Published online Sep 15, 2020. doi: 10.4251/wjgo.v12.i9.1044
Peer-review started: May 6, 2020
Revised: June 21, 2020
Accepted: August 15, 2020
Article in press: August 15, 2020
Published online: September 15, 2020
Processing time: 126 Days and 17.9 Hours
Surgical resection is considered the standard treatment option for long-term survival in colorectal cancer liver metastasis (CRLM) patients, but only a small number of patients are suitable for resection following diagnosis. Radiofrequency ablation (RFA) is an accepted alternative therapy for CRLM patients who are not suitable for resection. However, the relatively high rate of local tumor progression (LTP) is an obstacle to the more widespread use of RFA.
To determine the oncological outcomes and predictors of RFA in CRLM patients.
A retrospective analyze was performed on the clinical data of 85 consecutive CRLM patients with a combined total of 138 liver metastases, who had received percutaneous RFA treatment at our institution from January 2013 to December 2018. Contrast-enhanced computed tomography was performed the first month after RFA to assess the technique effectiveness of the RFA and to serve as a baseline for subsequent evaluations. The Kaplan-Meier method was used to calculate overall survival (OS) and LTP-free survival (LTPFS). The log-rank test and Cox regression model were used for univariate and multivariate analyses to determine the predictors of the oncological outcomes.
There were no RFA procedure-related deaths, and the technique effectiveness of the treatment was 89.1% (123/138). The median follow-up time was 30 mo. The LTP rate was 32.6% (45/138), and the median OS was 36 mo. The 1-, 3-, and 5-year OS rates were 90.6%, 45.6%, and 22.9%, respectively. Univariate analysis revealed that tumor size and ablative margin were the factors influencing LTPFS, while extrahepatic disease (EHD), tumor number, and tumor size were the factors influencing OS. Multivariate analysis showed that tumor size larger than 3 cm and ablative margin of 5 mm or smaller were the independent predictors of shorter LTPFS, while tumor number greater than 1, size larger than 3 cm, and presence of EHD were the independent predictors of shorter OS.
RFA is a safe and effective treatment method for CRLM. Tumor size and ablative margin are the important factors affecting LTPFS. Tumor number, tumor size, and EHD are also critical factors for OS.
Core Tip: Relatively high rate of local tumor progression (LTP) is an obstacle to more widespread use of radiofrequency ablation (RFA) in colorectal cancer liver metastasis (CRLM) patients. The purpose of this retrospective study was to determine the oncological outcomes and predictors of RFA in CRLM patients. The median overall survival (OS) of the 85 patients was 36 mo, and the rate of LTP was 32.6% in 138 lesions. Multivariate analysis showed that tumor size and ablative margin were independent predictors of LTP-free survival, while tumor number, tumor size, and extrahepatic disease were independent predictors of OS.
- Citation: Wang CZ, Yan GX, Xin H, Liu ZY. Oncological outcomes and predictors of radiofrequency ablation of colorectal cancer liver metastases. World J Gastrointest Oncol 2020; 12(9): 1044-1055
- URL: https://www.wjgnet.com/1948-5204/full/v12/i9/1044.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i9.1044
Colorectal cancer (CRC) is among the most common malignant tumors of the gastrointestinal system. In 2018, for example, more than 1.1 million individuals were diagnosed with CRC worldwide and the number of deaths exceeded 550000[1,2]. In CRC, most disease-related deaths are secondary to metastatic disease, with the liver being the most common site of metastasis[3]. It has been reported that more than half of CRC patients develop liver metastases during disease progression[4,5]. The survival and prognosis of patients therefore depend on how effective the treatment is. Surgical resection is considered the first-line treatment for the cure or long-term survival of colorectal cancer liver metastasis (CRLM) patients, with 5-year overall survival (OS) rates of 32%-58%[5-10]. Unfortunately, when the patient’s clinical state and the surgical resection criteria are taken into consideration, only 10%-20% of patients are suitable for resection at the time of diagnosis of liver metastasis[11,12].
Image-guided radiofrequency ablation (RFA) is widely used in clinical practice as an alternative to resection, especially for selected smaller tumors that can be ablated with margins[13,14]. RFA combined with chemotherapy increases the 5-year OS rate of patients with unresectable CRLM to 24%-48%, which is close to that of patients undergoing surgical resection[15-17]. Moreover, the left lobe of the liver can be well visualized using endoscopic ultrasound (EUS)[18-21], making it possible to perform EUS-PFA[22]. In addition, RFA has been shown to be safe, with few side effects; being a minimally invasive treatment, the rate of major complications of RFA is 0.9%–7.2% and the mortality rate is less than 1%[23,24]. Despite these gratifying results, however, the relatively high local tumor progression (LTP) rate is still an obstacle to the widespread use of RFA[25,26].
The aim of this study was to determine the oncological outcomes of CRLM after RFA, to evaluate the risk predictors affecting OS and LTP-free survival (LTPFS), to identify the group of CRLM patients who benefit most from RFA, and to provide a reference framework for personalized treatment strategies.
In compliance with the principles of the World Medical Association Declaration of Helsinki, patients in this retrospective study were exempt from the need to provide informed consent, and the study was approved by the Medical Ethics Committee of Shengjing Hospital of China Medical University. Between January 2013 and December 2018, 85 consecutive CRLM patients who received percutaneous RFA were enrolled in the study and followed until January 2019. The patients comprised 56 males and 29 females, with a mean age of 59.1 ± 10.9 years (range, 35-76 years). A total of 138 liver metastases were detected in these 85 patients, with a mean tumor size of 2.8 ± 1.0 cm (range, 0.8–5.0 cm). Of these 85 patients, 45 had a single lesion, 27 had two lesions, and 13 patients had three lesions, with a mean of 1.6 ± 0.7 lesions per patient. Twenty-two (25.9%) out of the 85 patients had imaging evidence of extrahepatic disease (EHD), which was located in the lungs (n = 14), lymph nodes (n = 4), lungs and lymph nodes (n = 3), and a solitary vertebral body (n = 1). Sixty-three (74.1%, 63/85) patients were considered unsuitable for hepatectomy, because of multiple liver metastases, EHD, unfavorable tumor location, or comorbidities. The other 22 (25.9%, 22/85) patients refused surgical intervention. Seventy-six (89.4%, 76/85) patients underwent systemic chemotherapy regimens prescribed by oncologists, such as irinotecan, leucovorin and 5-fluorouracil, and oxaliplatin, leucovorin, and 5-fluorouracil. We stopped chemotherapy for 2 wk or so before RFA treatment took place, without any intervention in the actual chemotherapy regimen.
All CRLM patients were treated with RFA under the following conditions: Surgical resection of colorectal tumors had been performed and histopathological results had confirmed primary colorectal malignant tumors; imaging evidence supported the diagnosis of liver metastasis; the number of intrahepatic metastases was 3 or less and the maximum diameter was 5 cm or smaller; there were no uncorrectable coagulation abnormalities; and they were not candidates for resection of the metastases or had refused surgical resection. All patients provided informed consent before undergoing RFA. RFA procedures were performed under computed tomography (CT) guidance, local anesthesia, analgesia, and hemodynamic monitoring. CelonLab POWER (Olympus Surgical Technologies Europe, Hamburg, Germany) or RF3000 Radio Frequency Generator (Boston Scientific, Natick, MA, United States) was used, depending on the tumor size, shape, location, and the operator’s preference. According to the manufacturer’s instructions, impedance-based control of the generator was adjusted to transmit the radiofrequency energy. For larger tumors, RFA was performed repeatedly to create overlapping ablation zones and safe ablative margins (≥ 5 mm, ideally > 10 mm).
Contrast-enhanced CT examination was performed the first month after RFA treatment to assess the technique effectiveness and to serve as a new baseline for future comparisons. Additional CT examinations were performed every 2 to 4 mo to evaluate the progression of the disease. Additional MRI or PET/CT were performed for those patients with an unclear diagnosis. According to standardized terminology and reporting criteria for tumor ablation[27,28], technique effectiveness is defined as no evidence of residual tumor within 1 cm of the ablation defect; LTP is defined as any new peripheral or nodular enhancement within 1 cm or enlargement of the baseline ablation defect. Patients with multiple new intrahepatic lesions and/or new extrahepatic lesions detected during follow-up were not considered for RFA re-treatment, while patients with a single new intrahepatic lesion and/or LTP lesions were considered for RFA re-treatment.
Complications were classified as either major or minor. Major complications were defined as those events that led to an increased level of care, prolonged hospital stay, or that caused permanent adverse sequelae, including any cases requiring blood transfusion or interventional drainage[28]. Any other complications were classified as minor.
SPSS 16.0 software (SPSS, Inc., Chicago, IL, United States) was used for data analyses. Continuous variables are presented as the mean ± SD and categorical variables are expressed as frequencies (percentages). The primary endpoints of the study were the OS for each patient and the time of LTPFS for each tumor. The Kaplan-Meier method was used to calculate OS and LTPFS from the time of RFA as well as to plot the survival curve. The log-rank test was used for univariate analysis. Variables with P < 0.05 in the univariate analysis were introduced into the Cox multivariate regression model to identify independent predictors affecting OS and LTPFS and to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). P < 0.05 was considered statistically significant.
A total of 140 RFA sessions were performed on 138 CRLM lesions in 85 patients, of whom 56 received one session of RFA, 12 received two sessions, ten received three sessions, five received four sessions, and two received five sessions. All RFA procedures were completed as planned. A total of 123 out of 138 lesions showed complete ablation at the first month of enhanced CT follow-up, with a technique effectiveness rate of 89.1%. The failures of the first ablation of 15 CRLM lesions were related to poor tumor coverage by the ablation area due to the large tumor volume, irregular morphology requiring overlapping ablation, or tumor proximity to larger vessels leading to heat loss.
The median follow-up time of 138 CRLM lesions in the 85 patients was 30 mo. The clinical features are shown in Tables 1 and 2. By the end of follow-up, 45 (32.6%, 45/138) lesions in 42 (49.4%, 42/85) patients developed LTP. Of these, 32 (23.2%, 32/138) LTP lesions were found in 29 (34.1%, 29/85) patients in the first year, and eight (5.8%, 8/138) LTP lesions were found in eight (9.4%, 8/85) patients in the second year. Thus, 71.1% (32/45) of LTP lesions occurred within the first year after RFA, and 88.9% (40/45) occurred by the end of the second year.
| Variable | Number of tumors (n = 138) | LTP rate (%) | Univariate | Multivariate | |
| P value | HR (95%CI) | P value | |||
| Gender | 0.052 | ||||
| Male | 91 | 27.5 | |||
| Female | 47 | 42.6 | |||
| Age (yr) | 0.653 | ||||
| ≤ 60 | 76 | 30.3 | |||
| > 60 | 62 | 35.5 | |||
| Location of primary tumor | 0.868 | ||||
| Colon | 99 | 32.3 | |||
| Rectum | 39 | 33.3 | |||
| TNM classification | 0.502 | ||||
| I, II, or III | 81 | 30.9 | |||
| IV | 57 | 35.1 | |||
| Tumor differentiation | 0.591 | ||||
| Well or moderate | 81 | 34.6 | |||
| Poor | 57 | 29.8 | |||
| Previous liver resection | 0.120 | ||||
| No | 105 | 29.5 | |||
| Yes | 33 | 42.4 | |||
| EHD | 0.522 | ||||
| No | 93 | 32.3 | |||
| Yes | 45 | 33.3 | |||
| Tumor number | 0.799 | ||||
| 1 | 45 | 35.6 | |||
| 2 or 3 | 93 | 31.2 | |||
| Tumor size | < 0.001 | 3.712 (1.894-7.277) | < 0.001 | ||
| ≤ 3 cm | 86 | 15.1 | |||
| > 3 to 5 cm | 52 | 61.5 | |||
| Ablative margin | < 0.001 | 3.077 (1.479-6.405) | 0.003 | ||
| ≤ 5 mm | 67 | 52.2 | |||
| > 5 mm | 71 | 14.1 | |||
| Variable | Number of patients (n = 85) | Median OS (mo) | Univariate | Multivariate | |
| P value | HR (95%CI) | P value | |||
| Gender | 0.341 | ||||
| Male | 56 | 42 | |||
| Female | 29 | 35 | |||
| Age (yr) | 0.504 | ||||
| ≤ 60 | 44 | 35 | |||
| > 60 | 41 | 36 | |||
| Location of primary tumor | 0.229 | ||||
| Colon | 62 | 42 | |||
| Rectum | 23 | 33 | |||
| TNM classification | 0.208 | ||||
| I, II, or III | 52 | 45 | |||
| IV | 33 | 33 | |||
| Tumor differentiation | 0.100 | ||||
| Well or moderate | 56 | 36 | |||
| Poor | 29 | 34 | |||
| Previous liver resection | 0.084 | ||||
| No | 65 | 36 | |||
| Yes | 20 | 34 | |||
| EHD | < 0.001 | 3.676 (1.730-7.811) | 0.001 | ||
| No | 63 | 50 | |||
| Yes | 22 | 26 | |||
| Tumor number | < 0.001 | 2.475 (1.099-5.573) | 0.029 | ||
| 1 | 45 | 50 | |||
| 2 or 3 | 40 | 26 | |||
| Maximum tumor diameter | < 0.001 | 3.641 (1.732-7.654) | 0.001 | ||
| ≤ 3 cm | 48 | 45 | |||
| > 3 to 5 cm | 37 | 26 | |||
| Minimum ablative margin | 0.367 | ||||
| ≤ 5 mm | 44 | 33 | |||
| > 5 mm | 41 | 36 | |||
Of the 45 LTP lesions in the 42 patients, 31 (68.9%, 31/45) LTP lesions in 29 (69.0%, 29/42) patients received RFA re-treatment; while 14 (31.1%, 14/45) LTP lesions in 13 (31.0%, 13/42) patients did not receive RFA re-treatment due to disease progression or patient preference. Among 31 re-treated LTP lesions, 17 (54.8%, 17/31) LTP lesions in 16 (55.2%, 16/29) patients were controlled, while 14 (45.2%, 14/31) LTP lesions in 13 (44.8%, 14/29) patients were not controlled. In summary, the CRLM lesions of 69.4% (59/85) patients were controlled by repeated RFA. The total control rate of CRLM lesions was 79.7% (110/138).
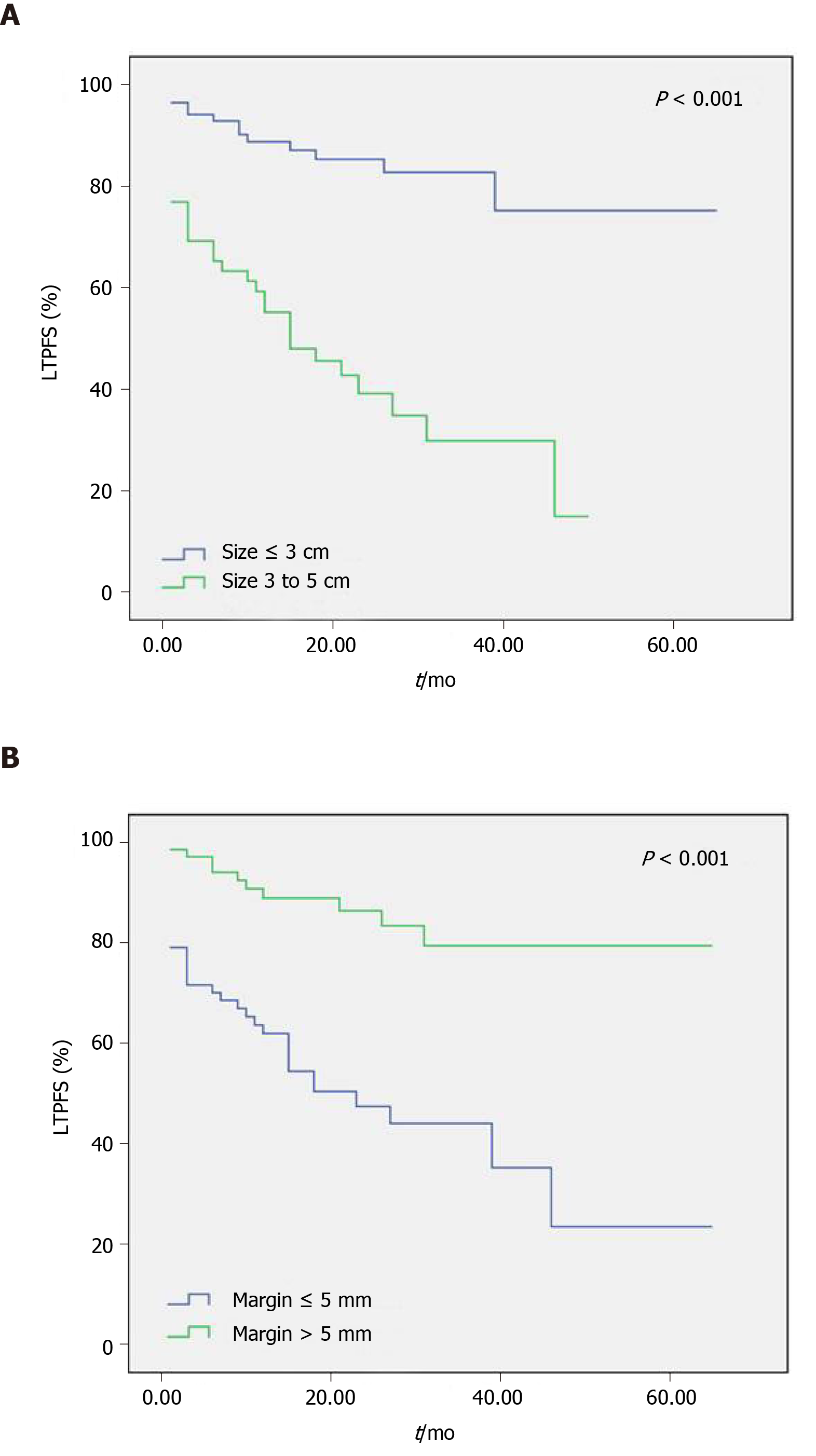
The log-rank univariate analysis showed that gender, age, location of primary tumor, TNM stage, tumor differentiation, liver resection history, EHD, and tumor number had no significant effect on LTPFS (Table 1, P > 0.05). However, tumor size larger than 3 cm and ablative margin of 5 mm or smaller were associated with shorter LTPFS (Figure 1, P < 0.05). By introducing the above two variables into the multivariate Cox model, tumor size larger than 3 cm (P < 0.001, HR = 3.712, 95%CI: 1.894-7.277) and ablative margin of 5 mm or smaller (P = 0.003, HR = 3.077, 95%CI: 1.479-6.405) were shown to be independent predictors of shorter LTPFS (Table 1). In addition, of the 15 lesions with ablative margin more than 10 mm, only one lesion developed LTP. The LTP rate was thus 6.7% (1/15). Among the 12 lesions with ablative margin of 0 mm, 11 lesions developed LTP, thus the LTP rate for no ablative margin was 91.7% (11/12).
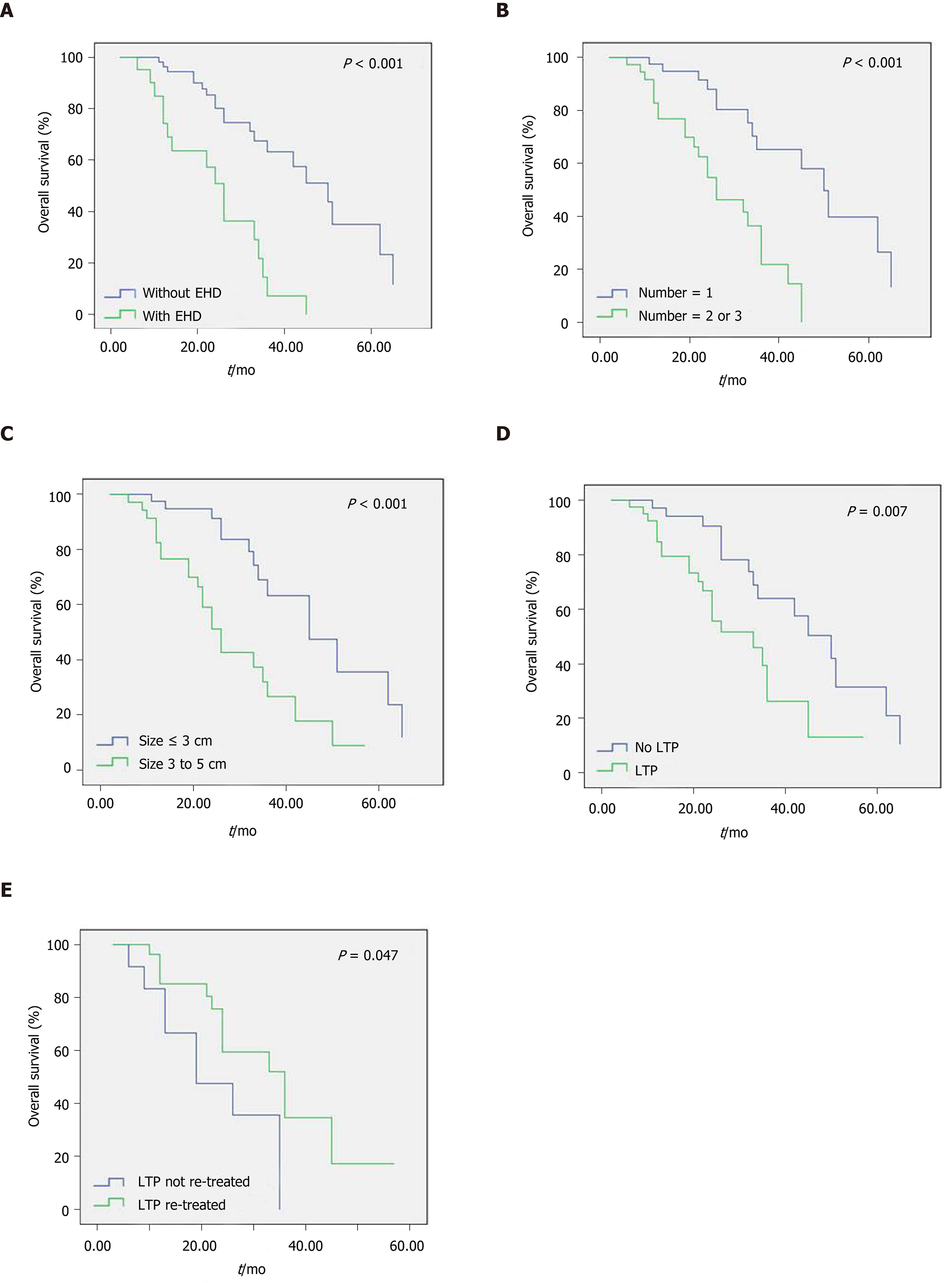
The median OS of the 85 patients was 36 mo. The 1-, 3-, and 5-year OS rates were 90.6%, 45.6%, and 22.9%, respectively. Log-rank univariate analysis indicated that gender, age, location of primary tumor, TNM stage, tumor differentiation, liver resection history, and tumor number had no significant influence on OS (Table 2, P > 0.05). However, tumor number greater than 1, tumor size larger than 3 cm, and presence of EHD were associated with shorter OS (Figure 2A-C, P < 0.05). The multivariate Cox model was used to analyze the above three variables, and the results showed that tumor number greater than 1 (P = 0.029, HR = 2.475, 95%CI: 1.099-5.573), tumor size larger than 3 cm (P = 0.001, HR = 3.641, 95%CI: 1.732-7.654), and presence of EHD (P = 0.001, HR = 3.676, 95%CI: 1.730-7.811) were the independent predictors of shorter OS (Table 2). In this study, the median OS of patients with a single tumor, size of 3 cm or smaller, and no EHD was up to 62 mo, and the 5-year OS rate was 55.5%.
The median OS of 42 patients with LTP and 43 patients who were LTP-free were 33 mo and 50 mo, respectively; the difference was statistically significant (Figure 2D, P = 0.007). Of the 42 patients with LTP, 13 who did not receive RFA re-treatment had a median OS of 19 mo, and 29 who received RFA re-treatment had a median OS of 36 mo; this difference was statistically significant (Figure 2E, P = 0.047).
Minor complication rate for all treatments in this study was 12.1% (17/140), and major complication rate was 4.3% (6/140). The major complications included pneumothorax (n = 2), pleural effusion (n = 1), biloma (n = 1), liver abscess (n = 1), and subcapsular hematoma (n = 1). All the major complications were improved by percutaneous catheter drainage combined with intravenous antibiotics. No technology-related deaths were reported.
Affected by various factors such as tumor location, size, and shape, CRLM is unevenly heated during the RFA process and the surrounding tissue may not reach the temperature required to cause the death of tumor cells. Thus, there may be residual tumor tissue, and this is the main cause of LTP[29]. In previous studies, the LTP rate in CRLM patients treated with RFA ranged from 3.6% to 60%[30]. This wide variability may be explained by the differences among study populations and inclusion criteria. Most researchers concur that LTP is an important factor affecting the efficacy of RFA, and early detection and intervention of LTP are crucial in improving treatment outcomes in CRLM patients[31]. We followed 138 CRLM lesions in 85 patients and found that the LTP rate was 32.6% (45/138) and LTP occurred more frequently in the first year after RFA (71.1%, 32/45).
As the ablation range of the applicator is limited, overlapping ablation is often required to cover large tumors, which increases the risk of LTP. In this study, multivariate analysis shows that tumor size larger than 3 cm (P < 0.001, HR = 3.769, 95%CI: 1.921-7.398) was an independent predictor of shorter LTPFS, which is consistent with the results of Shady et al[25] and Hamada et al[32]. Furthermore, surgical margin of liver metastasis is an important factor in predicting recurrence after tumor resection[33,34]. Similarly in this case, the radiologically estimated ablative margin was used to evaluate oncological outcomes after RFA; ablative margin of 5 mm or smaller (P = 0.002, HR = 3.175, 95%CI: 1.524-6.616) was an independent predictor of shorter LTPFS in this study. It is noteworthy that the LTP rate was 91.7% in 12 CRLM lesions with ablative margin of 0 mm, while the LTP rate was only 6.7% in 15 CRLM lesions with ablative margin more than 10 mm. Therefore, expanding the ablative margin is an effective method to prolong LTPFS and local tumor control. Interestingly, the ablative margin was not an independent predictor of OS in patients with CRLM. This result is similar to that of some surgical resections. As long as the surgical margin of hepatectomy was negative, the width of the margin did not affect the OS of CRLM patients[35-37].
The American Society of Clinical Oncology analyzed 73 publications on the RFA treatment of CRLM published from 1996 to 2007. The 1-, 3-, and 5-year OS rates were 72%-95%, 25%-68%, and 17%-31%, respectively, and the median OS was 18-35 mo[30]. In a 10-year follow-up of 99 patients treated with RFA including 202 small CRLM lesions, Solbiati et al[17] reported that the 5-, 7-, and 10-year OS rates were 47.8%, 25.0%, and 18.0%, respectively, and the median OS of the selected patients was 53.2 mo. Of the 85 patients in this study, 63 were not candidates for hepatectomy and 22 had refused resection. The 1-, 3-, and 5-year OS rates were 90.9%, 47.9%, and 24.3%, respectively, and the median OS was 36 mo.
Many studies have confirmed that the number and size of tumors are important factors affecting OS, regardless of whether surgical resection or RFA is employed[15,38,39]. Cox multivariate analysis in this study likewise confirmed that multiple metastases and large size (> 3 to 5 cm) were the independent predictors of shorter OS in patients treated with RFA. However, whether or not EHD affects the OS of CRLM patients has been controversial. Gillams et al[15] reported that the presence of EHD significantly affected the survival of CRLM patients after RFA and was an independent predictor of shorter OS, while Berber et al[40] concluded that limited amounts of EHD did not appear to adversely affect survival. Hamada et al[32] claimed that EHD kept under control is not a prognostic factor for OS whereas uncontrolled EHD is a poor survival prognostic factor. Our results show that the median OS was 50 mo for patients without EHD and 26 mo for patients with EHD (P < 0.001). The presence of EHD was also an independent predictor of shorter OS after RFA in CRLM patients. Therefore, patients with a single tumor, size of 3 cm or smaller, and no EHD benefit most from RFA, with a median OS of 62 mo and a 5-year OS rate of 55.5%. This result is almost consistent with the 5-year OS rate of 55.4% reported by Hur et al[41].
In addition, our study showed that LTP-free patients had the longest median OS (50 mo). The median OS in LTP patients who received re-treatment (36 mo) was significantly longer than those who did not (19 mo). These data would potentially advocate a more aggressive initial RFA strategy and they demonstrate the advantages of RFA as a repeatable, minimally invasive treatment[17]. Otto et al[42] compared the oncology results of resection and RFA in the treatment of solitary colorectal liver metastasis; although the LTP rate in the RFA group is higher than that of the surgical resection group, the 3-year OS rates are similar. The authors attribute similar ratios of tumor-free patients in both groups to the repeatability of RFA (61% in one and 62% in the other). LTP occurred in 49.4% of patients in this study, but repeated RFA improved the rate of tumor-free patients to 69.4%. Although LTP frequently occurred after RFA in CRLM patients, RFA was still an effective treatment for non-surgical candidates.
Our study had some limitations. First, it was a retrospective study in a single institution with a relatively small number of patients, especially some patients had a short follow-up period. Second, most patients received systemic chemotherapy; thus, OS and LTPFS could not be attributed solely to RFA. In addition, there may have been selection bias when comparing specific patient subgroups, for example, between LTP patients who received repeated RFA treatment and those who did not.
In conclusion, RFA is a safe and effective minimally invasive treatment that can be used as an alternative for patients with unresectable CRLM. Tumor size and ablative margin are important factors influencing LTP, and expanding the ablative margin can effectively reduce the incidence of LTP in these patients. In addition, our study suggests that multiple tumors, large size, and presence of EHD are poor prognostic factors in CRLM patients.
Colorectal cancer liver metastasis (CRLM) is a common secondary malignant tumor of the liver and an important cause of tumor-related death. Radiofrequency ablation (RFA) is an accepted alternative therapy for CRLM patients who are unsuitable for resection. However, the relatively high rate of local tumor progression (LTP) is an obstacle to the more widespread use of RFA.
We want to identify the group of CRLM patients who benefit most from RFA, and to provide a reference framework for personalized treatment strategies.
This study aimed to determine the oncological outcomes of RFA in CRLM patients, and to assess predictors that affect LTP-free survival (LTPFS) and overall survival (OS).
A retrospective study was conducted. One hundred and thirty-eight lesions in 85 consecutive CRLM patients received RFA treatment from January 2013 to December 2018. Contrast-enhanced computed tomography was performed the first month after RFA to serve as a baseline for subsequent evaluations. The Kaplan-Meier method was used to calculate OS and LTPFS. Univariate and multivariate analyses were performed to determine the predictors of the oncological outcomes.
There were no RFA procedure-related deaths, and the technique effectiveness rate of the treatment was 89.1% (123/138). The median OS was 36 mo, and the 1-, 3-, and 5-year OS rates were 90.6%, 45.6%, and 22.9%, respectively. Tumor size larger than 3 cm and ablative margin of 5 mm or smaller were the independent predictors of shorter LTPFS, while tumor number greater than 1, size larger than 3 cm, and presence of extrahepatic disease (EHD) were the independent predictors of shorter OS.
RFA is a safe and effective treatment method for CRLM. Tumor size and ablative margin are the important factors affecting LTPFS, while tumor number, tumor size, and EHD are also critical factors in OS.
RFA is an effective minimally invasive treatment that can be used as an alternative for patients with unresectable CRLM. Expanding the ablative margin is an effective method to control LTP after RFA. Patients with a single tumor, size of 3 cm or smaller, and no EHD benefit most from RFA.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kantsevoy S, Osuga T, Tokunaga Y S-Editor: Wang JL L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55814] [Article Influence: 7973.4] [Reference Citation Analysis (132)] |
| 2. | Sak K. A Hypothetical Approach on Gender Differences in Cancer Diagnosis. J Transl Int Med. 2019;7:90-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Tan HL, Lee M, Vellayappan BA, Neo WT, Yong WP. The Role of Liver-Directed Therapy in Metastatic Colorectal Cancer. Curr Colorectal Cancer Rep. 2018;14:129-137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Tsitskari M, Filippiadis D, Kostantos C, Palialexis K, Zavridis P, Kelekis N, Brountzos E. The role of interventional oncology in the treatment of colorectal cancer liver metastases. Ann Gastroenterol. 2019;32:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Helling TS, Martin M. Cause of death from liver metastases in colorectal cancer. Ann Surg Oncol. 2014;21:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Hackl C, Neumann P, Gerken M, Loss M, Klinkhammer-Schalke M, Schlitt HJ. Treatment of colorectal liver metastases in Germany: a ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer. 2014;14:810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 246] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 7. | Gomez D, Sangha VK, Morris-Stiff G, Malik HZ, Guthrie AJ, Toogood GJ, Lodge JP, Prasad KR. Outcomes of intensive surveillance after resection of hepatic colorectal metastases. Br J Surg. 2010;97:1552-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18:78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 299] [Cited by in RCA: 596] [Article Influence: 85.1] [Reference Citation Analysis (1)] |
| 9. | Fernandez FG, Drebin JA, Linehan DC, Dehdashti F, Siegel BA, Strasberg SM. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET). Ann Surg. 2004;240:438-47; discussion 447-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 437] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 10. | Djiambou-Nganjeu H. Hepatic Encephalopathy in Patients in Lviv (Ukraine). J Transl Int Med. 2018;6:146-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | de Jong MC, Pulitano C, Ribero D, Strub J, Mentha G, Schulick RD, Choti MA, Aldrighetti L, Capussotti L, Pawlik TM. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 590] [Article Influence: 36.9] [Reference Citation Analysis (0)] |
| 12. | Line PD, Hagness M, Dueland S. The Potential Role of Liver Transplantation as a Treatment Option in Colorectal Liver Metastases. Can J Gastroenterol Hepatol. 2018;2018:8547940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Meijerink MR, Puijk RS, van Tilborg AAJM, Henningsen KH, Fernandez LG, Neyt M, Heymans J, Frankema JS, de Jong KP, Richel DJ, Prevoo W, Vlayen J. Radiofrequency and Microwave Ablation Compared to Systemic Chemotherapy and to Partial Hepatectomy in the Treatment of Colorectal Liver Metastases: A Systematic Review and Meta-Analysis. Cardiovasc Intervent Radiol. 2018;41:1189-1204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 145] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 14. | Veltri A, Guarnieri T, Gazzera C, Busso M, Solitro F, Fora G, Racca P. Long-term outcome of radiofrequency thermal ablation (RFA) of liver metastases from colorectal cancer (CRC): size as the leading prognostic factor for survival. Radiol Med. 2012;117:1139-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol. 2009;19:1206-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 16. | Van Tilborg AA, Meijerink MR, Sietses C, Van Waesberghe JH, Mackintosh MO, Meijer S, Van Kuijk C, Van Den Tol P. Long-term results of radiofrequency ablation for unresectable colorectal liver metastases: a potentially curative intervention. Br J Radiol. 2011;84:556-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Solbiati L, Ahmed M, Cova L, Ierace T, Brioschi M, Goldberg SN. Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology. 2012;265:958-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 261] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 18. | Lisotti A, Serrani M, Caletti G, Fusaroli P. EUS liver assessment using contrast agents and elastography. Endosc Ultrasound. 2018;7:252-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 19. | Samarasena J, Chang KJ. Endo-hepatology: A new paradigm. Endosc Ultrasound. 2018;7:219-222. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Sharma M, Rameshbabu CS, Dietrich CF, Rai P, Bansal R. Endoscopic ultrasound of the hepatoduodenal ligament and liver hilum. Endosc Ultrasound. 2018;7:168-174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Bhatia V, Dhir V. Radial EUS imaging of the liver: A pictorial guide. Endosc Ultrasound. 2019;8:76-81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Armellini E, Leutner M, Stradella D, Ballarè M, Occhipinti P. EUS-guided radiofrequency ablation: an option for the extrapancreatic region. Endosc Ultrasound. 2018;7:282-283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1051] [Cited by in RCA: 932] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 24. | Stoltz A, Gagnière J, Dupré A, Rivoire M. Radiofrequency ablation for colorectal liver metastases. J Visc Surg. 2014;151 Suppl 1:S33-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Shady W, Petre EN, Gonen M, Erinjeri JP, Brown KT, Covey AM, Alago W, Durack JC, Maybody M, Brody LA, Siegelbaum RH, D'Angelica MI, Jarnagin WR, Solomon SB, Kemeny NE, Sofocleous CT. Percutaneous Radiofrequency Ablation of Colorectal Cancer Liver Metastases: Factors Affecting Outcomes--A 10-year Experience at a Single Center. Radiology. 2016;278:601-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 280] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 26. | Sofocleous CT, Petre EN, Gonen M, Brown KT, Solomon SB, Covey AM, Alago W, Brody LA, Thornton RH, D'Angelica M, Fong Y, Kemeny NE. CT-guided radiofrequency ablation as a salvage treatment of colorectal cancer hepatic metastases developing after hepatectomy. J Vasc Interv Radiol. 2011;22:755-761. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 27. | Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD, Dupuy DE, Gervais D, Gillams AR, Kane RA, Lee FT, Livraghi T, McGahan J, Phillips DA, Rhim H, Silverman SG; Society of Interventional Radiology Technology Assessment Committee; International Working Group on Image-Guided Tumor Ablation. Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology. 2005;235:728-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 531] [Cited by in RCA: 522] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 28. | Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, Chen MH, Choi BI, de Baère T, Dodd GD, Dupuy DE, Gervais DA, Gianfelice D, Gillams AR, Lee FT, Leen E, Lencioni R, Littrup PJ, Livraghi T, Lu DS, McGahan JP, Meloni MF, Nikolic B, Pereira PL, Liang P, Rhim H, Rose SC, Salem R, Sofocleous CT, Solomon SB, Soulen MC, Tanaka M, Vogl TJ, Wood BJ, Goldberg SN; International Working Group on Image-guided Tumor Ablation; Interventional Oncology Sans Frontières Expert Panel; Technology Assessment Committee of the Society of Interventional Radiology,; Standard of Practice Committee of the Cardiovascular and Interventional Radiological Society of Europe. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 628] [Cited by in RCA: 896] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 29. | Snoeren N, Jansen MC, Rijken AM, van Hillegersberg R, Slooter G, Klaase J, van den Tol PM, van der Linden E, Ten Kate FJ, van Gulik TM. Assessment of viable tumour tissue attached to needle applicators after local ablation of liver tumours. Dig Surg. 2009;26:56-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Wong SL, Mangu PB, Choti MA, Crocenzi TS, Dodd GD, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D, Marsland TA, Michelson R, Poston GJ, Schrag D, Seidenfeld J, Benson AB. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol. 2010;28:493-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 31. | Kingham TP, Tanoue M, Eaton A, Rocha FG, Do R, Allen P, De Matteo RP, D'Angelica M, Fong Y, Jarnagin WR. Patterns of recurrence after ablation of colorectal cancer liver metastases. Ann Surg Oncol. 2012;19:834-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Hamada A, Yamakado K, Nakatsuka A, Uraki J, Kashima M, Takaki H, Yamanaka T, Inoue Y, Kusunoki M, Takeda K. Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol. 2012;30:567-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 33. | Ito H, Are C, Gonen M, D'Angelica M, Dematteo RP, Kemeny NE, Fong Y, Blumgart LH, Jarnagin WR. Effect of postoperative morbidity on long-term survival after hepatic resection for metastatic colorectal cancer. Ann Surg. 2008;247:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 34. | Nuzzo G, Giuliante F, Ardito F, Vellone M, Giovannini I, Federico B, Vecchio FM. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery. 2008;143:384-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 35. | Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, Curley SA, Loyer EM, Muratore A, Mentha G, Capussotti L, Vauthey JN. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241:715-722, discussion 722-discussion 724. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 812] [Article Influence: 40.6] [Reference Citation Analysis (0)] |
| 36. | Mbah NA, Scoggins C, McMasters K, Martin R. Impact of hepatectomy margin on survival following resection of colorectal metastasis: the role of adjuvant therapy and its effects. Eur J Surg Oncol. 2013;39:1394-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Mitchell D, Puckett Y, Nguyen QN. Literature Review of Current Management of Colorectal Liver Metastasis. Cureus. 2019;11:e3940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Veltri A, Sacchetto P, Tosetti I, Pagano E, Fava C, Gandini G. Radiofrequency ablation of colorectal liver metastases: small size favorably predicts technique effectiveness and survival. Cardiovasc Intervent Radiol. 2008;31:948-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 39. | Siperstein AE, Berber E, Ballem N, Parikh RT. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg. 2007;246:559-65; discussion 565-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 40. | Berber E, Pelley R, Siperstein AE. Predictors of survival after radiofrequency thermal ablation of colorectal cancer metastases to the liver: a prospective study. J Clin Oncol. 2005;23:1358-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 41. | Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, Cho CH, Ko HK, Lee JT, Kim NK. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg. 2009;197:728-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 161] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Otto G, Düber C, Hoppe-Lotichius M, König J, Heise M, Pitton MB. Radiofrequency ablation as first-line treatment in patients with early colorectal liver metastases amenable to surgery. Ann Surg. 2010;251:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |