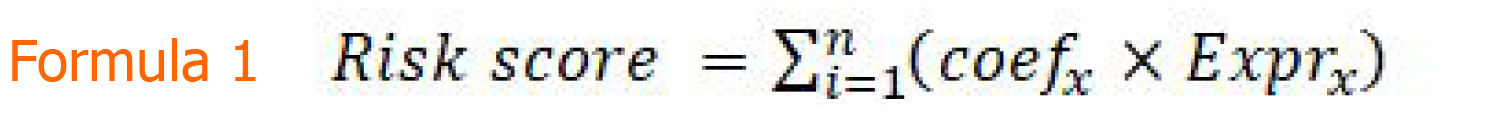Published online Sep 15, 2020. doi: 10.4251/wjgo.v12.i9.975
Peer-review started: April 5, 2020
First decision: May 15, 2020
Revised: May 15, 2020
Accepted: August 1, 2020
Article in press: August 1, 2020
Published online: September 15, 2020
Processing time: 155 Days and 1.6 Hours
Gastric carcinoma (GC) is one of the most aggressive primary digestive cancers. It has unsatisfactory therapeutic outcomes and is difficult to diagnose early.
To identify prognostic biomarkers for GC patients using comprehensive bioinformatics analyses.
Differentially expressed genes (DEGs) were screened using gene expression data from The Cancer Genome Atlas and Gene Expression Omnibus databases for GC. Overlapping DEGs were analyzed using univariate and multivariate Cox regression analyses. A risk score model was then constructed and its prognostic value was validated utilizing an independent Gene Expression Omnibus dataset (GSE15459). Multiple databases were used to analyze each gene in the risk score model. High-risk score-associated pathways and therapeutic small molecule drugs were analyzed and predicted, respectively.
A total of 95 overlapping DEGs were found and a nine-gene signature (COL8A1, CTHRC1, COL5A2, AADAC, MAMDC2, SERPINE1, MAOA, COL1A2, and FNDC1) was constructed for the GC prognosis prediction. Receiver operating characteristic curve performance in the training dataset (The Cancer Genome Atlas-stomach adenocarcinoma) and validation dataset (GSE15459) demonstrated a robust prognostic value of the risk score model. Multiple database analyses for each gene provided evidence to further understand the nine-gene signature. Gene set enrichment analysis showed that the high-risk group was enriched in multiple cancer-related pathways. Moreover, several new small molecule drugs for potential treatment of GC were identified.
The nine-gene signature-derived risk score allows to predict GC prognosis and might prove useful for guiding therapeutic strategies for GC patients.
Core Tip: A total of 95 differentially expressed genes were found by mining the datasets of Gene Expression Omnibus and the Cancer Genome Atlas databases. Overlapping differentially expressed genes were analyzed using univariate and multivariate Cox regression analyses. Receiver operating characteristic curve performance in the training and validation datasets demonstrated a robust prognostic value of the risk score model. Multiple database analyses for each gene provided evidence to further understand the nine-gene signature. Gene set enrichment analysis showed that the high-risk group was enriched in multiple cancer-related pathways. Moreover, several new small molecule drugs for potential treatment of gastric carcinoma (GC) were identified. A nine-gene signature was identified to predict GC prognosis and prove potentially useful for guiding therapeutic strategies for GC patients.
- Citation: Wu KZ, Xu XH, Zhan CP, Li J, Jiang JL. Identification of a nine-gene prognostic signature for gastric carcinoma using integrated bioinformatics analyses. World J Gastrointest Oncol 2020; 12(9): 975-991
- URL: https://www.wjgnet.com/1948-5204/full/v12/i9/975.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i9.975
Gastric carcinoma (GC) is a lethal digestive malignant tumor that ranks as the fifth most commonly occurring cancer and the third cause of cancer-related death worldwide. In 2018, the global incidence and mortality of GC were estimated at 1033000 and 783000, respectively[1]. Despite advances in various therapeutic strategies, the 5-year survival rate for GC is still less than 30% and 70% of patients with GC are usually diagnosed at an advanced stage[2,3]. Therefore, it is necessary to search for a multiple-gene signature-derived model for predicting prognosis and accurately identifying anti-cancer targeted therapies to improve the prognostic stratification and personalized therapy for GC patients.
With the popularization and advancement of high throughput sequencing technologies, there is a real possibility of establishing multiple-gene signatures based on data integration and bioinformatics analysis in cancer research. The Gene Expression Omnibus (GEO) database and The Cancer Genome Atlas (TCGA) project provide invaluable resources for researchers worldwide to query gene expression and other functional genomics data[4,5]. For example, Zhao et al[6] constructed a five-gene signature based on data from TCGA databases that accurately predicted GC prognosis. Similarly, an 11-microRNA signature-derived risk score module was demonstrated to effectively predict prognosis in GC via a comprehensive genome-wide profiling analysis[7]. Therefore, it is necessary to identify genes that are significantly correlated with progression in GC patients and to further establish robust multiple-gene signatures, which could provide early diagnosis and optimized therapy for GC patients.
In the current study, GC gene expression data from TCGA and GEO datasets were first evaluated using a comprehensive bioinformatics analysis that filtered out overlapping differentially expressed genes (DEGs). Multivariate Cox regression was applied to construct a nine-gene signature based on these identified DEGs to estimate prognosis and therapeutic outcomes in GC. The high-risk group was verified to be associated with tumor-associated pathways based on the nine-gene signature derived risk score model, which also identified promising small molecule drugs.
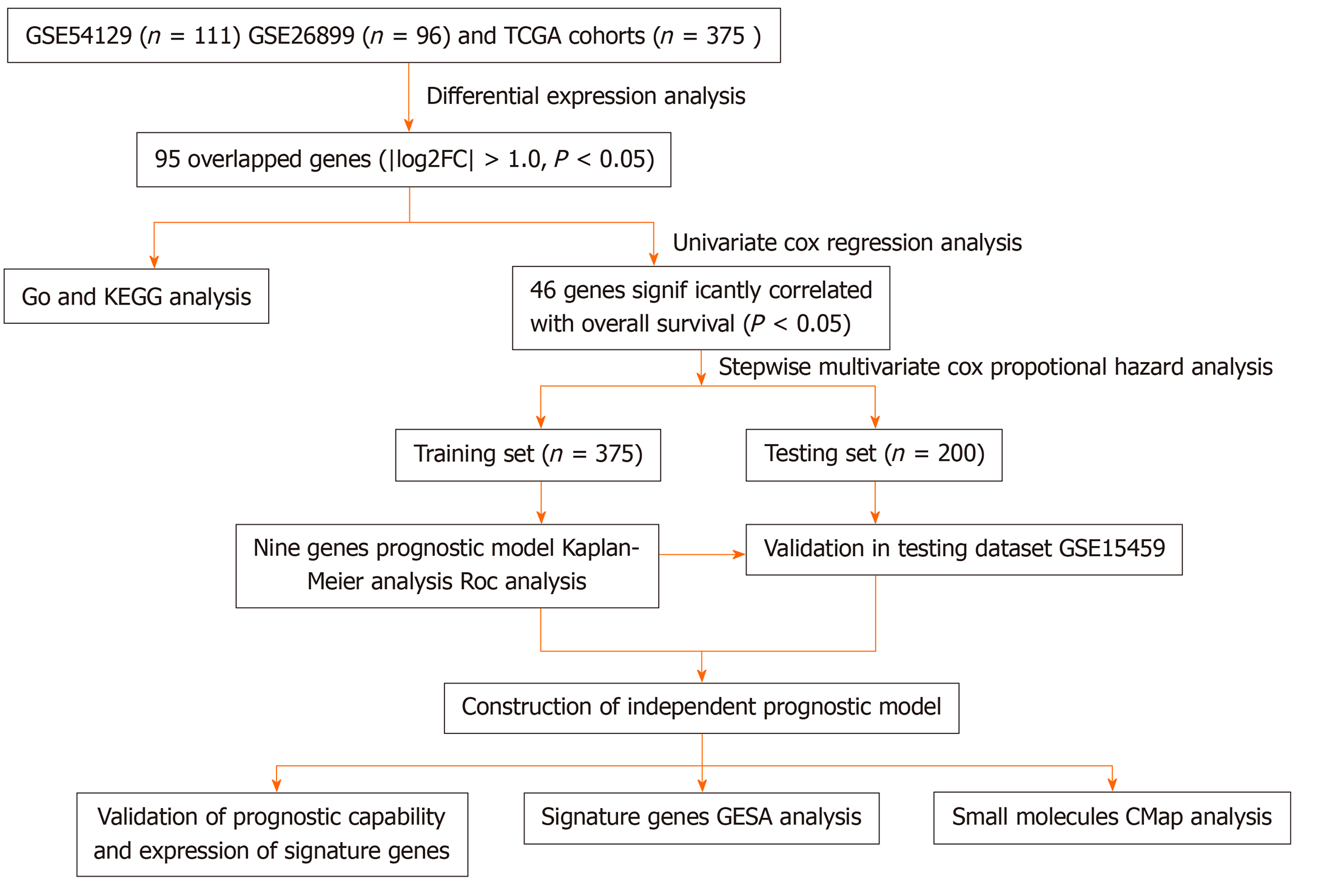
The two independent GC microarray datasets GSE54129 (containing 111 GC and 21 non-cancerous samples) and GSE26899 (including 96 GC and 12 non-cancerous samples) were obtained from the GEO database and normalized using the robust multi-array average method[8]. Gene sequencing data and corresponding clinical information containing 375 GC samples and 32 non-cancerous samples were extracted from the TCGA-STAD (stomach adenocarcinoma) database. Subsequently, DEGs were filtrated out from the three-gene expression datasets. A flowchart of this study is showed in Figure 1.
After standardization and log2 transformation of data from the original GEO datasets using the Affy package, the DEGs in GC were compared to normal gastric tissues and analyzed using the limma package in R software (version 3.2.1, https://www.r-project.org/)[9]. The |log2FoldChange (log2FC)| ≥ 1 and adjusted P value < 0.05 were defined as the cut-off criteria for identifying DEGs. In addition, the EdgeR package in R was used to explore DEGs for the RNA-Seq count from the TCGA database[10]. Data cut-off criteria were the same as described above. The upregulated/downregulated genes in the TCGA-GC cohort, GSE54129, and GSE26899 were overlapped to identify common and robust DEGs in GC.
Gene ontology functional enrichment analysis was performed to expound potential biological processes, molecular functions, and cellular components for the common DEGs. Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis was performed to explore potential signaling pathways associated with overlapping genes, which might influence GC survival. All of the above analyses were performed by utilizing the online Database for Annotation, Visualization, and Integrated Discovery (DAVID, version 6.8, https://david.ncifcrf.gov/)[11].
To further clarify the relationship between overlapping DEGs and the overall survival (OS) in GC patients, a univariate Cox proportional-hazards regression model in the TCGA-STAD cohort was utilized. Genes with a hazard ratio (HR) < 1 or > 1 were considered protective or risky, respectively. Subsequently, a multivariate Cox proportional-hazards regression analysis was performed to construct multiple DEG signatures. A risk score model was established using the Formula 1. In this equation, “coefx” represents the regression coefficient of gene X and “Exprx” is the expression value of gene X in the signature.
Gene expression data in the TCGA-STAD cohort were classified into high- and low-risk groups according to median cutoff of the risk score to evaluate the prognostic value of the risk score model. Survival differences between the two groups were compared using Kaplan-Meier (KM) survival analysis with the log-rank test. Reliability of the risk score model was assessed using the area under curve (AUC) of the receiver operating characteristic (ROC) curve.
Moreover, the reliability and prognostic value were validated using the ROC and Kaplan–Meier curves in an additional dataset GSE15459 containing 200 GC samples from the GEO database to explore whether the nine-gene signature functions as an independent prognostic factor.
The cBioPortal for the Cancer Genomics (http://www.cbioportal.org) database was utilized to verify a connection between genetic alterations and the nine genes. Then, Gene Expression Profiling Interactive Analysis (GEPIA, http://gepia.cancer-pku.cn/detail.php) was utilized to explore the expression of the nine genes at the transcriptional and translational levels, respectively. Furthermore, an OS analysis of each gene in patients with GC was analyzed using the KM plotter database (http://kmplot.com/analysis/).
Gene set enrichment analysis (GSEA) (http://software.broadinstitute.org/gsea) was used to identify the promising signaling pathways for the high-risk group based on the risk score module[12]. P value < 0.05 and |normalized enrichment score (NES)| > 0.65 were utilized to determine which functions to explore further.
The connectivity map (CMap) online database (http://www.broadinstitute.org) allows to investigate the interrelation among small molecule drugs, DNA microarray data, and diseases[13]. It was used to predict promising small molecule drugs involved in the overlapping DEGs from the GEO database and TCGA project that might be useful for treatment of GC.
Kaplan–Meier curves and log-rank method were utilized to validate the statistical criteria of observed differences in OS for low- and high-risk GC patients. The univariate and multivariate Cox proportional-hazards regression analyses were performed to estimate prognostic effects of independent genes and potential multiple-gene signatures. An ROC curve was used to evaluate the diagnostic performance of the nine-gene signature by calculating the AUC. All statistical analyses were performed using SPSS 20.0 (SPSS Inc., Chicago, IL, United States) and Prism 7.0 (GraphPad Software Inc., La Jolla, CA, United States) software. A P value < 0.05 was considered statistically significant.
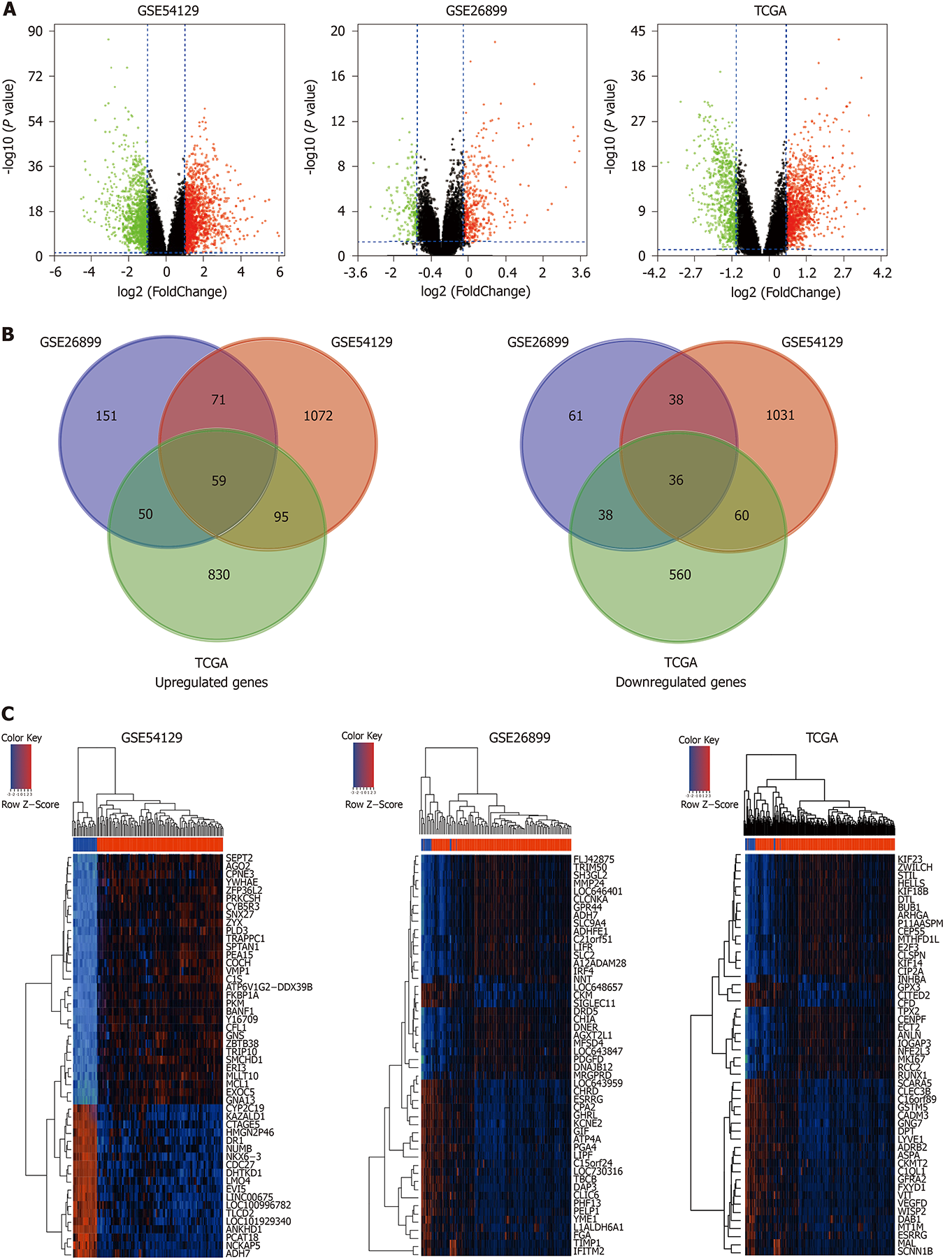
Using the cut-off criteria, where P < 0.05 and |log2FC| > 1.0, 1297 upregulated genes and 1165 downregulated genes in GSE54129, 331 upregulated genes and 173 downregulated genes in GSE26889, and 1034 highly expressed genes and 694 lowly expressed genes in the TCGA-STAD cohort were identified (Figure 2A). Furthermore, a total of 95 overlapping DEGs were screened out from the GEO microarray datasets and TCGA-STAD dataset, of which 59 were significantly upregulated and 36 were downregulated (Figure 2B). Hierarchical cluster heatmaps were used to explore DEG details between GC and non-cancerous tissues in each GC dataset (Figure 2C). Detailed information from the GEO datasets is shown in Supplementary Table 1.
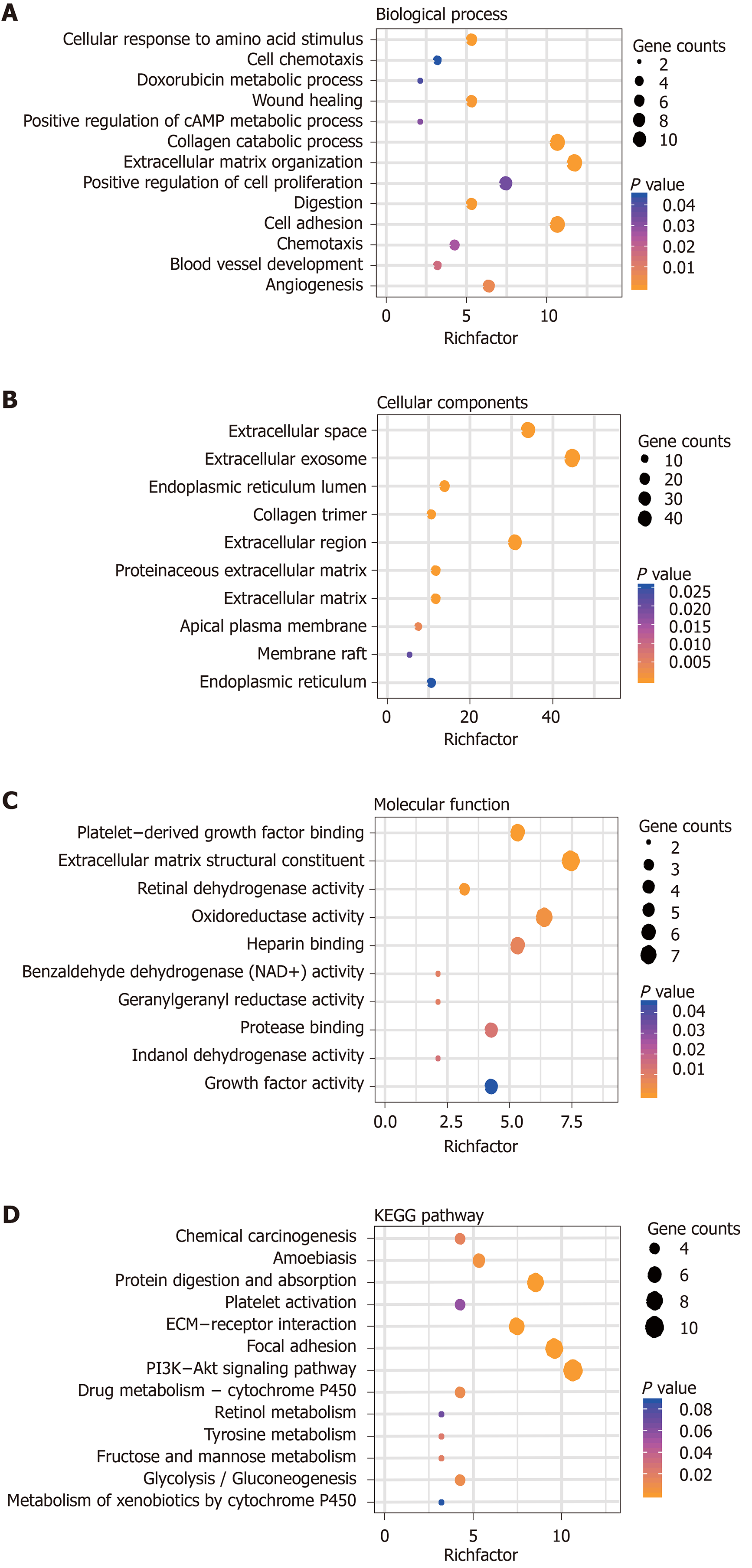
Gene Ontology and Genes and Genomes Pathway analyses were used to further elucidate the potential biological function and promising signaling pathways of the overlapping genes in GC. The biological processes analysis indicated that the most genes were enriched during the cellular response to amino acid stimulus, cell chemotaxis, doxorubicin metabolic process, and extracellular matrix organization. The cellular components analysis showed that the genes were enriched in the extracellular space, extracellular region, and proteinaceous extracellular matrix. The molecular functions analysis indicated that the genes were enriched in the extracellular matrix structural constituent, oxidoreductase activity, and protease binding. Biological pathways were mainly enriched with chemical carcinogenesis, focal adhesion, drug metabolism-cytochrome P450, and PI3K/Akt signaling pathways (Figure 3).
To determine promising biomarkers in connection with the prognosis of patients with GC, univariate Cox regression was performed to measure 95 overlapped genes in the TCGA-STAD cohort. A total of 46 genes (P < 0.05) were significantly correlated to OS in GC (Supplementary Table 2). These genes were then evaluated using multivariate Cox regression analysis.
Finally, a nine-gene signature (COL8A1, CTHRC1, COL5A2, AADAC, MAMDC2, SERPINE1, MAOA, COL1A2, and FNDC1) was constructed to assess the prognostic risk for each patient as follows: Risk score = βCOL8A1*ECOL8A1 + βCTHRC1*ECTHRC1 + βCOL5A2*ECOL5A2 + βAADAC*EAADAC + βMAMDC2*EMAMDC2 + βSERPINE1*ESERPINE1 + βMAOA*EMAOA + βCOL1A2*ECOL1A2 + ΒFNDC1*EFNDC1 (Table 1), where “E” is the expression level of the genes obtained from multivariate Cox regression analysis based on the TCGA-STAD dataset.
| Gene symbol | Description | Coef | HR | 95%CI | P value |
| COL8A1 | Collagen type VIII alpha 1 chain | -0.39134 | 0.6761 | 0.4974-0.9191 | 0.01247a |
| CTHRC1 | Collagen triple helix repeat containing 1 | 0.36470 | 1.4401 | 1.1432-1.8140 | 0.00196a |
| COL5A2 | Collagen type V alpha 2 chain | 0.45550 | 1.5770 | 0.9116-2.7278 | 0.10329 |
| AADAC | Arylacetamide deacetylase | 0.14823 | 1.1598 | 1.0437-1.2887 | 0.00585a |
| MAMDC2 | MAM domain containing 2 | 0.21034 | 1.2341 | 1.0514-1.4485 | 0.01006a |
| SERPINE1 | Serpin family E member 1 | 0.23183 | 1.2609 | 1.0583-1.5023 | 0.00949a |
| MAOA | Monoamine oxidase A | 0.17086 | 1.1863 | 1.0151-1.3865 | 0.03172a |
| COL1A2 | Collagen type I alpha 2 chain | -0.54104 | 0.5821 | 0.3462-0.9790 | 0.04135a |
| FNDC1 | Fibronectin type III domain containing 1 | 0.23264 | 1.2619 | 0.9808-1.6236 | 0.07041 |
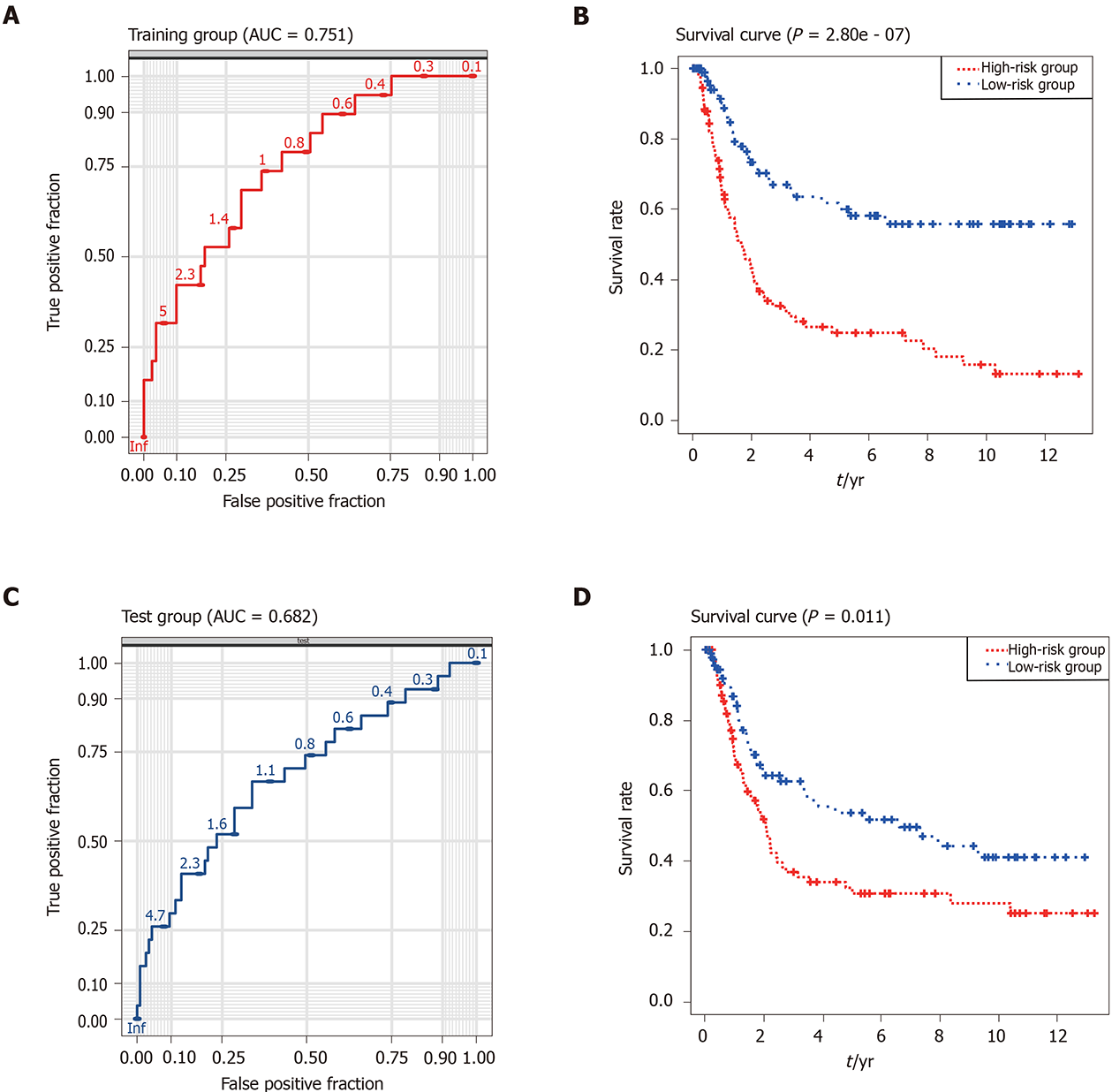
Subsequently, patients with GC were classified into high- and low-risk groups according to the median risk score of the nine-gene signature. The ROC and KM curves were used to evaluate prognostic capacity of the nine-gene signature in GC. The AUC reached 0.751, suggesting that this nine-gene signature was relatively sensitive and specific in prognostic prediction for GC patients (Figure 4A). Moreover, results of the Kaplan–Meier curve for the two collectives indicated that patients in the low-risk group had a better OS than those in the high-risk group (P < 0.001; Figure 4B). Taken together, the results demonstrated that the nine-gene signature-derived risk score was significantly different for prognosis and OS between the two groups.
To validate the repeatability and robustness of the nine-gene risk signature, an independent dataset GSE15459 was used as an external validation with the same formula. Patients in the dataset GSE15459 were divided into a high- or low-risk group with the same cutoff value as the training cohort. AUC for the nine-gene signature was calculated to be 0.682, which indicated that the model had a good prognostic capability for the survival of patients with GC in the testing collective (Figure 4C). Furthermore, in accordance with the training dataset, patients in the high-risk group had a significantly shorter OS than those in the low-risk group (P = 0.011; Figure 4D). These data further showed that the nine-gene signature could predict the prognosis of patients with GC.
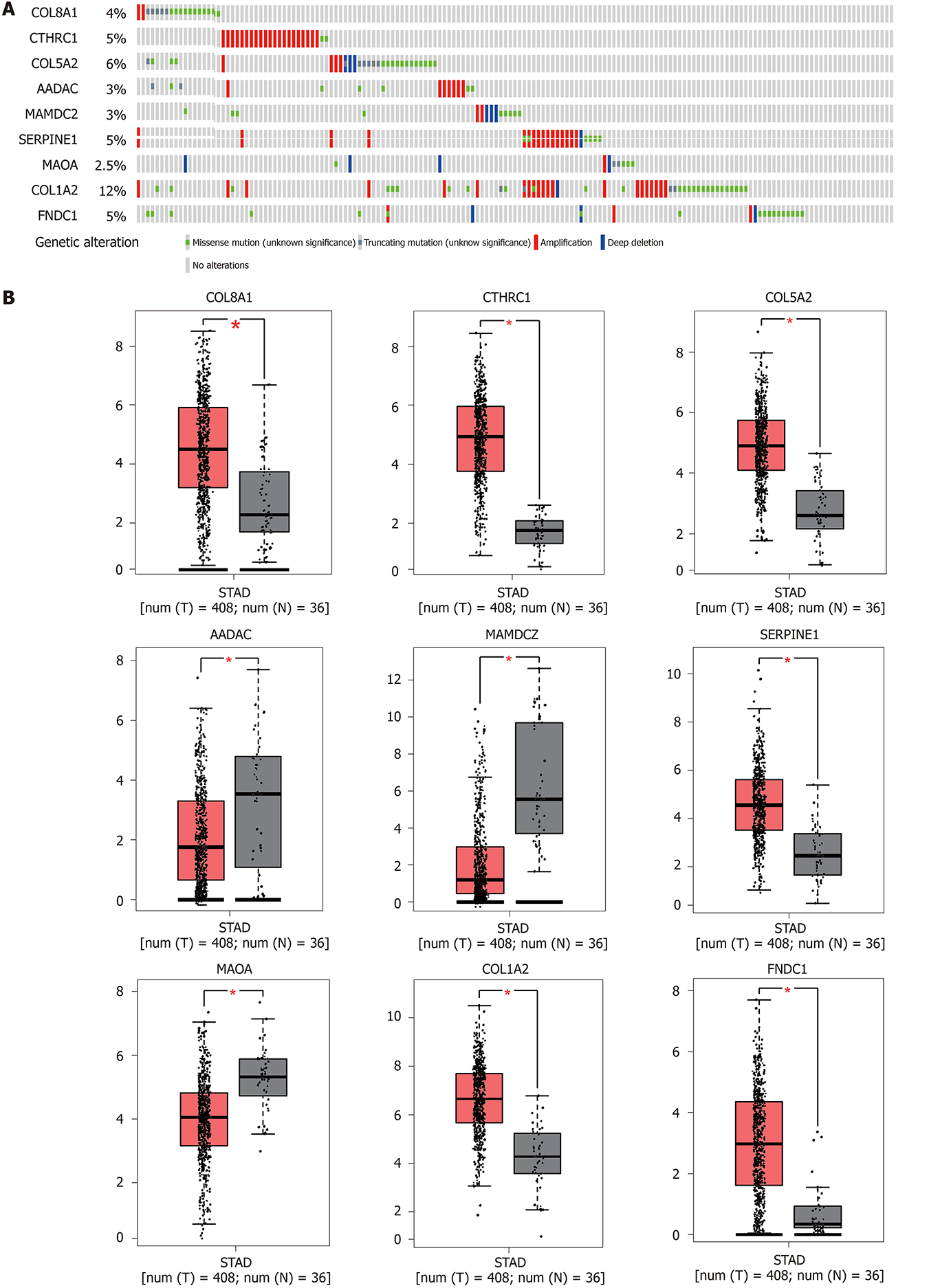
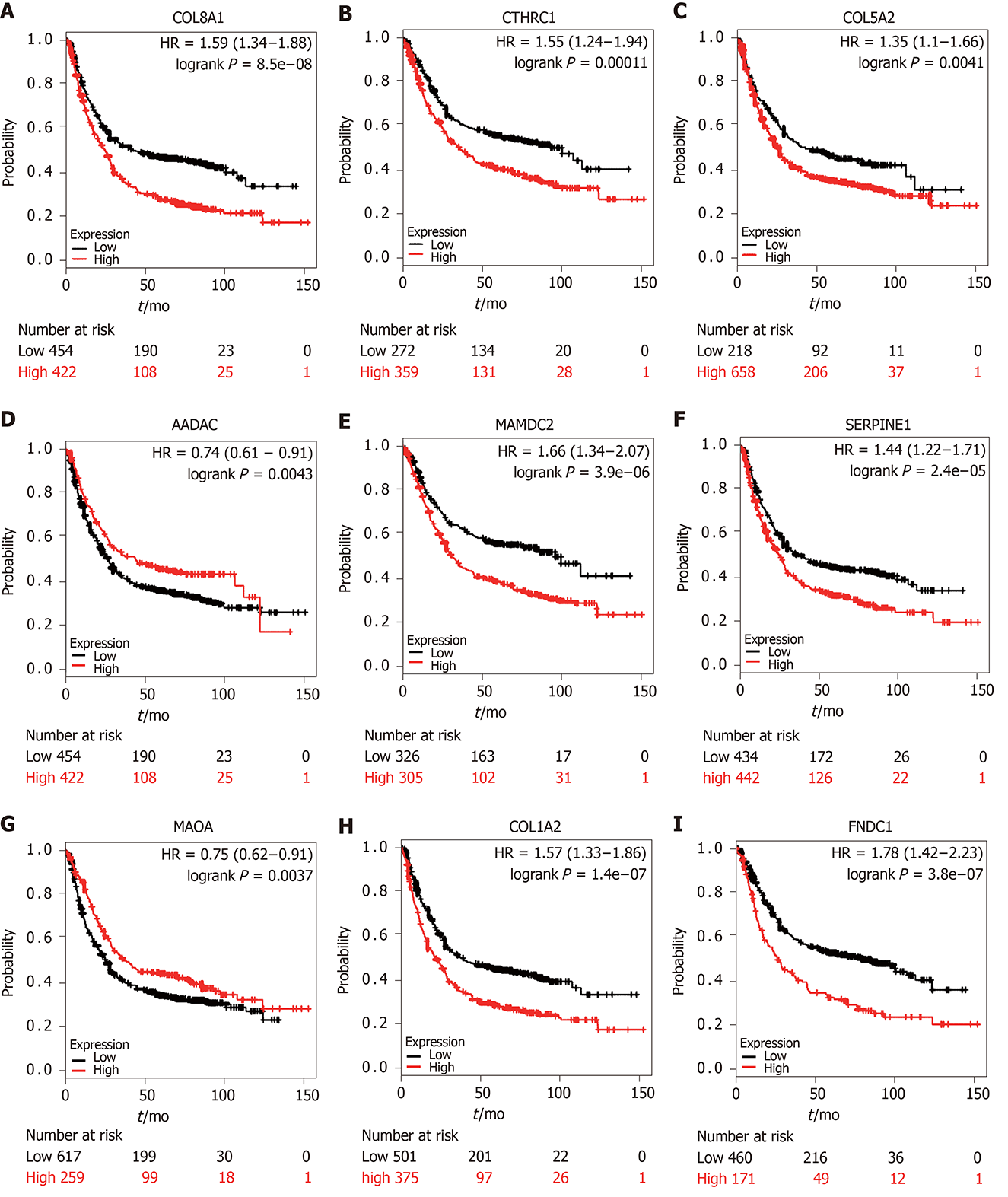
Genetic alterations in the nine genes were analyzed by exploring 375 GC samples in the cBioPortal database. The results indicated that 158 (44%) samples had genetic alterations in the nine genes. Sequence mutations for each gene are shown in Figure 5A. Furthermore, expression levels for the nine genes were significantly different (COL8A1, CTHRC1, COL5A2, SERPINE1, COL1A2, and FNDC1 were upregulated and AADAC, MAOA, and MAMDC2 were downregulated) in GC tumor tissues compared to non-cancerous tissues based on the GEPIA database (Figure 5B). KM plotter was used to study the prognostic performance of each gene in GC. The results identified that high COL8A1, CTHRC1, COL5A2, SERPINE1, COL1A2, MAMDC2, and FNDC1 expression was related to a worse prognosis, while high MAOA, and AADAC expression was related to a better prognosis in GC patients (Figure 6).
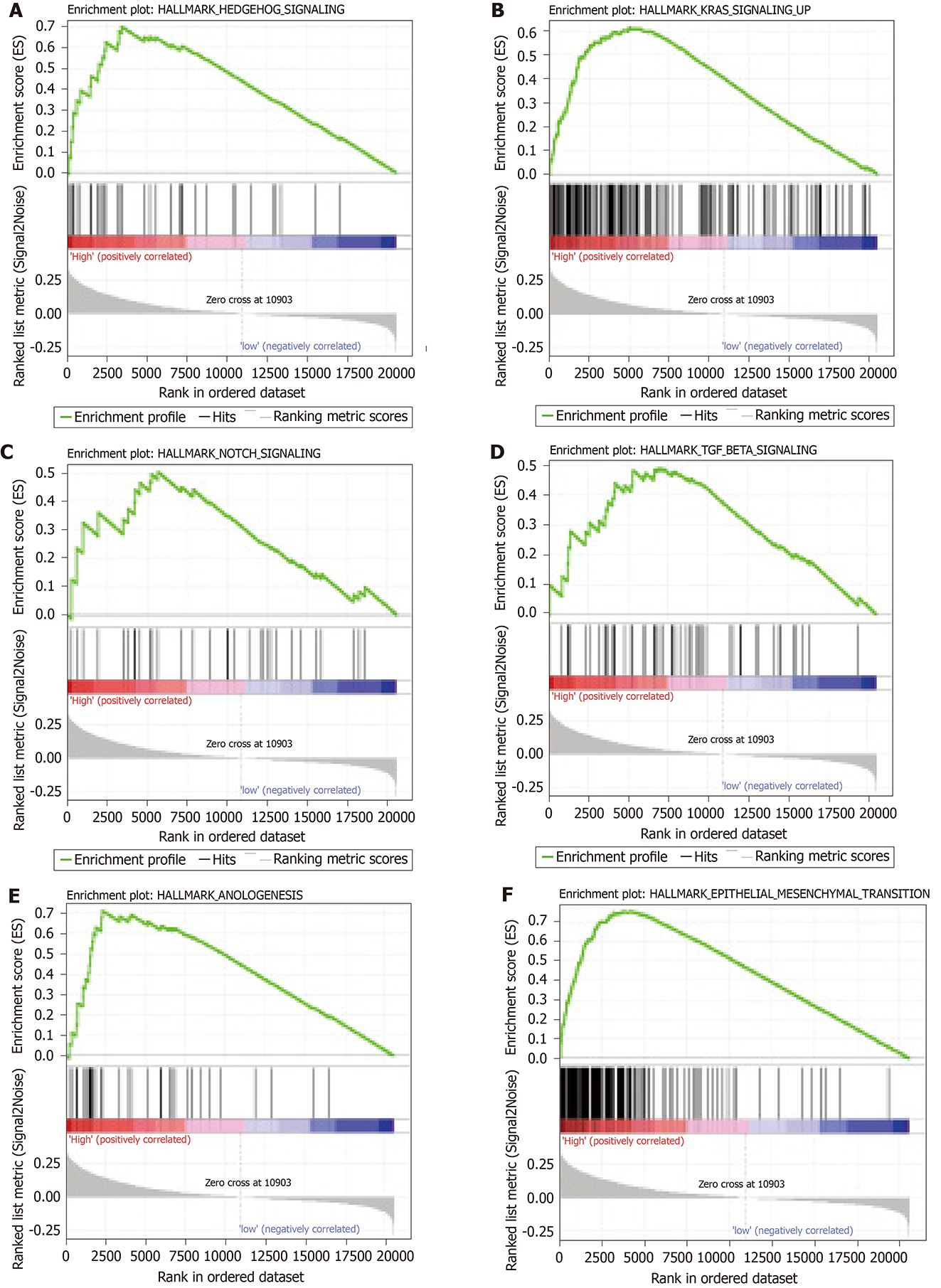
GSEA analysis was performed to explore potential signaling pathways associated with the high-risk group based on the nine-gene signature-derived risk score. The cut-off value was set at P < 0.05 and |enrichment score (ES)| > 0.65. Results showed that multiple tumor-associated pathways, such as angiogenesis, epithelial-mesenchymal transition, hedgehog signaling, Kirsten rat sarcoma viral oncogene homologue signaling, Notch signaling, and transforming growth factor (TGF)-β signaling, were enriched in the high-risk group GC patients (Figure 7).
The nine-gene signature was further analyzed in the CMap database to predict potential small molecule drugs for GC. Ten small molecule drugs were revealed using the high connectivity score and P value < 0.05 (Table 2). A total of nine small molecule candidates were negatively correlated. The 3D conformers for the top five most significant candidates are shown in Supplementary Figure 1. All findings indicated that these drugs had potential therapeutic applications in GC.
| Rank | CMap name | mean | n | Enrichment | P value | Specificity | Percent non-null |
| 1 | Trichostatin A | -0.344 | 182 | -0.364 | < 0.001 | 0.5545 | 50 |
| 2 | Thiamphenicol | -0.469 | 5 | -0.78 | 0.001 | 0.0333 | 60 |
| 3 | Vorinostat | 0.419 | 12 | 0.518 | 0.002 | 0.4221 | 66 |
| 4 | Levomepromazine | -0.245 | 4 | -0.814 | 0.002 | 0.0094 | 50 |
| 5 | Lasalocid | -0.299 | 4 | -0.789 | 0.004 | 0.0463 | 50 |
| 6 | Clorsulon | -0.303 | 4 | -0.76 | 0.007 | 0.0284 | 50 |
| 7 | Prestwick-1103 | -0.299 | 4 | -0.759 | 0.007 | 0.0397 | 50 |
| 8 | Aminobenzenesulfonamide | -0.389 | 4 | -0.712 | 0.014 | 0.0486 | 50 |
| 9 | Digoxigenin | -0.393 | 5 | -0.625 | 0.019 | 0.1071 | 60 |
| 10 | Disulfiram | -0.404 | 5 | -0.616 | 0.023 | 0.0935 | 60 |
Despite considerable development in the arena of various therapeutic GC strategies, including surgery, radiotherapy, chemotherapy, and targeted precise treatment, the OS of advanced GC patients has remained poor and the therapeutic effect is often unsatisfying. The current prognostic model established based on clinical prognostic factors, such as age, TNM stage, and pathology grade, is a routine predictive model for GC. However, because of the high GC heterogeneity, a conventional prognostic model cannot accurately predict the outcomes for GC patients. Multiple-gene assays, by contrast, are of great importance for precision medicine for GC patients[14]. Therefore, exploring potential molecular mechanisms and effective therapeutic targets is important for GC therapy and prevention. This study performed an integrated bioinformatics analysis to establish a nine-gene risk score model associated with prognosis and treatment response in GC patients. The high-risk group was identified to relate to tumor-associated signaling pathways based on the nine-gene signature-derived risk score and several novel small molecule drugs were discovered for potential GC treatment.
The initial step in this study was to identify the DEGs in GC using analysis of gene expression data from the TCGA and GEO datasets. These results showed that a total of 95 overlapping DEGs were identified compared to normal gastric tissue. The common genes were further evaluated using functional enrichment analyses. The results indicated that common DEGs play a crucial important role in cancerous development. Subsequently, univariate and multivariate Cox regression analyses were performed to explore the relationship between DEGs and GC survival. A total of six genes (COL8A1, CTHRC1, COL5A2, SERPINE1, COL1A2, and FNDC1) were upregulated in GC and inversely correlated with OS (β > 0, HR > 1), whereas three genes (AADAC, MAMDC2, and MAOA) were downregulated and positively correlated with survival (β < 0, HR < 1). A novel multi-gene signature-derived risk score model was constructed using these nine DEGs. A comprehensive examination of the nine-gene signature prognostic value in the training (TCGA-STAD) and testing (GSE15459) datasets was carried out. ROC curve and Kaplan–Meier analysis performance in the training and validation datasets underscored the robust prognostic value of the risk score model.
Among these nine genes, COL5A2, COL1A2, and COL8A1 are members of the collagen family, which is the main structural component of the extracellular matrix in tumors. COL5A2 encodes alpha 2 chain in type V collagen and is aberrantly expressed in ductal cancer in situ and invasive ductal cancer. It promotes the progression of cancer in situ to invasive cancer. Consistent with the present study, Hao et al[15] identified COL5A2 as a key gene in GC that serves as an oncogene associated with poor OS. Similarly, COL1A2 was reported to inhibit GC cell apoptosis and promote GC cell proliferation, invasion, and migration via the PI3k/Akt signaling pathway[16]. COL8A1 was found to be upregulated and relevant to the poor clinical outcomes in multiple carcinomas, such as colon adenocarcinoma and bladder cancer[17,18]. Its regulatory mechanism in GC remains unclear. CTHRC1 is a major glycosylated protein that has been demonstrated to be associated with cell proliferation, metastasis, and invasion via promoter demethylation and TGF-β1. It is also an independent prognostic predictor[19,20]. SERPINE1, also known as PAI-1, can increase GC metastasis and promote peritoneal tumor growth and formation of bloody ascites in a mouse model of GC metastasis, which serves as an important prognostic gene in GC[21,22]. FDNC1 is a principal component of the fibronectin structural domain that accelerates GC cell proliferation, differentiation, and metastasis via the epithelial-mesenchymal mechanism pathway. It also plays an important role in carcinogenesis in multiple cancers[23-25]. AADAC is a microsomal serine esterase that mainly exists in the liver and gastrointestinal tract. Its main function is involved in drug hydrolysis, as well as triglyceride metabolism and Gilles de la Tourette syndrome[26-28]. Liu et al[17] analyzed the prognostic genes in GC using bioinformatics analysis and found that ADACC is a significant tumor suppressor gene. MAOA degrades monoamine neurotransmitters and dietary amines and was also deemed to be a tumor suppressor in liver cancer[29], pancreatic cancer[30], and cholangiocarcinoma[31] and a tumor promoter in prostate carcinoma[32], breast carcinoma[33], and non-small cell lung carcinoma[34]. Its role in GC progression is poorly understood. There are few studies on the role of MAMDC2 in tumors. A meta-analysis study indicated that down-regulated MAMDC2 was related to a poor disease-free survival in breast carcinoma[35]. Another study reported that miR-196a promotes head and neck squamous cell cancer migration, invasion, and adhesion to fibronectin via MAMDC2[36]. The multiple database analysis of physiological and pathological functions for each gene in GC has provided important evidence helping to understand the prognostic and predictive capacity of the nine-gene signature.
GSEA analysis was utilized in order to provide a deeper insight into the molecular mechanisms for prognosis prediction of the nine-gene signature. Multiple cancer-associated signaling pathways were highlighted as a result, including angiogenesis, epithelial-mesenchymal transition, hedgehog signaling, Kirsten rat sarcoma viral oncogene homologue signaling, Notch signaling, and TGF-β signaling, which suggested that the nine-gene signature has predictive ability for prognosis and can reveal potential therapeutic targets in GC.
Therefore, the CMap database was utilized to explore promising small molecule drugs that have effective treatment response against GC. Levomepromazine, which belongs to antihistaminic compounds, is mainly used for treating breast cancer by binding to the translationally controlled tumor protein and induction of cell differentiation[37]. Lasalocid is a carboxylic ionophore antibiotic produced by Streptomyces lasaliensis that is recognized as a choice for prostate cancer therapy because it increases cytotoxic apoptosis and cytoprotective autophagy[38]. Trichostatin A is a histone deacetylase inhibitor that inhibits proliferation, migration, and invasion of GC cells and shows a good therapeutic effect in GC patients[39,40]. Similarly, vorinostat is a histone deacetylase inhibitor approved by the United States Food and Drug Administration for cutaneous T-cell lymphoma. It is a promising therapeutic candidate in GC when combined with chemotherapeutic agents[41]. Therefore, the present study suggested that these small molecule drugs could serve as novel therapeutic strategies for the high-risk GC group with a poor prognostic response.
One study reported that six genes related to GC prognosis based on DNA microarray data of 65 patients successfully prognosticated relapse in GC patients[42]. A recent study identified a three-gene signature, which could predict GC survival using DNA microarray data of 129 GC patients[43]. These studies were limited due to the small sample size or lack of suitable verification datasets, which limits the possibility of clinical application of the genes related to the GC prognosis. In the present study, a novel nine-gene signature was identified by examining the gene expression profile of 582 GC patients, which had a robustly effective prognostic capacity in GC.
However, this study includes some limitations. First, because the main sources of data in this study were downloaded from public databases that are constructed using available retrospective data, it is necessary to assess the probable utilization of molecular signatures for prognosis evaluation. Second, further studies including a greater number of GC patients are needed to verify the efficiency of the nine-gene signature in GC patients. A greater number of normal samples should also be included in the differential expression analyses. Moreover, multivariate Cox regression analysis was performed to obtain the expression level of multiple genes. More clinical events will be included to verify the prognosis effect of the nine-gene signature in further studies.
In conclusion, using a series of comprehensive bioinformatics analyses and validations, a novel nine-gene signature was constructed. The signature-derived risk score model had a robust prognostic capacity and therapeutic response in GC. Several small molecule drugs were identified to serve as potential therapeutic candidates for GC using bioinformatics. Further experimental studies are necessary to validate these findings and to elucidate the mechanisms for GC-related signaling pathways.
With the popularization and advancement of high throughput sequencing technologies, there is a real possibility of establishing multiple-gene signatures based on data integration and bioinformatics analysis in cancer research. The present study aimed to identify prognostic biomarkers for gastric carcinoma (GC) patients using comprehensive bioinformatics analyses.
GC is one of the most aggressive primary digestive tumors. It has unsatisfactory therapeutic outcomes and is difficult to diagnose early. Therefore, it is necessary to search for a multiple-gene signature-derived model for predicting prognosis and accurately identifying anti-cancer targeted therapies to improve the prognostic stratification and personalized therapy for GC patients.
We aimed to explore the potential multiple-gene prognostic biomarkers and effective therapeutic targets for GC. In this study, we performed integrated bioinformatics analysis to establish a nine-gene risk score model (COL8A1, CTHRC1, COL5A2, SERPINE1, COL1A2, FNDC1 AADAC, MAOA, and MAMDC2) associated with prognosis and treatment response in GC patients. The nine-gene signature-derived risk score allows to predict GC prognosis and might prove useful for guiding therapeutic strategies for GC patients.
Differentially expressed genes (DEGs) were screened using gene expression data from The Cancer Genome Atlas and GEO databases for GC. Overlapping DEGs were analyzed using univariate and multivariate Cox regression analyses. A risk score model was then constructed and signature prognostic values were validated utilizing an independent GEO dataset (GSE15459). CBioPortal, GEPIA, and KM-plotter databases were used to analyze each gene in the risk score model. Gene set enrichment analysis and the connectivity map database were used to predict high-risk score-associated pathways and therapeutic small molecule drugs, respectively.
A total of 95 overlapping DEGs were found and a nine-gene signature (COL8A1, CTHRC1, COL5A2, AADAC, MAMDC2, SERPINE1, MAOA, COL1A2, and FNDC1) was constructed for the GC prognosis prediction. Receiver operating characteristic curve performance in the training dataset (The Cancer Genome Atlas- stomach adenocarcinoma) and validation dataset (GSE15459) demonstrated a robust prognostic value of the risk score model. Multiple database analyses for each gene provided evidence to further understand the nine-gene signature. Gene set enrichment analysis showed that the high-risk group was enriched in multiple cancer-related pathways. Moreover, several new small molecule drugs for potential treatment of GC were identified.
Using a series of comprehensive bioinformatics analyses and validations, a novel nine-gene signature was constructed. The signature-derived risk score model had a robust prognostic capacity and therapeutic response in GC. Several small molecule drugs were identified to serve as potential therapeutic candidates for GC using bioinformatics. Further experimental studies are necessary to validate these findings and to elucidate the mechanisms for GC-related signaling pathways.
Multiple-gene assays are of great importance for precision medicine of GC patients. To further verify the prognostic capacity of the nine-gene signature, our future study may pay more attention to exploring the potential regulatory mechanisms how the nine-gene signature affects the development of GC.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Märkl B S-Editor: Zhang L L-Editor: Wang TQ P-Editor: Li JH
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55815] [Article Influence: 7973.6] [Reference Citation Analysis (132)] |
| 2. | Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3134] [Cited by in RCA: 3539] [Article Influence: 393.2] [Reference Citation Analysis (0)] |
| 3. | Zong L, Abe M, Seto Y, Ji J. The challenge of screening for early gastric cancer in China. Lancet. 2016;388:2606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 271] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 4. | Chandran UR, Medvedeva OP, Barmada MM, Blood PD, Chakka A, Luthra S, Ferreira A, Wong KF, Lee AV, Zhang Z, Budden R, Scott JR, Berndt A, Berg JM, Jacobson RS. TCGA Expedition: A Data Acquisition and Management System for TCGA Data. PLoS One. 2016;11:e0165395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 52] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 5. | Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH, Sherman PM, Holko M, Yefanov A, Lee H, Zhang N, Robertson CL, Serova N, Davis S, Soboleva A. NCBI GEO: archive for functional genomics data sets--update. Nucleic Acids Res. 2013;41:D991-D995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4527] [Cited by in RCA: 6777] [Article Influence: 521.3] [Reference Citation Analysis (0)] |
| 6. | Zhao L, Jiang L, He L, Wei Q, Bi J, Wang Y, Yu L, He M, Zhao L, Wei M. Identification of a novel cell cycle-related gene signature predicting survival in patients with gastric cancer. J Cell Physiol. 2019;234:6350-6360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 7. | Yang Y, Qu A, Zhao R, Hua M, Zhang X, Dong Z, Zheng G, Pan H, Wang H, Yang X, Zhang Y. Genome-wide identification of a novel miRNA-based signature to predict recurrence in patients with gastric cancer. Mol Oncol. 2018;12:2072-2084. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8090] [Cited by in RCA: 8275] [Article Influence: 376.1] [Reference Citation Analysis (0)] |
| 9. | Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22632] [Cited by in RCA: 29103] [Article Influence: 1818.9] [Reference Citation Analysis (0)] |
| 10. | McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288-4297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3211] [Cited by in RCA: 3659] [Article Influence: 281.5] [Reference Citation Analysis (0)] |
| 11. | Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24735] [Cited by in RCA: 27970] [Article Influence: 1748.1] [Reference Citation Analysis (0)] |
| 12. | Cheng W, Li M, Cai J, Wang K, Zhang C, Bao Z, Liu Y, Wu A. HDAC4, a prognostic and chromosomal instability marker, refines the predictive value of MGMT promoter methylation. J Neurooncol. 2015;122:303-312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Lamb J, Crawford ED, Peck D, Modell JW, Blat IC, Wrobel MJ, Lerner J, Brunet JP, Subramanian A, Ross KN, Reich M, Hieronymus H, Wei G, Armstrong SA, Haggarty SJ, Clemons PA, Wei R, Carr SA, Lander ES, Golub TR. The Connectivity Map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313:1929-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3474] [Cited by in RCA: 3730] [Article Influence: 196.3] [Reference Citation Analysis (0)] |
| 14. | Zuo S, Dai G, Ren X. Identification of a 6-gene signature predicting prognosis for colorectal cancer. Cancer Cell Int. 2019;19:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 15. | Hao S, Lv J, Yang Q, Wang A, Li Z, Guo Y, Zhang G. Identification of Key Genes and Circular RNAs in Human Gastric Cancer. Med Sci Monit. 2019;25:2488-2504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Ao R, Guan L, Wang Y, Wang JN. Silencing of COL1A2, COL6A3, and THBS2 inhibits gastric cancer cell proliferation, migration, and invasion while promoting apoptosis through the PI3k-Akt signaling pathway. J Cell Biochem. 2018;119:4420-4434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 17. | Liu X, Wu J, Zhang D, Bing Z, Tian J, Ni M, Zhang X, Meng Z, Liu S. Identification of Potential Key Genes Associated With the Pathogenesis and Prognosis of Gastric Cancer Based on Integrated Bioinformatics Analysis. Front Genet. 2018;9:265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 18. | Di Y, Chen D, Yu W, Yan L. Bladder cancer stage-associated hub genes revealed by WGCNA co-expression network analysis. Hereditas. 2019;156:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 19. | Wang P, Wang YC, Chen XY, Shen ZY, Cao H, Zhang YJ, Yu J, Zhu JD, Lu YY, Fang JY. CTHRC1 is upregulated by promoter demethylation and transforming growth factor-β1 and may be associated with metastasis in human gastric cancer. Cancer Sci. 2012;103:1327-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Gu L, Liu L, Zhong L, Bai Y, Sui H, Wei X, Zhang W, Huang P, Gao D, Kong Y, Lou G. Cthrc1 overexpression is an independent prognostic marker in gastric cancer. Hum Pathol. 2014;45:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Nishioka N, Matsuoka T, Yashiro M, Hirakawa K, Olden K, Roberts JD. Plasminogen activator inhibitor 1 RNAi suppresses gastric cancer metastasis in vivo. Cancer Sci. 2012;103:228-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Sakakibara T, Hibi K, Koike M, Fujiwara M, Kodera Y, Ito K, Nakao A. Plasminogen activator inhibitor-1 as a potential marker for the malignancy of gastric cancer. Cancer Sci. 2006;97:395-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 23. | Liu YP, Chen WD, Li WN, Zhang M. Overexpression of FNDC1 Relates to Poor Prognosis and Its Knockdown Impairs Cell Invasion and Migration in Gastric Cancer. Technol Cancer Res Treat. 2019;18:1533033819869928. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 24. | Das DK, Naidoo M, Ilboudo A, Park JY, Ali T, Krampis K, Robinson BD, Osborne JR, Ogunwobi OO. miR-1207-3p regulates the androgen receptor in prostate cancer via FNDC1/fibronectin. Exp Cell Res. 2016;348:190-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 25. | Bagordakis E, Sawazaki-Calone I, Macedo CC, Carnielli CM, de Oliveira CE, Rodrigues PC, Rangel AL, Dos Santos JN, Risteli J, Graner E, Salo T, Paes Leme AF, Coletta RD. Secretome profiling of oral squamous cell carcinoma-associated fibroblasts reveals organization and disassembly of extracellular matrix and collagen metabolic process signatures. Tumour Biol. 2016;37:9045-9057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 26. | Yuan L, Zheng W, Yang Z, Deng X, Song Z, Deng H. Association of the AADAC gene and Tourette syndrome in a Han Chinese cohort. Neurosci Lett. 2018;666:24-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Kobayashi Y, Fukami T, Shimizu M, Nakajima M, Yokoi T. Contributions of arylacetamide deacetylase and carboxylesterase 2 to flutamide hydrolysis in human liver. Drug Metab Dispos. 2012;40:1080-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Quiroga AD, Lehner R. Pharmacological intervention of liver triacylglycerol lipolysis: The good, the bad and the ugly. Biochem Pharmacol. 2018;155:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 29. | Li J, Yang XM, Wang YH, Feng MX, Liu XJ, Zhang YL, Huang S, Wu Z, Xue F, Qin WX, Gu JR, Xia Q, Zhang ZG. Monoamine oxidase A suppresses hepatocellular carcinoma metastasis by inhibiting the adrenergic system and its transactivation of EGFR signaling. J Hepatol. 2014;60:1225-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 30. | Jiang SH, Li J, Dong FY, Yang JY, Liu DJ, Yang XM, Wang YH, Yang MW, Fu XL, Zhang XX, Li Q, Pang XF, Huo YM, Li J, Zhang JF, Lee HY, Lee SJ, Qin WX, Gu JR, Sun YW, Zhang ZG. Increased Serotonin Signaling Contributes to the Warburg Effect in Pancreatic Tumor Cells Under Metabolic Stress and Promotes Growth of Pancreatic Tumors in Mice. Gastroenterology. 2017;153:277-291.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 206] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 31. | Huang L, Frampton G, Rao A, Zhang KS, Chen W, Lai JM, Yin XY, Walker K, Culbreath B, Leyva-Illades D, Quinn M, McMillin M, Bradley M, Liang LJ, DeMorrow S. Monoamine oxidase A expression is suppressed in human cholangiocarcinoma via coordinated epigenetic and IL-6-driven events. Lab Invest. 2012;92:1451-1460. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Wu JB, Shao C, Li X, Li Q, Hu P, Shi C, Li Y, Chen YT, Yin F, Liao CP, Stiles BL, Zhau HE, Shih JC, Chung LW. Monoamine oxidase A mediates prostate tumorigenesis and cancer metastasis. J Clin Invest. 2014;124:2891-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 33. | Sun WY, Choi J, Cha YJ, Koo JS. Evaluation of the Expression of Amine Oxidase Proteins in Breast Cancer. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Liu F, Hu L, Ma Y, Huang B, Xiu Z, Zhang P, Zhou K, Tang X. Increased expression of monoamine oxidase A is associated with epithelial to mesenchymal transition and clinicopathological features in non-small cell lung cancer. Oncol Lett. 2018;15:3245-3251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 35. | Meng L, Xu Y, Xu C, Zhang W. Biomarker discovery to improve prediction of breast cancer survival: using gene expression profiling, meta-analysis, and tissue validation. Onco Targets Ther. 2016;9:6177-6185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 36. | Darda L, Hakami F, Morgan R, Murdoch C, Lambert DW, Hunter KD. The role of HOXB9 and miR-196a in head and neck squamous cell carcinoma. PLoS One. 2015;10:e0122285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 37. | Seo EJ, Efferth T. Interaction of antihistaminic drugs with human translationally controlled tumor protein (TCTP) as novel approach for differentiation therapy. Oncotarget. 2016;7:16818-16839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Kim KY, Kim SH, Yu SN, Park SG, Kim YW, Nam HW, An HH, Yu HS, Kim YW, Ji JH, Seo YK, Ahn SC. Lasalocid induces cytotoxic apoptosis and cytoprotective autophagy through reactive oxygen species in human prostate cancer PC-3 cells. Biomed Pharmacother. 2017;88:1016-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Ma Y, Yue Y, Pan M, Sun J, Chu J, Lin X, Xu W, Feng L, Chen Y, Chen D, Shin VY, Wang X, Jin H. Histone deacetylase 3 inhibits new tumor suppressor gene DTWD1 in gastric cancer. Am J Cancer Res. 2015;5:663-673. [PubMed] |
| 40. | Wang YG, Wang N, Li GM, Fang WL, Wei J, Ma JL, Wang T, Shi M. Mechanisms of trichostatin A inhibiting AGS proliferation and identification of lysine-acetylated proteins. World J Gastroenterol. 2013;19:3226-3240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 41. | Seah KS, Loh JY, Nguyen TTT, Tan HL, Hutchinson PE, Lim KK, Dymock BW, Long YC, Lee EJD, Shen HM, Chen ES. SAHA and cisplatin sensitize gastric cancer cells to doxorubicin by induction of DNA damage, apoptosis and perturbation of AMPK-mTOR signalling. Exp Cell Res. 2018;370:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 42. | Cho JY, Lim JY, Cheong JH, Park YY, Yoon SL, Kim SM, Kim SB, Kim H, Hong SW, Park YN, Noh SH, Park ES, Chu IS, Hong WK, Ajani JA, Lee JS. Gene expression signature-based prognostic risk score in gastric cancer. Clin Cancer Res. 2011;17:1850-1857. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 257] [Cited by in RCA: 281] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 43. | Bao B, Zheng C, Yang B, Jin Y, Hou K, Li Z, Zheng X, Yu S, Zhang X, Fan Y, Qu X, Liu Y, Che X. Identification of Subtype-Specific Three-Gene Signature for Prognostic Prediction in Diffuse Type Gastric Cancer. Front Oncol. 2019;9:1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |