Published online Sep 15, 2020. doi: 10.4251/wjgo.v12.i9.992
Peer-review started: June 15, 2020
First decision: July 30, 2020
Revised: August 11, 2020
Accepted: August 31, 2020
Article in press: August 31, 2020
Published online: September 15, 2020
Processing time: 86 Days and 16.5 Hours
Borrmann classification (types I-IV) for the detection of advanced gastric cancer has been accepted worldwide, and lymphatic and/or blood vessel invasion (LBVI) status is related to the poor prognosis after gastric cancer.
To evaluate the significance of Borrmann type combined with LBVI status in predicting the prognosis of advanced gastric cancer.
We retrospectively studied the clinicopathological characteristics and long-term survival data of 2604 patients who were diagnosed with advanced gastric adenocarcinoma at Harbin Medical University Cancer Hospital from January 2009 to December 2013. Categorical variables were evaluated by the Pearson’s χ2 test, the Kaplan-Meier method was used to identify differences in cumulative survival rates, and the Cox proportional hazards model was used for multivariate prognostic analysis.
A total of 2604 patients were included in this study. The presence of LVBI [LBVI (+)] and Borrmann type (P = 0.001), tumor location (P < 0.001), tumor size (P < 0.001), histological type (P < 0.001), tumor invasion depth (P < 0.001), number of metastatic lymph nodes (P < 0.001), and surgical method (P < 0.001) were significantly correlated with survival. When analyzing the combination of the Borrmann classification and LBVI status, we found that patients with Borrmann type III disease and LBVI (+) had a similar 5-year survival rate to those with Borrmann IV + LBVI (-) (16.4% vs 13.1%, P = 0.065) and those with Borrmann IV + LBVI (+) (16.4% vs 11.2%, P = 0.112). Subgroup analysis showed that the above results were true for any pT stage and any tumor location. Multivariate Cox regression analysis showed that Borrmann classification (P = 0.023), vascular infiltration (P < 0.001), tumor size (P = 0.012), pT stage (P < 0.001), pN stage (P < 0.001), and extent of radical surgery (P < 0.001) were independent prognostic factors for survival.
Since patients with Borrmann III disease and LBVI (+) have the same poor prognosis as those with Borrmann IV disease, more attention should be paid to patients with Borrmann III disease and LBVI (+) during diagnosis and treatment, regardless of the pT stage and tumor location, to obtain better survival results.
Core Tip: Although the evolution of diagnostic methods has led to an increase in the diagnosis rate of early gastric cancer, most patients present with an advanced stage when they are diagnosed with gastric cancer. Comprehensive multimodal and multidisciplinary treatment systems, including chemotherapy and targeted therapy, are gradually improving. However, wise treatment choices must be made based on the clear clinical stage of the disease. Many studies have shown that Borrmann type and vessel invasion are independent risk factors for the prognosis of patients with advanced gastric cancer, but few studies have analyzed the prognostic signficance of the combination of the above two indexes in patients with advanced gastric cancer. Therefore, we analyzed whether Borrmann type combined with vessel invasion has prognostic significance in advanced gastric cancer, with an aim to provide a basis for clinicians to treat and predict the prognosis of these patients in the future.
- Citation: Zhai Z, Zhu ZY, Zhang Y, Yin X, Han BL, Gao JL, Lou SH, Fang TY, Wang YM, Li CF, Yu XF, Ma Y, Xue YW. Prognostic significance of Borrmann type combined with vessel invasion status in advanced gastric cancer. World J Gastrointest Oncol 2020; 12(9): 992-1004
- URL: https://www.wjgnet.com/1948-5204/full/v12/i9/992.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i9.992
At present, although the morbidity and mortality of gastric cancer (GC) are declining year by year, it is still an important public health burden worldwide[1]. In 2018, nearly 450000 cases of GC were recorded, accounting for 10.6% of all cancers. Furthermore, nearly 390000 patients died of this malignancy, accounting for 13.6% of all cancer-related deaths[2]. Although the evolution of diagnostic methods has led to an increase in the diagnosis rate of early GC, most patients present with an advanced stage when they are diagnosed with this malignancy. Comprehensive multimodal and multidisciplinary treatment systems, including chemotherapy and targeted therapy, are gradually improving[3]. However, wise treatment choices must be made based on the clear clinical stage of the disease. The depth of tumor invasion and number of metastatic lymph nodes are recognized as the most important prognostic factors for patients with advanced GC (AGC)[4,5]. However, the pT stage and pN stage can only be accurately obtained after surgery. Therefore, it is very important to establish a correct treatment strategy and evaluate patient prognosis by perfecting the classification based on the TNM stage.
The appearance-based Borrmann classification (type I-IV) is easily determined by preoperative endoscopy or macroscopic pathology after tumor resection, enabling it to be currently used by surgeons and endoscopists, and has been widely accepted by radiologists and considered an effective classification and valuable clinicopathological feature[6]. Although the clinicopathological features of each Borrmann type of GC have been studied before, the prognostic value of the macroscopic Borrmann classification for AGC is still not uniform. Lymphatic and/or blood vessel invasion (LBVI) is defined as the presence of tumor cells with fibrin clots or red blood cells in the gaps within the endothelial cells[7]. It includes vascular infiltration and lymphatic invasion. Previous studies have shown that LBVI, as a new biomarker related to survival, is closely related to the prognosis of GC and is considered to be an important step in the development of distant metastasis and lymph node metastasis[8,9]. Many studies have shown that Borrmann type and LBVI are independent risk factors for the prognosis of patients with AGC[9,10], but few studies have analyzed the prognostic significance of the combination of the two indexes in patients with AGC. Therefore, this study retrospectively analyzed the clinicopathological data of 2604 patients with AGC diagnosed by pathology from 2009 to 2013 at Harbin Medical University Cancer Hospital to explore whether Borrmann type combined with LBVI has prognostic significance in AGC, with an aim to provide a basis for clinicians to treat and predict the prognosis of these patients in the future.
This retrospective study was performed from November 2019 until March 2020, including 2604 patients who underwent surgical resection for AGC at the Department of Gastrointestinal Surgery of Harbin Medical University Cancer Hospital from January 2009 to December 2013. All the patients had complete clinical, pathological, and surgical data, including sex, age, Borrmann type, LBVI status, tumor location, tumor size, histological type, depth of invasion, number of metastatic lymph nodes, surgical method, treatment by combined resection, and 5-year survival rate. Tumor staging was based on the Eighth Edition of the American Joint Committee on Cancer/International Cancer Control Alliance (AJCC/UICC)[11]. According to the Japanese gastric cancer classification method, AGC is defined as a tumor that invades the muscular lamina or deeper, regardless of the presence of lymph node metastasis[12]. According to the standards of the International Union Against Cancer (UICC), R0 is defined as radical resection, and R1 and R2 are defined as residual tumors visible under a microscope or to the naked eye.
We excluded the following patients: (1) Patients with incomplete clinical or pathological data or follow-up information; (2) Patients undergoing neoadjuvant chemotherapy or perioperative radiochemotherapy; (3) Patients with other gastric tumors (lymphoma, stromal tumor, residual GC, etc.) or other malignant tumors (e.g., colorectal cancer); (4) Patients undergoing palliative surgery; (5) Patients with gastric stump cancer; and (6) Patients with Borrmann type V GC. After excluding these patients, a total of 2604 patients were included in the study.
The Borrmann classification and the determination of LBVI status were based on the Japanese gastric cancer classification[12]. The Borrmann type and LBVI status of each patient were independently evaluated by two pathologists, and any differences were determined by a third pathologist. The Borrmann type was defined as follows: Type I: Polypoid tumor, clearly demarcated from the surrounding mucosa; type II: Ulcerative carcinoma, with a clear border and elevation; type III: Ulcerative carcinoma without a clear boundary, infiltrating the surrounding stomach wall; and type IV: Diffuse invasive cancer, with no obvious ulcers, and no obvious margins that separate normal gastric tissue from the tumor.
Vascular infiltration was defined as tumor cells invading the blood vessel wall and/or the presence of tumor emboli in the space between endothelial cells. No attempt was made to distinguish between blood vessel and lymph vessel infiltration. GC tissue samples were all subjected to hematoxylin-eosin (HE) staining and immunohistochemical staining. All postoperative specimens were processed according to pathological procedures; vascular infiltration was detected by HE staining and immunohistochemical staining for CD34, and lymphatic vessel infiltration was detected by HE staining and immunohistochemical staining for S-100.
The clinical data related to the patients were input into commercially available SPSS 22.0 software, which was used for all statistical analyses. Categorical variables were evaluated by the Pearson’s χ2 test; continuous data are expressed as average values, and the Student's t-test was used to evaluate significant differences between average values. The Kaplan-Meier method was used to identify differences in cumulative survival rates; multivariate prognostic analysis was performed using the Cox proportional risk model. A P value < 0.05 was considered statistically significant.
The patients were followed through outpatient assessments and phone calls every 6 mo in the first 1-2 years after the operation and once every year in the 3-5 years after the operation. The follow-up period ended in June 2019. The median follow-up time was 68 mo.
Of the 2604 patients with AGC included in this study, 1939 (74.4%) were male and 665 (25.6%) were female. The median age was 60 years. Among these patients, 1586 (60.9%) underwent distal gastrectomy, and 234 (9.0%) and 784 (30.1%) underwent proximal gastrectomy and gastrectomy, respectively. There were 123 patients classified with Borrmann type I disease (4.7%), 464 with Borrmann type II (17.8%), 1663 with Borrmann type III (63.8%), and 354 with Borrmann type IV (13.5%). The overall positive rate of LBVI was 16.9%, and the incidence of LBVI (+) in Borrmann types I, II, III, and IV was 13.8%, 20.4%, 15.0%, and 22.5%, respectively (Table 1).
| Variable | LVBI | χ2 | P value | |
| Presence | Absence | |||
| Gender | 0.011 | 0.916 | ||
| Male | 330 (74.7) | 1609 (74.5) | ||
| Female | 112 (25.3) | 553 (25.5) | ||
| Age (yr) | 2.200 | 0.138 | ||
| < 60 | 233 (52.7) | 1056 (48.8) | ||
| ≥ 60 | 209 (47.3) | 1106 (51.2) | ||
| Borrmann type | 17.294 | 0.001 | ||
| I | 17 (3.8) | 106 (4.9) | ||
| II | 95 (21.5) | 369 (17.1) | ||
| III | 250 (56.6) | 1413 (65.4) | ||
| IV | 80 (18.1) | 274 (12.7) | ||
| Tumor location | 33.630 | < 0.001 | ||
| Upper third | 28 (6.3) | 273 (12.6) | ||
| Middle third | 62 (14.0) | 339 (15.7) | ||
| Lower third | 220 (49.8) | 1135 (52.5) | ||
| Whole stomach | 132 (29.9) | 415 (19.2) | ||
| Tumor size (mm) | 16.077 | < 0.001 | ||
| < 50 | 118 (26.7) | 793 (36.7) | ||
| ≥ 50 | 324 (73.3) | 1369 (63.3) | ||
| Histologic grade | 15.084 | < 0.001 | ||
| Well | 129 (29.2) | 843 (39.0) | ||
| Poor | 313 (70.8) | 1319 (61.0) | ||
| pT category | 213.269 | < 0.001 | ||
| T2 | 29 (6.6) | 294 (13.6) | ||
| T3 | 212 (48.0) | 358 (16.6) | ||
| T4a | 171 (38.7) | 1269 (58.7) | ||
| T4b | 30 (6.8) | 241 (11.1) | ||
| pN category | 96.976 | < 0.001 | ||
| N0 | 44 (10.0) | 522 (24.1) | ||
| N1 | 57 (12.9) | 409 (18.9) | ||
| N2 | 95 (21.5) | 529 (24.5) | ||
| N3 | 246 (55.7) | 702 (32.5) | ||
| Surgical approach | 37.183 | < 0.001 | ||
| Proximal gastrectomy | 23 (5.2) | 211 (9.8) | ||
| Distal gastrectomy | 235 (53.2) | 1351 (62.5) | ||
| Total gastrectomy | 184 (41.6) | 600 (27.8) | ||
| Combined resection | 1.251 | 0.263 | ||
| Presence | 19 (4.3) | 70 (3.2) | ||
| Absence | 423 (95.7) | 2092 (96.8) | ||
Table 2 shows the analysis results of the relationship between LVBI status and clinicopathological characteristics. The results showed that LVBI (+) and Borrmann type (P = 0.001), tumor location (P < 0.001), tumor size (P < 0.001), histological type (P < 0.001), depth of tumor invasion (P < 0.001), number of metastatic lymph nodes (P < 0.001), and surgical method (P < 0.001) were significantly related to survival.
| Variable | Patient number, n (%) | Survival period | P value |
| Gender | 0.619 | ||
| Male | 1939 (74.4) | 34 | |
| Female | 665 (25.6) | 32 | |
| Age (yr) | 0.705 | ||
| < 60 | 1289 (49.6) | 34 | |
| ≥ 60 | 1314 (50.4) | 32 | |
| Lymphatic and/or blood vessel invasion | < 0.001 | ||
| Presence | 442 (17) | 24 | |
| Absence | 2162 (83) | 36 | |
| Borrmann type | < 0.001 | ||
| I | 123 (4.7) | 60 | |
| II | 464 (17.8) | 45 | |
| III | 1663 (63.9) | 34 | |
| IV | 354 (13.6) | 16 | |
| Tumor location | < 0.001 | ||
| Upper third | 301 (11.6) | 32 | |
| Middle third | 401 (15.4) | 33 | |
| Lower third | 1355 (52) | 39 | |
| Whole stomach | 547 (21) | 22 | |
| Tumor size (mm) | < 0.001 | ||
| < 50 | 911 (35) | 45 | |
| ≥ 50 | 1693 (65) | 25 | |
| Histologic grade | < 0.001 | ||
| Well | 972 (37.3) | 40 | |
| Poor | 1632 (62.7) | 30 | |
| pT category | < 0.001 | ||
| T2 | 323 (12.4) | 60 | |
| T3 | 570 (21.9) | 37 | |
| T4a | 1440 (51.3) | 30 | |
| T4b | 271 (10.4) | 18 | |
| pN category | < 0.001 | ||
| N0 | 566 (21.7) | 60 | |
| N1 | 466 (17.9) | 45 | |
| N2 | 624 (24) | 33 | |
| N3 | 948 (36.4) | 18 | |
| Surgical approach | < 0.001 | ||
| Proximal gastrectomy | 234 (9) | 38 | |
| Distal gastrectomy | 1586 (60.9) | 39 | |
| Total gastrectomy | 784 (30.1) | 22 | |
| Radical surgery | < 0.001 | ||
| R0 | 2125 (81.7) | 39 | |
| R1/R2 | 479 (18.3) | 13 | |
| Combined resection | < 0.001 | ||
| Presence | 89 (3.4) | 18 | |
| Absence | 2125 (96.6) | 34 |
Regarding the prognostic survival of patients, univariate survival analysis demonstrated that some clinicopathological variables were significantly related to the survival rate, including Borrmann type (P < 0.001), LVBI (P < 0.001), tumor location (P < 0.001), tumor size (P < 0.001), histological type (P < 0.001), pT stage (P < 0.001), pN stage (P < 0.001), surgical method (P < 0.001), extent of radical surgery (P < 0.001), and treatment by combined resection (P < 0.001). Multivariate Cox regression analysis showed that Borrmann type (P = 0.023), vascular invasion (P < 0.001), tumor size (P = 0.012), pT stage (P < 0.001), pN stage (P < 0.001), and extent of radical surgery (P < 0.001) were independent prognostic factors (Table 3).
| Univariate analysis | Multivariate analysis | |||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Gender | 1.027 (0.925-1.139) | 0.619 | - | - |
| Age | 0.983 (0.898-1.076) | 0.705 | - | - |
| Lymphatic and/or blood vessel invasion | 0.733 (0.653-0.823) | < 0.001 | 0.869 (0.770-0.981) | < 0.001 |
| Borrmann type | 1.591 (1.481-1.710) | < 0.001 | 1.217 (1.130-1.312) | 0.023 |
| Tumor location | 1.120 (1.061-1.183) | < 0.001 | 0.990 (0.940-1.043) | 0.718 |
| Tumor size (mm) | 1.842 (1.667-2.036) | < 0.001 | 1.149 (1.031-1.280) | 0.012 |
| Histologic grade | 1.248 (1.135-1.372) | < 0.001 | 1.021 (0.927-1.125) | 0.667 |
| pT category | 1.413 (1.334-1.497) | < 0.001 | 1.180 (1.180-1.258) | < 0.001 |
| pN category | 1.622 (1.553-1.694) | < 0.001 | 1.447 (1.382-1.515) | < 0.001 |
| Surgical approach | 1.474 (1.360-1.598) | < 0.001 | 1.076 (0.988-1.172) | 0.094 |
| Radical surgery | 2.906 (2.607-3.239) | < 0.001 | 2.029 (1.810-2.276) | < 0.001 |
| Combined resection | 0.641 (0.508-0.809) | < 0.001 | 0.859 (0.677-1.090) | 0.212 |
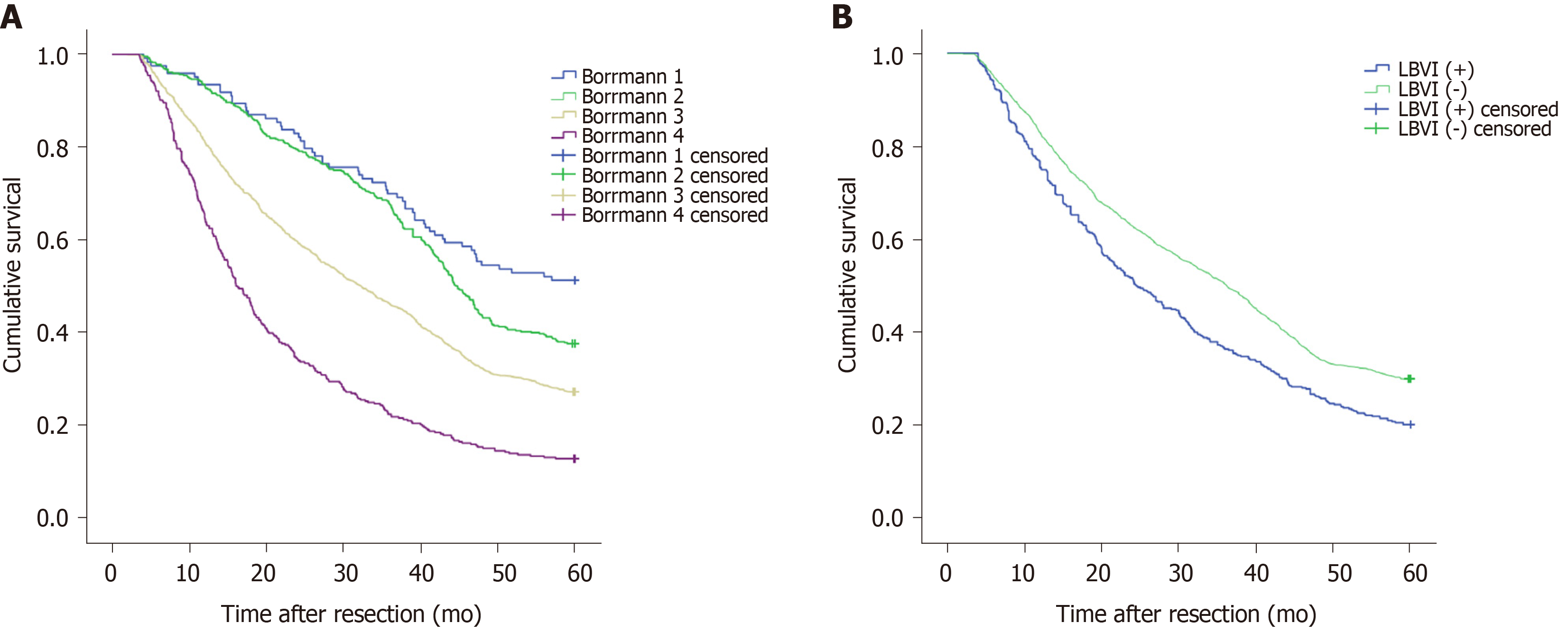
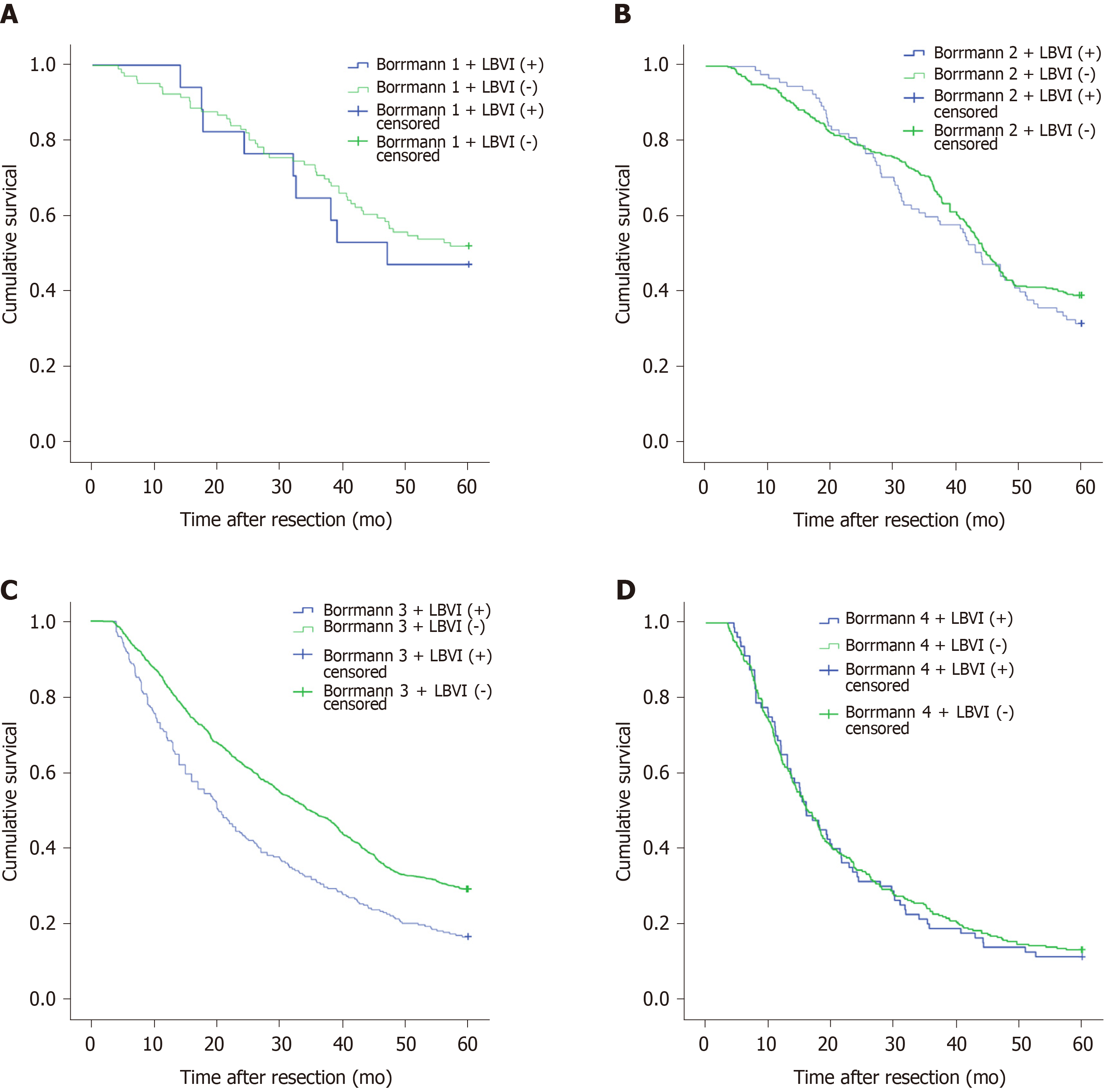
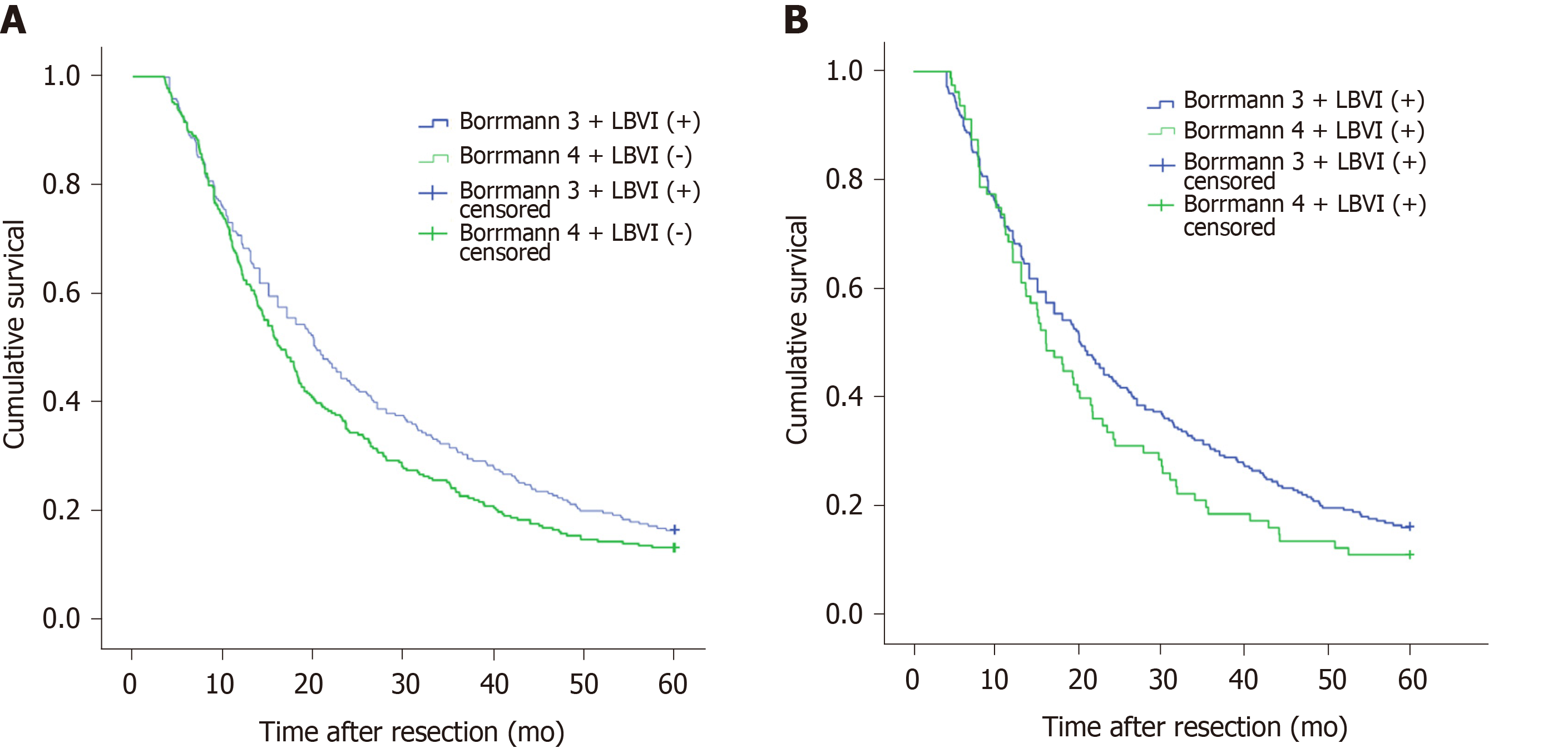
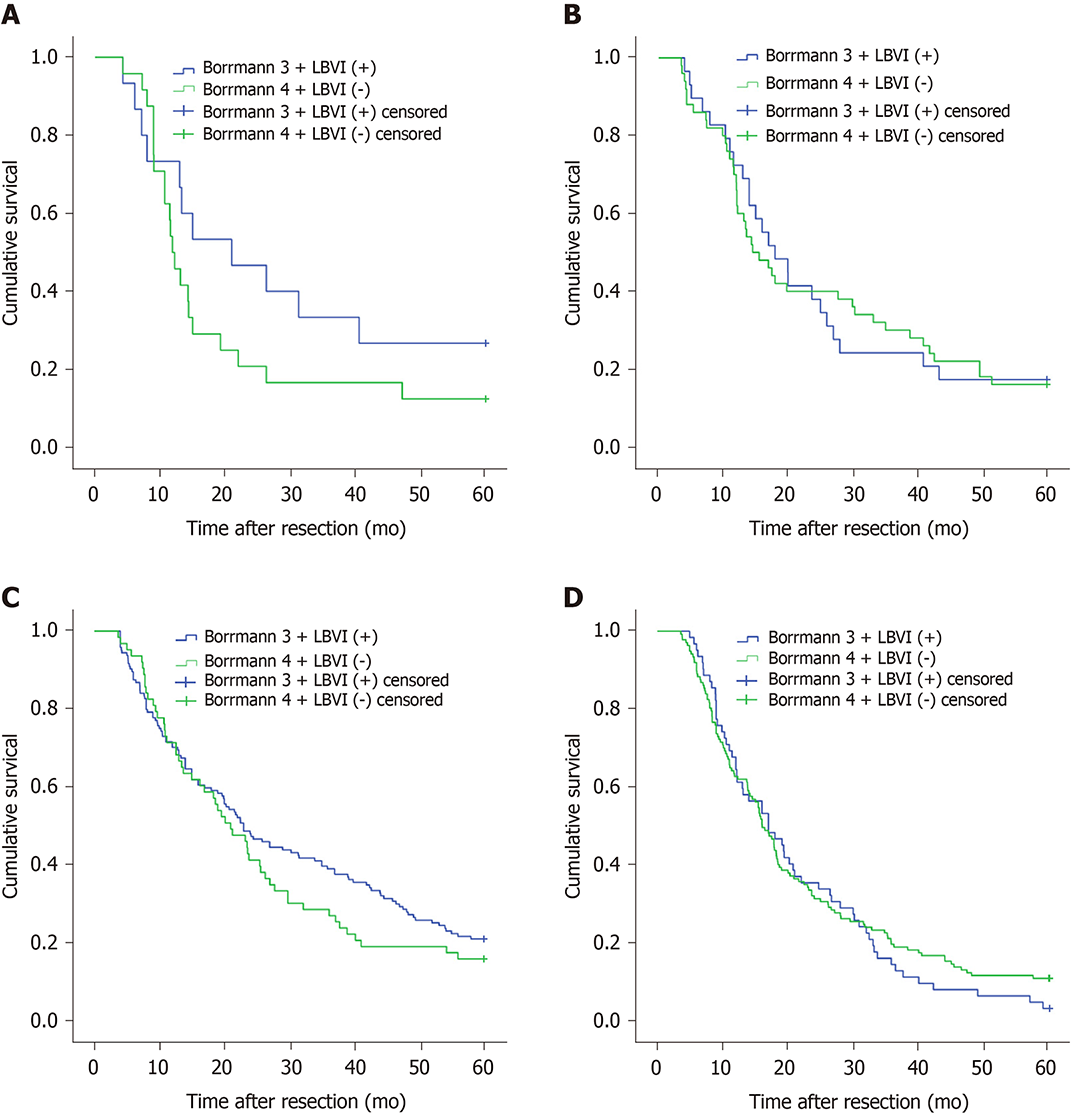
Figure 1A shows that the 5-year survival rate was significantly different among patients with Borrmann types I-IV diseases (P < 0.001), and Figure 1B shows that the 5-year survival rate of LBVI (+) patients was significantly lower than that of LBVI (-) patients (P < 0.001). Then, we analyzed the effect of the combination of Borrmann type and LBVI on the 5-year survival of patients. The results showed that when a patient was classified with Borrmann III disease, the presence or absence of LBVI had a significant impact on survival (16.4% vs 29.1%, P < 0.001; Figure 2C), and the presence or absence of LBVI did not result in a significant difference in the 5-year survival rates among patients with Borrmann types I, II, and IV disease (P = 0.660, 0.281, and 0.793, respectively; Figure 2A, B, and D). Interestingly, patients with Borrmann type III disease and LBVI (+) and those with Borrmann IV disease and LBVI (-) had similar 5-year survival rates (16.4% vs 13.1%, P = 0.065; Figure 3A). Furthermore, patients with Borrmann type IV disease and LBVI (+) had a similar 5-year survival rate to the two groups above (16.4% vs 11.2%, P = 0.112; Figure 3B).
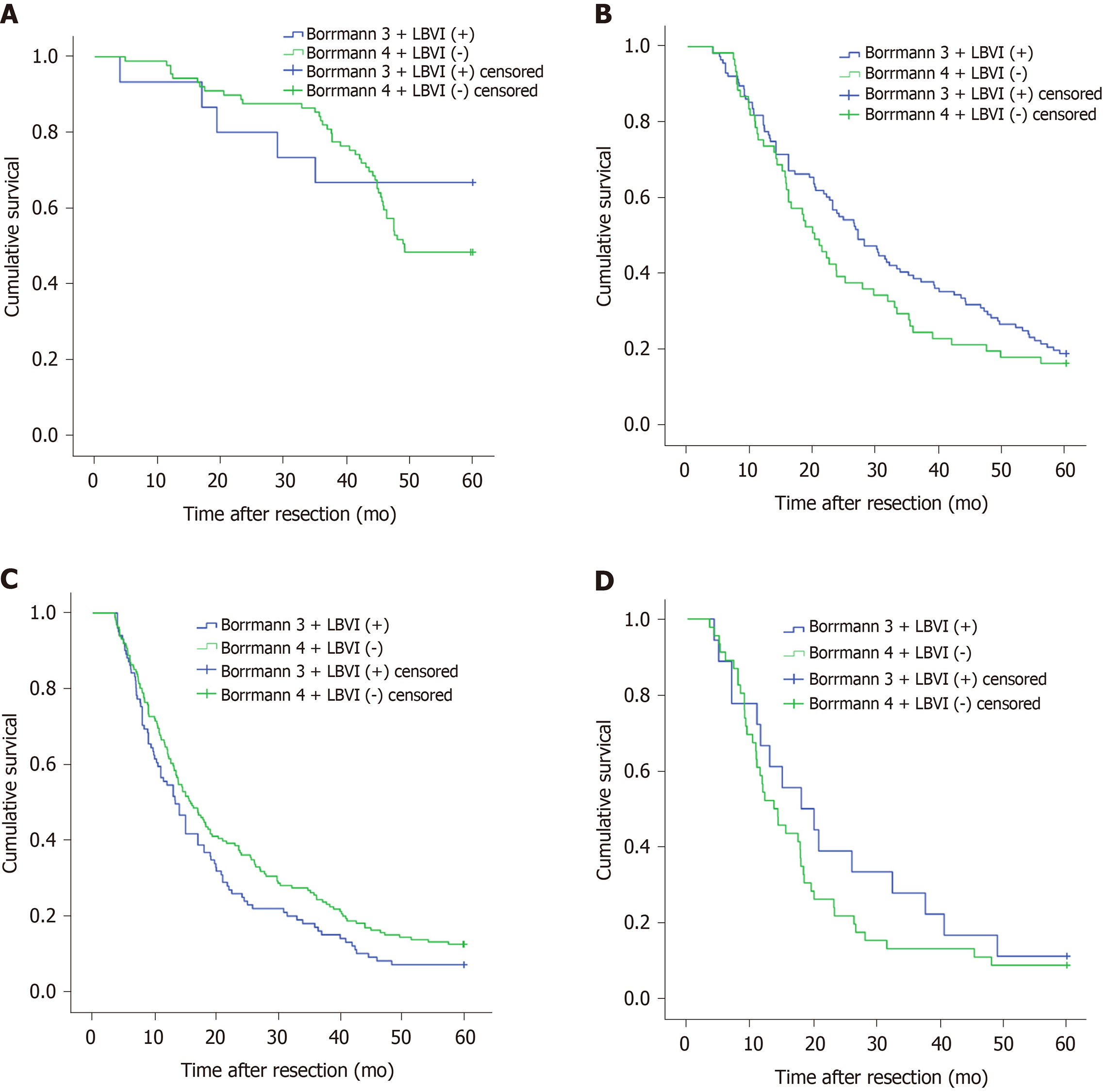
We also conducted a subgroup analysis to determine whether the depth of tumor invasion and tumor location affected the above results. The results showed that regardless of whether the patients had pT2, pT3, pT4a, or pT4b disease, the 5-year survival rates of patients with Borrmann type III disease and LBVI (+) and those with Borrmann type IV disease and LBVI (-) were not significantly different (P = 0.368, 0.202, 0.058, and 0.314, respectively; Figure 4). When the tumor was located in the upper 1/3, middle 1/3, or lower 1/3 of the stomach or when there were overlapping positions, there was a significant difference in the 5-year survival rate between patients with Borrmann type III disease and LBVI (+) and those with Borrmann type IV disease and LBVI (-) (P = 0.205, 0.928, 0.301, and 0.532, respectively; Figure 5).
At present, many researchers have published a large number of reports to explore the factors that affect the prognosis of GC. In general, some clinicopathological factors, such as tumor stage and grade, have been recognized as the most critical indicators that affect postoperative survival[13]. However, there are also a large number of articles showing that the Borrmann classification (types I-IV) has contributed to the macroscopic classification of AGC, and this classification has been accepted worldwide[14,15]. LBVI (+) is associated with a poor postoperative prognosis in GC patients with either positive or negative lymph nodes[16]. Previous studies have shown that the effectiveness of therapy for patients with early GC is relatively good, and the 5-year survival rate after surgery is higher than 90%. However, patients with AGC usually have a poor prognosis, and the 5-year survival rate is less than 30%[17]. Therefore, we aimed to predict the prognosis of GC patients by jointly analyzing two simple and effective clinical indicators, Borrmann type and LBVI status, thereby helping clinicians formulate more accurate treatment plans.
When jointly analyzing Borrmann type and LBVI status to predict the prognosis of patients with AGC, positive or negative LBVI status only resulted in a significant difference in the survival of patients with Borrmann type III disease, while the survival rate of the patients with Borrmann types I, II, and IV disease did not show a significant difference between LBVI statuses. We speculate that this may be due to the small sample size of patients with Borrmann types I, II, and IV disease. We also found that patients with Borrmann type III disease and LBVI (+) and those with Borrmann type IV disease and LBVI (-) had similar 5-year survival rates. The literature has shown that Borrmann type IV GC has a low rate of radical resection (31% to 52%) due to its special biological characteristics, and this rate is significantly lower than that for Borrmann type III GC. Radical resection is an important way for Borrmann type IV GC patients to achieve long-term survival. The 5-year survival rate after radical resection is 7.6%-38.4%, compared with the rate of only 0%-5% for nonradical resection[18]. The extent of radical resection was also an independent prognostic factor for survival among patients in this study. In addition, positive surgical margins are also one of the important causes of tumor residuals. In patients with Borrmann type IV GC, the boundary between the tumor and normal tissue is unclear, and the edge is usually difficult to judge. Studies have reported that the positive margin rate of Borrmann type IV GC is as high as 24.7%, much higher than the 2.2% of Borrmann type III GC[19]. Therefore, more attention should be paid to patients with Borrmann type III GC and LBVI (+). A number of studies have shown that LBVI (+)is associated with a high recurrence rate and low survival rate in many cancer types, and the most common recurrence pattern is peritoneal metastasis[8,9]. In addition, most of the patients in this study did not undergo routine ascites cytology. Positive ascites cytology is one of the important factors affecting the prognosis of patients and may be one of the reasons for peritoneal recurrence. To verify this hypothesis, more studies about the recurrence pattern of Borrmann type III GC with LBVI (+) should be conducted.
In the past 10 years, there have been a large number of studies on proximal GC. As reported by many researchers in Western countries, the number of proximal GC cases is increasing year by year, and a few studies have also observed similar results in Asian countries. Since proximal GC has more aggressive biological characteristics than distal GC, the prognosis of proximal GC is worse than that of distant GC[20-22]. The study by Gao et al[23] showed that in proximal GC, there is no significant difference in prognosis between patients with Borrmann type III disease and LBVI (+) and those with Borrmann type IV and LBVI (-). This is similar to the results of this study, which also included patients with GC in various locations. The depth of tumor invasion has always been an important factor in the prognosis of AGC, and pT status was an independent prognostic factor in patients with AGC in this study. In AGC, there is some overlap between the pT staging system and the Borrmann classification system, and tumor invasion in patients with Borrmann types III and IV GC is usually deeper. Therefore, we conducted a subgroup analysis and explored the effect of pT stage of GC on the results of this study. The results showed that among patients with pT2, pT3, pT4a, and pT4b disease, the 5-year survival rates of patients with Borrmann type III disease and LBVI (+), those with Borrmann type IV disease and LBVI (-), and those with Borrmann type IV disease and LBVI (+) were not significantly different. Therefore, we recommend that regardless of tumor location and tumor pT stage, patients with Borrmann type III AGC and LBVI (+) should be given more attention.
This study has some limitations. First, this is a retrospective study performed at a single center, which might lead to the existence of heterogeneity and internal deviations. Second, due to the small number of patients undergoing neoadjuvant chemotherapy at the time, we did not routinely analyze this variable. In addition, although many patients underwent postoperative systemic adjuvant chemotherapy during the study period, there was a lack of standardized treatment options for the patients. The differential feedback between patients may lead to changes in the treatment plan, so we have not provided sufficient evidence on postoperative adjuvant chemotherapy.
In conclusion, Borrmann type, LBVI status, tumor size, pT stage, pN stage, and extent of radical surgery all independently affected prognosis in this study. Patients with Borrmann type III AGC and LBVI (+) have similar 5-year survival rates to those with Borrmann type IV disease and LBVI (-) or LBVI (+). Therefore, we recommend that clinicians should formulate a comprehensive multidisciplinary, multimodal, and individualized treatment plan when they encounter patients with Borrmann type III GC and LBVI (+), regardless of the pT stage and tumor location, to obtain better survival results.
Gastric cancer (GC) is an important public health burden worldwide. Although the evolution of diagnostic methods has led to an increase in the diagnosis rate of early gastric cancer, most patients present with an advanced stage when they are diagnosed with gastric cancer. Comprehensive multimodal and multidisciplinary treatment systems, including chemotherapy and targeted therapy, are gradually improving. Many studies have shown that Borrmann type and lymphatic and/or blood vessel invasion (LBVI) are independent risk factors for the prognosis of patients with advanced gastric cancer, but few studies have analyzed the prognostic significance of the combination of the two indexes in patients with advanced gastric cancer.
Analyzing whether Borrmann type combined with LBVI has prognostic significance for advanced gastric cancer will provide a basis for clinicians to treat and predict the prognosis of these patients in the future.
To evaluate the significance of Borrmann type combined with LBVI status in evaluating the prognosis of advanced gastric cancer.
This retrospective study analyzed the clinicopathological characteristics and long-term survival data of 2604 patients with advanced gastric cancer, all of whom were diagnosed with advanced gastric adenocarcinoma at the Affiliated Tumor Hospital of Harbin Medical University from 2009 to 2013. Categorical variables were evaluated by the Pearson’s χ2 test, the Kaplan-Meier method was used to identify differences in cumulative survival rates, and the Cox proportional hazards model was used for multivariate prognostic analysis.
This retrospective study included a total of 2604 patients. The results showed that the 5-year survival rate of Borrmann types I-IV patients was significantly different (P < 0.001), and the 5-year survival rate of patients with LBVI (+) was significantly lower than that of LBVI (-) patients. When we combined Borrmann type and LBVI status, we found that patients with Borrmann type III disease and LBVI (+) had a similar 5-year survival rate to those with Borrmann type IV disease and LBVI (-) (16.4% vs 13.1%, P = 0.065) or LBVI (+) (16.4% vs 11.2%, P = 0.112). Subgroup analysis showed that the above results were true in any pT stage and any tumor location. Multivariate Cox regression analysis showed that Borrmann type (P = 0.023), LBVI (P < 0.001), tumor size (P = 0.012), pT staging (P < 0.001), pN stage (P < 0.001), and extent of radical surgery (P < 0.001) are independent prognostic factors.
Borrmann type, LBVI status, tumor size, pT stage, pN stage, and extent of radical surgery all independently affect prognosis. Patients with Borrmann type III disease and LBVI (+) have a similar 5-year survival rate to those with Borrmann type IV disease and LBVI (-) or LBVI (+)
We recommend that clinicians should formulate a comprehensive multidisciplinary, multimodal, and individualized treatment plan when they encounter patients with Borrmann type III GC and LBVI (+), regardless of the pT stage and tumor location, to obtain better survival results.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Krishnan A, Theiss AL, Yoo C S-Editor: Gong ZM L-Editor: Wang TQ P-Editor: Ma YJ
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun (Lond). 2019;39:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 733] [Cited by in RCA: 1122] [Article Influence: 187.0] [Reference Citation Analysis (1)] |
| 3. | Schwartz GK, Winter K, Minsky BD, Crane C, Thomson PJ, Anne P, Gross H, Willett C, Kelsen D. Randomized phase II trial evaluating two paclitaxel and cisplatin-containing chemoradiation regimens as adjuvant therapy in resected gastric cancer (RTOG-0114). J Clin Oncol. 2009;27:1956-1962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Marrelli D, Morgagni P, de Manzoni G, Coniglio A, Marchet A, Saragoni L, Tiberio G, Roviello F; Italian Research Group for Gastric Cancer (IRGGC). Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer: analysis of a large series from specialized Western centers. Ann Surg. 2012;255:486-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 5. | Huang JY, Xu YY, Li M, Sun Z, Zhu Z, Song YX, Miao ZF, Wu JH, Xu HM. The prognostic impact of occult lymph node metastasis in node-negative gastric cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:3927-3934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Borchard F. Classification of gastric carcinoma. Hepatogastroenterology. 1990;37:223-232. [PubMed] |
| 7. | del Casar JM, Corte MD, Alvarez A, García I, Bongera M, González LO, García-Muñiz JL, Allende MT, Astudillo A, Vizoso FJ. Lymphatic and/or blood vessel invasion in gastric cancer: relationship with clinicopathological parameters, biological factors and prognostic significance. J Cancer Res Clin Oncol. 2008;134:153-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Bu Z, Zheng Z, Li Z, Zhang L, Wu A, Wu X, Sun Y, Ji J. Lymphatic vascular invasion is an independent correlated factor for lymph node metastasis and the prognosis of resectable T2 gastric cancer patients. Tumour Biol. 2013;34:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Zhao LY, Chen XL, Wang YG, Xin Y, Zhang WH, Wang YS, Chen XZ, Yang K, Liu K, Xue L, Zhang B, Chen ZX, Chen JP, Zhou ZG, Hu JK. A new predictive model combined of tumor size, lymph nodes count and lymphovascular invasion for survival prognosis in patients with lymph node-negative gastric cancer. Oncotarget. 2016;7:72300-72310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Luo Y, Gao P, Song Y, Sun J, Huang X, Zhao J, Ma B, Li Y, Wang Z. Clinicopathologic characteristics and prognosis of Borrmann type IV gastric cancer: a meta-analysis. World J Surg Oncol. 2016;14:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Marano L, D'Ignazio A, Cammillini F, Angotti R, Messina M, Marrelli D, Roviello F. Comparison between 7th and 8th edition of AJCC TNM staging system for gastric cancer: old problems and new perspectives. Transl Gastroenterol Hepatol. 2019;4:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3). Gastric Cancer. 2011;14:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1723] [Cited by in RCA: 1897] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 13. | Lu J, Wang W, Zheng CH, Fang C, Li P, Xie JW, Wang JB, Lin JX, Chen QY, Cao LL, Lin M, Huang CM, Zhou ZW. Influence of Total Lymph Node Count on Staging and Survival After Gastrectomy for Gastric Cancer: An Analysis From a Two-Institution Database in China. Ann Surg Oncol. 2017;24:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 14. | Li C, Oh SJ, Kim S, Hyung WJ, Yan M, Zhu ZG, Noh SH. Macroscopic Borrmann type as a simple prognostic indicator in patients with advanced gastric cancer. Oncology. 2009;77:197-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Pan M, Huang P, Li S, Chen J, Wei S, Zhang Y. Double contrast-enhanced ultrasonography in preoperative Borrmann classification of advanced gastric carcinoma: comparison with histopathology. Sci Rep. 2013;3:3338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Li P, Ling YH, Zhu CM, Hu WM, Zhang XK, Luo RZ, He JH, Yun JP, Li YF, Cai MY. Vascular invasion as an independent predictor of poor prognosis in nonmetastatic gastric cancer after curative resection. Int J Clin Exp Pathol. 2015;8:3910-3918. [PubMed] |
| 17. | Katai H, Ishikawa T, Akazawa K, Isobe Y, Miyashiro I, Oda I, Tsujitani S, Ono H, Tanabe S, Fukagawa T, Nunobe S, Kakeji Y, Nashimoto A; Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer. 2018;21:144-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 358] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 18. | Zhu YL, Yang L, Sui ZQ, Liu L, Du JF. Clinicopathological features and prognosis of Borrmann type IV gastric cancer. J BUON. 2016;21:1471-1475. [PubMed] |
| 19. | Kitamura K, Beppu R, Anai H, Ikejiri K, Yakabe S, Sugimachi K, Saku M. Clinicopathologic study of patients with Borrmann type IV gastric carcinoma. J Surg Oncol. 1995;58:112-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Ze-Long Y, Guo-Hui M, Lin Z, Wei-Hong Y, Ke-Cheng Z, Yan-Wen J. Survival Trends of Patients With Surgically Resected Gastric Cardia Cancer From 1988 to 2015: A Population-based Study in the United States. Am J Clin Oncol. 2019;42:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Men W, Xiao H, Yang Z, Fan D. Agglomeration Effect of Medical Education: Based on the Web of Science Database. J Transl Int Med. 2018;6:165-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Wang G, Liu X, Wang S, Ge N, Guo J, Sun S. Endoscopic Ultrasound-guided Gastroenterostomy: A Promising Alternative to Surgery. J Transl Int Med. 2019;7:93-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 23. | Gao S, Cao GH, Ding P, Zhao YY, Deng P, Hou B, Li K, Liu XF. Retrospective evaluation of lymphatic and blood vessel invasion and Borrmann types in advanced proximal gastric cancer. World J Gastrointest Oncol. 2019;11:642-651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |