I-7 | F | 55 | Polyps | 38.94 ± 0.46 | 2.09 ± 0.06 | 3.00 ± 0.06 |
| I | I-8 | M | 62 | Polyps | 40.54 ± 0.52 | 5.56 ± 0.12 | 5.12 ± 0.13 |
| I | I-9 | M | 64 | Polyps | 43.86 ± 0.49 | 7.21 ± 0.16 | 3.44 ± 0.09 |
| I | I-10 | M | 68 | Polyps | 60.91 ± 0.75 | 8.23 ± 0.20 | 3.71 ± 0.11 |
| II | II-1 | M | 68 | Mild tubular adenocarcinoma | 55.32 ± 0.46 | 3.97 ± 0.12 | 4.06 ± 0.12 |
| II | II-2 | M | 72 | Mild tubular adenocarcinoma | 30.13 ± 0.36 | 13.81 ± 0.23 | 4.53 ± 0.18 |
| II | II-3 | F | 65 | Mild tubular adenocarcinoma | 52.54 ± 0.53 | 15.06 ± 0.25 | 4.89 ± 0.20 |
| II | II-4 | F | 70 | Mild tubular adenocarcinoma | 58.25 ± 0.49 | 5.68 ± 0.18 | 4.07 ± 0.17 |
| II | II-5 | M | 75 | Mild tubular adenocarcinoma | 35.02 ± 0.56 | 18.37 ± 0.29 | 4.61 ± 0.22 |
| II | II-6 | M | 77 | Mild tubular adenocarcinoma | 61.45 ± 0.67 | 21.35 ± 0.35 | 2.92 ± 0.15 |
| II | II-7 | M | 65 | Mild tubular adenocarcinoma | 62.38 ± 0.74 | 23.69 ± 0.37 | 4.82 ± 0.27 |
| II | II-8 | F | 63 | Mild tubular adenocarcinoma | 64.43 ± 0.48 | 24.92 ± 0.24 | 4.85 ± 0.26 |
| II | II-9 | M | 57 | Mild tubular adenocarcinoma | 68.67 ± 0.46 | 26.74 ± 0.30 | 5.68 ± 0.22 |
| II | II-10 | M | 62 | Mild tubular adenocarcinoma | 70.5 ± 0.71 | 28.32 ± 0.32 | 6.04 ± 0.17 |
| III | III-1 | M | 67 | Severe tubular adenocarcinoma | 72.53 ± 0.83 | 31.03 ± 0.26 | 6.36 ± 0.22 |
| III | III-2 | M | 65 | Severe tubular adenocarcinoma | 76.27 ± 0.96 | 37.17 ± 0.32 | 6.47 ± 0.20 |
| III | III-3 | F | 80 | Severe tubular adenocarcinoma | 79.81 ± 1.13 | 38.44 ± 0.41 | 7.53 ± 0.31 |
| III | III-4 | F | 76 | Severe tubular adenocarcinoma | 84.56 ± 1.41 | 43.68 ± 0.50 | 8.68 ± 0.25 |
| III | III-5 | M | 65 | Severe tubular adenocarcinoma | 86.01 ± 1.06 | 45.79 ± 0.55 | 4.70 ± 0.19 |
| III | III-6 | F | 71 | Severe tubular adenocarcinoma | 90.12 ± 1.28 | 49.31 ± 0.61 | 9.05 ± 0.35 |
| III | III-7 | M | 66 | Severe tubular adenocarcinoma | 93.17 ± 1.33 | 53.81 ± 0.53 | 9.52 ± 0.28 |
| III | III-8 | M | 78 | Severe tubular adenocarcinoma | 94.24 ± 1.57 | 54.68 ± 0.62 | 9.35 ± 0.34 |
| III | III-9 | F | 67 | Severe tubular adenocarcinoma | 93.08 ± 1.82 | 65.76 ± 0.72 | 9.41 ± 0.27 |
| III | III-10 | F | 72 | Severe tubular adenocarcinoma | 107.38 ± 2.34 | 85.91 ± 0.96 | 14.84 ± 0.38 |
We further determined the protein level of NGAL and MMP-9 using Western blotting. Typical samples (N-4, I-5, II-3, III-6) were selected for tested. According to the results, we found that the hybridization signal of NGAL was gradually enhanced from the control group to stage III, indicating the progression of tubular adenocarcinoma (Figure 2C), which was consistent with the results of qRT-PCR. The expression level of MMP-9 in stage I was slightly higher than that in the control group, and it continued to increase in stage II and III (Figure 2D), suggesting that MMP-9 was induced by NGAL, and the expression of MMP-9 might occur later than NGAL. More importantly, it was also found that MMP-9 increased as tubular adenocarcinoma of the colon progressed.
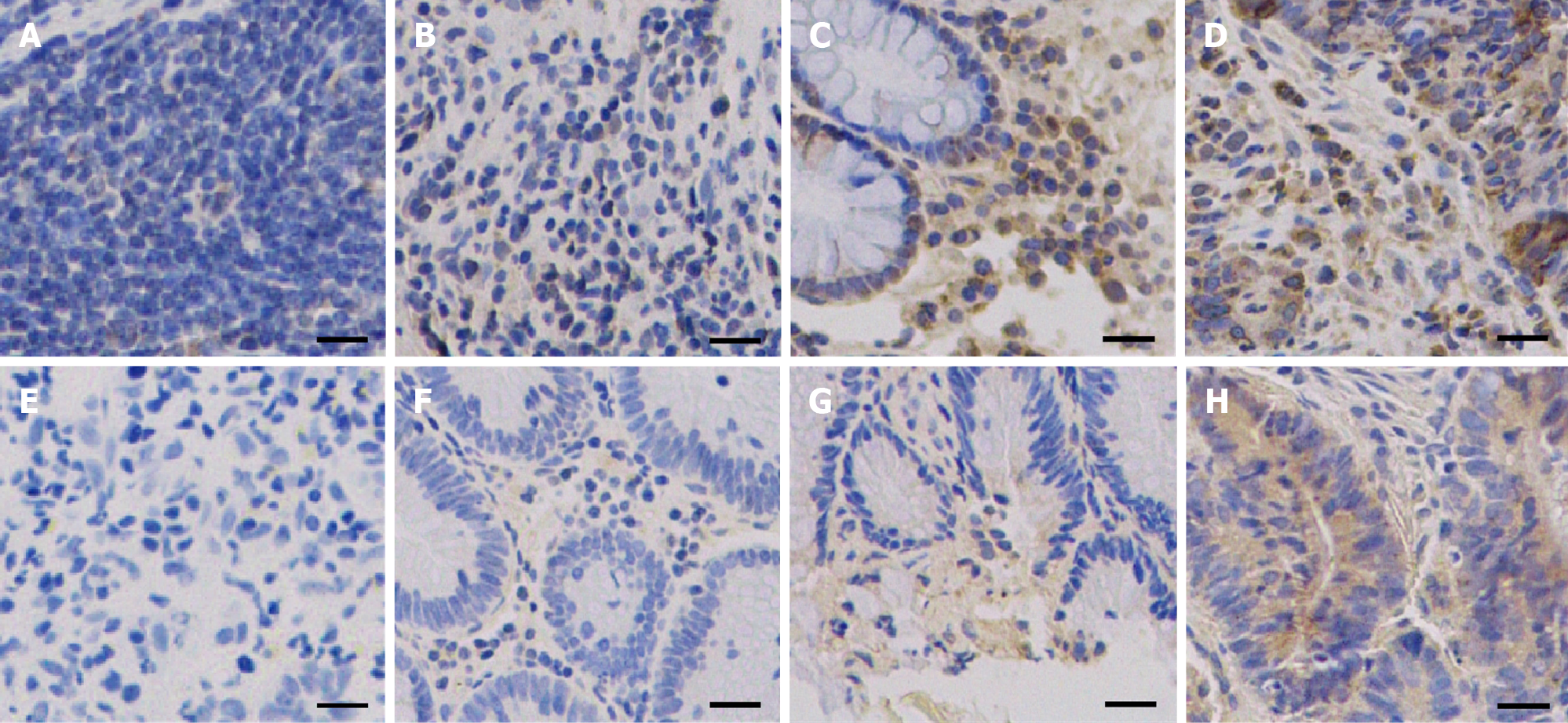
IHC staining was also used to detect the in situ expression of NGAL and MMP-9. The tissue samples used for IHC staining were from the same patients (N-4, I-5, II-3, III-6). Hybridization with anti-NGAL and anti-MMP-9 antibodies revealed that NGAL and MMP-9 in the lesion were both higher than those in the control. With progression of the disease, the signals became stronger (Figure 3). Therefore, it can be concluded that both NGAL and MMP-9 can be used as biomarkers to indicate the progression of tubular adenocarcinoma of the colon.
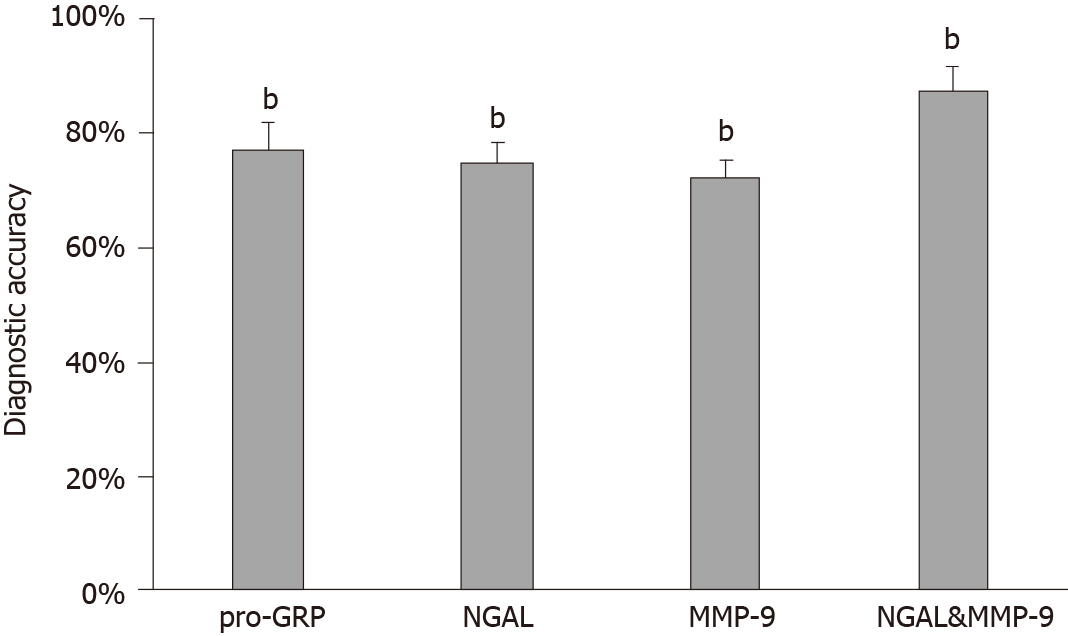
Although both NGAL and MMP-9 exhibited potential value as independent biomarkers in the detection of tubular adenocarcinoma of the colon, their accuracy was still a problem for clinical application. In addition, some of the cases in our study would be misdiagnosed if we measured only one of them. The expression of NGAL in 10 cases did not match the pathological examination results, and the accuracy of NGAL was 75% (Table 2). Similarly, the accuracy of MMP-9 was 72.5%. However, we found that the accuracy was improved to 87.5% when NGAL and MMP-9 were combined for the diagnosis of tubular adenocarcinoma of the colon. Only one of 40 cases was misdiagnosed (Table 2, Figure 4). Therefore, it is suggested that NGAL and MMP-9 may be used in combination to enhance diagnostic accuracy of tubular adenocarcinoma of the colon.
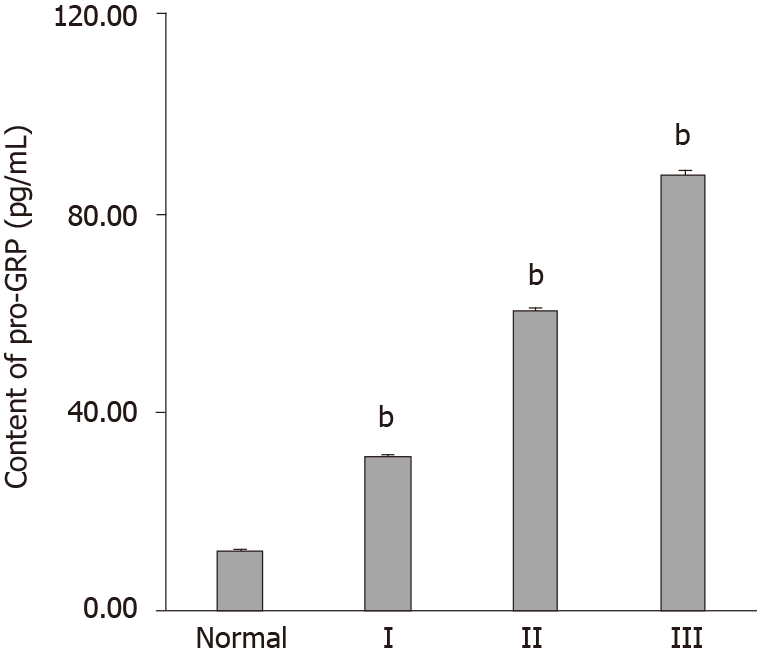
As pro-GRP is a commonly used tumor diagnostic marker, we measured the content of pro-GRP in these 40 cases, and 77.5% of the measured pro-GRP values correctly indicated the progression of tubular adenocarcinoma of the colon (Figure 5 and Table 2). By comparing the diagnostic results of the combination of NGAL and MMP-9, we found that most of results were consistent, indicating that the combination of NGAL and MMP-9 is reliable in clinical application. Moreover, although using pro-GRP was more accurate than using NGAL or MMP-9 alone, there was still a 22.5% probability of error, which was diagnosed correctly when using NGAL and MMP-9 together.
Despite significant medical progress in recent years, cancer is still one of the most common causes of death. Early-stage tubular adenocarcinoma of the colon is usually cured by surgical excision, but it is difficult to detect this disease in the early stage[10,11], which is due to the lack of effective biomarkers. More importantly, it is necessary to determine the progression of tubular adenocarcinoma of the colon during treatment, to allow efficient evaluation and adjustment of therapeutic strategies.
NGAL and MMP-9 have been reported to be involved in several types of cancers and have been used as biomarkers to indicate disease progression[10-15]. In our study, we found that the expression of NGAL was positively related to the occurrence of tubular adenocarcinoma of the colon using qRT-PCR. The expression of NGAL was closely related to the development of tubular adenocarcinoma of the colon, suggesting that NGAL could be used to indicate progression. The results for NGAL were consistent with those for pro-GRP, suggesting that NGAL is reliable for indicating disease progression. On the other hand, we also demonstrated that MMP-9 could be considered another potential biomarker for tubular adenocarcinoma of the colon. Our results showed that although the expression of MMP-9 in patients with stage I disease was only slightly higher than that in the control, it was lower than the expression of NGAL in stage I disease, which was consistent with the conclusion of a previous study which showed that the expression of MMP-9 was induced by NGAL[22]. The expression of MMP-9 continued to increase as tubular adenocarcinoma of the colon progressed, suggesting that MMP-9 is also suitable for indicating disease progression. We also detected protein expression and analyzed pathological tissue using Western blotting and IHC staining, respectively, and the findings were consistent with the results shown above and demonstrated the potential value of NGAL and MMP-9 as biomarkers for clinical application.
Most importantly, we found that the combination of NGAL and MMP-9 in the diagnosis of 40 clinical cases significantly improved diagnostic accuracy up to 87.5%, which was higher than that achieved using NGAL, MMP-9 or the traditional biomarker pro-GPR alone. This result suggested that combining NGAL and MMP-9 has great potential for detecting tubular adenocarcinoma of the colon. Only one case could not be identified by NGAL and MMP-9. The mechanism of tumorigenesis in this case may have been abnormal and complex, which requires further study.
In summary, our study demonstrated that both NGAL and MMP-9 could be used to detect tubular adenocarcinoma of the colon and that the combination of NGAL and MMP-9 showed greater diagnostic accuracy than either biomarker alone. As abnormal expression of NGAL/MMP-9 has been observed in several cancers, such as breast cancer, bladder cancer and gastric cancer, these two biomarkers described here for tubular adenocarcinoma of the colon may also be useful in other cancers.
Tubular adenocarcinoma of the colon, which originates from the epithelium of glands, is a major health concern worldwide. However, it is difficult to detect at an early stage. The lack of biomarkers is a main barrier in the diagnosis and treatment of tubular adenocarcinoma. Neutrophil gelatinase-associated lipocalin (NGAL) is a secreted protein, which induces the expression of matrix metalloproteinase-9 (MMP-9) and is involved in various tumors. NGAL and MMP-9 have been reported to be associated with tumorigenesis and tumor development. They may be potential biomarkers for the diagnosis of tubular adenocarcinoma of the colon.
The combination of NGAL and MMP9 are promising biomarkers for the early detection of tubular adenocarcinoma of the colon. To evaluate whether NGAL and MMP-9 can be used as potential biomarkers to indicate the progression of tubular adenocarcinoma of the colon, it may be beneficial to detect this tumor at the molecular level in the very early stage.
We evaluated whether NGAL and MMP-9 can be used as potential biomarkers to indicate the progression of tubular adenocarcinoma of the colon as patients with this disease will require early diagnosis and treatment.
Samples were collected from the colonic mucosa of various patients. Ten patients had polyps (I), 10 patients had mild tubular adenocarcinoma (II) and 10 patients had severe tubular adenocarcinoma (III), respectively, confirmed by a pathologist. In addition, 10 normal samples were included as controls. The content of pro-gastrin-releasing peptide (pro-GRP) in serum was measured by an electrochemiluminescence immunoassay. The mRNA expression of NGAL and MMP-9 was examined by quantitative real-time PCR (qRT-PCR) analysis, and their protein expression was examined by Western blotting and immunohistochemical (IHC) analysis. According to the status of tubular adenocarcinoma of the colon, the patients were divided into three groups. Thus, the clinical grouping in this research was novel, which has not adopted in other studies.
In this study, we found that NGAL and MMP-9 can be used as biomarkers for detecting tubular adenocarcinoma of the colon and their combination resulted in better diagnostic accuracy. By analyzing the expression of NGAL in tubular adenocarcinoma at different levels, we found that NGAL was significantly up-regulated in primary tubular adenocarcinoma compared with normal tissues. The up-regulation of NGAL was strongly correlated with both the degree of differentiation and the disease stage (I–III), indicating that NGAL could serve as a diagnostic biomarker for tubular adenocarcinoma. Using NGAL as a biomarker for diagnosis, the accuracy was similar to the widely used biomarker pro-GRP, suggesting that NGAL is a reliable biomarker. In addition, the expression of MMP-9 was also strongly correlated with differentiation and stage, demonstrating that MMP-9 can be used as a biomarker to indicate the progression of tubular adenocarcinoma of the colon. More importantly, the combination of NGAL and MMP-9 achieved greater diagnostic accuracy in tubular adenocarcinoma, and these results were further confirmed by immunohistochemical analysis of tissue sections.
In this study, the up-regulation of NGAL and MMP-9 was strongly correlated with both the degree of differentiation and stage of tubular adenocarcinoma of the colon. Both NGAL and MMP-9 can be used as biomarkers for detecting tubular adenocarcinoma of the colon and their combination achieved better diagnostic accuracy. Patients who develop tubular adenocarcinoma of colon will require early diagnosis and early treatment.
It is necessary to study more cases of tubular adenocarcinoma of the colon as other factors may be involved in the progression of this disease. A larger scale clinical study may be the best method for future research on this tumor.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Arville B, Shimizu Y S-Editor: Gong ZM L-Editor: Webster JR P-Editor: Liu JH
| 1. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20511] [Article Influence: 2051.1] [Reference Citation Analysis (20)] |
| 2. | Kontos CK, Tsiakanikas P, Avgeris M, Papadopoulos IN, Scorilas A. miR-15a-5p, A Novel Prognostic Biomarker, Predicting Recurrent Colorectal Adenocarcinoma. Mol Diagn Ther. 2017;21:453-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Yu L, Liu ZY, Jiao J, Shi XL, Cui WL, Zhang W, Li QX. [Polymorphisms of mTORC1 genes and risk of primary colorectal adenocarcinoma in Chinese populations]. Zhonghua Bing Li Xue Za Zhi. 2018;47:492-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Chen L, Zhu YY, Zhang XJ, Wang GL, Li XY, He S, Zhang JB, Zhu JW. TSPAN1 protein expression: a significant prognostic indicator for patients with colorectal adenocarcinoma. World J Gastroenterol. 2009;15:2270-2276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Lualdi M, Cavalleri A, Battaglia L, Colombo A, Garrone G, Morelli D, Pignoli E, Sottotetti E, Leo E. Early detection of colorectal adenocarcinoma: a clinical decision support tool based on plasma porphyrin accumulation and risk factors. BMC Cancer. 2018;18:841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | Luo C, Cen S, Ding G, Wu W. Mucinous colorectal adenocarcinoma: clinical pathology and treatment options. Cancer Commun (Lond). 2019;39:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 189] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 8. | Wu XL, Wang YY, Wang LK, Xue J, Yang DD, Qu M, Wang CY, Guo F, Yang RM, Liu B. Id-1 expression in colorectal adenocarcinoma tissues and its clinical significance. Rev Assoc Med Bras (1992). 2019;65:404-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Reynolds IS, O'Connell E, Fichtner M, McNamara DA, Kay EW, Prehn JHM, Furney SJ, Burke JP. Mucinous adenocarcinoma is a pharmacogenomically distinct subtype of colorectal cancer. Pharmacogenomics J. 2020;20:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Metallinou D, Lykeridou K, Karampas G, Liosis GT, Skevaki C, Rizou M, Papassotiriou I, Rizos D. Postpartum human breast milk levels of neutrophil gelatinase-associated lipocalin (NGAL) and matrix metalloproteinase-9 (MMP-9)/NGAL complex in normal and pregnancies complicated with insulin-dependent gestational diabetes mellitus. A prospective pilot case-control study. J Obstet Gynaecol. 2020;40:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Candido S, Di Maso M, Serraino D, McCubrey JA, Bortolus R, Zanin M, Battiston M, Salemi R, Libra M, Polesel J. Diagnostic value of neutrophil gelatinase-associated lipocalin/matrix metalloproteinase-9 pathway in transitional cell carcinoma of the bladder. Tumour Biol. 2016;37:9855-9863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Ricci S, Bruzzese D, DI Carlo A. Evaluation of MMP-2, MMP-9, TIMP-1, TIMP-2, NGAL and MMP-9/NGAL complex in urine and sera from patients with bladder cancer. Oncol Lett. 2015;10:2527-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1150] [Cited by in RCA: 2849] [Article Influence: 569.8] [Reference Citation Analysis (5)] |
| 14. | Cymbaluk-Płoska A, Chudecka-Głaz A, Pius-Sadowska E, Sompolska-Rzechuła A, Chudecka K, Bulsa M, Machaliński B, Menkiszak J. Clinical Relevance of NGAL/MMP-9 Pathway in Patients with Endometrial Cancer. Dis Markers. 2017;2017:6589262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | DI Carlo A. Evaluation of neutrophil gelatinase-associated lipocalin (NGAL), matrix metalloproteinase-9 (MMP-9) and their complex MMP-9/NGAL in sera and urine of patients with kidney tumors. Oncol Lett. 2013;5:1677-1681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Buonafine M, Martinez-Martinez E, Jaisser F. More than a simple biomarker: the role of NGAL in cardiovascular and renal diseases. Clin Sci (Lond). 2018;132:909-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 17. | Shalenkova MA, Mikhailova ZD, Klimkin PF. [NGAL as a marker for some extrarenal complications in acute coronary syndrome]. Kardiologiia. 2018;19-26. [PubMed] |
| 18. | Marchewka Z, Tacik A, Piwowar A. [KIM-1 and NGAL as potential biomarkers for the diagnosis and cancer progression]. Postepy Hig Med Dosw (Online). 2016;70:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Dahiya K, Gupta K, Dhankhar R, Atri R, Goyal N, Dalal D, Pal S, Ahlawat R, Kumar S. Chemoradiation in Lung Cancer: Effect on Levels of NGAL and Vitamin D in Serum. Clin Lab. 2020;66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Chappell WH, Candido S, Abrams SL, Russo S, Ove R, Martelli AM, Cocco L, Ramazzotti G, Cervello M, Montalto G, Steelman LS, Leng X, Arlinghaus RB, Libra M, McCubrey JA. Roles of p53, NF-κB and the androgen receptor in controlling NGAL expression in prostate cancer cell lines. Adv Biol Regul. 2018;69:43-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Falzone L, Candido S, Salemi R, Basile MS, Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M, Libra M. Computational identification of microRNAs associated to both epithelial to mesenchymal transition and NGAL/MMP-9 pathways in bladder cancer. Oncotarget. 2016;7:72758-72766. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Eilenberg W, Stojkovic S, Kaider A, Kozakowski N, Domenig CM, Burghuber C, Nanobachvili J, Huber K, Klinger M, Neumayer C, Huk I, Wojta J, Demyanets S. NGAL and MMP-9/NGAL as biomarkers of plaque vulnerability and targets of statins in patients with carotid atherosclerosis. Clin Chem Lab Med. 2017;56:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Mondal S, Adhikari N, Banerjee S, Amin SA, Jha T. Matrix metalloproteinase-9 (MMP-9) and its inhibitors in cancer: A minireview. Eur J Med Chem. 2020;194:112260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 332] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 24. | Cavalieri S, Morelli D, Martinetti A, Galli G, Nichetti F, de Braud F, Platania M. Clinical implications for pro-GRP in small cell lung cancer. A single center experience. Int J Biol Markers. 2018;33:55-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Kiseli M, Caglar GS, Yarci Gursoy A, Tasci T, Candar T, Akincioglu E, Pabuccu EG, Boran N, Tulunay G, Umudum H. Pro-Gastrin Releasing Peptide: A New Serum Marker for Endometrioid Adenocarcinoma. Gynecol Obstet Invest. 2018;83:540-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |