Published online Aug 15, 2020. doi: 10.4251/wjgo.v12.i8.931
Peer-review started: January 16, 2020
First decision: April 18, 2020
Revised: May 8, 2020
Accepted: July 1, 2020
Article in press: July 1, 2020
Published online: August 15, 2020
Processing time: 208 Days and 18.4 Hours
Portal pressure is of great significance in the treatment of hepatocellular carcinoma (HCC), but direct measurement is complicated and costly; thus, non-invasive measurement methods are urgently needed.
To investigate whether ultrasonography (US)-based portal pressure assessment could replace invasive transjugular measurement.
A cohort of 102 patients with HCC was selected (mean age: 54 ± 13 years, male/female: 65/37). Pre-operative US parameters were assessed by two independent investigators, and multivariate logistic analysis and linear regression analysis were conducted to develop a predictive formula for the portal pressure gradient (PPG). The estimated PPG predictors were compared with the transjugular PPG measurements. Validation was conducted on another cohort of 20 non-surgical patients.
The mean PPG was 17.32 ± 1.97 mmHg. Univariate analysis identified the association of the following four parameters with PPG: Spleen volume, portal vein diameter, portal vein velocity (PVV), and portal blood flow (PBF). Multiple linear regression analysis was performed, and the predictive formula using the PVV and PBF was as follows: PPG score = 19.336 - 0.312 × PVV (cm/s) + 0.001 × PBF (mL/min). The PPG score was confirmed to have good accuracy with an area under the curve (AUC) of 0.75 (0.68-0.81) in training patients. The formula was also accurate in the validation patients with an AUC of 0.820 (0.53-0.83).
The formula based on ultrasonographic Doppler flow parameters shows a significant correlation with invasive PPG and, if further confirmed by prospective validation, may replace the invasive transjugular assessment.
Core tip: The direct measurement of portal pressure is complicated; therefore, non-invasive measurement methods are urgently needed to guide the treatment of hepatocellular carcinoma. The combined measurements of portal vein velocity and portal blood flow could be clinically and economically useful in estimating portal pressure gradient.
- Citation: Zhang Y, Wang Z, Yue ZD, Zhao HW, Wang L, Fan ZH, Wu YF, He FL, Liu FQ. Accurate ultrasonography-based portal pressure assessment in patients with hepatocellular carcinoma. World J Gastrointest Oncol 2020; 12(8): 931-941
- URL: https://www.wjgnet.com/1948-5204/full/v12/i8/931.htm
- DOI: https://dx.doi.org/10.4251/wjgo.v12.i8.931
Hepatocellular carcinoma (HCC) is a significant public health problem worldwide and is currently the main event leading to death in patients with cirrhosis[1]. The current treatment modalities for HCC include liver resection (LR) and liver transplantation. Portal pressure accurately predicts the risk of peri-operative morbidity and mortality[1,2]. The European Association for the Study of the Liver and American Association for the Study of Liver Diseases guidelines for the management of HCC consider a hepatic venous pressure gradient (HVPG) ≥ 10 mmHg to be a contraindication for LR[3,4].
Portal pressure gradient (PPG), ranges between 1 mmHg and 5 mmHg in normal conditions, which represents the hepatic perfusion pressure of portal blood[5]. HVPG measurement has the advantages of simple measurement techniques and low risk, which is widely used to estimate PPG and is regarded as the gold standard for the diagnosis of portal hypertension. Based on HVPG, clinically significant portal hypertension (CSPH) is defined as an HVPG of at least 10 mmHg[6-8]. The limitations of HVPG measurement are that it is invasive and impractical for routine clinical practice. Many non-invasive portal pressure assessment techniques have been introduced in recent years[9-13]. Doppler sonography offers real-time observation of blood flow with qualitative and quantitative assessments, and the application of microbubble-based contrast agents has improved the detectability of peripheral blood flow. In addition, elastography of the liver and spleen covers a wider field beyond the original purpose of fibrosis assessment. These developments enhance the practical use of ultrasonography (US) in the evaluation of portal hemodynamic abnormalities[12,14]. However, none of these methods have gained extensive clinical acceptance, as a consequence of small sample size, lack of external validation, and/or their low accuracy in the prediction of CSPH.
The aim of this study was to clarify whether simple, non-invasive US parameters correlate with the invasive transjugular PPG measurement and to develop a formula to estimate PPG.
The present study was based on a retrospective analysis of prospectively collected data in our department. This study was compliant with the Health Insurance Portability and Accountability Act. Due to the retrospective nature of the study, informed consent was waived. This study was approved by the hospital ethics committee.
All consecutive patients who underwent transjugular PPG measurement from January 2016 to June 2018 were included.
The inclusion criteria were as follows: (1) Patients aged 18-70 years; (2) Patients who were diagnosed with HCC; (3) Patients who underwent transjugular portal pressure measurement, abdominal computed tomography (CT) angiography, and Doppler US; (4) Patients received no treatment for HCC at the time of PPG measurement, and underwent US examination at the same time as PPG measurement; and (5) Patients with a follow-up period of minimum 12 mo.
The exclusion criteria were: (1) Patients with portal vein thrombosis or hepatic vein thrombosis; (2) Those with massive ascites in which accurate measurements by Doppler US were not possible; and (3) Pregnant or lactating women.
Baseline demographic, clinical, and laboratory characteristics were retrieved from clinical records. All patients underwent hematological tests including complete blood counts, routine coagulation examination, and kidney and liver function tests at admission. Details pertaining to the use of alcohol and hepatotoxic drugs were recorded. Patient sera were tested for hepatitis B surface antigen and antibody to hepatitis C virus. Other appropriate tests for determining etiology were also performed, if required. The Child-Pugh and Model for End-stage Liver Disease (MELD) scores were calculated on the basis of clinical data. The severity of liver disease at inclusion and during follow-up was assessed by the Child–Pugh grade and MELD score. The ALBI grade was calculated using the following equation: Linear predictor = (log10 bilirubin μmol/L × 0.66) + (albumin g/L × -0.085).
US was performed before the hemodynamic investigation in patients fasted for 8 h. US examination was performed using a 3.5-MHz sector transducer (iU22 Ultrasound System; Philips Healthcare, Reedsville, PA, United States). The diameter of the portal vein was measured using B-mode US. In each patient, all measurements were carried out on a longitudinal section of the vessel and were repeated by one radiologist who had no knowledge of the hemodynamic values. These measurements included the diameter of the portal vein and portal blood velocity. All measurements were performed in triplicate and then averaged.
The portal blood flow was calculated as portal vein velocity (PVV, cm/s) × portal vein cross-sectional area × 0.57, and the congestion index (CI) of the portal vein was calculated as previously reported[15]: The “congestion index” is used to mean the ratio between the cross-sectional area (cm2) and the blood flow velocity (cm/s) of the portal vein, as determined by a duplex Doppler system.
Transjugular PPG and HVPG measurements were performed under general anesthesia in the angiography suite by an experienced radiologist. Pressure measurements were conducted using a balloon catheter (Edwards Lifesciences, Irvine, CA, United States) with a pressure transducer at the tip. A zero measurement with the transducer open to air was needed before transjugular catheterization. All measurements were performed in triplicate and then averaged.
Using an established technique to measure PPG[16], the portal vein was punctured with a modified transjugular liver biopsy needle under ultrasonographic and radiological guidance, and was aimed at the right portal vein branch 1-3 cm above the portal vein bifurcation. After successful puncture, the portal vein was catheterized using a 5F catheter, and baseline measurements of portal venous pressure, inferior vena cava pressure, and the PPG were obtained.
Transjugular HVPG measurement was conducted according to the standard protocol[17]. The free HVPG was measured in the right hepatic vein (approximately 1-3 cm from the IVC). Then, as the balloon was inflated for total occlusion of the right hepatic vein, the wedged hepatic venous pressure was measured. Continuous recording was necessary until the pressure reached a plateau. HVPG was calculated by subtracting the free venous hepatic pressure from the wedged hepatic pressure.
The CT-based portal pressure score was calculated as follows: 17.37-4.91 × ln (liver-to-spleen volume ratio) + 3.8 (if perihepatic ascites is present)[18].
Quantitative variables are expressed as the mean ± SD and qualitative data are expressed as percentages. The independent t test or analysis of variance was applied for comparisons of normally distributed variables. For non-normally distributed data, the Kruskal-Wallis test or Wilcoxon’s rank-sum (Mann-Whitney) test was used to analyze the statistical significance of intergroup differences. Pearson’s correlation for normally distributed variables and Spearman’s rank-correlation coefficient for non-normally distributed data were used, as appropriate. Linear regression analyses were performed according to the least-squares method. Spearman correlation coefficient analysis (R2 value) and the Bland-Altman plot were used to assess the correlation and the agreement between transjugular PPG and HVPG, and between estimated PPG and transjugular PPG, respectively. The proposed PPG predictive models were subsequently tested on a validation cohort, which included 20 patients (none of these patients underwent surgery or transplantation). The performance of the estimated PPG in predicting transjugular PPG was assessed using receiver operator characteristic curves and the area under the curve (AUC) was calculated. Two-sided P < 0.05 was considered statistically significant. Statistical analyses were performed with the SPSS 20.0 package (SPSS, Chicago, IL, United States) and Graphpad Prism 8.0 (Graphpad Software Inc., United States).
A total of 102 patients with HCC were included, and their demographics and clinicopathological parameters are shown in Table 1. The baseline liver function of these patients was as follows: Alanine aminotransferase, 24.4 ± 18.0 IU/L; aspartate aminotransferase, 35.0 ± 24.4 IU/L; and total bilirubin, 2.20 ± 3.61 mg/dL. No complications during the measurement of direct PPG were recorded in the present series.
| Index | Index | ||
| Age (yr) | 54 ± 13 | Globulin (g/dL) | 30.5 ± 8.9 |
| Gender (male/female) | 65/37 | Albumin (g/dL) | 34.9 ± 5.9 |
| Etiology, n | 102 | Total protein (g/dL) | 65.5 ± 10.0 |
| Virus | 48 | ALP (U/L) | 120.6 ± 86.7 |
| Alcohol | 20 | GGT (U/L) | 67.7 ± 82.3 |
| Cryptogenic | 5 | BUN (mmol/L) | 6.8 ± 5.4 |
| Multifactorial | 20 | Creatinine (μmol/L) | 88.7 ± 138.6 |
| Others | 9 | LDH (UL) | 194.3 ± 59.8 |
| GB history, n (%) | 76 (74.51) | K (mmol/L) | 4.0 ± 0.7 |
| Refractory ascites, n (%) | 78 (76.47) | Na (mmol/L) | 138.4 ± 14.5 |
| Encephalopathy, n (%) | 4 (3.92) | Cl (mmol/L) | 106.4 ± 5.1 |
| Red blood cells (1012/L) | 3.3 ± 1.6 | Ca (mmol/L) | 2.15 ± 0.15 |
| Hemoglobin (g/L) | 91.6 ± 25.3 | Blood ammonia (μmol/L) | 51.2 ± 30.0 |
| White blood cells (1012/L) | 4.1 ± 4.0 | FIB (n/L) | 2.3 ± 1.5 |
| Platelet count (109/L) | 106.7 ± 95.7 | APTT (s) | 34.5 ± 6.3 |
| ALT (U/L) | 24.4 ± 18.0 | TT (s) | 17.7 ± 5.4 |
| AST (U/L) | 35.0 ± 24.4 | D dimer level (μg/L) | 809 ± 1009 |
| TBIL (mg/dL) | 2.20 ± 3.61 | Child–Pugh class, n (A/B/C) | 26/58/18 |
| DBIL (mg/dL) | 1.41 ± 3.14 | ALBI score | -2.06 ± 0.47 |
| IBIL (mg/dL) | 0.67 ± 0.43 | MELD score | 7.66 ± 5.46 |
| PT(s) | 14.6 ± 3.6 | HVPG (mmHg) | 17.07 ± 4.78 |
| PT (%) | 61.1 ± 16.6 | PVP (mmHg) | 34.40 ± 5.95 |
| INR | 1.4 ± 0.3 | PPG (mmHg) | 17.32 ± 1.97 |
Doppler liver and abdominal vascular scans were performed for all patients. These US Doppler parameters are summarized in Table 2. The preoperative US Doppler parameters were as follows: Portal vein diameter, 1.20 cm ± 0.37 cm; portal vein velocity, 25.1 cm/s ± 11.4 cm/s; portal blood flow, 1729.9 mL/min ± 1003.1 mL/min; and CI, 0.11 ± 0.07.
| Parameter | Result |
| Portal vein diameter (cm) | 1.20 ± 0.37 |
| Portal vein velocity (cm/s) | 25.1 ± 11.4 |
| Portal blood flow (mL/min) | 1729.9 ± 1003.1 |
| Congestion index | 0.11 ± 0.07 |
| IVC diameter (cm) | 8.7 ± 2.9 |
| IVC blood velocity (cm/s) | 62.2 ± 31.0 |
| Spleen vein diameter (cm) | 1.12 ± 0.23 |
| Spleen vein velocity (cm/s) | 11.51 ± 3.23 |
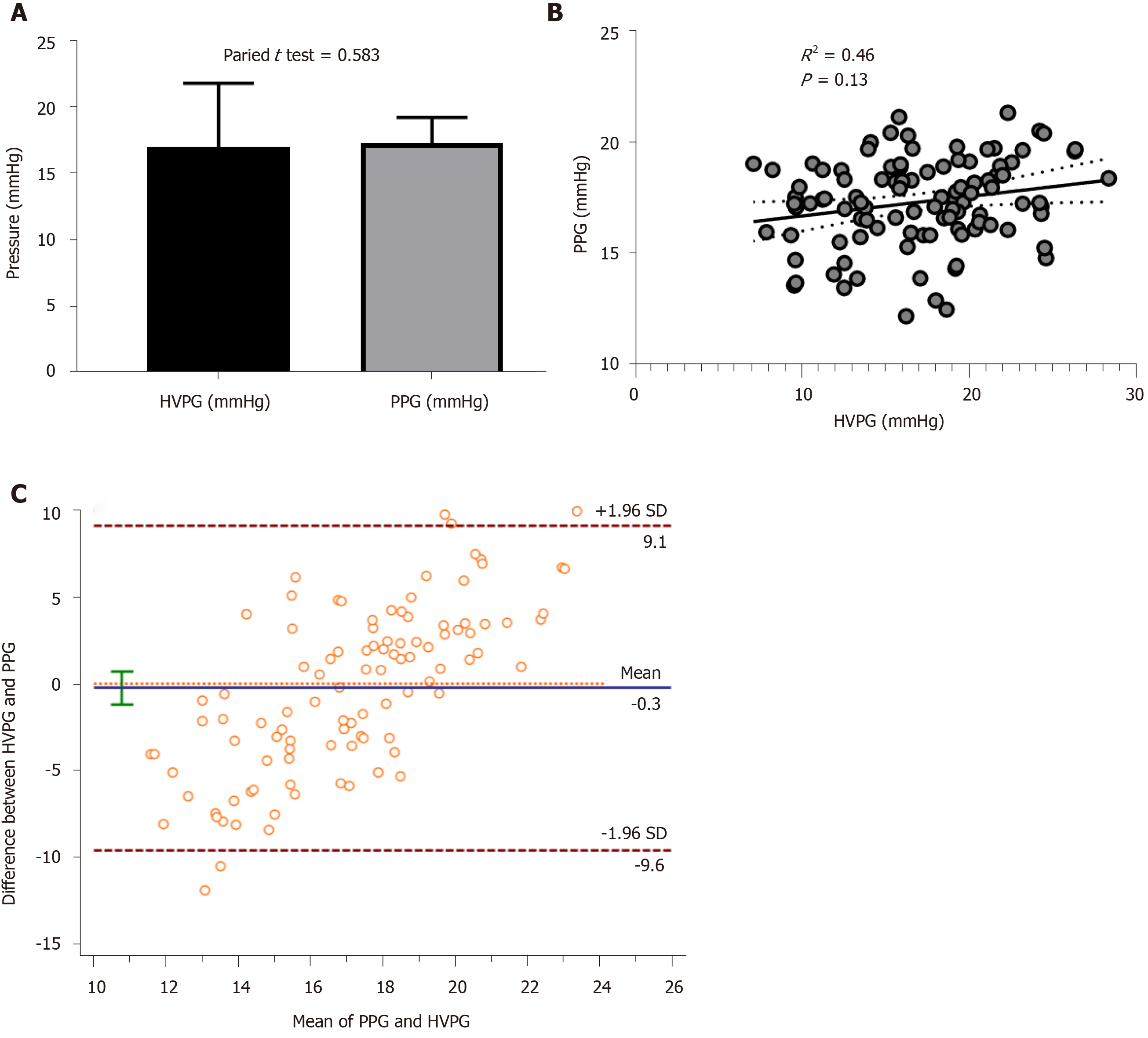
HVPG was 17.07 ± 4.78 mmHg and PPG was 17.32 ± 1.97 mmHg. The paired t test showed no significant difference between HVPG and PPG (Figure 1A). Correlation analysis showed that the correlation coefficient between HVPG and PPG was 0.51, and the R2 was 0.46 (P = 0.13, Figure 1B). The Bland-Altman plot showed a difference between HVPG and PPG (Figure 1C). These results indicated that the PPG had a good correlation with HVPG.
Table 3 shows the correlations between the PPG and other comparable parameters. The correlation analysis identified four variables as significantly negatively correlated with PPG: SV, PVD, PVV, and PBF (P < 0.05). Other parameters were not correlated with the PPG in these patients.
| Index | Correlation with PPG (γ) | P value |
| Age (yr) | 0.345 | 0.632 |
| Peri-hepatic ascites (yes vs no) | 0.753 | 0.233 |
| Platelet count (× 109/L) | -0.341 | 0.061 |
| Total bilirubin (mg/dL) | -0.231 | 0.487 |
| Serum albumin (g/dL) | 0.542 | 0.683 |
| AST (IU/L) | 0.452 | 0.712 |
| ALT (IU/L) | 0.028 | 0.652 |
| NH3 (μg/dL) | 0.126 | 0.515 |
| MELD score | 0.025 | 0.523 |
| Portal vein diameter (cm) | 0.102 | 0.019 |
| Portal vein velocity (cm/s) | -0.321 | 0.034 |
| Portal blood flow (mL/min) | -0.032 | 0.048 |
| PV-CI | 0.285 | 0.021 |
| IVC diameter (cm) | 0.129 | 0.496 |
| IVC blood velocity (cm/s) | 0.163 | 0.389 |
| Spleen vein diameter (cm) | 0.142 | 0.248 |
| Spleen vein velocity (cm/s) | -0.062 | 0.654 |
The four selected US parameters were examined for correlations with PPG using multiple linear regression analysis by the stepwise method (Table 4). Based on this result, the following regression equation was established: PPG score = 19.336 - 0.312 × PVV (cm/s) + 0.001 × PBF (mL/min).
| Model | Unstandardized coefficients | Standardized coefficient | T value | P value | 95% Confidence Interval | ||
| β | Standard error | β | |||||
| 1 (Constant) | 19.432 | 2.785 | 8.538 | 0.000 | 15.133-21.482 | ||
| SV (cm3) | -0.212 | 0.214 | -0.265 | -1.432 | 0.654 | -0.378-0.431 | |
| PVD (cm) | 0.322 | 0.254 | 0.331 | 0.085 | 0.723 | 0.134-0.564 | |
| PVV (cm/s) | -0.323 | 0.187 | -0.353 | -1.572 | 0.157 | -0.623-0.113 | |
| PBF (mL/min) | 0.001 | 0.056 | 0.274 | 1.431 | 0.197 | 0.023-0.422 | |
| 2 (Constant) | 19.345 | 2.634 | 8.634 | 0.000 | 15.268-21.372 | ||
| PVD (cm) | 0.312 | 0.262 | 0.232 | 0.079 | 0.654 | 0.211-0.592 | |
| PVV (cm/s) | -0.343 | 0.232 | -0.412 | -1.548 | 0.132 | -0.451-0.065 | |
| PBF (mL/min) | 0.001 | 0.067 | 0.283 | ||||
| 3 (Constant) | 19.336 | 2.543 | 8.634 | 0.000 | 16.235-22.354 | ||
| PVV (cm/s) | -0.312 | 0.134 | -0.532 | -2.645 | 0.032 | -0.454-0.001 | |
| PBF (mL/min) | 0.001 | 0.078 | 0.276 | 2,143 | 0.025 | 0.034-0.462 | |
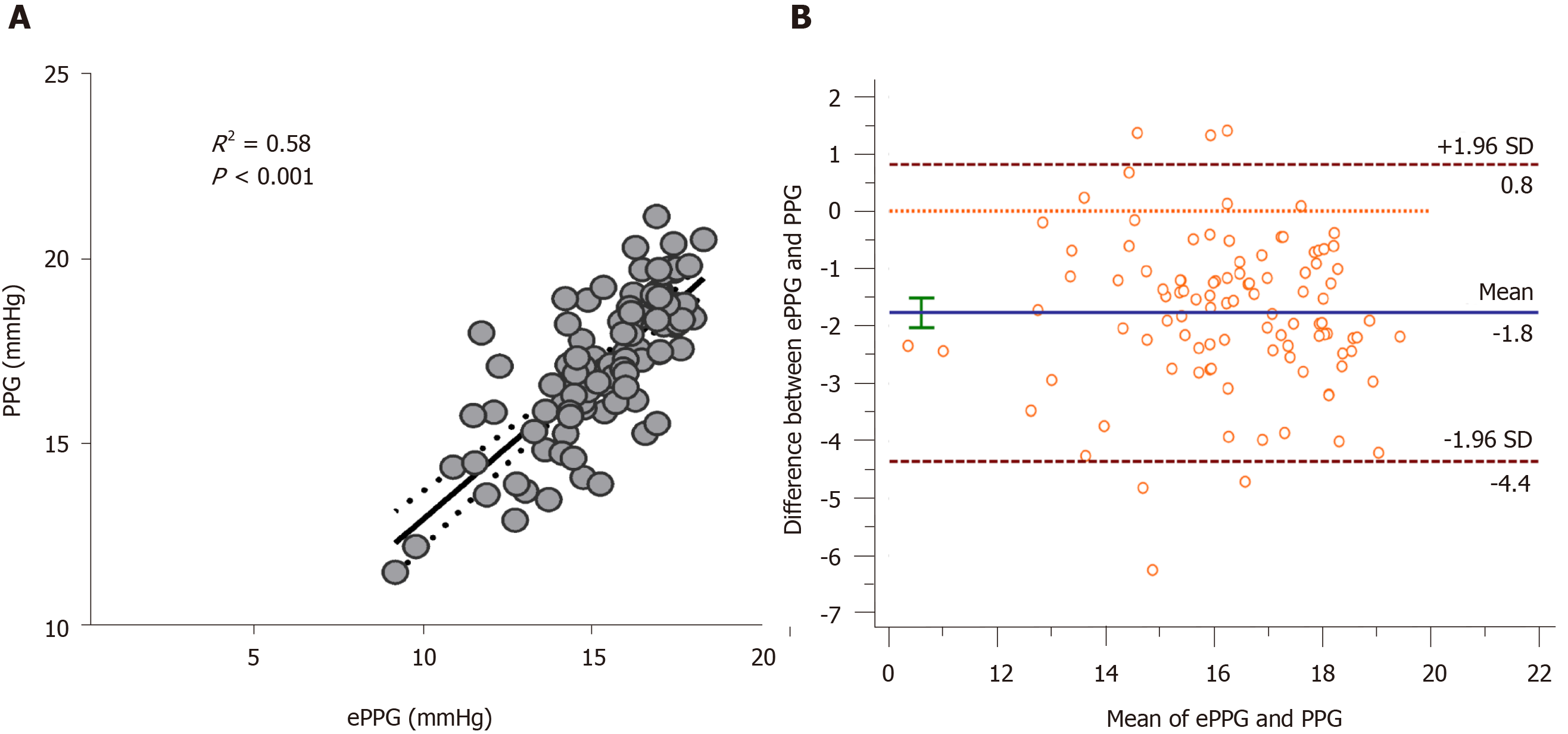
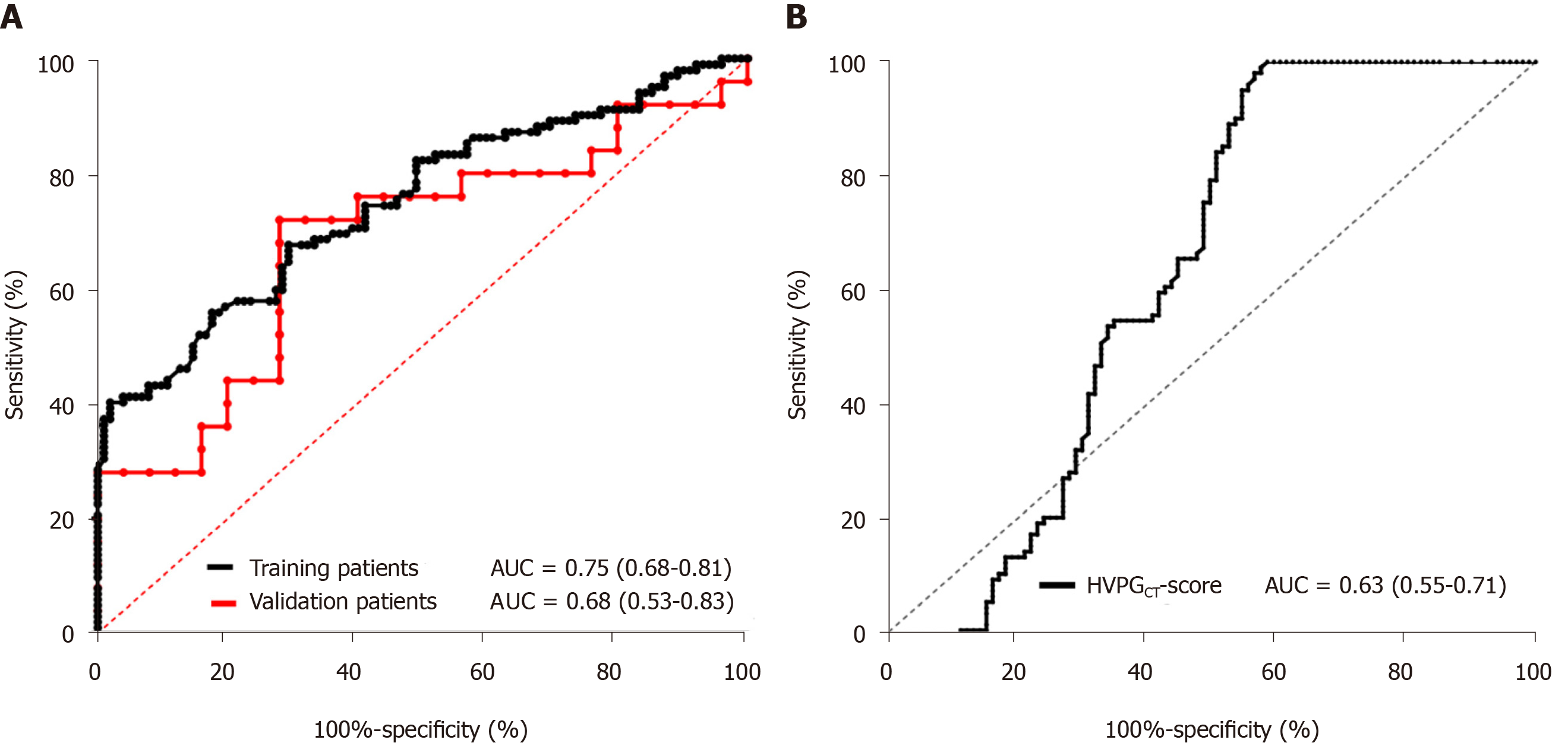
The mean estimated PPG using the predictive formula was 17.16 ± 1.92 mmHg (11.51-21.14 mmHg). There was a statistically significant correlation between the PPG score and PPG in overall participants (n = 102, R = 0.884, P < 0.001, Figure 2A). A similar result was achieved using the Bland-Altman plot (Figure 2B). The proposed PPG score was applied to the training patients, which confirmed its good accuracy with an AUC of 0.75 (0.68-0.81).
In addition, 20 patients were enrolled as the validation cohort, which included 12 with hepatic virus infection, 6 with alcoholic liver diseases, and 1 each with non-alcoholic liver disease and primary biliary cholangitis. The proposed PPG score was applied to the validation group and the results confirmed its good accuracy with an AUC of 0.68 (0.53-0.83, Figure 3A).
The CT-based HVPG score was applied to estimate HVPG, which confirmed its good accuracy with an AUC of 0.63 (0.55-0.71, Figure 3B). Compared with the estimated PPG formula proposed in this study, the power of the test was equivalent, but the ultrasound data in this study were relatively easy to obtain and there was no radiation damage during CT examination.
Currently, the golden standard for measuring portal hypertension and its severity is usually HVPG measurement[19,20]. Measuring this gradient is safe and relatively simple to perform, but it is invasive and costly. In this study, the PVV and PBF showed independent positive correlations with the PPG. Thus, we developed an US-based estimated PPG formula and further validated its performance in the non-invasive diagnosis of portal pressure in patients with HCC. As expected, the estimated PPG showed significant agreement with invasive PPG measurement.
Hepatic hemodynamic changes in patients with portal hypertension are often complicated. As a non-invasive method for assessing portal hypertension, Doppler US is economical, simple, and easy to repeat. Its development prospects are considerable. It is expected to become one of the development directions in the non-invasive diagnosis of portal hypertension. Some Doppler parameters have been proposed as candidate surrogates of the HVPG[21,22]. However, in validation studies, none of these parameters have proved to be accurate. A possible reason for this is that Doppler measurements can be influenced by many factors, such as respiration and vasoactive drugs, as well as by inter-observer and inter-equipment variability. However, measuring liver stiffness by ultrasound and dynamically detecting hemodynamic parameters can be used as non-invasive indicators for evaluating portal pressure and the presence or absence of portal hypertension[14]. Indeed, portal vein hemodynamics are predictive markers and lower velocity in the portal trunk in compensated cirrhosis is an indicator of decompensation[23]. As with any other vascular system, portal pressure is the product of two independent factors, namely, resistance to blood flow and amount of flow, as stated by Ohm’s law: Pressure = Resistance × Flow[24]. Liver stiffness measurement accurately reflects liver fibrosis in chronic liver diseases. However, the exact HVPG value cannot be reliably estimated by LSM (correlation R ranges from 0.59 to 0.70)[25].
In the present study, the combined measurements of the PVV and PBF were clinically and economically useful in distinguishing those patients who truly required further assessment for portal hypertension using more invasive and expensive procedures such as PPG determination. By comparing the calculated PPG with the actual PPG, a strong correlation was observed even though both the calculated PPG and the actual PPG were not always the same in each patient, and the calculated PPG was extremely accurate in the prediction of PPG (AUC = 0.75) in the training cohort. During the validation study, based on a cohort of 20 patients, the calculated score was slightly lower, but still showed good accuracy with an AUC of 0.68. In another study, the diagnostic accuracy of HVPG reached 0.83, but the non-invasive HVPG interpretation is relatively time-consuming (approximately 2.5 h per case)[26]. The formula can save time in each patient and may be used as a preliminary choice before the virtual evaluation of HVPG. However, based on the research conditions of this study, there may be the following restrictions when using this formula. The sample of this study is mainly the Chinese population. The cause of cirrhosis is mainly viral cirrhosis, which is different from the alcoholic cirrhosis in Western countries. When using this formula, we should consider the differences caused by different etiology.
There are several limitations to this study. Due to the limited sample size in this study, the detection index was also small, which affected the accuracy of the results to some extent. In future studies, prospective studies with a large sample size are required to increase the test indicators and identify indicators that can objectively and accurately reflect PPG. Despite the very good accuracies of the proposed model including PVV and PBF, a larger sample size may further improve the study power. A further external validation appears mandatory prior to potential wider clinical use.
In conclusion, PVV and PBF are independently and positively correlated with PPG, suggesting the usefulness of these parameters as non-invasive predictors of PPG. Monitoring of PVV and PBF may be clinically useful for the early detection and management of portal hypertension to distinguish those patients who require further invasive and expensive procedures such as PPG determination.
Portal pressure accurately predicts the risk of peri-operative morbidity and mortality in liver carcinoma. The limitations of HVPG measurement are that it is invasive and impractical for routine clinical practice. Thus, non-invasive measurement methods are urgently needed.
Doppler sonography offers real-time observation of blood flow with qualitative and quantitative assessments, and the application of microbubble-based contrast agents has improved the detectability of peripheral blood flow. The aim of this study was to clarify whether simple, non-invasive US parameters correlate with the invasive transjugular PPG measurement and to develop a formula to estimate PPG.
To investigate whether ultrasonography (US)-based portal pressure assessment could replace invasive transjugular measurement.
A cohort of 102 patients with HCC was selected (mean age: 54 ± 13 years, male/female: 65/37). Pre-operative US parameters were assessed by two independent investigators, and multivariate logistic analysis and linear regression analysis were conducted to develop a predictive formula for the portal pressure gradient (PPG). The estimated PPG predictors were compared with the transjugular PPG measurements. Validation was conducted on another cohort of 20 non-surgical patients.
The mean PPG was 17.32 ± 1.97 mmHg. Univariate analysis identified the association of the following four parameters with PPG: Spleen volume, portal vein diameter, portal vein velocity (PVV), and portal blood flow (PBF). Multiple linear regression analysis was performed, and the predictive formula using the PVV and PBF was as follows: PPG score = 19.336-0.312 x PVV (cm/s) + 0.001x PBF (mL/min). The PPG score was confirmed to have good accuracy with an area under the curve (AUC) of 0.75 (0.68-0.81) in training patients. The formula was also accurate in the validation patients with an AUC of 0.820 (0.53–0.83).
The formula based on ultrasonographic Doppler flow parameters shows a significant correlation with invasive PPG and, if further confirmed by prospective validation, may replace the invasive transjugular assessment.
The formula for the prediction of PPG should be verified on a larger and external validation cohort for widespread acceptance.
The investigators are grateful to all participants for their cooperation in the study.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmed M, Kreisel W, Sun WW, Tamori A S-Editor: Dou Y L-Editor: Wang TQ P-Editor: Wang LL
| 1. | Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut. 2014;63:844-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 929] [Cited by in RCA: 1106] [Article Influence: 100.5] [Reference Citation Analysis (1)] |
| 2. | Boleslawski E, Petrovai G, Truant S, Dharancy S, Duhamel A, Salleron J, Deltenre P, Lebuffe G, Mathurin P, Pruvot FR. Hepatic venous pressure gradient in the assessment of portal hypertension before liver resection in patients with cirrhosis. Br J Surg. 2012;99:855-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 3. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6569] [Article Influence: 469.2] [Reference Citation Analysis (1)] |
| 4. | European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4517] [Article Influence: 347.5] [Reference Citation Analysis (2)] |
| 5. | Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet. 2014;383:1749-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1139] [Cited by in RCA: 1311] [Article Influence: 119.2] [Reference Citation Analysis (0)] |
| 6. | Haj M, Rockey DC. Predictors of clinical outcomes in cirrhosis patients. Curr Opin Gastroenterol. 2018;34:266-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Abraldes JG, Sarlieve P, Tandon P. Measurement of portal pressure. Clin Liver Dis. 2014;18:779-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Suk KT. Hepatic venous pressure gradient: clinical use in chronic liver disease. Clin Mol Hepatol. 2014;20:6-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 9. | Stefanescu H, Procopet B. Noninvasive assessment of portal hypertension in cirrhosis: liver stiffness and beyond. World J Gastroenterol. 2014;20:16811-16819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Leung JC, Loong TC, Pang J, Wei JL, Wong VW. Invasive and non-invasive assessment of portal hypertension. Hepatol Int. 2018;12:44-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Procopeţ B, Tantau M, Bureau C. Are there any alternative methods to hepatic venous pressure gradient in portal hypertension assessment? J Gastrointestin Liver Dis. 2013;22:73-78. [PubMed] |
| 12. | Castera L, Pinzani M, Bosch J. Non invasive evaluation of portal hypertension using transient elastography. J Hepatol. 2012;56:696-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 13. | Thabut D, Moreau R, Lebrec D. Noninvasive assessment of portal hypertension in patients with cirrhosis. Hepatology. 2011;53:683-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Maruyama H, Yokosuka O. Ultrasonography for Noninvasive Assessment of Portal Hypertension. Gut Liver. 2017;11:464-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 15. | Moriyasu F, Nishida O, Ban N, Nakamura T, Sakai M, Miyake T, Uchino H. "Congestion index" of the portal vein. AJR Am J Roentgenol. 1986;146:735-739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 153] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Casado M, Bosch J, García-Pagán JC, Bru C, Bañares R, Bandi JC, Escorsell A, Rodríguez-Láiz JM, Gilabert R, Feu F, Schorlemer C, Echenagusia A, Rodés J. Clinical events after transjugular intrahepatic portosystemic shunt: correlation with hemodynamic findings. Gastroenterology. 1998;114:1296-1303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 315] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 17. | Groszmann RJ, Wongcharatrawee S. The hepatic venous pressure gradient: anything worth doing should be done right. Hepatology. 2004;39:280-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 390] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 18. | Iranmanesh P, Vazquez O, Terraz S, Majno P, Spahr L, Poncet A, Morel P, Mentha G, Toso C. Accurate computed tomography-based portal pressure assessment in patients with hepatocellular carcinoma. J Hepatol. 2014;60:969-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 79] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 19. | Chelliah ST, Keshava SN, Moses V, Surendrababu NR, Zachariah UG, Eapen C. Measurement of hepatic venous pressure gradient revisited: Catheter wedge vs balloon wedge techniques. Indian J Radiol Imaging. 2011;21:291-293. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 20. | Thalheimer U, Leandro G, Samonakis DN, Triantos CK, Patch D, Burroughs AK. Assessment of the agreement between wedge hepatic vein pressure and portal vein pressure in cirrhotic patients. Dig Liver Dis. 2005;37:601-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 21. | Baik SK, Kim JW, Kim HS, Kwon SO, Kim YJ, Park JW, Kim SH, Chang SJ, Lee DK, Han KH, Um SH, Lee SS. Recent variceal bleeding: Doppler US hepatic vein waveform in assessment of severity of portal hypertension and vasoactive drug response. Radiology. 2006;240:574-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 22. | Kim MY, Baik SK, Park DH, Lim DW, Kim JW, Kim HS, Kwon SO, Kim YJ, Chang SJ, Lee SS. Damping index of Doppler hepatic vein waveform to assess the severity of portal hypertension and response to propranolol in liver cirrhosis: a prospective nonrandomized study. Liver Int. 2007;27:1103-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 23. | Kondo T, Maruyama H, Sekimoto T, Shimada T, Takahashi M, Okugawa H, Yokosuka O. Impact of portal hemodynamics on Doppler ultrasonography for predicting decompensation and long-term outcomes in patients with cirrhosis. Scand J Gastroenterol. 2016;51:236-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Berzigotti A. Non-invasive evaluation of portal hypertension using ultrasound elastography. J Hepatol. 2017;67:399-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 25. | You MW, Kim KW, Pyo J, Huh J, Kim HJ, Lee SJ, Park SH. A Meta-analysis for the Diagnostic Performance of Transient Elastography for Clinically Significant Portal Hypertension. Ultrasound Med Biol. 2017;43:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 26. | Qi X, An W, Liu F, Qi R, Wang L, Liu Y, Liu C, Xiang Y, Hui J, Liu Z, Qi X, Liu C, Peng B, Ding H, Yang Y, He X, Hou J, Tian J, Li Z. Virtual Hepatic Venous Pressure Gradient with CT Angiography (CHESS 1601): A Prospective Multicenter Study for the Noninvasive Diagnosis of Portal Hypertension. Radiology. 2019;290:370-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |